Safety issues relating to nanomaterials for construction applications
Abstract:
Nanotechnology holds great promise for advancements in medicine, basic science and engineering, not the least for the construction industry. Here, nanomaterials are used in a variety of eco-efficient applications including improved mechanical properties, interior light control, renewable energy harvesting, and advanced durability. However, the long-term safety aspects of these novel materials are poorly understood. While toxicological liabilities have been discovered in the laboratory for all known classes of nanomaterials, it is unclear how much toxic potential for humans and the environment various types of nanoscale materials pose. The number of manufactured nanomaterials (MNMs) keeps growing at an exponential pace and traditional in vivo toxicity approaches are unable to keep up with the sheer number of MNMs and MNM-composite materials that will be used in construction. Fortunately, the application of high-throughput in vitro methodologies allows insight to be gained into the underlying nanotoxicity paradigms, which in turn will enable an understanding of the nano-properties that cause the toxic effects of a given nanomaterial and thus informs on safe design features, enabling the safe employment of this powerful technology. This chapter aims to give a bird’s eye view of nanomaterials used in construction, their potential toxicological liabilities, and high throughput methodologies that can be employed towards detecting nanotoxicity during the safety assessment of nanomaterials.
7.1 Introduction to nanotoxicity
Nanotechnology is at a critical juncture. Enough is known about the technology to see the enormous potential, but at the same time, the risk associated with this technology is not entirely clear (Damoiseaux et al., 2011). While in the last two decades nanotechnology has been applied to many diverse fields and has been included in many consumer products (Gopel, 1991; Fahy, 1993; Murphy et al., 1994; Ferrari, 2005), little is known about the long-term effects nanomaterials might have on health and the environment. Manufactured nanomaterials (MNMs) have already played a critical role in the medical and electronic fields, while the construction industry has only recently been exploring nanotechnologies for means to advance traditional construction methods and materials (Zhu et al., 2004; Ge and Gao, 2008; Alvarez et al., 2010). The reason for the implementation of MNMs in construction lies in the unique physical and chemical properties of these nanomaterials that can lead to the improvement of numerous characteristics of construction materials (Tans et al., 1998; Chan et al., 2002; Daniel and Astruc, 2004; Arico et al., 2005). It is the very same properties that make nanomaterials so unique that give rise to concern about their safety (Lee et al., 2009).
The diversity of MNMs is staggering. On a broad scale MNMs can be subdivided into classes such as metals, metal oxides, metal chalcogenides (‘quantum dots’), fullerenes, single-walled nanotubes (SWNT), multi-walled nanotubes (MWNT), dendrimers, etc. However, the members of these classes are typically fine-tuned toward their applications by the variation of properties, such as size, shape, aspect ratio, crystallinity, and surface modifications. At this point in time, we are only beginning to understand the interactions between nanomaterials and biomaterials that would enable us to rationally predict the positive or negative impact of MNMs. We are learning about the MNM properties that make an MNM safe or hazardous but are still forced to test each MNM for its safety profile empirically for the foreseeable future while over 1000 MNMs can be already found in consumer products – and the number is rising exponentially.
Traditional in vivo experiments have been performed to evaluate cytotoxicity of many MNMs using live organisms such as laboratory mammals. However, due to the sheer diversity of novel MNMs, this is not only financially unsustainable but time-consuming. More progressive methods employed are typically based on in vitro approaches or modeling of surface activity relationships. In vitro approaches allow for greater control over the experimental parameters, the assay readouts can be better tailored to answer specific questions regarding specific toxicity paradigms, and the process is generally less expensive. Most importantly, in vitro assays are amenable to high-throughput methodologies, which enable us to logistically manage the testing of this avalanche of novel MNMs in an effective manner, provide rapid feedback for the development of novel MNMs by enabling us to give the go-ahead to suitable MNMs and minimize hazardous MNMs.
Here, we discuss applications of MNMs in construction, review potential hazards of these materials and give an introduction to high-throughput nanotoxicology as an effective means toward the safety assessment of MNMs. We will also review an example of a safe design feature for zinc nanomaterials and review the current state of a priori prediction of nanotoxicity.
7.1.1 Naturally occurring nanomaterials
Nanomaterials are not necessarily manmade; many are created naturally through a variety of weather and geological phenomena. These include volcanic ash, ocean spray, forest fire smoke, and clouds (Goldman and Coussens, 2005). Some of these naturally occurring nanomaterials, such as nanoclays, are mined and have applications in composite building materials (Faruk and Matuana, 2008; Basak et al., 2010). Many nanoparticles (NPs) can also be found in biological systems such as lipoprotein particles (German et al., 2006) and biogenic magnetite in the human brain (Kirschvink et al., 1992). Generally, these nanomaterials are created incidentally, like in the case of volcanic ash, or for an evolutionary purpose, such as the transport of fat molecules by lipoproteins.
7.1.2 Engineered nanomaterials and their use in construction
Unlike naturally occurring nanomaterials, engineered nanomaterials are typically manufactured for specific properties. MNMs can be tailored for application to a wide range of products including personal care products, medical devices, and electrical conductors (Derno et al., 1995). For example, nano-TiO2 has been used in sunscreens and lotions as a UV absorber (Contado and Pagnoni, 2008). Other consumer products such as toys and clothing use Ag NPs as an antimicrobial agent (Benn et al., 2010). In the medical field, numerous advances in imaging and drug-delivery systems have been made using MNMs (Sosnik et al., 2010; Parveen et al., 2012). Quantum dots (QDs) are unique metallic nanomaterials with advanced electronic properties used in transistors and solar cells (Leobandung et al., 1995; Nozik, 2002).
In the construction industry, MNMs have been used to improve the mechanical strength of concrete and steel, fireproofing of windows, electricity generation, and corrosion resistance (Zhu et al., 2004; Mann, 2006; Abraham et al., 2008). Manufactured nanomaterials are used in a wide range of construction applications from concrete and steel to glass windows and paint (Irie et al., 2004; Sobolev and Gutierrez, 2005; Ge and Gao, 2008; Kumar et al., 2008; Rana et al., 2009; Raki et al., 2010). There are several distinct categories of potential benefits that may be gained from the use of these materials such as improved safety, user convenience, enhanced lifetime of the structure, and increased ease of construction. It is also possible for one nanomaterial to provide various benefits spanning multiple benefit categories. For example, SiO2-NPs incorporated in window glass confer flame resistance, anti-reflection, and self-cleaning, thus improving both safety and auxiliary properties (Mann, 2006; Rana et al., 2009).
Carbon-based MNMs, such as carbon nanotubes, are some of the strongest materials currently known (Hayashi et al., 2007). For this reason, they are often used in concrete and ceramics to improve the mechanical strength and durability (Becher, 1991; Luo et al., 2004; Sobolev and Gutierrez, 2005; de Ibarra et al., 2006; Ge and Gao, 2008; Raki et al., 2010). In addition, carbon nanotubes help to prevent cracks by strongly binding together cement and aggregates. Similarly, these materials are incorporated into ceramics to also prevent crack propagation, and improve strength and thermal properties. Alternate uses include nano- and micro-sensors and actuators implanted into the structure to monitor real-time health and environmental conditions such as overall wear, moisture content, and temperature (Zhang et al., 2006). These devices are known as nano- or microelectro-mechanical systems (NEMS/MEMS). Carbon-based MNMs are capable of enhanced electron shuttling and can be used to harvest renewable energy in solar cells (Girishkumar et al., 2005; Brown and Kamat, 2008).
In addition to carbon-based nanomaterials, metallic, non-metallic, and alloyed MNMs also have beneficial uses in the construction industry. Metal oxide NPs are used to reinforce the mechanical and compressive strength of concrete, generate non-utility electricity in solar cells, provide flame resistance to ceramics and windows, and increase the hydration ability of cement. Common metal oxide NPs used in construction are TiO2, SiO2, and Fe2O3. Often, SiO2 and Fe2O3 are used as filling materials in the pores of concrete to prevent weakening from road deicers, such as salt, that react with the concrete constituents. When incorporated into concrete, these NPs also enhance the mechanical strength.
Nano-scale TiO2 and SiO2 are also utilized in windows, pavements, walls, and roofs to gain several useful benefits. Layers of nano-silica between glass window panels can provide fireproofing, where antireflective coatings of SiO2 nanoparticles will control exterior light to improve energy conservation via reduction of air conditioning usage (Mann, 2006; Rana et al., 2009). Reactive oxygen species (ROS) can be generated through reactions between TiO2 and UV wavelengths from artificial or natural light, making TiO2 an excellent antimicrobial agent and dirt-repellent (Paz et al., 1995; Irie et al., 2004). By coating windows with TiO2, bacterial films and dirt buildup will be eliminated by these ‘self-cleaning’ windows. Titanium dioxide is also superhydrophilic, which aids in the prevention of hydrophobic dust accumulation. Similar results can be obtained on pavements, walls, and roofs, as TiO2 will also act as an antifouling agent under solar irradiation. Additionally, light-mediated TiO2 surface hydroxylation provides glass windows with antifogging properties (Irie et al., 2004; Kontos et al., 2007). Electricity generation is possible through the use of TiO2 and silicon-based flexible solar cells applied to roofs and windows (Zhu et al., 2004).
