Nanoscience and nanoengineering of cement-based materials
Abstract:
Concrete is the most widely used construction material and given the current population growth, economic development, and need for repair/replacement of aging infrastructure, its consumption is expected to increase. Unfortunately though, the production of one of its major constituents, cement, is associated with approximately 5–10% of the global anthropogenic carbon dioxide emissions and therefore the industry and the specific material is in urgent need for reevaluation. The chemical reactions and resulting products that are produced when cement is mixed with water create a material that is highly complex. The dominant component, C-S-H gel, has a local structure of a precipitate with nanoscale features that are difficult to model and understand. Consequently, the development of the material relied primarily on empirical knowledge obtained through macroscopic experimentation and little is known about the underlying mechanisms that control the response of the material when employed in engineering applications. Recent experimental and theoretical advancements in the field of nanoscience and nanotechnology provide optimistic expectations for a refined understanding of the material that will create the scientific basis for a more sustainable and eco-efficient construction.
2.1 Introduction
2.1.1 Macroscale: cement and concrete
Cement is a pulverized fine powder which develops into a strong binder when mixed with water. The best known hydraulic cement1 is ordinary Portland cement (OPC). Current production of cement (the main component of concrete and all other cement-based materials) is approximately over 3 billions tonnes per year which makes it the most widely used solid on earth (Fig. 2.1). This quantity is sufficient to produce over 30 billions tonnes of concrete or over 4 tonnes for every person currently alive. Given the abundance of its major natural constituents (calcium and silica) in the earth’s crust (responsible for its current low price), and technical advantages over other construction materials (timber, steel, composites, etc.), it is highly unlikely that any other material will displace concrete from the construction industry, at least in the foreseeable future.
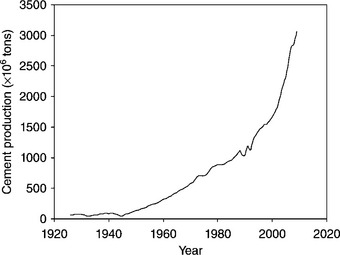
2.1 Worldwide annual cement production for the period 1925–2009. Data from Kelly and van Oss (2010).
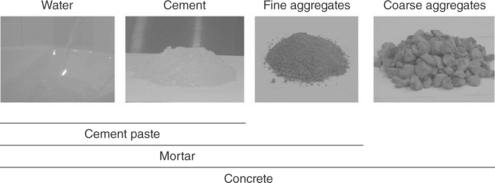
Concrete is essentially a composite consisting of a binding matrix (cement paste) with embedded particles (aggregates). Cement paste is the resulting product of the complex chemical reactions that take place between cement (see Table 2.1 for typical compositions) and water (Double and Hellawell, 1976). Aggregates could be sand, gravel, crushed stone, crushed blast-furnace slag, or demolition waste and are usually separated by their size. Aggregates with diameters larger than 4.75 mm (No. 4 sieve) are referred to as coarse, whereas particles with diameter in the range of 75 μm–4.75 mm (No. 200 to No. 4 sieve) are referred to as fine (Mehta and Monteiro, 2006). When fine aggregates are added to the initial mix, we term the resulting product as mortar (cement + water + fine aggregates), whereas concrete is created with the further addition of coarse aggregates (cement + water + fine/coarse aggregates), as illustrated in Fig. 2.2.
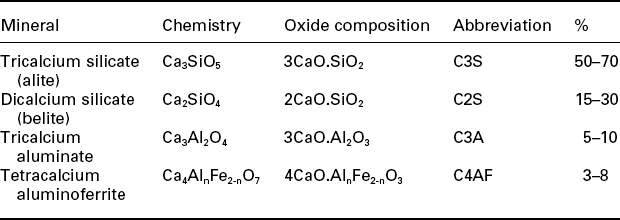
Table 2.1
Chemical formulae and cement nomenclature for major constituents of Portland cement. Cement chemistry abbreviation: C = CaO, S = SiO2, A = Al2O3, F = Fe2O3. Also water is abbreviated as H = H2O. (Hewlett, 2004)
2.1.2 Nanoscale: C-S-H
Cementitious materials are the product of complex chemical reactions that take place when cement (primarily tricalcium and dicalcium silicates) reacts with water to form various hydration products with nanoscale features that are arranged in a multi-scale fashion in a three-dimensional space (Feldman and Sereda, 1968; Taylor, 1990, 1993; Nonat, 2004; Richardson, 2008). As alite and belite (C3S and C2S) comprise over 80% of most cements, their hydration products dominate in terms of volume. Both silicate phases react with water to form a hydrated version of calcium silicates (C-S-H) and calcium hydroxide (CH or Portlandite):
The main constituent phase, C-S-H, which dominates in terms of volumetric proportions (>70%) and as a consequence governs the macroscopic response, manifests itself in the nm to μm length scale (Nonat, 2004; Jennings, 2008; Richardson, 2008). The poorly crystalline, highly porous and non-conductive nature of the material makes it difficult to study and as a result the actual mechanisms that govern the formation and its properties remain unidentified to date. In fact, the hyphens in C-S-H reflect its uncertain stoichiometry. Furthermore, this constituent phase cannot be recapitulated effectively ex-situ; one has to, therefore, access the properties of C-S-H in-situ at the length scale where it naturally occurs. Experimental data report C/S-ratios in the 1.2–2.1 range with an average around 1.75, while the H content fluctuates even more. The recent advent of innovative and powerful experimental techniques provides the cement and concrete community with an unprecedented opportunity to probe this phase in its natural environment, understand its behavior, and transfer these ideas to higher levels through multi-scale models that can deliver the composite concrete response.
2.1.3 Call for innovation
Despite the ubiquitous presence and extreme importance of concrete for modern societies, its development over the past decades has had a largely empirical basis. On one hand, the construction industry is characterized by risk aversion as the use of materials is dictated by codes or standards, and therefore it is difficult for new products to be introduced into the industry. On the other hand, the industry itself is highly fragmented2 and as a consequence very little effort and money is invested by companies on fundamental research and development. As a result, our knowledge of this very important material has remained largely on an empirical basis, and any fundamental research for understanding and innovation is developing from isolated efforts primarily in research and educational institutions.
The low cost and wide availability of the material, coupled with its excellent properties such as ability to be shaped, resistance to water and fire, and good mechanical properties, have been sufficient to maintain concrete as the most used material in the construction industry. Recent environmental publications, however, raise concerns over the ecological footprint of cement and as a consequence cement-based materials like concrete. Compared to steel, aluminum, plastics and other manufactured materials, Portland cement concrete is generally considered as environmentally friendly (Fig. 2.3). However, the large volumes of the material produced every year consume 2–3% of the global energy (Juenger et al., 2011) and approximately 12–15% of the industrial energy (Ali et al., 2011). Furthermore, there is a growing awareness that concrete production and construction practices of today are not sustainable (Worrell et al., 2001; Gartner, 2004; Phair, 2006; Damtoft et al., 2008; Mehta, 2009).
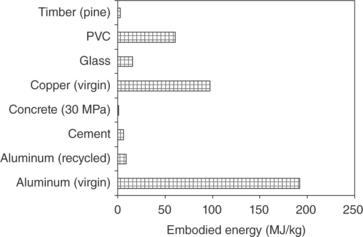
2.3 Embodied energy consumption of some common construction materials. Data from Alcorn (2003).
The production of cement involves heating clay and limestone to 1450°C which involves the burning of fossil fuels to generate such heat releasing in the process significant amounts of CO2 into the atmosphere. It is estimated that for every tonne of cement produced, approximately a tonne of CO2 is released in the atmosphere. About 60% of that is allocated to the chemical reactions that occur when limestone is heated in the kiln (calcination) with the remaining 40% admitted to energy generation through fossil fuels usage for kiln heating and clinker grinding. As a result, cement production is one of the major carbon dioxide contributors in the Earth’s atmosphere. Estimates suggest that the industry is responsible for approximately 7% of global CO2 emissions. Furthermore, while concrete structures are generally thought to be ‘eternal’, they have life-spans that range between 50 and 100 years, depending on the material quality and environmental conditions in use. As a result, the durability performance of the majority of the world’s infrastructure is gradually decaying, posing significant sustainability concerns for various governments.
These recent sustainability concerns provide important drivers for the further understanding and development of this very important material. Recent advances in experimental and theoretical nanomechanics provide new avenues for material decoding (nanoscience) and material optimization for specific applications (nanoengineering). The fundamental idea of the efforts that have been in effect over the last decade or so is to develop the material science approach that will link the initial synthesis and processing conditions with the evolving microstructure and the resulting macro-scopic response. An elegant scientific methodology equivalent to the one used in industrial metals and ceramics is currently lacking. The microstructure-property approach is adopted such as to provide scientific interpretations for the macroscopic mechanical, physical, and chemical responses observed in practice.
