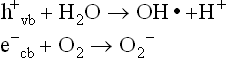Nanotechnology in manufacturing paints for eco-efficient buildings
C. Del Cacho, O. Geiss, P. Leva, S. Tirendi and J. Barrero-Moreno, European Commission, Joint Research Centre, Institute for Health and Consumer Protection, Italy
Abstract:
The addition of a photocatalyst provides decontamination properties to a paint. It is capable of continuously oxidizing both organic and inorganic pollutants and microorganisms under the influence of light during its lifetime. Photocatalytic paints are useful for degrading air pollutants, reducing the costs of maintenance of the exterior aspect and sterilizing the environment. TiO2 is by far the most widely used photocatalyst, but is only active under UV (e.g., solar) irradiation, thus limiting its applicability. In this sense, in order to use photocatalytic paints also in indoor environments, new photocatalysts with a higher activity under visible irradiation is needed. Aspects such as the formation of potentially harmful by-products should also be considered in order to keep the purifying benefits of photocatalytic paints.
15.1 Introduction
Photocatalytic paints are characterized by the addition of a photocatalyst which, when irradiated, favours the oxidation of both inorganic and organic air pollutants, and provides self-cleaning properties to the paint and presents bactericidal and antimicrobial properties.
With a production of more than 3,500 million tonnes worldwide in 2011, the paint industry is one of the largest chemical industries, being the biggest markets located in Asia, Europe and North America. The 4,500 paint companies in Europe employ around 175,000 people (WPCIA, 2011).
Basically, photocatalysis is based on the creation of holes on the electronic structure of the photocatalyst surface by the absorption of light of the appropriate wavelength (generally, UV-A):
where h+vb and e−cb represent the hole and the electron created on the catalyst surface as a consequence of the excitation of an electron present in the valence band of the photocatalyst to the conducting band during the absorption of the incident light.
The optimum wavelength for the activation of a certain photocatalytic material depends on its band gap, as the electrons must, under excitation by absorption of incident radiation, pass into the conducting band. Table 15.1 presents the optimum wavelength of the most commonly used photocatalysts.
Table 15.1
Band gap and optimum excitation wavelength of different photocatalysts
| Photocatalyst | Band gap (eV) | Optimal wavelength (nm) |
| ZnS | 3.6 | 345 |
| SnO2 | 3.6 | 345 |
| Anatase TiO2 | 3.2 | 390 |
| SrTiO3 | 3.2 | 390 |
| ZnO | 3.2 | 390 |
| α-Fe2O3 | 3.1 | 400 |
| CaBi2O4 | 3.1 | 400 |
| Rutile TiO2 | 3.0 | 415 |
| Brookite TiO2 | 3.0 | 415 |
| In2O3 | 2.9 | 430 |
| WO3 | 2.8 | 445 |
| CdS | 2.5 | 495 |
| V2O5 | 2.2 | 565 |
| CdSe | 1.8 | 690 |
The creation of such holes provides a highly reactive and oxidizing material which is thus able to catalyse the oxidation of chemicals deposited or adsorbed on the photocatalyst surface. Particular attention should be paid to the case of adsorbed water and oxygen molecules. These molecules can react with the holes and excited electrons of the photocatalyst, and are transformed into highly reactive compounds such as hydroxyl radicals (OH•) and superoxide anions (O2–):
The most commonly used photocatalyst is TiO2. In nature, TiO2 can be present in three different crystalline structures: rutile, anatase and brookite. TiO2, and especially rutile TiO2, has been extensively used since the nineteenth century as a white pigment in the formulation of paints. Even if the chemical reactions induced by irradiated TiO2 had already been observed, it was not until 1938 that Dooedeve and Kitchener understood the photocatalytic mechanism by which TiO2 was capable of producing the photobleaching of organic dyes and solvents when irradiated with UV light from a mercury lamp (Dooedeve and Kitchener, 1938). Further experiments demonstrated that the anatase crystalline structure of TiO2 possessed a considerably higher photoactivity than that of rutile and brookite TiO2 (Hashimoto et al., 2005).
Different protocols have been described in the literature for the preparation of TiO2 photocatalysts involving sol-gel processes, vapour decomposition of titanium alkyloxides and TiCl4 and calcination of titanium oxysulphate at 400–600 °C (Kaneko and Okura, 2002; Wang et al., 2004; Sun et al., 2008; Akpan and Hameed, 2010).
Once prepared, the photocatalyst is added to the paint formulation, in a concentration typically ranging from 5 to 40% which, depending on the type of application of the paint, is equivalent to a critical pigment volume concentration (CPVC) of around 10% w/v (US Patent, 2009). The CPVC represents the maximum amount of pigment that can be used for a certain amount of non-volatile solvents in the paint formulation without causing a deterioration of the paint properties.
Paints are homogeneous mixtures which contain the following:
• Binder or resin: The resin imparts adhesion and binds the pigments together. It has a direct influence on properties such as gloss, durability, flexibility and toughness. Typical binders include alkyl-, chloride-, vinyl-, epoxy- and polyurethane resins. The paint is often named after the main binder used in its formulation. In most cases, the resin is cured after the application of the paint, which generally involves the polymerization of its constituents.
• Pigments: Pigments are the materials responsible for the colour and opacity of the paint, and they have a direct influence on its mechanical resistance. Pigments include compounds such as minerals (e.g., mica, talc), inorganic salts (Fe, Cr, Cd, Mo, Ti or Pb oxides and hydroxides, calcium carbonate, zinc phosphate, etc.) and organic dyes (toluidines, phthalocyanines). Some pigments confer antioxidant properties on the paint, thereby increasing its stability towards the degradation of paint constituents.
• Solvents and diluents: The appropriate mixture of solvent and diluents is selected in terms of both their volatility and the time required for the curing process of the resin. In this sense, an inappropriate match between these two parameters would result in paints drying rapidly on their surface, but remaining wet in the interior coating. Additionally, solvents have a crucial role in controlling the viscosity of the paint and, thus, the ease of the painting process. The most commonly used solvents are aliphatic solvents, aromatic solvents, alcohols, ketones and esters, which in some cases (e.g., low or zero–VOC paints) are mixed with water.