Popular metallic MNMs used in construction include copper and silver. The most common use for Cu NPs is incorporation into steel to improve weldability and provide resistance to corrosion (Ge and Gao, 2008). Like TiO2, Ag NPs are also strong antimicrobials and can be used in paints and wall coatings to inactivate pathogenic microbes (Kumar et al., 2008). Silver NPs can also be utilized indoors since their antimicrobial activity is not photo-assisted. This is particularly useful in hospitals and childcare facilities. Copper and CuO have been shown to have antimicrobial properties as well, but this may be a species-specific phenomenon (Ruparelia et al., 2008). Nonetheless, Cu and CuO are sometimes used as a biocide instead of silver.
Many MNM alloys also have a niche in construction. These materials include metallic carbon or nitrogen compounds, QDs, and nanoclays. When uniformly dispersed through a steel matrix, metallic carbonitrides increase strength against creep by two orders of magnitude (Taneike et al., 2003). Similar to metal oxide NPs like TiO2 and SiO2, QDs can also be used in windows to control interior light by being translucent in the visible spectrum to increase intensity and being reflective in the infrared spectrum to impede thermal transfer (Anikeeva et al., 2009). A variety of non-metal polymeric NPs are used in matrices as constituents in windows, antibacterial coatings, and nano-clay composites (Chauhan et al., 2006). Incorporation of polymeric MNMs can increase tensile strength and flexibility of construction materials (Podsiadlo et al., 2007). Nanoclays and polymer-clay nanocomposites are used as filler materials or to increase compressive strength (Podsiadlo et al., 2007). When nanoclays are incorporated into wood/plastic composites, the resulting material gains enhanced mechanical properties and improved rot-resistance (Faruk and Matuana, 2008).
7.2 Potential nano-hazards of manufactured nanomaterials (MNMs) utilized in construction
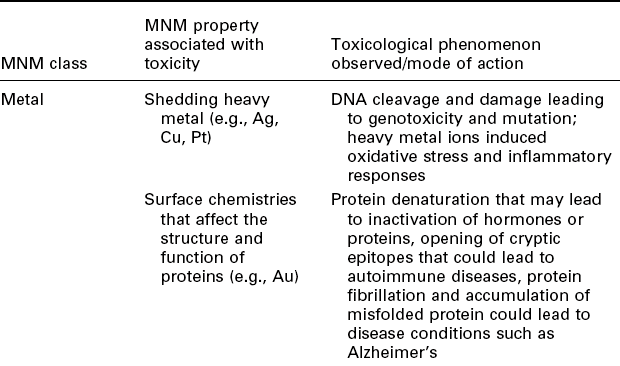
The unique characteristics of MNMs that allow them to enhance construction are frequently the very source of their hazardous properties. Carbonbased, metal-containing, and non-metallic MNMs, including carbon fullerenes, metal oxide and metallic NPs, quantum dots, and nanoclays, have been shown to have toxic effects in a variety of studies (Shiohara et al., 2004; Lam et al., 2006; Buzea et al., 2007; Hagens et al., 2007; Karlsson et al., 2008; Xia et al., 2009; Zolnik et al., 2009). It has been further suggested that MNMs have the potential to modulate the immune system in unpredictable ways (Dobrovolskaia and McNeil, 2007; Dwivedi et al., 2011). It is useful to know that a given class of nanomaterials frequently shares its toxicological traits with many of its members. Table 7.1 shows the properties of each MNM class that are frequently associated with their toxic effects. Table 7.2 gives the reverse view: shown are the nano-hazards of MNMs frequently utilized in construction. The reader should keep in mind that the field is very much in flux and thus our tables are unlikely to be comprehensive. We discuss some of the nanotoxicological issues of individual classes of MNMs in order to demonstrate the range of different toxicity paradigms that can be encountered when dealing with different MNM classes.
Table 7.1
Overview of MNM classes and examples of properties which are associated with toxicity responses to the respective MNM class and the observed phenomenon or mode of action

7.2.1 Carbon-based nanomaterials
Frequently used MNMs containing primarily carbon atoms enclosing a hollow interior include carbon nanotubes (CNTs) and fullerenes such as C20, C60, and C60 derivatives. Specifically, carbon nanotubes, C60 fullerenes, and C60 derivatives are currently raising health concerns as they have been shown to have adverse effects on bacterial, mammal and human cells (Jia et al., 2005; Park et al., 2010). CNTs are frequently contaminated with heavy metals, which is due to their particular production process. This brings up a common issue of MNMs: MNMs can be contaminated with other elements that might have toxicological relevance. Stringent quality control of the nanomaterials for such contaminations is essential.
Carbon nanotubes
Available in both single-walled (SWCNT) and multi-walled (MWCNT) forms, carbon nanotubes have been shown to exert bacterial toxicity via direct cell wall damage or oxidative stress (Kang et al., 2007, 2008a, 2008b, 2009). More relevantly, however, studies have shown that both types can cause pulmonary inflammation, fibrosis, and epithelioid granulomas in mammalian cells when respired (Ding et al., 2005; Jia et al., 2005; Wei et al., 2007). CNTs are likely to enter unprotected lungs because they are of breathable size and weight (Soto et al., 2008; Herzog et al., 2009). In a study performed on rats, it was concluded that MWCNTs pose a carcinogenic threat, inducing mesothelioma in exposed organisms (Basak et al., 2010). Other toxicological implications include damage to mitochondrial DNA (Derno et al., 1995), cellular apoptosis and necrosis (Hoffmann et al., 1995, Hoffmann, 1995), and reproductive toxicity (Hansen et al., 2008).
Additionally, heavy metal ions often become imbedded in the CNTs during the production phase. A common method for nanotube synthesis is chemical vapour deposition, which employs the use of a metal or alloyed catalyst such as iron, cobalt, or nickel. Ions from these metals will incidentally become bound within the CNTs. These metallic impurities can lead to toxic effects, which are not specifically ‘nano’ in nature; they still contribute to overall toxicological liability of CNTs (Vecitis et al., 2010).
C60 fullerenes and their derivatives
C60 fullerenes may be respired during the preparation process, causing lung inflammation (Park et al., 2010). In addition to their use as raw nanomaterials, these fullerenes are often suspended as water-stable aggregates. The resulting fullerene solution has been shown to have broad antimicrobial potential (Lyon et al., 2005, 2006). While there have been hypotheses that this cytotoxicity is mediated by oxidative stress from ROS, recently it has been shown that direct cell membrane oxidation from C60 contact is likely responsible (Lyon and Alvarez, 2007; Lyon et al., 2008). In eukaryotes, this oxidative stress is also responsible for cell death, leading to lipid peroxidation (Oberdörster, 2004; Sayes et al., 2005). Derivatives of C60 fullerenes, such as fullerol and carboxyfullerene, can cause cytotoxicity by physical membrane damage (Tsao et al., 2002) as well as by oxidative routes (Faruk and Matuana, 2008).
7.2.2 Metal-containing nanoparticles
Metals and metalloids are common components in manufactured nanomaterials and include titanium, copper, silver, iron, and zinc. These elements can be used in pure nanoparticle form as with copper and silver, or as metal oxides like titanium dioxide and copper oxide.
Titanium dioxide nanoparticles
Titanium dioxide (TiO2) NPs can cause cell death, inflammation, and DNA damage in mammalian cells by producing ROS under the presence of sunlight or UV light (Oberdorster et al., 1995; Zhang et al., 1998; Sayes et al., 2006; Park et al., 2007; Handy et al., 2008; Karlsson et al., 2008; Reeves et al., 2008; Zhu et al., 2008). While this irradiation gives TiO2 its antibacterial property, it can potentially damage human cells by direct chemical oxidation (Dunford et al., 1997). Additionally, TiO2 NPs inhibit growth and suppress photosynthetic activity in algal cells. For these reasons, TiO2 NPs are considered to be acutely lethal to microorganisms. In mammalian cells, a reduction in metabolic and mitochondrial activities has been observed when exposed to TiO2 NPs.
Copper and copper oxide nanoparticles
Copper and CuO nanoparticles are shown to induce oxidative stress and cause damage to DNA via single strand breaks in a variety of organism and mammalian cells including bacteria, algae, yeast, as well as mouse and humans cell lines. In the mouse model, acute toxicity to the spleen, liver, and kidneys was observed (Chen et al., 2006). Additional effects include lipid peroxidation and necrosis of hepatocytes. Studies indicate that the toxic effects resulting from exposure to Cu NPs are most likely mediated by ion release.
Silver nanoparticles
Silver NPs have been used in a wide range of applications as an antimicrobial agent. These NPs are known to have cytotoxic effects in algae, bacteria, mammalian, and yeast cells. Additionally, the extent of toxicity, including developmental toxicity and genotoxicity, has been observed to be dependent on cell type and particle size (Park et al., 2011). In vitro studies show that oxidative stress from the production of ROS is likely the largest contributor to the Ag NP toxic effects. Like Cu NPs, ions released from Ag NPs are the facilitators of these toxic effects, including the aforementioned ROS generation.
7.2.3 Non-metal nanoparticles
Like many other nanomaterials, both silicon dioxide (SiO2) and nanoclays have been shown to produce toxic effects in microorganisms and mammalian cells. While these particular nanomaterials do not contain heavy metals, toxic effects have nonetheless been associated with them, as demonstrated by several studies: bacteria, algae, and mammalian cells are all targets of its ROS mediated cytotoxicity and membrane damage and carcinogenic effects have been observed.
