The use of nanotechnology to improve the bulk and surface properties of steel for structural applications
Abstract:
This chapter discusses the utilization of nanotechnology to greatly enhance the properties of steels, by modifying the chemical composition and/or microstructure of bulk material or surface layer. It starts with a brief discussion of the relevant knowledge base, including: microstructure and chemical composition of steel, influence of nano-modification and processing approaches, and modeling of nanocomposite steel. Then, it provides a review of technological advances in the use of nanotechnology to produce high performance steels with outstanding mechanical properties or corrosion resistance. The chapter concludes with a discussion of future developments expected on the subject.
5.1 Introduction
Steel is a versatile material of technological importance and its outstanding recyclability and ease of manufacturing greatly contribute to eco-efficient construction. Steel is a type of widely used engineering material in many industries, e.g., transportation, construction, defense, healthcare, energy, aeronautics, manufacturing, and chemical processing. It has found diverse applications in transport vehicles and components, ships, buildings, civil structures, offshore structures, aircraft landing gears, bulletproof sheets and vests, household appliances, medical tools, pipelines, heat exchangers, solar panels, equipment, nuts and bolts, bearings, packaging, etc. Steel is the most widely used metallic alloy in modern industries, with its usage approximately 80% by weight of all alloys (Smith, 1981).
Over the last two decades, great strides have been made to improve the engineering properties of steels through research, development and implementation. In supporting such innnovative technological advances, nanotechnology has been playing an increasingly important role. For instance, Lesuer et al. (2010) reported the fabrication of Fe-C alloys with ultrahigh strength (4600 MPa) through quenching and severe plastic deformation (SPD). The strengthening was attributed to the nano-size effect of lath and plate martensite grains and the small interparticle spacing. As reviewed by Kolpakov et al. (2007), metallurgy approaches to the production of high performance steels with a fine-grain structure and/or self-organization of strengthening nanophases (carbides, nitrides, carbonitrides, intermetallides) have been burgeoning under the guide of nanotechnological principles, including nanoprocesses for steel smelting and microalloying, mechanical pressure treatment (e.g., SPD), and heat treatment (e.g., superfast quenching of melts). One such technology commercialized in the US produces high performance carbon steels that feature a ‘three-phase microstructure consisting of grains of ferrite fused with grains that contain dislocated lath structures in which laths of martensite alternate with thin films of austenite’ (Kusinski et al., 2004).
Recent years have seen the fabrication of high performance steels desirable for light weight construction and other engineering applications. As detailed later, these steels typically feature an ultrafine or nano-grained microstructure, which leads to excellent properties in both strength and ductility. In addition, nanotechnology has been employed to enhance the durability of the steel bulk material or surface layer, in terms of resistance to wear, fatigue, and/or corrosion. This is made possible by achieving the desirable finely crystalline microstructure of steel (e.g., nanocrystallization) or by modifying its chemical composition at the nanometer scale. Formation of Cu nanoparticles at the steel grain boundaries (GBs) has been used to improve the corrosion resistance of steel. Addition of Cu nanoparticles has also been reported to mitigate the fatigue cracking of steel, by reducing the surface roughness of steel and associated stress risers (Mann, 2006).
This chapter synthesizes the findings of some of the major research efforts in this area and presents a discussion of utilizing nanotechnology to greatly enhance the properties of steels, by modifying the chemical composition and/or microstructure of bulk material or surface layer.
5.2 Research relating to nanocomposite steel
5.2.1 Microstructure and chemical composition of steel
While Fe is the main element in steels, other elements (e.g., C, alloying elements, and impurities) define the manifold properties of steels, including: tensile strength, fatigue strength, ductility, hardness, toughness, wear resistance, formability, weldability, fire resistance, corrosion resistance, etc. The microstructure of steel is inherently heterogeneous, generally consisting of grains (or ‘phases’, considered to be homogeneous in physiochemical nature), dislocations, GBs, precipitates, and lattice defects. Figure 5.1 presents a portion of the equilibrium phase diagram of the Fe-C system under atmospheric pressure (Chipman, 1972), which illustrates the thermodynamics of three main phases in carbon steel, i.e., autensite (γ), ferrite (α), and cementite (Fe3C), as a function of temperature and C content. Note that equilibrium phases tend to form when there is sufficient time to allow diffusion of atoms and molecules. In many cases, the processing of steel may include quenching or application of mechanical stress, which leads to the formation of non-equilibrium phases such as martensite (α'). For steels with a significant amount of alloying elements (e.g., stainelss steels), their microstructure may include many phases other than γ, α, and α' (Lo et al., 2009).
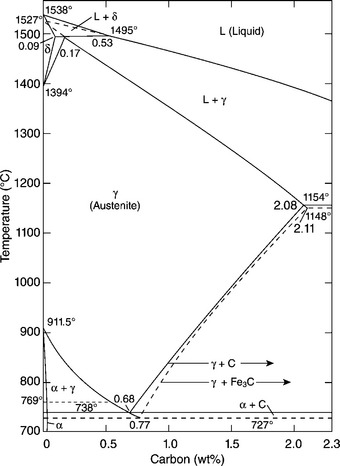
Fig. 5.1 Portion of the phase diagram Fe-C relevant to carbon steel. Metastable γ-range and system Fe-Fe3C shown by dashed lines. Curie temperature dotted. With kind permission from Springer Science and Business Media. (adopted from Chipman, 1972)
High-strength steels are often martensitic; as the C content increases, their strength increases but ductility and weldability tend to decrease. Martensite can serve as an effective starting phase for acquiring ultrafine or nano-grained microstructure with small strains. For instance, Tsuji and Maki (2009) reported the formation of microstructure in steel characteristic of equiaxed α grains (~ 200 nm), by cold-rolling and annealing of a starting α' phase. Austenitic stainless steels (SS) generally have low yield strength (150–300 MPa) yet excel at corrosion and oxidation resistance, work-hardening rate, and formability. Their strengthening by grain refinement can be achieved via reverse α' transformation or SPD, and greatly enhances strength and resistance to wear, pitting, cavitation, cavitation-erosion, and radiation-induced damage (Lo et al., 2009). The grain refinement of γ SS may compromise ductility and work-hardening. The strengthening of γ SS by martensite or dispersed precipitates can compromise their corrosion resistance, formability and ductility (Lo et al., 2009).
Alloying elements in steel ‘may change the transformation temperature, or form more stable special carbides instead of cementite, . . . (or) change the sequence of nucleation and growth processes by affecting the interface energies or the diffusion processes’ (Eisenhüttenleute, 1992). In addition, the microstructure of steel can be controlled by manipulating the kinetics of its formation, via thermal and mechanical treatments. For instance, the following solid–solid state transformation can occur (Fig. 5.1): γ → α + Fe3C (Branagan et al., 2006). Each phase may feature multiple allotropes, i.e., various structural forms of the same chemical composition. For instance, cementite can be in the form of ‘pearlite, bainite, and/or tempered martensite, depending on steel composition and preceding cooling conditions’ (Eisenhüttenleute, 1992). The ultimate microstructure of a steel with given alloy chemistry is often defined by the γ-state grain size and homogeneity, transformations induced by heat treatment (e.g., quenching and annealing), and physical interactions induced by mechanical processing (e.g., SPD).
