Solar Pasteurization
Abstract
Heating water to a sufficiently high temperature for a certain period destroys harmful microorganisms; this process is often termed “pasteurization” after the scientist Louis Pasteur (1822–1895). Solar pasteurization is a well-known water purification technique in developing countries as it can be performed using only household objects and the heat of the sun. Devices can range from cheap, homemade devices that take multiple hours to pasteurize small amounts of water to expensive, complicated devices that can pasteurize larger amounts of water in shorter amounts of time.
3.1 Microbiology of Water Pasteurization
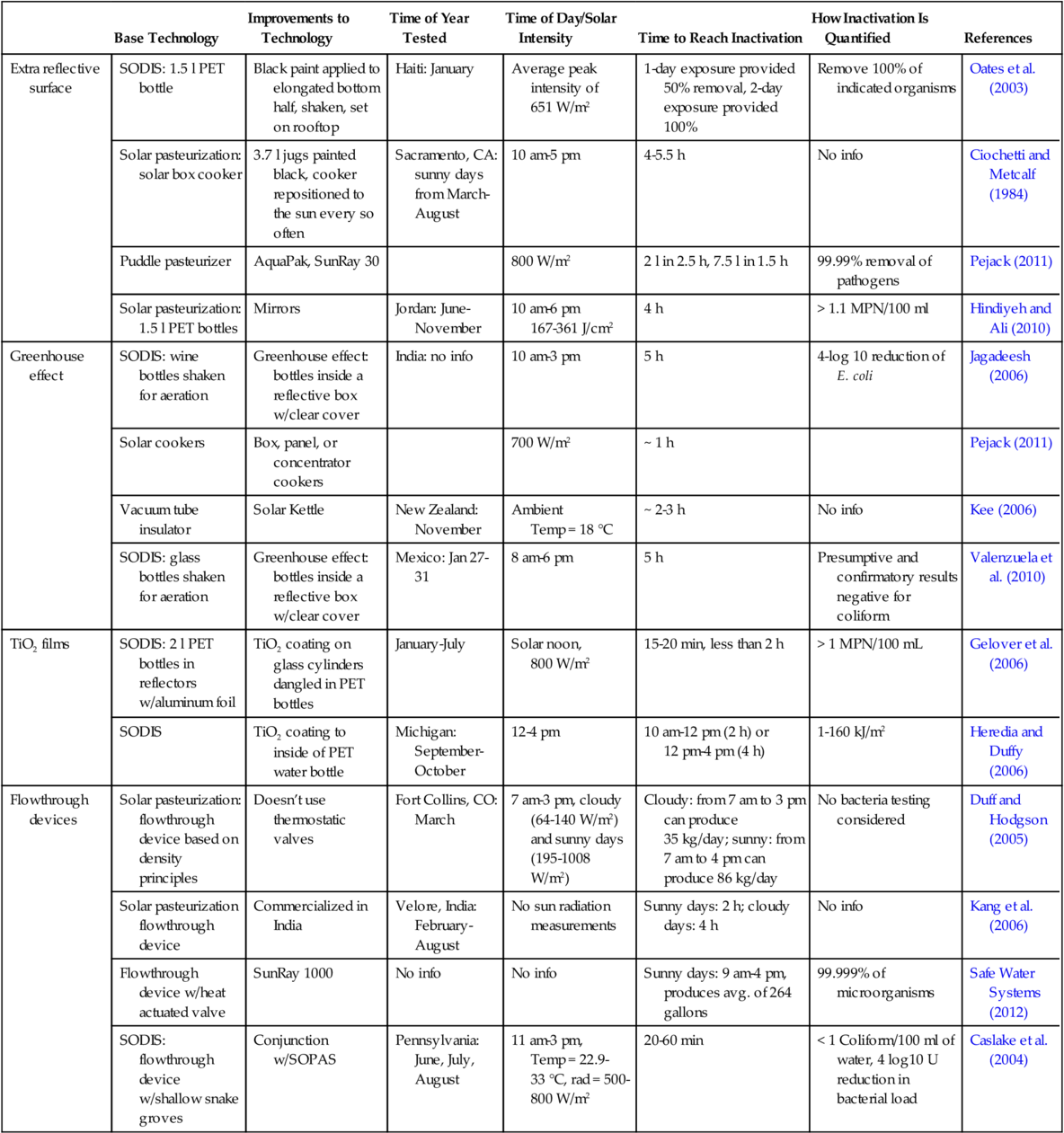
Heating water to a sufficiently high temperature for a certain period destroys harmful microorganisms; this process is often termed “pasteurization” after the scientist Louis Pasteur (1822-1895). The required temperatures and corresponding periods for the destruction of microbes are shown in Table 3.1 (Ciochetti and Metcalf, 1984). The D value is the time to kill 90% of the organisms. A time of 5D would kill 99.999% of the microbes.