Silicon dioxide nanoparticles
Studies indicate that exposure to SiO2 NPs can cause lipid peroxidation and membrane damage to human lung cell lines (Lin et al., 2006). In the rodent model, experiments indicate that these particles may induce tumor necrosis genes and might have carcinogenic activity. The generation of ROS appears to be a large contributor to the toxicity of SiO2. Interestingly, the relative surface area of the particles is related to the toxicity effects in as much as a larger surface area caused stronger cytotoxicity effects in mammalian and algal cells. In microorganisms, experiments suggest that the direct interactions of SiO2 particles attached to cell membranes are related to the mechanism of cellular toxicity. Additionally, the cell division of microalgae is hindered by the presence of SiO2 NPs.
Nanoclay particles
Nanoclays are an umbrella term for a diverse group of MNMs and range in chemical makeup and crystalline structure. These particles are often used in polymer matrices to increase flexibility, durability, and strength but can be found as well as filler materials in construction nanocomposites. Studies in the rodent model indicate toxic effects via cell membrane damage. Specifically, sepiolite nanoclays have been found to elicit multinucleated macrophage agglomerates. In vitro studies on human cell lines demonstrate intracellular ROS generation, leading to oxidative stress and cell death.
7.2.4 Bimetallic alloys
In construction, this category of nanomaterials consists of semiconducting alloys in a core/shell configuration called quantum dots, which usually contain toxic heavy metals like cadmium. These quantum dots can dissolve in the digestive tract of rats upon ingestion and release these toxic components (Karabanovas et al., 2008).
Quantum dots
Quantum dots may be toxic due to a variety of mechanisms depending on their composition. Most common is the release of toxic ions from the heavy metals contained within the QDs core. These heavy metals may include cadmium, lead, and zinc and are extremely toxic to both mammalian cells and bacteria. Experiments performed on human cells indicate that cytotoxicity results from ROS damage to organelles and inflammation caused by the release of cytokines. Quantum dots can accumulate in the spleen, liver, and kidneys in mice, creating localized toxic effects. In microorganisms, other toxicity mechanisms include growth inhibition, lipid peroxidation, oxidative stress. In addition to the cellular toxicity caused by the heavy metal core, some shell materials have also been identified as toxic. Gene expression studies in algae show that toxic responses to intact QDs differ significantly from responses to internal ions: in eukaryotic cells, oxidative stress, damage to nucleic acids contribute to cytotoxicity independently of the release of internal heavy metal ions from their core. Additionally, QD toxicity experiments on mice neural cells demonstrate impaired calcium influx and exocytotic mechanisms.
7.3 Lifecycle of nano-enabled structures
Manufactured nanomaterials may be released into the environment over the entire lifecycle of the structure, from the time of construction, throughout the use of the structure, and after demolition and disposal. It is important to realize that these materials may transform over time via physical, chemical, or biological processes.
It is important to recognize that much research needs to be conducted to fill in the knowledge gaps regarding aging MNMs in structures. Few studies currently published have investigated long-term physical and chemical changes of imbedded MNMs and the associated hazards. For example, if an MNM is imbedded into a concrete floor or pavement, continual traffic and abrasion will inevitably cause the release of nanomaterials.
7.3.1 Manufacturing of nanomaterials and use in construction
One of the common human exposure routes for nanomaterials is through inhalation. This threat is prevalent largely during the periods of manufacturing and construction due to the high levels of airborne or aerosolized particles. Though unintentional, carbon fullerenes may be aerosolized during the aqueous suspension process, which might require sonication. These and other types of nanomaterials can become airborne when exposed to open air for weighing. Sepiolite nanoclay, which may be used as filler in construction nanocomposites, is lost to the air during mining, transportation, and the manufacturing process.
The most vital step to ensure exposure prevention is an accurate assessment of potential chemical or physical reactions that may occur during the lifecycle of the MNM. In other words, it is the responsibility of the manufacturing and construction industries to prevent any transformations of the MNM so that the creation of hazardous byproducts can be avoided. In addition, it is undesirable to incidentally modify the unique properties of the MNMs being used since this will likely make them behave less efficiently than design has intended, and perhaps cause more adverse structural or health effects than if they were not incorporated at all.
Since there are many factors involved in determining the relationships between the structure and the MNM, thorough physicochemical studies should be made by the manufacturer during the development process to identify any unfavorable conditions and reactions. Here, the fate, behavior, and environmental reactions such as adsorption and desorption, particle aggregation, reduction-oxidation reactions, deposition, or ion dissolution should be investigated for each MNM. Similarly, the construction companies using these new materials should consider the findings provided by the manufacturer and determine the safest way to incorporate the MNMs into the structure without taking away from their desired benefits. This may include encapsulation or coatings to reduce dangerous interactions with other structural components. Contractors should also consider minimizing the amount of MNMs used in a project when one material may do the work of many. It may be safer to use the same MNM for a variety of purposes than to use a different MNM for each purpose so that potential damaging reactions may be minimized and an increase in adverse health effects is kept low.
7.3.2 Useful life of the structure
Throughout the use of the structure, MNMs may still be released even when proper construction practices have been used. Unanticipated environmental conditions, weather phenomena, vandalizing, and wear and tear can cause cracks to form, paint to peel, and internal structural components to be exposed. When such damage occurs, MNMs may be released into the environment to be taken up by users via inhalation or ingestion, or they may be chemically/physically transformed by new unfavorable conditions. In addition to allowing MNMs to release from the construction, structural flaws also permit water and other reactants to enter through openings to transform the MNMs. Depending on the material used, hazardous byproducts may be created or unveiled, reducing the usefulness of the nanomaterial.
7.3.3 Demolition, disposal, and recycling
To reduce the consumption of raw MNMs and minimize waste, recycling and reusing MNMs from construction materials is a safe alternative. Demolition must be controlled and monitored to prevent the release of MNMs into the environment and community. As with the manufacturing and construction phases, MNMs are capable of becoming airborne during deconstruction and disposal, posing potential health risks for workers. Dust can contain MNMs that may be inhaled, ingested, or cause eye irritation.
Prior to demolition, efficient strategies for collecting the used MNMs from complex media should be developed so that the unique properties of the MNMs remain intact and human or environmental exposure is minimized. The MNMs should be characterized and evaluated for their ability to be reactivated and reused. The ease and costs of extraction should also be considered in determining the recycling potential of an MNM. The remaining MNM-containing construction wastes must be disposed of properly to prevent release and/or transformation of MNMs (Bystrzejewska-Piotrowska et al., 2009). Each MNM may have special disposal requirements according to the manufacturer or other regulatory frameworks so a thorough investigation of these procedures should be performed in advance. Reinforcement barriers in landfills are recommended to prevent MNM leachate from contaminating groundwater and underlying aquifers. Once the appropriate disposal measures have been taken, continual interception and remediation methods must be developed to monitor secondary MNM release into the environment.
7.4 Toxicity profiling for nanomaterials
Currently, there is considerable debate about how to proceed with engineered nanomaterials (ENM) toxicity testing, with the major discussion points centering around which toxicological endpoints to screen for, the rigor of the screening effort, the correct balance of in vitro (cellular and molecular) versus in vivo (animal or whole organism) testing, the cost of the effort, and who should be responsible for overseeing this nano-EHS development. Attempts to use traditional toxicological assays and models have resulted in some advancement of our knowledge about nanotoxicology but we are still experiencing at times conflicting results and we are far away from the implementation of a generally accepted screening platform. While much of the knowledge about MNM toxicity has been generated using fairly straightforward single read-out plate reader based screening assays, each chosen assay represents typically only a single specific reaction to a toxic stimulus and thus is of limited predictive value. Since toxic effects are always a function of the presence or absence of targets for a given toxic stimulus in the organism in question, toxic effects of MNMs can differ between species. For comprehensive MNM toxicity testing, it is thus necessary to consider the analysis of multiple species such as, e.g., human, fish, bacteria, algae, etc.
The biggest challenge for the evaluation of nanomaterial toxicity and hazard is the sheer number of different materials – we can expect in excess of 100,000 MNM enabled products in the next decade (Service, 2008). A solution to the bottleneck in MNM testing is high throughput screening (HTS). HTS became the leading paradigm in drug discovery in the late 1980s and early 1990s and generated many more lead compounds (Pereira and Williams, 2007) than the labor-intensive 40–50-year-old descriptive toxicology platforms used up to this point could handle. The toxicology departments had to adopt HTS methodologies (Dimasi, 2001) to keep up with the number of chemical candidates that came out of HTS campaigns. The resulting arsenal of HTS toxicology assays and platforms is large and much of it can be transferred to ENM toxicity screening. It is interesting to note that the National Academy of Sciences (NAS) has recognized the need for novel methodologies which can support carrying out a large number of toxicological tests without relying primarily on animal testing and put forth a vision and strategy paper in which HTS methodologies are prominently featured as one feasible approach for turning the vision into reality (Gibb, 2008). Similarly, the European Union has enacted the REACH program under which all chemicals have to undergo toxicology testing and takes notes from the EPA ToxCast program in which HTS toxicology methods are applied towards screening chemicals for their toxicological properties according to the NAS vision paper (Judson et al., 2010).