• Additives: A wide range of additives are added to the formulation of the paint in order to favour the drying and curing process, remove the bubbles formed during painting, increase the chemical and mechanical resistance, etc.
Several parameters have been observed to affect the photocatalytic efficiency of paints for the oxidation of air pollutants under irradiation of the photocatalytic paint (Mo et al., 2009). The most critical parameters are:
• Wavelength of incident radiation: The wavelength of the incident radiation has to be appropriate in order to excite the electrons of the photocatalyst and initiate the photocatalytic process.
• Light intensity: The reaction rate increases with increasing light intensity.
• Effective surface area of the photocatalyst: A higher surface area of the photocatalyst increases the photocatalytic rate.
• Paint constituents and interaction with the photocatalyst: It has recently been observed that paint constituents (e.g., binder) have a considerable influence on the performance of the photocatalytic paint (Aguia et al., 2010). In this sense, it has been observed that the nature and proportion of the different paint constituents can have a significant effect in terms of enhancing or diminishing the photocatalytic efficiency of the catalyst. Further research is still needed in order to understand the mechanism by which each of the single paint constituents affects the photocatalytic efficiency of photocatalytic paints, in order to better optimize their formulation.
• Relative humidity: Water molecules adsorbed on the photocatalytic paint seem to enhance the photocatalytic efficiency of TiO2 through the formation of hydroxyl radicals which, in turn, oxidize the air pollutants. However, an excessive relative humidity (> 70%) inhibits the photocatalytic degradation of air pollutants, due to the competition for adsorption sites on the photocatalyst surface (Pichat, 2010).
• Temperature: A rise in the temperature speeds up the kinetics of the reaction between the pollutants and the photocatalyst, while at the same time decreasing the adsorption of the pollutants on the surface of the photocatalytic paint. Since the net photocatalytic reaction rate is a combination of both processes, a maximum photocatalytic oxidation rate is obtained at the optimum temperature (Obee and Hay, 1997).
• Concentration of the pollutant: The relationship between the concentration of the pollutant and the photocatalytic rate is generally governed by the Langmuir–Hinselwood model (Shiraisi et al., 2007), according to which the reaction rate increases with the concentration as described in the equation:
where r represents the reaction rate, k the rate constant, K the adsorption coefficient of the pollutant on the photocatalytic paint and [Pollutant] the concentration of the pollutant of concern.
• Presence of mixtures of pollutants: Due to the competition on the adsorption of the different pollutants on the surface of the photocatalyst, the photocatalytic reaction rate for a single component is normally lower in the presence of different kinds of pollutants (Ao et al., 2004; Chen and Zhang, 2008).
The present chapter describes the different fields in which photocatalytic paints are used, providing a critical overview of both their advantages and drawbacks, and outlining the areas in which further research is still needed, such as the evaluation of the potential loss in the catalytic efficiency during the lifecycle of photocatalytic paints.
15.2 Application of photocatalytic paints in an outdoor environment
15.2.1 Investigation into the photocatalytic efficiency of active outdoor paints at the laboratory scale
Several experiments corroborated the initial observations carried out by Fujishima and Honda (1972), and demonstrated that TiO2 powder, and more particularly anatase TiO2, was capable of degrading air pollutants (e.g., N2O, NO, NO2, SO2, BTEX, carbonyl compounds, alcohols, CO, CH4, CFCs, etc.) (Wang et al., 2007; Mo et al., 2009; Laufs et al., 2010; De Richter and Caillol, 2011) when exposed to solar-like radiation. Considering the promising results obtained in laboratory-scale experiments, TiO2 has since been incorporated into building materials, such as concrete, glass or paint (Allen et al., 2009; Pacheco-Torgal and Jalali, 2011; PICADA n.d.).
The typical experimental set-up consists of a chamber test in which a controlled atmosphere with a known concentration of pollutant is created (Fig. 15.1). The paint, installed inside the chamber, is irradiated with a light source of a well characterized emission spectra, while the pollutant is flushed through the chamber and its concentration is monitored at both the entrance (before the photocatalysis) and the exit (after the photocatalysis) of the chamber.
In order to quantify the photocatalytic efficiency of the paint accurately, it is necessary to assess for influence of competitive depletion mechanisms that could affect the concentration of the pollutant of concern inside the chamber. Thus, the experiments involve the evaluation of:
• the adsorption of the pollutant on the chamber surface
• the adsorption of the pollutant on the photocatalytic paint itself in the absence of irradiation
• the photolytic degradation of the pollutant through decomposition under UV radiation
Photocatalytic paints are generally activated by irradiating the paint in the test chamber and flushing clean air (i.e., without pollutant) through it for at least 12 hours (Aguia et al., 2011). It is important to stress that, when evaluating the photocatalytic efficiency of paints, the activation conditions are the critical parameters affecting the time required to reach the steady state, after which the photoactivity of the paint either remains constant or diminishes in the event of inactivation by the adsorption of intermediate products on the active catalytic sites.
Aguia et al. (2010, 2011) evaluated the photocatalytic efficiency and selectivity towards the photodegradation of NO of 10 different anatase-based photocatalytic paints using a typical outdoor base paint to which they added different commercial TiO2 photocatalysts in a concentration of 9% w/v. They were quite surprised to observe that there was no direct correlation between the photocatalytic activity of the pure photocatalyst and the corresponding derived photocatalytic paint, in the sense that the most efficient photocatalyst did not yield the optimum photocatalytic paint. Even if further research is needed in order to understand the mechanism by which paint components have such a critical influence on the activity of a photocatalytic paint, this result suggests that the paint components and the way in which the photocatalyst is mixed in the paint matrix play a crucial role in the photoactivity of the final product.
