5
Photo‐Control of Molecular Alignment for Photonic and Mechanical Applications
Miho Aizawa1, Christopher J. Barrett1,2 and Atsushi Shishido1,3,*
1 Tokyo Institute of Technology, Laboratory for Chemistry and Life Science, R1‐12, 4259 Nagatsuta, Midori‐ku, Yokohama, 226‐8503, Japan
2 McGill University, Department of Chemistry, 801 Sherbrooke Street West, Montreal, QC, H3A 0B8, Canada
3 PRESTO, JST, 4‐1‐8 Honcho, Kawaguchi, Saitama, 332‐0012, Japan
5.1 Introduction
The development of stimuli‐responsive functional materials is among the key goals of modern materials science. The structure and properties of such switchable materials can be designed to be controlled by various stimuli, among which light is frequently the most powerful trigger. Light is a gentle energy source that can target materials remotely, with extremely high spatial and temporal resolution, easily and cheaply. Light control over molecular alignment in particular has in recent years attracted particular interest, due to potential applications as reconfigurable photonic elements and optical‐to‐mechanical energy conversion. In this chapter, we highlight some recent examples and emerging trends in this exciting field of research, focusing on liquid crystals (LCs), liquid‐crystalline polymers, and photo‐chromic organic materials, which we hope will help stimulate more interest toward the development of light‐responsive materials and their successful application to a wide variety of current and future high‐tech applications in optics, photonics, and energy harvesting and conversion.
Materials based on LCs have emerged as an especially versatile host material for optical effects; often described as a “fourth state of matter,” LCs are now ubiquitous in our everyday lives, as they lie at the heart of practically all of our present visual display devices. Lying in between isotropic liquids and crystalline solids, LCs combine both liquid‐like high mobility and the orientational order of solids achievable. They can also “communicate” with each other via molecular cooperative motions, and sympathetic orientation of LC molecules can be readily controlled externally, which enables their use as “macroscopic molecular switches” and actuators with precise control over refractive index and anisotropy. LC materials thus possess immense technological potential because of this inducible and reversible anisotropy and cooperative motion [1–4]. By incorporation of cleverly designed photo‐responsive molecules into the LCs, this molecular alignment can be precisely controlled over large areas and enables the fabrication of specialty light‐responsive materials for photonic applications such as optical switching and signal processing, lasing, and actuation [5–8]. Light‐controlled functional switching processes in particular have been attracting great attention from both Materials Chemistry and Optical Physics research scientists. Specific advantages of photo‐induced functional systems for application in devices include facile non‐contact influence, superior spatio‐temporal resolution, and multifunctional operability. Effectively, it is these new functionalities and advantages of photo‐responsive molecular materials that have enabled many of the devices we now use daily [9–12].
One of the most elegant and effective of these light‐control schemes devised is based on reversible photo‐switching through incorporation of photo‐chromic units doped or functionalized into a host LC material system. Azobenzene derivatives, for example, through their reversible trans‐cis geometric isomerization [13], can be used to construct molecular‐level switchable systems [14] to control self‐assembly and aggregation of various supramolecular systems [15] or the structure and function of biomolecules [16, 17] and to design stimuli‐responsive macroscopic actuators, micro‐motors, and photo‐deformable materials. In other examples, similar simple absorbing dyes can be used to bring about enhanced response of the material system to optical fields [18, 19]. This is especially effective in the important field of photorefractive systems, where photo‐alignment has been developed effectively to become a major component of NLO photo‐functional materials research [20].
Reversible photo‐induced molecular alignment enabled by azobenzene dates back to early holographic recording experiments by the groups of Todorov [21] and Wendorff [22] in the 1980s. Ichimura et al. were then the first to propose a “command surface” concept to control the orientation of bulk LC molecules by means of an adjacent photo‐responsive surface [23, 24]. Tazuke et al. then demonstrated photo‐chemical phase transitions achievable with light, that is, that a photo‐isomerization process can disrupt the LC phase and turn the material isotropic [25]. In the early 2000s, cross‐linked LC systems emerged as a new mechanically improved class of materials for photo‐alignment control. Finkelmann et al. demonstrated the photo‐contraction of LC elastomers [26], and the Ikeda group demonstrated a large photo‐induced bending of cross‐linked LC films [27, 28]. Key to those macroscopic LC actuators is an amplification of the molecular‐level switching motion through cooperative effects that take place in LCs. Interestingly, such photo‐induced cooperative effects were also reported in photo‐chromic crystals by the Irie group [29]. Compared with elastomers, crystals can exhibit faster photo‐mechanical actuation because of their higher Young's modulus, enabling them as an emerging new class of “harder” but flexible photo‐mechanical materials.
The goal of this chapter is to review and highlight, through selected recent examples, some significant leading examples of photo‐control that has achieved over molecular alignment and motion in the design of macroscopic photo‐switchable materials and light‐driven actuators, and some emerging trends evolving in new directions of mechanical applications. Several lengthy comprehensive reviews on both photo‐responsive LCs and photo‐mechanical materials have appeared during the past few years [6, 30–36], which we recommend for more detailed research reading, and along with this chapter, we hope will serve to demonstrate that this field has both a long history, yet is also still timely and exciting for new directions of application. We start by outlining some recent advances in photo‐chemical photo‐alignment control using dyes, then present some new trends in photo‐physico‐chemical alignment control in dye‐free systems, and then conclude with some key reports of application of these systems for actuation and devices, finally summarizing some remaining challenges and future perspectives.
5.2 Photo‐Chemical Alignment
It has been more than 30 years since early research toward light‐induced molecular alignment control in photo‐responsive azobenzene‐containing polymer films started receiving wide attention and the first practical demonstrations for various photonic applications. It was 1984 when Todorov et al. first observed that birefringence could be induced reversibly by linearly polarized light and thus demonstrated polarization‐holographic gratings in amorphous poly(vinyl alcohol) films doped with methyl orange dye [21], and in 1987 Eich and Wendorff et al. extended these studies to liquid‐crystalline polymers that enabled reversible holographic gratings inscribed with higher diffraction efficiency [22]. The first demonstration of photo‐alignment control using nondye LC materials via surface photochemistry was reported by Ichimura et al. in 1988 [23], where they observed that the trans‐cis isomerization of azobenzene molecules anchored on a 2D substrate could induce a homeotropic‐to‐planar reorientation of 3D bulk LC molecules adjacent. They termed this clever functional surface a “command surface,” evoking the image of many transparent “soldier” LC molecules in bulk following the orientational orders of the azo “commanders” from the surface (Figure 5.1). Shortly after this first report, Gibbons et al. [38], Dyadyusha et al. [39, 40], and Schadt et al. [41] nearly simultaneously demonstrated similar photo‐chemical alignment success using an azo‐dye‐doped polyimide and a photo‐cross‐linkable polymer surface, respectively. Inspired by these pioneering early studies, synthesis of new photo‐aligning materials and development of new photo‐alignment technologies has become one of the hottest recent topics of applied LC science. Following these pioneering studies, many other photo‐chromic and photo‐cross‐linkable materials were reported shortly thereafter, as reviewed well in 2000 and then again in 2002 [42, 43].
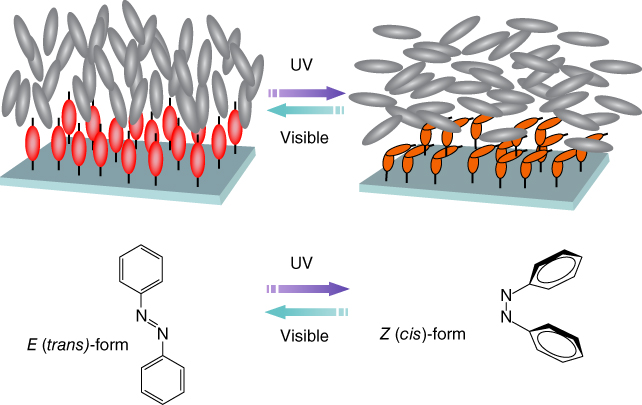
Figure 5.1 Schematic illustration of reversible photo‐alignment by a “command surface.”