5.2.2 Influence of nano-modification
In the last decades, grain refinement has been confirmed as an effective way to concurrently enhance strength and toughness of polycrystalline materials and has been used to remarkably improve the comprehensive mechanical properties of steels. Zhao et al. (2011) found the grain size of large-grain-size regions to be responsible for the ductile-to-brittle transition temperature of an ultrafine grained ferrite/cementite steel. As the average grain size or weighted average grain size decreases, the yield strength and hardness of polycrystalline materials (e.g., steels) are expected to increase greatly, according to the well-known Hall–Petch relation (Lo et al., 2009). This can be attributed to the high concentration of GBs, which serve as barriers to pin the motion of dislocations. Once the grain size is reduced to the nanometer range, an inverse Hall–Petch relation can be observed. This can be credited to the extremely high density of GBs in the microstructure of nano-grained steel, which leads to ‘role-exchange of the grain bulk and the GB in the deforming mechanism’ and ‘a transition from intragranular to GB-mediated deformation’ (Weertman, 1993; Song et al., 1999; Frontán et al., 2012). Nonetheless, as reviewed by Lesuer et al. (2010), the tensile strength of steels generally exhibits a negative linear relationship with the interparticle spacing, despite the significant variations in their chemical composition and thermomechanical history (Fig. 5.2). In other words, grain refinement has been the main pathway to achieve high strength for steels. The only exception was a case where a lath martensite nano-structure greatly enhanced the strength of the steel, thus deviating from this linear relation (Fig. 5.2).
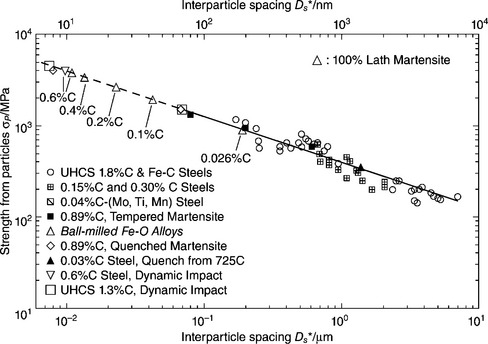
Fig. 5.2 Tensile strength of steels as a function of interparticle spacing. With kind permission from Springer Science and Business Media (adopted from Lesuer et al., 2010)
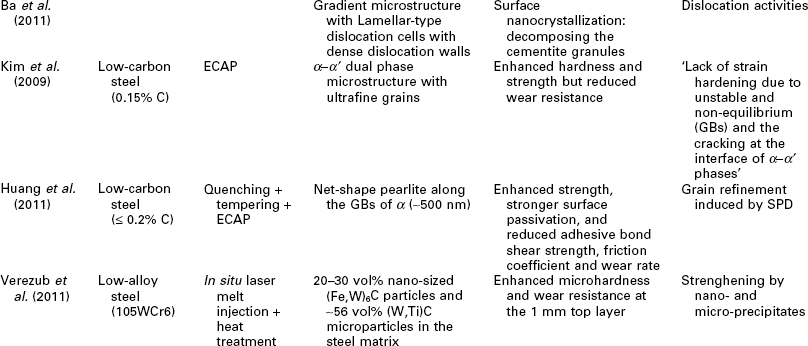
One potential issue related to microstructure ultrafining of steels is that their heat affected zones (HAZ) tend to exhibit various degrees of embrittlement and localized softening as a result of localized grain coarsening. This can be addressed by inducing the nucleation of intragranular acicular ferrite around oxide inclusions, following the oxide-dispersed-strengthened (ODS) steel technology (Lei et al., 2007). Another way to refine the HAZ grains and improve weld toughness is to add Mg and Ca nanoparticles in the steel (Mann, 2006). Grain coarsening at elevated temperatures can be inhibited via GB segregation and various drag effects (Malow and Koch, 1997; Matsuia et al., 2006), or via GB modification or manipulation of nanotwin boundaries inside the grains (Lu et al. 2009; Li et al. 2010). All of these mechanisms serve to preserve the ductility of high strength steel. Gonsalves et al. (1994) suggested that carbide particles a few microns in diameter may act as initiation sites of fatigue cracks. Lo et al. (2009) suggested that the precipitation of carbides at GBs can cause localized depletion of Cr content in SS and increase their risk of corrosion and sensitization They also reported the use of nitrogen to inhibit the formation of the M23C6 carbide so as to minimize its undesirable effects on GB serration and creep-fatigue resistance. As detailed in Table 5.1, the formation of nano-sized grains, twins, or inclusions can mitigate such risks of microstructure ultrafining.
Table 5.1
Recent research on the utilization of nanotechnology to improve the mechanical properties of steel bulk
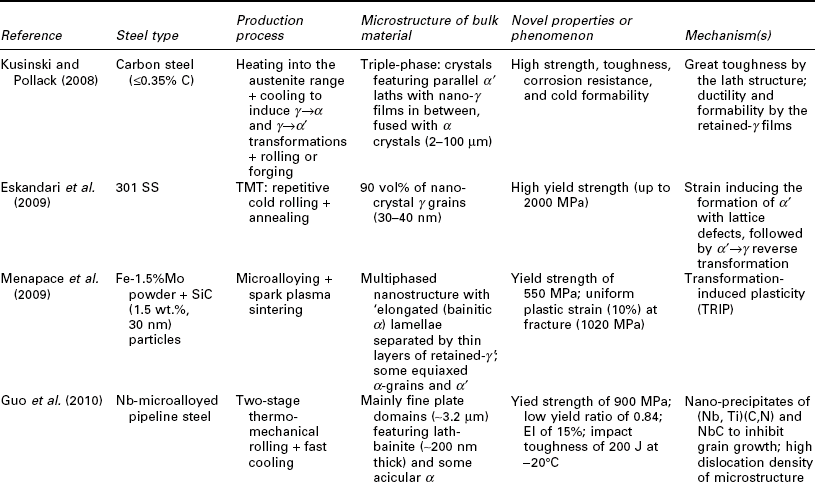
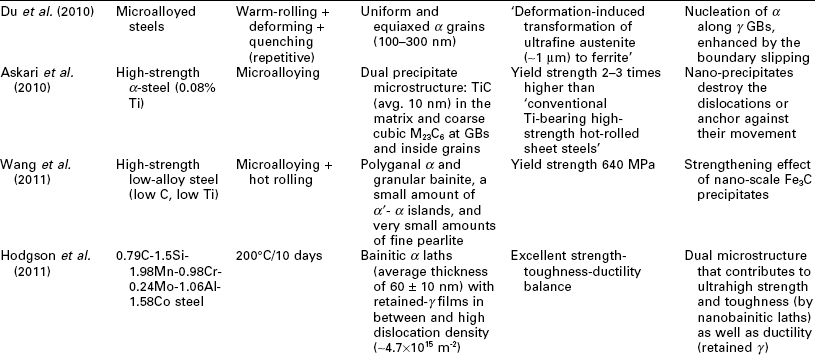

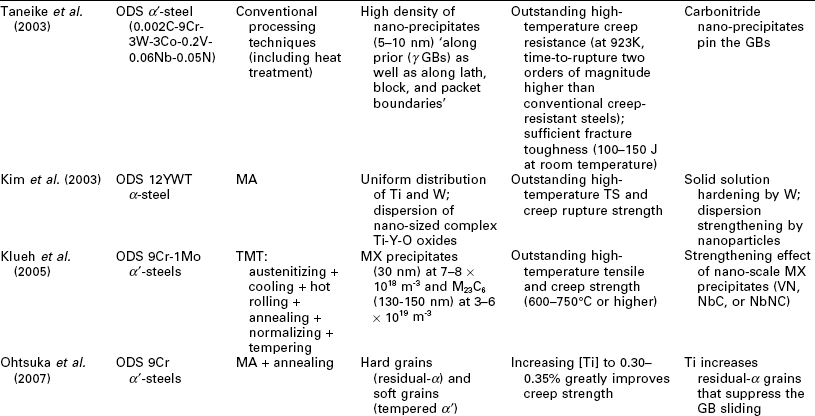
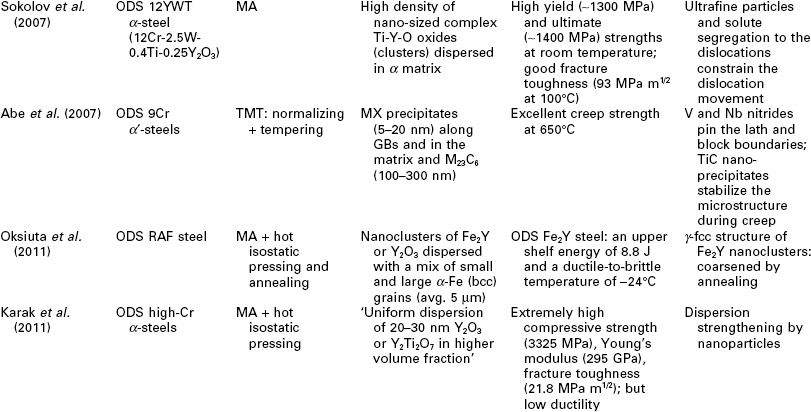

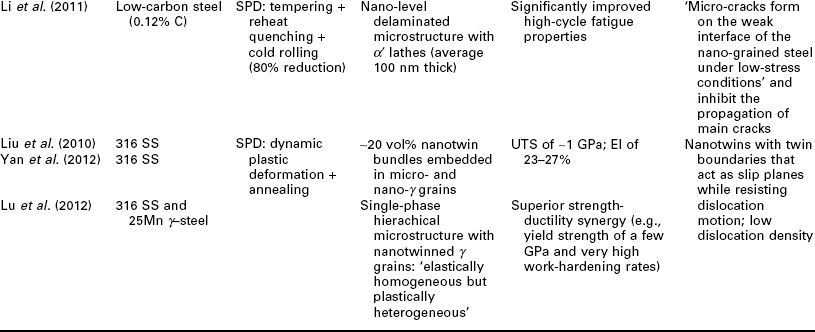
BOF: basic oxygen furnace; CSP: compact strip production; HE: hydrostatic extrusion.