Table 3.1
Effect of Temperature on Microbes (Ciochetti and Metcalf, 1984)
| Temperature | Microbes Destroyed | Time Required for Inactivation | Total Time for Pasteurization (h) |
| 55 °C (131 °F) | Worms, protozoa | 1 min | 4.1 |
| 60 °C (140 °F) | E. coli, rotavirus, Salmonella typhi, Vibrio cholera, Shigella | 1 min | 4 |
| 65 °C (149 °F) | Hepatitis A virus | 12 s | 4.5 |

At temperatures higher than those listed in Table 3.1, the D value decreases significantly; for example, for the microbes in Table 3.1 with a D-value of ~ 1 min at 60 °C, the D-value decreases to ~ 12 s at 65 °C. Also, considering that water as it heats toward 65 °C also spends time at 64 °C, 63 °C, and so on (at which temperatures additional fractions of microbes are killed), it is widely agreed that pasteurizing water to 65 °C will make it safe to drink. Many pasteurization devices use a safety factor of several degrees above 65 °C to allow for variations in measurement and equipment.
The time required to pasteurize water decreases exponentially with an increase in temperature (Feachem et al., 1983), as illustrated in the semi-log plot of time versus temperature shown in Figure 3.1.
It is a common misconception that water pasteurization requires boiling water (sometimes it is even stated that the water must boil for 20 min). Unnecessary boiling wastes a significant amount of fuel, which is already an expensive and scarce item in much of the world. Boiling water requires about twice the energy as heating it to 65 °C, plus the extra time for monitoring it and the time and expense of obtaining fuel. The idea that water must be boiled to be pasteurized may have arisen because it is easy to tell when water boils, and one can then be certain that the water has been heated above 65 °C.
Note that the type of pasteurization discussed in this chapter is concerned with the destruction of microbes only. Toxic chemicals, metal salts, and other contaminants are not removed by pasteurization. Insoluble material such as silt can be decreased by prefiltering or settling the water.
Pasteurization as discussed in this chapter is a thermal process, relying on the destruction of microbes by temperature (achieved with solar energy). A related but different water treatment concept is solar disinfection (acronym SODIS), where microbes are destroyed by the direct action of certain wavelengths of the solar spectrum, independent of temperature. It is the ultraviolet range of the spectrum (200-400 nm) that is most effective in destroying microbes; consequently, transparent containers that have good transmittance in this wavelength band should be used. The interested reader can find many reports of SODIS studies and trials, for example, Caslake et al. (2004) and Meierhoffer and Wegelin (2002).
Methods of solar pasteurization (SOPAS) discussed in this chapter include:
2. Bottles and reflectors
3. Commercially produced devices
4. Flowthrough devices
5. Devices that use the greenhouse effect
6. SOPAS used in conjunction with SODIS
7. SOPAS used in conjunction with titanium dioxide
3.2 Use of Solar Cookers for Drinking Water Production
There is a widely disseminated body of knowledge and literature on the subject of solar cooking and solar cookers, and several international conferences have been held on this subject in the past 20 years; proceedings of these conferences have been archived by Solar Cookers, International (Sacramento, CA). Because many foods are cooked in the range of 80-100 °C, it is not surprising that solar cookers are also used to pasteurize water at 65 °C.
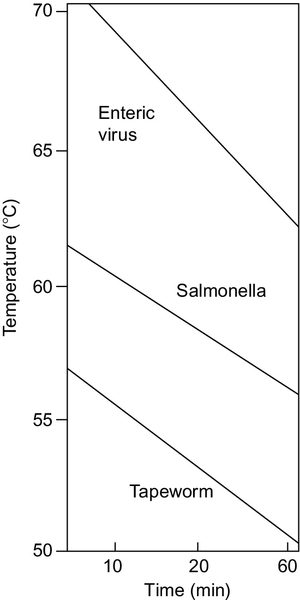
Perhaps the first type of practical solar cooker, the “box cooker” is commonly used around the globe. In its simplest form, it is a box insulated on its sides and bottom that has a transparent top cover. The box also frequently has a “booster reflector” at the top to augment the solar power entering the top cover, as shown in Figure 3.2a.

In recent decades, numerous analyses of box cookers have been made, and clever design attributes have been developed, which will not be discussed here. Some of the relevant design and operating parameters are:
• Optical properties of top cover
• Surface characteristic of box interior
• Booster reflectors
• Solar power, cloudiness
• Time (hour, day, month)
• Latitude
• Device orientation, tracking/nontracking
• Mass and type of food
• Pot size, surface, lid
• Wind, ambient temperature
Various box cookers are in use around the world; some are small and hold one pot, others hold more than 10 pots. Box cookers (Figure 3.2) have been made from cardboard (one of the first was the famous Kerr-Cole cooker), wood, metal, plastic, and mud. Thermal insulation for the box is necessary, and, again, very many materials have been used, including crumpled paper, air space, paper baffles, feathers, rice hulls, and foam.
The simplest way to use a box cooker to pasteurize water is to place a pot of water in the cooker as if cooking food. Often, a reasonably designed box (able to cook a variety of foods in 2 h) with a solar radiation of approximately 700 W/m2 can pasteurize a liter of water in about an hour.
Another popular cooker design, the “panel cooker,” consists of several flat reflective surfaces (panels) adjacent to a pot, which is enclosed in a transparent bag or enclosure. There is no insulated box; rather, the heat loss from the pot is minimized by the layer of hot air between the pot and the bag. Bags are commonly made of polypropylene film, but other transparent films have been used (such as polyethylene and nylon). The bag should not fit tightly on the pot; this prevents the bag from melting from the hot pot and prevents too much heat loss. If the bag has too large an air space, the insulating ability of the air space is decreased. An air gap of about 1-2 cm is recommended. Ideally, the bag’s material would have a high transmittance for solar radiation in wavelengths of 0.3-1 nm and low transmittance for thermal radiation in wavelengths of 5-14 nm (around 100 °C). Two types of panel cookers are shown in Figure 3.3.
