Nanotechnology for domestic water purification
Abstract:
Water, a nonsubstitutional natural resource, is best described by Leonardo Da Vinci as ‘the vehicle of nature’ (‘vetturale di natura’). This is the single most essential commodity responsible for the existence and sustenance of life on the planet earth. It is not at all an exaggeration to state that water is primarily responsible for the restoration of health, environment and prosperiy of human civilization. Unfortunately, this most precious natural resource is becoming increasingly scarce day by day. Water scarcity is among the main problems facing many societies around the world in the twenty-first century. Water use has been growing at more than twice the rate of population increase in the last century. According to a report from the United Nations, by 2025, 1800 million people will be living in countries or regions with absolute water scarcity, and two-thirds of the world’s population could be under stress conditions. As emphasized in one of the UN’s Millenium Development Goals (MDGs), water scarcity calls for strengthened international cooperation in the fields of technologies for enhanced water productivity. Recent years have witnessed impressive breakthroughs towards application of nanostructured materials such as carbon nanotubes (CNTs), metal/metal-oxide nanoparticles, zeolites, and dendrimers in the field of water purification. The present chapter aims to give an overview of the developments in the application of nanotechnology in water treatment, with a special emphasis on domestic water purification. The focus is oriented to the fact that the ultimate practical realization of this new technology is based on the assessment of the risks as well as benefits posed by nanostructured materials. The challenges involved in producing a well-defined integrated nano-based water purification device are discussed.
The authors would like to thank Mr Nitesh Goswami, Scientific Officer C, Bhabha Atomic Research Centre, India for providing sincere assistance toward careful editing of this chapter.
16.1 Introduction
Water resources are becoming increasingly scarce worldwide. Global water consumption is increasing at more than double the rate of the world’s population growth. Population growth, pollution and climate change, which are all accelerating, are likely to combine to produce a drastic decline in water supply in the coming decades. At present 1.1 billion people lack access to safe drinking water and 2.4 billion people lack access to proper sanitation, nearly all of them in developing countries. At present, a third of the world’s population live in water-stressed countries, and by 2025, this is expected to rise to two-thirds. Table 16.1 (Gleick, 2000; Hillie et al., 2007a) shows that water consumption is greatest in irrigated agriculture, accounting for 70% of global water withdrawals in 2000, while the respective shares of industrial and domestic usage were 20% and 10%. Agricultural use dominated globally (70%) and in developing countries (88%). Meanwhile, the share of industrial and domestic use has increased with rising country incomes, while agricultural use has declined.
Figure 16.1 shows the percentage of the population without access to reliable water sources, by region, and the predicted values for 2015 (World Bank, 2005). Lack of drinking water and sanitation kills about 4,500 children a day. Many children are missing school because neither their homes nor schools have adequate drinking water and sanitation facilities. Sustainable water management is therefore a critical aspect in addressing poverty, equity and related issues. The UN Millennium Development Goal of ensuring environmental sustainability commits governments to ‘reduce by half the proportion of people without sustainable access to safe drinking water’ by 2015, a goal closely linked with the separate goal of access to sanitation and basic hygiene education.
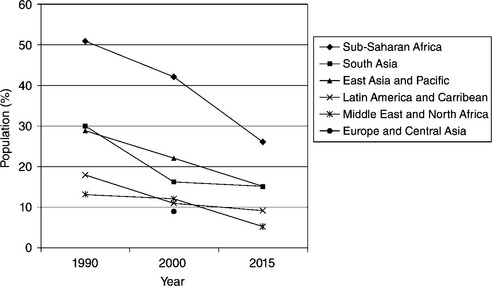
Fig. 16.1 Population without access to reliable water sources. (actual values 1990, 2000; estimated 2015). Source: World Bank (2005).
Different countries and regions face different environmental, social and economic conditions and have different needs with regard to water use, water quality, access to technology, field conditions, and the types of technologies that may be appropriate in different circumstances. Water treatment technologies include filtration using membranes, chemical treatment, heat and ultraviolet treatment and distillation. They seek to remove solid and other contaminants, or to neutralize them, and many treatments have a long history of use in systems for producing water for domestic, industrial and agricultural use (OECD, 2011). Most current approaches are materials intensive, have a large ecological footprint and are not in a position to comply with the increasing water quality standards of developing industrialized nations. The conventional methods of disinfection such as chlorination and ozonation can produce harmful byproducts due to reactions with various constituents of wastewater, which may call for a tradeoff between optimum disinfection and harmful byproduct formation.
The possibility of having ready access to safe drinking water is becoming increasingly rare because of over-exploitation of existing water resources, alarming effects of global warming (causing highly uneven rainfall patterns in particular places, which in turn disturbs the tendency for water harvesting), increasing levels of contamination as a result of rapid industrialization, leaky water distribution systems and deterioration of water quality upon ageing (though water has been treated at the point of entry, POE). On the other hand, funding, governance, trained engineers and skilled labour are commonly recognized obstacles to establishing regional and national-scale water treatment systems.
The difficulty and lack of success in overcoming obstacles to regional and national water supplies has led, in part, to increased interest in point-of-use (POU) water treatment methods at the household and community level (Hillie et al., 2007b). Community and household level POU water treatment methods avoid many of the barriers associated with large-scale water supply projects because they are relatively inexpensive, can be purchased by the unit, and/or constructed using readily available materials. For these reasons, POU technologies avoid the need for large capital investments, management systems and governance structures.
Nanotechnology is increasingly being identified as an area of science and technology that could play a role in addressing some of the shortcomings of conventional POU devices. Studies by Brame et al. (2011), Theron et al., (2008) and Watlington (2005) suggest that nanotechnology-based materials could lead to cheaper, more durable, and more efficient water treatment technologies that meet the needs of developing countries. Several water treatment methods and devices that incorporate nanoscale materials are already commercially available, and others are being developed. These nanotechnology-based products include water filters, filtration membranes, catalysts, and nanoparticles for groundwater remediation. However, a well-defined and well-engineered nano-based product needs to address significant challenges before a suitable POU device for household use can be commercialized to take care of the safe-water needs of the poor as well as the rich.
The present chapter aims to provide readers with a review of the practical applications of nanomaterials in the water sector. In the following sections, an overview of the different types of nanomaterials (e.g., metal and metal oxide nanoparticles, carbon nanotubes, zeolites, dendrimers, etc.) presently in use for water treatment applications is provided along with the different methods of nanomaterials synthesis. Subsequent sections discuss the environmental and health implications of the use of nanomaterials. Finally, the chapter focuses on the challenges associated with development of practical nano-based water purification systems.
16.2 Nanomaterials and water purification
There are a number of studies (Diallo et al., 2009; Cloete et al., 2010; Hotze and Lowry, 2010) highlighting the importance of nanostructured materials in the field of water purification, desalination, wastewater treatment, water recycle and reuse. Nanomaterials have shown their potential not only in water treatment, but also in water quality monitoring through sensing and detection. However, we shall restrict our discussion to water treatment applications within the confinement of water purification only. The specific issues regarding application modalities with respect to a household (domestic) scale are addressed in Section 16.7.
16.3 The need for nanomaterials in water purification
The variation in the quality and quantity of water available across the globe is drastically different and hence it is mandatory to have different kinds of region-specific solutions. The already scarce water resource is becoming increasingly scarce because of rapid population growth and ceaseless industrialization. Coupled to this is the alarming issue of global warming and climate change which is potentially responsible for uneven distribution of rainfall. All these factors in turn lead to increasing levels of contamination and, moreover, newer types of contamination than encountered previously. The permissible limits of contaminants in a proposed safe drinking water are becoming less and less with the passage of time (e.g., the recommended maximum permissible value for arsenic in drinking water according to WHO international standards has been reduced from 200 ppb to 10 ppb through a number of revisions in the last 50 years. The case is the same with lead, where the limits have been brought down to 10 ppb from 50 ppb).
Given the deteriorating water resource situation, it is inevitable that newer, more efficient and more selective water purification technologies are required to take care of the specific contaminants at a very low level. The technology to remove these contaminants should also reach molecular limits so that capture can be highly efficient in a minimum residence time. On the other hand, such a process should be environmentally friendly, economically feasible and with minimum or no use of electricity for rural adaptability.
‘Nanotechnology’ has a tremendous role to play in such a precarious situation, where the reactions can take place at ionic/atomic/molecular scale in a very selective manner with amazingly high efficiency. Because of the significant increase in surface-to-volume ratio in a nano-dimension, the contaminant uptake capacity becomes many fold. Compared to the conventional water treatment technologies such as membrane-based treatment, activated carbon, UV-based filtration, electrodialysis and distillation, the nano-based systems would provide the following advantages (Pradeep and Anshup, 2009a):
• higher efficiency of removal even at low concentration of adsorbents
• functionalization capability of nanomaterials leads to specific uptake
Two of the major distinctions that define types of conventional remediation technologies also apply to nanotechnologies for remediation: adsorptive versus reactive and in situ versus ex situ. Absorptive remediation technologies remove contaminants (especially metals) by sequestration, whereas reactive technologies affect degradation of contaminants, sometimes all the way to harmless products (e.g., CO2 and H2O in the case of organic contaminants). In situ technologies involve treatment of contaminants in place, whereas ex situ refers to treatment after removing the contaminated material to a more convenient location (e.g., pumping contaminated groundwater to the surface and treatment above ground). In situ degradation of contaminants, when feasible, is often preferred over other approaches because it has the potential to be more cost effective (Tratnyek and Johnson, 2006). However, in situ remediation requires delivery of the treatment to the contamination and this has proven to be a major obstacle to expanded development of in situ remediation technologies. With respect to this issue, nanotechnology has special relevance because of the potential for injecting nanosized (reactive or absorptive) particles into contaminated water. In this manner, it should be possible to create either: (i) in situ reactive zones with nanoparticles that are relatively immobile; or (ii) reactive nanoparticle plumes that migrate to contaminated zones if the nanoparticles are sufficiently mobile.
The current understanding of the basic processes involved in this technology is still evolving and incomplete. In addition to making it difficult to move forward with the engineering of full-scale implementations, these uncertainties make it very difficult to assess the risks that this technology might bear to human and/or ecological health. Recognizing this, some groups have adopted the ‘precautionary’ position that in situ applications of nanoparticles for remediation should be prohibited (The Royal Society and The Royal Academy of Engineering, 2004), whereas others have recommended, in effect, that research on all fronts should proceed in parallel (EPA, 2005).
16.4 Types, properties and uses of nanomaterials in water purification
Nanoparticles have two key properties that make them particularly attractive as sorbents. On a mass basis, they have much larger surface areas than bulk particles. Nanoparticles can also be functionalized with various chemical groups to increase their affinity towards target compounds. It has been found that the unique properties of nanoparticles enable the development of high capacity and selective sorbents for metal ions and anionic contaminants. To make the discussion on the application of nanomaterials in the field of water purification easy to comprehend and follow, the nanomaterials can be categorized under four different classes:
The literatures encompassing the research and development work involving these nanomaterials are cited very briefly in the sub-sections that follow. To make the discussion meaningful and somewhat exhaustive, at different places (application of nanomaterials like TiO2 nanoclays and magnetic nanoparticles), excerpts from the Annual Review of Nano Research (Mansoori et al., 2008) are reproduced.
16.4.1 Zeolites
Zeolites are microporous crystalline solids with well-defined structures. Generally they contain silicon, aluminium and oxygen in their framework and cations, water and/or other molecules within their pores. Many occur naturally as minerals and they are extensively mined in many parts of the world. Others are synthetic and are made commercially for specific uses. Synthetic zeolites are usually made from silicon-aluminium solutions or coal fly ash, and are used as sorbents in column filters. Zeolites are widely used as ion-exchange beds for domestic and commercial water purification, and water softening where alkali metals such as sodium or potassium prefer to exchange out of the zeolite, being replaced by the ‘hard’ calcium and magnesium ions from the water. Commercial wastewater containing heavy metals can also be cleaned up using such zeolites. Zeolites are generally used for the removal of metal contaminants. Natural zeolites from Mexico and Hungary have been shown to reduce arsenic from drinking water sources to levels deemed acceptable by the World Health Organization (Elizalde-González et al., 2001).
Zeolites made from coal fly ash can adsorb a variety of heavy metals including lead, copper, zinc, cadmium, nickel, and silver from wastewater. Under some conditions, fly ash zeolites can also adsorb chromium, arsenic and mercury. NaP1 zeolites (Na6Al6 Si10O32, 12H2O) have a high density of Na (I) ion exchange sites. They can be inexpensively synthesized by hydrothermal activation of fly ash with low Si/Al ratio at 150 °C in 1.0–2.0 M NaOH solutions. NaP1 zeolites have been evaluated as ion exchange media for the removal of heavy metals from acid mine wastewaters. Alvarez-Ayuso et al. (2003) reported the successful use of synthetic NaP1 zeolites to remove Cr (III), Ni (II), Zn (II), Cu (II) and Cd (II) from metal electroplating waste-water. The adsorptive capacity of zeolites is influenced by several factors including their composition, the water pH, and the concentrations and types of contaminants. For example, the water pH influences whether the ash surface is positively or negatively charged. Also, because lead and copper are more easily adsorbed by fly ash, high concentrations of these metals decrease the amount of cadmium and nickel removed (Wang et al., 2004).
Zeolite-silver compound has been proven effective against microorganisms, including bacteria and mould. Additionally, the silver in this compound provides residual protection against regrowth of these biological contaminants. Zeolites do not adequately remove organic contaminants. Also, air moisture contributes to zeolites’ saturation and makes them less effective. Zeolites were reported to result in high flux reverse osmosis nanocomposite membrane (the utilities of having a membranous structure have been discussed in the last section) without compromise in selectivity (Jeong et al., 2007).
16.4.2 Dendrimers
Dendrons are dendritic wedges that comprise one type of functionality (such as chemical bonding) at their core and another at the periphery. To obtain a dendrimer structure, several dendrons are reacted with a multifunctional core to yield a dendrimer. Using different synthetic strategies, over 100 compositionally different dendrimer families have been synthesized and over 1000 differentiated chemical surface modifications have been reported (Bosman et al., 1999; Fischer and Vögtle, 1999; Tomalia and Majoros, 2003). One such dendrimer structure is shown in Fig. 16.2 (Jang et al., 2009).