RAFM: reduced activation ferritic/martensitic; UTS: ultimate tensile strength.
Nanotechnology provides a potential solution to considerably enhancing the ductility of high strength steel, as the nanocomposite steels deform by GB mechanisms, e.g., sliding and diffusion, rather than dislocation motion. Grain refinement can be coupled with phase transformation induced plasticity (TRIP) to form γ grains of micron or nano-size, endowing the steel with outstanding synergy in strength, ductility, and work-hardening ability. While coarse MC carbides degrade creep-fatigue resistance and fracture resistance of SS, fine MC carbides can inhibit the growth of grains and greatly improve the impact property and strength of SS (Lo et al., 2009). Often, a second phase or nanostructure can be uniformly dispersed in the relatively brittle bulk matrix in order to introduce plasticity. Alternatively, nano-grains or nanotwin boundaries inside the grains can be introduced to delocalize the micro- and nano-scale deformation and to improve toughness. Zhao et al. (2011) suggested high-angle GBs to be most effective in hindering the propagation of cleavage cracks. Manipulation of GB structures (i.e., GB engineering) can be utilized to enhance the bulk properties of steels, e.g., by reducing carbide precipitation at GBs (Lo et al., 2009).
5.2.3 Influence of processing approaches
Conventionally, there are four main pathways to achieve a fine-grained microstructure (e.g., grain size of 1 mm): repetitive short austenitizing at low temperatures; ‘deformation in the austenite range followed by recrystallization’; deformation of austenite at temperatures featuring a low recrystallization rate; or cooling to induce transformation at low temperatures (Eisenhüttenleute, 1992).
Recent decades have seen the increased use of metallurgical refining, SPD, thermomechanical treatment (TMT), and liquid-state treatments to produce steels with ultrafine grained microstructure. The reduction of grain size from millimeter scale to micron scale is achieved by increasing the rate of grain nucleation while reducing the rate of grain growth. The grain refinement in the liquid state, for instance, may involve the use of pulsed magnetic fields, pulsed current, or ultrasonic energy to induce refined microstructure of SS during its solidification (Lo et al., 2009). Okamura et al. (1995) reported the formation of ultrafine grained bainite structure in new HT780 steels (0.05–0.06% C; B-free) by direct quenching and tempering. These steels exhibit outstanding mechanical properties: tensile strength (TS) of 825 MPa and total elongation (El) of 26% at fracture. They also exhibit excellent welding properties (e.g., low HAZ hardness) and good fatigue properties, attributable to precipitation hardening and grain refinement by Cu, Nb, and V. Some alloying elements (e.g., Tb, Ni, and V) can form nano-precipitates with the C or N in steel and greatly inhibit the growth of grains while forming a high concentration of non-uniform grains. There are also alloying elements (e.g., Mn and Cr) that can reduce the phase transformation temperature and refine the grains during or after the phase transformation. Lei et al. (2007) suggested that the best way of grain refining was to couple alloying with TMT or SPD.
While nano-grained or nano-amorphous steels may be synthesized by the ‘bottom-up’ approach such as gas condensation or chemical analysis (Averback, 1993; Gonsalves et al., 1994), these production processes do not lend themselves to industrial manufacturing. In contrast, the ‘top-down’ approach such as mechanical alloying (MA), TMT and SPD can be readily implemented at large industrial scale. TMT can be as simple as the conventional cold rolling followed by annealing, or may consist of advanced treatment sequences. Bhadeshia (2008) reported the use of TMT to induce phase transformation, forming nanostructured bainitic α and γ plates (~ 20 nm) in steels with a high C concentration. In addition to phase transformation, TMT may induce other solid–solid reactions such as recrystallization and precipitation. SPD involves intensive straining processes. SPD can induce the formation of nanocrystalline grains in bulk material (with equal channel angular pressing – ECAP, high pressure torsion, accumulative roll bonding, repetitive corrugation and straightening, constrained groove rolling or pressing, etc.) or in surface layer (100 μm to 30 mm thick, with surface mechanical attrition treatment – SMAT, ball milling, slide wearing, wire brushing, ultrasonic or high-energy shot peening, supersonic fine particles bombardment – SFPB, severe cold drawing, ultrasonic cold forging – UCFT, etc.). SPD concurrently improves the strength and toughness of steels by substantially refining the microstructure of steel, increasing the density of dislocations, dislocation walls, and vacancies, forming non-equilibrium GBs, and stabilizing austensite phases (Valiev, 2004). Tsuji and Maki (2009) demonstrated that nanostructures in steel can be produced by different ways of combining phase transformation and plastic deformation. They also revealed the effective role of plastic deformation in increasing nucleation sites for subsequent phase transformation.
5.2.4 Modeling of nanocomposite steel
Modeling can be used as a powerful tool to advance the fundamental knowledge pertinent to nanocomposite steel, especially when integrated with or validated by experimental investigation. Thermodynamic modeling can provide insights into phase stability and transformations. For instance, a Pourbaix diagram (potential–pH diagram) can be used to predict and guide the synthesis and application of steels under given working conditions (Kaufman et al., 2009). Molecular dynamics modeling can shed light on mechanisms underlying mechanical properties of steels, by simulating the interactions between dislocations with GBs, twins, precipitates and other barriers during deformation (Li et al., 2010; Wu et al., 2009, 2011). Mechanistic models have also been established for predicting the fatigue life of SS under various conditions (Lo et al., 2009). In addition, improved understanding of corrosion and inhibition mechanisms has been continually achieved through characterization and modeling of the steel surface and corrosion products at various length scales down to the nanometer scale (Murayama et al., 2008).