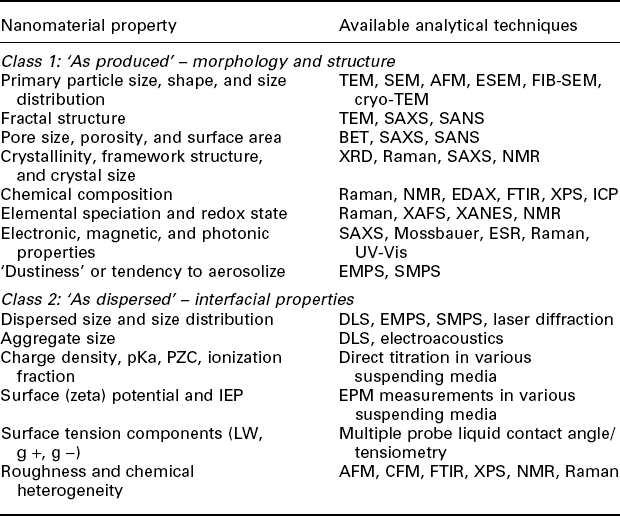
7.4.1 Characterization of MNMs before toxicity screening
Before the actual toxicity testing, a thorough characterization of the MNMs in ‘dry’ and ‘as dispersed’ form is necessary (see Table 7.3 for an overview of MNM properties and frequently used techniques): while MNMs are typically delivered as a dry powder, when they come into contact with a living entity and exert their toxicity, they will be in aqueous phase – be this now in, e.g., a waste water matrix, or the lung fluid of a mammal. While the ‘dry’ characterization typically includes assessment of important metrics such as size and shape as well as phase and crystallinity, one has to bear in mind that MNMs can change quite drastically in aqueous phase: aggregation, changes in surface charge, absorption of, e.g., proteins present in the aqueous phase are quite common and influence the nano-toxicity. Hence, it is important to determine not only the properties of an MNM ‘as produced’ in dry powder form but also, e.g., the size, size distribution, state of dispersal/aggregation, stability and the zeta-potential of the MNM in aqueous phase, as these important parameters are dependent on the media matrix they are in.
Table 7.3
Overview characterization of ENMs. While Class 1 properties are intrinsic to a nanomaterial itself, the Class 2 properties are dependent on the interplay between ENM and assay system
For example, ions play an important role for the aggregation behaviour of MNMs (Jin et al., 2010). Moreover, the MNM dispersions are typically not stable and much effort has been put into the discovery of suitable dispersal agents, which are compatible with the biological system in which they are to be tested. In this context it has to be mentioned that dispersion agents used for the stabilization during testing of MNMs do modify the surface of the material and may therefore interfere in toxicity screening: for example, DPPC in relatively high doses has been shown to completely suppress the cytotoxicity and pro-apoptotic effects of quartz nanoparticles (Gao et al., 2001). In this context it should be noted that, although nanoparticle dispersion has been extensively researched, only a few studies have addressed the stability of the resulting dispersions and only limited information is available from systematic studies on the effect of proteins and other chemical surfactants on nanoparticle stability in assay media such as cell culture media. A methodic approach is imperative when performing nanotoxicology in any assay system to exclude false results. Therefore, it becomes urgent to develop standard protocols for evaluating nanomaterial stability during in vitro or in vivo nanotoxicity studies.
We will now review existing approaches for small molecule HTS toxicology screening as many of these approaches and workflows currently used in drug discovery can be potentially adapted towards ENM toxicology screening.
7.4.2 General considerations for MNM toxicity profiling using HTS
HTS approaches for toxicology typically utilize microtiter plates of the 96 or 384 well plate format. These plate formats are formalized by the Society of Biomolecular Screening and all existing equipment is compatible with the plate formats.
The screening approaches themselves are either plate reader or high content screening (HCS) based. Plate reader based assays rely on one of the classic standard readouts such as luminescence, fluorescence, absorption, time-resolved fluorescence and fluorescence polarization and measure the whole well at once. TR-FRET and fluorescence polarization (FP) are readouts that are less commonly used for toxicity screening as their cost is higher than the other three assay readouts mentioned and especially FP is very sensitive to interference from contaminants and temperature variations. While plate reader based assays typically have a single parameter, which is measured at a point in time, HCS offers a complementary approach, which can be understood as automated microscopy coupled with image analysis software. It enables the measurement of multiple parameters with cellular resolution in the same well at the same time and offers a more detailed view. Moreover, HCS enables the detection of rare cellular events, segregation of sub-populations of cells from a single well and has the potential to be more sensitive and also pick up sub-lethal effects. Another important factor is that HCS enables phenotypic screening, which can be based on, e.g., cell morphology or translocation event of a given protein.
HCS-based toxicity screening has significant advantages over classical plate reader based assays. In a 2006 study (O’Brien et al., 2006), conventional single plate reader based readouts were compared to a five parameter HCS assay for toxicology prediction power on a set of reference compounds: the single parameter readouts had a very limited sensitivity (< 25%) but high specificity (about 90%), but HCS had a sensitivity of 93% and a specificity of 98%. What is more interesting is the fact that although hepatocyte HepG2 cells were used, 92% of the toxic drugs, which had other organ toxicities, but no hepatotoxic properties, were detected.
While many assays are available for toxicity screening of small organic molecules, it remains to be seen whether all possible toxicology paradigms can be covered with already existing assays as ENM-triggered toxicity has the potential to follow different paradigms than small molecule toxicity. Another argument in favor of custom development of novel assays is their usability for testing the hypothesis of the mode of action of ENM toxicity. To accomplish this, novel assay platforms are needed for the evaluation of ENMs in environmentally relevant systems such as, e.g., algae or other aquatic species, which will require tailoring of assays to ENM toxicity. The strength of plate reader based assays is their speed. HCS-based assays can be slow to read on the imaging systems, so at times a balance between information required and the needed acquisition speed has to be found.
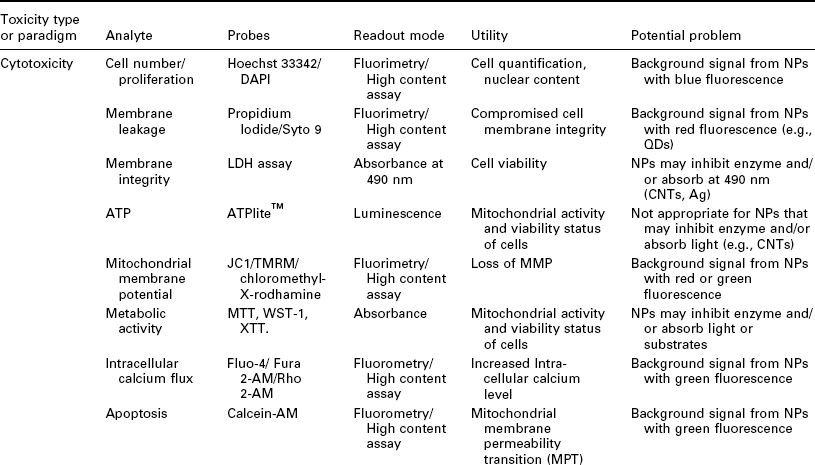
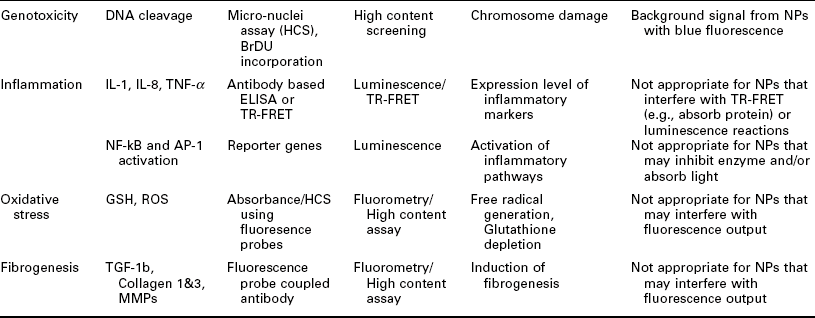
Importantly, it has to be noted that each assay has its artifacts and other liabilities. This is especially true for nanotoxicology where it is very common for, e.g., dyes used in an assay to interact with a given MNM leading to unreliable or even false results. Table 7.4 gives an overview of MNM toxicity paradigms, corresponding assays/readouts as well as possible interference of the MNM with the assay readout. Typically, it is advisable to confirm results on toxic MNMs with another assay, which uses a different readout paradigm in order to exclude artifacts.
Table 7.4
Examples of toxicity paradigms, possible analytes, readout modes and potential problems when using various readouts for MNM toxicity screening
While, for example, some toxicity assays rely on cell-free systems such as detection of ROS generation (Gabriel et al., 1997) by the MNM using a fluorescent dye or co-incubation of an MNM with an enzyme and subsequent measurement of enzymatic activity, it is the cell-based assays which offer the most insight into MNM toxicity as a living cell contains all potential targets for MNM toxicity (Damoiseaux et al., 2011).
As the lowest functional unit of an organism, cells represent the ultimate target for an intruding MNM and from a biochemical perspective the cells respond to foreign materials in essentially similar ways to that of the whole organism. Although primary cell lines are ideal candidates for toxicity screening, the cells are frequently not available in sufficient quantities for use in HTS, hence cell lines are used. A cell-based assay in the context of nanotoxicity can be regarded as an analytical procedure that assesses the biological outcome resulting from the interaction of nanomaterials with a given cell. However, the manifestation of biological outcome is generally faster in a cell-based in vitro system (often in the order of minutes to hours) than in vivo, thus providing a rapid readout of MNM toxicity. In order to achieve the true toxicological significance of a cellular injury response, it is necessary to correctly pick the cell line type and in vitro end point/readout in order to generate high quality information about the potential impact of the nanomaterial.