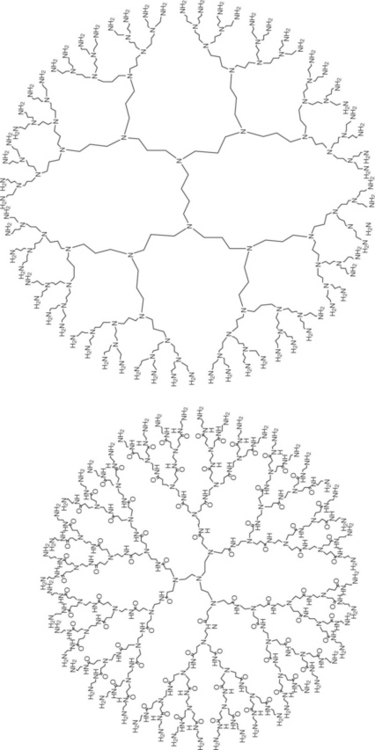
Fig. 16.2 Commercially available G5 PAMAM (a) and G4 PPI (b) dendrimers. (adapted with permission from Jang et al., 2009)
Dendritic polymers exhibit many features that make them particularly attractive as functional materials for water purification. These ‘soft’ nanoparticles, with sizes in the range of 1–20 nm, can be used as high capacity and recyclable water soluble ligands for toxic metal ions, radionuclide and inorganic anions (Ottaviani et al., 2000). The environmental applications of dendrimers were first explored by Diallo et al. (2005). They reported the effective removal of copper from water via different generations of poly(amidoamine) (PAMAM) dendrimers. Later, Diallo et al. (2005) studied the feasibility of using dendrimer improved ultrafiltration to recover Cu (II) from aqueous solution. The dendrimer-Cu (II) complexes can be efficiently separated from aqueous solutions by ultrafiltration. Dendritic polymers can also be used as (i) recyclable unimolecular micelles for recovering organic solutes from water (Arkas et al., 2003) and (ii) scaffolds and templates for the preparation of redox and catalytically active nanoparticles. Dendritic polymers have also been used successfully as delivery vehicles or scaffolds for antimicrobial agents such as Ag (I) and quaternary ammonium chlorides (Balogh et al., 2001).
PAMAM-based silver complexes and nanocomposites have proved to be effective antimicrobial agents in vitro. Rether and Schuster (2003) made a water-soluble benzoylthiourea modified ethylenediamine core-polyamidoamine dendrimer for the selective removal and enrichment of toxicologically relevant heavy metal ions. They studied complexation of Co (II), Cu (II), Hg (II), Ni (II), Pb (II) and Zn (II) by the dendrimer ligand and using the polymer-supported ultrafiltration process.
One of the novel systems for encapsulating organic pollutants is cross-linked dendritic derivatives. In the research carried out by Arkas et al. (2005) for the preparation of ultra pure water, the amino groups of polypropyleneimine dendrimer and hyperbranched polyethylene imine were interacted under extremely mild conditions with 3-(triethoxysilyl) propyl isocyanate. They produced porous ceramic filters and employed these dendritic systems for water purification. In this experimental work, the concentration of polycyclic aromatic compounds in water was reduced to a few ppb’s by continuous filtration of contaminated water through these filters. Then, the filters loaded with pollutants were effectively regenerated by treatment with acetonitrile.
In another work, Arkas et al. (2006) developed a method that permits removal of organic pollutants by employing a simple filtration step, which can be easily scaled up. They used the long-alkyl chain functionalized polypropylene imine dendrimers, polyethylene imine hyperbranched polymers and β-cyclodextrin derivatives which are completely insoluble in water.
16.4.3 Metal-containing nanoparticles
Metal and metal oxide nanoparticles have been studied extensively for water treatment applications. Most notable among the metal nanoparticles are the noble metals (such as silver and gold which are treated as famous biocides) and nano zero-valent iron (NZVI) used for treatment of water containing pesticides. Among metal oxides, oxides of iron, aluminium, zinc, magnesium and titanium have been made use of significantly in groundwater remediation. We first discuss the category of metals and then case studies pertaining to metal oxides are discussed.
Zero-valent iron (ZVI)
Among the popular nanosorbents at the present time, the most exploited is the nano ZVI having applications ranging from removal of halogenated organics, arsenic, nitrate and heavy metals. Nanoparticles could provide very high flexibility for both in situ and ex situ remediations. They can also be anchored onto a solid matrix such as carbon, zeolite or membrane for enhanced treatment of water. ZVI removes aqueous contaminants by reductive dechlorination, in the case of chlorinated solvents or by reducing them to an insoluble form, in the case of aqueous metal ions.
Different material and engineering aspects of ZVI nanoparticles used for removal of environmental pollutants were discussed by Li et al. (2006b). These nanoparticles were used for separation and immobilization of Cr (VI) and Pb (II) from aqueous solution by reduction of chromium to Cr (III) and Pb to Pb (0) (Ponder et al., 2000). Nanopowder of ZVI was used for the removal of nitrate in water (Choe et al., 2000). Nanoscale ZVI was employed by Lowry and Johnson (2004) for dechlorination of polychlorinated biphenyl (PCB) to lower-chlorinated products under ambient conditions. It was demonstrated that nano-sized ZVI oxidizes organic compounds in the presence of oxygen (Feitz et al., 2005). The high surface area of nanoscale ZVI may allow for more efficient generation of oxidants. A decrease in reactivity is expected with the build-up of iron oxides on the surface, particularly at high pH.
The EZVI (emulsified zero-valent iron) technology with nanoscale or microscale iron was enhanced to address this limitation associated with the conventional use of ZVI. Quinn et al. (2005) evaluated the performance of nanoscale emulsified zero-valent iron (nEZVI) to improve in-situ dehalogenation of dense, non-aqueous phase liquids (DNAPLs) containing trichloroethene (TCE) from groundwater. One of the applications of ZVI is the removal and sorption of arsenic contamination from groundwater (Kanel et al., 2006). Nanopowder of ZVI as a fine powder cannot be used in fixed-bed columns unless they have granular shape (Guo and Chen, 2005).
Xu and Zhao (2007) used carboxy methyl cellulose (CMC) stabilized ZVI nanoparticles to reduce Cr (VI) in aqueous media through batch and continuous flow column study. They found that the stabilized ZVI nanoparticle is more effective than the non-stabilized one for the removal of Cr (VI). In the batch experiments, the reduction of Cr (VI) was improved from 24% to 90% as the dosage of ZVI increased from 0.04 to 0.12 g/L. In another work, Xiong et al. (2007) studied the degradation of perchlorate in water and illustrated the stabilized ZVI nanoparticles could increase perchlorate reduction rate by 53% in saline water (with concentration of NaCl up to 6% w/w).
Giasuddin et al. (2007) investigated the removal of humic acid (HA) with ZVI nanoparticles and also their interaction with As (III) and As (V). Cheng et al. (2007) also applied ZVI nanoparticle and commercial form of ZVI powder with different mesh sizes for the dechloronation of p-chlorophenol from water. Comparison between those particles indicated that the nanoscale was more effective for the reduction process.
Noble metal nanoparticles
The first detailed report on the interaction of noble metal nanoparticles with halocarbons appeared in 2003 (Nair and Pradeep, 2003). It was found that noble metals at nanodimensions react with halocarbons in a manner similar to other metals (i.e., reductive dehalogenation) leading to the formation of metal halide with no reaction byproducts. The reaction was later extended to several halocarbons and was found to be completely efficient at room temperature.
The reaction of noble metal nanoparticles was studied with widely used pesticides such as endosulfan (Nair et al., 2003), malathion (Nair and Pradeep, 2007) and chlorpyrifos (Nair and Pradeep, 2007). The noble metal nanoparticles supported on alumina were very effective for the removal of pesticides from solution. Realizing the fact that a number of pesticides found in drinking water are organochlorine (e.g., simazine, lindane, atrazine, etc.) or organosulfur pesticides (e.g., triazophos, quinalphos, etc.) or contain nitrogen-based functional groups (e.g., carbaryl, carbofuran, monochrotofos, etc.), the chemistry of supported noble metal nanoparticles can comfortably be utilized for the complete removal of such pesticides from drinking water. This aspect of complete removal of a wide variety of pesticides makes the chemistry of supported noble metal nanoparticles unique for drinking water purification.
Noble metal nanoparticles are also found extremely important for ultra-low concentration sensing of pesticides. Overall, there are two approaches followed for ultra-low concentration detection of pesticides using gold nanoparticles:
• changes in the signature properties of a functional group attached to noble metal nanoparticle surface in the presence of organic molecules: reported to reach a detection limit in ppt level (T. J. Lin et al., 2006; Rajan et al., 2007; Sun et al., 2008);
• changes in the optical properties of noble metal nanoparticles upon interaction with pesticides (Burns et al., 2006; Dubas and Pimpan, 2008).
Another interesting application area of noble metal nanoparticles in drinking water purification is the sequestration of heavy metals (Henglein, 1998). Also the functionalized noble metal nanoparticle surfaces can be exploited for the detection of heavy metals (Ono and Togashi, 2004; Lee et al., 2007; Lu and Liu, 2007).
The anti-microbial effects of silver, in zerovalent and ionic form, have been widely studied in great detail (Aymonier et al., 2002; Sondi and Sondi, 2004; Jain and Pradeep, 2005; Sambhy et al., 2006). Due to the numerous scientific investigations published on this topic and its consequences in different applications, review articles may be consulted for a detailed understanding (Silver, 2003; Silver et al., 2006; Neal 2008; Rai et al., 2009; Sharma et al., 2009). The chemistry behind the biocidal activity of silver nanoparticles, and the way silver ions act against micro-organisms are discussed in the review by Pradeep and Anshup (2009a). While the precise details are not yet elucidated, protein inactivation and loss of replication ability of DNA are suggested. A few important observations are highlighted by Pradeep and Anshup (2009a).
Metal oxide nanoparticles
Wang et al. (2007) investigated the effect of size, fabrication method, and morphology of ZnO nanoparticles as photocatalysts on the decomposition of methyl orange. It was found that the preparation method was the most important step and ZnO nanoparticle, 50 nm in diameter synthesized via thermal evaporation method, provided the highest photocatalyst activity.
Alumina nanoparticles have been utilized for the removal of heavy metals from drinking water (Kasprzyk-Hordern, 2004). The suggested mechanism involves the metal ion induced flocculation of negatively charged alumina nanoparticles (alkaline pH conditions). Alumina is used as support for heterogeneous catalysis (Nair and Pradeep, 2007). The major reasons behind the use of oxides for water purification are: high surface area for adsorption, mesoporous structure, presence of surface charge, stability and low solubility in water.
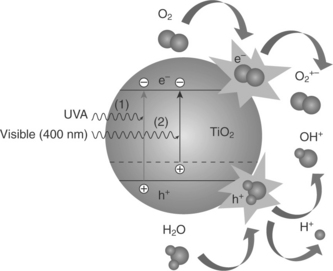
Titanium dioxide (TiO2) is one of the most important materials used in water treatment applications because of its photocatalytic properties. Two different types of photocatalytic applications can be distinguished in water treatment: solar photocatalysis and photocatalytic systems equipped with artificial ultraviolet (UV) light. Both systems can be applied at ambient temperature to degrade various chemical and microbiological pollutants in water and air. As it makes use of sunlight, solar photocatalysis technology is inexpensive, environmentally friendly and universally applicable. The equipment needed is minimal and also appropriate for developing countries or remote sites with no access to electricity. Nanoparticles that are activated by light, such as the large band-gap semiconductors titanium dioxide (TiO2) and zinc oxide (ZnO), are frequently studied for their ability to remove organic contaminants from various wastewater. These nanoparticles have the advantages of ready availability, being inexpensive, and having low toxicity. The rapid recombination of photo-generated electron hole pairs and the non-selectivity of the system are the main problems that limit the application of photocatalysis processes. The specific chelating agents such as arginine, lauryl sulfate and salicylic acid can modify the surface properties of nanocrystal TiO2 and inhibit rapid recombination of photo-generated electron hole pairs. A comprehensive review of photocatalytic nano-TiO2 for environmental applications is found in Kwon et al., (2008).
Conventional TiO2 photocatalysts are utilized only under UV light due to its wide bandgap of 3.2 eV and, therefore, cannot be used indoors or inside vehicles. In order to obtain the photocatalytic activity under visible light, various types of TiO2-based photocatalysts have been created (Sato, 1986; Kisch et al., 1998; Zang et al., 1998; Asahi et al., 2001; Umebayashi et al., 2002; Irie et al., 2003; Sakthivel and Kisch, 2003; Miyauchi et al., 2004); and nitrogen-doped TiO2 (TiO2–xNx) is regarded as one of the most effective and practical catalysts (Irie et al., 2003; Miyauchi et al., 2004; Irokawa et al., 2006). Nitrogen doping has extended the photoactive wavelengths up to 520 nm as a result of bandgap narrowing, realizing the equivalent photoactivity in conventional TiO2 under UV light since the number of carrier-recombination centres was minimized. The change in band gap and generation of free radicals are shown in Fig. 16.3.
Synthesized titanium dioxide nanoparticles of both anatase and rutile forms were used for wet oxidation of phenols by hydrothermal treatment (Andersson et al., 2002). In another study, a novel composite reactor with combination of photochemical and electrochemical system was used for the degradation of organic pollutant such as Rhodamine 6G (R-6G) (Chen et al., 2003). Fine TiO2 particles have shown better efficiency than the immobilized catalysts, but complete separation and recycling of fine particles (less than 0.5 μm) from the treated water, are very expensive. Therefore, from an economical point of view, this method is not suitable for the industrial scale. This problem was solved by fixing the carbonblack-modified nano-TiO2 (CB-TiO2) on aluminium sheet as a support (L. Li et al., 2003). The photocatalytic activity of CB-TiO2 thin films was observed to be 1.5 times greater than that of TiO2 thin films in the degradation of reactive Brilliant Red X-3B.
Decomposition of parathion with the nanometer rutile titanium dioxide (TiO2) powder as the sonocatalyst after treatment of high-temperature activation was carried out by Wang et al., (2006). In the study by Li et al. (2006a), carbon grain coated with activated nano-TiO2 (20–40 nm) (TiO2/AC) was prepared and used for the photodegradation of methyl orange (MO) dyestuff in aqueous solution under UV irradiation. Mahmoodi et al. (2007) immobilized TiO2 nanoparticles for the degradation and mineralization of two agricultural pollutants (Diazinon and Imidacloprid as N-heterocyclic aromatics).
The photocatalytic efficiency of immobilized TiO2 nanoparticle with 6 nm diameter (supported by glass substrate) as well as conventional suspended catalysts has been investigated recently by Mascolo et al. (2007) for the degradation of methyl red dye. Although the mechanism for dye degradation was found to be the same for both cases (suspended as well as immobilized nanoparticles), lowering of photodegradation performance in the immobilized case was due to reduction in active surface area for adsorption and subsequent catalyst action. However, the recovery of nanoparticles is easy when the particles are immobilized.
Degradation of nitrobenzene by using nano-TiO2 and ozone was studied by Yang et al. (2007). They compared the effect of nano-TiO2 catalysed plus ozone and ozone only and found that the catalysed ozonation was more efficient than ozone alone. Sobana et al. (2006) prepared silver nanoparticles doped with TiO2 and used them for the photodegradation of direct azo dyes.
16.4.4 Carbon nanotubes
A carbon nanotube (CNT) is a one-atom thick sheet of graphite (called graphene) rolled up ino a seamless cylinder with diameter of the order of a nanometer and capped at both ends by hemispheres of fullerene. This results in a nanostructure where the length-to-diameter ratio exceeds 10,000. CNTs can be categorized by their structures as single-walled nanotubes (SWNT) and multi-walled nanotubes (MWNT). In the most general way, the CNT can be shown as composed of a concentric arrangement of several cylinders (Fig. 16.4). The high curvature of the graphene sheets increases the total energy of the tubules per carbon atom, but this is more than offset by a lowering of the energy because of the absence of dangling bonds at the edges of the graphene sheets. Such cylindrical carbon molecules have novel properties that make them potentially useful in a wide variety of applications in water and health care.