5.3 Properties of nanocomposite steel
5.3.1 Nanotechnology to improve mechanical properties of steel bulk
Nanotechnology has been employed to enhance the mechanical properties of the steel bulk itself, by achieving the desirable finely crystalline micro- structure of steel or by modifying its chemical composition or morphology at the nano- or micro-scale. Table 5.1 summarizes recent research on this subject, involving various classes of steel: carbon steel (≤ 2.1% C, low alloy), SS (≥ 10.5% Cr), alloy steel, ODS steel, etc. A wide variety of processing approaches have been investigated for producing nanocomposite steels. TMT, MA, and SPD have been used individually or synergistically, to induce nanostructure via phase transformation and deformation processes. There is also a high level of diversity in the resulting microstructure of nanocomposite steel, ranging from single-phase, dual-phase, to multi-phase and ranging from nano-grains of α or γ, to α' laths or bainitic α lamellae with nano-γ films in between, to nano-precipitates/clusters in or along nano- or micro-grains of α' or α. These nano-modified steel bulk feature novel mechanical properties, characteristic of considerable improvements in strength (e.g., TS up to 2000 MPa) alone or in strength as well as toughness (e.g., El of 15%; impact toughness of 200 J at − 20 °C) and high-temperature creep resistance (e.g., at 923 K, time-to-rupture 2 orders of magnitude higher than conventional creep-resistant steels), etc. There are multiple mechanisms underlying the outstanding strength–ductility synergy of nanocomposite steels. Often the toughness is introduced by the lath or lamella structure or high percentage of high-angle GBs; ductility and formability are introduced by the retained γ films or tempered α'; and strength is enhanced by the nano-grains, nano-precipitates of carbides, nitrides, or carbonitrides, or other nano-inclusions.
One recent advance in this field is the use of dynamic plastic deformation (DPD) followed by annealing to produce SS and alloy steels with superior strength–ductility synergy, such as TS of 1 GPa, El of 27%, and very high work-hardening rates (Liu et al., 2010; Lu et al., 2012; Yan et al., 2012). These nanocomposite steels feature a unique single-phase hierachical microstructure with ~ 20 vol% nanotwin (NT) bundles embedded in micro- and nano-γ grains and low dislocation density. Their mechanical behavior is ‘elastically homogeneous but plastically heterogeneous’. It was hypothesized that the NT boundaries act as slip planes while resisting dislocation motion. Figure 5.3 presents typical bright-field transmission electron microscopy (TEM) images of 316 SS after DPD and annealing, showing the early-stage static recystallization (SRX) in shear bands between NT bundles. The SRX grains and NT bundles introduce a great amount of ductility into the steel bulk, while enhancing its strength simultaneously (Yan et al., 2012).

Fig. 5.3 Typical bright-field TEM images (a and b) of 316 SS with DPD ε = 1.6, annealing @ 730 °C / 20 min, showing the early-stage SRX in shear bands between NT bundles. With kind permission from Elsevier Science. (Yan et al., 2012)
5.3.2 Nanotechnology to improve mechanical properties of steel surface
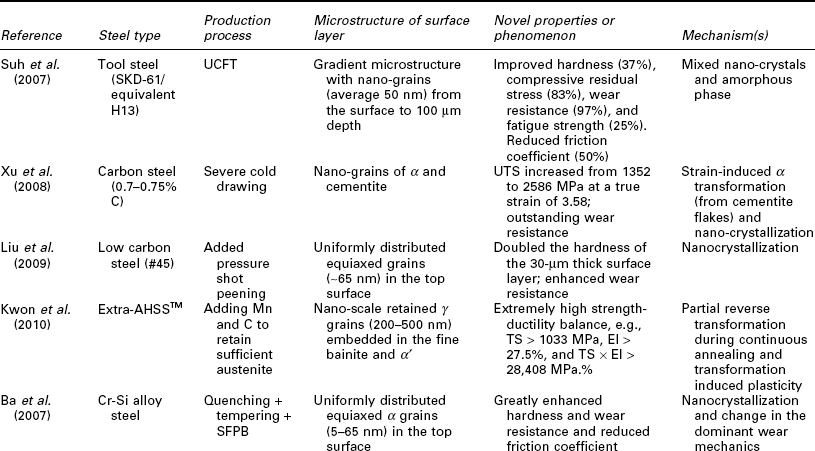
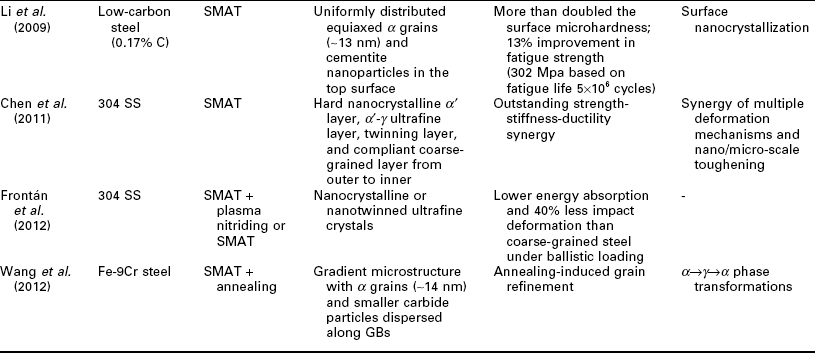
Nanotechnology has also been employed to enhance the mechanical properties of the steel surface layer, by achieving the desirable finely crystalline microstructure of steel or by modifying its chemical composition and morphology at the nano- or micro-scale (Lo et al., 2009). Table 5.2 summarizes recent research on this subject, involving various classes of steel: carbon steel, SS, alloy steel, etc. A variety of processing approaches have been investigated for producing a nanocomposite surface on steels, typically via surface SPD or SPD coupled with TMT. While there tends to be a gradient microstructure from the treated surface to the bulk of steel, there is also a high level of diversity in the resulting surface microstructure, ranging from nano-grains of α or α and cementite, to nano-scale retained γ grains embedded in the fine bainite and α', to net-shape pearlite along the nano-grains of α, to nano-twinned ultrafine crystals. These nano-modified steel surfaces feature novel mechanical properties, characteristic of considerable improvements in strength (e.g., TS by 91% and fatigue strength by 13%), hardness (by 100%), wear resistance (by 97%), and fatigue strength (by 25%), and toughness (e.g., El of 27.5%) and reduced friction coefficient (by 50%). There are multiple mechanisms underlying the outstanding strength–stiffness–ductility synergy of the nanocomposite surface. Often the toughness and ductility are introduced by phase transformation and dislocation distribution, whereas strength and stiffness are enhanced by surface nanocrystallization and/or nano-precipitation and change in the dominant deformation or wear mechanics.
Table 5.2
Recent research on the utilization of nanotechnology to improve the mechanical properties of steel surface
One recent advance in this field is the use of SMAT to produce 304 SS with outstanding strength–stiffness–ductility synergy, by forming hard nanocrystalline α' layer, α'–γ ultrafine layer, twinning layer, and compliant coarse-grained layer from outer to inner of the stainless steel. This bio-inspired design aims to benefit from the synergy of multiple deformation mechanisms and nano/micro-scale toughening. Figure 5.4 presents typical TEM images of 304 SS after SMAT and its corresponding selected area electron diffraction (SAED) patterns at four different depths (Chen et al., 2011).

Fig. 5.4 Typical TEM images of 304 SS after SMAT and its corresponding selected area electron diffraction (SAED) patterns at four different depths: 5, 100, 250, and 400 μm for (a)-(d) respectively. The lower inset of (a) is the grain size distribution. With kind permission from Elsevier Science (Chen et al., 2011)
Another advance in this field deals with the use of surface-modified nano-Cu as effective lubrication additive (at 10 vol% in oil). It works by ‘forming a chemical reaction film on the steel’ surfaces, thus improving their wear resistance and anti-fatigue performance of carbon steel (Zhang et al., 2010).
5.3.3 Nanotechnology to improve corrosion resistance of steel
The corrosion of steel as a result of chemical or electrochemical reaction with its service environment is a spontaneous process, which can compromise the integrity of the materials and impact assets, environment, and people if no measures are taken to prevent or control it. The corrosion of steel is generally electrochemical in nature, and may take many forms such as uniform corrosion, galvanic corrosion, pitting corrosion, crevice corrosion, underdeposit corrosion, dealloying, stress corrosion cracking (SCC), corrosion fatigue, corrosion wear, and microbially influenced corrosion (MIC).