15.2.2 Real-life examples of the use of outdoor photocatalytic paints
Photocatalytic paints possess several properties, the most attractive of which are the following:
• photocatalysis of both inorganic and organic pollutants
• self-cleaning of building materials, reducing the maintenance costs and enabling a longer pristine view of the building
• antimicrobial and antifungal properties, as photocatalytic paints avoid bacterial and fungal growth on their surface
• anti-fogging properties: the contact angle with water decreases to nearly 0° when the photocatalyst is irradiated, avoiding the formation of droplets on the photocatalyst surface.
Some real-life examples of the applications of photocatalytic paints include:
• Music and Art City Hall (Chambery, France)
• Umberto I tunnel (Rome, Italy) (Fig. 15.2)
A significant reduction of both inorganic pollutants (NO, NO2, SO2) and organic pollutants (benzene, toluene) in the surrounding outdoor air has been observed as a result of the application of photocatalytic paint (Marolt et al., 2011; Guerrini, 2012).
15.3 Application of photocatalytic paints in an indoor environment
On average, people in Europe and North America spend 90% of their time in confined indoor environments (Leech et al., 2002; Brasche and Bischof, 2005). Indoor air pollutants are either emitted from numerous indoor sources (e.g., furniture, building materials, cleaning agents, electronic equipment), originate from human activities (e.g., cooking) or enter from the outdoor environment by the ventilation system and doors or windows that are insufficiently airtight. Depending on peoples’ sensitivity, inappropriate indoor air quality can present several health risks, generically referred to as ‘sick building syndrome’ symptoms, such as irritation of the eyes, nose and throat, headaches, dizziness, fatigue, asthma, hypersensitivity, pneumonitis, etc. (EPA, 1991). The basic strategies to improve indoor air quality involve the monitoring and eventual substitution of the emission sources, improvement of the ventilation scheme and purification of the air.
Photocatalytic degradation of air pollutants therefore appears to be a promising technique for air purification. As already mentioned, the most widely used photocatalyst is TiO2, which is only activated by means of UV-A light, the intensity of which is generally low in an indoor environment. Thus, the development of other types of photocatalysts activated by visible light is of the utmost importance in order to apply photocatalytic paints effectively as a methodology for the efficient purification of indoor air.
Apart from the general parameters already mentioned in Section 15.1, the photocatalytic efficiency of indoor paints is greatly influenced by both the nature and the concentration of the photosensitizer that is used to enable the activation of the photocatalyst by visible light. Here, the nature of the photosensitizer used has a direct influence on the change in the band gap of TiO2 and the wavelength required for the activation of the photocatalyst. The concentration of the photosensitizer, on the other hand, needs to be carefully optimized, in order to fine tune the wavelength required for the activation of the modified photocatalyst. Finally, the photodecomposition of the sensitizer itself should be considered.
15.3.1 Strategies for the preparation of visible light active photocatalytic materials
TiO2 photocatalytic materials activated by visible light
Two different alternatives have been employed to prepare TiO2-based photocatalytic materials that can be activated by visible light: sensitization and doping.
The sensitization approach consists of the anchoring of an organic coloured dye (e.g., rhodamine B, eosin, erythrosin B, thionine, chlorophyllin or methylene blue) (Abe et al., 2000; Chatterjee and Mahata, 2001, 2002; Mele et al., 2003; Moon et al., 2003; Kaur and Singh, 2007), a polymer (e.g., polyfluorine-co-thiophene) (Song et al., 2007; Qiu et al., 2008) or a narrow band gap semiconductor (e.g., Bi2S3, CdS, CdSe or V2O5) (Bessekhouad et al., 2004; Ho and Yu, 2006; Jianhua et al., 2006; Wu et al., 2006) to the TiO2 surface. The sensitizer acts as an intermediary and, by enhancing the absorption of visible light, it enables the activation of the photocatalyst by electron transfer between the excited sensitizer and TiO2.
TiO2 sensitized with meso-tetrakis(4-sulfonatephenyl) porphyrin has been applied successfully to the photo-oxidation of acetaldehyde in indoor air under visible light irradiation (Ismail and Bahnemann, 2010).
The doping approach involves the modification of the electronic structure of TiO2 by adding a dopant that modifies the band structure either by increasing the energy of the valence band or by minimizing the energy of the conduction band. The ultimate effect is a minimization of the band gap, thus enabling the doped photocatalyst to be activated by means of visible radiation.
Doped TiO2 photocatalysts prepared by doping with either non-metals (e.g., N, C or S) (Ao et al., 2009; Jo and Kim, 2009; Rockafellow et al., 2009) or transition metals (e.g., Fe, Co, Cu, Au or Mn) (Andronic et al., 2009; Bengtsson et al., 2009; Kafizas et al., 2009; Song et al., 2009; Cacho et al., 2011) have been applied successfully for the degradation of indoor air pollutants when activated by visible light.
Non-TiO2 photocatalytic materials activated by visible light
Only a few attempts at the development of non-TiO2-based photocatalytic materials that can be activated directly by visible light have been successful, due to their narrower band gap. In this sense, metal calcogenides (e.g., CdS, CdSe) (Reutergardh and Iangphasuk, 1997; Green and Rudham, 2003), metal oxides (α-Fe2O3, In2O3 SnO2, WO3, ZnO) (Faust et al., 1989; Kormann et al., 1989; Pulgarin and Kiwi, 1995; Mazellier and Bolte, 2000; Bandara et al., 2001) and mixed metal oxides (SrTiO3, CaBi2O4) (Rothenberger et al., 1985; Tang et al., 2004) have been evaluated as potential photocatalysts. However, the formation of short lived metal-to-ligand and ligand-to-metal charge transfer states, and the narrower band gap, make these materials considerably less stable towards their own photocorrosion.
15.3.2 Investigation into the efficiency of photocatalytic indoor paints at the laboratory scale
Photocatalysis of NO and NO2
Photocatalytic decomposition of NO and NO2 has been widely investigated using pure TiO2 film surfaces, photocatalytic paints, photocatalytic concrete and photocatalytic glass surfaces (Ohko et al., 2009; Huang et al., 2010; Laufs et al., 2010).