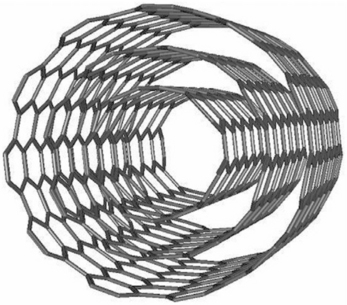
Fig. 16.4 Multi-walled carbon nanotube shown as composed of a concentric arrangement of several graphene cylinders.
The as-grown or acidified CNTs have shown potential as a sorbing media for removal of various contaminants from water. The as-grown CNTs have got defect sites (originated during synthesis), which make them a suitable adsorbent for uptake of contaminants. However, it is noteworthy that such CNTs do not pose any selectivity towards uptake of specific contaminants. On the other hand, the surface area of as-grown CNTs lies in the range of 50–100 m2/g, which is not significant in comparison to activated charcoal or activated alumina (where the surface area is up to 1000 m2/g), which are being extensively used in water decontamination because of being cheaper and having easy availability. However, some of the work carried out by researchers using as-grown and functionalized CNTs are cited below.
Peng et al. (2005) developed a novel adsorbent, ceria supported on CNTs (CeO2-CNTs), for the removal of arsenate from water. Under natural pH conditions, an increase from 0 to 10 mg/L in the concentration of Ca (II) and Mg (II) results in an increase from 10 to 81.9 and 78.8 mg/g in the amount of As (V) adsorbed, respectively. The adsorption was shown to be pH-dependent. The efficient regeneration of the loaded adsorbent was carried out and the adsorption mechanism was suggested.
Y. H. Li et al. (2003a) used aligned carbon nanotubes (ACNTs) for the removal of fluoride from water. The adsorption slightly depends on the solution pH value. The highest adsorption capacity of ACNTs occurs at pH 7 and reaches 4.5 mg/g at equilibrium fluoride concentration of 15 mg/L. Y. H. Li et al. (2003b) studied the removal of cadmium (II) with as-grown and surface-oxidized CNTs. Cadmium (II) adsorption capacities for three kinds of oxidized CNTs increase owing to the functional groups introduced by oxidation compared with the as-grown CNTs. The cadmium (II) adsorption capacity of the as-grown CNTs is only 1.1 mg/g, while it reaches 2.6, 5.1 and 11.0 mg/g for the H2O2-, HNO3- and KMnO4-oxidized CNTs, respectively, at the cadmium (II) equilibrium concentration of 4 mg/L. Adsorption of cadmium (II) by CNTs was strongly pH-dependent and the increase of adsorption capacities for HNO3- and KMnO4-oxidized CNTs is more obvious than that of the as-grown and H2O2-oxidized CNTs at lower pH regions. Analysis revealed that the KMnO4-oxidized CNTs hosted manganese residuals, and these surely contributed to cadmium sorption to a yet-undefined extent.
Li et al. (2002) found that CNTs show exceptional adsorption capability and high adsorption efficiency for lead removal from water. The adsorption is significantly influenced by the pH value of the solution and the nanotube surface status, which can be controlled by their treatment processing. The adsorption isotherms are well described by both the Langmuir and Freundlich models.
The first data on multi-wall nanotubes (MWNTs) as sorbent for dioxin removal was reported by Long and Yang (2001). A technique based on temperature-programmed desorption (TPD) was used to study dioxin adsorption. The amount adsorbed on CNTs is 1034 higher than on activated carbon. Hence, significantly higher dioxin removal efficiency is expected with CNTs than with activated carbon. The strong interaction between dioxin and CNTs may be attributed to the unique structure and electronic properties of CNTs. The CNTs consist of hexagonal arrays of carbon atoms in graphene sheets that surround the tube axis. Strong interactions between the two benzene rings of dioxin and the surface of the CNTs are expected.
Srivastava et al. (2004) reported the fabrication of freestanding macroscopic hollow cylinders having radially aligned CNT (ACNT) walls, with diameters and lengths up to several centimetres. These cylindrical membranes are used as filters in the elimination of multiple components of heavy hydrocarbons from petroleum – a crucial step in post-distillation of crude oil – with a single-step filtering process, and the filtration of bacterial contaminants such as Escherichia coli or the nanometre-sized poliovirus (~ 25 nm) from water. These macro filters can be cleaned for repeated filtration through ultrasonication and autoclaving.
Techniques have been developed to synthesize CNTs in significant quantities using three different methods:
An overview of these different routes is summarized in Table 16.2 (Balasubramanian and Burghard, 2005).
Table 16.2
Overview of the important synthesis procedures for CNTs
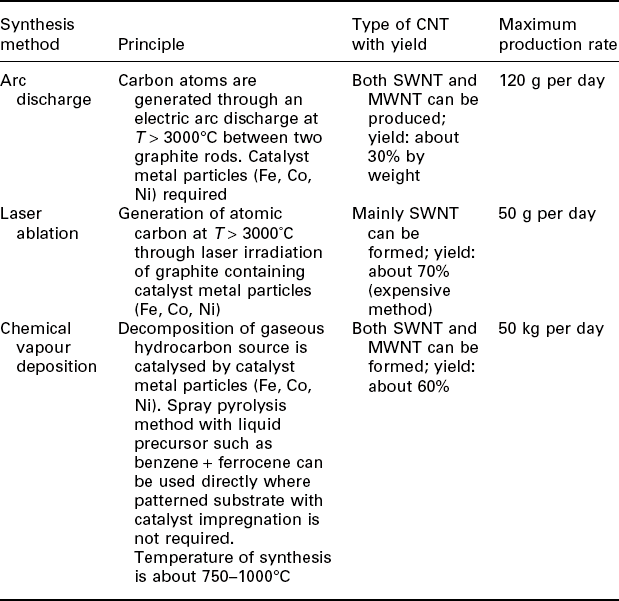
(adapted with permission from Balasubramanian and Burghard, 2005)
Of the various methods of CNT synthesis, CVD has got tremendous potential for scaleup. In addition, the growth of CNTs can take place without having patterned substrate (with impregnation of catalyst particles). A special case of CVD is called spray pyrolysis, where liquid precursors like mixture of benzene and ferrocene can be used. The experimental setup is shown in Fig. 16.5 (Dasgupta et al., 2008). It consists of a sprayer, a container for the liquid precursor and a quartz tube (inner diameter 10 mm). The sprayer is made up of a pyrex nozzle (inner diameter 0.4 mm) and an outer pyrex tube with an exit diameter of 2 mm. The inner nozzle carries the liquid precursor and the outer one carries the nitrogen gas. The sprayer is attached to the quartz tube kept inside a resistive furnace. An optimized combination of composition and the flow rate of liquid precursor solution, the carrier gas flow rate, the diameter of inner and outer nozzles of sprayer and the temperature of growth helps in the synthesis of CNTs with particular length, diameter and alignment. Each of the parameters is critical toward the nature as well as yield of CNTs (Dasgupta et al., 2008). This synthesis route provides CNTs with a high degree of alignment because the catalyst particle (iron in the case of ferrocene precursor) is generated in situ. Also the method is economical with no requirement of substrate preparation as well as less involvement of trained manpower compared to the other two synthesis routes.
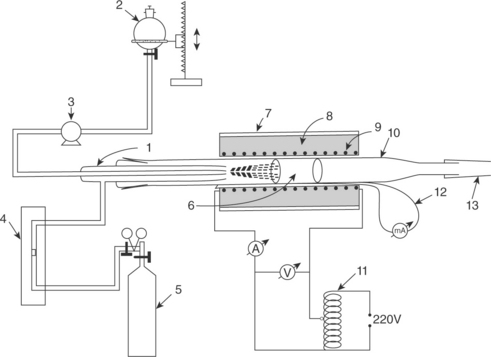
Fig. 16.5 Schematic of the spray pyrolysis setup: (1) pyrex sprayer, (2) container for ferrocene-benzene solution, (3) peristaltic pump, (4) nitrogen gas flow meter, (5) nitrogen gas cylinder, (6) quartz template, (7) furnace outer shell, (8) thermal and electrical insulation, (9) heating element, (10) quartz tube, (11) power supply, (12) thermocouple, (13) outlet to exhaust. (adapted with permission from Dasgupta et al., 2008)
A self-standing tubular macrogeometry of aligned carbon nanotubes with very high surface area (~ 90 m2/g) was developed with optimization of spray pyrolysis parameters. The macrogeometry of aligned CNTs is shown in Fig. 16.6, and Fig. 16.7 shows the scanning electron microscope (SEM) image depicting the highly aligned CNTs along the thickness of cylinder. It has the usual advantages of a membrane where, based upon the principle of size exclusion, the water purification can take place.

Fig. 16.6 The stereoscopic micrograph of the self-standing macrotube made up of aligned carbon nanotubes. (adapted with permission from Dasgupta et al., 2008)
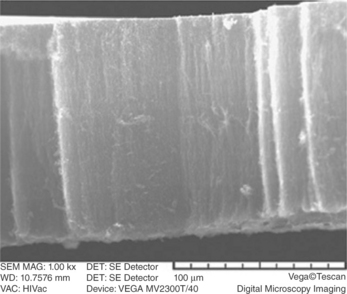
Fig. 16.7 SEM image of the self-standing tube showing the alignment of CNT in the radial direction. (adapted with permission from Dasgupta et al., 2008)
A noteworthy breakthrough in the area of development of nanotube chemistry is the oxidation of CNT in concentrated nitric acid (Rosca et al., 2005). Such a drastic condition helps in opening of the CNT tips as well as oxidative etching along the sidewalls enabling the decoration of walls with various oxygen-containing groups (mainly carboxyl group). The incorporation of carboxyl group exposes various useful sites in CNTs for further modification as per requirements (ester or amide bond formations can take place). In addition, the formation of anhydride at the tube ends can take place through which the rings of CNTs are accessible (Sano et al., 2001). The most important implication of the introduction of a carboxyl group lies in the fact that the van der Waals forces existing between the individual CNTs are reduced and hence the CNTs can be made water soluble (as-grown CNTs are not soluble in any solvent) by addition/substitution of new moieties.
On the other hand, addition reactions help in direct coupling of functional groups onto the π-conjugated carbon framework. A series of addition reactions like fluorination, hydrogenation and cycloaddition can be possible. The fluorine atoms of fluorinated CNTs can be replaced through nucleophilic substitution reaction, and thus, functional groups of alcohols, amines and Grignard reagent, etc., can be successfully incorporated onto the CNT sidewall.
So far we have been concerned mainly with the functionalization of the sidewall and tip of CNTs. But the inner hollow cavity of CNTs offers tremendous opportunity to materials scientists, chemists and engineers to do excellent R&D in the nanoscale test tube. The critical issue is the wetting properties of the CNTs. The wettability determines what liquid would fill the tube by capillary action and cover the inner surface. The Young–Laplace relation relates the pressure difference AP across the liquid-vapour interface in a capillary to the surface tension of the liquid (γ) and contact angle (θ) between the solid and the liquid as shown by:
where r is the radius of curvature of the meniscus. The contact angle θ is an indicator of the strength of the interaction between the liquid and the solid interface relative to the cohesive forces in the liquid. If θ is smaller than 90°, the contact between the liquid and the surface is said to be wetting and ΔP is positive. Therefore the liquid will be pulled into the capillary spontaneously, as there is an energy gain in the wetting process. If θ is larger than 90°, the contact angle is said to be non-wetting and ΔP will be negative. Therefore, when θ > 90°, the only way to introduce liquid into a capillary is to apply pressure larger than ΔP.
Extremely high aspect ratios, molecularly smooth hydrophobic graphitic walls, and nanoscale inner diameters of carbon nanotubes give rise to the unique phenomenon of ultra-efficient transport of water through these ultra-narrow tubes. The idea of water occupying such confined hydrophobic channels is somewhat difficult to comprehend, though experimental evidence has confirmed that water can indeed occupy these channels (Naguib et al., 2004; Kolesnikov et al., 2006). The proposed water transport mechanism has a distinct similarity to the transport mechanisms of biological ion channels. In recent years, numerous simulations (Hummer et al., 2001; Kalra, 2003) of water transport through SWNT have suggested that fast molecular transport takes place, far in excess of what continuum hydrodynamic theories would predict if applied on this length scale. There have been many efforts to define the boundary between bulk water and confined water transport, and it was found sensible to set a threshold for the continuum treatment of liquid as around 7.5 nm.
A no slip boundary condition is typically used in continuum fluid dynamics. It constrains a fluid closest to a solid boundary to obtain the same tangential velocity as the solid. When the tangential velocity of the fluid differs from that of the solid, we usually say the surface imposes a slip boundary condition as depicted in Fig. 16.8 (Choi et al., 2011). The slip boundary condition helps us describe non-continuum behaviour of water transport inside the CNT in the framework of continuum dynamics. For example, slip length, Ls, may serve as a good indicator for the molecular interaction between water molecules and CNT via provision of information about the degree of departure the transport innately has from the hydrodynamic Hagen–Poiseuille flow. Also, the slip length indicator can compare with results of molecular dynamics (MD) simulations often used for the exact prediction of water flow under the CNT nanoconfinement.
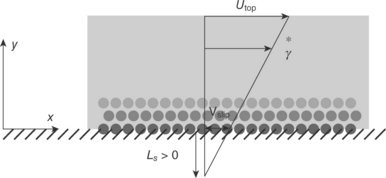
Fig. 16.8 Hydrodynamic slip flow profile characterized by slip length Ls. (adapted from Choi et al., 2011)
Slip length, Ls, is convenient to explain the hydrodynamic boundary condition at the interface of fluid and wall, which is defined according to the Navier boundary condition:
where n and t denote normal and tangential directions of the wall, vt is the velocity of a fluid tangential to the wall, and vwall is the velocity of the wall. vt,wall – vwall is denoted as a slip velocity.
The solutions for the velocity and the corresponding volume flow rate in the flow direction, z, with respect to the distance from the centre, r, have a parabolic profile given by:
where μ, p and R represent viscosity, pressure, and tube radius, respectively.
There are two primary reasons a continuum theory of a molecular scale slip is investigated. On the one hand, it helps to understand the behaviour of a flow in a nanoscale tube. On the other hand, the slip length theory can serve as a reference to explain large water flow enhancements, compared with no-slip Hagen-Poiseuille formalism, observed by several experimental studies (Majumder et al., 2005a; Holt et al., 2006; Whitby et al., 2008) using CNT membranes.
Although the ultrafast water transport in the CNT could be explained by the slip length model to some extent, results of MD simulations, especially on the water configuration, are investigated as an effective way to understand many fundamental nanofluidic characteristics. In fact, lots of physical properties of water depend on the coherence and the number of hydrogen bonds. The hydrogen bond is useful in explaining water configuration or properties inside CNT, since water has a dipole and an electrostatic interaction via hydrogen bonding.
The effect of CNTs on bacteria and viruses has not received particular attention, probably due to the difficulty of dispersing CNTs in water. Surfactants or polymers such as polyvinylpyrolidone (PVP) or Triton-X are generally used to facilitate the dispersion. The few studies available credited SWNTs with antimicrobial activity towards Gram-positive and Gram-negative bacteria, and the damage inflicted was attributed to either a physical interaction or oxidative stress that compromise cell membrane integrity (Narayan et al., 2005; Kang et al., 2008). However, the degre of aggregation and the bioavailability of the nanotube will have to be considered to exploit the antimicrobial properties effectively.