Nanotechnology has also been employed to enhance the corrosion resistance of the steel bulk or surface layer. Table 5.3 provides a snapshot of recent research on this subject, involving various classes of steel: weathering steel, SS, and alloy steel, whereas the following sections review recent inventions on this subject. The improved resistance to pitting corrosion, corrosion, wear, or corrosion wear can be derived from nano-phases in the passive film, high density of GBs as nucleation sites for growing passive film, hardening by nano-precipitates or nano-grains, etc. As summarized by Lo et al. (2009), laser surface melting (LSM) can reduce the size of carbides and impurities (e.g., MnS) and alter the microstructure in the surface layer of SS, often leading to improved resistance to intergranular corrosion and pitting.
Table 5.3
Recent research on the utilization of nanotechnology to improve the corrosion resistance of steel

It should be cautioned that some production processes of steels may alter their microstructure and increase their susceptibility to cavitation erosion and hydrogen embrittlement (Lo et al., 2009). Amarnath and Namboodhiri (2003) revealed that for a high strength low alloy steel, heating followed by normalizing (air cooling) coarsened the grain sizes and lowered the dislocation density, which in turn decreased the number of hydrogen trap sites and increased the risk of hydrogen embrittlement. Relative to the as-received steel, its water quenching followed by tempering also led to higher risk of hydrogen embrittlement. It is hypothesized that a microstructure with high dislocation density and high concentration of GBs and other interfaces would be less prone to hydrogen embrittlement in steel. Addition of Mo and V nanoparticles has been reported to mitigate the delayed fracture of steel, by reducing the intergranular cementite and associated hydrogen embrittlement (Mann 2006). Addition of some alloying elements (e.g., Cu, Cr, Ni, Mo, W) can improve the corrosion resistance of steels.
First, nanotechnology has been utilized in endowing the steel bulk materials with excellent corrosion resistance, mainly by refining their crystal grains to nano-scale. The steel substrate with a nano-phased grain structure tends to have less defects or inhomogeneities where corrosion attack traditionally initiates and/or propagates. Miura et al. (2010) disclose a steel with an improved corrosion resistance and ultra-hardness and toughness, comprising an aggregate of α nano-crystal grains containing a solid-solution type N (0.1–2.0% by mass). The steel is prepared by MA of fine powders of α steel-forming components (e.g., Fe, Cr, Ni, Mn, and C) and an N-containing substance (e.g., N2, NH3, and nitride of Fe, Cr, and Mn), followed by forming-by-sintering treatment and subsequent annealing. The crystal grains are more finely divided on a nanometer scale by mixing a particle dispersant (e.g., AlN, NbN, TaN, Si3N4, or TiN) or mixing a metal oxide or a semimetal oxide in the MA process. Furthermore, an oxide, nitride, carbide, silicide, or boride of a metal or semimetal exists as a crystal grain growth inhibitor between and/or in the nano-crystal grains. Such SS, having a high N concentration (in place of expensive Ni or Mn), were reported to feature much improved resistance to corrosion and particularly to pitting corrosion as well as significantly reduced sensitivity to SCC.
Buck (2008) discloses the use of TMT to create a fine-grained microstructure imparting the steel good corrosion resistance, high strength, and high toughness. In one example, a 15 cm thick steel slab was first soaked at 1230 °C for 2 h ‘such that the structure is mostly face-centered-cubic (fcc) γ throughout the alloy’, before being hot-worked on a reversing rolling mill at a temperature between 1230 °C and 1150 °C. During the forming process, a true strain of 0.22–0.24 per pass was utilized to induce recrystallization. The resulting plate was then air-cooled to room temperature with or without further heat treatment, ultimately transformed into a fine-grained α' SS.
Wright and Jung (2006) disclose the invention of Cr-Ni-Co-Mo-Ti-Al SS with an excellent combination of strength, toughness, and corrosion resistance across a variety of strength levels. The SS feature ‘a predominantly lath-α' microstructure essentially without topologically close packed intermetallic phases and strengthened primarily by a dispersion of intermetallic particles primarily of the η-Ni3Ti phase’. The Ti and C levels are controlled ‘such that C can be dissolved during a homogenization step and subsequently precipitated during forging to provide a grain-pinning dispersion’ of carbides of Ti, V, Nb, or Ta.
Second, nanotechnology has been utilized in surface treatments to improve the performance and service life of steels in oxidizing and corrosive environments. A recent invention (Kerber, 2007) is directed to nanoparticle surface treatments and methods of providing such treatments for forming a thin oxide coating on alloys, thereby providing the substrate with enhanced corrosion and oxidation resistance. The disclosed method relates to such nanoparticles as CeO2, nanoceria, or an oxide of an element selected from the group consisting of Al, Si, Ti, Y, Nb, Zr, and other rare earth elements. One possible mechanism is that these elements exhibit a reactive element effect that decreases the oxide scale growth rate and reduces scale spallation by improving the scale–alloy adhesion. The invention suggests exemplary applications of this technology in protecting SS and Ni or Al alloys at high temperatures and in steam environments. The effectiveness of nanoparticle surface treatments in managing metallic oxidation and corrosion was demonstrated. For instance, the steel samples were dip-coated with nanoparticles in their respective solutions once or several times with intermediate drying at 200 °C. After heating to 1000 °C for 34 h, the 316 SS treated with nanoceria had a self-protective, thin, and adherent oxide film formed on its surface, whereas the sample without the nanoparticle treatment had thick, spalled oxide scale on its surface. Similarly, beneficial effects of nanoceria surface treatment for 430 and 410 SS were observed after heating to 800 °C in air for some time. Tests of nanocrystalline-coated and uncoated SS confirmed the corrosion resistance of the self-protective surfaces to humid air, to direct contact with liquid in the temperature range of 150 °C to 350 °C, to submerged service in high salinity solutions, and to the vapor phase above these solutions.
Another invention in this category (Sugama, 2009) presents methods of endowing the alloy surfaces with outstanding corrosion resistance by forming an ultrathin (preferably less than 10 nm), Cr-free film comprising an at least partially crosslinked amido-functionalized silanol component and nanoparticles of rare-earth metal oxide. The formed coatings were reported to provide better coverage of the substrate metal and similar or superior corrosion resistance than Cr-based coatings. For instance, one such coating demonstrated to extend the lifetime of the steel substrate under salt-fog test at 35 °C from approximately 10 h to approximately 768 h.
Third, nanotechnology has been utilized in decorative and protective coatings that provide the steel substrate with superior abrasion resistance and good corrosion resistance. Chen (2008) discloses the use of cathodic arc evaporation (CAE) for physical vapor deposition (PVD). For Zr, the resulting strike layer exists either as amorphous to nano-size crystals up to 50 nm or as preferentially-oriented crystals up to 80 nm in size, with a small percentage of amorphous refractory oxide acting as precipitation hardening particles. By maintaining the flow ratio of oxygen to argon into the vacuum chamber during CAE, a stoichiometric ZrO2 layer (preferably between 10 and 30 nm thick) is then deposited on the strike layer, which provides another non-conductive barrier layer to improve resistance to corrosion and pitting.
Chan (2006) discloses novel methods of depositing a nanocomposite coating of SS and a metallic carbide or metallic nitride onto a solid metallic substrate (e.g., SS) to increase its surface hardness. Unlike the continuous deposition process, very thin layers (e.g., 5–10 nm per layer) of such nanocomposite coating are deposited by reactive sputtering in a C or N2 gas plasma, using pure SS and Cr targets or their alloy targets. When the substrate is away from the deposition locations, the deposited SS and CrC (or CrN) phases were reported to relax into ‘their most thermodynamically suitable sites’. The hardness improvement was achieved by forming the nanocomposite structure in which the CrC nanophases precipitated along the SS GBs. This method features a clean process for obtaining a hard, wear-resistant, and corrosion-resistant coating with an SS-like appearance.
