4
Design of Multiproton‐Responsive Metal Complexes as Molecular Technology for Transformation of Small Molecules
Shigeki Kuwata
Tokyo Institute of Technology, School of Materials and Chemical Technology, Department of Chemical Science and Engineering, 2‐12‐1‐E4‐1 Ookayama, Meguro‐ku, Tokyo, 152‐8552, Japan
4.1 Introduction
The highly efficient and selective catalysis by metalloenzymes in nature relies on the integrated functional groups in their active site. The metal and surrounding peptide residues and other prosthetic groups cooperate in substrate binding, activation, and transformation through multiple weak, noncovalent interactions including hydrogen bonds [1]. Such sophisticated biological catalysis has motivated considerable efforts of synthetic chemists to design new polydentate ligands that allow to locate proton‐responsive sites at appropriate positions around the metal center [2, 3]. These ligands are directed not only to emulate the structures and functions of the metalloenzymes but to develop novel man‐made catalysts. This chapter aims to discuss the future prospects of multiproton‐responsive complexes thus designed as a molecular technology for chemical transformation of inert molecules, emphasizing contributions from our research group. First, some representative biological systems, where the participation of the functional groups around the metal center is evident or suggested in their catalysis, are portrayed. The following sections are dedicated to the synthesis and reactivities of bioinspired complexes with two or more proton‐responsive sites fixed around the metal by rigid chelate scaffolds such as pincer‐type and tripodal ligands. Chemical transformations with this class of compounds are also described.
4.2 Cooperation of Metal and Functional Groups in Metalloenzymes
The active site of metalloenzymes generally contains versatile proton‐responsive units ligating to the metal or residing in the proximity of the metal without direct bonding (i.e. in the second coordination sphere). In most cases, these functional groups are essential components in the catalysis. This section briefly describes the catalytic roles of the proton‐responsive functional groups in some representative metalloenzymes.
4.2.1 [FeFe] Hydrogenase
Hydrogenases are metalloenzymes that transform protons into molecular hydrogen reversibly at remarkable reaction rates for using hydrogen as a source of low‐potential electrons [4, 5]. Hydrogenases are classified by the metal composition in their active site; [FeFe] hydrogenases feature an azadithiolato‐bridged FeFe core with a dangling Fe4S4 cubane‐type unit ( Scheme 4.1). In the catalytic cycle, the Fe atom distal to the cubane unit in Hox first undergoes coordination of dihydrogen. Subsequent heterolytic cleavage of the H−H bond would be assisted by the Brønsted basic amine moiety, which is placed appropriately in the second coordination sphere thanks to the chelate structure. Finally, protons and electrons are released to regenerate Hox. As each step including the proton shuttling with the pendant amine is reversible, both H2 production and H2 oxidation are catalyzed by the [FeFe] hydrogenases, which are often more active in H2 production.
![Schematic catalytic cycle proposed for [FeFe]-hydrogenases.](http://images-20200215.ebookreading.net/4/1/1/9783527341634/9783527341634__molecular-technology-volume__9783527341634__images__c04h001.jpg)
Scheme 4.1 Catalytic cycle proposed for [FeFe] hydrogenases.
4.2.2 Peroxidase
Peroxidases are heme‐containing enzymes that catalyze oxidation of various substrates with hydrogen peroxide, a by‐product from aerobic metabolism [6–8]. A currently accepted mechanism is depicted in Scheme 4.2. The uncoordinated, distal histidine operates as a Brønsted acid‐base catalyst to promote proton migration in the coordinated hydrogen peroxide (1 to 2). Following heterolytic cleavage of the O−O bond leads to the formation of a high‐valent oxo intermediate known as compound I, which then oxidizes the substrate. This step can be recognized as a proton‐coupled electron transfer (PCET) from the histidine and heme to the hydrogen peroxide substrate. The arginine residue in the second coordination sphere additionally facilitates the coordination of hydrogen peroxide and stabilizes the intermediate 2 and compound I through hydrogen bonds. The mechanism nicely illustrates the participation of plural protic groups in the chemical transformation without their direct coordination.

Scheme 4.2 Mechanism of peroxidases.
4.2.3 Nitrogenase
While industrial production of ammonia has been performed by the Haber‐Bosch process at high temperature and pressure, nitrogenase enzymes in nature fix atmospheric nitrogen at room temperature according to Scheme 4.3a, in a catalytic manner [9, 10]. The molybdenum‐dependent nitrogenases contain an Fe7MoS9C cluster unit known as the FeMo cofactor (FeMo‐co) in their active site ( Scheme 4.3b). Although the mechanism of the reduction of N2 to ammonia on the FeMo‐co is not yet fully understood, initial binding of N2 to Fe sites is strongly implicated. A leading mechanism recently proposed is illustrated in Scheme 4.3c [11]. Consecutive fourfold e−/H+ transfer to the FeMo‐co first takes place to give the E4 intermediate having two bridging hydrides and two protic hydrosulfido ligands. Subsequent reductive elimination of H2 would generate an electron‐rich iron center, which binds dinitrogen through π‐back donation. The π‐acidity of the N2 ligand may be further enhanced by the neighboring hydrosulfido protons, and at the same time, the π‐back donation increases the Brønsted basicity of the N2 ligand. Ultimately, PCET from the FeMo‐co to chemically inert dinitrogen occurs to afford the diazene intermediate E4(N2H2). This species would further undergo multiple e−/H+ transfer to produce ammonia as the final product. This mechanism implies the significance of the metal‐ligand cooperation to avoid high barriers on the reaction coordinate, which are often encountered in challenging chemical transformations such as dinitrogen reduction.

Scheme 4.3 (a) Enzymatic nitrogen fixation. (b) Structure of FeMo‐co in molybdenum‐dependent nitrogenases. (c) Proposed mechanism for reduction of N2 on FeMo‐co. E n denotes the FeMo‐co, where n gives the number of electrons transferred.
4.3 Proton‐Responsive Metal Complexes with Two Appended Protic Groups
Multidentate ligands furnished with two or more proton‐responsive pendant groups effectively introduce multifunctionality around the metal, which is found in the active site of metalloenzymes. The numbers, acidity, and relative orientation of the appended functional groups built in the ligand should greatly affect the properties of the crafted molecular architecture. This section focuses on bidentate and tridentate ligands furnished with two protic pendant groups. Protic pyrazole [12, 13] and 2‐hydroxypyridine [14, 15] are mainly discussed as the appended functional group because these units with some resemblance to biomolecules can locate a reasonably acidic hydrogen atom in the second coordination sphere. In addition, their delocalized π‐system would allow a facile and bidirectional proton transfer that exerts a significant impact on the metal‐centered reactivity through smooth switching between a dative L‐type and monoanionic X‐type modes ( Scheme 4.4). The metal‐ligand cooperating reactivities of the proton‐responsive complexes are also described.

Scheme 4.4 Reversible deprotonation of pyrazole and hydroxypyridine ligands.