In 1998, Negishi et al. (1998) proposed a mechanism by which NO is photochemically oxidized into NO2, and NO2 is either further oxidized to HNO3 on a generally slower step or photolytically reverts into the formation of NO and hydroxyl radicals:
Such a mechanism was further confirmed by two different kinds of experiments. Thus, Dalton et al. (2002) evaluated the adsorption of nitric acid and nitrate ions on the surface of the photocatalyst. Ohko et al. (2009), on the other hand, confirmed the formation of a mixture of NO and NO2 in the gaseous phase when the photocatalysis of either pure NO or NO2 was tested.
Photooxidation of volatile organic compounds
It is well known that volatile organic compounds (VOCs) are photocatalytically oxidized by TiO2 when exposed to UV radiation. The potential for cleaning indoor air has been demonstrated for pollutants such as formaldehyde, acetaldehyde, acetone, benzene or toluene in photochemical reactors using UV radiation and anatase TiO2 powdered films and building materials (Obee and Brown, 1995; Stevens et al., 1998; Pichat et al., 2000; Strini et al., 2005; Everaert and Baeyens, 2004; Maggos et al., 2007). Furthermore, doping of TiO2 photocatalyst with N has proved valuable for the degradation of acetaldehyde, 2-propanol, acetone, toluene and ethylene under visible irradiation (Asahi et al., 2001; Ihara et al., 2003; Miyauchi et al., 2004; Irokawa et al., 2006). As we will show in Section 15.4.2, the use of photocatalytic paints is more complex than the use of powdered films due to the potential formation of secondary emissions from photocatalytic paint constituents.
One of the parameters that critically affects the performance of photocatalytic paints is the interaction between the paint components and the photocatalyst. Thus, when incorporated into the paint matrix, the photocatalyst loses around 90% of its overall photocatalytical efficiency (Aguia et al., 2011) which, in the case of the degradation of volatile organic compounds, can be critical.
Salthammer and Fuhrmann (2007) have recently evaluated the efficiency of different photocatalytic indoor paints in terms of their degradation of organic and inorganic air pollutants. In general, all the evaluated photocatalytic paints showed the same pattern. In this sense, even if NO2 abatement was satisfactory when irradiating the photocatalytic paints with a typical tungsten light bulb, the efficiencies obtained for the degradation of volatile organic compounds were very low. Thus, degradation of formaldehyde and terpene compounds (pinene, limonene) was possible only at high concentration levels, while CO was not observed to be significantly degraded under any of the evaluated conditions.
Antimicrobial and antifungal effect
Hochmannova and Vytrasova (2010) demonstrated the inhibition of the potential cell growth on plates coated with photocatalytic paints prepared using ZnO and TiO2 Degussa as photocatalysts. ZnO-based photocatalytic paints were able to completely inhibit cell growth for Escherichia coli, Pseudomonas aureaginosa, Staphylococcus aureus, Aspergillus niger e Penicillium chrysogenum, and were therefore suggested as optimum photocatalysts for the preparation of antimicrobial photocatalytic paints, where a highly sterile environment (e.g., in hospitals) is required.
This antimicrobial effect may be due to the attack of the microorganisms by the hydroxyl radicals and superoxide anions created during the irradiation of the photocatalyst surface (Section 15.1).
15.4 Potential formation of by-products
By-products formed during the photocatalysis can be either intermediate products or secondary emissions. Some of these by-products might even be more harmful compared to the original pollutant degraded by the photocatalytic paint, and their formation should therefore be avoided wherever possible.
• Intermediate products are formed due to the incomplete photocatalysis of a certain pollutant.
• Secondary emissions are formed due to the photo-oxidation of the supporting material in which the photocatalyst is embedded. Previous studies indicate that various compounds are released due to the oxidation of the organic constituents of the material supporting the photocatalyst during the photocatalytic process.
15.4.1 Formation of intermediate products
Recent studies have shown that the reactions achieved by the degradation of pollutants on photocatalytic paints are generally not complete, and the reaction stops along the way, giving rise to the formation of intermediate products. It is known that NO2 is formed as an intermediate product by the incomplete degradation of NO to nitrate (Ohko et al., 2009), while degradation of organic pollutants gives rise to the formation of aliphatic compounds, aromatic compounds, carbonyl compounds, alcohols, alkoxyalcohols and carboxylic acids (Huang and Li, 2011), which may even be more harmful than the original pollutant. In this sense, the formation of compounds such as benzaldehyde, phenol, formic acid, acetic acid, hexamethylene, heptane or benzene as intermediate products during the photocatalytic degradation of toluene has been observed (Sun et al., 2010).
Some of these intermediate products (e.g., carboxylic acids) are strongly adsorbed on the photocatalyst surface (Huang and Li, 2011), and reduce the number of active catalyst sites in the photocatalytic paint, which can in critical cases considerably minimize its efficiency.
The photocatalytic surface can be regenerated by washing the surface with water or irradiating it with UV light. However, these methodologies are not easy to apply in the case of indoor usage, and further research will be needed in order to develop new methodologies to remove adsorbed intermediate products from indoor photocatalytic paints.
15.4.2 Secondary emissions from paint constituents
Formation of carbonyls while irradiating photocatalytic paints under either UV or visible light has been repeatedly observed as part of a set of investigations to assess the decontamination efficiency of photocatalytically active paints in the past. The main source of such carbonyl compounds was assumed to be the photoinduced decomposition of paint binders (Salthammer and Fuhrmann, 2007; Auvinen and Whirtanen, 2008; Pichat, 2010; Geiss et al., 2012).
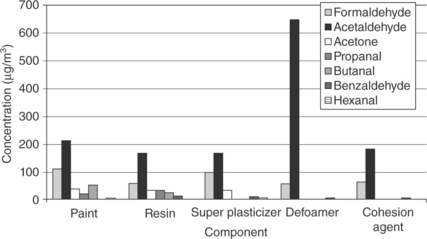
An exhaustive investigation has recently been carried out in order to evaluate the impact of the different paint components on the formation of carbonyl compounds when irradiating photocatalytic paints. Thus, both the photocatalytic paint and each of its individual components was irradiated in the presence of 5% pure anatase TiO2 (Geiss et al., 2012). The components evaluated included cohesion agents, super plasticizers, defoaming agents and redispersable resins.