2.1.4 Chapter outline
This chapter provides an overview of the recent contributions of nanotechnology to the cement and concrete industry. Space constraints dictate that we explore promising ideas and contributions in a non-exhaustive way, while the reader is directed throughout the text to journal publications for a more detailed exposition. Some recent reviews can also serve as introduction to the topic (Mann, 2006; Scrivener and Kirkpatrick, 2008; Sanchez and Sobolev, 2010; Black et al., 2010; Raki et al., 2010; Jennings and Bullard, 2011; Pacheco-Torgal and Jalali, 2011). The content is presented in two main parts:
1. Section 2.2, ‘Nanoscience of cement-based materials’, deals with the fundamental understanding recently obtained through the application of novel techniques that probe the nanoscale structure of concrete (C-S-H). This refined understanding provides opportunities for more delicate modeling that can transfer nanoscale knowledge to the level where the material is applied to engineering applications.
2. Section 2.3, ‘Nanoengineering of cement-based materials’, is devoted to recent attempts to modify the nanoscale of cementitious materials utilizing advances in nanoscale synthesis, chemistry, and manufacturing in order to develop a stronger, more durable, and environmentally friendly material.
2.2 Nanoscience of cement-based materials
2.2.1 Experimental micro/nano-mechanics
In the past few decades the scientific community has experienced a rapid advancement in the availability of experimental tools for monitoring, manipulating and synthesizing nanoscale features. This provided an unprecedented opportunity for revisiting ubiquitous materials like cementitious systems and refining our fundamental understanding of the underlying mechanisms that control their macroscopic response while at the same time opening avenues for science-based innovation and materials optimization. Several new techniques have been developed, some of which have already been exploited on cementitious materials and some of which remain unexplored. These include atomic force microscopy (AFM), nuclear magnetic resonance (NMR), X-ray microscopy, focused ion beam (FIB), scanning electron microscopy (SEM/ESEM), transmission electron microscopy (TEM), small angle neutron scattering (SANS), small angle X-ray scattering (SAXS). The application of some of these techniques to the benefit of cement and concrete nanoscience is presented below.
Atomic force microscopy (AFM)
Seeing the nanoscale of cementitious materials has been challenging primarily because the C-S-H matrix is highly porous and thus non-conducting. The advent of AFM has provided an opportunity to ‘visualize’ the nanoscale of this important material as it relies on contact rather than any other conduction-related mechanism. AFM, which is generally considered the successor of scanning tunnelling microscopy (STM), was developed by Gerd Binnig and Heinrich Rohrer3 in the early 1980s at IBM Research Labs. The technique, which works by scanning a very sharp metal wire tip over the surface of interest, is utilizing quantum mechanical effects of tunneling and piezoelectric effects to develop a nanoscale image of the material surface. AFM was introduced a few years later in 1985 by Binnig et al. surface. AFM was introduced a few years later in 1985 by Binnig et al. (1986) mainly to overcome the main drawback of STM, its requirement for conductive surfaces. The technique which utilizes interatomic van der Waals forces as monitoring mechanism consists of a sharp tip, usually sharp silicon with nanoscale curvatures, to scan the surface and provide a digital threedimensional morphological profile. Through the contact interaction mechanism, the technique can be applied on virtually any type of surface, these being polymers, metals, ceramics, biomaterials, composites or concrete.
Early attempts (to the best of the author’s knowledge) to apply nanotechnology tools to cementitious materials date back to 1996 when Mitchell et al. (1996) applied AFM to study the physicochemical changes that occur during the hydration process of cement. The ability to image in a liquid environment was found to be beneficial for this material system whose formation is the resulting product of a chemical reaction. This innovative approach presented for the first time the morphological details of the main hydration product, the C-S-H phase. It was observed that C-S-H forms nanocrystalline domains of the order of a few nanometers that agglomerate into a larger porous domain, the main connecting matrix of all cementitious composites. This nanogranular microstructural arrangement was suggested by several other subsequent publications (Lesko et al., 2001; Nonat, 2004). Plassard et al. (2005) managed to isolate atomically smooth domains of recrystallized C-S-H nanoparticles through long-term exposure to saturated calcium hydroxide solution specimens with various C/S ratios. The atomic scale characteristics and nanomechanical responses (elastic modulus) via AFM indentation have been studied by Plassard et al. (2004). Other AFM studies have concentrated on the origin of C-S-H strength and cohesion which remains unresolved. Interaction forces between C-S-H and probes have been measured (Finot et al., 1999; Lesko et al., 2001; Plassard et al., 2005) and the growth process has been monitored (Garrault et al., 2005) shedding some light on this intriguing material system.
The technique further contributed to the study of other chemical constituents of cement paste as well as their chemomechanical stability in time. The carbonation of calcium hydroxide crystals, prepared using the micareplication method, exposed to various environments (combinations of N2, O2, H2O, and CO2), has been studied using AFM images. Spherules of CaCO3, the chemical product of the carbonation reactions, have been observed and the necessary conditions for their formation have been recorded (Yang et al., 2003). The technique currently provides reliable means of seeing at the nanoscale of C-S-H and assists in the quantification of either modification attempts (e.g., Weiguo et al., 2011) or in the fundamental understanding of chemical formation or degradation phenomena.
Nanoindentation
The advent of instrumented indentation enabled fundamental studies of the nanomechanical response of metals, ceramics, polymers, and composites (see, e.g., Oliver and Pharr, 1992, 2004; Fischer-Cripps, 2011). Current technology allows for contact-based deformation of nanoscale load and displacement resolution and has been leveraged for both general mechanical characterization of small materials volumes and unprecedented access to the physics and deformations processes of materials. While nanoindentation was originally developed for homogeneous metals and ceramics, it was quickly appreciated that nanoscale resolution can be of significant use to the decoding of C-S-H structure, the binding phase of all cementitious materials (Constantinides et al., 2003; Constantinides and Ulm, 2004). However, accurate nanomechanical analysis of composites requires advanced analysis that takes into consideration the multi-phase, multi-scale nature of the material and its pressure-sensitive mechanical response (Constantinides et al., 2003; Constantinides and Ulm, 2004, 2007; Ulm et al., 2005, 2007, 2010; Ganneau et al., 2006; Trtik et al. 2009; Randall et al., 2009).
A typical nanoindentation test consists of establishing contact between an indenter (typically diamond) and a smooth sample (Miller et al., 2008), while continuously measuring the load, P and the penetration depth h (Plate I between pages 162 and 163). Analysis of the P-h response proceeds by applying a continuum scale model (Fischer-Cripps, 2011) to derive the indentation modulus ![]() , and indentation hardness H,
, and indentation hardness H, ![]() , where S is the unloading slope at maximum depth hmax, Pmax is the maximum indentation force, and Ac is the projected contact area at hmax. Several empirical means to estimate Ac exist, either through postindentation inspection, geometric idealizations of the probe, or more commonly through analysis of the indentation response for a material of ostensibly known E and H to determine this area as a function of Ac = A(hc). Equations for M and H rely on the assumption of a semi-infinite half-space and therefore caution should be exercised when testing highly heterogeneous materials. In particular, the number of tests should be significantly increased and the choice of indentation depth should be carefully made (Constantinides et al., 2006; Ulm et al., 2007). The two indentation properties measured during a test (M and H) can then be linked to the elastic M = M(E, v) and plastic H = H(c, φ) properties of the indented materials, through advanced continuum scale models (Ganneau et al., 2006). The extracted mechanical properties of the two types of C-S-H measured on hundreds of specimens where found to be intrinsic to all cement-based materials E = 20–30 GPa and H = 400–1000 MPa, where the range relates to the local density variations (nanoscale porosity) observed in cementbased materials.
, where S is the unloading slope at maximum depth hmax, Pmax is the maximum indentation force, and Ac is the projected contact area at hmax. Several empirical means to estimate Ac exist, either through postindentation inspection, geometric idealizations of the probe, or more commonly through analysis of the indentation response for a material of ostensibly known E and H to determine this area as a function of Ac = A(hc). Equations for M and H rely on the assumption of a semi-infinite half-space and therefore caution should be exercised when testing highly heterogeneous materials. In particular, the number of tests should be significantly increased and the choice of indentation depth should be carefully made (Constantinides et al., 2006; Ulm et al., 2007). The two indentation properties measured during a test (M and H) can then be linked to the elastic M = M(E, v) and plastic H = H(c, φ) properties of the indented materials, through advanced continuum scale models (Ganneau et al., 2006). The extracted mechanical properties of the two types of C-S-H measured on hundreds of specimens where found to be intrinsic to all cement-based materials E = 20–30 GPa and H = 400–1000 MPa, where the range relates to the local density variations (nanoscale porosity) observed in cementbased materials.
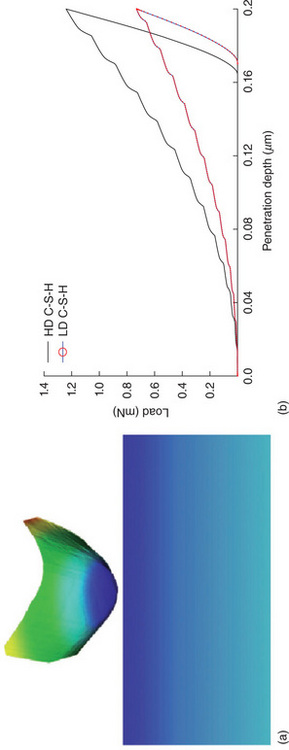
Plate I (a) Nanoindentation principle: a diamond nanoindenter is brought in contact with the material of interest and the force (P) and penetration depth (h) are monitored continuously. (b) Typical P-h curves for a low-density (LD) and a high-density (HD) Version of C-S-H. Results are from finite element simulations.