The toxicity profile of NMs may vary from one cell type to another because of the possible difference in the cellular uptake and processing of nanomaterials. A simple solution to this liability is to incorporate more than one cell type (often different cell lineage) and to conduct multiple cytotoxicity assays simultaneously for a range of reasonable doses and durations of exposure. Recently, Shaw et al. (2008) demonstrated that the predictive power of an in vitro assay could be greatly improved by including multiple cell lines (4 different cell lines) and multiple doses of nanomaterials. It should be emphasized that the data quality from cell-based HTS assays is to a strong degree dependent on the equipment and the experimental conditions. Special attention has to be paid to artifacts stemming from the use of microtiter plates such as edge effects due to the evaporation of culture media or irregularities arising from liquid handling errors. Hence, any cellbased assay has to be thoroughly optimized for HTS, validated on the equipment used for the toxicity screening and it is necessary to standardize the experimental conditions employing appropriate negative and positive controls on each microtiter plate. We will now review in detail HTS approaches toward three of the most important nanotoxicity paradigms: mutagenicity, cytotoxicity, and oxidative stress.
7.4.3 HTS for mutagenicity, cytotoxicity and oxidative stress effects of MNMs
The first in vitro toxicology assay was the Ames test, which dates back to the 1970s and it is still in use today. In this test, several strains of bacteria – Salmonella typhimurium with various mutations in the histidine production pathway – are being exposed to a mutagenic material in question. Mutations resulting in a frame shift mutation or reversing a point mutation results in a restoration of the respective histidine production pathway gene and results in the production of histidine. The readout is growth on histidine deficient media. Several commercial HTS versions of this test exist for plate readers. For example, damaging effects of MNMs on DNA can be detected, triggering using a reporter gene assay system in which luciferase is put under the recN promoter which gets triggered by the SOS DNA repair response. The commercial name of this test is ‘VitoTox’ test (Verschaeve et al., 1999).
Care should be taken when comparing the results from assays: while the Ames test detects mutagenicity, the VitoTox test detects genotoxicity. GreenScreen is another assay similar in principle to the VitoTox test. In GreenScreen (Van Gompel et al., 2005), the RAD54 promotor drives green fluorescent protein (GFP) expression in yeast. RAD54 is involved in DNA double strand breakage response and the activation of the repair pathway by a genotoxic MNMs leads to GFP expression. In the commercial product RadarScreen, the GFP is replaced with β-galactosidase. Galactosidase can be read on a plate reader using various colorigenic or luminogenic substrates. Going up in the evolutionary chain, the GreenScreen HC assay relies on a TK6 lymphoblastoma cell line with a growth arrest and damage gene promoter GADD45α in driving the luciferase reporter.
It is interesting to note that drugs, which are normally tested in these assays, are exposed to liver enzymes that metabolize the compound in question before their use in these assays. The reasoning is that frequently the metabolites are more toxic than the parent compound. At this point it is frequently unclear where and how MNMs are processed or broken down in an organism and it is unclear if exposure to liver enzyme will be useful for MNM toxicity testing. While results from assays for mutagenicity testing are quite universally applicable since the carrier of genetic information, DNA, is universal, this is not the case for cytotoxicity. Cytotoxicity is very dependent on the presence of a target mediating the cytotoxic effect. Hence precise knowledge of the mode of action (MOA) is necessary to fully understand and appreciate the cytotoxic potential of any given MNM.
For drugs, dyes allowing for measuring DNA content such as Hoechst 33342 are frequently used for the detection of, e.g., pro-mitotic effects using HCS approaches. Cell cycle analysis using various dye combinations can be very useful as well for the detection of hazardous MNMs and are best executed using HCS. Membrane integrity can be assessed using propidium iodide (PI) and/or Calcein-AM dyes. While the nucleic acid dye PI does not pass intact membranes, Calcein AM is a conjugated membrane permeable, fluorogenic fluoresceine derivative that once in the cell and processed by esterases can no longer leave the cell – except if the membrane integrity is compromised. This assay can be performed in a plate reader or HCS system.
Cytotoxicity of an MNM can show itself as well in the collapse of the gluthathion level of a cell or radical oxygen species (ROS) formation, which can be detected in the presence or absence of cells. Gluthathione depletion can be easily assessed using monochloimane and ROS formation can be easily measured using dichlorofluoresceine. Both of these dyes can be used in a plate reader format. The redox potential of a cell – i.e. gluthathion level – can be followed using dyes such as MTT or Alamar Blue (Nakayama et al., 1997). While these dyes are relatively cheap, the fluorescent character of the nanomaterials might interfere with their detection. Hence the use of CytoLite (measures NADH) (Chan et al., 2001), ATP-Lite or CellTiter Glo (measure ATP) might be preferable (Hannah et al., 2001). A combination of these three assays should enable a first picture of the MOA of the toxicity of any given nanomaterial.
An interesting twist on apoptosis detection, which is a frequent liability of toxic MNMs, is the use of Z-DEVD-aminoluciferin for Caspase 3/7 and LETD-aminoluciferin for Caspase 8/9 (O’Brien et al., 2005). Both substrates have in common that upon activation of the Caspases in question, the peptide part is cleaved off the aminoluciferin hence making it convertible by firefly luciferase. While Capase 3/7 would indicate mitochondrial impairment, Caspase 8/9 activity might point more at inflammation and activation of TNFα and interleukin-1β secretion.
Injury responses due to oxidative stress from MNM exposure have been documented extensively in the literature and oxidative stress is one of the best-understood mechanisms of MNM mediated toxicity (Oberdorster et al., 2005; Nel et al., 2006, 2009). MNM induced oxidative stress can be divided into three tiers: antioxidant defense, pro-inflammatory effects and cytotoxicity. MNM toxicity is typically mediated by ROS generation which can be extra- or intracellular and in both cases depletes the gluthathion redox-equilibrium of the living cell. Each of these response tiers is initiated by specific biological sensors and activation mechanisms. In Tier 1, the transcription factor Nrf2 is activated to enhance the expression of phase II enzymes, which attempts to restore redox equilibrium. If the level of oxidant injury cannot be recovered by phase II enzymes, Tier 2 response commences, signaling pathways such as the mitogen-activated protein kinase (MAPK), and nuclear factor kappa B (NF-κB) cascades are activated and cells express proinflammatory cytokines. These inflammatory effects contribute to disease processes such as asthma and atherosclerosis.
At the highest level of oxidative stress (Tier 3), the mitochondrial integrity is compromised and a resulting drop in ATP synthesis and release of calcium and other pro-apoptotic factors ultimately leads to cell death. Cytotoxicity (Tier 3 response) is the most extreme response to particle effects involving ROS production. Modes of action can involve the shedding of toxic metal ions that trigger intracellular ROS generation or cationic nanoparticles or dissolved transition metal interference with the mitochondrial electron transduction. As is evident, it is necessary to select multiple readouts to capture cellular events specific to Tiers 1–3.
Oxidative stress can be detected very easily using HCS. For example, a cocktail of Hoechst 33342 and the mitochondrial membrane potential dye JC1 detects Tier 2 responses based on mitochondrial membrane depolarization. A dye cocktail of Hoechst 33342, fluo-4 (detecting cytosolic Ca2 +) and propidium iodide (detecting membrane damage) is very useful for the detection of Tier 3 responses in which the mitochondrial membrane loses integrity, calcium enters the cytosol and the cell membrane disintegrates. In the oxidative stress paradigm, in vitro and in vivo are correlated extremely well: for example, ZnO nanoparticles elicit responses in vitro that are analogous to a disease called ‘metal fume fever’ (Duffin et al., 2007). Metal fume fever is an acute inflammatory condition of the lung in welders who are exposed to aerosolized metal oxide nanoparticles. In the workers, one finds the expression of Tier 2-like responses in macrophages and epithelial cells as demonstrated by the detection of secreted interleukin 8 (IL-8) and TNF-α in the lungs and bronchoalveolar lavage fluid of exposed workers (Xia et al., 2008).
7.5 Future trends and conclusions
With increased attention on MNM health hazards, there has been a need to develop new methods for toxicity profiling, safer manufacturing procedures, and ‘greener’ MNMs as alternatives to more harmful materials. While engineered nanomaterials provide many benefits to ‘green’ construction, uncertainty remains about the long-term effects of nano-enhanced structures. In this context, it will be important to ensure MNMs are not released into the environment until these MNMs are proven harmless. Or course, the ultimate goal is to use safe, next generation MNMs for future construction projects. Ultimately, methods for predicting MNM toxicity a priori must be explored. Since no definitively hazard-free nanomaterial presently exists, there is much work to be done in this area to alleviate public concern and move forward with new nano-enabled construction.
7.5.1 Nanomaterial toxicity and green nanomaterials
Our ability to generate safer MNMs will depend on our understanding of the toxicological liabilities of current MNMs, our ability to tie toxicological liabilities to MNM properties, and design of greener MNM, which are devoid of any toxicological liability while building on our conclusions. A prime example of this approach is the design of environmentally friendlier, ‘greener’ metal oxide MNMs (Meng et al., 2009). One of the main toxicity paradigms of metal oxide MNMs is oxidative stress (Xia et al., 2006). For example, it has been shown that the overlap of conduction band energy (E(c)) levels with the cellular redox potential (− 4.12 to − 4.84 eV) is strongly correlated to the ability of various nanoparticles to induce oxygen radicals, oxidative stress, and inflammation (Zhang et al., 2012).
Iron doping is one way to tune down the toxicity of ZnO particles, which are notorious for their ability to generate ROS through ion shedding. The addition of 1–10% iron results in ZnO particles which are less soluble, which in consequence are less toxic in the rodent lung and towards zebrafish embryos. We can be hopeful that we will see many more examples of greener MNMs in the years to come.