4.3.1 Pincer‐Type Bis(azole) Complexes
The meridional, tridentate pincer‐type ligands have been used as templates for predictable construction of rigid coordination environments with notable stability [16]. 2,6‐Bis(pyrazol‐3‐yl)pyridines 3, which furnish two proton‐responsive pendant arms, are thus attractive modules to construct a metal‐ligand cooperating platform [17–19].
Thiel [20] and we [21] independently demonstrated that the bis(pyrazolyl)pyridines 3a and 3b reacted with [RuCl2(PPh3)3] to give the pincer‐type complexes 4 with two protic NH groups at the position β to the metal ( Scheme 4.5a). Crystallographic study revealed the presence of multiple hydrogen bonds depicted in Scheme 4.5b, suggesting the Brønsted acidic nature of the NH groups in the second coordination sphere. Interestingly, the analogous reaction of the protic pincer‐type ligand 3c without substituents at the 5‐positions of the pyrazole rings affords the dinuclear complex 5, where two molecules of 3c bridge the two metal atoms with an unexpected tautomeric form. The selective formation of 5 may be rationalized by the reduced steric hindrance at the pyrazole nitrogen atoms distal to the pyridine as well as the increased numbers of intramolecular NH⋯Cl and NH⋯pyridine hydrogen bonds.
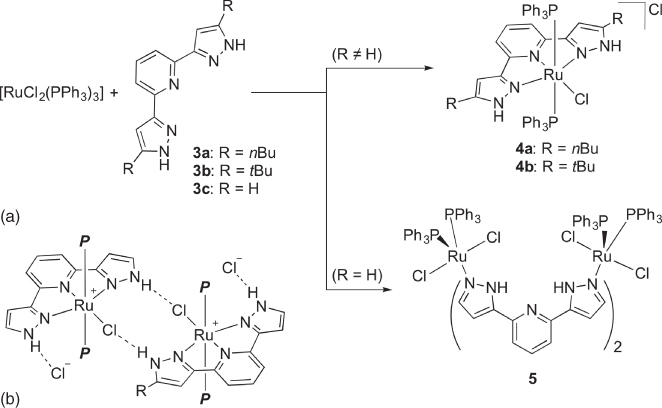
Scheme 4.5 (a) Synthesis of ruthenium complexes 4 and 5 bearing protic NNN pincer‐type ligands 3. (b) Hydrogen bond interactions in protic pincer‐type complexes 4. P = PPh3.
Complexes 4 and their derivatives catalyze hydrogenation [20, 22] and transfer hydrogenation [20, 23, 24] of acetophenone. Direct participation of the NH groups in the catalysis, however, remains debatable because an N‐allylated homologue of 4a also exhibits catalytic activity toward transfer hydrogenation [25]. Nevertheless, some stoichiometric reactions have clearly substantiated the proton‐responsive nature of 4. Thus, reversible deprotonation of the cationic complex 4b has been achieved by a weak base to give the uncharged pyrazolato‐pyrazole complex6 ( Scheme 4.6) [21]. Even twofold deprotonation of 4b in methanol takes place to afford the bis(pyrazolato) methanol complex 7. X‐ray analysis of 7 has disclosed that hydrogen bonds including a co‐crystalized methanol molecule in the second coordination sphere stabilize the methanol coordination, as illustrated in Scheme 4.6. Still the methanol ligand is so labile that it readily replaced by O2 and even N2 to yield the side‐on peroxo complex 8 and end‐on dinitrogen complex 9, respectively. Coordination of a weak π‐accepting ligand N2 suggests the increased electron donation of the proton‐responsive ligand upon deprotonation, although the high N−N stretching frequency of 2160 cm−1 of 9 implies that the π‐back donation is insufficient for further reductive transformation of N2.
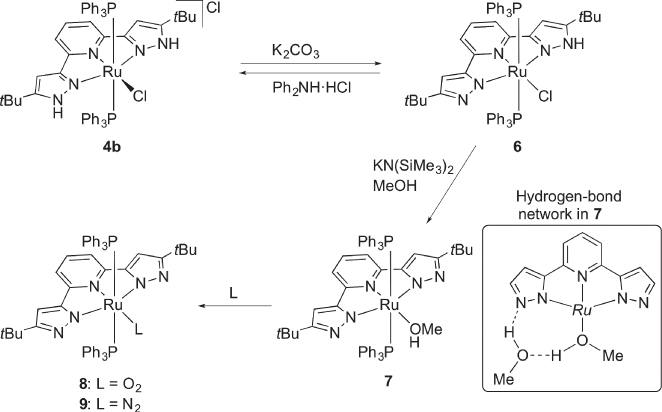
Scheme 4.6 Reversible deprotonation and subsequent transformations of protic pincer‐type complex 4b. Ru = Ru(PPh3)2.
The stoichiometric deprotonation reactions described above show that the pincer‐type bis(pyrazolyl)pyridine ruthenium complexes 4 provide two protons outward. Given the increased electron density of the deprotonated complex, the proton transfer to the substrate in the second coordination sphere may well be coupled with electron transfer from the metal center to the substrate, as in the PCET process proposed in the nitrogenase catalysis (vide supra).
We have recently demonstrated that the iron complex 10a bearing the bis(pyrazolyl)pyridine ligand 3b catalyzes the N−N bond cleavage of a nitrogenase substrate, hydrazine ( Scheme 4.7) [26]. The reactions with the mono‐ and dimethylated homologues 10b and 10c produce less than stoichiometric amounts of the product, implying the significance of the NH groups provided by the multiproton‐responsive pincer‐type ligand 3b. The plausible mechanism for the catalytic disproportionation is shown in Scheme 4.8. The pyrazole NH group in the second coordination sphere facilitates heterolytic N−N bond cleavage of the coordinated hydrazine in 11 through a hydrogen bond to afford the Fe(IV) amido species 12 and ammonia. This process can be compared with the O−O cleavage through PCET in peroxidases (vide supra). The second pyrazole NH group in the protic pincer ligand operates as an acid‐base catalyst to promote substitution of the amido ligand in 12 by the hydrazine to give the hydrazido(1–) species 13. Subsequent PCET from the hydrazido ligand to the pyrazolato Fe(IV) unit would afford the Fe(II) diazene complex 14. The phenyldiazene complex 14b has been actually isolated when phenylhydrazine (R = Ph, R′ = H) was used as the substrate. The crystal structure of 14b revealed the phenyldiazene ligand benefits from stabilization by a hydrogen‐bonding network with the two pyrazole NH units and counteranion. On the other hand, the reaction of 1,1‐diphenylhydrazine (R = R′ = Ph) with 10a, wherein the distal hydrogen for the PCET is unavailable, yields the hydrazinophosphonium salt 15 as a result of reductive elimination from 13. Finally, substitution of the diazene ligand in 14 by hydrazine completes the catalytic cycle, and the free diazene immediately undergoes bimolecular disproportionation into dinitrogen and hydrazine. This biorelevant catalysis by the multiproton‐responsive complex 10a provides insights into the catalytic role of the two well‐positioned NH groups in the second coordination sphere, including participation in bidirectional PCET.