Instrumented indentation provides mechanical access to the C-S-H phases and has been exploited apart from fundamental studies (Constantinides et al., 2003; Constantinides and Ulm, 2004, 2007; Mondal et al.,, 2007, 2008; Vandamme and Ulm, 2009; Vandamme et al. 2010; Chen et al., 2010; Ulm et al., 2010; Xu and Yao, 2011; Song et al., 2011) but also for the evaluation of chemical degradation phenomena (Constantinides and Ulm, 2004; DeJong and Ulm, 2007), and the nanomechanical quality of various cementbased systems like alkali activated aluminosilicates (Nemecek et al., 2010), ultra high performance concrete (Sorelli et al., 2008), fiber reinforced systems (Wang et al., 2009; Sakulich and Li, 2011), nanosilica concrete (Zyganitidis et al., 2011), and carbon nanotube reinforced concrete (Sáez de Ibarra et al., 2006; Konsta-Gdoutos et al., 2010a). The technique can potentially serve as a nanomechanical screening tool in the search for a more durable and environmentally friendly material.
Small angle neutron and x-ray scattering (SANS/SAXS)
Small angle neutron and X-ray scattering (SANS/SAXS) are powerful techniques for characterizing the microand nanostructures of disordered heterogeneous materials on the 1–100 nm length scale (Winslow and Diamond, 1974; Winslow et al., 1994, 1995; Allen et al.,, 1982, 1987; Volkl et al., 1987; Allen, 1991; Eichhorn et al., 1993; Beddoe and Lang, 1994; Allen and Thomas, 2007). Macro structures like polymers, precipitates in metallurgical specimens, biological molecules, micelles and magnetic systems like ferrofluids can be identified. The drawback, however, is that SANS requires a neutron source which is expensive and available only in a handful of laboratories around the world associated with research nuclear reactors. Around 37 neutron sources exist, the majority of which (>60%) are in Europe. Neutron scattering has an advantage over X-ray scattering (SAXS) due to selective absorption and scattering cross section of neutrons across the periodic table. SAS is particularly suitable for cement-based materials as it does not require drying and can be conducted in situ during hydration. Furthermore, it circumvents the need for specimen preparation and any associated interference that might be incorporated, as with microscopy techniques.
The technique involves measuring the intensity of neutron or X-ray scattered (due to heterogeneities) through small angles, usually less than 1°. By employing a suitable model for data interpretation, one can determine information on the geometrical characteristics like size distribution, volume fractions, surface area, fractal characteristics, etc. In general, and owing to their neutrality, neutrons can penetrate the specimen far better than X-ray scattering techniques, thus probing the material on higher length-scales. In fact, recent instrument modifications, namely ultrasmall-angle scattering (USANS and USAXS), allow through crystal diffraction optics material data to be received from much lower scattering vectors, thus enabling microstructure characterization to extend to larger domains, i.e. >1 μm for USAXS and >10 μm for USANS. The combination of all these techniques suggest that C-S-H is a nanogranular material with a fundamental unit on the order of 5 nm in the vertical direction, a chemical formula of (CaO)1.7(SiO2)(H2O)1.8 and a density of 2604 kg/m3 (Allen et al., 2007; Jennings et al., 2007). This unit appears to agglomerate, possibly with fractal characteristics, into larger domains with local spatial variability in particle packing and therefore densities. Information on the larger CH crystals can be obtained through inelastic neutron scattering (INS), whereas the different states of free and bound water found in cement systems can be probed when employing quasielastic neutron scattering techniques (QENS). Apart from a fundamental understanding of the C-S-H phase, the techniques have been employed for monitoring the hydration mechanism and its alteration through accelerators or decelerators, the effect of cement additives and cement replacement materials, and the effect of calcium leaching.
The microstructure of concrete revisited
It is now clear that at the centimeter scale, concrete or mortar can be considered as a two-phase composite with a (usually) weak interface generated by the local packing of the cement particles during hydration that generates what is known as an interfacial transition zone (ITZ). While this macroscopic visualization is quite accurate at that level, it cannot capture all the peculiarities that cement-based materials exhibit, e.g., access to aggressive ions, time-dependent deformation, sensitivity to humidity, response to low and high temperatures, pressure sensitivity of strength, and many more. All these require understanding of the percolated C-S-H phase which controls the macroscopic response. The very nature of C-S-H has been the subject of many experimental and theoretical attempts over the last 100 years. It is now well accepted that it is a colloidal network gel with local density variability (Scherer, 1999; Jennings, 2000, 2008; Tennis and Jennings, 2000). While C-S-H continues to be the subject of significant research towards its fundamental understanding, there is now ample experimental data on which to build preliminary predictive models. The models aim to rationalize as many experimental data reported as possible. Among the many reported over the years, we refer to the models by Feldman and Sereda, and Jennings (Fig. 2.4).
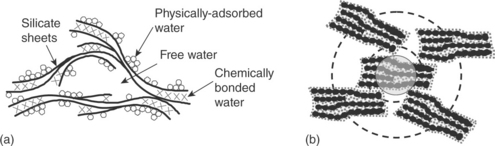
2.4 Two of the many C-S-H models proposed in the literature: (a) Feldman and Sereda layered model and (b) Jennings colloidal model.
The Feldman and Sereda model suggests that C-S-H, similar to clay particles, forms a three-dimensional assemblage of layer silicate sheets which locally tend to form parallel network groups with entrapped water and pore space (Fig. 2.4a). The Jennings nanoscale model for the structure of C-S-H rationalizes the differences in the density and surface area values obtained from different experimental techniques. The smallest distinct units of C-S-H are ‘globules’ just under 5 nm in the smallest dimension, which pack together into two distinct structures, called high density (HD) C-S-H and low density (LD) C-S-H. The average packing density of the HD structure is 74%, while the LD C-S-H structure is more complex, with a packing density that varies with scale such that the structure is fractal over length scales up to a maximum of about 60 nm. The LD C-S-H structure was found to vary with the curing conditions, aging, load, and environment. The mechanical response of these two phases (LD and HD C-S-H) has been measured by nanoindentation (Constantinides and Ulm, 2004) and serves as the input of multi-scale micromechanical models.
Fundamental questions remain regarding the physics of C-S-H that need to be decoded to aid significant advancements. One such nagging question is the very nature of water in nanoconfined spaces and how this contributes to the strength of this material (Kalinichev et al., 2007; Pellenq et al., 2008; Xu et al., 2009).
2.2.2 Theoretical nano/micro-mechanics
Atomistic simulations
The exponential growth of computer power over the past decades has springboarded the development of computational methods and code interfaces that provide the potential of material simulations for a variety of scientific problems. The community of concrete science and engineering was reluctant to employ these techniques primarily because the important characteristics of C-S-H were poorly understood.
A realistic molecular model of hydrating cement has recently been presented in Pellenq et al. (2009). Starting from a monoclinic periodic computational cell of dry Tobermorite and subtracting SiO2 groups, they created a defected silicate chain that achieve C/S ratios close to the experimentally obtained values from NMR (Cong and Kirkpatrick, 1996). The resulting molecular model was then enriched, using grand canonical Monte Carlo simulations, with water molecules to reach a crystal structure of (CaO)1.65(SiO2)(H2O)1.75 and a density of 2.56 g/cm3 which are in close agreement with the experimental values reported through SANS/SAXS by Allen et al. (2007) of (CaO)u(SiO2)(H2O)1.8 and 2.6 g/cm3, respectively. The model was then contrasted to experimental data on hydrated cement, namely fine structures X-ray absorption spectroscopy signals, X-ray diffraction intensities, nanoindentation results, and vibrational density from infrared spectroscopy with very good agreement.
The studies that have already been presented in the literature include the calculation of elastic and chemical bonding properties of the most common cement analoges, Tobermorite and Jennite (Churakov, 2008, 2009; Shahsavari et al., 2009), the atomistic modeling of the major hydration products of cement (Manzano et al., 2006, 2007, 2009), the development of an empirical force field (CSH-FF) for calcio-silicate hydrates (Shahsavari et al., 2011), the impact of chemical impurities on the hydration/structure/mechanical performance of alite (Ca3SiO5 or in short C3S) and belite (Ca2SiO4 or in short C2S) clinker phases (Manzano et al., 2011), and the chloride binding on the various hydration products (Kalinichev and Kirkpatrick, 2002), to name a few. The development of a robust computational model at the nanoscale will allow scientists and engineers to fine-tune and optimize the nanostructure of material through cost-effective virtual simulations. Proper benchmarking against detailed nanoscale experiments will be an essential part of the development process.