It is noteworthy here that CNTs are the only nanostructured material bestowed with so many unusual (however essentially interesting) attributes which can serve as a wonderful global water filter. The critical factor is to put the CNTs in a membranous structure to exploit all their potential benefits, which are discussed in Section 16.7.
16.4.5 Other nanomaterials (nanoclays, micelles, magnetic nanoparticles)
Clays are alumino silicates with a planar silicate structure. There are three main categories of clay: kaolinite, montmorillonite–smectite and illite, amongst which the first two are the most widely studied (Pradeep and Anshup, 2009b). Usually the structure contains silicate sheets (Si2O5) bonded to aluminium oxide/hydroxide layers (Al2(OH)4) called gibbsite layers. The primary structural unit of this group is a layer composed of one octahedral sheet with one tetrahedral sheet (kaolinite is 1 : 1 clay mineral). The condensation of two sheets happens by coordination of an oxygen atom with one silicon atom in the tetrahedral sheet and two aluminium atoms in the octahedral sheet. Clays undergo exchange interactions of adsorbed ions with the outside too.
Although clays are very useful for many applications, they have one main disadvantage, they lack permanent porosity. To overcome this problem, researchers have been looking for a way to prop and support the clay layers with molecular pillars. Most of the clays can swell and thus increase the space in between their layers to accommodate the adsorbed water and ionic species. Ding et al. (1999) and Ooka et al. (2004) prepared different kinds of TiO2 pillared clays from different raw clays and the adsorption and photocatalytic-decomposition performance were evaluated. It was found that surface hydrophobicity of pillared clays (especially TiO2) largely varied with the host clay. Since the TiO2 particles in the pillared clays are too small to form a crystal phase, they presented poor photocatalytic activity.
Nanocomposite of iron oxide and silicate was also synthesized for degradation of azo-dye orange (II) (Feng et al., 2003). A new class of nano-sized large porous titanium silicate (ETAS-10) and aluminium-substituted ETAS-10 with different Al2O3/TiO2 ratios were successfully synthesized by Choi et al. (2006) and applied to the removal of heavy metals, in particular Pb (II) and Cd (II).
Micelles are self-assembled surfactant materials in a bulk solution. Surfactants or ‘surface active agents’ are usually organic compounds that are amphipathic, meaning they contain both hydrophobic groups (tails) and hydrophilic groups (heads). Therefore, they are typically soluble in both organic solvents and water. Surfactant-enhanced remediation techniques have shown significant potential in their application for the removal of polycyclic aromatic hydrocarbon (PAHs) pollutants. Molecular self-assembly is gathering of molecules without guidance or management from an outside source. There are two types of self assembly: intramolecular and intermolecular. Attaching a monolayer of molecules to mesoporous ceramic supports gives materials known as self-assembled monolayers on mesoporous supports (SAMMS). The highly ordered nanostructure of SAMMS is the result of three molecular self-assembly stages. SAMMS of silica-based materials are highly efficient sorbents for target species, such as heavy metals, tetrahedral oxometalate anions and radionuclides (Mansoori et al., 2008).
Magnetic nanoparticles are generally studied as adsorbents and nanocatalysts for water treatment. One of the major applications of magnetic particles is in the area of magnetic separation. In this case, it is possible to separate a specific substance from a mixture of different other substances, called ‘magnetically assisted chemical separation’ (MACS). Hu et al. (2005) developed an innovative process combining nanoparticle adsorption and magnetic separation for the removal and recovery of Cr (VI) from waste-water. Chang et al. (2006) prepared the magnetic chitosan nanoparticles with an average diameter of 13.5 nm as a magnetic nano-adsorbent. Magnetic chitosan nano-adsorbent was shown to be quite efficient for the fast removal of Co (II) ions at the pH range of 3–7 and the temperature range of 20–45 °C.
Ngomsik et al. (2006) have studied the removal of nickel ions from the aqueous solution using magnetic alginate microcapsules. Also, magnetic particles in the microcapsules allowed easy isolation of the microcapsule beads from aqueous solutions after the sorption process. Mayo et al. (2007) also studied the effect of particle sizes in the adsorption and desorption of As (III) and As (VI). Different kinds of magnetic nanoparticles were also employed for the removal of organic pollutants, such as sorption of methylene blue on polycyclic acid-bound iron oxide from an aqueous solution (Mak and Chen, 2004).
16.5 Synthesis of nanomaterials
There are a number of techniques available to fabricate different nanomaterials (Tiwari et al., 2008). Nanoparticles can be produced from larger structures (top-down) by use of ultrafine grinders, lasers and vapourization followed by cooling. For complex particles, nanotechnologists generally prefer to synthesize nanostructures by a bottom-up approach by arranging molecules to form complex structures with new and useful properties. The detailed views on different synthesis routes is beyond the scope of this chapter and hence they are listed below with references:
• layer-by-layer deposition (Philips et al., 2006)
• self-assembly (Graveland and Kruif, 2006; Lorenceau et al., 2005)
• gas phase synthesis and sol-gel processing (Siegel, 1991, 1994; Uyeda, 1991)
• crystallization (Boanini et al., 2006)
• microbial synthesis (Bhainsa and Souza, 2006; Bhattacharya and Gupta, 2005)
• other methods (sonochemical processing, cavitation processing, microemulsion processing and high-energy ball milling).
16.6 Nanotechnology: health, safety and environment
Nanotechnology is a potential provider of unprecedented technological solutions to many environmental problems including climate change, pollution and clean drinking water. It is claimed that it enables economic growth through more efficient and durable products and new markets. However, the applicability of such a system has to be perceived after due consideration of the process and product in its entirety, with serious attention being paid to the probable health and environmental risks.
While it is perceived that nanotechnology will deliver cleaner production (e.g., through green chemistry, synthesis and processing of nanoscale materials that will reduce consumption of raw materials and natural resources such as water and energy, and improved chemical reactions and catalysis), in reality it is very difficult to ensure these propositions unless there is a proper life cycle analysis of the nanomaterials through validated nano-specific risk assessment methodologies.
Although there are now only a limited number of products in the marketplace that contain engineered nanomaterials, the pace of nanotechnology development assures that the market soon is going to be flooded with nano-based products. In such a case, it is essential to have a grasp of the attribute-related concerns, associated health and environment risks and the extent of the population going to be affected.
The following attributes of nanoparticles create a number of unknown exposures:
• size of particles: the size of nanoparticles necessitates usage of sophisticated analytical tools.
• increased reactivity and conductivity: nanoparticles are more reactive and conductive than the same material in bulk.
• routes of exposure: because of their very small size, nanoparticles can be inhaled or ingested; in addition, they are capable of crossing the blood-brain barrier, which protects the brain against contamination (Oberdörster et al., 2004).
16.6.1 Evidence for toxicity of nanomaterials
Nanoparticles have the ability to induce lung injuries because of their small size, a large surface area, and an ability to generate reactive oxygen species (ROS).1 Section 16.6.1 is adapted from Wani et al., 2011: Nanotoxicity: dimensional and morphological concerns, Advances in Physical Chemistry, Volume 2011, doi:10.1155/2011/450912. The short-term pulmonary toxicity studies in rats with ultrafine and fine carbon black, nickel and TiO2 particles have established enhanced lung inflammatory strength of the ultrafine particles in comparison to fine-sized particulates of similar composition (Warheit et al., 2006; Grassian et al., 2007; Pettibone et al., 2008). Low toxicity nanoparticles such as carbon black and polystyrene stimulate the macrophages via reactive oxygen species and calcium signalling, to make proinflammatory cytokines such as tumour necrosis factor alpha (Brown et al., 2004). The cationic nanoparticles, including gold and polystyrene, have shown to cause haemolysis and blood clotting, while usually anionic particles are quite non-toxic. High exposures to diesel exhaust particles (DEPs) by inhalation caused altered heart rate in hypertensive rats interpreted as a direct effect of DEP on the pacemaker activity of the heart (Hansen et al., 2007). Exposure to singlewalled carbon nanotubes has also resulted in cardiovascular effects (Li et al., 2007). The nanoparticles inhaled can gain access to the brain by means of two different mechanisms, namely, transsynaptic transport after inhalation through the olfactory epithelium and uptake through the blood-brain barrier (Lockman et al., 2004; Jallouli et al., 2007). In vitro studies have shown that multiwalled carbon nanotubes are capable of localizing within and initiating an irritation response in human epidermal keratinocytes, which are a primary route of occupational exposure (Baroli et al., 2007; Zvyagin et al., 2008).
The change in the structural and physicochemical properties of nanoparticles with a decrease in size could be responsible for numerous material interactions that could lead to toxicological effects (Nel et al., 2006); for example, shrinkage in size may create discontinuous crystal planes that increase the number of structural defects as well as disrupt the electronic configuration of the material and give rise to altered electronic properties (Fig. 16.9). These changes could establish specific surface groups that could function as reactive sites. Chemical composition of the materials is particularly responsible for these changes and their importance. The surface groups can make nanoparticles hydrophilic or hydrophobic, lipophilic or lipophobic, or catalytically active or passive. These surface properties can lead to toxicity by the interaction of electron donor or acceptor active sites (chemically or physically activated) with molecular oxygen (O2), and electron capture can lead to the formation of the superoxide radical, which generates additional reactive oxygen species (ROS) through Fenton chemistry (Fig. 16.9).
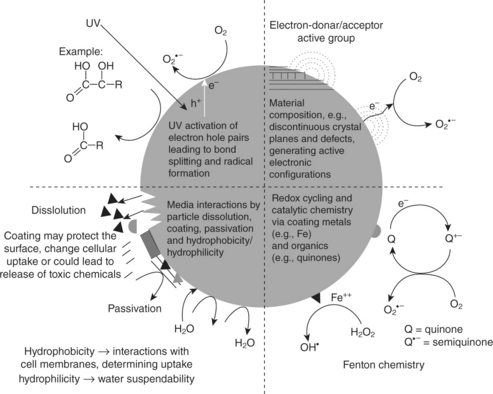
Fig. 16.9 Effect of reactive oxygen species in cells. (adapted from Wani et al., 2011)
Various studies have been carried out to investigate the adverse effects of nanoparticle on the biological systems (N. Li et al., 2003; Hoshino et al., 2004; Li et al., 2007; Baker et al., 2008; Lyon et al., 2006; Peters et al., 2006) and the interaction of nanoparticles with biological systems is represented in Fig. 16.10. It has been demonstrated that carbon black nanoparticles produce their increased inflammatory effects via mechanisms other than the leaching of soluble components from the particle surface. Transition metals are an important source of free radicals, which are important in PM10-stimulated lung inflammation. Therefore, it is clear that nanoparticles may exert their increased proinflammatory effects, at least in part, by modulating intracellular calcium.

Fig. 16.10 Interaction and the adverse effects of nanoparticles on biological systems. (adapted from Wani et al., 2011)
Nanomaterials themselves constitute a new generation of toxic chemicals. As particle size decreases, in many nanomaterials the production of free radicals increases, as does toxicity. Studies have shown that nanomaterials now in commercial use can damage human DNA, negatively affect cellular function and even cause cell death. There is a small but growing body of scientific studies (termed as nanotoxicology) showing that some nanomaterials are toxic to commonly used environmental indicators such as algae, invertebrate and fish species (Hund-Rinke and Simon, 2006; Lovern and Klaper, 2006; Templeton et al., 2006; Federici et al., 2007; Lovern et al., 2007). There is also evidence that some nanomaterials could impair the function or reproductive cycles of earthworms which play a key role in nutrient cycling that underpins ecosystem function (Fordsmand et al., 2008).
Studies demonstrated that when introduced into the lungs of rodents, certain carbon nanotubes cause inflammation, granuloma development, fibrosis, artery ‘plaque’ responsible for heart attacks and DNA damage (Donaldson et al., 2006; Lam et al., 2006; Muller et al., 2006). Two independent studies have shown that some carbon nanotubes can also cause the onset of mesothelioma – cancer previously thought to be only associated with asbestos exposure (Poland et al., 2008; Takagi et al., 2008). We now discuss in brief the toxicity of those nanomaterials (such as silver, CNT, TiO2 and silica) that are being used substantially in water purification applications.
16.6.2 Toxicity of silver nanoparticles
Silver nanoaprticles (Ag NPs) are, due to their antimicrobial properties, the most widely used NPs in commercial products. Ag NPs are incorporated into medical products like bandages as well as textiles and household items. The toxicity of Ag NPs has also been shown by a number of in vitro studies (Kawata et al., 2009; Kim et al., 2009; Foldbjerg et al., 2009, 2011). Toxicological investigations of NPs imply that, for example, size, shape, chemical composition, surface charge, solubility, their ability to bind and affect biological sites as well as their metabolism and excretion influence the toxicity of NPs (Schrand et al., 2010; Castranova, 2011). The high surface area of metal-based NPs increases the potential that metal ions are released from these NPs (Bian et al., 2011; Mudunkotuwa and Grassian, 2011), yet it is not clear to what degree the toxicity of Ag NPs results from released silver ions and how much toxicity is related to the Ag NPs themselves. It is quite possible that free silver ions in Ag NP preparations play a considerable role in the toxicity of Ag NP suspensions. While the contribution of free silver ion to the measured toxicity of Ag NP suspensions is an important determinant for the toxicity, a combined effect of Ag ion and Ag NP appears for lower concentrations of Ag ions.
16.6.3 Toxicity of carbon nanotubes
Despite their many advantages, CNTs represent a hazard to the environment and human health. Like asbestos, the aspect ratio (length : diameter) and metal components of CNTs are known to have an effect on the toxicity of carbon nanotubes. Kim et al. (2011) evaluated the toxic potential of CNTs in relation to their aspect ratio and metal contamination, in vivo and in vitro genotoxicity tests were conducted using high aspect ratio (diameter: 10–15 nm, length: ~ 10 μm) and low aspect ratio multi-wall carbon nanotubes (MWCNTs, diameter: 10–15 nm, length: ~ 150 nm) according to OECD test guidelines 471 (bacterial reverse mutation test), 473 (in vitro chromosome aberration test), and 474 (in vivo micronuclei test). High aspect ratio MWCNTs were found to be more toxic than the low aspect ratio MWCNTs. Thus, while high aspect ratio MWCNTs do not induce direct genotoxicity or metabolic activation-mediated genotoxicity, genotoxicity could still be induced indirectly through oxidative stress or inflammation.
A recent in vivo cancer therapy study using CNTs originally designed as drug delivery enhancers was able to demonstrate that tumour cells respond to toxicity differently than do wild type cells (Liu et al., 2008). Lam et al. (2004) tested a variety of SWCNT samples with varying amounts of metal impurities and concluded that all SWCNT preparations induced dose-dependent lung granulomas in mice. Warheit et al. (2004) reported a mild and transient pulmonary inflammatory response in rats instilled intratracheally with SWCNTs, with subsequent development of multifocal granulomas in the lungs after 1 month in a mouse instillation study using highly purified SWCNTs.
Shvedova et al. (2005) found granulomas, lung fibrosis and a significant elevation in markers of toxicity in bronchoalveolar lavage (BAL) fluid and concluded that SWCNTs exerted greater toxicity on a mass basis than crystalline silica. A critical review of carbon nanotube toxicity and assessment of potential occupational and environmental health risks was provided by Lam et al. (2006), where the toxicological hazard assessment of potential human exposures to airborne CNTs and occupational exposure limits for these novel compounds are discussed in detail.