Scheme 4.7 Catalytic disproportionation of hydrazine with protic pincer‐type iron complex 10a.
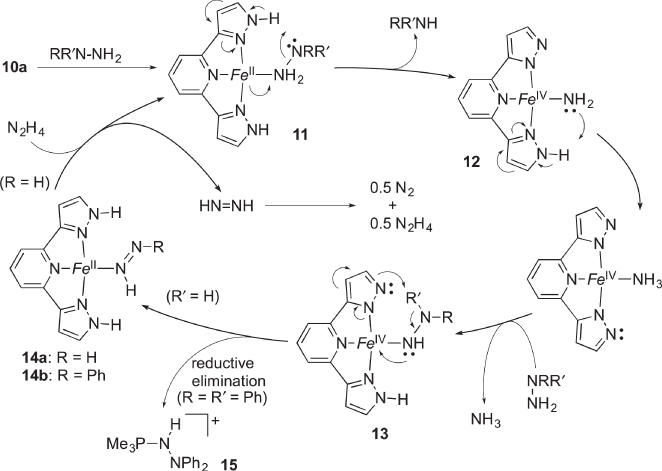
Scheme 4.8 Proposed mechanism for catalytic disproportionation of hydrazine with protic pincer‐type complex 10a. Fe = Fe(PMe3)2 2+. The tert‐butyl groups in the pincer ligand have been omitted in the mechanistic scheme.
Thanks to the modularity of the pincer‐type ligand [16, 27], unsymmetrical pincer‐type complexes bearing two different protic pendants have been developed and their proton‐responsive character has been evaluated. The protic pincer‐type complexes 17, which contain a protic pyrazole and isoelectronic N‐heterocyclic carbene (protic NHC [12, 13, 28, 29]) arms, are synthesized by chelation‐assisted formal tautomerization of the imidazole proligands 16 ( Scheme 4.9) [30, 31]. Complexes 17 undergo reversible deprotonation upon treatment with an equimolar amount of a base, giving the pyrazolato‐NHC complexes 18. The selective loss of the pyrazole proton in 17, which has been confirmed unambiguously by the 1H NMR spectra with the aid of 15N‐labeling and X‐ray analysis, indicates that the protic pyrazole is more Brønsted acidic than the protic NHC pendant.
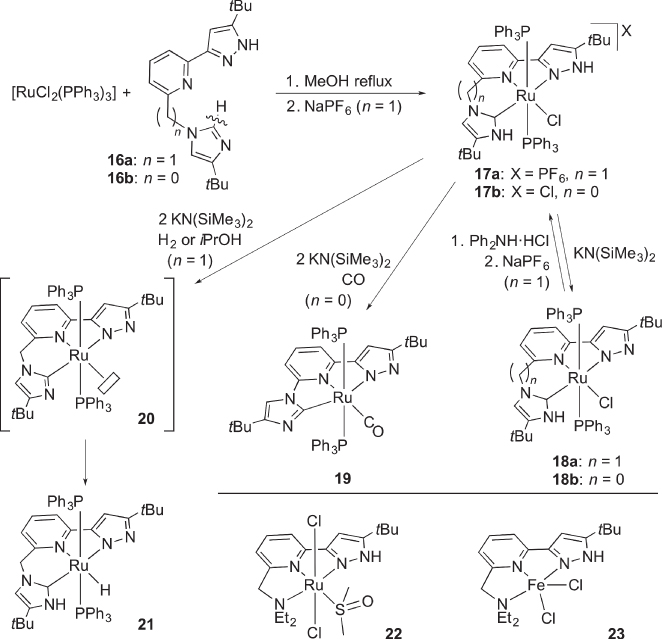
Scheme 4.9 Synthesis and reactivities of unsymmetric, multiproton‐responsive pincer‐type complexes.
Twofold deprotonation of the five‐membered chelate complex 17b under carbon monoxide yields the pyrazolato‐imidazolyl carbonyl complex19 ( Scheme 4.9). Comparison of the CO stretching frequencies between 19 and the related bis(pyrazolato) complexes derived from 4b shows that the imidazolyl unit is more electron‐donating than the pyrazolato group. On the other hand, the six‐membered chelate complex 17a reacted with a base in the presence of H2 or 2‐propanol to afford the hydrido complex 21 most likely through metal‐ligand cooperating hydrogenation or hydrogen transfer to the coordinatively unsaturated pyrazolato‐imidazolyl complex 20 [32, 33].
We have also synthesized the unsymmetrical protic pincer‐type ruthenium and iron complexes 22 and 23 featuring a tertiary amine pendant [22]. The amino group does not appear to operate as a hemilabile, proton‐responsive site because its dissociation in the ruthenium complex 22 is not observed even at an elevated temperature in the 1H NMR criteria. Complex 22 catalyzes hydrogenation of acetophenone, although the catalytic activity is lower than that of the bis(pyrazole) complex 4b.
Pyrazole‐assisted cyclometalation is also applicable for the synthesis of protic NCN pincer‐type complexes, which feature the electron‐donating central aryl group. The reactions of 1,3‐bis(pyrazolyl)benzenes 24 with [RuCl(OAc)(PPh3)3] result in selective C−H metalation at the 2‐position of the central benzene ring to afford the NCN pincer‐type ruthenium complexes 25 ( Scheme 4.10) [24]. The iridium complex 26 is obtained in a similar manner. The stronger trans influence of the monoanionic NCN pincer‐type ligand is evidenced by the longer M‐Cl distance in comparison with those in the NNN pincer complex 27. The enhanced electron donation of the pincer ligand also enables facile conversion of the ruthenium complexes 25 to the carbonyl complexes 28. In addition, comparison of the CO stretching frequencies with the NNN analogues indicates the increased electron density in the NCN pincer‐type complexes.

Scheme 4.10 Synthesis of protic NCN pincer‐type complexes.
4.3.2 Bis(2‐hydroxypyridine) Chelate Complexes
A 2‐hydroxypyridine moiety is known to exist in the active site of [Fe] hydrogenases, which catalyze only H2 heterolysis without redox, and to be involved in H2 activation as a cooperating unit as in the azadithiolato ligand in [FeFe] hydrogenases (vide supra) [4, 5, 14]. In parallel with the recognition, a number of complexes containing 2‐hydroxypyridine derivatives have been developed as metal‐ligand bifunctional catalysts [14, 15].