Continuum micromechanical modeling
There have been significant developments in the field of micromechanics over the last 50 years (i.e., Torquato, 2001; Dormieux et al., 2006). As the tools of nanoscale synthesis and manipulation became available, there was a pressing need for concurrent development of the predictive modeling techniques. Continuum micromechanics represent the systematic approach for upscaling properties (mechanical, physical, etc.) of composite materials based on the constituents’ properties that compose the heterogeneous microstructure. Typical models incorporate the fundamental mechanical properties of C-S-H together with estimates of the volumetric proportions (fi) of all constituent phases (i) in a multi-scale homogenization scheme that can predict the composite macroscopic elastic (Ehom, vhom = F(Ei,vi,fi)) (Constantinides and Ulm, 2004; Ulm et al., 2004; Sanahuja et al., 2007) and strength (chom, φhom = F(ci,φi,fi)) behavior (Pichler et al., 2009; Pichler and Hellmich, 2011), where the only input requirements are the elastic (Ei, vi) and plastic (ci, φi) properties of the individual constituents and their volumetric proportions (fi). The morphological arrangement of the phases in space is taken into consideration in the choice of the continuum micromechanical models. It has been found that for cementitious materials, a combination of Mori–Tanaka and self-consistent schemes appears to deliver robust results (Constantinides, 2006). Figure 2.5 demonstrates the predictive capabilities of continuum-based micromechanical models, suggesting that they can effectively transfer the Information across several orders of magnitude in length scale starting from the atomistic C-S-H level to the decimeter level of concrete structures. While the above described approaches rely on analytical formulation of the problem, numerical schemes are also employed which provide more powerful possibilities to tackle with non-linear phenomena, like strength, creep, permeability, etc. (Hain and Wriggers, 2008; Smilauer and Bazant, 2010).
2.2.3 Concluding remarks: a framework for science-based innovation
The field of experimental and theoretical nanomechanics is advancing at a rapid pace. As the tools of experimentation and the resulting theoretical frameworks of data analysis are developing, new opportunities for understanding and characterization of materials arise. This provides an unprecedented opportunity to probe long-used ubiquitous construction materials, like concrete, that have not been rigorously characterized and modeled in the past and create a science-based platform for material optimization. Some ideas and recent developments in the field of cement-based materials are presented below.
2.3 Nanoengineering of cement-based materials
The process of tailoring the microstructure of cementitious materials to achieve desirable properties for specific applications is termed materials engineering. When nanoscale features are involved, we refer to this process as nanoengineering. From the above discussion it becomes apparent that cement-based materials, which essentially depend on the performance of C-S-H, are nanoscale materials. Nanoengineering can occur by modifying this nanoscale phase directly, through some macroscopic synthesis and processing means, or by introducing nanoscale additives that can either be inert or chemically interact with the material in certain ways. Recent developments in nanotechnology provide ample routes for concrete nanoengineering, some of which are discussed below.
2.3.1 Empirical routes for material development
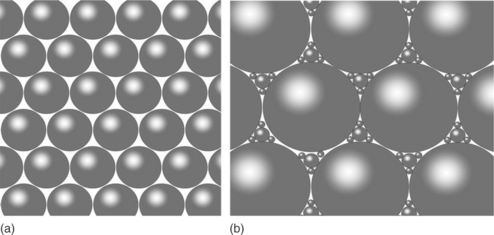
When reviewing the historical evolution of cementitious materials, it can be identified that much of their development relied on empirical knowledge that was achieved through trial and error experimentation. In retrospect, the degree of optimization that has been achieved in this fashion is rather impressive. Traditionally, the most used variable for the material characterization has been the mass ratio of water to cement in the initial mix. It has been argued that this ratio is ultimately related to the spacing achieved within cement particles (Bentz and Aitcin, 2008) and as a result of the amount of porosity and all related parameters. It is common knowledge in the concrete community that the lower the w/c ratio the higher the elasticity, strength, and durability. Of course the process cannot be continued indefinitely: as the distance between particles is reduced, the particles tend to agglomerate with a subsequent loss of workability. To circumvent this problem, a higher particle packing can be achieved by playing with the particle size distribution of the dry mix (Fig. 2.6). Furthermore, the development of superplasticizers that could create workable mixes for low w/c ratios resulted in high strength and performance materials. As for most materials, the stronger they become, the more brittle they tend to be. The development of fiber reinforcements (steel, polymeric, etc.) added ductility to the system and created a material as attractive as metallic systems. Cementitious materials with strength as great as that of mild steel have been reported in the literature (e.g., Richard and Cheyrezy, 1995). Other empirical techniques that alleviated some of the problems associated with these high performance systems, like heat treatment, etc., have been proposed.
2.3.2 Cementitious nanocomposites
Nanoparticles have sizes with at least one of their dimensions in the 1–100 nm range. As the particle size decreases, a larger proportion of atoms is situated on the free surface compared to those atoms in the bulk, resulting in properties which can be significantly different from their larger scale counterparts. Several nanoscale particles are currently being considered as nanoscale additives with the aim of improving macroscopic performance or adding functionality to the material. Among the candidates are nanosilica, titanium dioxide, carbon nanotube, nanoscale limestone, haematite nanoparticles, pigments, clay particles, etc. (Meng et al., 2008; Sato and Diallo, 2010; Tregger et al., 2010a,b; Sato and Beaudoin, 2011).
Microand nanosilica (μ/n-SiO2)
Silica fume, also known as microsilica, is an amorphous polymorph of silicon dioxide, silica. It is a by-product of the carbothermic reduction of high purity quartz in electric arc furnaces in the production of silicon and ferrosilicon alloys. The resulting product is a grey powder of surface area on the order of 20 m2/g and particle sizes in the 100–200 nm range, approximately one hundredth of the cement particles. When silica fume is added to Portland cement concrete, it increases its compressive strength, tensile strength and abrasion resistance. These improvements stem from the closed packing achieved in the cement paste system that reduces the overall porosity and improves the interfacial transition zone. Furthermore, the material is involved in the pozzolanic reaction (Lin et al., 2011) with the calcium hydroxide crystals producing additional cementing material (C-S-H) and eliminating areas of stress concentrations and prone to failure initiation (Ca(OH)2 crystals). The reduction of porosity produces at the same time a material more durable and resistant to chemical degradation processes like chloride ion diffusion, alkali silica reaction and calcium leaching, which preserves the material from mechanical degradation and protects reinforcing steel from corrosion (Zhang and Li, 2011). Several recent publications report on the significant advantages of nano-SiO2 as compared to microsilica (Jo et al., 2007; Ye et al., 2007; Senff et al., 2010).
The already reported benefits persist in an amplified fashion. The highly reactive and large surface area provided by colloidal silica accelerates cement hydration and has a significant impact on the hydration process at early ages (Bjornstrom et al., 2004). The high surface area, however, decreases the amount of lubricating water and interferes with the flowing characteristics of fresh concrete. It is therefore essential to ensure proper workable conditions, i.e., with the use of superplasticizers, so as to avoid air entraining in the fresh system and benefiting from the above described performance (Senff et al., 2009). The use of silica reinforced cementitious materials might find applications in a variety of systems ranging from oil well cement (Choolaei et al., 2012) to pavement (Zhang and Li, 2011) and high performance compacting applications (Nazari and Riahi, 2011).
Carbon nanotubes
Carbon nanotubes (CNT) are the strongest and stiffest material known to date4 and hold great promise for reinforcing cement-based composites (Makar and Beaudoin, 2004; Makar et al., 2005; Nasibulin et al., 2009; Chaipanich et al., 2010; Konsta-Gdoutos et al., 2010b; Manzur and Yazdani, 2010; Metaxa et al., 2010; Wille and Loh, 2010; Kumar et al., 2012). They are allotropes of carbon with a cylindrical-tubular geometry of diameter that ranges from 1 to 100 nm and lengths up to a few millimeters. Aspect ratios (lengthto-diameter ratio) beyond 100,000,000:1 have been reported in the literature which allows crack-bridging capabilities at finer scales. Owing to their geometry and covalent sp2 bonds formed between the individual carbon atoms, they exhibit superb mechanical, electrical, and thermal properties (among others) coupled with impressive deformation characteristics which makes them excellent candidates for additives to various structural materials, including concrete (Salvetat et al., 1999). It is in fact impressive that with only a tiny portion of reinforcement the composite system can potentially influence the mechanical response. CNTs can occur as a single layer of graphite rolled in a tubular shape (SWCNT) or multiple wall concentric tubes (MWCNT, also known as CNF, whereas F stands for fiber). SWCNT have reported values of 1 TPa for elasticity and 60 GPa for strength whereas the respective values for CNF are in the order of 400 GPa and 7 GPa respectively. The mechanical reduction of MWCNTs is due to the weak shear interactions between adjacent tubes. This limitation has recently been addressed by applying high-energy electron irradiation, which crosslinks inner shells and tubes, and can effectively increase the strength of these materials to ~60 GPa.