Source: From Seki 2014 [37]. Reproduced with permission of Nature Publishing Group.
A wide range of applications were proposed and demonstrated by many groups in the two decades following these first reports, for example, optical storage, holography, optical switching, and display technologies, in response to evolving technology needs [44–47]. More recently, photo‐alignment methods were introduced into the industrial production of LC displays as a facile alternative to alignment via rubbing techniques [48], and today, these advanced surface photo‐alignment techniques for controlling the orientation of LCs are now standard in industry as strong competition for conventional rubbing‐based alignment layers in display technologies [49]. Compared with physical techniques, photo‐alignment layers enable remote and reversible control over anisotropic molecular arrangement, which in turn allows patterning and better control over optical properties, for example, light propagation through LCs [20, 37, 50–52]. From the standpoint of fundamental research, photo‐alignment control in polymeric LC films is still of great interest in next‐generation photonic applications [53]. Toward both these applied and more fundamental research efforts, we now highlight recent examples of current state‐of‐the‐art photo‐alignment control of LC polymers and then some new photo‐alignment materials that undergo other photo‐chemical reactions.
Generally speaking, the photo‐alignment control of LC materials has been based on either photo‐induction of anisotropy in thin alignment layers, as so‐called “command surfaces” [24] or by doping small amounts of photo‐responsive units into the bulk of the LC material [48]. Either way, an azo or other dye needs to be incorporated into the device to transduce the incident light into molecular orientation. A new strategy, employing a molecular command system at the free surface as opposed to the substrate, was recently proposed by the Seki group [54], which can offer some distinct advantages. Here, they blended a small amount of an azobenzene‐containing block copolymer (azo‐BCP) into a non‐photo‐responsive LC polymer. The LC polymer segregates to the LC‐air interface and spontaneously assumes a homeotropic alignment on clean quartz slides and then acts as a “free‐surface command layer” that allows for in‐plane photo‐alignment control of the LC polymer with linearly polarized light. With this film system, in‐plane photo‐patterning can thus be achieved with linearly polarized light. The free‐surface command layers can then be removed after the photo‐alignment process, effectively providing a dye‐free system after this removal step, which can then be reapplied onto secondary material surfaces. Using a standard inkjet printing process, for example, onto various material surfaces, they demonstrated that photo‐aligned fine patterning and arbitrary “designer” images of aligned mesogens in the polymer films are possible and facile, with high spatial resolution, as demonstrated in Figure 5.2. This clever and general strategy is applicable to a wide variety of material systems and importantly requires no pretreatment or modification of the substrate surface. Building on this first report, Nakai et al. then further demonstrated a reversible out‐of‐plane photo‐switching effect from a free‐surface command skin layer [55], using a homeotropic‐planer reversible alignment of a cyanobiphenyl side chain polymer film, controlled by the photo‐isomerization of an azobenzene command polymer placed at the free surface.

Figure 5.2 (a) Schematic illustration of a photo‐alignment system using a photo‐responsive layer at the free surface. (b) Illustration of the inkjet printing procedure of a free‐surface command layer on a homeotropically aligned, non‐photo‐responsive LC polymer. (c and d) Examples of birefringence patterning by this inkjet alignment method. Scale bar, 200 μm.
Source: From Fukuhara et al. 2014 [54]. https://www.nature.com/articles/ncomms4320. Licensed under CC BY 4.0.
A further recent development in photo‐alignment control that should be highlighted is the use of azobenzene‐containing ferroelectric LC polymers as switchable second‐order nonlinear optical (NLO) materials. Here, photo‐induced changes in molecular alignment can provide several advantageous secondary functions, and we next introduce the use of azobenzene‐containing ferroelectric LC polymers as switchable second‐order NLO materials [56]. Such NLO effects can be difficult to achieve in bulk because second‐order NLO materials must necessarily possess a non‐centrosymmetric molecular alignment of the molecular constituents. Organic materials have further advantages for use as NLO materials because unlike their traditional inorganic crystal counterparts, the NLO response of organic molecules can be reversibly switched by photo‐chemical or electro‐chemical stimuli. Toward this goal, alignment methods based on azobenzene‐triggered macroscopic order‐disorder molecular phase changes in cross‐linked ferroelectric LC polymers were proposed recently that provide both high‐contrast and reversible switching in cross‐linked ferroelectric LC polymers, as shown in Figure 5.2. To address the significant problem of the large optical extinction coefficient of azobenzene moieties, two‐photon excitation was applied for efficient switching through the bulk of the material [56], which also offers a significant improvement on the reversibility and repeatability of the switching and also affords high switching contrast that can be achieved via two‐photon methods.
New materials have also been developed to accommodate specification requirements demanded from various specialized photonic applications. Photo‐chemically reactive materials with requirements of extremely low color, for example, or extremely high thermal stability, can be crucial for display technologies with greater color fidelity needs or for use in more extreme environments [48]. Another thrust of photo‐chemical alignment research is in developing new photo‐responsive materials beyond traditional azobenzene dyes. Schadt et al., for example, have reported photo‐alignment of LCs with polyvinylcinnamate films with linearly polarized light [41, 57], and the Kawatsuki group has also systematically investigated the photo‐alignment behavior of photo‐cross‐linkable LC polymers containing cinnamate and cinnamic acid (CA) derivatives [58]. Recently, they reported a new photo‐reactive liquid‐crystalline polymer composed of 4‐methoxy‐N‐benzylideneaniline (NBA) side groups that showed photo‐induced molecular reorientation for holographic applications [59], where a large birefringence of 0.11 was obtained in a perfectly colorless system. Moreover, they explored this photo‐induced reorientation of composite films consisting of a photo‐inactive polymethacrylate with benzoic acid (BA) side groups and photo‐responsive monomeric materials such as CA or NBA derivatives [60, 61]. H‐bonding between BA and CA/NBA side groups was encouraged and played an important role in achieving a sufficient amplification of the photo‐alignment. In other similar reports, the same group demonstrated a facile fabrication of a photo‐alignable polymer film achieved by coating two non‐photo‐reactive materials employing the free surface [62, 63]. Here, they produced photo‐alignable NBA side groups in selective areas by coating phenylamine derivatives onto a polymethacrylate film with phenylaldehyde side groups, where neither coating materials have photo‐reactivity. Most recently, the same group further achieved a control of homeotropic/homogeneous alignment by means of top‐coating aromatic molecules combined with irradiation with linearly polarized light [64]. Via this process, they demonstrated a precise control of in‐plane and out‐of‐plane orientations using cheap and facile inkjet printing of aromatic molecules.
Stumpe and coworkers have recently demonstrated other novel types of colorless photo‐active molecules for the induction of anisotropy, with impressive dichroism values reported of up to 0.2 [65]. Here, “photo‐rotors” containing a photo‐sensitive ethane unit flanked by donor and acceptor substituents were used, elongated by cyclohexane ring systems for forming rod‐like LCs. This photo‐rotor molecule has practically no absorption in the visible region; therefore, high‐quality colorless anisotropic optical films can be fabricated by this process, with high induced dichroism. Finally, Kosa et al. reported various naphthopyran‐containing LCs that can undergo order‐increasing photo‐induced phase transitions with large photo‐induced dichroism, with potential implications in ophthalmic applications [66]. These naphthopyran‐based compound exhibited photo‐induced conformational change of the photo‐switchable molecule from a closed form to an open form, which leads to order‐increasing phase transitions. By doping the naphthopyran molecules into a LC host, it was observed that the phase transition temperature of the host materials was shifted to lower temperature with the closed form shape of the naphthopyran. These photo‐induced shifts of phase transition induced by the photo‐responsive molecular conformational changes then leads to the order‐increasing phase transitions. Isotropic‐to‐nematic and nematic‐to‐smectic phase transitions have both been demonstrated by UV light irradiation as shown in Figure 5.3. Furthermore, the naphthopyran molecules of this system demonstrated unprecedented changes in the order parameter of the dye, with potential applications in ophthalmic devices such as photo‐chromic and polarized variable transmission sunglasses.