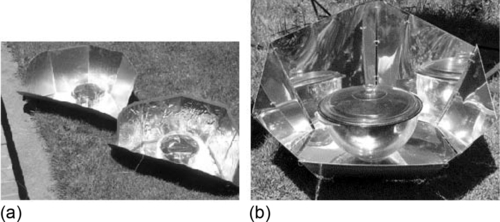
The panels are reflective surfaces that vary in number and orientation. The panels’ function is to reflect solar rays onto the bag, increasing the concentration and solar power to the bag (and the water pot). The number of panels ranges from 1 to a 12 or more, and the panels are usually flat planes for ease of construction and foldability; however, curved, hemispherical, and other modified panels have been employed.
A particular version of the panel cooker, the “CooKit” (Figure 3.3), developed by Solar Cookers International (SCI, 2009a,b), has the desirable attributes of low cost and weight, ease of construction, and foldability. It has seven flat panels when unfolded, and the front flap is adjusted upward to reflect sunlight on the pot or bag. One useful characteristic of this device is that it does not need to be re-aimed at the sun for several hours because the panels compensate for the relative solar motion. A pot can be filled with polluted water and, with sufficient solar radiation and time, the CooKit can pasteurize the water in approximately the same amount of time as the box cooker.
A large variety of panel cookers have been developed and produced in recent years. Design variations include the number and shape of the panels, overall size, type of container, materials used in construction, and method of folding/unfolding.
A third class of solar cooker, called a “concentrator,” concentrates the solar radiation much more than a box or panel cooker. This type uses parabolic surfaces (simple or compound), Fresnel lenses, or multiple aimed reflectors. Heat rates are much higher for concentrators than for box or panel cookers. Thus, users must be careful not to place their arms or face into the concentrated solar rays. Because the radiation to the pot is so high, a bag or cover around the pot is usually unnecessary. To use the reflective properties of parabolic surfaces most effectively, concentrators should be tracked to the sun more often than box or panel cookers. Examples of concentrators are shown in Figure 3.4.

3.3 Devices Designed Specifically for Water
While devices that are used for solar cooking can be used to pasteurize water, devices that are designed specifically for water treatment can be more efficient. These devices incorporate factors that minimize the time required to pasteurize water in their design.
The time required to pasteurize water is decreased by:
• Increasing solar input power by operating when the solar power is highest (e.g., around solar noon or at a sunny location) or by using reflectors to achieve concentration.
• Decreasing the mass of water in the device. If the initial mass is too great, the pasteurization temperature may not be achieved before the solar flux becomes too low.
• Decreasing the initial temperature difference between the pasteurization temperature (e.g., 65 °C) and the initial temperature of the polluted water. Starting with warmer water decreases the time required to pasteurize it.
• Minimizing heat loss from the water as it heats by using thermal insulation, paying attention to thermal radiation from the device, and avoiding windy locations.
This simple model of a pasteurizing device ignores the effect of water evaporation. If water vapor is allowed to escape the device, this can represent a huge energy loss, increasing the time to pasteurize the water. As noted above, minimizing the heat loss decreases the time required to pasteurize water. A very low loss factor can be achieved by vacuum insulation, which practically eliminates heat loss by convection. One of several such systems (Figure 3.5) is the Solar Kettle (Kee, 2006), where the water is contained in an insulated glass vacuum tube 0.75 m in length. The outer surface of the inner tube is coated with a selective material (such as aluminum nitrate) to create a selective surface (as shown in Figure 3.5), enhancing the absorbance of solar rays and minimizing re-radiation of thermal radiation.

3.4 Simple Devices from Common Materials
Surprisingly simple devices made from common materials can pasteurize small quantities of water in 1 h. Here we discuss simple, common, inexpensive pasteurization devices because, often, poor, distressed peoples do not have safe water nor do they have the resources to employ sophisticated, complex water treatment devices. Perhaps the simplest pasteurization device is a simple flat puddle of water with a transparent cover that is exposed to sunlight, with or without adjacent planar reflectors (Andreatta, 2009; Pejack et al., 1996).
Researchers obtained experimental results using this simple flat type of puddle; a transparent polypropylene bag to contain the water; and zero, one, or two adjacent vertical reflectors each made of 0.30 m × 0.30 m (1 ft × 1 ft) of aluminum foil. When two reflectors were used, they were oriented 90° to each other with the water between the reflectors. The size of the flat water puddle was 0.25 m × 0.41 m (10 in. × 16 in.), and the puddle rested on a foam surface to minimize heat loss from the bottom. The experiments were performed in June in Stockton, California, on a sunny day when the noon solar flux was 980 W/m2.
Andreatta (2009) describes an amazingly simple method to pasteurize the flat puddle, called the “sack pasteurizer.” Starting with a circular sheet of transparent plastic about 1 m in diameter, the plastic is bunched up to form a sack that holds 3 l of water and then tied closed. The sack is placed on various surfaces (black plastic on grass, foam, or bubble wrap, grass alone, black foam), which shapes the water into a somewhat flat puddle, and the sack is then covered with a separate sheet of transparent plastic similar to that which forms the sack. An air gap is left between the sack and the cover. Experiments at 40°N latitude (Ohio) in late August on a strong sunny day achieved pasteurization temperatures (Table 3.2).