In order to harness the real benefits of the filler, any fiber-reinforced composite should ensure the efficient transfer of the stresses between the hosting matrix and the reinforcing material. To ensure this condition, two requirements should be met: (a) the fibers should be uniformly dispersed in the matrix thus avoiding possible agglomeration of particles, through van der Waals forces, in the system, and (b) the fiber–matrix interface should be strong enough such as to avoid pull-out phenomena during service. The selection of chemicals that will ensure proper dispersion and bonding is complicated by the requirement to comply with the chemical system, i.e., not to interfere in a negative way with the hydration process. As a result, many of the well-known surfactants that have been used effectively to disperse CNTs in other systems have been discarded for use in concrete. While the actual protocol that will allow proper dispersion of CNT in the matrix and good bonding qualities is still the subject of intense research, some preliminary results have already been reported in the literature (Gay and Sanchez, 2010; Yazdanbakhsh et al., 2010; Collins et al., 2012). Approaches to disperse the fibers include their surface modification through functionalization usually in conjunction with sonication (Cwirzen et al., 2008). Surface treatment with ozone gas (Chung, 2005), sulphuric and nitric acids (Li et al., 2005), use of gum Arabic (Sáez de Ibarra et al., 2006), polyacrylic acid and polyallylamine hydrochloride (Grunlan et al., 2008), growing CNTs on cement particles (Nasibulin et al., 2009; Nasibulina et al., 2010) and polymer grafting on CNT (Li et al., 2005) have also been suggested.
Admixtures that are commonly used in cementitious materials have also been tested with the polycarboxylate and lignosulfate to show good results (Collins et al., 2012). Cwirzen et al. (2008) demonstrated that stable and homogeneous water dispersions of MWCNTs can be obtained by using functionalized CNTs with COOH and additional treatment with polyacrylic acid polymers. The mixing method included stirring combined with sonication at 50 Hz. The cement paste specimens produced revealed an increase in compressive strength values in comparison with the reference pure cement paste specimens. The highest increase in the compressive strength was nearly 50% in cement paste incorporating only 0.045% of the polyacrylic acid polymer-treated MWCNTs. These results indicate the existence of chemical bonds between the OH groups and probably the C-S-H phase of the cement matrix which enabled the stress transfer.
The quality of the interface characteristics is an even more complicated field and is still in its infancy. CNTs have been treated using a H2SO4/HNO3 mixture solution which potentially leads to the formation of carboxyl acid groups on their surfaces (Li et al., 2005). The presence of carboxylic acid groups enhances the interface efficiency through a series of chemical reactions. In general, when properly dispersed and well bonded, CNTs enhance compressive, tensile, and flexural performance by creating bridging mechanisms between the fibers and matrix, thus capturing microcracks and causing more material to go into plastic deformation prior to failure. Furthermore, there appears to be a densification of the C-S-H matrix. Through nanoindentation experiments, it has been suggested that CNT promote the formation of the HD C-S-H (Shah et al., 2009). The CNT can potentially be employed in any cement-based material ranging from fly-ash mix (Chaipanich et al., 2010) to ultra high performance composites (Sáez de Ibarra et al., 2006). Finally the exploitation of CNTs in the matrix for monitoring and sensing properties is currently under investigation (Wansom et al., 2006; Li et al., 2007; Han et al., 2011).
Titanium dioxide
Titanium dioxide nanoparticles in the form of anatase have received considerable attention in the construction industry in recent years due to their potential to add new functionalities to infrastructures, i.e., self-cleaning properties and the ability to remove air pollutants through photocatalysis (Fig. 2.7). TiO2 are semiconductors that behave as photocatalysts when irradiated by ultraviolet (UV) light in the presence of gas or liquid (Serpone and Pelizzetti, 1989). When mixed with cement, it can photocatalytically degrade organic pollutants that are, after neutralization, washed away through the hydrophilic nature of the surface, maintaining at the same time the aesthetic characteristics of concrete structures, particularly those constructed with white cement (Cassar, 2004; Ruot et al., 2009). TiO2 has proven very efficient in the removal of pollutants such as NOx, aromatics, aldehydes, and ammonia, and currently finds applications in various infrastructure projects like pavements, tunnels, buildings, etc. (Guerrini, 2012). A more detailed exposition of photocatalytic applications is provided in a separate chapter of this book.
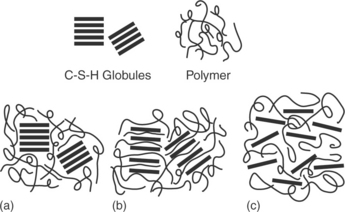
Cement-polymer nanocomposites
While considerable research effort has been devoted to the study of organic composites with small amounts of inorganic reinforcements, like polymerclay systems, little is known about the consequences of adding small amounts of organic material in an inorganic matrix, like the C-S-H/polymer system. This approach constitutes a rather recent and alternative route for concrete nanocomposites which stems from biomimicry approaches (synthesis of composites from aqueous routes like many natural biocomposites, e.g., tooth, bone, and shells) and relates to the attempt to modify the nanoscale C-S-H material through organic hybridization (Matsuyama and Young, 1999a,b; Minet et al., 2006, Franceschini et al., 2007; Alizadeh et al., 2011; Fan et al., 2012). The process, which has been verified computationally (Pellenq et al., 2008), involves grafting organic moieties on the silica layers through controlled hydrolysis of organo-silane precursor mixtures or by hydration of anhydrous silicates in silanized polymer solutions. Additional benefits are expected when the organic molecules intercalate the interlayer C-S-H spaces (Fig. 2.8). The real breakthrough, however, is expected when (and if) an exfoliation of the C-S-H layer is achieved within the organic matrix. The actual chemistries and processing conditions involved are the topic of extreme importance for cement chemists. Recently, a few promising articles have presented experimental data on organic–inorganic hybrid composites that have been synthesized through the sol–gel method. Intercalations has been reported for specific polymers and C-S-H stoichiometric conditions suggesting that the initial chemistry of the inorganic material can act as a template for material tuning. The degree of modification of properties and actual structures involved remains to be quantified.
2.4 Conclusion
Over the last decade, the scientific community has witnessed rapid progress on available experimental, theoretical, and technological developments that allow revisiting this ubiquitous material and examining its environmental footprint. We have presented in this chapter some recent developments in the field of concrete science and engineering which provide a refined understanding, especially of the nanostructure that is responsible for what we experience in the macroscopic world of engineering applications, and allow us to devise methodologies for a stronger, more durable, and environmentally friendly material. While a lot has been achieved so far, even more has to be contributed until a sustainable and ecological, friendly construction material is developed. The necessary tools are being developed which makes us optimistic for the future. It is hoped that the construction industry will invest in a scientific approach in order to utilize (or even invent) new technologies so as to lead concrete into a sustainable future.
2.5 References
Alcorn, A. Embodied energy and CO2 coefficients for NZ building materials, Centre for Building Performance Research Report. New Zealand: Victoria University of Wellington; 2003.
Ali, M.B., Saidur, R., Hossain, M.S. A review on emission analysis in cement industries. Renewable and Sustainable Energy Reviews. 2011; 15(5):2252–2261.
Alizadeh, R., Beaudoin, J.J., Raki, L., Terskikh, V. C-S-H/polyaniline nanocomposites prepared by in situ polymerization. Journal of Materials Science. 2011; 46(2):460–467.
Allen, A.J. Time-resolved phenomena in cements, clays and porous rocks. Journal of Applied Crystallography. 1991; 24(5):624–634.
Allen, A.J., Thomas, J.J. Analysis of C-S-H gel and cement paste by smallangle neutron scattering. Cement and Concrete Research. 2007; 37(3):319–324.
Allen, A.J., Windsor, C.G., Rainey, V.S., Pearson, D., Double, D.D., Alfor, N. McN. A small-angle scattering study of cement porosities. Journal of Physics D: Applied Physics. 1982; 15(9):1817–1833.
Allen, A.J., Oberthur, R.C., Pearson, D., Schofield, P., Wilding, C.R. Development of the fine porosity and gel structure of hydrating cement systems. Philosophical Magazine Part B. 1987; 56(3):263–288.
Allen, A.J., Thomas, J.J., Jennings, M.H. Composition and density of nano scale calcium silicate hydrate in cement. Nature Materials. 2007; 35(6):311–316.
Beddoe, R.E., Lang, K. Effect of moisture on fractal dimension and specific surface of hardened cement paste by small-angle X-ray-scattering. Cement and Concrete Research. 1994; 24(4):605–612.
Bentz, D.P., Aitcin, P.C. Hidden meaning of water-cement ratio. Concrete International. 2008; 30(5):51–54.
Binnig, G.K., Quate, C.F., Gerber, Ch. Atomic force microscope. Physical Review Letters. 1986; 56(9):930–933.
Bjornstrom, J., Martinelli, A., Matic, A., Borjesson, L., Panas, I. Accelerating effects of colloidal nano-silica for beneficial calcium-silicate-hydrate formation in cement. Chemical Physics Letters. 2004; 392(1–3):242–248.
Black, L., Purnell, P., Hill, J. Current themes in cement research. Advances in Applied Ceramics. 2010; 109(5):253–259.
Cassar, L. Photocatalysis of cementitious materials: clean buildings and clean air. MRS Bulletin. 2004; 29(5):328–331.