Figure 5.3 Polarized optical micrographs of phototropic phase transitions. Illustrations of the photo‐induced phase transitions from isotropic to nematic (a–c), nematic to smectic (d–f), and isotropic to nematic (g–i).
Source: From Kosa et al. 2012 [66]. Reproduced with permission of Nature Publishing Group.
5.3 Photo‐Physical Alignment
Research toward photo‐physical systems, that is, materials in which the photo‐alignment control is achieved by using non‐photo‐chromic groups, has also enjoyed strong recent progress. Compared with photo‐chemical systems, non‐photo‐isomerizable dyes provide several unique advantages: First, the optical molecular reorientation only takes place when the incident light intensity is above a well‐defined threshold, which allows writing and reading using the same wavelength without disturbing the molecular alignment. Second, photo‐physical processes use an order‐to‐order process, which is more simple and facile, and also allows more precise control over LC alignment. Third, a non‐photo‐isomerizable system is more stable in many cases compared with azobenzene‐containing systems because the conformational changes taking place in the photo‐stationary state of azobenzene‐doped LCs may give rise to reorientation instabilities and fluctuations. Photo‐physical alignment methods have been developed primarily with NLO systems. The NLO class describes the behavior of light propagation through nonlinear media, meaning that optical output effects can depend strongly nonlinearly on the optical input and can be completely absent before a threshold is reached [67, 68]. As a result of the mass introduction of laser technology in the 1960s, various new and advanced applications enabled by NLO processes have been developed, such as frequency conversion [69, 70], multi‐photon absorption [71, 72], self‐phase modulation [73, 74], and self‐focusing [75, 76]. However, the generation of such optical nonlinearities typically is challenging for soft organic materials because of the requirement of very high light intensities and costly for the high‐power laser sources required. Several groups have thus worked with crystals [77, 78], high T g polymers [79, 80], and robust LCs [81] that exhibit high optical nonlinearity to induce NLO effects with inexpensive and low‐power lasers. In particular, some LC systems received great attention as NLO devices due to the strong LC order amplifying an optical nonlinearity [82]. So photo‐physical reorientation has thus been extensively studied in dye‐doped LCs because of such strong light‐intensity‐dependent refractive index changes that can be induced due to high LC molecular alignment. This “orientational optical nonlinearity” gives rise to interesting NLO phenomena such as self‐phase modulation of light beams and the potential to generate optical solitons [68, 73, 81]. Compared with photo‐chemical systems, non‐photo‐isomerizable systems may provide some unique characteristics, allowing for molecular reorientation only above a clear threshold intensity and reduced reorientation instabilities and fluctuations in the photo‐stationary state. Several material systems with high optical nonlinearity have been reported [83–86].
Around 1980, three research groups almost simultaneously developed nematic liquid crystals (NLCs) that showed remarkably high optical nonlinearity, of up to nine orders of magnitude higher than that what had previously observed for usual materials [87–89]. This phenomenon was caused by an increase of the photo‐induced molecular director orientation of LCs and the resultant huge change in the refractive index [87]. Marrucci offered a physical mechanism for this photo‐induced NLCs molecular reorientation [82], by suggesting that when a homeotropic LC is vertically irradiated with linearly polarized light, the NLC molecular orientation directs along the polarization direction, which leads to the strong homogeneous orientation. At the same time, rotation of the molecular director prevented by surface anchoring and bulk elasticity is opposed. The final molecular director was thus determined based on the balance between these opposing torques, and the rotation of the molecular director leads to a rotation of birefringence axes, thus to a net high refractive index change. Following these first discoveries of remarkably high optical nonlinearity of NLCs, Janossy et al. reported that a large optical nonlinearity improvement in NLCs was possible by doping a small amount of anthraquinone dye [90]. The enhanced optical nonlinearity in such dye‐doped NLC was two orders magnitude larger than that of non‐doped NLC, as the molecular polarizability of photo‐excited dyes enhanced the torque to rotate NLC. Thanks to this dye‐induced optical nonlinearity enhancement, the input power‐level threshold necessary to induce the reorientation was decreased to safer (and cheaper) levels for NLO effects [18].
Various dyes were then developed to further enhance the nonlinearity of LCs, where it was discovered that the chemical structure of dyes strongly affected the molecular reorientation efficiency [18, 84]. In 2000, Zhang et al. showed that oligothiophene (TR5) could also work successfully as photo‐functional dye for the enhancement of optical nonlinearity of LCs [19]. Small amounts of TR5 were doped into both polar LCs (5CB) and nonpolar LCs (MBBA), and the threshold light intensity for photo‐induced molecular reorientation was dramatically decreased in both systems. This research provides a novel way to control the LC orientation with light of conveniently low intensity. Moreover, because TR5 is a molecule with high fluorescence, this system can be expected to be useful for other interesting photonic applications requiring emission. In 2004, Lucchetti et al. reported a strong refractive index change caused by molecular reorientation that was increased with low light intensities in the range of nW cm−2 [85]. They employed azobenzene‐doped LC glass cells with several types of surface treatment and investigated their optical nonlinearity. In this report, the highest value of nonlinear refractive index reported so far in LC materials was achieved with untreated cells, suggesting that nonlinear response is enhanced by weaker anchoring conditions. These record‐setting optical nonlinearities were determined via a holographic grating setup, which restricted the practical use of the optical nonlinearity due to its requirement for a two‐beam interference. In contrast, a self‐focusing effect for evaluating the threshold intensity is triggered more simply by single‐beam irradiation, so can be expected to have a wider variety of applications [81]. In these systems, optical properties were evaluated using the diffraction ring pattern formed by self‐phase modulation. The number of rings indicated a refractive index modulation at different light intensities, and the threshold intensity was defined as that reached when the first diffraction ring formed [89].
More recently, a conceptually new approach to enhance the nonlinearity of doped LCs, based on polymer stabilization, was proposed. Here, Aihara et al. found that TR5‐doped LCs stabilized by photo‐polymerization reduced the threshold intensity [91]. They combined oligothiophene molecules as low‐molecular‐weight absorbing dopants with polymer‐stabilized LCs (PSLC) and investigated optical nonlinearity using the self‐focusing effect. In this system, they employed homeotropic‐aligned cells and observed that the threshold intensity became six times lower when compared with conventional homeotropic LCs. Moreover, Wang et al. investigated the NLO effects of hybrid‐aligned oligothiophene‐doped PSLC films [92], where again, self‐diffraction rings were formed by photo‐induced molecular reorientation at the lowest light intensity of 400 mW cm−2, which indicates that hybrid‐aligned PSLC decreases the threshold intensity by a factor of 8.5 compared with conventional homeotropic‐aligned LCs. The decreasing threshold intensity was explained by a decrease in total surface anchoring. They also demonstrated that the self‐focusing effect due to molecular reorientation could be induced with a simple 1‐mW‐battery‐operated laser pointer as shown in Figure 5.4. Most recently, Usui et al. investigated a different approach for reducing light intensity for reorientation by modifying a substrate surface that controls initial molecular orientation in polymer‐stabilized nematic LCs doped with oligothiophene [93]. This report showed that the threshold intensity for inducing molecular reorientation of PSLCs was greatly reduced by carefully controlling the surface treatment of glass substrates. The optical nonlinearity owing to molecular reorientation was quantitatively evaluated here by the measurement of the self‐diffraction rings arising from self‐focusing and self‐phase modulation effects. The threshold intensity decreased as surface anchoring was weakened, as for the cells treated with a 0.003 wt% silane coupler solution, the threshold intensity was reduced by 30% compared with the highest silane coupler concentration. Further decreases in the threshold intensity of NLO in dye‐doped LCs or PSLCs will lead to the development of photonic materials and devices, such as a novel material that shields only high‐intensity light, of great interest to wearable optics, sunshields, and smart windows.