Table 3.2
The Test Results of a Puddle Pasteurizer
| Time to Pasteurize (min) | Water Depth (cm) | Water Volume (l) | Reflectors |
| 25 | 0.40 | 0.41 | Two |
| 79 | 0.98 | 1.00 | Two |
| 54 | 0.98 | 1.00 | One |
| 109 | 0.98 | 1.00 | Zero |

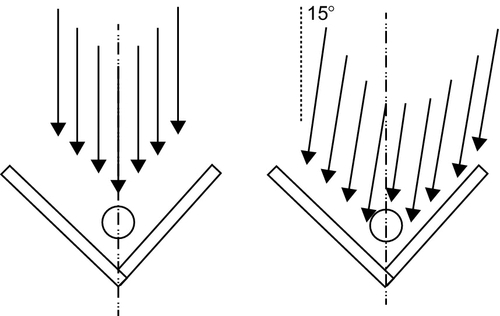
Another simple water pasteurizer can be made with easily obtained cylindrical bottles. The configuration consists of two vertical reflective surfaces of aluminum foil oriented 90° to each other, plus a third reflector on the ground (Figures 3.6 and 3.7). The total reflector area was 0.36 m2 (3.9 ft2).

The water containers used were:
• Bottle 1: 0.36 l glass, spray painted black • Bottle 2: 1.0 l soda (polyethylene terephthalate, PET), painted black • Bottle 3: 0.5 l brown glass beer bottle |

Transparent covers for the bottles were made in these ways:
• Cover 2: Two 2-l PET bottles slit around the middle, then one bottom half slit 6 × lengthwise and the other bottom half slid over the other • Cover 3: No cover |
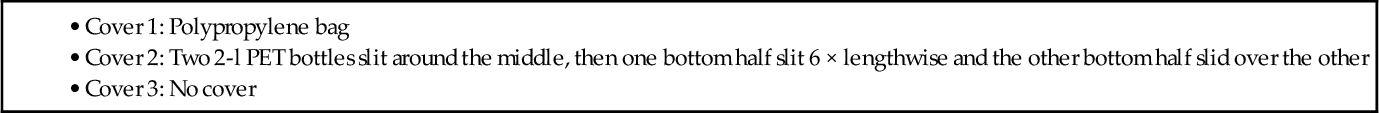
Results of the experiments (on the same day as the flat puddle experiment discussed above) were:
| Time to Pasteurize (min) | Container |
| 116 | Bottle 1, Cover 3 |
| 82 | Bottle 2, Cover 2 |
| 72 | Bottle 2, Cover 1 |
| 51 | Bottle 3, Cover 1 |
Sometimes a question is raised concerning whether exposing PET plastic bottles to sunlight could cause plasticizers to enter the water. Studies have indicated that SODIS devices using PET are safe with respect to human exposure to the plasticizers di(2-ethylhexyl)adipate (DEHA) and di(2-ethylhexyl)phthalate (DEHP) (Yegian and Andreatta, 1996).
Reflectors can also be used in conjunction with PET bottles. Research found that two 90° reflector planes could be oriented with an azimuth of 15°; then, for 2 h, the relative solar movement would result in the concentration decreasing from 3.6 to 3.0 for the first hour, then increasing from 3.0 back to 3.6 for the second hour, without any adjustment of the reflectors. In this case, the water cylinder was placed two bottle diameters from the intersection of the two reflectors. These studies suggest that 0.5-1 l of water may be pasteurized in 1-1.5 h using simple materials and simple geometry. Too often, large numbers of people such as refugees and victims of catastrophes are congested in a confined area with contaminated drinking water. In those situations, methods similar to those described above may be very advantageous. Travelers in remote regions where safe drinking water is unobtainable may also find these methods very useful.
Many variations of simple pasteurization devices have been developed using various configurations of water containers, covers, and reflectors (Cengel, 1998; Duff and Hodgson, 1999; Yegian and Andreatta, 1996).
3.5 Commercial Devices in Production
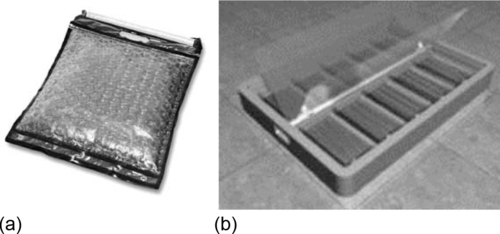
A form of the puddle-type pasteurizer described above is commercially produced as AquaPak® (Figure 3.8a), which is a small (0.35 m × 0.35 m), flat polyethylene plastic bag with one side made of transparent bubble pack insulation, and the other side black. Its ability to pasteurize water depends on the ambient temperature, solar power, and the quantity of water to be pasteurized (2-5 l). It is reported to have been used in 30 countries. When the solar radiation was 800 W/m2 and the initial water temperature was 25 °C, 2 l of water were pasteurized in 2.2 h. The lid of the AquaPak has a small, wax-filled capsule that indicates (by displacement of the melted wax) whether the water has been pasteurized. A larger capacity water pasteurization system, the SunRay 30 (Figure 3.8b), is a flat collector (79 cm × 61 cm × 9 cm) that contains 10 black bottles. It pasteurized 7.5 l of water in 1.5 h.