Namavar (2006) discloses an invention that provides metallic components (e.g., SS) with integrally formed, homo-metallic protective coatings on their surfaces. The deposited substance and the bulk substrate have at least one metallic constituent element in common; and the formed coatings feature crystalline grains preferably in a range of about 10–200 nm and thus an enhanced hardness and a high degree of resistance to corrosion and wear. To improve the adhesion of the coating to the substrate, the average crystalline grain size can decrease continuously from the substrate to the coating within the transition zone.
Detor and Schuh (2006) disclose the use of bipolar pulsed current (BPP) to produce alloy deposits with a specified nanocrystalline average grain size and thus superior macroscopic quality and/or resistance to corrosion and abrasion. Polarity ratio (characterized by the amplitude and/or duration of the negative pulse relative to those of the positive pulse) was used to enable ‘grading and layering of nanocrystalline crystal size and/or composition within a deposit’ without introducing voids and cracks. Relative to traditional microcrystalline metals, the nanocrystalline metal coatings with nano-grains are expected to show exceptional combination of properties such as excellent corrosion and wear resistance, enhanced yield strength and ductility, and desirable magnetic properties.
Finally, nanotechnology has been employed to alter the steel/electrolyte interface, by forming nano-modified polymeric coating on steel. The incorporation of nano-sized particles (e.g., SiO2, Fe2O3, and halloysite clay) into conventional polymer coatings can significantly enhance the anti-corrosive performance of such coatings on steel substrates (Shi et al., 2009). A comprehensive review on this subject, however, is beyond the scope of this chapter. A recent review in 2007 by Saji and Thomas discussed the incorporation of nanoparticles in ceramic coatings, polymer coatings, and hybrid sol–gel systems for improved properties (e.g., resistance to corrosion and high-temperature oxidation, self-cleaning, and anti-fouling).
5.4 Future trends
The use of nanotechnology has led to remarkable improvements in the mechanical properties and corrosion resistance of steels, by achieving the desirable microstructure of steels via phase transformation or deformation kinetics or by controlling the key chemistry down to the nanometer scale. By controlling the grain size and distribution and the heterogeneity and gradation of microstructure (e.g., quantity, morphology, and distribution of nano-phases), strength/toughness synergy and other properties of steels could be greatly enhanced. One interesting research need is to optimize the distribution and motion of dislocations in nanocomposite steels. Many of the R&D efforts detailed in previous sections will undoubtedly continue and more cost-effective solutions will emerge as a result of the advanced knowledge base and continuous improvements in R&D and in production technologies (e.g., high N-content SS). The great potential of nanotechnology in this field has not yet been fully achieved and in the near future new techniques and new applications can be expected along with new products to be introduced into the market. Multi-scale modeling of nanocomposite steels under mechanical or chemical stresses is crucial to unravel the role of dislocations, GBs, precipitates, and lattice defects and their interactions at various length scales. Continued developments in design and processing technologies can be expected to address needs in long-term durability, minimized maintenance, environmental sustainability, and/or competitive performance (e.g., high strength-to-weight ratio). To maximize the sustainability of using steels for construction, it is important to match the steel type to the working conditions and user requirements.
Nanotechnology has demonstrated its clear benefits and will continue to play a key role in the production of high performance steels. Future developments will be centered on furthering the understanding of why and how superior properties of steels can be achieved by the design and control of their chemical composition and morphology at the micro- and nanometer scales. Progress in steel production technology will open up the possibility of rapid change in steel metallurgy and increase the competitiveness of nano-enabled products. More research is needed to advance the knowledge base relevant to using nanotechnology to modify the surface layer or bulk material of steels and to shed light on the mechanisms and pathways defining the cause-and-effect relations that link metallurgy and production processes with microstructure and properties of steels.
The research in this domain is ongoing and the interdisciplinary nature of nanotechnology requires experts from a variety of disciplines working together to produce viable solutions. While the authors anticipate many evolutionary and revolutionary developments in this specific field, the ultimate market share of nanocomposite steels will depend on continued investment and efforts in R&D as well as market-driven product strategies (Osman et al., 2006). A multitude of technical and cost barriers remain for many of the inventions. For instance, concerns have surfaced about the responsible development, production, use, and disposal of some nanomaterials and related technologies. These are generally sparked by the nanosize effect and present unique challenges to be addressed before the successful commercialization of nanocomposite steels in some applications. For instance, Khettabi et al. (2008) indicated that the formation of nano- and micro-sized particles during metal cutting may pose a health risk.
5.5 References
Abe, F., Taneike, M., Sawada, K. Alloy design of creep resistant 9Cr steel using a dispersion of nano-sized carbonitrides. International Journal of Pressure Vessels and Piping. 2007; 84:3–12.
Ahmadabadi, M.N., Shirazi, H., Ghasemi-Nanesa, H., Nedjad, S.H., Poorganji, B., Furuhara, T. Role of severe plastic deformation on the formation of nanograins and nano-sized precipitates in Fe-Ni-Mn steel. Materials and Design. 2011; 32:3526–3531.
Amarnath, G.A.N., Namboodhiri, T.K.G. Effect of heat treatments on the hydrogen embrittlement susceptibility of API X-65 grade line-pipe steel. Bulletin of Materials Science. 2003; 26(4):435–439.
Askari, H.A., Shen, Y.F., Wang, C.M., Sun, X., Zbib, H.M., The effect of nano-precipitates on strength in a micro-alloyed ferritic steel. MRS Proceedings 1296. 2010, doi: 10.1557/opl.2011.1463. [mrsf10-1296-o05-04].
Averback, R.S. Sintering and deformation of nano-grained materials. Zeitschrift für Physik D: Atoms, Molecules and Clusters. 1993; 26:84–88.
Ba, D.M., Ma, S.N., Meng, F.J., Li, C.Q. Friction and wear behaviors of nanocrystalline surface layer of chrome-silicon alloy steel. Surface and Coatings Technology. 2007; 202:254–260.
Ba, D.M., Ma, S.N., Meng, F.J. The effect of initial microstructure on surface nanocrystallization of quenched and tempered steel. Advanced Materials Research. 2011; 148–149:778–782.
Bhadeshia, H.K.D.H. Properties of fine-grained steels generated by dis-placive transformation. Materials Science and Engineering A. 2008; 481–482:36–39.
Branagan, D.J., Sergueeva, A.V., Mukherjee, A.K. Towards the development of a new Iron Age. Advanced Engineering Materials. 2006; 8(10):940–943.
Buck, R.F. Method of producing fine-grained martensitic stainless steel. US patent. 2008; 7:470,336.
Chan, W.S.Y. Method of forming a nanocomposite coating. US patent. 2006; 7:001,675.
Chen, A.Y., Ruan, H.H., Zhang, J.B., Liu, X.R., Lu, J. Introducing a hierarchical structure for fabrication of a high performance steel. Materials Chemistry and Physics. 2011; 129:1096–1103.
Chen, G. Decorative and protective coating, 2008. [US patent 20080206580A1.].
Chipman, J. Thermodynamics and phase diagram of the Fe-C system. Metallurgical and Materials Transactions B. 1972; 3(1):55–64.
Detor, A.J., Schuh, C.A. Method for producing alloy deposits and controlling the nanostructure thereof using negative current pulsing electro-deposition, and articles incorporating such deposits, 2006. [US patent 20060272949A1.].
Du, L., Yao, S., Xiong, M., Liu, X., Wang, G. Austenite grain ultrarefinement and the formation of nanocrystallized structure through transformation of low carbon steels. Materials and Manufacturing Provesses. 2010; 25:26–32.