16.6.4 Toxicity of titanium dioxide and silica (SiO2) nanoparticles
Studies with fine and ultrafine (< 100 nm) TiO2 particles demonstrate some respiratory toxicity and epithelial inflammation of the lung in rodents (Ferin and Oberdörster, 1985; Ferin et al., 1991; Oberdörster et al., 1992; Bermudez et al., 2002, 2004; Warheit et al., 2005, 2006). Silica nanoparticles have been shown to have a low toxicity when administered in moderate doses (W Lin et al., 2006; Chang et al., 2007; Jin et al., 2007). Unfortunately, silica nanoparticles also tend to agglomerate and have been demonstrated to lead to protein aggregation in vitro at a dose of 25 μg/mL (Barik et al., 2008). Oxidative stress has been implicated as an explanation behind silica nanoparticles cytotoxicity both in vitro and in vivo (Chen and von Mikecz, 2005; Yang et al., 2009; Wang et al., 2009). All these studies have reported cytotoxicity and oxidative stress, as determined by increasing lipid peroxidation (LPO), reactive oxygen species (ROS), and decreasing cellular glutathione (GSH level), but no similarity exists regarding dose response.
Very little is known about the safety risks presented by engineered nanomaterials. Given their unique properties, particularly their increased reactivity and electrical conductivity, safety concerns are focusing on whether nanomaterials could cause fires or explosions. Because nanoparticles behave differently from larger particles, questions have arisen about whether they can pollute the water supply or damage crops during processes that release these particles into the air, soil or water. Again, studies in this area are in their infancy.
In the short term, the major health and safety risks will be to researchers in laboratories and production staff exposed during the manufacture of nanomaterials. People in these occupations must be aware of the potential hazards of using materials that have unknown properties, and they must take measures to mitigate their risks. However, their activities are contained and generally do not pose a threat to the public or to the environment.
Owing to the highly interdisciplinary nature of nanotechnology, it can be viewed as an enabling technology that is a sincere augmentation of the existing technologies in the field of water purification, textile, aerospace, health care and electronics. Keeping in view the unknown behaviour and fate of nanomaterials in the environment, nanotechnology may pose tremendous challenges to the existing waste management systems. Knowledge on the mobility, persistence and bioaccumulation potential in the environment is hardly available. Hence risk assessment on the possible impact of nanowastes is critical and needs to be made.
Regulators in the United States, the European Union and elsewhere around the world believe that nanoparticles represent an entirely new risk and that it is necessary to carry out an extensive analysis of the risk. Such studies then can form the basis for government and international regulations. As a proper and comprehensive risk and life cycle analysis, encompassing production, application and waste management strategies of nanomaterials, is of urgent need for a successful commercialization of a technology with proven societal benefits, otherwise environmental costs could be high and the technology as a whole could be distrusted or rejected by the public.
16.7 Domestic water purification: challenges to bring about an integrated system
According to a WHO (2007) report on ‘combating waterborne disease at the household level’ by (The International Network to Promote Household Water Treatment and Safe Storage):
• 1.1 billion lack access to an ‘improved’ drinking water supply; many more drink water that is grossly contaminated.
• 4 billion cases of diarrhoea occur annually, of which 88% is attributable to unsafe water, and inadequate sanitation and hygiene.
• 1.8 million people die every year from diarrhoeal diseases, the vast majority children under 5.
• Lack of safe water perpetuates a cycle whereby poor populations become further disadvantaged, and poverty becomes entrenched.
• WHO estimates that 94% of diarrhoeal cases are preventable through modifications to the environment, including through interventions to increase the availability of clean water, and to improve sanitation and hygiene.
The major drinking water contaminants are shown in Table 16.3 (Daniels and Mesner, 2005). The most serious amongst them are microbiological contamination. WHO guidelines make reference to the water supply situation common in many countries where water must be collected from a well or standpipe, transported home and then stored for domestic use. In such circumstances: ‘Water that is transported or stored unhygienically may be recontaminated, which represents a public health risk. Most recontamination is the result of behavioural patterns; if these can be changed, the health risk can be reduced or eliminated’ (WHO, 1997). In their Water Handbook, UNICEF observe: ‘There are many cases of water which is bacteria-free at the source becoming contaminated during transportation, storage and consumption. Any water supply project that neglects this aspect will be ineffective’ (UNICEF, 1999).
There is now conclusive evidence that simple, acceptable, low-cost interventions at the household and community level are capable of dramatically improving the microbial quality of household stored water and reducing the attendant risks of diarrhoeal disease and death. Treating water at the household level has been shown to be one of the most effective and cost-effective means of preventing waterborne disease in development and emergency settings. Promoting household water treatment and safe storage (HWTS) helps vulnerable populations to take charge of their own water security by providing them with the knowledge and tools to treat their own drinking water (UNICEF, 2008). Household-level approaches to drinking water treatment and safe storage are also commonly referred to as managing the water at the ‘point-of-use’. This term or its abbreviation ‘POU’ typically describes the same procedures as other abbreviations derived from household water treatment, like ‘HHWT’ or ‘HWT’ or ‘HWTS’. (The ‘S’ in ‘HWTS’ refers to safe storage.) ‘Household water management’ is also commonly used, and can encompass both treatment and storage. All these terms can refer to a variety of treatment procedures, for example, with chlorine or other chemical disinfectants, sunlight or UV lamps, various filters, or flocculation-disinfection formulations (WHO, 2007).
A comparison of POU conventional water filtration technologies is shown in Table 16.4. It compares the performance of the ceramic-based filters with that of activated carbon, granular media. From all perspective (efficiency of decontamination, capacity and ease of use), activated carbon has been found to be the best conventional household water treatment method.
Table 16.4
Comparison of POU conventional water filtration technologies


* Cysts: Giardia and cryptosporidium cysts.
**V: Variable depending on.
***U: Exact amount unspecified.
(adapted from Hillie et al., 2007b)
Table 16.5 provides a comparative chart of conventional UV treatment and chemical treatment technologies. It has been observed that coagulation-flocculation outweighs UV-based and chemical-based water treatment options in terms of type and range of contaminants that can be addressed. However, it requires the involvement of trained manpower.
Table 16.5
Comparative chart of POU conventional UV and chemical treatment technologies


*Cysts: Giardia and cryptosporidium cysts.
**V: Variable depending on.
***HH: Household (4–5 people).
(adapted from Hillie et al., 2007b)
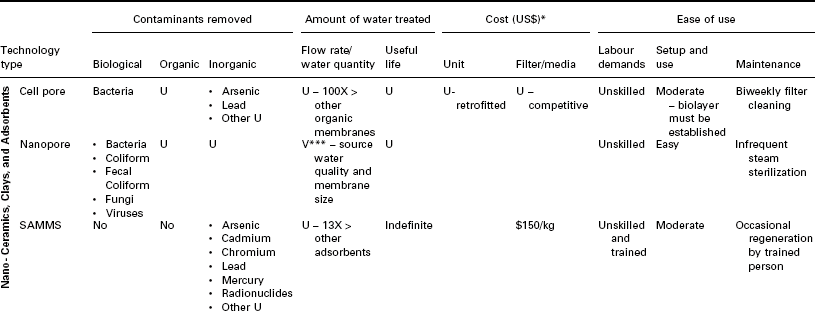
A comparison of POU nanotechnology-based water purification technologies is given in Table 16.6. It is evident from the table that nanoparticle embedded membranes have all the essential features that can be tuned to address the specific contaminant. In addition, it is scalable and widely deployable.
Table 16.6
Comparison of POU nanotechnology-based water purification technologies

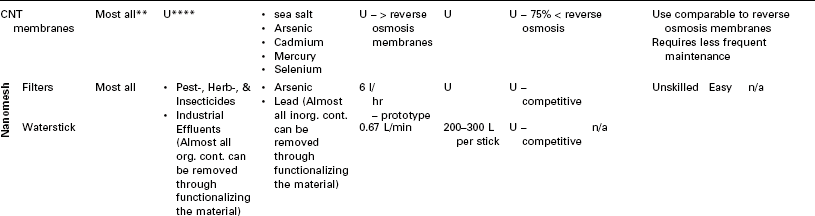

*Costs assume mass production.
**Biological contaminants: Bacteria, Bacterial Spores, Giardia & Cryptosp. Cysts, Coliform, Fecal Coliform, DNA & RNA, Fungi, Mold, Parasites, Protozoa, and Viruses Organic contaminants: Pest-, Herb-, & Insecticides, Industrial Effluents, MTBE, PAHS, PCBs, VOCs, and others. Inorganic contaminants: Heavy Metals, Nitrites, Salts, Asbestos, Radionuclides, Calcium, Magnesium, and others.
***V: Variable depending on.
****U: Exact amount unspecified.
(adapted from Hillie et al., 2007b)
16.7.1 Sustainability of a water purification technology
There are thousands of types of water filters that have the capability to purify contaminated water. However, most filters are too expensive for the nations with the greatest need for potable water. Technologically advanced filters have no real application in countries without the capability to sustain them, which is why more basic filtering methods are needed to truly have an impact on the global clean water shortage. Such organizations as the UN and WHO are currently pushing the water filter industry to develop sustainable solutions to empower many rural nations with the ability to filter their own water in cheaper, more environmentally friendly ways. These sustainable technologies are innovative, simple, and incorporate combinations of basic science and local materials to create usable and efficient filters. Sustainable, or appropriate, technology is defined as, ‘Technology that can be made at an affordable price by ordinary people using local materials to do useful work in ways that do the least possible harm to both human society and the environment’ (Cunningham et al., 1999).
The following guidelines are suggested based on an evaluation of the sustainable technology definition, the ethical standards, and information from sustainable development of technologies (Skye McAllister, 2005). It is important to keep in mind that these steps are to be used in addition to traditional engineering standards and ethics.
16.7.2 Challenges with development of integrated nano-based systems for water purification
There are three different categories of challenges associated with development of successful nano-based technologies and products:
• availability of nanomaterials
• integration of nanomaterials into water purification systems
• societal implications because of health and environment risks.
Availability of nanomaterials
From the databank on different routes of production and usage of nanomaterials, it is strongly believed that there will not be any dearth of nanomaterials either in present day or in the near/far future. As demand rises, the production would automatically try to cope up with the demand. The Freedonia Group has completed a study of the nanomaterials industry (Freedonia Group Inc., 2005). The study provides data on the demand for nanomaterials in the US for the years 2000 and 2003. It also includes forecasts of demand to the years 2008, 2013 and 2020 by classes of materials (e.g., metal oxides, clays, metals, polymers and chemicals, nanotubes, dendrimers, etc.) and by applications (e.g., abrasives; coatings, thin films, sunscreens; biocides; pharmaceutical fillers and reinforcements; catalysts; structural materials, etc.). These forecasts anticipate that most nanomaterials will be nanoscale versions of established products such as silica, titanium dioxide, clays and metal powders. Larger quantities of carbon nanotubes, fullerenes and dendrimers will also be available as these nanomaterials become key components of several application industries in water, electronics and health care.
Integration of nanomaterials into water purification systems
This is the most serious challenge that has to be overcome for exploiting the potential benefits of nanomaterials with exposition of minimum health and environment risks. Most of the usage of nanomaterials at present is based on the applications identified with fine powders. As we have discussed in the previous section regarding the sustainability of a technology/product, the nanoparticles have to be impregnated in a chemically compatible and physically suitable host matrix so that a robust device can emerge with the potential for global applicability. The embedding of nanopowders in a host matrix would have the following advantages:
• The agglomeration of the nanoparticles can be minimized and hence better utilization of the desirable properties can be possible.
• The support can provide a high concentration environment of target contaminant species around the loaded nanoparticles by adsorption. Therefore, the rate of reaction/sorption is enhanced.
• The contaminants, after being reacted/oxidized on the nano surfaces, the resulting toxic intermediates can also get adsorbed on the support and as a result, they are not released in the air atmosphere to cause secondary pollution.
• The potential sorption capability of the nanoparticle–support hybrid can be maintained for a long time.
• Most important, the recovery of nanoparticles would be easy from the host matrix and therefore their reusability can be improved.
• The possibility of secondary contamination of purified product water with the nanomaterials can be minimized.
Another most important property that can be incorporated in a hybrid matrix is the option for treatment of contaminants of different kind by a single system instead of addressing the contaminant sequentially. Here the challenge will be to develop cost-effective and environmentally acceptable separation and reactive media that can be deployed in composite packed-bed reactors (Savage and Diallo, 2005) as shown in Fig. 16.11 for purification of water contaminated by mixtures of metal ions, organic solutes and bacteria.
Fig. 16.11 Schematic of composite nanomaterial packed-bed reactor for water purification. (adapted with permission from Savage and Diallo, 2005)
When we discuss the incorporation of nanomaterials onto a host matrix, the immediate process/technology that comes to our mind is ‘membranes’. Membranes have gained an important place in chemical technology and are used in a broad range of applications. The key property that is exploited is the ability of a membrane to control the permeation rate of a chemical species through the membrane. In separation applications, the goal is to allow one component of a mixture to permeate across the membrane freely, while hindering permeation of other components. A membrane system separates an influent stream into two streams known as the permeate and the concentrate. The permeate is the portion of the fluid that has passed through the semi-permeable membrane, whereas the concentrate stream contains the constituents that have been rejected by the membrane. Membrane separation processes enjoy numerous industrial applications with the following advantages:
• clean technology with operational ease
• replaces conventional processes like filtration, distillation, ion exchange and chemical treatment systems
Pressure driven membrane processes such as reverse osmosis (RO), nanofiltration (NF) and ultrafiltration (UF) are becoming the ‘standard’ water purification technologies for public utilities and industry because they are flexible, scalable, modular and relatively easy to operate and maintain.
The impregnation of nanomaterials onto polymeric membrane host matrix would help fabrication of a nanocomposite membrane having synergistic effects on water purification performances in the manner that membrane by the principle of size exclusion would take care of the unwanted contaminants, while the nanomaterials can add on selectivity to the system. This can be illustrated by the following real research examples being carried out in the workplace of the authors.
Take the case of a domestic polysulfone ultrafiltration membrane candle with polysulfone membrane which takes care of the suspended solids, colour, odour and most importantly microbial contamination. The impregnation of silver nanoparticles into it would assure the product water totally disinfected and in addition help in restoring the life of the membrane by making the membrane surface biofouling resistant. Fig. 16.12 shows the domestic water purification candle with and without silver coating. Using a similar approach, nanocomposite membrane coatings can be provided for removal of arsenic using zero-valent iron and removal of pesticides using titanium dioxide nanoparticles. Also the membranes can be impregnated with multiple nanoparticles to ensure removal of multiple contaminants and in turn the system can be designed for region- or field-specific water conditions.

Fig. 16.12 Domestic water purification candles showing the silver coating for disinfection. The white candle is without silver coating and the grey one with silver coating.