Figure 5.4 (a) Schematic diagram of the optical setup for the observation of laser‐pointer‐driven nonlinear optical effects in a hybrid‐aligned cell. (b) Photograph of a ring pattern generated from a hybrid‐aligned cell with a common 1 mW handheld laser pointer.
Source: From Wang et al. 2015 [92]. https://www.nature.com/articles/srep09890. Licensed under CC BY 4.0.
In addition to enhancing the optical nonlinearity (and decreasing the light intensity at which NLO self‐phase modulation takes place), in such systems, it is also possible to lock in the molecular alignment through photo‐polymerization, enabling the inscription of fixed photonic elements. Here, Yaegashi et al. created micro‐lens arrays by combining photo‐induced reorientation of dye‐doped LC‐LC mixtures and simultaneous photo‐polymerization [94]. These fabricated microlens arrays have a polarization‐selectivity and arrays with various lattice patterns were obtained by controlling the polarization directions of the incident beam. Conventional photo‐alignment control has traditionally been triggered by photo‐chemical or photo‐cross‐linkable molecules, and their further development depends on new fundamental developments in molecular design. Additionally, new strategies in material design, including free‐surface optimization in azobenzene systems, and polymer‐stabilization‐enhanced photo‐physical processes, have the potential for more flexibility in optimization of material performance and may well offer new routes for next‐generation photo‐alignment control.
5.4 Photo‐Physico‐Chemical Alignment
As described in the previous section, photo‐induced molecular alignment methods using both photo‐chemical and photo‐physical systems have been extensively studied by many research groups, who have demonstrated various successful applications. These methods, however, still have some difficult challenges to overcome, such as a requirement of complicated (thus tedious, costly) procedures for fabricating molecular alignment layers commercially and subsequently aligning the LCs in a high‐throughput mass production facility. In addition, specific photo‐reactive compounds are needed for induction of the molecular alignment, which can often introduce unwanted mechanical or optical properties. The required irradiation with linearly polarized light is also not always desired or optimal. Thus, to address these concerns, there is great interest in developing novel photo‐control methods of molecular alignment method by using photo‐physico‐chemical systems, that is, using light to induce an orientation, yet in completely dye‐free systems that can be optimized instead for optical and mechanical properties, and amenable to cheap and facile high‐throughput existing fabrication facilities [95].
The first of these new general dye‐free photo‐physico‐chemical methods was proposed in 2016 [95]. In this new method, molecular alignment was induced by masked photo‐polymerization of a monomer and cross‐linker in a completely dye‐free system, where masked photo‐polymerization brings about molecular transport toward irradiated or unirradiated regions, depending on gradients in the chemical potential (Figure 5.5). This alignment process is common to a wide range of existing photo‐polymerizable materials and does not require alignment layers or polarized light. This new paradigm builds upon some previous research that was conducted to find such an integration of molecules is achieved by polymers using photo‐polymerization systems. Historically, starting in 1976, Tomlinson et al. first fabricated volume‐phase holograms and grating devices with photo‐polymer systems [96]. The two‐way diffusion induced by polymerization of the more reactive monomers at the exclusion of the less reactive species from the irradiated regions led to a gradient of the chemical composition and enabled the inscription of the gratings. The Bunning group also demonstrated holographic Bragg grating materials inscribable in a polymer dispersed LC system in 1993 [97]. Gratings were formed here by the changes in the chemical potential of the system induced by photo‐polymerization. The LC separates as a distinct phase in submicrometer droplets in the regions of polymerization. The fabricated grating is thus a superposition of a polymer density grating and a LC droplet grating. Following this, Broer et al. investigated similar photo‐polymerization‐induced‐diffusion systems. In 1995, they produced stable optical filters by cross‐linking cholesteric molecules by photo‐polymerization [98]. They balanced monomer diffusion by controlling the photo‐polymerization rate for the formation of a pitch gradient and obtained wide‐band reflective polarizers. More recently, they fabricated various polymeric relief microstructures by photo‐polymerization with photomasks [99]. In this paper, they investigated the influence of various conditions such as structure period, energy dose, development temperature, film thickness, and photo‐polymer blend composition. The same group then rationalized the phenomenology of this system by a diffusion‐polymerization model [100]. According to this model, the polymerization‐induced monomer‐concentration gradient together with diffusivity differences, cross‐linking properties, interaction between the different components, and the surface free energy together determine the migration of monomer and therefore the final relief structure. Despite these previous reports, however, still no one has reported the direct induction of molecular alignment through diffusion, with no alignment layer.
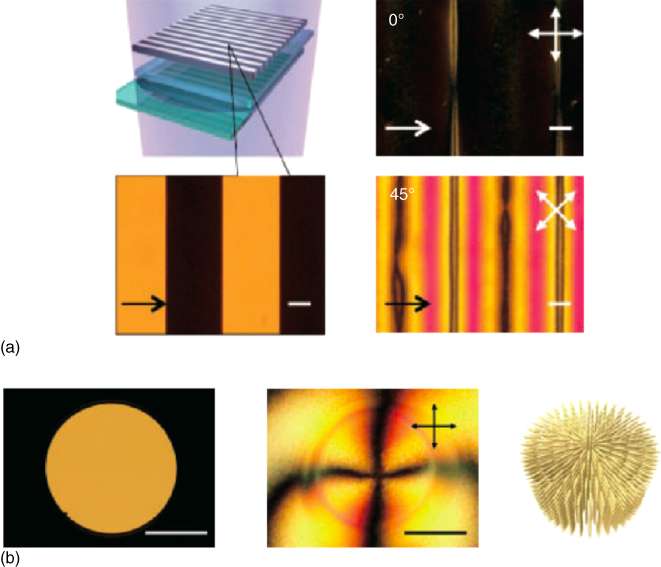
Figure 5.5 (a) Unidirectional molecular alignment behavior of the polymer film photo‐polymerized with a line‐space photomask. Illustration of the photo‐polymerization process and a micrograph of the line‐space photomask (left). Polarized optical micrographs of the polymerized film (right). (b) Two‐dimensional (2D) molecular alignment by photo‐polymerization with a pinhole photomask. Micrographs of the pinhole (left) and 2D aligned film (center). Illustration of the alignment direction of the fabricated polymer film (right).
Source: From Hisano et al. 2016 [95]. http://iopscience.iop.org/article/10.7567/APEX.9.072601/meta. Licensed under CC BY 4.0.