Taking MNM production from ‘green by trial and error’ to ‘green by design’ requires us to be able to predict MNM toxicity a priori. Such a prediction of MNM toxicity will depend on our ability to generate a nanostructure activity relationship (nano-SAR) (Liu et al., 2012) in which MNM toxicity properties are correlated with MNM descriptors (Zhang et al., 2012). This nano-SAR will provide models of the toxicity profile of an MNM at the design stage without actually having to test the material in the laboratory. Liu et al. have succeeded in generating such a nano-SAR for metal oxides. A set of 14 descriptors for MNMs was used in order to correlate the toxicity of a set of nine metal oxide MNMs on BEAS-2B lung epithelial cells measured as membrane degradation in a high content screening assay using propidium iodide. The best-performing nano-SAR model resulted in a 100% classification accuracy. This model was based on three descriptors: atomization energy of the metal oxide, period of the nanoparticle metal, and nanoparticle primary size, in addition to nanoparticle volume fraction (in solution). While the sample library of MNM was very small, the results are very encouraging and we may hope that the integration of much larger datasets will eventually yield universal design rules for green MNMs.
The nano revolution has tremendous potential to address some of the world’s most pressing needs and it is our obligation to implement this disruptive technology safely. High throughput screening will play a decisive role, as it is the only technology which enables us to test a multitude of nanomaterials in various doses, time points and assay systems in a very short period at a reasonable cost. The necessary HTS infrastructure is accessible at centers such as the Molecular Screening Shared Resource (MSSR), which is part of the NSF/EPA sponsored multi-disciplinary University of California Center for the Environmental Impact of Nanotechnology (UC-CEIN) at the University of California, Los Angeles.
To work toward creating safe nanomaterials, it is important to coordinate research on nanotoxicology and cooperate on the development of new nanotechnology materials and applications. Rather than working independently, these areas must be closely collaborative to begin identifying health hazards during the developmental phase before new nanomaterials are used commercially. This research network will also facilitate the creation of nanomaterials with fewer environmental and health impacts and ways to better maintain their stability throughout their lifecycle. The future of the nanotechnology revolution and ‘green’ construction lies in the ability to understand potential hazards and mitigate these hazards before use. The answer is in a tightly cooperative network of researchers equally interested in advancing nanotechnology applications and the associated health concerns.
7.6 References
Abraham, V.C., Towne, D.L., Waring, J.F., Warrior, U., Burns, D.J. Application of a high-content multiparameter cytotoxicity assay to prioritize compounds based on toxicity potential in humans. J Biomol Screen. 2008; 13:527–537.
Alvarez, P.J.J., Lee, J., Mahendra, S. Nanomaterials in the construction industry: a review of their applications and environmental health and safety considerations. ACS Nano. 2010; 4:3580–3590.
Anikeeva, P.O., Halpert, J.E., Bawendi, M.G., Bulovic, V. Quantum dot light-emitting devices with electroluminescence tunable over the entire visible spectrum. Nano Letters. 2009; 9:2532–2536.
Arico, A.S., Bruce, P., Scrosati, B., Tarascon, J.M., Van Schalkwijk, W. Nanostructured materials for advanced energy conversion and storage devices. Nature Materials. 2005; 4:366–377.
Basak, G.C., Kumar, K.D., Bandyopadhyay, A., Bhowmick, A.K. Elegant way of strengthening polymer-polymer interface using nanoclay. ACS Applied Materials & Interfaces. 2010; 2:2933–2943.
Becher, P.F. Microstructural design of toughened ceramics. J Am Ceram Soc. 1991; 74:255–269.
Benn, T., Cavanagh, B., Hristovski, K., Posner, J.D., Westerhoff, P. The release of nanosilver from consumer products used in the home. J Environ Qual. 2010; 39:1875–1882.
Brown, P., Kamat, P.V. Quantum dot solar cells. Electrophoretic deposition of CdSe-C60 composite films and capture of photogenerated electrons with nC60 cluster shell. J Am Chem Soc. 2008; 130:8890–8891.
Buzea, C., Pacheco Blandino, I.I., Robbie, K. Nanomaterials and nanoparticles: sources and toxicity. Biointerphases. 2007; 2:MR17–172.
Bystrzejewska-Piotrowska, G., Golimowski, J., Urban, P.L. Nanoparticles: their potential toxicity, waste and environmental management. Waste Manag. 2009; 29:2587–2595.
Chan, J.H., Dua, H.S., Powell-Richards, A., Jones, D.R., Harris, I.M. Effect of ABO blood group mismatching on corneal epithelial cells: an in vitro study. Br J Ophthalmol. 2001; 85:1104–1109.
Chan, J., Bayliss, P.E., Wood, J.M., Roberts, T.M. Dissection of angiogenic signaling in zebrafish using a chemical genetic approach. Cancer Cell. 2002; 1:257–267.
Chauhan, R.S., Chaturvedi, R., Gutch, P.K. Polymer-clay nano composites. Defence Sci J. 2006; 56:649–664.
Chen, Z., Meng, H.A., Xing, G.M., Chen, C.Y., Zhao, Y.L., Jia, G.A., Wang, T.C., Yuan, H., Ye, C., Zhao, F., Chai, Z.F., Zhu, C.F., Fang, X.H., Ma, B.C., Wan, L.J. Acute toxicological effects of copper nanoparticles in vivo. Toxicol Lett. 2006; 163:109–120.
Contado, C., Pagnoni, A. TiO2 in commercial sunscreen lotion: flow fieldflow fractionation and ICP-AES together for size analysis. Anal Chem. 2008; 80:7594–7608.
Damoiseaux, R., George, S., Li, M., Pokhrel, S., Ji, Z., France, B., Xia, T., Suarez, E., Rallo, R., Madler, L., Cohen, Y., Hoek, E.M., Nel, A. No time to lose – high throughput screening to assess nanomaterial safety. Nanoscale. 2011; 3:1345–1360.
Daniel, M.C., Astruc, D. Gold nanoparticles: assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem Rev. 2004; 104:293–346.
De Ibarra, Y.S., Gaitero, J.J., Erkizia, E., Campillo, I. Atomic force microscopy and nanoindentation of cement pastes with nanotube dispersions. Phys Stat Sol A – Appl Mater Sci. 2006; 203:1076–1081.
Derno, M., Jentsch, W., Hoffmann, L. Effect of long-time exposure to different environmental temperatures on heat-production of growing pigs. Livestock Production Science. 1995; 43:149–152.
Dimasi, J.A. Risks in new drug development: approval success rates for investigational drugs. Clin Pharmacol Ther. 2001; 69:297–307.
Ding, L.H., Stilwell, J., Zhang, T.T., Elboudwarej, O., Jiang, H.J., Selegue, J.P., Cooke, P.A., Gray, J.W., Chen, F.Q.F. Molecular characterization of the cytotoxic mechanism of multiwall carbon nanotubes and nano-onions on human skin fibroblast. Nano Lett. 2005; 5:2448–2464.
Dobrovolskaia, M.A., McNeil, S.E. Immunological properties of engineered nanomaterials. Nat Nanotechnol. 2007; 2:469–478.
Duffin, R., Tran, L., Brown, D., Stone, V., Donaldson, K. Proinflammogenic effects of low-toxicity and metal nanoparticles in vivo and in vitro: highlighting the role of particle surface area and surface reactivity. Inhal Toxicol. 2007; 19:849–856.
Dunford, R., Salinaro, A., Cai, L.Z., Serpone, N., Horikoshi, S., Hidaka, H., Knowland, J. Chemical oxidation and DNA damage catalysed by inorganic sunscreen ingredients. FEBs Letters. 1997; 418:87–90.
Dwivedi, P.D., Tripathi, A., Ansari, K.M., Shanker, R., Das, M. Impact of nanoparticles on the immune system. J Biomed Nanotechnol. 2011; 7:193–194.
Fahy, G.M. Molecular nanotechnology. Clin Chem. 1993; 39:2011–2016.
Faruk, O., Matuana, L.M. Nanoclay reinforced HDPE as a matrix for wood-plastic composites. Compos Sci Technol. 2008; 68:2073–2077.
Ferrari, M. Cancer nanotechnology: opportunities and challenges. Nat Rev Cancer. 2005; 5:161–171.
Gabriel, C., Camins, A., Sureda, F.X., Aquirre, L., Escubedo, E., Pallas, M., Camarasa, J. Determination of nitric oxide generation in mammalian neurons using dichlorofluorescin diacetate and flow cytometry. J Pharmacol Toxicol Methods. 1997; 38:93–98.
Gao, N., Keane, M.J., Ong, T., Ye, J., Miller, W.E., Wallace, W.E. Effects of phospholipid surfactant on apoptosis induction by respirable quartz and kaolin in NR8383 rat pulmonary macrophages. Toxicol Appl Pharmacol. 2001; 175:217–225.
Ge, Z., Gao, Z. Applications of nanotechnology and nanomaterials in construction. First Inter Confer Construc Develop Countries. 2008; 235–240.
German, J.B., Smilowitz, J.T., Zivkovic, A.M. Lipoproteins: when size really matters. Curr Op Colloid & Interface Sci. 2006; 11:171–183.
Gibb, S. Toxicity testing in the 21st century: a vision and a strategy. Reprod Toxicol. 2008; 25:136–138.