Figure 15.3 shows the amount of the secondary emission of carbonyl compounds during the irradiation of the different paint components in the presence of pure anatase TiO2 as photocatalyst. As can be observed, formaldehyde, acetaldehyde and acetone appear to be the main degradation compounds for all the paint components evaluated. Lower amounts of longer chain carbonyl compounds such as propanal, butanal or hexanal were formed in the initial stages. A radical mechanism based on the β-scission has been proposed for the degradation of longer-chain carbonyl compounds (Geiss et al., 2012).
15.4.3 Possible solutions to diminish the accumulation of by-products during photocatalysis
Indoor environments are characterized by the coexistence of multiple emission sources responsible for overall indoor air pollution. Formaldehyde is a well-known and ubiquitous indoor pollutant emitted from a wide variety of indoor sources, such as household products, furniture or smoke. Similarly, NO2 is commonly found in indoor environments and originates mainly from combustion and gas appliances. Reported indoor concentrations of formaldehyde and NO2 in dwellings range from 15 to 30 μg m–3 and from 15 to 50 μg m–3, respectively (WHO, 2010).
The formation of NO2 and/or carbonyl compounds as by-products is an undesirable effect, contributing to the chemical load in indoor environments, and should therefore be eliminated or minimized as far as possible, as they might contribute as a relevant additional source which might exceed the guideline values.
Two different approaches are commonly used to improve indoor air quality and avoid the accumulation of indoor pollutants: they are source control and eventual substitution of the emission sources, and increasing the ventilation.
Studies carried out on the formation of by-products during the photocatalytic degradation of both inorganic and organic pollutants indicate that a relatively large amount of by-products is accumulated, even under the realistic conditions of an air exchange rate of 0.5 h–1 and typical indoor concentration levels of the various pollutants. In this sense, experiments carried out on exposure chambers with a synthetic atmosphere containing 50 μg m–3 NO, in order to simulate an indoor environment, resulted in the formation of up to 12 μg m–3 NO2. Similarly, the formation of 30 and 7.5 μg m–3, respectively, of formaldehyde and acetaldehyde was observed, as a result of the decomposition of the paint components.
In order to minimize the formation of intermediate products during the photocatalytic degradation of indoor air pollutants by photocatalytic active paints, the parameters affecting the photocatalytic process need to be optimized, in order to achieve the maximum possible rate of photocatalytic oxidation. Thus, particular attention should be paid to the development of more active photocatalysts in order to optimize the nature and concentration of the sensitizers/dopants used to enable activation by visible light.
Two possible alternative approaches that attempt to minimize the secondary emission of potentially harmful compounds from the degradation of paint constituents during the photocatalytic process have been proposed.
Development of more stable supporting materials
An increase in the stability of the supporting material towards the photocatalytic oxidation should considerably diminish the amount of carbonyl compounds released. In order to increase this stability, a careful selection of the ingredients used in the preparation of the supporting materials should be made. As an example, Fig. 15.4 presents the reduction obtained for the formation of carbonyl compounds during the irradiation of photocatalytic paints, following the appropriate selection of the paint components.
Pretreatment of the photocatalytic paint prior to commercial distribution
It has been repeatedly observed that the amount of carbonyl compounds emitted diminishes with repeated uses of the photocatalytic material, indicating that the emitted carbonyl compounds depend on the extent of the previous irradiation of the supporting material. In order to confirm this effect, the photocatalytic paint has been irradiated for a long period of time, during which periodic measurements have been taken of the amount of carbonyl compounds. As can be observed in Fig. 15.5, the amount of emitted formaldehyde diminished exponentially with the irradiation time. A similar effect was found for all the carbonyl compounds.
As a consequence of this effect, it would be possible to minimize the amount of emitted carbonyl compounds by pre-treating the photocatalytic paint. It could therefore be irradiated with UV light prior to its commercial distribution, given the emission of carbonyl compounds. Recent experiments carried out under controlled conditions indicate that the secondary emission of carbonyl compounds after continuous irradiation of 3 weeks can be considered to be negligible (Geiss et al., 2012).
15.5 Future trends
The present chapter has outlined the current state of the art in the development of photocatalytic paints. Further research is still needed in order to achieve a breakthrough, in the following areas:
• The incorporation of the nano-catalysts into photocatalytic paints, which would theoretically enhance the photocatalytic efficiency by drastically increasing the specific catalyst surface. Special attention should be attached to the evaluation of the potential health risks derived from the usage of such reactive nano-materials.
• The development of more active catalysts that can be activated by visible radiation.
• The increase in the stability of paint components to minimize the formation of secondary emissions during photocatalysis.
15.6 References
Abe, R., Hara, K., Sayamaa, K., Domen, K., Arakawa, H. Steady hydrogen evolution from water on eosin Y–fixed TiO2 photocatalyst using a silane coupling reagent under visible irradiation. Journal of Photochemistry and Photobiology. 2000; 137:63–69.
Aguia, C., Angelo, J., Madeira, L.M., Mendes, A. Influence of photocatalytic paint components on the photoactivity of P25 towards NO abatement. Catalysis Today. 2010; 151:77–83.
Aguia, C., Angelo, J., Madeira, L.M., Mendes, A. Photo-oxidation of NO using an exterior paint. Screening of various commercial titania in powder pressed and paint films. Journal of Environmental Monitoring. 2011; 92:1724–1732.
Akpan, U.G., Hameed, B.H. The advancements in sol-gel method of doped TiO2 photocatalysts. Applied Catalysis A. 2010; 375:1–11.
Allen, N.S., Edge, M., Verran, J., Caballero, L., Abrusci, C., Stratton, J., Maltby, J., Bygott, C. Photocatalytic surfaces: environmental benefits of nanotitania. The Open Materials Science Journal. 2009; 3:6–27.
Andronic, L., Hristache, B., Enesca, A., Visa, M., Duta, A. Studies on titanium dioxide catalyst doped with heavy metals (cadmium, copper and nickel). Environmental Engineering Management. 2009; 8:747–751.