Kar et al. (2011) focused on development of nanoparticle embedded membrane with better biofouling resistant behaviour to be used in waste-water treatment. Nanocomposite polysulfone (PS) membranes with incorporation of nanoparticles of silver (Ag), copper (Cu) and silver-copper mixture (Ag + Cu) were synthesized (as per the composition shown in Table 16.7) and characterized. Representative cross sections were punched from each membrane after they are flushed with bacteria culture, and placed on Luria agar plates and incubated at 37 °C overnight. The results (Fig. 16.13) showed growth of bacteria in PS and PS + Cu while no bacterial growth was detected in PS + Ag and PS + Ag + Cu. This confirmed that the impregnation of silver on to the polysulfone prone to biofouling, and hence an anti-biofouling membrane was developed.
Fig. 16.13 Biofouling-resistant property of membrane surfaces showing no bacterial growth in the silver impregnated nanocomposite membranes: (a) polysulfone; (b) polysulfone + copper; (c) polysulfone + silver + copper; (d) polysulfone + silver.
The potential attributes of CNTs were discussed previously. Again the emphasis is upon the fact that CNT is the only nanostructured material which, on impregnation onto a membrane host matrix (Hinds et al., 2004; Holt et al., 2006), can help in the formulation of the next generation of membranes with improved flux, slectivity and anti-fouling. The fast mass transport of water through CNT would ensure high flux (Majumder et al., 2005a). The functionalization of the CNT tip, as shown in Fig. 16.14, would confirm the gate-keeper controlled chemical separation (Majumder et al., 2005b). In addition to the foregoing, the CNT itself or appropriately functionalized CNTs would make the membrane surface anti-biofouling (Kang et al., 2008; Narayan et al., 2005). However, there are numerous challenges associated with each step of membrane making, starting from growth of CNTs to membrane performance evaluation and scale up. Primarily, there are four approaches (Majumdar and Ajayan, 2010) to the synthesis of membranes based on CNTs as shown in Fig. 16.15.

Fig. 16.14 Functionalization of the CNT membrane via coupling chemistry between amine groups (circled) of the functional molecule and the carboxylic acid with (a) short (C9)-chain amine, (b) long (C22)-chain amine, (c) charged dye molecule with SO32- groups, and (d) long-chain polypeptide resulting in change of transport properties through the CNT membrane (e). Figures (a)–(e) adapted with permission from Majumder et al., (2005a). These experiments indicate the feasibility of functionalizing CNT membranes and should offer avenues for imparting antifouling, biocompatible, molecular recognition or selective adsorption properties, whenever desired.
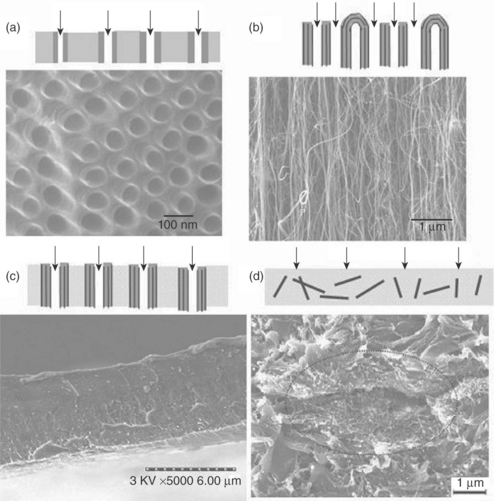
Fig. 16.15 Different approaches to carbon nanotube (CNT) membrane synthesis. (a) Template synthesis approach – carbonaceous material deposited inside anodized alumina template; (below) scanning electron micrograph (SEM) of the nanotubes after dissolution of the template. (b) Dense-array outer-wall CNT membrane; (below) SEM image demonstrating the dense array of CNTs. The fluid transport is through the interstice between the nanotubes, although some transport can occur through some open-ended tubes (Srivastava et al., 2004). (c) Open-ended CNT membrane; (below) SEM image showing the cross section of the membrane with aligned CNTs in an impervious polymer matrix; transport in this membrane structure occurs through the inner core of the CNT (Hinds et al., 2004). Image taken with permission from Majumder et al. (2007). (d) Mixed-matrix membrane composed of CNTs in a polymer matrix; (below) SEM image of the composite membrane structure. Image taken with permission from Geng et al. (2007).
1. Deposition of carbonaceous materials inside pre-existing ordered porous membranes, such as anodized alumina, also known as the template synthesized CNT membranes (Miller et al., 2001).
2. Membranes based on the interstice between nanotubes in a vertical array of CNTs, subsequently referred to as the dense-array outer-wall CNT membrane (Srivastava et al., 2004).
3. Encapsulation of as-grown vertically aligned CNTs by a space-filling inert polymer or ceramic matrix followed by opening up of the CNT tips using plasma chemistry, or the open-ended CNT membrane (Hinds et al., 2004; Holt et al., 2006).
4. Membranes composed of nanotubes as fillers in a polymer matrix, also known as mixed-matrix membranes.
Although CNT-based membranes possess excellent properties to emerge as next generation membranes, still a significant number of challenges remain to be tackled. To grow 12–13 order of magnitude CNTs per square centimetre is a real technological challenge, though chemical vapour deposition methodology offers excellent parameters to achieve this objective. It is very difficult to obtain the yield of CNTs in a particular batch of synthesis beyond 90% reproducibly. Tedious purification steps to remove the sooty deposits and to make the CNT wall defect free make things more complicated. The nanocomposite membrane fabrication route has to confirm that CNTs are well dispersed and well aligned, which is highly challenging. It may require functionalization of CNTs to have better dispersion. Functionalization of CNTs with desired functional groups requires substantial knowledge of Chemistry, for CNT is not soluble in any solvent. The most critical step, that is opening of the CNT tips with either acid treatment or plasma-based oxidation, is not that trivially simple to be adopted. Moreover, the tip-opening step may cause thinning of the CNT wall and disruption of tube integrity and subsequent failure of membrane channels. Finally, scale-up with respect to CNT growth, CNT alignment, nanocomposite formation, CNT tip or side-wall functionalization, are all highly complex and many material as well as process challenges are involved. It is proposed that aligned CNTs may be grown in cylindrical macrogeometry instead of growth of CNTs on rectangular supports. The former path seems to be technically a more efficient means of finding a durable CNT-based device bearing in mind the conditions prevalent in the actual field as shown in Fig. 16.16.
Societal implications
As yet the potential for nanomaterials to exert deleterious effects on humans or the environment is poorly understood, but data on their possible effects are needed so that expanded development and use of nanotechnology can proceed. The environmental fate and toxicity of a material are critical issues in materials selection and design for water purification. There are no systematic investigations of the hydrolytic, oxidative, photochemical and biological stability of nanomaterials (e.g., dendrimers, carbonaceous nanoparticles, metal oxides, etc.) in natural and engineered environmental systems. Assessing the risk of using nanomaterials presents some unique challenges because there is little published research on which to base conclusions and recommendations. As with any new technology that offers significant benefits to humankind, there are also risks of adverse and unintended consequences with nanotechnology. Interdisciplinary discussions of ethical and social dimensions of nanotechnology must be respected. Investment in nanotechnology by governments as well as careful attention to consequences to human health and the environment are both necessary for the public to accept and benefit from commercial products with nanomaterials. Nanotechnology will present opportunities to integrate science and technology with social science and humanities. Education must provide mechanisms for updating scientists and engineers on new technologies as well as helping to organize intelligent debates about societal effects of nanotechnology (Doyle, 2006).
16.9 References
Alvarez-Ayuso, E., Sánchez, A.G., Querol, X. Purification of metal electroplating waste waters using zeolites. Water Res. 2003; 37:4855–4862.
Andersson, M., Osterlund, L., Ljungstrom, S., Palmqvist, A. Preparation of nanosize anatase and rutile TiO2 by hydrothermal treatment of microemulsions and their activity for photocatalytic wet oxidation of phenol. J Phys Chem B. 2002; 106:10674–10679.
Arkas, M., Tsiourvas, D., Paleos, C.M. Functional dendrimeric “nanosponges” for the removal of polycyclic aromatic hydrocarbons from water. Chem Mater. 2003; 14:2844–2847.
Arkas, M., Tsiourvas, D., Paleosc, M. Organosilicon dendritic networks in porous ceramics for water purification. Chem Mater. 2005; 17:3439–3444.
Arkas, M., Allabashi, R., Tsiourvas, D., Mattausch, E.M., Perfler, R. Organic/inorganic hybrid filters based on dendritic and cyclodextrin “nanosponges” for the removal of organic pollutants from water. Environ Sci Technol. 2006; 40:2771–2777.
Asahi, R., Morikawa, T., Ohwaki, T., Aoki, K., Taga, Y. Visible-light photocatalysis in nitrogen-doped titanium oxides. Science. 2001; 293:269–271.
Aymonier, C., Schlotterbeck, U., Antonietti, L., Zacharias, P., Thomann, R., Tiller, J.C., Mecking, S. Hybrids of silver nanoparticles with amphiphilic hyperbranched macromolecules exhibiting antimicrobial properties. Chem Comm. 2002; 24:3018–3019.
Baker, G.L., Gupta, A., Clark, M.L., Valenzuela, B.R., Staska, L.M., Harbo, S.J., Pierce, J.T., Dill, J.A. Inhalation toxicity and lung toxicokinetics of C60 fullerene nanoparticles and microparticles. Toxicol Sci. 2008; 101(1):122–131.
Balasubramanian, K., Burghard, M. Chemically functionalized carbon nanotubes. Small. 2005; 1:180–192.
Balogh, L., Swanson, D.R., Tomalia, D.A., Hagnauer, G.R., McManus, A.T. Dendrimersilver complexes and nanocomposites as antimicrobial agents. Nano Lett. 2001; 1:18–21.
Barik, T.K., Sahu, B., Swain, V. Nanosilica – from medicine to pest control. Parasitol Res. 2008; 103:253–258.
Baroli, B., Ennas, M.G., Loffredo, F., Isola, M., Pinna, R., López-Quintela, M.A. Penetration of metallic nanoparticles in human full-thickness skin. J Invest Dermatol. 2007; 127:1701–1712.
Bermudez, E., Mangum, J.B., Asgharian, B., Wong, B.A., Reverdy, E.E., Janszen, D.B., Hext, P.M., Warheit, D.B., Everitt, J.I. Long-term pulmonary responses of three laboratory rodent species to subchronic inhalation of pigmentary titanium dioxide particles. Toxicol Sci. 2002; 70:86–97.
Bermudez, E., Mangum, J.B., Wong, B.A., Asgharian, B., Hext, P.M., Warheit, D.B., Everitt, J.I. Pulmonary responses of mice, rats, and hamsters to subchronic inhalation of ultrafine titanium dioxide particles. Toxicol Sci. 2004; 77:347–357.
Bhainsa, K.C., Souza, S.F. Extracellular biosynthesis of silver nanoparticles using the fungus Aspergillus fumigates. Colloids Surfaces B: Biointerfaces. 2006; 47:160–164.
Bhattacharya, D., Gupta, R.K. Nanotechnology and potential of microorganisms. Crit Rev Biotechnol. 2005; 25:199–204.
Bian, S.W., Mudunkotuwa, I.A., Rupasinghe, T., Grassian, V.H. Aggregation and dissolution of 4 nm ZnO nanoparticles in aqueous environments: influence of pH, ionic strength, size, and adsorption of humic acid. Langmuir. 2011; 27:6059–6068.
Boanini, E., Torricelli, P., Gazzano, M., Giardino, R., Bigi, A. Nanocomposites of hydroxyapatite with aspartic acid and glutamic acid and their interaction with osteoblast-like cells. Biomaterials. 2006; 27:4428–4433.
Bosman, A.W., Janssen, H.M., Meijer, E.W. About dendrimers: structure, physical properties and applications. Chem Rev. 1999; 99:1665–1688.
Brame, J., Li, Q., Alvarez, P.J.J. Nanotechnology enabled water treatment and reuse: emerging opportunities and challenges for developing countries. Trends Food Sci & Tech. 2011; 22:618–624.
Brown, D.M., Donaldson, K., Borm, P.J., Schins, R.P., Dehnhardt, M., Gilmour, P., Jimenez, L.A., Stone, V. Calcium and ROS-mediated activation of transcription factors and TNF-α cytokine gene expression in macrophages exposed to ultrafine particles. Am J Physiol. 2004; 286:L344–L353.
Burns, C., Spendel, W.U., Puckett, S., Pacey, G.E. Solution ionic strength effect on gold nanoparticle solution color transition. Talanta. 2006; 69:873–876.
Castranova, V. Overview of current toxicological knowledge of engineered nanoparticles. J Occup Environ Med. 2011; 53:S14–S17.
Chang, J.S., Chang, K.L.B., Hwang, D.F., Kong, Z.L. In vitro cytotoxicitiy of silica nanoparticles at high concentrations strongly depends on the metabolic activity type of the cell line. Environ Sci Technol. 2007; 41:2064–2068.
Chang, Y.C., Chang, S.W., Chen, D.H. Magnetic chitosan nanoparticles: studies on chitosan binding and adsorption of Co (II) ions. Reactive Func Poly. 2006; 66:335–341.
Chen, J., Liu, M., Zhang, L., Zhang, J., Jin, L. Application of nano TiO2 towards polluted water treatment combined with electro-photochemical method. Water Res. 2003; 37:3815–3820.
Chen, M., von Mikecz, A. Formation of nucleoplasmic protein aggregates impairs nuclear function in response to SiO2 nanoparticles. Experimental Cell Res. 2005; 305:51–62.
Cheng, R., Wang, J.L., Zhang, W.X. Comparison of reductive dechlorination of p-chlorophenol using FeO and nanosized FeO. J Hazard Mater. 2007; 144:334–339.
Choe, S., Chang, Y.Y., Hwang, K.Y., Khim, J. Kinetics of reductive denitrification by nanoscale zero-valent iron. Chemosphere. 2000; 41:1307–1314.
Choi, J.H., Kim, S.D., Noh, S.H., Oh, S.J., Kim, W.J. Adsorption behaviors of nano-sized ETS-10 and Al-substituted-ETAS-10 in removing heavy metal ions, Pb2 + and Cd2 +. Micro Meso Mater. 2006; 87:163–169.
Choi, J.W., Alexandrova, M.A., Park, H.G., Carbon nanotube nanofluidics, 2011. Available at. http://cdn.intechopen.com/pdfs/17301/InTech-Carbon_nanotube_nanofluidics.pdf
Cloete, T.E., de Kwaadsteniet, M., Botes, M., López-Romero, J.M. Nanotechnology in Water Treatment Applications. Norfolk: Caister Academic Press; 2010.
Cunningham, W.P., Cunningham, M.A., Saigo, B.W. Environmental Science: A Global Concern, 7th edn. New York: McGraw-Hill; 1999.
Daniels, B., Mesner, N. ‘Drinking water treatment systems’, Water Quality, Utah State University Extension. Available at: http://extension.usu.edu/smac/files/uploads/DW_Bacteria_Jan2011.pdf, 2005.
Dasgupta, K., Kar, S., Ramani, V., Bindal, R.C., Prabhakar, S., Tewari, P.K., Bhattacharya, S., Gupta, S.K., Sathiyamoorthy, D. Self-standing geometry of aligned carbon nanotubes with high surface area. Mater Lett. 2008; 62:1989–1992.
Diallo, M., Christie, S., Swaminathan, P., Johnson, J.H., Jr., Goddard, W.A. Dendrimer enhanced ultrafiltration. 1. Recovery of Cu (II) from aqueous solutions using PAMAM dendrimers with ethylene diamine core and terminal NH2 groups. Environ Sci Technol. 2005; 39:1366–1377.