Chaipanich, A., Nochaiya, T., Wongkeo, W., Torkittikul, P. Compressive strength and microstructure of carbon nanotubes–fly ash cement composites. Materials Science and Engineering: A. 2010; 527(4–5):1063–1067.
Chen, J.J., Sorelli, L., Vandamme, M., Ulm, F.-J., Chanvillard, G. A coupled nanoindentation/SEM-EDS study on low water/cement ratio Portland cement paste: evidence for C-S-H/Ca(OH)2 nanocomposites. Journal of the American Ceramic Society. 2010; 93(5):1484–1493.
Choolaei, M., Rashidi, A.M., Ardjmand, M., Yadegari, A., Soltanian, H. The effect of nanosilica on the physical properties of oil well cement. Materials Science and Engineering: A. 2012; 538:288–294.
Chung, D.D. Dispersion of short fibers in cement. Journal of Materials in Civil Engineering. 2005; 17(4):379–383.
Churakov, S.V. Hydrogen bond connectivity in Jennite from ab initio simulations. Cement and Concrete Research. 2008; 38(12):1359–1364.
Churakov, S.V. Structure of the interlay in normal 11A Tobermorite from ab initio study. European Journal of Mineralogy. 2009; 21(1):261–271.
Collins, F., Lambert, J., Duan, W.H. The influences of admixtures on the dispersion, workability, and strength of carbon nanotube-OPC paste mixtures. Cement and Concrete Composites. 2012; 34(2):201–207.
Cong, X.D., Kirkpatrick, R.J. Si-29 MAS NMR study of the structure of calcium silicate hydrate. Advanced Cement Based Materials. 1996; 3(3–4):144–156.
Constantinides, G.Invariant mechanical properties of calcium silicate hydrates (C-S-H) in cement-based materials: instrumented nanoindentation and microporomechanical modeling. MIT, 2006. [PhD Thesis].
Constantinides, G., Ulm, F.-J. The effect of two types of C-S-H on the elasticity of cement-based materials: results from nanoindentation and micromechanical modeling. Cement and Concrete Research. 2004; 34(1):67–80.
Constantinides, G., Ulm, F.-J. The nanogranular nature of C-S-H. Journal of the Mechanics and Physics of Solids. 2007; 55(1):64–90.
Constantinides, G., Ulm, F.-J., van Vliet, K.J. On the use of nanoindentation for cementitious materials. Materials and Structures. 2003; 36(205):191–196.
Constantinides, G., Ravichandran, K.S., Ulm, F.-J., van Vliet, K.J. Grid indentation analysis of composite microstructure and mechanics: Principles and validation. Materials Science and Engineering: A. 2006; 430(1–2):189–202.
Cwirzen, A., Habermehl-Cwirzen, K., Penttala, V. Surface decoration of carbon nanotubes and mechanical properties of cement/carbon nanotube composites. Advances in Cement Research. 2008; 20(2):65–73.
Damtoft, J.S., Lukasik, J., Herfort, D., Sorrentino, D., Gartner, E.M. Sustainable development and climate change initiatives. Cement and Concrete Research. 2008; 38(2):115–127.
DeJong, M.J., Ulm, F.-J. The nanogranular behavior of C-S-H at elevated temperatures (up to 700 degrees C). Cement and Concrete Research. 2007; 37(1):1–12.
Dormieux, L., Kondo, D., Ulm, F.-J. Microporomechanics. New York: Wiley; 2006.
Double, D.D., Hellawell, A. The hydration of Portland cement. Nature. 1976; 261(10):486–488.
Eichhorn, F., Haussler, F., Baumbach, H. Structural studies on hydrating cement pastes. Journal de Physique IV. 1993; 03(C8):369–372.
Fan, W., Stoffelbach, F., Rieger, J., Regnaud, L., Vichot, A., Bresson, B., Lequeux, N. A new class of organosilane-modified polycarboxylate superplasticizers with low sulfate sensitivity. Cement and Concrete Research. 2012; 42(1):166–172.
Feldman, R.F., Sereda, P.J. A model for hydrated Portland cement paste as deduced from sorption-length change and mechanical properties. Materials and Structures. 1968; 1(6):509–520.
Finot, E., Lesniewska, E., Mutin, J.C., Goudonnet, J.P. Investigations of surface forces between gypsum crystals in electrolytic solutions using microcantilevers. Journal of Chemical Physics. 1999; 111(14):6590–6598.
Fisher-Cripps, A.C. Nanoindentation. New York: Springer; 2011.
Franceschini, A., Abramson, S., Mancini, V., Bresson, B., Chassenieux, C., Lequeux, N. New covalent bonded polymer-calcium silicate hydrate composites. Journal of Materials Chemistry. 2007; 17(9):913–922.
Ganneau, F.P., Constantinides, G., Ulm, F.-J. Dual-indentation technique for the assessment of strength properties of cohesive-frictional material. International Journal of Solids and Structures. 2006; 43(6):1727–1745.
Garrault, S., Finot, E., Lesniewska, E., Nonat, A. Study of C-S-H growth on C3S surface during its early hydration. Materials and Structures. 2005; 38(278):435–442.
Gartner, E. Industrially interesting approaches to ‘low-CO2’ cements. Cement and Concrete Research. 2004; 34(9):1489–1498.
Gay, C., Sanchez, F. Performance of carbon nanofiber-cement composites with a high-range water reducer. Transportation Research Record. 2010; 2142:109–113.
Grunlan, C., Liu, L., Regev, O. Weak polyelectrolyte control of carbon nanotube dispersion in water. Journal of Colloid and Interface Science. 2008; 317(1):346–349.
Guerrini, G.L. Photocatalytic performances in a city tunnel in Rome: NO(x) monitoring results. Construction and Building Materials. 2012; 27(1):165–175.
Hain, M., Wriggers, P. Numerical homogenization of hardened cement paste. Computational Mechanics. 2008; 42(2):197–212.
Han, B., Yu, X., Ou, J. Multifunctional and smart carbon nanotube reinforced cement-based materials. In: Gopalakrishnan K., Birgisson B., Taylor P., Attoh-Okine N.O., eds. Nanotechnology in Civil Infrastructure. Berlin: Springer; 2011:1–47.
Hewlett P., ed. Lea’s Chemistry of Cement and Concrete, 4th ed, London: Butterworth-Heinemann, 2004.
Jennings, H.M. A model for the microstructure of calcium silicate hydrate in cement paste. Cement and Concrete Research. 2000; 30(1):101–116.
Jennings, M.H. Refinements to colloid model of C-SH in cement: CM-II. Cement and Concrete Research. 2008; 38(2):275–289.
Jennings, M.H., Bullard, J.W. From electrons to infrastructure: engineering concrete from the bottom up. Cement and Concrete Research. 2011; 41(7):727–735.
Jennings, H.M., Gevrenov, J.S., Thomas, J.J., Constantinides, G., Ulm, F.-J. A multi-technique investigation of the nanoporosity of cement paste. Cement and Concrete Research. 2007; 37(3):329–336.
Jo, B.W., Kim, C.H., Tae, G.H., Park, J.B. Characteristics of cement mortar with nano-SiO2 particles. Construction and Building Materials. 2007; 21(6):1351–1355.
Juenger, M.C.G., Winnefeld, F., Provis, J.L., Ideker, J.H. Advances in alternative cementitious binders. Cement and Concrete Research. 2011; 41(12):1232–1243.
Kalinichev, A.G., Kirkpatrick, R.J. Molecular dynamics modeling of chloride binding to the surfaces of calcium hydroxide, hydrated calcium aluminate, and calcium silicate phases. Chemistry of Materials. 2002; 14(8):3539–3549.
Kalinichev, A.G., Wang, J.W., Kirkpatrick, R.J. Molecular dynamics modeling of the structure, dynamics and energetics of mineral–water interfaces: application to cement materials. Cement and Concrete Research. 2007; 37(3):337–347.
Kelly, T.D., van Oss, H.G. Cement Statistics. Reston, VA: US Geological Survey; 2010.
Konsta-Gdoutos, M.S., Metaxa, Z.S., Shah, S.P. Highly dispersed carbon nanotube reinforced cement based materials. Cement and Concrete Research. 2010; 40(7):1052–1059.
Konsta-Gdoutos, M.S., Metaxa, Z.S., Shah, S.P. Multi-scale mechanical and fracture characteristics and early-age strain capacity of high performance carbon nanotube/cement nanocomposites. Cement and Concrete Composites. 2010; 32(2):110–115.
Kumar, S., Kolay, P., Malla, S., Mishra, S. Effect of multiwalled carbon nanotubes on mechanical strength of cement paste. Journal of Materials in Civil Engineering. 2012; 24(1):84–91.
Lesko, S.I., Lesniewska, E., Nonat, A., Mutin, J.C. Investigation by atomic force microscopy of forces at the origin of cement cohesion. Ultramicroscopy. 2001; 86(1):11–16.
Li, G.Y., Wang, P.M., Zhao, X. Mechanical behaviour and microstructure of cement composites incorporating surface-treated multi-walled carbon nanotubes. Carbon. 2005; 43(6):1239–1245.