Ao, C.H., Lee, S.C., Yu, J.Z., Xu, J.H. Photodegradation of formaldehyde by TiO2 photocatalyst. Effect of NO, SO2 and VOCs. Applied Catalysis B. 2004; 54:41–50.
Ao, Y., Su, D., Fu, C., Yuan, S. Synthesis of C,N,S-tridoped mesoporous titania with enhanced visible light induced photocatalytic activity. Microporous and Mesoporous Materials. 2009; 122:1–6.
Asahi, R., Morikawa, T., Ohwaki, T., Aoki, K., Taga, Y. Visible light photocatalysis in nitrogen-doped titanium dioxides. Science. 2001; 293:269–271.
Auvinen, J., Whirtanen, L. The influence of photocatalytic interior paints on indoor air quality. Atmospheric Environment. 2008; 42:4101–4112.
Bandara, J., Mielczarski, J.A., Lopez, A., Kiwi, J. Sensitized degradation of chlorophenols on iron oxides induced by visible light – comparison with titanium dioxide. Applied Catalysis B. 2001; 34:321–333.
Bengtsson, N., Castellote, M., López-Muñoz, M.J., Cerro, L. Preparation of co-doped TiO2 for photocatalytic degradation of NOx in air under visible light. Journal of Advanced Oxidation Technologies. 2009; 12:55–64.
Bessekhouad, Y., Robert, D., Weber, J.V. Bi2S3/TiO2 and CdS/TiO2 heterojunctions as an available configuration for photocatalytic degradation of organic pollutant. Journal of Photochemistry and Photobiology A. 2004; 163:569–580.
Brasche, S., Bischof, W. Daily time spent indoors in German homes – baseline data for the assessment of indoor exposure of German occupants. International Journal of Environmental Health and Hygiene. 2005; 208:247–253.
Cacho, C., Geiss, O., Barrero-Moreno, J., Binas, V.D., Kiriakidis, G., Bottalico, L., Kotzias, D. Studies on photo-induced NO removal by Mn-doped TiO2 under indoor-like illumination conditions. Journal of Photochemistry and Photobiology A. 2011; 222:304–306.
Chatterjee, D., Mahata, A. Photosensitized detoxification of organic pollutants on the surface of modified TiO2 semiconductor particulate system. Catalysis Communications. 2001; 2:1–3.
Chatterjee, D., Mahata, A. Visible light induced photodegradation of organic pollutants on dye adsorbed TiO2 surface. Journal of Photochemistry and Photobiology A. 2002; 153:199–204.
Chen, W.H., Zhang, J.S. Photocatalytic oxidation of multi-component systems. An investigation using toluene/ethylbenzene, octane/decane/dodecane and formaldehyde/acetaldehyde. Journal of Advanced Oxidation Technologies. 2008; 11:163–173.
Dalton, J.S., Janes, P.A., Jones, N.G., Nicholson, J.A., Hallam, K.R., Allen, G.C. Photocatalytic oxidation of NOx gases using TiO2: a surface spectroscopic approach. Environmental Pollution. 2002; 120:415–422.
De Richter, R., Caillol, S. Fighting global warming: the potential of photocatalysis against CO2, CH4, N2O, CFCs, tropospheric O3, BC and other major contributors to the climate change. Journal of Photochemistry and Photobiology C. 2011; 12:1–19.
Dooedeve, C.F., Kitchener, A. The mechanism of photosensitization by solids. Transactions of the Faraday Society. 1938; 34:902–908.
Environmental Protection Agency. Indoor Air Facts no. 4: Sick Building Syndrome. available at: http://www.epa.gov/iaq/pdfs/sick_building_factsheet.pdf, 1991. [(accessed August 2012)].
Everaert, K., Baeyens, J. Catalytic combustion of volatile organic compounds. Journal of Hazardous Materials. 2004; 109:113–139.
Faust, B.C., Hoffamnn, M.R., Bahnemann, D.W. Photocatalytic oxidation of sulphur dioxide in aqueous suspensions of α-iron oxide (Fe2O3). Journal of Physical Chemistry. 1989; 93:6371–6381.
Fujishima, A., Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature. 1972; 238:37–38.
Geiss, O., Cacho, C., Barrero-Moreno, J., Kotzias, D. Photocatalytic degradation of organic paint constituents – formation of carbonyls. Building and Environment. 2012; 48:107–112.
Green, K.J., Rudham, R. Photocatalytic oxidation of 2-propanol by semiconductor–zeolite composites. Journal of the Chemical Society Faraday Transactions. 2003; 89:1867–1870.
Guerrini, G.L. Photocatalytic performances in a city tunnel in Rome: NOx monitoring results. Construction and Building Materials. 2012; 27:165–175.
Hashimoto, K., Irie, H., Fujushima, A. TiO2 photocatalysis: a historical overview and future prospects. Japanese Journal of Applied Physics. 2005; 44:8269–8285.
Ho, W., Yu, J.C. Sonochemical synthesis and visible light photocatalytic behaviour of CdSe and CdSe/TiO2 nanoparticles. Journal of Molecular Catalysis A. 2006; 247:268–274.
Hochmannova, L., Vytrasova, J. Photocatalytic and antimicrobial effects of indoor paints. Progress in Organic Coatings. 2010; 67:1–5.
Huang, C.H., Wang, I.K., Lin, Y.M., Tseng, Y.H., Lu, C.M. Visible light degradation of nitric oxides on PtOx-modified TiO2 via sol-gel and impregnation method. Journal of Molecular Catalysis A. 2010; 316:163–170.
Huang, H., Li, W. Destruction of toluene by ozone–enhanced photocatalysis: performance and mechanism. Applied Catalysis B. 2011; 102:449–453.
Ihara, T., Miyoshi, M., Iriyama, Y., Matsumoto, O., Sugihara, S. Visible light active titanium dioxide photocatalyst realized by an oxygen-deficient structure and by nitrogen doping. Applied Catalysis B. 2003; 42:403–409.