Diallo, M., Duncan, J., Savage, N., Street, A., Sustich, R. Nanotechnology solutions for improving water quality. In: Savage N., Diallo M., Dancan J., Street A., Sustich R., eds. Nanotechnology Applications for Clean Water. NY, William Andrew: Norwich; 2009:585–588.
Ding, Z., Zhu, H.Y., Lu, G.Q., Greenfield, P.F. Photocatalytic properties of titania pillared clays by different drying methods. J Colloid Interf Sci. 1999; 209:193–199.
Donaldson, K., Aitken, R., Tran, L., Stone, V., Duffin, R., Forrest, G., Alexander, A. Carbon nanotubes: a review of their properties in relation to pulmonary toxicology and workplace safety. Toxicol Sci. 2006; 92(1):5–22.
Doyle, M.E. FRI Briefings: ‘Nanotechnology: A Brief Literature Review’. Available at: http://fri.wisc.edu/docs/pdf/FRIBrief_Nanotech_Lit_Rev.pdf, 2006.
Dubas, S.T., Pimpan, V. Humic acid assisted synthesis of silver nanoparticles and its application to herbicide detection. Mater Lett. 2008; 62:2661–2663.
Elizalde-González, M.P., Mattusch, J., Wennrich, R. Application of natural zeolites for preconcentration of arsenic species in water samples. J Environ Monit. 2001; 3:22–26.
EPA. Nanotechnology Workgroup: ‘Nanotechnology White Paper’, Science Policy Council, US EPA. Available at: http://www.epa.gov/osa/pdfs/nanotech/epa-nanotechnology-whitepaper-0207.pdf, 2005.
Federici, G., Shaw, B., Handy, R. Toxicity of titanium dioxide nanoparticles to rainbow trout (Oncorhynchus mykiss): gill injury, oxidative stress, and other physiological effects. Aquatic Toxicol. 2007; 84:415–430.
Feitz, A.J., Joo, S.H., Guana, J., Suna, Q., Sedlak, D.L., Waite, T.D. Oxidative transformation of contaminants using colloidal zero-valent iron. Colloids Surf A: Physicochem Eng Aspects. 2005; 265:88–94.
Feng, J., Hu, X., Yue, P.L., Zhu, H.Y., Lu, G.Q. Degradation of azo-dye orange II by a photoassisted fenton reaction using a novel composite of iron oxide and silicate nanoparticles as a catalyst. Ind Eng Chem Res. 2003; 42:2058–2066.
Ferin, J., Oberdörster, G. Biological effects and toxicity assessment of titanium dioxides: anastase and rutile. Am Ind Hyg Assoc J. 1985; 46:69–72.
Ferin, J., Oberdörster, G., Soderholm, S.C., Gelein, R. Pulmonary tissue access of ultrafine particles. J Aerosol Med. 1991; 4:57–68.
Fischer, M., Vögtle, F. Dendrimers: from design to application: a progress report. Angew Chem Intl Ed Engl. 1999; 38:884–905.
Foldbjerg, R., Olesen, P., Hougaard, M., Dang, D.A., Hoffmann, H.J., Autrup, H. PVP-coated silver nanoparticles and silver ions induce reactive oxygen species, apoptosis and necrosis in THP-1 monocytes. Toxicol Lett. 2009; 190:156–162.
Foldbjerg, R., Dang, D.A., Autrup, H. Cytotoxicity and genotoxicity of silver nanoparticles in the human lung cancer cell line, A549. Arch Toxicol. 2011; 85:743–750.
Fordsmand, J.C., Krogh, P., Schaefer, M., Johansen, A. The toxicity testing of double-walled nanotubescontaminated food to Eisenia veneta earthworms. Ecotoxicol Environ Safety. 2008; 71:616–619.
Freedonia Group, Inc. Nanomaterials – market size, market share, market leaders, demand forecast, sales, company profiles, market research, industry trends. Available at: www.freedoniagroup.com, 2005.
Geng, H.Z., Kim, K.K., So, K.P., Lee, Y.S., Chang, Y., Lee, Y.H. Effect of acid treatment on carbon nanotube-based flexible transparent conducting films. J Am Chem Soc. 2007; 129:7758–7759.
Giasuddin, A.B.M., Kanel, S.R., Choi, H. Adsorption of humic acid onto nanoscale zerovalent iron and its effect on arsenic removal. Environ Sci Technol. 2007; 47:2022–2027.
Gleick, P.H. The World’s Water 2000–2001: The Biennial Report on Freshwater Resources. Washington, DC: Island Press; 2000.
Grassian, V.H., O’Shaughnessy, P.T., Adamcakova-Dodd, A., Pettibone, J.M., Thorne, P.S. Inhalation exposure study of titanium dioxide nanoparticles with a primary particle size of 2 to 5 nm. Environ Health Perspect. 2007; 115:397–402.
Graveland, B.J.F., Kruif, C.G. Unique milk protein based nanotubes: food and nanotechnology meet. Trends Food Sci Technol. 2006; 17:196–203.
Guo, X., Chen, F. Removal of arsenic by bead cellulose loaded with iron oxyhydroxide from groundwater. Environ Sci Technol. 2005; 39:6808–6818.
Hansen, C.S., Sheykhzade, M., Møller, P., Folkmann, J.K., Amtorp, O., Jonassen, T., Loft, S. Diesel exhaust particles induce endothelial dysfunction in apoE −/− mice. Toxicol Appl Pharmacol. 2007; 219:24–32.
Henglein, A. Colloidal silver nanoparticles: photochemical preparation and interaction with O2, CCL4, and some metal ions. Chem Mater. 1998; 10:444–450.
Hillie, T., Munasinghe, M., Hlope, M., Deraniyagala, Y., Nanotechnology, water and development, Global Dialogue on Nanotechnology and the Poor: Opportunities and Risks. Meridian Institute’s project website. 2007 Available at: http://www.merid.org/nano/waterpaper
Hillie, T., Munasinghe, M., Hlope, M., Deraniyagala, Y. Overview and comparison of conventional water treatment technologies and nano-based water treatment technologies. Global Dialogue on Nanotechnology and the Poor: Opportunities and Risks. 2007 Meridian Institute’s project website. Available at: http://www.merid.org/nano/watertechpaper
Hinds, B.J., Chopra, N., Andrews, R., Gavalas, V., Bachas, L. Aligned multi-walled carbon nanotube membranes. Science. 2004; 303:62–65.
Holt, J.K., Park, H.G., Wang, Y., Stadermann, M., Artyukhin, A.B., Grigoropoulos, C.P., Noy, A., Bakajin, O. Fast mass transport through sub-2-nanometer carbon nanotubes. Science. 2006; 312:1034–1037.
Hoshino, A., Fujioka, K., Oku, T., Nakamura, S., Suga, M., Yamaguchi, Y., Suzuki, K., Yasuhara, M., Yamamoto, K. Quantum dots targeted to the assigned organelle in living cells. Microbiol Immunol. 2004; 48:985–994.
Hotze, M., Lowry, G. Nanotechnology for sustainable water treatment. In: Hester R.E., Harrison R.M., eds. Sustainable Water. London: RSC; 2010:138–164.
Hu, J., Lo, I.M.C., Chen, G. Fast removal and recovery of Cr (VI) using surface-modified jacobsite (MnFe2O4) nanoparticles. Langmuir. 2005; 21:11173–11179.
Hummer, G., Rasaiah, J.C., Noworyta, J.P. Water conduction through the hydrophobic channel of a carbon nanotube. Nature. 2001; 414(6860):188–190.
Hund-Rinke, K., Simon, M. Ecotoxic effect of photocatalytic active nanoparticles (TiO2) on algae and daphnids. Environ Sci Poll Res. 2006; 13(4):225–232.
Irie, H., Watanabe, Y., Hashimoto, K. Nitrogen-concentration dependence on photocatalytic activity of TiO2-xNx powders. J Phys Chem B. 2003; 107:5483–5486.
Irokawa, Y., Morikawa, T., Aoki, K., Kosaka, S., Ohwaki, T., Taga, Y. Photo-degradation of toluene over TiO2-xNx under visible light irradiation. Phys Chem Chem Phys. 2006; 8:1116–1121.
Jain, P., Pradeep, T. Potential of silver nanoparticle-coated poly-urethane foam as an antibacterial water filter. Biotechnol Bioeng. 2005; 90:59–63.
Jallouli, Y., Paillard, A., Chang, J., Sevin, E., Betbeder, D. Influence of surface charge and inner composition of porous nanoparticles to cross blood-brain barrier in vitro. Int J Pharm. 2007; 344:103–109.
Jang, W.D., Selim, K.M.K., Lee, C.H., Kang, I.K. Bioinspired application of dendrimers: from bio-mimicry to biomedical applications. Prog Polym Sci. 2009; 34:1–23.
Jeong, B.-H., Hoek, E.M.V., Han, Y., Subramani, A., Huang, X., Hurwitz, G., Ghosh, A.K., Jawor, A. Interfacial polymerization of thin film nanocomposites: a new concept for reverse osmosis membranes. J Membr Sci. 2007; 294:1–7.
Jin, Y., Kannan, S., Wu, M., Zhao, J.X. Toxicity of luminescent silica nanoparticles to living cells. Chem Res Toxicol. 2007; 20:1126–1133.
Kalra, A. From the cover: osmotic water transport through carbon nanotube membranes. Proceedings of the National Academy of Sciences. 2003; 100:10175–10180.
Kanel, S.R., Greneche, J.M., Choi, H. Arsenic(V) removal from ground-water using nano scale zero-valent iron as a colloidal reactive barrier material. Environ Sci Technol. 2006; 40:2045–2050.
Kang, S., Herzberg, M., Rodrigues, D.F., Elimelech, M. Antibacterial effects of carbon nanotubes: size does matter. Langmuir. 2008; 24:6409–6413.
Kar, S., Subramanian, M., Ghosh, A.K., Bindal, R.C., Prabhakar, S., Nuwad, J., Pillai, C.G.S., Chattopadhyay, S., Tewari, P.K. Potential of nanoparticles for water purification: a case-study on anti-biofouling behaviour of metal based polymeric nanocomposite membrane. Desl Water Treat. 2011; 27:224–230.
Kasprzyk-Hordern, B.K. Chemistry of alumina, reactions in aqueous solution and its application in water treatment. Adv Colloid Interface Sci. 2004; 110:19–48.
Kawata, K., Osawa, M., Okabe, S. In vitro toxicity of silver nanoparticles at noncytotoxic doses to HepG2 human hepatoma cells. Environ Sci Technol. 2009; 43:6046–6051.
Kim, S., Choi, J.E., Choi, J., Chung, K.H., Park, K., Yi, J., Ryu, D.Y. Oxidative stress dependent toxicity of silver nanoparticles in human hepatoma cells. Toxicol InVitro. 2009; 23:1076–1084.
Kim, J.S., Lee, K., Lee, Y.H., Cho, H.S., Kim, K.H., Choi, K.H., Lee, S.H., Song, K.S., Kang, C.S., Yu, I.J. Aspect ratio has no effect on genotoxicity of multi-wall carbon nanotubes. Arch Toxicol. 2011; 85:775–786.
Kisch, H., Zang, L., Lange, C., Maier, W.F., Antonius, C., Meissner, D. Modified amorphous titania – a hybrid semiconductor for detoxification and current generation by visible light. Angew Chem Int Ed. 1998; 37:3034–3036.
Kolesnikov, A.I., Loong, C.K., De Souza, N.R., Burnham, C.J., Moravsky, A.P. Anomalously soft dynamics of water in carbon nanotubes. Physica B: Condensed Matter. 2006; 385–386(Part 1):272–274.
Kwon, S., Fan, M., Cooper, A. Photocatalytic applications of micro- and nano-TiO2 in environmental engineering. Crit Rev Environ Sci Technol. 2008; 38:197–226.
Lam, C.W., James, J.T., McCluskey, R., Hunter, R.L. Pulmonary toxicity of single-wall carbon nanotubes in mice 7 and 90 days after intractracheal instillation. Toxicol Sci. 2004; 77:126–134.
Lam, C.W., James, J., McCluskey, R., Arepalli, S., Hunter, R. A review of carbon nanotube toxicity and assessment of potential occupational and environmental health risks. Crit Rev Toxicol. 2006; 36:189–217.
Lee, J.S., Han, M.S., Mirkin, C.A. Colorimetric detection of mercuric ion (Hg2 +) in aqueous media using DNA-functionalized gold nanoparticles. Angew Chem Int Ed. 2007; 46:4093–4096.
Li, L., Zhu, W., Zhang, P., Chen, Z., Han, W. Photocatalytic oxidation and ozonation of catechol over carbon-black-modified nano-TiO2 thin films supported on Al sheet. Water Res. 2003; 37:3646–3651.
Li, N., Sioutas, C., Cho, A., Schmitz, D., Misra, C., Sempf, J., Wang, M., Oberley, T., Froines, J., Ne, Andre. Ultrafine particulate pollutants induce oxidative stress and mitochondrial damage. Environ Health Perspect. 2003; 111:455–460.
Li, Y., Li, X., Li, J., Yin, J. Photocatalytic degradation of methyl orange by TiO2-coated activated carbon and kinetic study. Water Res. 2006; 40:1119–1126.
Li, X.Q., Elliott, D.W., Zhang, W.X. Zero-valent iron nanoparticles for abatement of environmental pollutants: materials and engineering aspects. Crit Rev Solid State Mater Sci. 2006; 31:111–122.
Li, Y.H., Wang, S., Zhang, X., Wei, J., Xu, C., Luan, Z., Wu, D. Adsorption of fluoride from water by aligned carbon nanotubes. Mater Res Bull. 2003; 38:469–476.
Li, Y.H., Wang, S., Luan, Z., Ding, J., Xu, C., Wu, D. Adsorption of cadmium (II) from aqueous solution by surface oxidized carbon nanotubes. Carbon. 2003; 41:1057–1062.
Li, Y.H., Wang, S., Wei, J., Zhang, X., Xu, C., Luan, Z., Wu, D., Wei, B. Lead adsorption on carbon nanotubes. Chem Phys Lett. 2002; 357:263–266.
Li, Z., Hulderman, T., Salmen, R., Chapman, R., Leonard, S.S., Young, S.H., Shvedova, A., Luster, M.I., Simeonova, P.P. Cardiovascular effects of pulmonary exposure to single-wall carbon nanotubes. Environ Health Perspect. 2007; 115(3):377–382.
Lin, T.J., Huang, K.T., Liu, C.Y. Determination of organophosphorous pesticides by a novel biosensor based on localized surface plasmon resonance. Biosens Bioelectron. 2006; 22:513–518.
Lin, W., Huang, Y.W., Zhou, X.D., Ma, Y. In vitro toxicity of silica nanoparticles in human lung cancer cells. Toxicol Appl Pharmacol. 2006; 217:252–259.
Liu, Z., Chen, K., Davis, C., Sherlock, S., Cao, Q., Chen, X., Dai, H. Drug delivery with carbon nanotubes for in vivo cancer treatment. Cancer Res. 2008; 68:6652–6660.
Lockman, P.R., Koziara, J.M., Mumper, R.J., Allen, D. Nanoparticle surface charges alter blood-brain barrier integrity and permeability. J Drug Targ. 2004; 12:635–641.