Girishkumar, G., Rettker, M., Underhile, R., Binz, D., Vinodgopal, K., McGinn, P., Kamat, P. Single-wall carbon nanotube-based proton exchange membrane assembly for hydrogen fuel cells. Langmuir. 2005; 21:8487–8494.
Goldman, L., Coussens, C. Implications of Nanotechnology for Environmental Health Research. Washington, DC: The National Academies Press; 2005.
Gopel, W. Chemical sensing, molecular electronics and nanotechnology – interface technologies down to the molecular scale. Sensors and Actuators B-Chemical. 1991; 4:7–21.
Hagens, W.I., Oomen, A.G., De Jong, W.H., Cassee, F.R., Sips, A.J. What do we (need to) know about the kinetic properties of nanoparticles in the body? Regul Toxicol Pharmacol. 2007; 49:217–229.
Handy, R.D., Henry, T.B., Scown, T.M., Johnston, B.D., Tyler, C.R. Manufactured nanoparticles: their uptake and effects on fish – a mechanistic analysis. Ecotoxicology. 2008; 17:396–409.
Hannah, R.B.M., Moravec, R., Riss, T. Cell Titer-GloTM luminescent cell viability assay: a sensitive and rapid method for determining cell viability. Promega Cell Notes. 2001; 11–13.
Hansen, S.F., Michelson, E.S., Kamper, A., Borling, P., Stuer-Lauridsen, F., Baun, A. Categorization framework to aid exposure assessment of nanomaterials in consumer products. Ecotoxicology. 2008; 17:438–447.
Hayashi, T., Kim, Y.A., Natsuki, T., Endo, M. Mechanical properties of carbon nanomaterials. Chem Phys Chem. 2007; 8:999–1004.
Herzog, E., Byrne, H.J., Casey, A., Davoren, M., Lenz, A.G., Maier, K.L., Duschl, A., Oostingh, G.J. SWCNT suppress inflammatory mediator responses in human lung epithelium in vitro. Toxicol Appl Pharmacol. 2009; 234:378–390.
Hoffmann, K.D. Population-growth poverty and environmental destruction in the third-world. Gegenwartskunde Gesellschaft Staat Erziehung. 1995; 44:393–425.
Hoffmann, M.R., Martin, S.T., Choi, W.Y., Bahnemann, D.W. Environmental applications of semiconductor photocatalysis. Chem Rev. 1995; 95:69–96.
Irie, H., Sunada, K., Hashimoto, K. Recent developments in TiO2 photocatalysis: novel applications to interior ecology materials and energy saving systems. Electrochem. 2004; 72:807–812.
Jia, G., Wang, H.F., Yan, L., Wang, X., Pei, R.J., Yan, T., Zhao, Y.L., Guo, X.B. Cytotoxicity of carbon nanomaterials: single-wall nanotube, multi-wall nanotube, and fullerene. Environ Sci Technol. 2005; 39:1378–1383.
Jin, X., Li, M., Wang, J., Marambio-Jones, C., Peng, F., Huang, X., Damoiseaux, R., Hoek, E.M. High-throughput screening of silver nanoparticle stability and bacterial inactivation in aquatic media: influence of specific ions. Environ Sci Technol. 2010; 44:7321–7328.
Judson, R.S., Houck, K.A., Kavlock, R.J., Knudsen, T.B., Martin, M.T., Mortensen, H.M., Reif, D.M., Rotroff, D.M., Shah, I., Richard, A.M., Dix, D.J. In vitro screening of environmental chemicals for targeted testing prioritization: the ToxCast project. Environ Health Perspect. 2010; 118:485–492.
Kang, S., Pinault, M., Pfefferle, L.D., Elimelech, M. Single-walled carbon nanotubes exhibit strong antimicrobial activity. Langmuir. 2007; 23:8670–8673.
Kang, S., Herzberg, M., Rodrigues, D.F., Elimelech, M. Antibacterial effects of carbon nanotubes: size does matter. Langmuir. 2008; 24:6409–6413.
Kang, S., Mauter, M.S., Elimelech, M. Physicochemical determinants of multiwalled carbon nanotube bacterial cytotoxicity. Environ Sci Technol. 2008; 42:7528–7534.
Kang, S., Mauter, M.S., Elimelech, M. Microbial cytotoxicity of carbonbased nanomaterials: implications for river water and wastewater effluent. Environ Sci Technol. 2009; 43:2648–2653.
Karabanovas, V., Zakarevicius, E., Sukackaite, A., Streckyte, G., Rotomskis, R. Examination of the stability of hydrophobic (CdSe)ZnS quantum dots in the digestive tract of rats. Photochem Photobiol Sci. 2008; 7:725–729.
Karlsson, H.L., Cronholm, P., Gustafsson, J., Moller, L. Copper oxide nanoparticles are highly toxic: a comparison between metal oxide nanoparticles and carbon nanotubes. Chem Res Toxicol. 2008; 21:1726–1732.
Kirschvink, J.L., Kobayashi-Kirschvink, A., Woodford, B.J. Magnetite biomineralization in the human brain. Proc Nat Acade Sci USA. 1992; 89:7683–7687.
Kontos, A.I., Kontos, A.G., Tsoukleris, D.S., Vlachos, G.D., Falaras, P. Superhydrophilicity and photocatalytic property of nanocrystalline titania sol-gel films. Thin Solid Films. 2007; 515:7370–7375.
Kumar, A., Vemula, P.K., Ajayan, P.M., John, G. Silver-nanoparticleembedded antimicrobial paints based on vegetable oil. Nature Mater. 2008; 7:236–241.
Lam, C.W., James, J.T., McCluskey, R., Arepalli, S., Hunter, R.L. A review of carbon nanotube toxicity and assessment of potential occupational and environmental health risks. Crit Rev Toxicol. 2006; 36:189–217.
Lee, J., Mahendra, S., Alvarez, P.J.J. Potential environmental impacts of nanomaterials used in the construction industry. In: Bittnar Z., Zeman J., Nemecek J., Smilauer V., Bartos P.J.M., eds. Nanotechnology in Construction – 3. Berlin: Springer Verlag, 2009.
Leobandung, E., Guo, L.J., Chou, S.Y. Single hole quantum-dot transistors in silicon. Appl Phys Lett. 1995; 67:2338–2340.
Lin, W.S., Huang, Y.W., Zhou, X.D., Ma, Y.F. Toxicity of cerium oxide nanoparticles in human lung cancer cells. Int J Toxicol. 2006; 25:451–457.
Liu, R., Rallo, R., George, S., Ji, Z., Nair, S., Nel, A.E., Cohen, Y. Classification NanoSAR development for cytotoxicity of metal oxide nanoparticles. Small. 2012; 7:1118–1126.
Luo, T.Y., Liang, T.X., Li, C.S. Addition of carbon nanotubes during the preparation of zirconia nanoparticles: influence on structure and phase composition. Powder Technology. 2004; 139:118–122.
Lyon, D.Y., Alvarez, P.J. How a fullerene water suspension kills bacteria: exploring three possible mechanisms. Chem Res Toxicol. 2007; 20:1991.
Lyon, D.Y., Fortner, J.D., Sayes, C.M., Colvin, V.L., Hughes, J.B. Bacterial cell association and antimicrobial activity of a C60 water suspension. Environ Toxicol Chem. 2005; 24:2757–2762.
Lyon, D.Y., Adams, L.K., Falkner, J.C., Alvarez, P.J.J. Antibacterial activity of fullerene water suspensions: effects of preparation method and particle size. Environ Sci Technol. 2006; 40:4360–4366.
Lyon, D.Y., Brunet, L., Hinkal, G.W., Wiesner, M.R., Alvarez, P.J.J. Antibacterial activity of fullerene water suspensions (nC(60)) is not due to ROS-mediated damage. Nano Letters. 2008; 8:1539–1543.
Mann, S., Nanotechnology and Construction. Nanoforum Report. 2006. [May 30].
Meng, H., Xia, T., George, S., Nel, A.E. A predictive toxicological paradigm for the safety assessment of nanomaterials. ACS Nano. 2009; 3:1620–1627.
Murphy, J., Carr, B., Atkinson, T. Nanotechnology in medicine and the biosciences. The UK National Symposium on Nanotechnology in Medicine and the Biosciences, London, UK, 16–18 March l994. Trends Biotechnol. 1994; 12:289–290.
Nakayama, G.R., Caton, M.C., Nova, M.P., Parandoosh, Z. Assessment of the alamar blue assay for cellular growth and viability in vitro. J Immunol Methods. 1997; 204:205–208.
Nel, A., Xia, T., Madler, L., Li, N. Toxic potential of materials at the nanolevel. Science. 2006; 311:622–627.
Nel, A.E., Madler, L., Velegol, D., Xia, T., Hoek, E.M.V., Somasundaran, P., Klaessig, F., Castranova, V., Thompson, M. Understanding biophysicochemical interactions at the nano-bio interface. Nat Mater. 2009; 8:543–557.
Nozik, A.J. Quantum dot solar cells. Physica E – Low-Dimensional Systems & Nanostructures. 2002; 14:115–120.
O’Brien, M.A., Daily, W.J., Hesselberth, P.E., Moravec, R.A., Scurria, M.A., Klaubert, D.H., Bulleit, R.F., Wood, K.V. Homogeneous, bioluminescent protease assays: caspase-3 as a model. J Biomol Screen. 2005; 10:137–148.