Molecular alignment induced by masked photo‐polymerization of a monomer and cross‐linker in a completely dye‐free system could now be considered a leading technique for future applications, where masked photo‐polymerization brings about molecular transport toward irradiated or unirradiated regions, depending on gradients in the chemical potential [95]. This is the first report that masked polymerization with nonpolarized light enables the precise control of molecular alignment without using a conventional alignment layer. In this process, they used a mixture of an optically anisotropic acrylate monomer and an isotropic dimethacrylate cross‐linker. The molecular alignment direction depends on the shape of the photomask because the alignment direction is controlled by the vector direction normal to the boundary between the irradiated and unirradiated regions and the resultant molecular diffusion. As shown in Figure 5.5, uniform 1D or 2D molecular alignment was achieved by this photo‐physico‐chemical alignment technique. Moreover, they investigated the effect of photo‐polymerization temperature, and it was revealed that this alignment is realized not by the cooperative effect of molecules or elongation of the film surface arising from embossing but by the shear stress arising from molecular diffusion. This novel alignment method revealed that photo‐polymerization‐induced molecular alignment can be a unique and improved alternative approach for precisely aligning molecules with various complex patterns.
5.5 Application as Photo‐Actuators
In addition to all of the applications as orientable materials, one interesting side‐use of many of these light‐responsive systems, especially those that rely on reversible shape changes, is the mechanical forces and stresses that can result reversibly from irradiation. If cleverly applied, these molecular forces can be amplified into macroscopic shape‐shifting, actuating “artificial muscles,” or “molecular machines.” Actuators are systems in which energy is converted from any input stimulus into useful mechanical motion, and light‐activated photo‐reversible systems in particular can often be easily designed and synthesized, and many are able to undergo large deformation upon relatively low input stimulus [101, 102]. Photo‐mechanical actuation, where light energy is converted into mechanical shape changes of the material, is particularly promising for devices, due to the possibility for precisely defined, noncontact actions triggered by low‐cost light sources or even by sunlight. Various light‐induced shape changes have been achieved in shape‐memory polymers [103], carbon‐nanotube‐containing composites and bilayers [104, 105], and cross‐linked polymers and elastomers incorporating photo‐chromic molecules [106–108]. Now, we focus on photo‐chromic actuators, in the same materials as those of the photo‐chemical molecular alignment techniques that have developed orientation applications. One example of interesting photo‐mechanical behavior is a bending of twisted‐nematic elastomers to act as light actuators.
Efficient control over the molecular alignment is a key for achieving and optimizing large‐scale photo‐mechanical effects, yet can be different when comparing the photo‐mechanical effect in amorphous and liquid‐crystalline polymer systems. In amorphous azobenzene‐containing polymers, due to the lack of cooperative molecular motions, photo‐induced dimensional changes only in the range of 1% can be achieved, and in general, they photo‐expand with light. In LC polymers and elastomers, however, reversible uniaxial photo‐contraction may reach 15–20% [26, 109, 110], due to coupling between photo‐modification of molecular alignment and large mechanical deformation provided by the cross‐linked polymer network, and in general, these systems photo‐contract. The light‐induced forces generated both systems, which are brought about by surface strains in three‐dimensional deformed films [111], are often large enough to do significant work against external load, and are being able to fuel, for example, plastic motors [112], robotic‐arm movements [113], and catapult motions [114]. An interesting new development in the design of photo‐actuators is the expansion from linear contraction and in‐plane bending to out‐of‐plane twisting and helical motions. An inspiration for increasing the complexity of the photo‐induced motions perhaps derives from nature: Various biological “engines” are built upon twisting or helical motions [115, 116], and several animals combine bending, twisting, and sweeping motions to generate power for efficient flight [117, 118]. White and coworkers were the first to emulate and achieve such combined in‐plane oscillation and out‐of‐plane twisting in artificial azo‐containing LC polymer networks. Oscillation (or bending) coupled with out‐of‐plane twisting occurs at intermediate angles, due to the combined strain and shear gradients caused by nonuniform light absorption through the thickness of the cantilever [119].
Twisted‐nematic elastomers have proven particularly interesting in terms of their photo‐mechanical behavior, as demonstrated by Harris et al. that the chirality associated with the twisted molecular alignment may produce a coiling motion of elastomeric cantilevers [120]. Broer and coworkers also reported an azobenzene‐doped photo‐polymerized film with twisted networks that showed uniaxial bending or helical coiling deformation modes after UV irradiation [120]. The initial shape of twisted‐nematic cantilevers is sensitive to their dimensions: Depending on the aspect ratio, they can adopt either helicoidal or spiral shapes [121, 122]. The light‐triggered twisting/coiling motions were further extended by Wie et al. who reported photo‐mechanical responses of both twisted‐nematic and hybrid‐aligned cantilevers [123]. A further step toward biomimicry was recently taken by Katsonis et al., who fabricated spring‐like photo‐actuators and demonstrated various types of complex motions such as helix winding, unwinding, and inversion, all photo‐induced as shape changes of azobenzene‐containing LC polymer films (Figure 5.6) [124]. Here, using spring‐like polymeric films, molecular movement was converted and amplified into controlled and reversible twisting motions, that by careful sample preparation techniques these LC polymer photo‐actuators comprised regions that exhibit different dynamic behavior, where these light‐induced conformation changes were determined and controlled by their alignment directions. Such motion mimics the movement of plant tendrils found, for example, in wild cucumbers. Moreover, they reported photo‐switching behavior and thermal stability of photo‐activated molecular deformation systems on a larger macroscopic scale [125]. By employing fluorinated azobenzenes as basic switching elements to slow the thermal reconversion from cis back to trans, the fluorinated switches activated by visible light retained their photo‐chemical shape for more than 8 days.
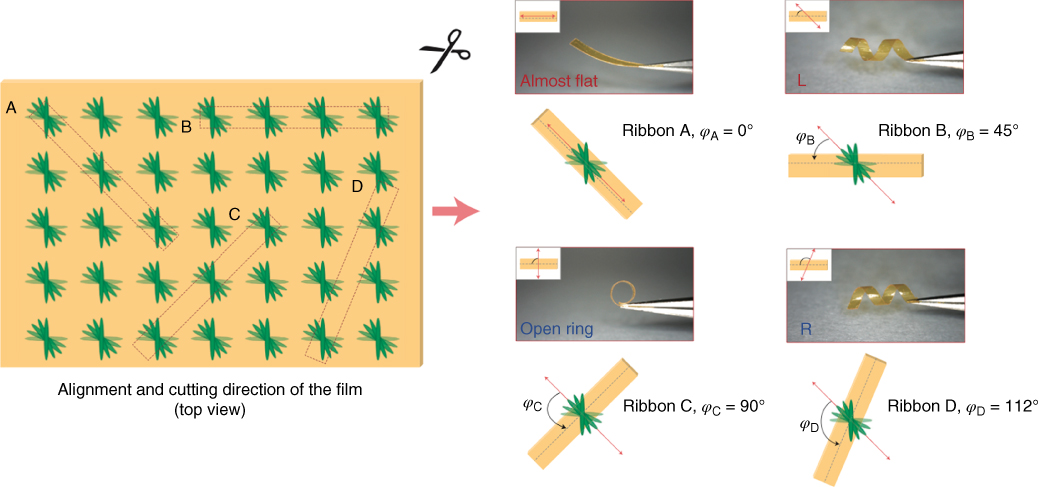
Figure 5.6 Photo‐actuation modes of azobenzene‐containing cross‐linked LC polymer ribbons, exhibiting a complex twisting photo‐mechanical response.
Source: From Iamsaard et al. 2014 [124]. Reproduced with permission of Nature Publishing Group.