3.6 Devices with Recovery Heat Exchange
In the devices discussed above, pasteurized water is either removed at the pasteurization temperature or allowed to cool in the device for use later. The energy in the pasteurized water, once used to heat the water to pasteurization temperature, can be transferred to water not yet pasteurized, thereby requiring less solar energy to heat more water. Such a device uses a heat exchanger, which makes the device a flowthrough device, as opposed to a batch-process device. The savings in energy or the increase in quantity of pasteurized water can be quite significant.
The flowthrough pasteurizer works as follows: As shown in Figure 3.9, polluted water at ambient temperature T0 enters one side of the heat exchanger at the mass flow rate ṁ and exits the heat exchanger at the higher temperature T1, where it enters the solar device. In the solar device, solar power Qs enters and some power QL is lost to the ambient environment. The flowing water achieves pasteurization temperature Tp in the device, exits the device, and enters the other leg of the heat exchanger. The water flows counter-currently, transferring heat to the incoming stream, and finally exiting the heat exchanger at temperature T2, which is lower than Tp but slightly higher than T0.

A reader interested in heat exchanger theory, design, and analysis may consult any of the many references on the subject (Duff and Hodgson, 2005). Other analyses of pasteurizers with heat exchangers can be found in Stevens et al. (1998) and Duff and Hodgson (2005).
Whereas the batch-process pasteurizers function with a fixed mass of water, the mass flow rate in the flowthrough process can be adjusted during operation to account for the variable of solar power. The batch process in most cases uses a fixed mass of water, and, if set at too high a value or if the solar power decreases during heating, the entire batch may not be pasteurized. Similarly, if the solar power is higher than expected, the mass of water pasteurized could be less than is possible with a larger starting mass.
The flowthrough pasteurizer often uses a thermostatic valve, which opens and closes in response to temperature, to control the water flow rate. Some applications have adapted the thermostatic valve from the cooling systems of automobiles, with the notion that the valve would allow flow only when the temperature is above the pasteurization temperature.
The valves operate from closed to open over a temperature range, making the mass flow rate vary with temperature, opening more at the higher temperatures and closing more at the lower end of the range, sometimes with cyclic operation. This cyclic operation can lead to inefficiencies in the device as the valve is not able to successfully control the flow of the heat exchanger throughout the entire day. Changes in ambient temperature cause the valve to increase flow before water has been pasteurized or decrease the flow causing boiling and contamination issues. (Duff and Hodgson, 2005). Issues with thermostatic valves have led to research on more robust techniques for flow-through solar pasteurization.
Duff and Hodgson (1999, 2005) have designed and operated several versions of a heat exchanger pasteurization system in which the flow is controlled by the density difference between hot (pasteurized) water and colder (unpasteurized) water, rather than using valves. Heated water is directed to a vertical riser tube, and, when the water there is hot enough, the lower density (and higher water column) allows the pasteurized water to “spill over” and flow through the heat exchanger. The system eliminates the operation problems of thermostatic valves. Some of the performance data reported includes an average hourly production rate of 16.4 kg/h (1 kg water = 1 l water) for a 2-h period when the normal solar radiation averaged 913 W/m2 on a mostly sunny day. The production of pasteurized water for that entire day, from 8:00 am to 3:00 pm, was 86 l. Duff and Hodgson’s collector was a set of five evacuated heat pipes with a total area of 0.45 m2, which had a relatively low heat loss coefficient.
Many heat exchangers are the concentric tube or flat type (Figure 3.10). Yegian and Andreatta (1996) discuss their experiences with a concentric tube heat exchanger consisting of an inner and an outer annulus separated by a copper tube. The outer annulus contains wrapped wire to enhance the heat transfer. These authors also discuss a flat heat exchanger made with sandwich-like layers of copper sheets and wood. Rubber strips force the water to take a serpentine path to increase heat transfer. Heat transfer effectiveness values for the two designs were 60-80%, depending on the water mass flow rate. The mass of water pasteurized using these devices compared to the batch-process-type device was increased by a factor of eight. A system can be assembled using a solar collector originally designed for service water (e.g., for heating buildings) and adapting a counterflow heat exchanger. However, there are several disadvantages to using a flowthrough pasteurization device. For example, the thermostatic valve can break.

3.7 Water Pasteurization Indicators
A useful attribute of many batch-process solar water pasteurization devices is that once they are positioned in the sun, they may be left unattended. However, suppose that a device is left alone in strong sun, and the user returns late in the day. Did the water, now cool, reach pasteurization temperature during the day? Unless a recording thermometer has been installed, the temperature history of the water is unknown. What is needed is a sensor that can indicate, and preserve that indication, whether the pasteurization temperature was achieved during the day. Such an indicator could be based on a number of physical phenomena, such as:
1. Melting of a material from solid to liquid: A material that melts at the pasteurization temperature could be arranged so that once melted, it changes shape or location and maintains that change when the temperature later decreases.
2. Differential thermal expansion: Two materials with different coefficients of thermal expansion could be made to interact so that a detectable change in geometry is produced at the pasteurization temperature, and that change is maintained when the temperature later decreases.