O’Brien, P.J., Irwin, W., Diaz, D., Howard-Cofield, E., Krejsa, C.M., Slaughter, M.R., Gao, B., Kaludercic, N., Angeline, A., Bernardi, P., Brain, P., Hougham, C. High concordance of drug-induced human hepatotoxicity with in vitro cytotoxicity measured in a novel cell-based model using high content screening. Arch Toxicol. 2006; 80:580–604.
Oberdörster, E. Manufactured nanomaterials (fullerenes, C60) induce oxidative stress in the brain of juvenile largemouth bass. Environ Health Perspectives. 2004; 112:1058–1062.
Oberdorster, G., Gelein, R.M., Ferin, J., Weiss, B. Association of particulate air-pollution and acute mortality – involvement of ultrafine particles. Inhal Toxicol. 1995; 7:111–124.
Oberdorster, G., Oberdorster, E., Oberdorster, J. Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles. Environ Health Perspectives. 2005; 113:823–839.
Park, K., Park, E.J., Kim, H., Kim, Y., Yi, J., Choi, K. Carbon fullerenes (C60s) can induce inflammatory responses in the lung of mice. Toxicol Appl Pharmacol. 2010; 244:226–233.
Park, M.V.D.Z., Neigh, A.M., Vermeulen, J.P., De La Fonteyne, L.J.J., Verharen, H.W., Briede, J.J., Van Loveren, H., De Jong, W.H. The effect of particle size on the cytotoxicity, inflammation, developmental toxicity and genotoxicity of silver nanoparticles. Biomaterials. 2011; 32:9810–9817.
Park, S., Lee, Y.K., Jung, M., Kim, K.H., Chung, N., Ahn, E.K., Lim, Y., Lee, K.H. Cellular toxicity of various inhalable metal nanoparticles on human alveolar epithelial cells. Inhal Toxicol. 2007; 19:59–65.
Parveen, S., Misra, R., Sahoo, S.K. Nanoparticles: a boon to drug delivery, therapeutics, diagnostics and imaging. Nanomed – Nanotechnol Biol Med. 2012; 8:147–166.
Paz, Y., Luo, Z., Rabenberg, L., Heller, A. Photooxidative self-cleaning transparent titanium-dioxide films on glass. J Mater Res. 1995; 10:2842–2848.
Pereira, D.A., Williams, J.A. Origin and evolution of high throughput screening. Br J Pharmacol. 2007; 152:53–61.
Podsiadlo, P., Kaushik, A.K., Arruda, E.M., Waas, A.M., Shim, B.S., Xu, J.D., Nandivada, H., Pumplin, B.G., Lahann, J., Ramamoorthy, A., Kotov, N.A. Ultrastrong and stiff layered polymer nanocomposites. Science. 2007; 318:80–83.
Raki, L., Beaudoin, J., Alizadeh, R., Makar, J., Sato, T. Cement and concrete nanoscience and nanotechnology. Materials. 2010; 3:918–942.
Rana, A.K., Rana, S.B., Kumari, A., Kiran, V. Significance of nanotechnology in construction engineering. Int J Recent Trends in Engineering. 2009; 1:46–48.
Reeves, J.F., Davies, S.J., Dodd, N.J.F., Jha, A.N. Hydroxyl radicals (•OH) are associated with titanium dioxide (TiO2) nanoparticle-induced cytotoxicity and oxidative DNA damage in fish cells. Mutat Res. 2008; 640:113–122.
Ruparelia, J.P., Chatteriee, A.K., Duttagupta, S.P., Mukherji, S. Strain specificity in antimicrobial activity of silver and copper nanoparticles. Acta Biomaterialia. 2008; 4:707–716.
Sayes, C.M., Gobin, A.M., Ausman, K.D., Mendez, J., West, J.L., Colvin, V.L. Nano-C-60 cytotoxicity is due to lipid peroxidation. Biomaterials. 2005; 26:7587–7595.
Sayes, C.M., Wahi, R., Kurian, P.A., Liu, Y.P., West, J.L., Ausman, K.D., Warheit, D.B., Colvin, V.L. Correlating nanoscale titania structure with toxicity: a cytotoxicity and inflammatory response study with human dermal fibroblasts and human lung epithelial cells. Toxicol Sci. 2006; 92:174–185.
Service, R. Nanotechnology – can high-speed tests sort out which nanomaterials are safe? Science. 2008; 321:1036–1037.
Shaw, S.Y., Westly, E.C., Pittet, M.J., Subramanian, A., Schreiber, S.L., Weissleder, R. Perturbational profiling of nanomaterial biologic activity. Proc Nat Acad Sci USA. 2008; 105:7387–7392.
Shiohara, A., Hoshino, A., Hanaki, K., Suzuki, K., Yamamoto, K. On the cyto-toxicity caused by quantum dots. Microbiol Immunol. 2004; 48:669–675.
Sobolev, K., Gutierrez, M.F. How nanotechnology can change the concrete world. Am Ceram Soc Bull. 2005; 84:16–20.
Sosnik, A., Carcaboso, A.M., Glisoni, R.J., Moretton, M.A., Chiappetta, D.A. New old challenges in tuberculosis: potentially effective nanotechnologies in drug delivery. Adv Drug Deliv Rev. 2010; 62:547–559.
Soto, K.F., Garza, K.M., Shi, Y., Murr, L.E. Direct contact cytotoxicity assays for filter-collected, carbonaceous (soot) nanoparticulate material and observations of lung cell response. Atmos Environ. 2008; 42:1970–1982.
Taneike, M., Abe, F., Sawada, K. Creep-strengthening of steel at high temperatures using nano-sized carbonitride dispersions. Nature. 2003; 424:294–296.
Tans, S.J., Verschueren, A.R.M., Dekker, C. Room-temperature transistor based on a single carbon nanotube. Nature. 1998; 393:49–52.
Tsao, N., Luh, T., Chou, C., Chang, T., Wu, J., Liu, C., Lei, H. In vitro action of carboxyfullerene. J Antimicrob Chemotherapy. 2002; 49:641–649.
Van Gompel, J., Woestenborghs, F., Beerens, D., Mackie, C., Cahill, P.A., Knight, A.W., Billinton, N., Tweats, D.J., Walmsley, R.M. An assessment of the utility of the yeast GreenScreen assay in pharmaceutical screening. Mutagenesis. 2005; 20:449–454.
Vecitis, C.D., Zodrow, K.R., Kang, S., Elimelech, M. Electronic-structuredependent bacterial cytotoxicity of single-walled carbon nanotubes. ACS Nano. 2010; 4:5471–5479.
Verschaeve, L., Van Gompel, J., Thilemans, L., Regniers, L., Vanparys, P., Van Der Lelie, D. VITOTOX bacterial genotoxicity and toxicity test for the rapid screening of chemicals. Environ Mol Mutagen. 1999; 33:240–248.
Wei, W., Sethuraman, A., Jin, C., Monteiro-Riviere, N.A., Narayan, R.J. Biological properties of carbon nanotubes. J Nanosci Nanotechnol. 2007; 7:1284–1297.
Xia, T., Kovochich, M., Brant, J., Hotze, M., Sempf, J., Oberley, T., Sioutas, C., Yeh, J.I., Wiesner, M.R., Nel, A.E. Comparison of the abilities of ambient and manufactured nanoparticles to induce cellular toxicity according to an oxidative stress paradigm. Nano Letters. 2006; 6:1794–1807.
Xia, T., Kovochich, M., Liong, M., Madler, L., Gilbert, B., Shi, H., Yeh, J.I., Zink, J.I., Nel, A.E. Comparison of the mechanism of toxicity of zinc oxide and cerium oxide nanoparticles based on dissolution and oxidative stress properties. ACS Nano. 2008; 2:2121–2134.
Xia, T., Li, N., Nel, A.E. Potential health impact of nanoparticles. Annu Rev Public Health. 2009; 30:137–150.
Zhang, H., Ji, Z., Xia, T., Meng, H., Low-Kam, C., Liu, R., Pokhrel, S., Lin, S., Wang, X., Liao, Y.P., Wang, M., Li, L., Rallo, R., Damoiseaux, R., Telesca, D., Madler, L., Cohen, Y., Zink, J.I., Nel, A.E. Use of metal oxide nanoparticle band gap to develop a predictive paradigm for oxidative stress and acute pulmonary inflammation. ACS Nano. 2012; 6:4349–4368.
Zhang, Q.W., Kusaka, Y., Sato, K., Nakakuki, K., Kohyama, N., Donaldson, K. Differences in the extent of inflammation caused by intratracheal exposure to three ultrafine metals: role of free radicals. J Toxicol Environ Health – Part a – Current. Issues. 1998; 53:423–438.
Zhang, W., Suhr, J., Koratkar, N. Carbon nanotube/polycarbonate composites as multifunctional strain sensors. J Nanosci Nanotechnol. 2006; 6:960–964.
Zhu, W., Bartos, P.J.M., Porro, A. Application of nanotechnology in construction – summary of a state-of-the-art report. Mater Struct. 2004; 37:649–658.
Zhu, X.S., Zhu, L., Duan, Z.H., Qi, R.Q., Li, Y., Lang, Y.P. Comparative toxicity of several metal oxide nanoparticle aqueous suspensions to Zebrafish (Danio rerio) early developmental stage. J Environ Sci Health Part A. 2008; 43:278–284.
Zolnik, B.S., Gonzalez-Fernandez, A., Sadrieh, N., Dobrovolskaia, M.A. Nanoparticles and the immune system. Endocrinology. 2009; 151:458–465.