Newer interesting developments of azobenzene photo‐mechanical systems derive from the use of either complex LC order or LC elastomer microstructures. Complex‐ordered and patterned LC polymer networks have been most notably studied by Broer and coworkers [126, 127], and engineering of complex molecular ordered and patterned LC polymer networks and elastomers into proof‐of‐principle real devices has been studied by both Broer and White groups [128]. Here, they used LC cells containing cinnamate‐based photo‐alignment layers that were irradiated through a photomask with a wedge‐shaped opening while slowly rotating the cells, using a programming of LCN and LCE materials to localize their mechanical response to generate surface features or local shape changes. Using these cells for photo‐polymerization, various complex‐ordered free‐standing films with continuous change of the LC alignment direction were achieved. And most recently, Broer et al. reported a heat‐driven mechanical effect of azimuthal and radial aligned actuator films with an IR lamp (Figure 5.7) [126]. By using patterned molecular alignment, interesting photo‐thermal actuators exhibiting, for example, checkerboard patterns upon stimulus, were fabricated, where the deformation directions of the actuator films were observed to be different, depending on the alignment patterns [127].

Figure 5.7 Actuation behavior of cross‐linked LC polymer films with the azimuthal and radial alignment upon heating with an IR lamp. The arrows along the radius and the azimuth indicate the direction of deformation.
Source: From de Haan et al. 2012 [126]. Reproduced with permission of John Wiley and Sons.
Topological defects of similar liquid‐crystalline polymer networks were also investigated and reported by White and coworkers [129]. They then demonstrated several types of surface topographies fabricated by exposing the azobenzene‐functionalized LCN films in a controlled manner, applying these patterned alignment techniques to liquid‐crystalline elastomers [130, 131], where the polymer films with voxelated circular director profiles showed a thermomechanical response. Upon heating, conical actuation was observed in the polymer film, and on cooling, the deformation recovers, yielding the initial flat film reversibly (Figure 5.8). Most recently, they reported a photo‐responsive and reversible shape change in elastic films prepared with azo‐LCE [132]. Here, photo‐induced reversible shape morphing between 2D and 3D shapes was demonstrated. This complex alignment method can be addressed both remotely and selectively, which has great advantages for a variety of applications. Finally and most recently, Yu et al. reported a clever new strategy to manipulate fluid slugs by light‐driven tubular microactuators fabricated from photo‐responsive liquid‐crystalline polymers [133]. Photo‐responsive asymmetric deformation of the actuator induces capillary forces for liquid propulsion as shown in Figure 5.9. They fabricated several shapes of micro‐actuators such as straight, serpentine, helical, and “Y”‐shaped from a mechanically robust linear LC polymer. Moreover, they also created a light‐driven micro‐swimming “robot” with a gripper based on this new functional material to realize more complex movements like swimming, grabbing, carrying, and transport [134]. These materials represent remotely light‐driven effects achieving complex driving and controlling of various micro‐robotic motions and structures, and therefore, these materials are expected to hold great potential for applications as micro‐machines and light‐powered robotics.
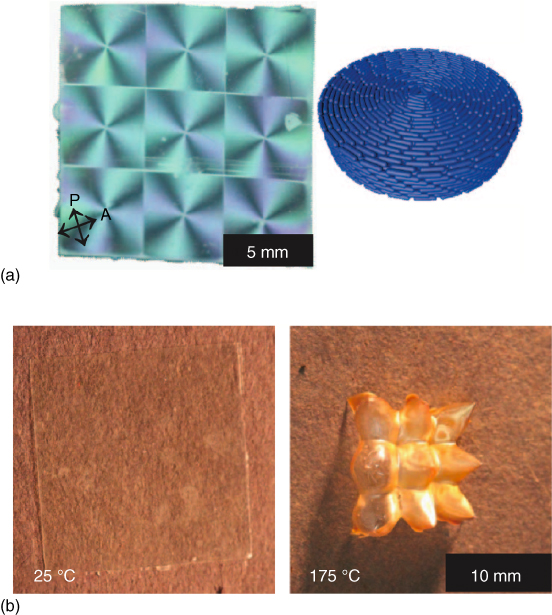
Figure 5.8 (a) Photograph of LCE film with nine +1 topological defects between crossed polarizers and illustration of the alignment direction around the defect. (b) Actuation behavior of the LCE film. Nine cones arise from the LCE film upon heating, and the film becomes reversibly flat upon cooling.
Source: From Ware et al. 2015 [130]. Reproduced with permission of The American Association for the Advancement of Science.
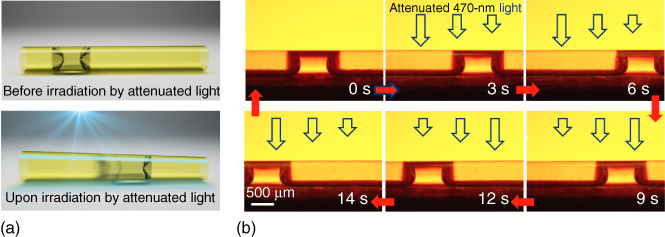
Figure 5.9 (a) Illustrations showing a light‐driven motion of liquid “slugs” inside a tubular microactuator driven by photo‐deformation. (b) Photographs of the reversible motion of a silicone oil slug inside a microtube actuated by 470 nm light.
Source: From Lv et al. 2016 [133]. Reproduced with permission of Nature Publishing Group.
5.6 Conclusions and Perspectives
Precise and reversible control over molecular alignment and shape has emerged in recent years as a crucial key requirement for a wide array of optics applications such as reconfigurable photonic elements and optical‐to‐mechanical energy conversion. Soft materials such as LCs and liquid‐crystalline polymers have also emerged as some of the most promising and exciting classes of host materials to achieve such photo‐switching effectively and efficiently, where molecular properties can be separately tailored and tuned for each specific application or device. As a stimulus for reversible control over these properties, light can be considered as superior, for a direct transfer of photon energy into mechanical motion with no moving parts. Light is also an ideal triggering mechanism as it can be localized in time and space to allow for remote activation of a system, is cheap and easy to employ, and visible light is also an inherently gentle non‐damaging stimulus. Thus, for alignment control, actuation, and mechanical motion, photo‐functional soft materials are of greatest recent interest. Azobenzene is the emerging leader among the small class of photo‐reversible molecules, and soft azobenzene‐containing materials show great promise for next‐generation photonic and photo‐mechanical devices. In this chapter, we have introduced some key current research areas of photo‐driven molecular alignment methods and highlighted some of their recent applications using photo‐chemical, photo‐physical, and photo‐physico‐chemical systems. Photo‐chemical and photo‐physical alignment processes especially benefit from well‐established theoretical understanding. The latest class, photo‐physico‐chemical alignment methods, where alignment shear stress arises from molecular diffusion, are also now just being approached theoretically to help rationalize and thus optimize this new type of molecular alignment system. Twisting, aligning, and bending materials with light is an exciting effect in particular that can offer important and significant advantages to many applied fields and warrants much further study and application. Continued efforts toward the development of molecular alignment control with light can open new possibilities and opportunities for new future applications of functional soft materials for next‐generation effects and devices.
References
- 1 Fleischmann, E.K. and Zentel, R. (2013). Angew. Chem. Int. Ed. 52: 8810.
- 2 Bisoyi, H.K. and Kumar, S. (2011). Chem. Soc. Rev. 40: 306.
- 3 Goodby, J.W. (2011). Liq. Cryst. 38: 1363.
- 4 Kato, T., Mizoshita, N., and Kishimoto, K. (2006). Angew. Chem. Int. Ed. 45: 38.
- 5 De Sio, L., Tabiryan, N., Bunning, T. et al. (2013). Prog. Optics 58: 1.
- 6 Yu, H. and Ikeda, T. (2011). Adv. Mater. 23: 2149.
- 7 White, T.J., McConney, M.E., and Bunning, T.J. (2010). J. Mater. Chem. 20: 9832.
- 8 Coles, H. and Morris, S. (2010). Nat. Photon. 4: 676.
- 9 Priimagi, A., Barrett, C.J., and Shishido, A. (2014). J. Mater. Chem. C 2: 7155.
- 10 Seki, T. (2016). J. Mater. Chem. C 4: 7895.