As a (2‐hydroxypyridine)‐based multiproton‐responsive complex, Papish and coworkers reported the half‐sandwich‐type ruthenium complex 29 bearing a 6,6′‐dihydroxy‐2,2′‐bipyridine (6‐DHPB) ligand ( Scheme 4.11) [34]. The two OH groups are free from coordination but engaged in hydrogen bonds with the counteranion and solvated methanol molecule in the crystal. Complex 29 catalyzes transfer hydrogenation of acetophenones with 2‐propanol. Notably, the protic complex 29 exhibits a catalytic activity superior to those of the aprotic dimethoxy derivative 30 and unsubstituted bipyridine complex in transfer hydrogenation with sodium formate in MeOH–H2O (1 : 9), implicating a significant role of the proton‐responsive sites in the catalysis.

Scheme 4.11 Synthesis of N,N‐chelate bis(hydroxypyridine) complexes 29 and 31.
Independently, Fujita and Yamaguchi published the isoelectronic iridium complex 31 [35] as a logical extension of their intensive studies on the catalysis of mono(hydroxypyridine) iridium complexes ( Scheme 4.11). They demonstrated that 31 and the doubly deprotonated complex 32 [36] catalyze dehydrogenative oxidation of primary and secondary alcohols to the corresponding carbonyl compounds without base additives. The proposed mechanism for the acceptorless alcohol dehydrogenation by the much superior catalyst 32 (TON of up to 275 000) is shown in Scheme 4.12 [37]. After dissociation of the aqua ligand in 32, the resultant coordinatively unsaturated intermediate 34 undergoes a hydride transfer from the substrate alcohol coupled with a proton transfer to the pyridonato oxygen in the second coordination sphere (35), which gives the hydrido‐hydroxypyridine complex 36. Owing to the assistance of an external alcohol to the proton shift (37), the dihydrogen complex 38 would be formed. Finally, liberation of hydrogen gas regenerates 34. In line with the mechanism, the 4,4′‐dihydroxy‐2,2′‐bipyridine (4‐DHPB) complex 33 without proton‐responsive sites in the second coordination sphere exhibits lower catalytic activity [35]. Fujita, Yamaguchi [38, 39], and others [40, 41] have further applied this class of multiproton‐responsive complexes for various dehydrogenative transformations of alcohols and amines.

Scheme 4.12 Proposed mechanism for catalytic dehydrogenative oxidation of benzyl alcohol with bis(pyridonato) complex 32.
Since their pioneering work on hydrogen storage using formic acid and formate by 4‐DHPB complexes [42], Himeda and coworkers have extensively investigated the catalysis of hydroxypyridine chelate complexes [43, 44]. Hull, Himeda, and Fujita disclosed that the tetrahydroxybipyrimidine‐bridged dinuclear iridium complex 39 containing both 4‐ and 6‐DHPB motifs catalyzes dehydrogenation of formic acid under acidic conditions ( Scheme 4.13) [45]. Meanwhile, the reaction in the reverse direction, hydrogenation of CO2 to formate, is catalyzed by the deprotonated form 40 under basic conditions. Following these findings, Himeda and Fujita investigated a series of 6‐DHPB complexes capable of hydrogenation of CO2 and dehydrogenation of formic acid, as mononuclear prototypes of the excellent catalysts 39 and 40. Schemes Scheme 4.14 illustrates the proposed mechanism for the catalytic dehydrogenation of formic acid with [Cp*Ir(6‐dhpb)]2+ (the cationic part of 31) [46]. The mechanism quite resembles that for the dehydrogenative oxidation of alcohols shown in Scheme 4.12. The coordinatively unsaturated intermediate 34 generated by deprotonation undergoes hydrogen transfer from formic acid with the aid of the pyridonato oxygen atom in the second coordination sphere, which affords the hydrido complex 41 along with CO2. Subsequent water‐assisted proton relay leads to the formation of H2 and 34. The reverse reaction, CO2 hydrogenation catalyzed by the bis(pyridonato) complex 32, would take place in a similar manner [46]. Quite recently, the role of alkali metal in the CO2 hydrogenation as well as the absence of significant outer‐sphere interaction in the formic acid dehydrogenation under acidic conditions has been proposed for these catalyst systems [47].
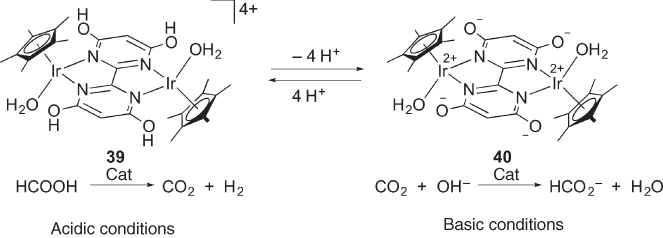
Scheme 4.13 Dehydrogenation of formic acid and hydrogenation of CO2 catalyzed by tetrahydroxybipyrimidine complexes 39 and 40.
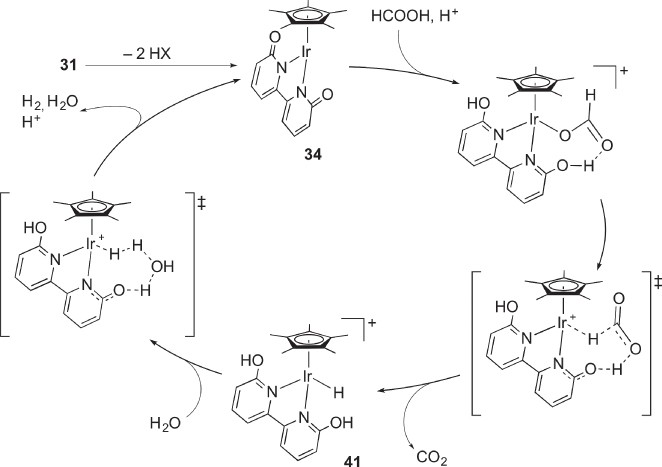
Scheme 4.14 Proposed mechanism for catalytic dehydrogenation of formic acid with bis(hydroxypyridine) complex 31.
Recently, Lin [48] and Papish [49] reported that the 6‐DHPB copper complexes catalyze electrochemical water oxidation ( Scheme 4.15). Some iridium complexes related to 31 are also known to catalyze this reaction [50–52].

Scheme 4.15 Electrochemical water oxidation catalyzed by bis(hydroxypyridine)copper complexes.
Szymczak recently synthesized the multiproton‐responsive ruthenium complex 42 bearing two hydroxypyridine moieties on a pincer‐type platform ( Scheme 4.16) [53]. The two OH groups form intramolecular hydrogen bonds with the chlorido ligand. Complex 42 catalyzes transfer hydrogenation of ketones with 2‐propanol. On the basis of experiments using the carbonyl derivative 43 with higher stability during the catalysis, a mechanism shown in Scheme 4.17 has been proposed [54]. The base‐ and cation‐dependence of the catalysis as well as NMR detection of a hydrido ligand trans to the phosphine supports initial formation of 44. Subsequent hydrido transfer to the ketone substrate would be assisted by electrostatic interaction between the carbonyl oxygen atom and the dangling alkaline metal in the second coordination sphere of the ruthenium atom (45). After exchange of alcohol on the alkaline metal to release the reduction product, hydride transfer to the ruthenium atom regenerate 44. H2 heterolysis on a related bis(hydroxypyridine) pincer‐type ruthenium complex has also been reported recently [55].