3. Color change: Certain materials change color at specific temperatures. If the material changes color at a certain temperature, that could indicate the pasteurization temperature has been reached.
One must be aware that, in a volume of water, there will likely be temperature variations. Temperatures at the bottom of a container may be several degrees colder than at the top. Therefore, the temperature indicator should be placed at a lower level of the water. In cases where the container is heated from the bottom (as for example, in a concentrator), the spatial temperature variation may be quite different. In any particular situation, spatial temperature variations should be measured, and the indicators should be placed at the colder regions.
A device based on a material that melts from a solid to a liquid has been widely used in a variety of SOPAS devices, in several forms. The WAPI (water pasteurization indicator) (SCI, 2009a,b) is comprised of a small quantity of wax (soybean wax, for example, which melts at ~ 70 °C) inside a plastic (or glass) tube sealed at both ends, as shown in Figure 3.11. Initially, the wax is at one end of the tube. If the WAPI is suspended vertically with the wax end on top, then as the melting temperature of the wax is reached, the wax melts and flows by gravity to the lower end of the tube. Thus, finding the wax at the bottom indicates that the water has been pasteurized, even after the water has cooled. The WAPI can easily be reset for further use by inverting it. It has a flexible string and a weight so it can be suspended vertically in water. The WAPI is currently used in many locations worldwide.

The water temperature indicator shown in Figure 3.12 is based on differential thermal expansion of dissimilar metals and is called the Snap Disk (Saye and Pejack, 1994). It is constructed of two dissimilar stainless steel disks that are pressed together and bonded to form a 2.54-cm disk. At any temperature, the disk is concave on one side and convex on the other. As the temperature is raised and reaches the snap temperature, the disk snaps into reverse concavity (an example of what is called “snap buckling” in mechanics of materials). The disk remains in the new snapped position even when the temperature decreases below the snap temperature. The snap temperature is quite precise and repeatable over millions of cycles. Snap disks are manufactured in many countries. Because this is a relatively mature technology, disks can be made with any snap temperature and tolerance.

To use the snap disk, one snaps the disk to the starting position and places it in the water container. When the water is heated to the snap temperature, the disk automatically snaps to the opposite side. Then, at any time later, when the water has been cooled, the user can see if the disk has snapped and therefore tell if the water was pasteurized because the disk does not snap back when cooled. To reuse the disk, users push on the disk with their fingers to snap it back to the starting condition. The disk is fast acting, and, if lowered into water hotter than the snap temperature, it immediately snaps with a distinct audible “pop.”
Of course, the user must be able to distinguish between the two sides of the disk. In the Snap Disk shown, the disk was placed in a folded stainless steel housing that is open on one side and has a hole on the other side for manually snapping the disk, making the two sides of the disk readily discernible. The housing also prevents users from overstressing the disk when they manually reset it. The disk has a snap temperature of 68 °C with a manufacturer’s tolerance of 2.8 °C, but tests on samples showed the tolerance to be much less. In actual use, after a time the Snap Disk would experience some corrosion from being exposed to the water chemistry as well as the intimate contact of dissimilar metals. Therefore, the disk should be dried when not in use. The manufacturer also reports that other metal combinations could provide better corrosion resistance.
3.8 Multi-use Systems
In addition to the solar water pasteurization applications (~ 65 °C) described above, other uses for solar thermal devices include:
• Autoclaving for medical sterilization (> 122 °C)
• Producing distilled water for medical use
• Producing service hot water (50 °C)
• High temperature steaming and cooking (> 150 °C)
It is possible to create a multi-use system designed for several of the purposes listed above. Such a multi-use system, using solar collectors, heat exchangers, and associated valves and piping, conceivably could be more practical to design and construct than individual systems for all the uses given above. The higher temperatures needed would likely require low loss collectors (e.g., vacuum tube collectors) and solar concentrators, and the design and operation would be considerably more complex and expensive than simple pasteurization devices.
3.9 The Greenhouse Effect
Several SOPAS devices use the greenhouse gas effect (GHE) to create a more efficient heating process. A typical example of a SOPAS device is a water bottle filled with contaminated water and left in the sun for several hours. Sealing the water bottle inside a clear plastic bag uses the greenhouse effect to make the SOPAS process more efficient. Several authors have researched GHE devices that consist of lids made out of glass and sides made out of reflective materials to create a chamber to hold clear water bottles (Jagadeesh, 2006; Valenzuela et al., 2010). The glass lid traps heat inside the chamber, keeping the water bottles warm and protecting them from wind. See Figures 3.13 and 3.14 for examples of GHE devices. An advantage to using the GHE is that this technology can operate with lower intensity radiation, that is, on cloudy or winter days. PET bottles are generally used to pasteurize water because they are easily accessible in many rural areas, but empty wine bottles have also been used (Jagadeesh, 2006). While health concerns have been raised regarding the leaching of toxins into water from heating PET bottles using sunlight, Schmid et al. (2008) notes the health risk does not reach critical levels. The internal water temperature needs to exceed 66 °C for a certain period for inactivation of Hepatitis A (Kang et al., 2006). When this temperature is met, other bacteria and pathogens will also be inactivated. There are discrepancies in the research about how long inactivation temperatures must be sustained, varying from 1 min to more than 1 h (Jagadeesh, 2006; Kang et al., 2006; Valenzuela et al., 2010). Maintenance for this technology is minimal and includes cleaning the PET bottles or replacing the bottles when they become brown or scratched (Jagadeesh, 2006). GHE devices operate similarly to solar cookers although they are designed specifically to pasteurize water in bottles. Just as with solar cookers, a GHE device does not require skilled operators though the bottles require careful handling because improper water storage could lead to the growth of microbes (Kang et al., 2006). Costs of these devices were not discussed although all materials were noted to be accessible in developing countries and easily assembled.