Li, G.Y., Wang, P.M., Zhao, X. Pressure-sensitive properties and microstructure of carbon nanotube reinforced cement composites. Cement and Concrete Composites. 2007; 29(5):377–382.
Lin, Q., Xu, Z.Z., Lan, X.H., Ni, Y.R., Lu, C. The reactivity of nano silica with calcium hydroxide. Journal of Biomedical Materials Research Part B: Applied Biomaterials. 2011; 99B(2):239–246.
Makar, J.M., Beaudoin, J.J. Carbon nanotubes and their application in the construction industry. In: Bartos P.J.M., Hughes J.J., Trtik P., Zhu W., eds. Nanotechnology in Construction. Cambridge: Royal Society of Chemistry; 2004:331–341.
Makar, J., Margeson, J., Luh, J. Carbon nanotube/cement composites Early results and potential applications. Proceedings of the 3rd International Conference on Construction Materials: Performance, Innovations and Structural Implications. 2005:1–10. [Vancouver, BC, August 22–24].
Mann, S. Nanoforum report on Nanotechnology and Construction, 2006. www.nanoforum.org/dateien/temp/Nanotech and Construction Nanoforum report.pdf?09052012123958 [(accessed November 2012).].
Manzano, H., Dolado, J.S., Ayuela, A. On the formation of cementitious C-S-H nanoparticles. Journal of Computer Aided Material Design. 2006; 14(1):45–51.
Manzano, H., Dolado, J.S., Guerrero, A., Ayuela, A. Mechanical properties of crystalline calcium-silicate-hydrates: comparison with cementitious C-S-H gels. Physica Status Solidi A. 2007; 204(6):1775–1780.
Manzano, H., Dolado, J.S., Ayuela, A. Elastic properties of the main species present in Portland cement pastes. Acta Materialia. 2009; 57(5):1666–1674.
Manzano, H., Durgun, E., Abdulhosseine Quomi, M.J., Ulm, F.-J., Pellenq, R., Grossman, J. Impact of the chemical impurities on the crystalline cement clinker phases determined by atomistic simulations. Crystal Growth and Design. 2011; 11(7):2964–2979.
Manzur, T., Yazdani, N. Strength enhancement of cement mortar with carbon nanotubes: early results and potential. Transportation Research Record. 2010; 2142(2):102–108.
Matsuyama, H., Young, J.F. Intercalation of polymers in calcium silicate hydrate: a new synthetic approach to biocomposites? Chemistry of Materials. 1999; 11(1):16–19.
Matsuyama, H., Young, J.F. Synthesis of calcium silicate hydrate/polymer complexes: Part II. Cationic polymers and complex formation with different polymers. Journal of Materials Research. 1999; 14(8):3389–3396.
Mehta, P.K. Global concrete industry sustainability. Concrete International. 2009; 32(2):45–48.
Mehta, P.K., Monteiro, P.J.M. Concrete: Microstructure, Properties and Materials, 3rd edn. Maidenhead: McGraw-Hill; 2006.
Meng, T., Qian, K.L., Qian, X.Q., Zhan, S.L. Effect of the nano-CaCO3 on hydrated properties and interface of cement paste. Rare Metal Materials and Engineering. 2008; 37(2):667–669.
Metaxa, Z.S., Konsta-Gdoutos, M.S., Shah, S.P. Carbon nanofiber-reinforced cement-based materials. Transportation Research Record. 2010; 2142:114–118.
Miller, M., Bobko, C., Vandamme, M., Ulm, F.-J. Surface roughness criteria for cement paste nanoindentation. Cement and Concrete Research. 2008; 38(4):467–476.
Minet, J., Abramson, S., Bresson, B., Franceschini, A., Van Damme, H., Lequeux, N. Organic calcium silicate hydrate hybrids: a new approach to cement based nanocomposites. Journal of Materials Chemistry. 2006; 16(14):1379–1383.
Mitchell, L.D., Prica, M., Birchall, J.D. Aspects of Portland cement hydration studied using atomic force microscopy. Journal of Materials Science. 1996; 31(16):4207–4212.
Mondal, P., Shah, S.P., Marks, L. A reliable technique to determine the local mechanical properties at the nanoscale for cementitious materials. Cement and Concrete Research. 2007; 37(10):1440–1444.
Mondal, P., Shah, S.R., Marks, L.D. Nanoscale characterization of cementitious materials. ACI Materials Journal. 2008; 105(2):174–179.
Nasibulin, A.G., Shandakov, S.D., Nasibulina, L.I., Cwirzen, A., Mudimela, P.R., Habermehl-Cwirzen, K., Grishin, D.A., Gavrilov, Y.V., Malm, J.E.M., Tapper, U., Tian, Y., Penttala, V., Karppinen, M.J., Kauppinen, E.I. A novel cementbased hybrid material. New Journal of Physics. 2009; 11:023013.
Nasibulina, L.I., Koltsova, T.S., Joentakanen, T., Nasibulin, A.G., Tolochko, O.V., Malm, J.E.M., Karppinen, M.J., Kauppinen, E.I. Direct synthesis of carbon nanofibers on cement particles. Transportation Research Record. 2010; 2142:96–101.
Nazari, A., Riahi, S. The effects of SiO2 nanoparticles on physical and mechanical properties of high strength compacting concrete. Composites Part B: Engineering. 2011; 42(3):570–578.
Nemecek, J., Smilauer, V., Kopecky, L. Nanoindentation characteristics of alkali-activated aluminosilicate materials. Cement and Concrete Composites. 2010; 33(2):163–170.
Nonat, A. The structure and stoichiometry of C-S-H. Cement and Concrete Research. 2004; 34(8):1521–1528.
Oliver, W.C., Pharr, G.M. An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. Journal of Materials Research. 1992; 7(6):1564–1583.
Oliver, W.C., Pharr, G.M. Measurement of hardness and elastic modulus by instrumented indentation: advances in understanding and refinements to methodology. Journal of Materials Research. 2004; 19(1):3–20.
Pacheco-Torgal, F., Jalali, S. Nanotechnology: advantages and drawbacks in the field of construction and building materials. Construction and Building Materials. 2011; 25(2):582–590.
Pellenq, R.J.-M., Lequeux, N., Van Damme, H. Engineering the bonding scheme in C-S-H: the iono-covalent framework. Cement and Concrete Research. 2008; 38(2):159–174.
Pellenq, R.J.-M., Kushima, A., Shahsavari, R., Buehler, M.J., Van Vliet, K.J., Yip, S., Ulm, F.-J. A realistic molecular model of cement hydrates. Proceedings of the National Academy of Sciences of the United States of America. 2009; 106(38):16102–16107.
Phair, J.W. Green chemistry for sustainable cement production and use. Green Chemistry. 2006; 8(9):763–780.
Pichler, B., Hellmich, C. Upscaling quasi-brittle strength of cement paste and mortar: a multi-scale engineering mechanics model. Cement and Concrete Research. 2011; 41(5):467–476.
Pichler, B., Hellmich, C., Eberhardsteiner, J. Spherical and acicular representation of hydrates in a micromechanical model for cement paste: prediction of early-age elasticity and strength. Acta Mechanica. 2009; 203(3–4):137–162.
Plassard, C., Lesniewska, E., Pochard, I., Nonat, A. Investigation of the surface structure and elastic properties of calcium silicate hydrates at the nanoscale. Ultramicroscopy. 2004; 100(3–4):331–338.
Plassard, C., Lesniewska, E., Pochard, I., Nonat, A. Nanoscale experimental investigation of particle interactions at the origin of the cohesion of cement. Langmuir. 2005; 21(16):7263–7270.
Raki, L., Beaudoin, J., Alizadeh, R., Makar, J., Sato, T. Cement and concrete nanoscience and nanotechnology. Materials. 2010; 3(2):918–942.
Randall, N.X., Vandamme, M., Ulm, F.-J. Nanoindentation analysis as a two-dimensional tool for mapping the mechanical properties of complex surfaces. Journal of Materials Research. 2009; 24(3):679–690.
Richard, P., Cheyrezy, M. Composition of reactive powder concretes. Cement and Concrete Research. 1995; 25(7):1501–1511.
Richardson, I.G. The calcium silicate hydrates. Cement and Concrete Research. 2008; 38(1):137–142.
Ruot, B., Plassais, A., Olive, F., Guillot, L., Bonafous, L. TiO2-containing cement pastes and mortars: measurements of the photocatalytic efficiency using a rhodamine B-based colourimetric test. Solar Energy. 2009; 83(10):1794–1801.
Sáez de Ibarra, Y., Gaitero, J.J., Erkizia, E., Campillo, I. Atomic force microscopy and nanoindentation of cement pastes with nanotube dispersions. Physica Status Solidi (A). 2006; 203(6):1076–1081.
Sakulich, A.R., Li, V.C. Nanoscale characterization of engineered cementitious composites (ECC). Cement and Concrete Research. 2011; 41(2):169–175.