Scheme 4.16 Synthesis of ruthenium complex 42 bearing hydroxypyridine‐based protic pincer‐type ligand.
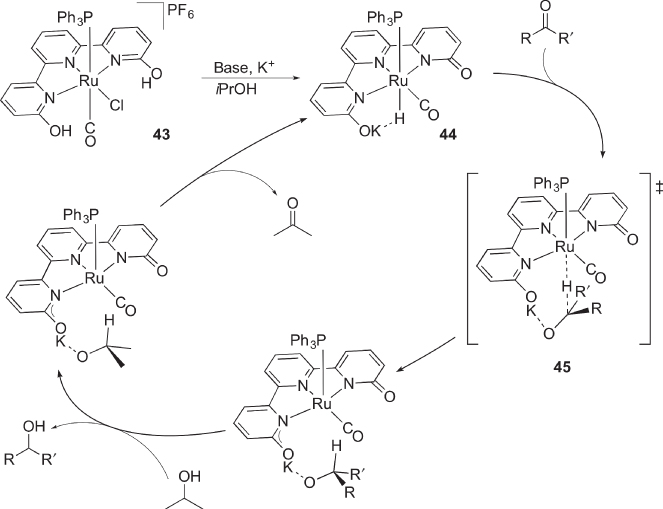
Scheme 4.17 Proposed mechanism for catalytic transfer hydrogenation of ketone with protic pincer‐type complex 43.
4.4 Proton‐Responsive Metal Complexes with Three Appended Protic Groups on Tripodal Scaffolds
Efficient accumulation of more than two proton‐responsive units to mimic the complicated biological coordination sphere would require new ligand topology other than the bidentate chelate and meridional pincer‐type skeleton. For this purpose, tertiary amine‐based tripodal ligands furnished with three proton‐responsive arms have been used widely owing to their accessibility and structural diversity.
We have recently developed a tris(pyrazol‐3‐ylmethyl)amine as a new entry of the multiproton‐responsive ligands ( Scheme 4.18) [56]. The chlorido‐bridged dinuclear ruthenium complex 46 serves as a useful synthetic precursor. When 46 is heated with triphenylphosphine in toluene, the mononuclear phosphine complex 47 is obtained. Interestingly, the reaction in methanol affords the isomer 48, in which the incoming phosphine ligand resides in the position cis to the amine nitrogen atom in the tripodal ligand [57]. The notable dependence of the product on the reaction solvent may be explained by the hydrogen bond network including the protic solvent, which renders the chlorido ligand in the pyrazole rings inert toward ligand substitution. The Brønsted acidity of the proton‐responsive ligand has been substantiated by, for example, reversible deprotonation of 47, which gives the pyrazolato‐bis(pyrazole) complex 49 [56]. When an excess amount of 1,2‐diphenylhydrazine (DPH) is added to 46, catalytic N−N bond cleavage of DPH takes place to afford azobenzene and aniline, along with the aniline complex 50. The aprotic tris(pyridylmethyl)amine (TPA) complex [Ru(tpa)(MeCN)2]2+ shows no catalytic activity, indicating the significance of the proton‐responsive sites in the second coordination sphere.
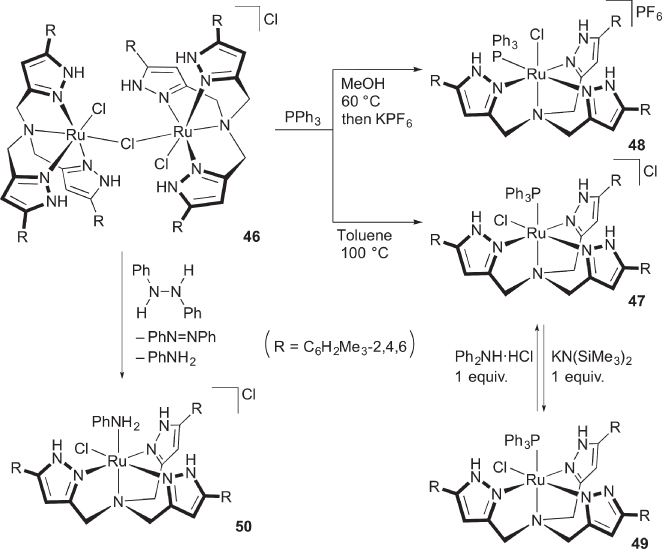
Scheme 4.18 Reactions of tris(pyrazolylmethyl)amine ruthenium complex 46.
Szymczak reported the copper complexes 51 and 52 bearing a hydroxypyridine‐based protic tripodal ligand ( Scheme 4.19) [58, 59]. The crystallographic analysis and 19F NMR spectroscopy revealed that the Cu(I)‐F interaction in 52a is very weak, whereas the three strong hydrogen bonds with the multiproton‐responsive ligand capture the fluoride anion [59]. The fluoride ion in the second coordination sphere of 52a is successfully replaced upon treatment with a silyl nitrite to give the Cu(II) aqua complex 55 along with nitric oxide [60]. Importantly, both of the aprotic [CuF(PPh3)3] and electron‐deficient Cu(II) complex 51a do not yield sufficient amounts of nitric oxide under otherwise identical reaction conditions. These control experiments suggest that the reaction involves 2H+/e− transfer from the multiproton‐responsive copper complex to the nitrite anion through intermediates like 53 and 54. The result would also provide some insight into the mechanism of copper nitrite reductases that catalyze the one‐electron reduction of nitrite to nitric oxide.

Scheme 4.19 Reactions of tris(hydroxylpyridylmethyl)amine copper complexes 52.
In their seminal work on the coordination chemistry of multiproton‐responsive tripodal ligands [61], Borovik demonstrated that the unsymmetric tris(carboxamide)manganese complex 56 catalyzes the O2 reduction with DPH ( Scheme 4.20) [62]. The isolable Mn(II) complex 56 first reacts with O2 to give the unstable superoxo Mn(III) adduct 57. Subsequent PCET from DPH leads to the formation of azobenzene and the peroxo Mn(III) complex 58, in which the carboxamide arms are all protonated. Complex 58 further undergoes PCET‐induced, homolytic O−O bond cleavage to afford bis(hydroxo) species 59. Proton shift from one of the carboxamide arm to the hydroxo ligand would release water to give the hydroxo‐carboxamide or oxo‐carboxamidato Mn(III) complex 60, which has been isolated and crystallographically characterized. Finally, PCET from DPH again takes place to regenerate the Mn(II) carboxamidato complex 56. The multiproton‐responsive coordination sphere crafted by the protic tripodal ligand is thus involved in the catalysis through stabilization of intermediates and promotion of proton‐electron transfer.