Eisenhüttenleute, V.D., Steel: A Handbook for Materials Research and EngineeringVolume 1: Fundamentals. New York: Springer-Verlag, 1992.
Eskandari, M., Kermanpur, A., Najafizadeh, A. Formation of nano-grained structure in a 301 stainless steel using a repetitive thermal-mechanical treatment. Materials Letters. 2009; 63:1442–1444.
Foroozmehr, F., Najafizadeh, A., Shafyei, A. Effects of carbon content on the formation of nano/ultrafine grained low-carbon steel treated by martensite process. Materials Science and Engineering A. 2011; 528:5754–5758.
Frontán, J., Zhang, Y., Dao, M., Lu, J., Gálvez, F., Jérusalem, A. Ballistic performance of nanocrystalline and nanotwinned ultrafine crystal steel. Acta Materialia. 2012; 60:1353–1367.
Gonsalves, K.E., Xiao, T.D., Chow, G.M., Law, C.C. Synthesis and processing of nanostructured M50 type steel. NanoStructured Materials. 1994; 4(2):139–147.
Guo, A., Misra, R.D.K., Xu, J., Guo, B., Jansto, S.G. Ultrahigh strength and low yield ratio of niobium-microalloyed 900 MPa pipeline steel with nano/ultra- fine bainitic lath. Materials Science and Engineering A. 2010; 527:3886–3892.
Hidaka, H., Kawasaki, K., Tsuchiyama, T., Takaki, S. Effect of carbon on nano-crystallization in steel during mechanical milling treatment. Materials Transactions. 2003; 44(10):1912–1918.
Hodgson, P., Timokhina, I., Xiong, X., Adachi, Y., Beladi, H. Understanding of the banite transformation in a nano-structured bainitic steel. Solid State Phenomena. 2011; 172–174:123–128.
Hosseini, S.M., Najafizadeh, A., Kermanpur, A. Producing the nano/ultrafine grained low carbon steel by martensite process using plane strain compression. Journal of Materials Processing Technology. 2011; 211:230–236.
Huang, S.-J., Semenov, V.I., Shuster, L., Lin, P.-C. Tribological properties of the low-carbon steels with different micro-structure processed by heat treatment and severe plastic deformation. Wear. 2011; 271(5–6):705–711.
Karak, S.K., Chudoba, T., Witczak, Z., Lojkowski, W., Manna, I. Development of ultra high strength nano-Y2O3 dispersed ferritic steel by mechanical alloying and hot isostatic pressing. Materials Science and Engineering A. 2011; 528:74757483.
Kaufman, L., Perepezko, J.H., Hildal, K., Farmer, J., Day, D., Yang, N., Branagan, D. Transformation, stability and Pourbaix diagrams of high performance corrosion resistant (HRCRM) alloys. CALPHAD: Computer Coupling of Phase Diagrams and Thermochemistry. 2009; 33:89–99.
Kerber, S.J. Nanoparticle surface treatment, 2007. [US patent 20070141370A1.].
Khettabi, R., Songmene, V., Masounave, J., Zaghbani, I. Understanding the formation of nano and micro particles during metal cutting. International Journal of Signal System Control and Engineering Application. 2008; 1(3):203–210.
Khodabakhshi, F., Kazeminezhad, M. The effect of constrained groove pressing on grain size, dislocation density and electrical resistivity of low carbon steel. Materials and Design. 2010; 32:3280–3286.
Khodabakhshi, F., Kazeminezhad, M., Kokabi, A.H. Constrained groove pressing of low carbon steel: nano-structure and mechanical properties. Materials Science and Engineering A. 2010; 527:4043–4049.
Kim, I.-S., Kchoi, C.-Y., Kang, C.-Y., Okuda, T., Maziasz, P.J., Miyahara, K. Effect of Ti and W on the mechanical properties and microstructure of 12% Cr base mechanical-alloyed nano-sized ODS ferritic alloys. ISIJ International. 2003; 43(10):1640–1646.
Kim, Y.-S., Yu, H.-S., Shin, D.-H. Low sliding wear resistance of ultrafine grained Al alloys and steel having undergone severe plastic deformation. Intl. J. Mater. Res.. 2009; 6:871–874.
Klueh, R.L., Hashimoto, N., Maziasz, P.J. Development of new nanoparticle-strengthened martensite steels. Scripta Materialia. 2005; 53:275–280.
Kolpakov, S.V., Parshin, V.A., Chekhovoi, A.N. Nanotechnology in the metallurgy of steel. Steel in Translation. 2007; 37(8):716–721.
Kozikowski, P.M., Krawczynska, A.T., Kulczyk, M., Lewandowska, M., Kurydlowski, K.J. Tailoring mechanical properties of nano-structured Eurofer 97 steel for fusion applications. Physica Status Solidi C: Current Topics in Solid State Physics. 2010; 7(5):1388–1390.
Kusinski, G.J., Pollack, D. Triple-phase nano-composite steels, 2008. [International patent application PCT/US2002/040126.].
Kusinski, G.J., Pollack, D., Thomas, G. Process for making triple-phase nanocomposite steels, 2004. [US patent 6,827,797.].
Kwok, C.T., Cheng, F.T., Man, H.C., Ding, W.H. Corrosion characteristics of nanostructured layer on 316 L stainless steel fabricated by cavitation-annealing. Materials Letters. 2006; 60:2419–2422.
Kwon, O., Lee, K., Kim, G., Chin, K.-G. New trends in advanced high strength steel developments for automotive application. Materials Science Forum. 2010; 638–642:136–141.
Lei, Y., Yu, X., Yu, S., Liu, Z. Present status and development direction of high performance structural material-oriented ultra-fine grained steel. Journal of China University of Petroleum. 2007; 31(2):155–162.
Lesuer, D., Syn, C., Sherby, O. Nano-scale strengthening from grains, sub- grains and particles in Fe-C alloys. Journal of Materials Science. 2010; 45:4889–4894.
Li, D., Chen, H.N., Xu, H. The effect of nanostructured surface layer on the fatigue behaviors of a carbon steel. Applied Surface Science. 2009; 255:3811–3816.
Li, X., Wei, Y., Lu, W., Lu, K., Gao, H. Dislocation nucleation governed softening and maximum strength in nano-twinned metals. Nature. 2010; 464(8):877–880.
Li, X., Jing, T.F., Lu, M.M., Xu, R., Liang, B.Y., Zhang, J.W. Fatigue property of nano-grained delaminated low-carbon steel sheet. Journal of Materials Science and Technology. 2011; 27(4):364–368.
Liu, G.Z., Tao, N.R., Lu, K. 316 L austenite stainless steels strengthened by means of nano-scale twins. Journal of Materials Science and Technology. 2010; 26(4):289–292.
Liu, L., Jie, X., Yu, N., Mai, Y. Study on surface nano-crystallization by adding pressure shot peening and its effect on wear resistance. Hot Working Technology. 2009; 38(14):124–126.
Lo, K.H., Shek, C.H., Lai, J.K. Recent developments in stainless steels. Materials Science and Engineering R. 2009; 65:39–104.
Lu, K., Lu, L., Suresh, S. Strengthening materials by engineering coherent internal boundaries at the nanoscale. Science. 2009; 324(5925):349–352.
Lu, K., Yan, F.K., Wang, H.T., Tao, N.R. Strengthening austenitic steels by using nanotwinned austenitic grains. Scripta Materialia. 2012; 66(11):878–883.
Malow, T.R., Koch, C.C. Grain growth in nanocrystalline iron prepared by mechanical attrition. Acta Materialia. 1997; 45(5):2177–2186.
Mann, S., Nanotechnology and ConstructionEuropean Nanotechnology Gateway – Nanoforum Report. Institute of Nanotechnology, 2006. [2–10].