Irokawa, Y., Morikawa, T., Aoki, K., Kosaka, S., Ohwaki, T., Taga, Y. Photodegradation of toluene over TiO2-xNx under visible light irradiation. Phys Chem Chem Phys. 2006; 8:1116–1121.
Ismail, A.A., Bahnemann, D.W. Metal–free porphyrin–sensitized mesoporous titania films for visible light indoor air oxidation. Chemical and Sustainability Energy and Materials. 2010; 3:1057–1062.
Jianhua, L., Rong, Y., Songmei, L. Preparation and characterization of the TiO2/ V2O5 photocatalyst with visible light activity. Rare Metals. 2006; 25:636–642.
Jo, W.K., Kim, J.T. Application of visible light photocatalysis with nitrogen-doped or unmodified titanium dioxide for control of indoor-level volatile organic compounds. Journal of Hazardous Materials. 2009; 164:360–366.
Kafizas, A., Kellici, S., Darr, J.A., Parkin, L.P. Titanium dioxide and composite metal/metal oxide titania films on glass: a comparative study of photocatalytic activity. Journal of Photochemistry and Photobiology A. 2009; 204:183–190.
Kaneko, M., Okura, I. Photocatalysis: Science and Technology. New York: Springer; 2002.
Kaur, S., Singh, V. Visible light induced sonophotocatalytic degradation of reactive red dye 198 using dye sensitized TiO2. Ultrasonics Sonochemistry. 2007; 14:531–537.
Kormann, C., Bahnemann, D.W., Hoffmann, M.R. Environmental photochemistry: is iron oxide (hematite) an active photocatalyst? A comparative study: α-Fe2O3, ZnO, TiO2. Journal of Photochemistry and Photobiology A. 1989; 34:161–169.
Laufs, S., Burgeth, G., Duttlinger, W., Kurtenbach, R., Maban, M., Thomas, C., Wiesen, P., Kleffmann, J. Conversion of nitrogen oxides on commercial photocatalytic dispersion paints. Atmospheric Environment. 2010; 44:2341–2349.
Leech, J.A., Nelson, W.C., Burnett, R.T., Aaron, S., Raizenne, M.E. It’s about time: a comparison of Canadian and American time–activity patterns. Journal of Exposure Analysis and Environmental Epidemiology. 2002; 12:427–432.
Maggos, T., Bartzis, J.G., Leva, P., Kotzias, D.K. Application of photocatalytic technology for NOx removal. Applied Physics A. 2007; 89:81–84.
Marolt, T., Sever, A., Bernard, J., Zivec, P., Gaberscek, M. Photocatalytic activity of anatase-containing façade coatings. Surface & Coatings Technology. 2011; 206:1355–1361.
Mazellier, P., Bolte, M. Heterogeneous light induced transformation of 2,6-dimethylphenol in acqueous suspensions containing goethite. Journal of Photochemistry and Photobiology A. 2000; 132:129–135.
Mele, G., del Sole, R., Vasapollo, G., Lopez, E.G., Palmisano, L., Schiavello, M. Photocatalytic degradation of 4-nitrophenol in aqueous suspension using polycrystalline TiO2 impregnated with Cu(II)-porphyrin and Cu(II)-phthalocyanine. Journal of Catalysis. 2003; 217:334–342.
Miyauchi, M., Ikezawa, A., Tobimatsu, H., Irie, H., Hashimoto, K. Zeta-potential and photocatalytic activity of nitrogen–doped TiO2 thin films. Phys Chem Chem Phys. 2004; 6:865–870.
Mo, J., Zhang, Y., Xu, Q. Joaquin Lamson J and Zhao R, Photocatalytic purification of volatile organic compounds in indoor air: a literature review. Atmospheric Environment. 2009; 43:2229–2246.
Moon, J., Yun, C.Y., Chung, K.W., Kang, M.S., Yi, J. Photocatalytic activation of TiO2 under visible light using acid red 44. Catalysis Today. 2003; 87:77–86.
Negishi, N., Takeuchi, K., Ibusuki, T. Surface structure of the TiO2 thin film photocatalyst. Journal of Materials Science. 1998; 33:5789–5794.
Obee, T.N., Brown, R.T. TiO2 photocatalysis for indoor air applications. Effects of humidity and trace contaminant levels on the oxidation rates of formaldehyde, toluene and 1,3-butadiene. Environmental Science and Technology. 1995; 29:1223–1231.
Obee, T.N., Hay, S.O. Effects of moisture and temperature on the photooxidation of ethylene on titania. Environmental Science and Technology. 1997; 31:2034–2038.
Ohko, Y., Nakamura, Y., Negishi, N., Matsuzawa, S., Takeuchi, K. Photocatalytic oxidation of nitrogen monoxide using TiO2 thin films under continuous UV light illumination. Journal of Photochemistry and Photobiology A. 2009; 205:28–33.
Pacheco-Torgal, F., Jalali, S. Nanotechnology: advantages and drawbacks in the field of construction and building materials. Construction and Building Materials. 2011; 25:582–590.
PICADA, n.d. Photocatalytic Innovative Coverings Applications for Depollution Assessment (PICADA Project), Available at: http://www.picada-project.com/domino/SitePicada/Picada.nsf?OpenDataBase (accessed August 2012).
Pichat, P. Some views about indoor air photocatalytic treatment using TiO2: conceptualization of humidity effects, active oxygen species, problem of C1-C3 carbonyl pollutants. Applied Catalysis B. 2010; 99:428–434.
Pichat, P., Disdier, J., Hoang-Van, C., Mas, D., Goutailler, G., Gaysse, C. Purification/deodorization of indoor air and gaseous effluents by TiO2 photocatalysis. Catalysis Today. 2000; 63:363–369.
Pulgarin, C., Kiwi, J. Iron oxide mediated degradation, photodegradation and biodegradation of aminophenols. Langmuir. 1995; 11:519–526.