- 11 Xiao, K., Kong, X.‐Y., Zhang, Z. et al. (2016). J. Photochem. Photobiol. C 26: 31.
- 12 Bisoyi, H.K. and Li, Q. (2014). Acc. Chem. Res. 47: 3184.
- 13 Dhammika Bandara, M.H. and Burdette, S.C. (2012). Chem. Soc. Rev. 41: 1809.
- 14 Russew, M.M. and Hecht, S. (2010). Adv. Mater. 22: 3348.
- 15 Yagai, S. and Kitamura, A. (2008). Chem. Soc. Rev. 37: 1520.
- 16 Beharry, A.A. and Woolley, G.A. (2011). Chem. Soc. Rev. 40: 4422.
- 17 Goulet‐Hanssens, A. and Barrett, C.J. (2013). J. Polym. Sci. Part A 51: 3058.
- 18 Janossy, I. and Lloyd, A.D. (1991). Mol. Cryst. Liq. Cryst. 203: 77.
- 19 Zhang, H., Shiino, S., Shishido, A. et al. (2000). Adv. Mater. 12: 1336.
- 20 Yaroshchuk, O. and Reznikov, Y. (2012). J. Mater. Chem. 22: 286.
- 21 Todorov, T., Nikolova, L., and Tomova, N. (1984). Appl. Opt. 23: 4309.
- 22 Eich, M., Wendorff, J.H., Reck, B., and Ringsdorf, H. (1987). Makromol. Chem. Rapid Commun. 8: 59.
- 23 Ichimura, K., Suzuki, K., Seki, T. et al. (1988). Langmuir 4: 1214.
- 24 Ichimura, K. (2000). Chem. Rev. 100: 1847.
- 25 Tazuke, S., Kurihara, S., and Ikeda, T. (1987). Chem. Lett. 16: 911.
- 26 Finkelmann, H., Nishikawa, E., Pereira, G.G., and Warner, M. (2001). Phys. Rev. Lett. 87 (015501).
- 27 Ikeda, T., Nakano, M., Yu, Y. et al. (2003). Adv. Mater. 15: 201.
- 28 Yu, Y., Nakano, M., and Ikeda, T. (2003). Nature 425: 145.
- 29 Kobatake, S., Takami, S., Muto, H. et al. (2007). Nature 446: 778.
- 30 Yu, H. (2014). Prog. Polym. Sci. 39: 781.
- 31 Seki, T. (2013). Macromol. Rapid. Commun. 35: 271.
- 32 Mahimwalla, Z., Yager, K.G., Mamiya, J. et al. (2012). Polym. Bull. 69: 967.
- 33 Irie, M., Fukaminato, T., Matsuda, K., and Kobatake, S. (2014). Chem. Rev. 114: 12174.
- 34 Abendroth, J.M., Bushuyev, O.S., Weiss, P.S., and Barrett, C.J. (2015). ACS Nano 9: 7746.
- 35 Naumov, P., Chizhik, S., Panda, M.K. et al. (2015). Chem. Rev. 115: 12440.
- 36 Nagano, S. (2016). Chem. Rec. 16: 378.
- 37 Seki, T. (2014). Polym. J. 46: 751.
- 38 Gibbons, W.M., Shannon, P.J., Sun, S.‐T., and Swetlin, B.J. (1991). Nature 351: 49.
- 39 Dyadyusha, A., Kozinkov, V., Marusii, T. et al. (1991). Ukr. Fiz. Zh. 36: 1059.
- 40 Dyadyusha, A.G., Marusii, T.Y., Reshetnyak, V.Y. et al. (1992). JETP Lett. 56: 17.
- 41 Schadt, M., Schmitt, K., Kozinkov, V., and Chigrinov, V. (1992). Jpn. J. Appl. Phys. 31: 2155.
- 42 Natansohn, A. and Rochon, P. (2002). Chem. Rev. 102: 4139.
- 43 Delaire, J.A. and Nakatani, K. (2000). Chem. Rev. 100: 1817.
- 44 Natansohn, A. and Rochon, P. (1999). Adv. Mater. 11: 1387.
- 45 Ikeda, T. (2003). J. Mater. Chem. 13: 2037.
- 46 Shibaev, V., Bobrovsky, A., and Boiko, N. (2003). Prog. Polym. Sci. 28: 729.
- 47 Shishido, A. (2010). Polym. J. 42: 525.
- 48 Chigrinov, V.G., Kozenkov, V.M., and Kwok, H.S. (2008). Photoalignment of Liquid‐Crystalline Materials: Physics and Applications, Wiley SID Series in Display Technology. Wiley.
- 49 Miyachi, K., Kobayashi, K., Yamada, Y., and Mizushima, S. (2010). SID Sym. Dig. Tech. Papers 41: 579.
- 50 Seki, T., Nagano, S., and Hara, M. (2013). Polymer 54: 6053.
- 51 Wei, B.Y., Hu, W., Ming, Y. et al. (2014). Adv. Mater. 26: 1590.
- 52 Tabiryan, N.V., Nersisyan, S.R., Steeves, D.M., and Kimball, B.R. (2010). Opt. Photonics News 21: 40.
- 53 Yu, H. (2014). J. Mater. Chem. C 2: 3047.
- 54 Fukuhara, K., Nagano, S., Hara, M., and Seki, T. (2014). Nat. Commun. 5: 3320.
- 55 Nakai, T., Tanaka, D., Hara, M. et al. (2016). Langmuir 32: 909.
- 56 Priimagi, A., Ogawa, K., Virkki, M. et al. (2012). Adv. Mater. 24: 6410.
- 57 Schadt, M., Seiberle, H., and Schuster, A. (1996). Nature 381: 212.
- 58 Kawatsuki, N. (2011). Chem. Lett. 40: 548.
- 59 Kawatsuki, N., Matsushita, H., Kondo, M. et al. (2013). APL Materials 1: 022103.
- 60 Minami, S., Kondo, M., and Kawatsuki, N. (2016). Polym. J. 48: 267.
- 61 Fujii, R., Kondo, M., and Kawatsuki, N. (2016). Chem. Lett. 45: 673.
- 62 Kawatsuki, N., Miyake, K., and Kondo, M. (2015). ACS Macro Lett. 4: 764.
- 63 Kawatsuki, N., Miyake, K., Ikoma, H. et al. (2015). Polymer 77: 239.
- 64 Miyake, K., Ikoma, H., Okada, M. et al. (2016). ACS Macro Lett. 5: 761.
- 65 Rosenhauer, R., Kempe, C., Sapich, B. et al. (2012). Adv. Mater. 24: 6520.
- 66 Kosa, T., Sukhomlinova, L., Su, L. et al. (2012). Nature 485: 347.
- 67 Shen, Y.R. (1984). The Principle of Nonlinear Optics. New York: John Wiley & Sons.
- 68 Tabiryan, N.V., Sukhov, A.V., and Zel'dovich, B.Y. (1986). Mol. Cryst. Liq. Cryst. 136: 1.
- 69 Dalton, L.R., Sullivan, P.A., and Bale, D.H. (2010). Chem. Rev. 110: 25.
- 70 Kumar, R.A. (2013). J. Chem. 2013: 154862.
- 71 Pawlicki, M., Collins, H.A., Denning, R.G., and Anderson, H.L. (2009). Angew. Chem. Int. Edit. 48: 3244.
- 72 Park, S.H., Yang, D.Y., and Lee, K.S. (2009). Laser Photo. Rev. 3: 1.
- 73 Peccianti, M. and Assanto, G. (2012). Phys. Rep. 516: 147.
- 74 Dudley, J.M., Genty, G., and Coen, S. (2006). Rev. Mod. Phys. 78: 1135.