3.10 Use of SOPAS in Conjunction with SODIS
SODIS uses the sun’s radiation (UV-A rays) rather than heat to inactivate microorganisms in water. The thermal and irradiation processes can be used in conjunction to make water treatment quicker and more efficient. SODIS is dependent on the intensity of solar radiation, a large surface area exposure, and shallow water depths. The effectiveness of SODIS is also dependent on low turbidity because particulates in water cause the sun’s rays to scatter. Using both SOPAS and SODIS creates a synergistic effect. Once the temperature 45 °C is surpassed, inactivation of microorganisms starts to occur; in SOPAS, temperatures need to be in the high 50 or 60 °C range. Maintaining a water temperature of approximately 50 °C requires only one-third of the typical solar irradiation intensity needed for inactivation (Oates et al., 2003). Because SODIS is dependent on the intensity of solar radiation, the material of the container used to purify the water is important. Some polymers can block UV-A rays, so the material needs to be a UV-transparent, colorless plastic or glass. Several small-scale technologies have been studied for using SODIS/SOPAS in conjunction, including water bottles (Hindiyeh and Ali, 2010), solar cookers (Saitoh and El-Ghetany, 2002), and specially adapted flowthrough devices (Caslake et al., 2004). A disadvantage to this procedure is that no indicator exists to show when disinfection has been achieved. The current indicator of disinfection is given as a length of time the water needs to irradiate depending on latitude and solar conditions. These general guidelines may not always be applicable to every situation. While SOPAS has various indicators for when the critical temperature is reached (including melting wax, shown in Figure 3.11, and popping metal, shown in Figure 3.12), no indicators have been developed to distinguish disinfected water from untreated water.
3.11 SODIS and Titanium Dioxide
The efficiency of SODIS can be improved through use of titanium dioxide (TiO2). TiO2 has been found effective in water purification because TiO2 works as an advanced oxidizer that operates in conjunction with the sun’s UV-A rays. A PET water bottle is suggested as a container. A TiO2 film can be created by acidifying TiO2 with HClO4; then the inside of the bottle can be coated with the TiO2 solution or the solution can be applied to glass rings that are then suspended in the bottle. In developing countries, coating the inside of the bottle is easier than coating glass rings. The TiO2 film makes the irradiation process more efficient when the water temperature is high and when there are high quantities of dissolved oxygen (DO) in the water. High temperatures can be achieved by placing the water container in direct sunlight and adding reflective surfaces. Shaking the PET bottle vigorously when three-quarters full and then completely filling the bottle can create the necessary high DO values. Low-turbidity water provides a clear medium for penetration of UV-A rays, so high-turbidity water should be filtered. The time recommended for sun exposure varies. Exposure time should be approximately 2 h when starting radiation in the morning on a sunny day. On cloudy days, exposure needs to last around 7 h (Heredia and Duffy, 2006). These times decrease when reflectors are added to the system (Gelover et al., 2006). While the length of needed sun exposure is still long, the TiO2 does decrease the time needed for inactivation compared to simple SODIS. Several advantages to TiO2 films exist apart from increasing the rate of inactivation. Adding a TiO2 film can oxidize other chemicals in the drinking water, such as arsenic. Another advantage of using TiO2 films is that there is no regrowth of bacteria after treatment and during storage. Although TiO2 can have advantages, several improvements still need to be made to the process. Acidifying the TiO2 can be dangerous for those who are not used to handling strong acids. Heredia and Duffy (2006) suggested that a medical technician in the town could be the one to handle the acid; however, future advances are needed to make this technology more manageable. A disadvantage of using a coating in a bottle is that the reaction rate of oxidation is limited because only the water close to the edge of the bottle interacts with the thin coating. Similar to SODIS, this method also has no indicator to tell the user when the water has been disinfected; such an indicator needs to be developed. No cost estimates were found for the use of TiO2, and there was no mention of how much powder would be needed for a community’s water treatment processes.
3.12 SOPAS and SODIS Technology Evaluation
A SOPAS system can have many forms, as seen from the above discussion, and some thought, analysis, and planning is required to decide what is appropriate, practical, and feasible for any particular situation.
When choosing a solar water pasteurization system, keep in mind that there is a trade-off between using commercially available devices and adapting raw materials to construct custom devices using the basic concepts outlined in this chapter. Commercial SOPAS devices have desirable attributes in that they are more likely perform as intended, have a history of operation, have a robust design, have clear operation procedures, and reduce the time needed for disinfection. However, because of variable local conditions, even well-developed commercial devices need to have some support from the manufacturers. Commercial devices may not be feasible in some cases because of purchase cost, transportation, and availability of spare parts. In those cases, a custom design and unique construction may serve the users better and may be more environmentally appropriate.