Qiu, R., Zhang, D., Mo, Y., Song, L., Brewer, E., Huang, X., Xiong, Y. Photocatalytic activity of polymer modified ZnO under visible light irradiation. Journal of Hazardous Materials. 2008; 156:80–85.
Reutergardh, L.B., Iangphasuk, M. Photocatalytic decoloration of reactive azo dye: a comparison between TiO2 and US photocatalysis. Chemosphere. 1997; 35:585–596.
Rockafellow, E.M., Stewart, L.K., Jenks, W.S. Is sulphur-doped TiO2 an effective visible light photocatalyst for remediation? Applied Catalysis B. 2009; 91:554–562.
Rothenberger, G., Moser, J., Gratzel, M., Serpone, N., Sharma, D.K. Charge carrier trapping and recombination dynamics in small semiconductor particles. Journal of the American Chemical Society. 1985; 107:8054–8059.
Salthammer, T., Fuhrmann, F. Photocatalytic surface reactions on indoor wall paint. Environmental Science and Technology. 2007; 41:6573–6578.
Shiraisi, F., Nomura, T., Yamaguchi, S., Ohbuchi, Y. Rapid removal of trace HCHO from indoor air by an air purifier consisting of a continuous concentrator and photocatalytic reactor and its computer simulation. Chemical Engineering Journal. 2007; 127:157–165.
Song, H.M., Ko, J.M., Park, J.H. Hybrid photoreactive magnet obtained from Fe3O4/ TiO2 composite nanoparticles. Chemical Letters. 2009; 38:612–613.
Song, L., Qiu, R., Mo, Y., Zhang, D., Wei, H., Xiong, Y. Photodegradation of phenol in a polymer-modified TiO2 semiconductor particulate system under the irradiation of visible light. Catalysis Communications. 2007; 8:429–433.
Stevens, L., Lanning, J.A., Anderson, L.G., Jacoby, W.A., Chomet, N. Investigation of the photocatalytic oxidation of low level carbonyl compounds. Journal of Air and Waste Management Association. 1998; 48:979–984.
Strini, A., Cassese, S., Schiavi, L. Measurement of benzene, toluene, ethylbenzene and o-xylene gas phase photodegradation by titanium dioxide dispersed in cementitious materials using a mixed flow reactor. Applied Catalysis B. 2005; 61:90–97.
Sun, H., Wang, C., Pang, S., Li, X., Tao, Y., Tang, H., Liu, M. Photocatalytic TiO2 films prepared by chemical vapour deposition at atmospheric pressure. Journal of Non Crystalline Solids. 2008; 354:1440–1443.
Sun, L., Li, G., Wan, S., An, T. Mechanistic study and mutagenicity assessment of intermediates in photocatalytic degradation of gaseous toluene. Chemosphere. 2010; 78:313–318.
Tang, J., Zou, Z., Ye, J. Efficient photocatalytic decomposition of organic contaminants over CaBi2O4 under visible light irradiation. Angewandte Chemie International Edition. 2004; 43:4463–4466.
US Patent 2009/0061246 A1. Photocatalytic content. 2009.
Wang, S., Ang, H.M., Tade, M.O. Volatile organic compounds in indoor environment and photocatalytic oxidation: state of the art. Environment International. 2007; 33:694–705.
Wang, W., Gu, B., Liang, L., Hamilton, W.A., Wesolowski, D.J. Synthesis of rutile nanocrystals with highly controlled size and shape by low temperature hydrolysis: effects of solvent competition. Journal of Physical Chemistry B. 2004; 108:14789–14792.
WHO. WHO guidelines for indoor air quality: selected pollutants, chapters 3 (formaldehyde) and 5 (nitrogen dioxide). http://www.euro.who.int/data/assets/pdf_file/0009/128169/e94535.pdf, 2010. [Available at: (accessed August 2012)].
WPCIA. World’s Top Ten Paint Companies 2011 Annual Report. Available at: http://www.wpcia.org/News/2011report.html, 2011. [(accessed August 2012)].
Wu, L., Yu, J.C., Fu, X. Characterization and photocatalytic mechanism of nanosized CdS coupled TiO2 nanocrystals under visible light irradiation. Journal of Molecular Catalysis A. 2006; 244:25–32.
15.7 Appendix: acronyms and definitions
| Bi2S3 | Bismuth sulphide |
| BTEX | Mixture of benzene, toluene, ethylbenzene and xylenes |
| CCaBi2O4 | Calcium bismuthate |
| CdS | Cadmium sulphide |
| CdSe | Cadmium selenide |
| CFCs | Chlorofluorocarbon compounds |
| CH4 | Methane |
| CO | Carbon monoxide |
| CPVC | Critical pigment volume concentration |
| e–cb | Excited electron in the conducting electronic band of the catalyst as a consequence of the absorption of incident radiation |
| hv | Radiation (nm) |
| h+vb | Hole created in the valence electronic band of the catalyst due to the excitation of an electron as a consequence of the absorption of the incident radiation |
| H2O | Water |
| HNO3 | Nitric acid |
| In2O3 | Indium oxide (III) |
| k | Photocatalytic rate constant |
| K | Adsorption coefficient, μg (adsorbed compound)/g (sorbent) |
| N2O | Nitrous oxide |
| NO | Nitric oxide |
| NO2 | Nitrogen dioxide |
| O2– | Superoxide anion |
| OH• | Hydroxyl radical |
| r | Photocatalytic reaction rate, (μg/m3 catalyzed compound)/h |
| SnO2 | Tin oxide (IV) |
| SO2 | Sulphur dioxide |
| SrTiO3 | Strontium titanium oxide |
| TiCl4 | Titanium chloride (IV) |
| TiO2 | Titanium dioxide |
| UV | Ultraviolet radiation with wavelengths ranging from 1 to 400 nm |
| UV-A | UV radiation with wavelengths ranging from 320 to 400 nm |
| V2O5 | Vanadium oxide (V) |
| VOCs | Volatile organic compounds |
| WO3 | Tungsten oxide (VI) |
| ZnO | Zinc oxide |
| ZnS | Zinc sulphide |
| α-Fe2O3 | α-ferric oxide |