- 75 McLaughlin, D.W., Muraki, D.J., and Shelley, M. (1996). J. Phys. D 97: 471.
- 76 Wan, W., Dylov, D.V., Barsi, C., and Fleischer, J.W. (2010). Opt. Lett. 35: 2819.
- 77 Sasaki, T., Mori, Y., Yoshimura, M. et al. (2002). Mater. Sci. Eng. R 30: 1.
- 78 Vijayan, N., Babu, R.R., Gopalakrishnan, R. et al. (2004). J. Cryst. Growth 262: 490.
- 79 Yesodha, S.K., Sadashiva Pillai, C.K., and Tsutsumi, N. (2004). Prog. Poly. Sci. 29: 45.
- 80 Cho, M.J., Choi, D.H., Sullivan, P.A. et al. (2008). Prog. Poly. Sci. 33: 1013.
- 81 Khoo, I.C. (2009). Phys. Rep. 471: 221.
- 82 Marrucci, L. (2002). Liq. Cryst. Today 11: 1.
- 83 Khoo, I.C. (1996). IEEE J. Quantum Elect. 32: 525.
- 84 Marrucci, L., Paparo, D., Maddalena, P. et al. (1997). J. Chem. Phys. 107: 9783.
- 85 Lucchetti, L., Di Fabrizio, M., Francescangeli, O., and Simoni, F. (2004). Opt. Commun. 233: 417.
- 86 Budagovsky, I.A., Zolot'ko, A.S., Ochkin, V.N. et al. (2008). J. Exp. Theor. Phys. 106: 172.
- 87 Zel'dovich, B.Y., Pilipetskii, N.F., Sukhov, A.V., and Tabiryan, N.V. (1980). JETP Lett. 31: 263.
- 88 Zolot'ko, A.S., Kitaeva, V.F., Kroo, N. et al. (1980). JETP Lett. 32: 158.
- 89 Durbin, S.D., Arakelian, S.M., and Shen, Y.R. (1981). Phys. Rev. Lett. 47: 1411.
- 90 Jánossy, I., Lloyd, A.D., and Wherrett, B.S. (1990). Mol. Cryst. Liq. Cryst. 179: 1.
- 91 Aihara, Y., Kinoshita, M., Wang, J. et al. (2013). Adv. Opt. Mater. 1: 787.
- 92 Wang, J., Aihara, Y., Kinoshita, M. et al. (2015). Sci. Rep. 5 (9890).
- 93 Usui, K., Katayama, E., Wang, J. et al. (2017). Polym. J. 49: 209.
- 94 Yaegashi, M., Kinoshita, M., Shishido, A., and Ikeda, T. (2007). Adv. Mater. 19: 801.
- 95 Hisano, K., Kurata, Y., Aizawa, M. et al. (2016). Appl. Phys. Express 9: 072601.
- 96 Tomlinson, W.J., Chandross, E.A., Weber, H.P., and Aumiller, G.D. (1976). Appl. Opt. 15: 534.
- 97 Sutherland, R.L., Natarajan, L.V., Tondiglia, V.P., and Bunning, T.J. (1993). Chem. Mater. 5: 1533.
- 98 Broer, D.J., Lub, J., and Mol, G.N. (1995). Nature 378: 467.
- 99 Sánchez, C., de Gans, B.J., Kozodaev, D. et al. (2005). Adv. Mater. 17: 2567.
- 100 Leewis, C.M., de Jong, A.M., van IJzendoorn, L.J., and Broer, D.J. (2004). J. Appl. Phys. 95: 4125.
- 101 Mirfakhrai, T., Madden, J.D.W., and Baughman, R.H. (2007). Mater. Today 10: 30.
- 102 Ohm, C., Brehmer, M., and Zentel, R. (2010). Adv. Mater. 22: 3366.
- 103 Lendlein, A., Jiang, H.Y., Junger, O., and Langer, R. (2005). Nature 434: 879.
- 104 Ahir, S.V. and Terentjev, E.M. (2005). Nat. Mater. 4: 491.
- 105 Zhang, X., Yu, Z., Wang, C. et al. (2014). Nat. Commun. 5: 2983.
- 106 Ikeda, T., Mamiya, J., and Yu, Y. (2007). Angew. Chem. Int. Ed. 46: 506.
- 107 Kroener, H., White, T.J., Tabiryan, N.V. et al. (2008). Mater. Today 11: 34.
- 108 van Oosten, C.L., Bastiaansen, C.W.M., and Broer, D.J. (2009). Nat. Mater. 8: 677.
- 109 Hogan, P.M., Tajbakhsh, A.R., and Terentjev, E.M. (2002). Phys. Rev. E 65 (041720).
- 110 Li, M.H., Keller, P., Li, B. et al. (2003). Adv. Mater. 15: 569.
- 111 Akamatsu, N., Tashiro, W., Saito, K. et al. (2014). Sci. Rep. 4 (5377).
- 112 Yamada, M., Kondo, M., Mamiya, J. et al. (2008). Angew. Chem. Int. Ed. 47 (4986).
- 113 Cheng, F., Yin, R., Zhang, Y. et al. (2010). Soft Matter 6: 3447.
- 114 Lee, K.M., Kroener, H., Vaia, R.A. et al. (2011). Soft Matter 7: 4318.
- 115 Armon, S., Efrati, E., Kupferman, R., and Sharon, E. (2011). Science 333: 1726.
- 116 Gerbode, S.J., Puzey, J.R., McCormick, A.G., and Mahadevan, L. (2012). Science 337: 1087.
- 117 Warrick, D.R., Tobalske, B.W., and Powers, D.R. (2005). Nature 435: 1094.
- 118 Hendrick, T.L., Cheng, B., and Deng, X. (2009). Science 324: 252.
- 119 Lee, K.M., Smith, M.L., Kroener, H. et al. (2011). Adv. Funct. Mater. 21: 2913.
- 120 Harris, K.D., Cuypers, R., Scheibe, P. et al. (2005). J. Mater. Chem. 15: 5043.
- 121 Sawa, Y., Ye, F., Urayama, K. et al. (2011). Proc. Nat. Acad. Sci. 108: 6364.
- 122 Lee, K.M., Bunning, T.J., and White, T.J. (2012). Adv. Mater. 24: 2839.
- 123 Wie, J.J., Lee, K.M., Smith, M.L. et al. (2013). Soft Matter 9: 9303.
- 124 Iamsaard, S., Asshoff, S.J., Matt, B. et al. (2014). Nat. Chem. 6: 229.
- 125 Iamsaard, S., Anger, E., Abhoff, S.J. et al. (2016). Angew. Chem., Int. Ed. 55: 9908.
- 126 de Haan, L.T., Sánchez‐Somolinos, C., Bastiaansen, C.W.M. et al. (2012). Angew. Chem. Int. Ed. 51: 12469.
- 127 de Haan, L.T., Gimenez‐Pinto, V., Konya, A. et al. (2013). Adv. Funct. Mater. 24: 1251.
- 128 White, T.J. and Broer, D.J. (2015). Nat. Mater. 14: 1087.
- 129 McConney, M.E., Martinez, A., Tondiglia, V.P. et al. (2013). Adv. Mater. 25: 5880.
- 130 Ware, T.H., McConney, M.E., Wie, J.J. et al. (2015). Science 347: 982.
- 131 Ware, T.H. and White, T.J. (2015). Polym. Chem. 6: 4835.
- 132 Ahn, S., Ware, T.H., Lee, K.M. et al. (2016). Adv. Funct. Mater. 26 (5819).
- 133 Lv, J., Liu, Y., Wei, J. et al. (2016). Nature 537: 179.
- 134 Huang, C., Lv, J., Tian, X. et al. (2015). Sci. Rep. 5 (17414).