An example of a SOPAS system for sustainable drinking water is the Safe Water Project (Kenya, 2008), which was initiated by Solar Cookers, International (Sacramento, CA), and directed by Dr. R. Metcalf (SCI, 2009a,b). Among the important factors to consider are the end users, who can be:
• One or two persons (at home, hiking, or trekking)
• One family
• An extended family
• A village
• A commercial enterprise
SODIS is currently used by 4.5 million people in developing countries, the majority of those very low income (McGuigan et al., 2012). Although SODIS is recommended by the World Health Organization as a short-term emergency water treatment (McGuiellen et al., 2012), very few studies have actually assessed the use of SODIS during an acute emergency (Lantagne and Clasen, 2009). Alternatively, McGuiellen et al. (2012) argues that the effective use of SODIS requires behavioral changes that are only effective in the acute phase of an emergency. These behavioral changes include cleaning, filling, organizing, and collecting the SODIS water that must be drunk consistently to decrease diarrhea. It is easier to encourage behavioral changes during an emergency, but these changes are typically short lived and are not always sustainable in the long term. For areas that are already familiar with SODIS, it might be possible to effectively implement the technique in the short term and the long term.
Someone implementing a solar water pasteurization system needs to consider factors relating to the acute emergency phase response as well as continued, sustainable operation that is based on the needs of the area. For example, the availability of materials to construct additional devices or to repair and replace parts should be included in the evaluation. Finally, the end users need to have some training to ensure successful operation, repair, and evaluation of the system.
A summary of the technologies discussed above can be found in Table 3.3.
Table 3.3
Summary of Simple Solar Technologies
| Base Technology | Improvements to Technology | Time of Year Tested | Time of Day/Solar Intensity | Time to Reach Inactivation | How Inactivation Is Quantified | References | |
| Extra reflective surface | SODIS: 1.5 l PET bottle | Black paint applied to elongated bottom half, shaken, set on rooftop | Haiti: January | Average peak intensity of 651 W/m2 | 1-day exposure provided 50% removal, 2-day exposure provided 100% | Remove 100% of indicated organisms | Oates et al. (2003) |
| Solar pasteurization: solar box cooker | 3.7 l jugs painted black, cooker repositioned to the sun every so often | Sacramento, CA: sunny days from March-August | 10 am-5 pm | 4-5.5 h | No info | Ciochetti and Metcalf (1984) | |
| Puddle pasteurizer | AquaPak, SunRay 30 | 800 W/m2 | 2 l in 2.5 h, 7.5 l in 1.5 h | 99.99% removal of pathogens | Pejack (2011) | ||
| Solar pasteurization: 1.5 l PET bottles | Mirrors | Jordan: June-November | 10 am-6 pm 167-361 J/cm2 | 4 h | > 1.1 MPN/100 ml | Hindiyeh and Ali (2010) | |
| Greenhouse effect | SODIS: wine bottles shaken for aeration | Greenhouse effect: bottles inside a reflective box w/clear cover | India: no info | 10 am-3 pm | 5 h | 4-log 10 reduction of E. coli | Jagadeesh (2006) |
| Solar cookers | Box, panel, or concentrator cookers | 700 W/m2 | ~ 1 h | Pejack (2011) | |||
| Vacuum tube insulator | Solar Kettle | New Zealand: November | Ambient Temp = 18 °C | ~ 2-3 h | No info | Kee (2006) | |
| SODIS: glass bottles shaken for aeration | Greenhouse effect: bottles inside a reflective box w/clear cover | Mexico: Jan 27-31 | 8 am-6 pm | 5 h | Presumptive and confirmatory results negative for coliform | Valenzuela et al. (2010) | |
| TiO2 films | SODIS: 2 l PET bottles in reflectors w/aluminum foil | TiO2 coating on glass cylinders dangled in PET bottles | January-July | Solar noon, 800 W/m2 | 15-20 min, less than 2 h | > 1 MPN/100 mL | Gelover et al. (2006) |
| SODIS | TiO2 coating to inside of PET water bottle | Michigan: September-October | 12-4 pm | 10 am-12 pm (2 h) or 12 pm-4 pm (4 h) | 1-160 kJ/m2 | Heredia and Duffy (2006) | |
| Flowthrough devices | Solar pasteurization: flowthrough device based on density principles | Doesn’t use thermostatic valves | Fort Collins, CO: March | 7 am-3 pm, cloudy (64-140 W/m2) and sunny days (195-1008 W/m2) | Cloudy: from 7 am to 3 pm can produce 35 kg/day; sunny: from 7 am to 4 pm can produce 86 kg/day | No bacteria testing considered | Duff and Hodgson (2005) |
| Solar pasteurization flowthrough device | Commercialized in India | Velore, India: February-August | No sun radiation measurements | Sunny days: 2 h; cloudy days: 4 h | No info | Kang et al. (2006) | |
| Flowthrough device w/heat actuated valve | SunRay 1000 | No info | No info | Sunny days: 9 am-4 pm, produces avg. of 264 gallons | 99.999% of microorganisms | Safe Water Systems (2012) | |
| SODIS: flowthrough device w/shallow snake groves | Conjunction w/SOPAS | Pennsylvania: June, July, August | 11 am-3 pm, Temp = 22.9-33 °C, rad = 500-800 W/m2 | 20-60 min | < 1 Coliform/100 ml of water, 4 log10 U reduction in bacterial load | Caslake et al. (2004) |