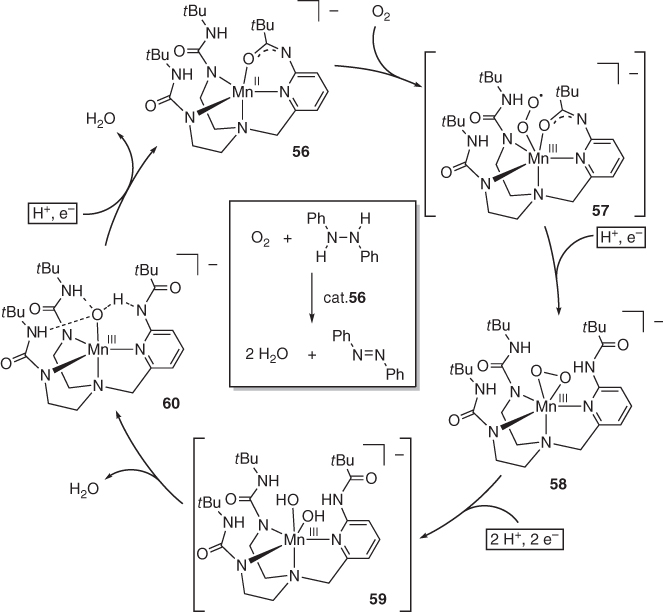
Scheme 4.20 Dioxygen reduction catalyzed by protic tripodal manganese complex 46. Protons and electrons required in each step are provided by 1,2‐diphenylhydrazine.
Fout recently developed a C 3‐symmetric tripodal amine ligand featuring three pyrrole‐imine arms as depicted in Scheme 4.21. The tautomerism of the pendant arm to the (amino)azafulvene form has some similarity with the character of pyrazole and hydroxypyridine, and hence, the multiproton‐responsive reactivities would be expected for this tripodal ligand. The reaction of [Fe(OTf)2(MeCN)2] with the ligand precursor leads to the formation of the triflato complex 61, in which the arms tautomerize to the (amino)azafulvene form and the NH groups rotate away from the apical triflato ligand [63]. Treatment of 61 with an equimolar amount of (nBu4N)NO2 yields the Fe(III) oxo complex 62, wherein the NH groups in the arms all face to the terminal oxo ligand and are engaged in intramolecular hydrogen bonding [64]. Loss of nitric oxide under mild conditions has been confirmed by the reaction with only half amount of the nitrite salt, which resulted in the formation of 62 and an equimolar amount of the nitrosyl complex 63. Considering isolation of the (nitrito)zinc complex 64b, the release of nitric oxide most likely occurs through the nitrito intermediate 64a involving an intramolecular hydrogen bond in the second coordination sphere. As an elegant extension of this chemistry, quite recently Fout disclosed the catalytic reduction of nitrate and perchlorate ions with DPH [65].

Scheme 4.21 Reactions of iron complex 61 bearing protic tripodal ligand.
4.5 Summary and Outlook
The significance of highly organized multifunctionality in the active sites of metalloenzymes is now well recognized. Multiproton‐responsive ligands provide a powerful strategy to construct bioinspired coordination spaces featuring directed and multiple noncovalent interactions in a well‐defined manner. In addition to structural replication of the biological systems, some intriguing reactivities toward ubiquitous and biologically relevant molecules such as H2, CO2, and O2, and hydrazines have been elicited from multiproton‐responsive complexes. Coupled proton/electron transfer appears to be a characteristic of these complexes. Further development of multiproton‐responsive ligands will open new avenues for crafting molecular architectures for challenging chemical transformations under mild reaction conditions. These studies should include quantitative evaluation of the thermodynamic parameters, such as pK a, of this class of ligands.
Acknowledgments
This work was supported by the PRESTO program on “Molecular Technology and Creation of New Functions” from JST (Grant Number JPMJPR14K6). I wish to thank all my coworkers who have contributed to the results presented here.
References
- 1 (a) Bertini, I., Gray, H.B., Stiefel, E.I., and Valentine, J.S. (2007). Biological Inorganic Chemistry. Structure and Reactivity. Sausalito: University Science Books.(b) Crichton, R.R. (2012). Biological Inorganic Chemistry. A New Introduction to Molecular Structure and Function, 2ee. Oxford: Elsevier.
- 2 Crabtree, R.H. (2011). Multifunctional ligands in transition metal catalysis. New J. Chem. 35 (1): 18–23.
- 3 Khusnutdinova, J.R. and Milstein, D. (2015). Metal‐ligand cooperation. Angew. Chem. Int. Ed. 54 (42): 12236–12273.
- 4 Schilter, D., Camara, J.M., Huynh, M.T. et al. (2016). Hydrogenase enzymes and their synthetic models: the role of metal hydrides. Chem. Rev. 116 (15): 8693–8749.
- 5 Lubitz, W., Ogata, H., Rüdiger, O., and Reijerse, E. (2014). Hydrogenases. Chem. Rev. 114 (8): 4081–4148.
- 6 Erman, J.E. and Vitello, L.B. (2002). Yeast cytochrome c peroxidase: mechanistic studies via protein engineering. Biochim. Biophys. Acta 1597 (2): 193–220.
- 7 Hiner, A.N.P., Raven, E.L., Thorneley, R.N.F. et al. (2002). Mechanisms of compound I formation in heme peroxidases. J. Inorg. Biochem. 91 (1): 27–34.
- 8 Poulos, T.L. (2014). Heme enzyme structure and function. Chem. Rev. 114 (7): 3919–3962.
- 9 Hu, Y. and Ribbe, M.W. (2016). Nitrogenases ‐ a tale of carbon atom(s). Angew. Chem. Int. Ed. 55 (29): 8216–8226.
- 10 Hoffman, B.M., Lukoyanov, D., Yang, Z.‐Y. et al. (2014). Mechanism of nitrogen fixation by nitrogenase: the next stage. Chem. Rev. 114 (8): 4041–4062.
- 11 Hoffman, B.M., Lukoyanov, D., Dean, D.R., and Seefeldt, L.C. (2013). Nitrogenase: a draft mechanism. Acc. Chem. Res. 46 (2): 587–595.
- 12 Kuwata, S. and Ikariya, T. (2011). β‐Protic pyrazole and N‐heterocyclic carbene complexes: synthesis, properties, and metal‐ligand cooperative bifunctional catalysis. Chem. Eur. J. 17 (13): 3542–3556.
- 13 Kuwata, S. and Ikariya, T. (2014). Metal‐ligand bifunctional reactivity and catalysis of protic N‐heterocyclic carbene and pyrazole complexes featuring β‐NH units. Chem. Commun. 50 (92): 14290–14300.
- 14 Moore, C.M., Dahl, E.W., and Szymczak, N.K. (2015). Beyond H2: exploiting 2‐hydroxypyridine as a design element from [Fe]‐hydrogenase for energy‐relevant catalysis. Curr. Opin. Chem. Biol. 25: 9–17.