Salvetat, J.-P., Bonard, J.-M., Thomson, N.H., Kulik, A.J., Forró, L., Benoit, W., Zuppiroli, L. Mechanical properties of carbon nanotubes. Applied Physics A. 1999; 69(3):255–260.
Sanahuja, J., Dormieux, L., Chanvillard, G. Modelling elasticity of a hydrating cement paste. Cement and Concrete Research. 2007; 37(10):1427–1439.
Sanchez, F., Sobolev, K. Nanotechnology in concrete a review. Construction and Building Materials. 2010; 24(11):2060–2071.
Sato, T., Beaudoin, J.J. Effect of nano-CaCO3 on hydration of cement containing supplementary cementitious materials. Advances in Cement Research. 2011; 23(1):33–43.
Sato, T., Diallo, F. Seeding effect of Nano-CaCO3 on the hydration of tricalcium silicate. Transportation Research Record. 2010; 2141:61–67.
Scherer, G.W. Structure and properties of gels. Cement and Concrete Research. 1999; 29(6):1149–1157.
Scrivener, K.L., Kirkpatrick, R.J. Innovation in use and research on cementitious materials. Cement and Concrete Research. 2008; 38(2):128–136.
Senff, L., Labrinca, J.A., Ferreira, V.M., Hotza, D., Repette, W.L. Effect of nano-silica on rheology and fresh properties of cement pastes and mortars. Construction and Building Materials. 2009; 23:2487–2491.
Senff, L., Hotza, D., Repette, W.L., Ferreira, V.M., Labrinca, J.A. Mortars with nano-SiO2 and micro-SiO2 investigated by experimental design. Construction and Building Materials. 2010; 24:1432–1437.
Serpone, N., Pelizzetti, E. Photocatalysis: Fundamentals and Applications. NewYork: Wiley; 1989.
Shah, S.P., Konsta-Gdoutos, M.S., Metaxa, Z.S., Mondal, P. Nanoscale modification of cementitious materials. Nanotechnology in Construction. 2009; 3:125–130.
Shahsavari, R., Buehler, M.J., Pellenq, R.J.M., Ulm, F.-J. First-principles study of elastic constants and interlayer interactions of complex hydrated oxides: case study of Tobermorite and Jennite. Journal of the American Ceramic Society. 2009; 92(10):2323–2330.
Shahsavari, R., Pellenq, R.J.M., Ulm, F.-J. Empirical force fields for complex hydrated calcio-silicate layered materials. Physical Chemistry Chemical Physics. 2011; 13(3):1002–1011.
Smilauer, V., Bazant, Z.P. Identification of viscoelastic C-S-H behavior in mature cement paste by FFT-based homogenization method. Cement and Concrete Research. 2010; 40(2):197–207.
Song, D., Yao, W., Liang, K., He, L. Nanoindentation characterization of calcium-silicate-hydrate with different curing conditions. Advanced Materials Research. 2011; 177:613–616.
Sorelli, L., Constantinides, G., Ulm, F.-J., Toutlemonde, F. The nanomechanical signature of ultra high performance concrete by statistical nanoindentation techniques. Cement and Concrete Research. 2008; 38:1447–1456.
Taylor, H.F.W. Cement Chemistry. London: Academic Press; 1990.
Taylor, H.F.W. Nano scale microstructure of C-S-H: current status. Advanced Cement Materials. 1993; 1(1):38–46.
Tennis, P.D., Jennings, H.M. A model for two types of C-S-H in the microstructure of Portland cement pastes. Cement and Concrete Research. 2000; 30(6):855–863.
Torquato, S. Random Heterogeneous Materials. Berlin: Springer; 2001.
Tregger, N., Pakula, M., Shah, S.P. Influence of micro- and nanoclays on fresh state of concrete. Transportation Research Record. 2010; 2141:68–74.
Tregger, N., Pakula, M., Shah, S.P. Influence of clays on the rheology of cement paste. Cement and Concrete Research. 2010; 40(3):384–391.
Trtik, P., Münch, B., Lura, P. A critical examination of nanoindentation on model materials and hardened cement pastes based on virtual experiments. Cement and Concrete Composites. 2009; 31(10):705–714.
Ulm, F.-J., Constantinides, G., Heukamp, F.H. Is concrete a poro-mechanics material? A multi-scale investigation of poro-elastic properties. Materials and Structures. 2004; 37(1):43–58.
Ulm, F.-J., Delafargue, A., Constantinides, G. Experimental microporomechanics. In: Dormieux L., Ulm F.-J., eds. Applied Microporomechanics of Porous Materials, CISM Lecture notes no 580. New York: Springer Wien, 2005.
Ulm, F.-J., Vandamme, M., Bobko, C., Ortega, J.A. Statistical indentation techniques for hydrated nanocomposites: concrete, bone, and shale. Journal of the American Ceramic Society. 2007; 90(9):2677–2692.
Ulm, F.-J., Vandamme, M., Jennings, H., Vanzo, J., Bentivegna, M., Krakowiak, K., Constantinides, G., Bobko, C., Van Vliet, K. Does microstructure matter for statistical nanoindentation techniques? Cement and Concrete Composites. 2010; 32(1):92–99.
Vandamme, M., Ulm, F.-J. Nanogranular origin of concrete creep. Proceedings of the National Academy of Sciences of the United States of America. 2009; 106(26):10552–10557.
Vandamme, M., Ulm, F.-J., Fonollosa, P. Nanogranular packing of C-S-H at substochiometric conditions. Cement and Concrete Research. 2010; 40(1):14–26.
Volkl, J.J., Beddoe, R.E., Setzer, M.J. The specific surface of hardened cement paste by small-angle X-ray-scattering effect of moisture-content and chlorides. Cement and Concrete Research.. 1987; 17(1):81–88.
Wang, X.H., Jacobsen, S., He, J.Y., Zhang, Z.L., Lee, S.F., Lein, H.L. Application of nanoindentation testing to study of the interfacial transition zone in steel fiber reinforced mortar. Cement and Concrete Research. 2009; 39(8):701–715.
Wansom, S., Kidner, N.J., Woo, L.Y., Mason, T.O. AC-impedance response of multi-walled carbon nanotube/cement composites. Cement and Concrete Composites. 2006; 28(6):509–519.
Weiguo, S., Gejin, G., Rui, D., Yu, T., Hu, C., Liqi, X. Fabrication and characterization of nano colloid surfaced concrete. Materials and Structures. 2011; 44(9):1559–1564.
Wille, K., Loh, K.J. Nanoengineering ultra-high-performance concrete with multiwalled carbon nanotubes. Transportation Research Record. 2010; 2142:119–126.
Winslow, D.N., Diamond, S. Specific surface of hardened cement paste as determined by small-angle X-ray scattering. Journal of the American Ceramic Society. 1974; 57(5):193–197.
Winslow, D.N., Bukowski, J.M., Young, J.F. The early evolution of the surface of hydrating cement. Cement and Concrete Research. 1994; 24(6):1025–1032.
Winslow, D., Bukowski, J.M., Young, J.F. The fractal arrangement of hydrated cement paste. Cement and Concrete Research. 1995; 25(1):147–156.
Worrell, E., Price, L., Martin, N., Hendriks, C., Meida, L.O. Carbon dioxide emissions from the global cement industry. Annual Review of Energy and the Environment. 2001; 26:303–329.
Xu, J., Yao, W. Nano-scratch as a new tool for assessing the nanotribological behavior of cement composite. Materials and Structures. 2011; 44(9):1703–1711.
Xu, S.Y., Simmons, G.C., Mahadevan, T.S., Scherer, G.W., Garofalini, S.H., Pacheco, C. Transport of water in small pores. Langmuir. 2009; 25(9):5084–5090.
Yang, T., Keller, B., Magyari, E., Hametner, K., Gunther, D. Direct observation of the carbonation process on the surface of calcium hydroxide crystals in hardened cement paste using an atomic force microscope. Journal of Materials Science. 2003; 38(9):1909–1916.
Yazdanbakhsh, A., Grasley, Z., Tyson, B., Al-Rub, R.K.A. Distribution of carbon nanofibers and nanotubes in cementitious composites. Transportation Research Record. 2010; 2142:89–95.
Ye, Q., Zhang, Z.N., Kong, D.Y., Chen, R.S. Influence of nano-SiO2 addition on properties of hardened cement paste as compared with silica fume. Construction and Building Materials. 2007; 21(3):539–545.
Zhang, M.H., Li, H. Pore structure and chloride permeability of concrete containing nano-particles for pavement. Construction and Building Materials. 2011; 25(2):608–616.
Zyganitidis, I., Stefanidou, M., Kalfagiannis, N., Logothetidis, S. Nanomechanical characterization of cement-based pastes enriched with SiO2 nanoparticles. Materials Science and Engineering: B. 2011; 176(19):1580–1584.
1By the term ‘hydraulic cement’, we refer to those materials whose products are stable in aqueous environments.
2According to (Mann, 2006) about 97% of EU construction companies employ fewer than 20 people.
3The two researchers were awarded a Nobel Prize in Physics in 1986 for their development.
4The recent invention of graphene, an allotrope of carbon, might relegate CNT to second in rank.