Matsuia, K., Ohmichi, N., Ohgai, M., Yoshida, H., Ikuhara, Y. Effect of alumina-doping on grain boundary segregation-induced phase transformation in yttria-stabilized tetragonal zirconia polycrystal. Journal of Materials Research. 2006; 21:2278–2289.
Menapace, C., Lonardelli, I., Tait, M., Molinari, A. Nanostructured/ultrafine multiphase steel with enhanced ductility obtained by mechanical alloying and spark plasma sintering of powders. Materials Science and Engineering A. 2009; 517:1–7.
Miura, H., Miyao, N., Ogawa, H., Oda, K., Katsumura, M., Mizutani, M. Nano-crystal austenitic steel bulk material having ultra-hardness and toughness and excellent corrosion resistance, and method for production thereof, 2010. [US patent 7,662,207.].
Murayama, M., Nishimura, T., Tsuzaki, K. Nano-scale chemical analysis of rust on a 2% Si-bearing low alloy steel exposed in a coastal environment. Corrosion Science. 2008; 50(8):2159–2165.
Namavar, Namavar, F., Nano-crystalline, homo-metallic, protective coatings, 2006. [US patent 7,048,767.].
Ohtsuka, S., Ukai, S., Sakasegawa, H., Fujiwara, M., Kaito, T., Narita, T. Nano-mesoscopic structural characterization of 9Cr-ODS martensitic steel for improving creep strength. Journal of Nuclear Materials. 2007; 367–370:160–165.
Okamura, Y., Okushima, M., Tamehiro, H., Kasuya, T., Tanaka, M., Yamaba, R., Inoue, H., Seto, A. Development of copper precipitation-hardened 780 N/ mm2 high-strengh steel with lower preheating temperature characteristics. Nippon Steel Technical Report. 1995; 66(7):65–76.
Oksiuta, Z., Lewandowska, M., Unifantowicz, P., Baluc, N., Kurzydlowski, K.J. Influence of Y2O3 and Fe2Y additions on the formation of nano-scale oxide particles and the mechanical properties of an ODS RAF steel. Fusion Engineering and Design. 2011; 86:2417–2420.
Osman, F.M., Rardon, D.E., Friedman, L.B., Vega, L.F. The commercialization of nanomaterials: today and tomorrow. JOM. 2006; 4:21–24.
Saji, V.S., Thomas, J. Nanomaterials for corrosion control. Current Science. 2007; 92(1):51–55.
Shi, X., Nguyen, T.A., Suo, Z., Liu, Y., Avci, R. Effect of nanoparticles on the anticorrosion and mechanical properties of epoxy coating. Surface and Coatings Technology. 2009; 204(3):237–245.
Smith, W.F. Structure and Properties of Engineering Alloys. New York: McGraw-Hill; 1981.
Sokolov, M.A., Hoelzer, D.T., Stoller, R.E., McClintock, D.A. Fracture toughness and tensile properties of nano-structured ferritic steel 12YWT. Journal of Nuclear Materials. 2007; 367–370:213–216.
Song, H.W., Guo, S.R., Hu, Z.Q. A coherent polycrystal model for the inverse Hall–Petch relation in nanocrystalline materials. NanoStructured Materials. 1999; 11(2):203–210.
Sugama, T. Corrosion-resistant metal surfaces, 2009. [US patent 7,507,480.].
Suh, C.-M., Song, G.-H., Suh, M.-S., Pyoun, Y.-S. Fatigue and mechanical characteristics of nano-structured tool steel by ultrasonic cold forging technology. Materials Science and Engineering A. 2007; 443:101–106.
Taneike, M., Abe, F., Sawada, K. Creep-strengthening of steel at high temperatures using nano-sized carbonitride dispersions. Nature. 2003; 424:294–296.
Tsuji, N., Maki, T. Enhanced structural refinement by combining phase transformation and plastic deformation in steels. Scripta Materialia. 2009; 60:1044–1049.
Valiev, R.Z. Materials science: nanomaterial advantage. Nature Materials. 2004; 3:511–516.
Vaynman, S., Fine, M.E., Asfahani, R.I., Bormet, D.M., Hahin, C., High performance copper-precipitation-hardened steel. Microalloyed Steels 2002: Proceedings of the International Symposium on Microalloyed Steels. 2002:43–48. [Columbus, OH].
Verezub, O., Kálazi, Z., Sytcheva, A., Kuzsella, L., Buza, G., Verezub, N.V., Fedorov, A., Kaptay, G. Performance of a cutting tool made of steel matrix surface nano-composite produced by in situ laser melt injection technology. Journal of Materials Processing Technology. 2011; 211:750–758.
Wang, J., Li, G., Xiao, A., Fu, J. Nano-scaled Fe3C precipitation strengthening in hot rolled low carbon high strength titanium microalloyed steel. Advanced Materials Research. 2011; 146–147:838–843.
Wang, L., Wang, Z., Guo, S., Lu, K. Annealing-induced grain refinement in a nanostructured ferritic steel. Journal of Materials Science and Technology. 2012; 28(1):41–45.
Wang, X.Y., Li, D.Y. Mechanical, electrochemical and tribological properties of nano-crystalline surface of 304 stainless steel. Wear. 2003; 255(7):836–845.
Weertman, J.R. Hall–Petch strengthening in nanocrystalline metals. Materials Science and Engineering A. 1993; 166:161–167.
Wright, J.A., Jung, J.-W. Martensitic stainless steel strengthened by Ni3Ti h-phase precipitation, 2006. [WO patent 2006/081401A3.].
Wu, Z.X., Zhang, Y.W., Srolovitz, D.J. Dislocation-twin interaction mechanisms for ultrahigh strength and ductility in nanotwinned metals. Acta Materialia. 2009; 57:4508–4518.
Wu, Z.X., Zhang, Y.W., Srolovitz, D.J. Deformation mechanisms, length scales and optimizing the mechanical properties of nanotwinned metals. Acta Materialia. 2011; 59:6890–6900.
Xu, L., Shi, J., Cao, W.Q., Wang, M.Q., Hui, W.J., Dong, H. Improved mechanical properties in Ti-bearing martensitic steel by precipitation and grain refinement. Journal of Materials Science. 2011; 46:6384–6389.
Xu, Y.H., Peng, J.H., Fang, L. Nano-crystallization of steel wire and its wear behavior. Materials Science and Engineering A. 2008; 483–484:688–691.
Yan, F.K., Liu, G.Z., Tao, N.R., Lu, K. Strength and ductility of 316 L austenitic stainless steels strengthened by nano-scale twin bundles. Acta Materialia. 2012; 60:1059–1071.
Zhang, T., Wu, Y., Yi, Z., Zhang, X., Wang, X. Nano-phases and corrosion resistance of C + Mo dual implanted steel. Science in China E. 2001; 44(4):383–388.
Zhang, Y.D., Yan, J.S., Yu, L.G., Zhang, P.Y. Effect of nano-Cu lubrication additive on the contact fatigue behavior of steel. Tribology Letters. 2010; 37:203–207.
Zhang, Z.W., Liu, C.T., Guo, S., Cheng, J.L., Chen, G., Fujita, T., Chen, M.W., Chung, Y.-W., Vaynman, S., Fine, M.E., Chin, B.A. Boron effects on the ductility of a nano-cluster-strengthened ferritic steel. Materials Science and Engineering A. 2011; 528:855–859.
Zhao, M.-C., Zeng, T.-Y., Li, J.-L., Huang, X., Zhao, Y.-C., Atrens, A. Identification of the effective grain size responsible for the ductile to brittle transition temperature for steel with an ultrafine grain size ferrite/cementite microstructure with a biomodal ferrite grain size distribution. Materials Science and Engineering A. 2011; 528:4217–4221.