- 15 Wang, W.‐H., Muckerman, J.T., Fujita, E., and Himeda, Y. (2013). Hydroxy‐substituted pyridine‐like N‐heterocycles: versatile ligands in organometallic catalysis. New J. Chem. 37 (7): 1860–1866.
- 16 (a) Szabó, K.J. and Wendt, O.F. ed. (2014). Pincer and Pincer‐Type Complexes: Applications in Organic Synthesis and Catalysis. Weinheim: Wiley‐VCH;(b) van Koten, G. and Milstein, D. ed. (2013). Organometallic Pincer Chemistry. Heidelberg: Springer.
- 17 Cook, L.J.K., Mohammed, R., Sherborne, G. et al. (2015). Spin state behavior of iron(II)/dipyrazolylpyridine complexes. New insights from crystallographic and solution measurements. Coord. Chem. Rev. 289–290: 2–12.
- 18 Halcrow, M.A. (2014). Recent advances in the synthesis and applications of 2,6‐dipyrazolylpyridine derivatives and their complexes. New J. Chem. 38 (5): 1868–1882.
- 19 Craig, G.A., Roubeau, O., and Aromi, G. (2014). Spin state switching in 2,6‐bis(pyrazol‐3‐yl)pyridine (3‐bpp) based Fe(II) complexes. Coord. Chem. Rev. 269: 13–31.
- 20 Jozak, T., Zabel, D., Schubert, A., and Thiel, W.R. (2010). Ruthenium complexes bearing N−H acidic pyrazole ligands. Eur. J. Inorg. Chem. (32): 5135–5145.
- 21 Yoshinari, A., Tazawa, A., Kuwata, S., and Ikariya, T. (2012). Synthesis, structures, and reactivities of pincer‐type ruthenium complexes bearing two proton‐responsive pyrazole arms. Chem. Asian. J. 7 (6): 1417–1425.
- 22 Toda, T., Kuwata, S., and Ikariya, T. (2015). Synthesis and structures of ruthenium and iron complexes bearing an unsymmetrical pincer‐type ligand with protic pyrazole and tertiary aminoalkyl arms. Z. Anorg. Allg. Chem. 641 (12–13): 2135–2139.
- 23 Roberts, T.D. and Halcrow, M.A. (2016). Supramolecular assembly and transfer hydrogenation catalysis with ruthenium(II) complexes of 2,6‐di(1H‐pyrazol‐3‐yl)pyridine derivatives. Polyhedron 103: 79–86.
- 24 Toda, T., Saitoh, K., Yoshinari, A. et al. (2017). Synthesis and structures of NCN pincer‐type ruthenium and iridium complexes bearing protic pyrazole arms. Organometallics 36 (6): 1188–1195.
- 25 Ghoochany, L.T., Farsadpour, S., Sun, Y., and Thiel, W.R. (2011). New N,N,N‐donors resulting in highly active ruthenium catalysts for transfer hydrogenation at room temperature. Eur. J. Inorg. Chem. (23): 3431–3437.
- 26 Umehara, K., Kuwata, S., and Ikariya, T. (2013). N−N bond cleavage of hydrazines with a multiproton‐responsive pincer‐type iron complex. J. Am. Chem. Soc. 135 (18): 6754–6757.
- 27 Younus, H.A., Ahmad, N., Su, W., and Verpoort, F. (2014). Ruthenium pincer complexes: ligand design and complex synthesis. Coord. Chem. Rev. 276: 112–152.
- 28 Jahnke, M.C. and Hahn, F.E. (2015). Complexes with protic (NH,NH and NH,NR) N‐heterocyclic carbene ligands. Coord. Chem. Rev. 293–294: 95–115.
- 29 Jahnke, M.C. and Hahn, F.E. (2015). Complexes bearing protic N‐heterocyclic carbenes: synthesis and applications. Chem. Lett. 44 (3): 226–237.
- 30 Toda, T., Kuwata, S., and Ikariya, T. (2014). Unsymmetrical pincer‐type ruthenium complex containing β‐protic pyrazole and N‐heterocyclic carbene arms: comparison of Brønsted acidity of NH groups in second coordination sphere. Chem. Eur. J. 20 (31): 9539–9542.
- 31 Toda, T., Yoshinari, A., Ikariya, T., and Kuwata, S. (2016). Protic N‐heterocyclic carbene versus pyrazole: rigorous comparison of proton‐ and electron‐donating abilities in a pincer‐type framework. Chem. Eur. J. 22 (46): 16675–16683.
- 32 Miranda‐Soto, V., Grotjahn, D.B., DiPasquale, A.G., and Rheingold, A.L. (2008). Imidazol‐2‐yl complexes of Cp*Ir as bifunctional ambident reactants. J. Am. Chem. Soc. 130 (40): 13200–13201.
- 33 Miranda‐Soto, V., Grotjahn, D.B., Cooksy, A.L. et al. (2011). A labile and catalytically active imidazol‐2‐yl fragment system. Angew. Chem. Int. Ed. 50 (3): 631–635.
- 34 Nieto, I., Livings, M.S., Sacci, J.B. III et al. (2011). Transfer hydrogenation in water via a ruthenium catalyst with OH groups near the metal center on a bipy scaffold. Organometallics 30 (23): 6339–6342.
- 35 Kawahara, R., Fujita, K., and Yamaguchi, R. (2012). Dehydrogenative oxidation of alcohols in aqueous media using water‐soluble and reusable Cp*Ir catalysts bearing a functional bipyridine ligand. J. Am. Chem. Soc. 134 (8): 3643–3646.
- 36 Kawahara, R., Fujita, K., and Yamaguchi, R. (2012). Cooperative catalysis by iridium complexes with a bipyridonate ligand: versatile dehydrogenative oxidation of alcohols and reversible dehydrogenation–hydrogenation between 2‐propanol and acetone. Angew. Chem. Int. Ed. 51 (51): 12790–12794.
- 37 Zeng, G., Sakaki, S., Fujita, K. et al. (2014). Efficient catalyst for acceptorless alcohol dehydrogenation: interplay of theoretical and experimental studies. ACS Catal. 4 (3): 1010–1020.
- 38 Fujita, K., Kawahara, R., Aikawa, T., and Yamaguchi, R. (2015). Hydrogen production from a methanol‐water solution catalyzed by an anionic iridium complex bearing a functional bipyridonate ligand under weakly basic conditions. Angew. Chem. Int. Ed. 54 (31): 9057–9060.
- 39 Fujita, K., Tanaka, Y., Kobayashi, M., and Yamaguchi, R. (2014). Homogeneous perdehydrogenation and perhydrogenation of fused bicyclic N‐heterocycles catalyzed by iridium complexes bearing a functional bipyridonate ligand. J. Am. Chem. Soc. 136 (13): 4829–4832.
- 40 Wang, R., Fan, H., Zhao, W., and Li, F. (2016). Acceptorless dehydrogenative cyclization of o‐aminobenzyl alcohols with ketones to quinolines in water catalyzed by water‐soluble metal‐ligand bifunctional catalyst [Cp*(6,6′‐[OH]2bpy)(H2O)][OTf]2. Org. Lett. 18 (15): 3558–3561.
- 41 Roy, B.C., Chakrabarti, K., Shee, S. et al. (2016). Bifunctional Ru(II) ‐complex‐catalysed tandem C−C bond formation: efficient and atom economical strategy for the utilization of alcohols as alkylating agents. Chem. Eur. J. 22 (50): 18147–18155.
- 42 Himeda, Y., Onozawa‐Komatsuzaki, N., Sugihara, H. et al. (2004). Half‐sandwich complexes with 4,7‐dihydroxy‐1,10‐phenanthroline: water‐soluble, highly efficient catalysts for hydrogenation of bicarbonate attributable to the generation of an oxyanion on the catalyst ligand. Organometallics 23 (7): 1480–1483.
- 43 Onishi, N., Xu, S., Manaka, Y. et al. (2015). CO2 hydrogenation catalyzed by iridium complexes with a proton‐responsive ligand. Inorg. Chem. 54 (11): 5114–5123.
- 44 Wang, W.‐H., Himeda, Y., Muckerman, J.T. et al. (2015). CO2 hydrogenation to formate and methanol as an alternative to photo‐ and electrochemical CO2 reduction. Chem. Rev. 115 (23): 12936–12973.
- 45 Hull, J.F., Himeda, Y., Wang, W.‐H. et al. (2012). Reversible hydrogen storage using CO2 and a proton‐switchable iridium catalyst in aqueous media under mild temperatures and pressures. Nat. Chem. 4 (5): 383–388.
- 46 Ertem, M.Z., Himeda, Y., Fujita, E., and Muckerman, J.T. (2016). Interconversion of formic acid and carbon dioxide by proton‐responsive, half‐sandwich Cp*IrIII complexes: a computational mechanistic investigation. ACS Catal. 6 (2): 600–609.
- 47 Siek, S., Burks, D.B., Gerlach, D.L. et al. (2017). Iridium and ruthenium complexes of N‐heterocyclic carbene‐ and pyridinol‐derived chelates as catalysts for aqueous carbon dioxide hydrogenation and formic acid dehydrogenation: the role of the alkali metal. Organometallics 36 (6): 1091–1106.
- 48 Zhang, T., Wang, C., Liu, S. et al. (2014). A biomimetic copper water oxidation catalyst with low overpotential. J. Am. Chem. Soc. 136 (1): 273–281.
- 49 Gerlach, D.L., Bhagan, S., Cruce, A.A. et al. (2014). Studies of the pathways open to copper water oxidation catalysts containing proximal hydroxy groups during basic electrocatalysis. Inorg. Chem. 53 (24): 12689–12698.
- 50 Lewandowska‐Andralojc, A., Polyansky, D.E., Wang, C.‐H. et al. (2014). Efficient water oxidation with organometallic iridium complexes as precatalysts. Phys. Chem. Chem. Phys. 16 (24): 11976–11987.
- 51 DePasquale, J., Nieto, I., Reuther, L.E. et al. (2013). Iridium dihydroxybipyridine complexes show that ligand deprotonation dramatically speeds rates of catalytic water oxidation. Inorg. Chem. 52 (16): 9175–9183.
- 52 Zhang, T., deKrafft, K.E., Wang, J.‐L. et al. (2014). The effects of electron‐donating substituents on [Ir(bpy)Cp*Cl]+: water oxidation versus ligand oxidative modifications. Eur. J. Inorg. Chem. 2014 (4): 698–707.
- 53 Moore, C.M. and Szymczak, N.K. (2013). 6,6′‐Dihydroxy terpyridine: a proton‐responsive bifunctional ligand and its application in catalytic transfer hydrogenation of ketones. Chem. Commun. 49 (4): 400–402.
- 54 Moore, C.M., Bark, B., and Szymczak, N.K. (2016). Simple ligand modifications with pendent OH groups dramatically impact the activity and selectivity of ruthenium catalysts for transfer hydrogenation: the importance of alkali metals. ACS Catal. 6 (3): 1981–1990.
- 55 Geri, J.B. and Szymczak, N.K. (2015). A proton‐switchable bifunctional ruthenium complex that catalyzes nitrile hydroboration. J. Am. Chem. Soc. 137 (40): 12808–12814.
- 56 Yamagishi, H., Nabeya, S., Ikariya, T., and Kuwata, S. (2015). Protic ruthenium tris(pyrazol‐3‐ylmethyl)amine complexes featuring a hydrogen‐bonding network in the second coordination sphere. Inorg. Chem. 54 (24): 11584–11586.
- 57 Yamagishi, H., Konuma, H., and Kuwata, S. (2017). Stereoselective synthesis of chlorido‐phosphine ruthenium complexes bearing a pyrazole‐based protic tripodal amine ligand. Polyhedron 125: 173–178.
- 58 Moore, C.M., Quist, D.A., Kampf, J.W., and Szymczak, N.K. (2014). A 3‐fold‐symmetric ligand based on 2‐hydroxypyridine: regulation of ligand binding by hydrogen bonding. Inorg. Chem. 53 (7): 3278–3280.
- 59 Moore, C.M. and Szymczak, N.K. (2015). Redox‐induced fluoride ligand dissociation stabilized by intramolecular hydrogen bonding. Chem. Commun. 51 (25): 5490–5492.
- 60 Moore, C.M. and Szymczak, N.K. (2015). Nitrite reduction by copper through ligand‐mediated proton and electron transfer. Chem. Sci. 6: 3373–3377.
- 61 Cook, S.A. and Borovik, A.S. (2015). Molecular designs for controlling the local environments around metal ions. Acc. Chem. Res. 48 (8): 2407–2414.
- 62 Shook, R.L., Peterson, S.M., Greaves, J. et al. (2011). Catalytic reduction of dioxygen to water with a monomeric manganese complex at room temperature. J. Am. Chem. Soc. 133 (15): 5810–5817.
- 63 Matson, E.M., Bertke, J.A., and Fout, A.R. (2014). Isolation of iron(II) aqua and hydroxyl complexes featuring a tripodal H‐bond donor and acceptor ligand. Inorg. Chem. 53 (9): 4450–4458.
- 64 Matson, E.M., Park, Y.J., and Fout, A.R. (2014). Facile nitrite reduction in a non‐heme iron system: formation of an iron(III)‐oxo. J. Am. Chem. Soc. 136 (50): 17398–17401.
- 65 Ford, C.L., Park, Y.J., Matson, E.M. et al. (2016). A bioinspired iron catalyst for nitrate and perchlorate reduction. Science (New York, N.Y.) 354 (6313): 741–743.