Long, R.Q., Yang, R.T. Carbon nanotubes as superior sorbent for dioxin removal. J Am Chem Soc. 2001; 123:2058–2059.
Lorenceau, E., Utada, A.S., Link, D.R., Cristobal, G., Joanicot, M., Weitz, D.A. Generation of polymerosomes from double-emulsions. Langmuir. 2005; 21:9183–9186.
Lovern, B., Klaper, R. Daphnia magna mortality when exposed to titanium dioxide and fullerene (C60) nanoparticles. Environ Toxicol Chem. 2006; 25(4):1132–1137.
Lovern, S., Strickler, J., Klaper, R. Behavioural and physiological changes in daphnia magna when exposed to nanoparticle suspensions (titanium dioxide, nano-C60, and C60HxC70Hx). Environ Sci Technol. 2007; 41:4465–4470.
Lowry, G.V., Johnson, K.M. Congener-specific dechlorination of dissolved PCBs by microscale and nanoscale zerovalent iron in a water/methanol solution. Environ Sci Technol. 2004; 38:5208–5216.
Lu, Y., Liu, J. Smart nanomaterials inspired by biology: dynamic assembly of error-free nanomaterials in response to multiple chemical and biological stimuli. Acc Chem Res. 2007; 40:315–323.
Lyon, D.Y., Adams, L.K., Falkner, J.C., Alvarez, P.J.J. Antibacterial activity of fullerene water suspensions: effects of preparation method and particle size. Environ Sci Technol. 2006; 40:4360–4366.
Mahmoodi, N.M., Arami, M., Yousefi, L.N., Gharanjig, K. Photocatalytic degradation of agricultural N-heterocyclic organic pollutants using immobilized nanoparticles of titania. J Hazard Mater. 2007; 145:65–71.
Majumder, M., Ajayan, P.M., Carbon nanotube membranes: a new frontier in membrane scienceDrioli, E., Giorno, L., eds. Comprehensive Membrane Science and Engineering; Volume 1. Oxford, Elsevier, 2010:291–310.
Majumder, M., Chopra, N., Hinds, B.J. Effect of tip functionalization on transport through vertically oriented carbon nanotube membranes. J Am Chem Soc. 2005; 127:9062–9070.
Majumder, M., Chopra, N., Andrews, R., Hinds, B.J. Nanoscale hydrodynamics – enhanced flow in carbon nanotubes. Nature. 2005; 438:44.
Majumder, M., Zhan, X., Andrews, R., Bruce, H.J. Voltage gated carbon nanotube membranes. Langmuir. 2007; 23:8624–8631.
Mak, S.Y., Chen, D.H. Fast adsorption of methylene blue on polyacrylic acid-bound iron oxide magnetic nanoparticles. Dyes and Pigments. 2004; 61:93–98.
Mansoori, G.A., Bastami, T.R., Ahmadpour, A., Eshaghi, Z. Environmental application of nanotechnology. Annual Review of Nano Research. 2008; 2:1–73.
Mascolo, G., Comparelli, R., Curri, M.L., Lovecchio, G., Lopez, A., Agostiano, A. Photocatalytic degradation of methyl red by TiO2: comparison of the efficiency of immobilized nanoparticles versus conventional suspended catalyst. J Hazard Mater. 2007; 142:130–137.
Mayo, J.T., Yavuz, C., Yean, S., Cong, L., Shipley, H., Yu, W., Falkner, J., Kan, A., Tomson, M., Colvin, V.L. The effect of nanocrystalline magnetite size on arsenic removal. Sci Technol Adv Mater. 2007; 8:71–75.
Miller, S.A., Young, V.Y., Martin, C.R. Electroosmotic flow in template- prepared carbon nanotube membranes. J Am Chem Soc. 2001; 123:12335–12342.
Miyauchi, M., Ikezawa, A., Tobimatsu, H., Irie, H., Hashimoto, K. Zeta potential and photocatalytic activity of nitrogen doped TiO2 thin films. Phys Chem Chem Phys. 2004; 6:865–870.
Mudunkotuwa, I.A., Grassian, V.H. The devil is in the details (or the surface): impact of surface structure and surface energetics on understanding the behavior of nanomaterials in the environment. J Environ Monit. 2011; 13:1135–1144.
Muller, J., Huaux, F., Lison, D. Respiratory toxicity of carbon nanotubes: how worried should we be. Carbon. 2006; 44:1048–1056.
Naguib, N., Haihui, Y., Yury, G., Almila, G.Y., Constantine, M.M., Yoshimura, M. Observation of water confined in nanometer channels of closed carbon nanotubes. Nano Lett. 2004; 4:2237–2243.
Nair, A.S., Pradeep, T. Halocarbon mineralization and catalytic destruction by metal nanoparticles. Curr Sci. 2003; 84:1560–1564.
Nair, A.S., Pradeep, T. Extraction of chlorpyrifos and malathion from water by metal nanoparticles. J Nanosci Nanotechnol. 2007; 7:1871–1877.
Nair, A.S., Tom, R.T., Pradeep, T. Detection and extraction of endosulfan by metal nanoparticles. J Environ Monit. 2003; 5:363–365.
Narayan, R.J., Berry, C.J., Brigmon, R.L. Structural and biological properties of carbon nanotube composite films. Mater Sci Eng B. 2005; 123:123–129.
Neal, A.L. What can be inferred from bacterium–nanoparticle interactions about the potential consequences of environmental exposure to nanoparticles? Ecotoxicology. 2008; 17:362–371.
Nel, A., Xia, T., Madler, L., Li, N. Toxic potential of materials at the nano-level. Science. 2006; 311:622–627.
Ngomsik, A.F., Bee, A., Siaugue, J.M., Cabuil, V., Cote, G. Nickel adsorption by magnetic alginate microcapsules containing an extractant. Water Res. 2006; 40:1848–1856.
Oberdörster, G., Ferin, J., Gelein, R., Soderholm, S.C., Finkelstein, J. Role of the alveolar macrophage in lung injury: studies with ultrafine particles. Environ Health Perspect. 1992; 97:193–199.
Oberdörster, G., Sharp, Z., Atudonrei, V., Elder, A., Gelein, R., Kreyling, W., Cox, C. Translocation of inhaled ultrafine particles to the brain. Inhalation Toxicol. 2004; 16:453–459.
OECD. Fostering nanotechnology to address global challenges: water. Available at: http://www.oecd.org/dataoecd/22/58/47601818.pdf, 2011.
Ono, A., Togashi, H. Highly selective oligonucleotide-based sensor for mercury (II) in aqueous solutions. Angew Chem Int Ed. 2004; 43:4300–4302.
Ooka, C., Yoshida, H., Suzuki, K., Hattori, T. Highly hydrophobic TiO2 pillared clay for photocatalytic degradation of organic compounds in water. Micro Meso Mater. 2004; 67:143–150.
Ottaviani, M.F., Favuzza, P., Bigazzi, M., Turro, N.J., Jockusch, S., Tomalia, D.A. A TEM and EPR investigation of the competitive binding of uranyl ions to star-burst dendrimers and liposomes: potential use of dendrimers as uranyl ion sponges. Langmuir. 2000; 19:7368–7372.
Peng, X., Luan, Z., Ding, J., Di, Z., Li, Y., Tian, B. Ceria nanoparticles supported on carbon nanotubes for the removal of arsenate from water. Mater Lett. 2005; 59:399–403.
Peters, A., Veronesi, B., Calderón-Garcidueñas, L., Gehr, P., Chen, L.C., Geiser, M., Reed, W., Rothen-Rutishauser, B., Schürch, S., Schulz, H. Translocation and potential neurological effects of fine and ultrafine particles: a critical update. Part Fiber Toxicol. 2006; 3:13.
Pettibone, J.M., Adamcakova-Dodd, A., Thorne, P.S., O’Shaughnessy, P.T., Weydert, J.A., Grassian, V.H. Inflammatory response of mice following inhalation exposure to iron and copper nanoparticles. Nanotoxicology. 2008; 2(4):189–204.
Phillips, K.S., Han, J.H., Martinez, M., Wang, Z.Z., Carter, D., Cheng, Q. Nanoscale glassification of gold substrates for surface plasmon resonance analysis of protein toxins with supported lipid membranes. Anal Chem. 2006; 78:596–603.
Poland, C., Duffin, R., Kinloch, I., Maynard, A., Wallace, Q., Seaton, A., Stone, V., Brown, S., MacNee, S.W., Donaldson, K. Carbon nanotubes introduced into the abdominal cavity of mice show asbestos like pathogenicity in a pilot study. Nat Nanotechnol. 2008; 3:423–428.
Ponder, S.M., Darab, J.G., Mallouk, T.E. Remediation of Cr (VI) and Pb (II) aqueous solutions using supported nanoscale zero valent iron. Environ Sci Technol. 2000; 34:2564–2569.
Pradeep, T., Anshup. Noble metal nanoparticles for water purification. Thin Solid Films. 2009; 517:6441–6478.
Pradeep, T., Anshup. Detection and extraction of pesticides from drinking water using nanotechnologies. In: Savage N., Diallo M., Duncan J., Street A., Sustich R., eds. Nanotechnology Applications for Clean Water. New York: William Andrew, 2009.
Quinn, J., Geiger, C., Clausen, C., Brooks, K., Coon, C., O’Hara, S., Krug, T., Major, D., Yoon, W.S., Gavaskar, A., Holdswoth, T. Field demonstration of DNAPL dehalogenation using emulsified zero-valent iron. Environ Sci Technol. 2005; 39:1309–1318.
Rai, M., Yadav, A., Gade, A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol Adv. 2009; 27:76–83.
Rajan, Chand S., Gupta, B.D. Surface plasmon resonance based fiberoptic sensor for the detection of pesticide. Sensor Actuator B Chem. 2007; 123:661–666.
Rether, A., Schuster, M. Selective separation and recovery of heavy metal ions using water-soluble N-benzoylthiourea modified PAMAM polymers. React Func Poly. 2003; 57:13–21.
Rosca, I.D., Watari, F., Uo, M., Akasaka, T. Oxidation of multiwalled carbon nanotubes by nitric acid. Carbon. 2005; 153124–153131.
Sakthivel, S., Kisch, H. Daylight photocatalysis by carbon-modified titanium dioxide. Angew Chem Int Ed. 2003; 42:4908–4911.
Sambhy, V., MacBride, M.M., Peterson, B.R., Sen, A. Silver bromide nanoparticle/polymer composites: dual action tunable antimicrobial materials. J Am Chem Soc. 2006; 128:9798–9808.
Sano, M., Kamino, A., Okamura, J., Shinkai, S. Ring closure of carbon nanotubes. Science. 2001; 293:1299–1301.
Sato, S. Photocatalytic activity of NOx-doped TiO2 in the visible light region. Chem Phys Lett. 1986; 123:126–128.
Savage, N., Diallo, M.S. Nanomaterials and water purification: opportunities and challenges. J Nanopart Res. 2005; 7:5331–5342.
Schrand, A.M., Rahman, M.F., Hussain, S.M., Schlager, J.J., Smith, D.A., Syed, A.F. Metal-based nanoparticles and their toxicity assessment. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2010; 2:544–568.
Sharma, V.K., Yngard, R.A., Lin, Y. Silver nanoparticles: green synthesis and their antimicrobial activities. Adv Colloid Interface Sci. 2009; 145:83–96.
Shvedova, A.A., Kisin, E.R., Mercer, R., Ashley, R., Murray, A.R., Johnson, V.J., Potapovich, A.I., Tyurina, Y.T., Gorelik, O., Arepalli, S., Berry, D.S., Hubbs, A.F., Antonini, J., Evans, D.E., Ku, B.K., Ramsey, D., Maynard, A., Kagan, V.E., Castranova, V., Baron, P. Unusual inflammatory and fibrogenic pulmonary responses to singlewalled carbon nanotubes in mice. Am J Physiol. 2005; 289:L698–L708.
Siegel, R.W. Nanomaterials: synthesis, properties and applications. Ann Rev Mater Sci. 1991; 21:559–578.
Siegel, R.W. ‘Physics of new materials’, in Fujita F E, Springer Series in Materials Science, 27. Berlin: Springer; 1994.
Silver, S. Bacterial silver resistance: molecular biology and uses and misuses of silver compounds. FEMS Microbiol Rev. 2003; 27:341–353.
Silver, S., Phung, L.T., Silver, G. Silver as biocides in burn and wound dressings and bacterial resistance to silver compounds. J Ind Microbiol Biotechnol. 2006; 33:627–634.
McAllister, Skye. EPD 397 Technical report: ‘Analysis and comparison of sustainable water filters’. Available at: http://potterswithoutborders.com/manual-uploads/UploadedDocuments/Studies/analysis-and-comparison-of-sustainable-water-filters.pdf, 2005.
Sobana, N., Muruganadham, M., Swaminathan, M. Nano-Ag particles doped TiO2 for efficient photodegradation of direct azo dyes. J Molecular Catalysis A: Chemical. 2006; 258:124–132.
Sondi, I., Sondi, B.S. Silver nanoparticles as antimicrobial agent: a case study on E. coli as a model for Gram-negative bacteria. J Colloid Interf Sci. 2004; 275:177–182.
Srivastava, A., Srivastava, O.N., Talapatra, S., Vajtai, R., Ajayan, P.M. Carbon nanotube filters. Nat Mater. 2004; 3:610–614.
Sun, X., Xia, K., Liu, B. Design of fluorescent self-assembled multilayers and interfacial sensing for organophosphorus pesticides. Talanta. 2008; 76:747–751.
Takagi, A., Hirose, A., Nishimura, T., Fukumori, N., Ogata, A., Ohashi, N., Kitajima, S., Kanno, J. Induction of mesothelioma in p53 +/- mouse by intraperitoneal application of multi-wall carbon nanotube. J Toxicol Sci. 2008; 33(1):105–116.
Templeton, R., Ferguson, P., Washburn, K., Scrivens, W., Chandler, G. Life-cycle effects of single-walled carbon nanotubes (SWNTs) on an estuarine meio- benthic copepod. Environ Sci Technol. 2006; 40:7387–7393.
Theron, J., Walker, J.A., Cloete, T.E. Nanotechnology and water treatment: applications and emerging opportunities. Crit Rev Microbiol. 2008; 34:43–69.
The Royal Society, The Royal Academy of Engineering Nanoscience and Nanotechnologies: Opportunities and Uncertainties. The Royal Society and The Royal Academy of Engineering, London, 2004. http://www.raeng.org.uk/news/publications/list/reports/Nanoscience_nanotechnologies.pdf [Available from URL:].
Tiwari, D.K., Behari, J., Sen, P. Application of nanoparticles in waste water treatment. World Applied Sciences Journal. 2008; 3:417–433.
Tomalia, D.A., Majoros, I. Dendrimeric supramolecular and supramacro-molecular assemblies. J Macro Sci. 2003; 43:411–477.
Tratnyek, P.G., Johnson, R.L. Nanotechnologies for environmental clean up. Nano Today. 2006; 1:44–48.
Umebayashi, T., Yamaki, T., Itoh, H., Asai, K. Band gap narrowing of titanium dioxide by sulfur doping. Appl Phys Lett. 2002; 81:454–456.
UNICEF, Towards better programming: a water handbook. Water, Environment and Sanitation Technical Guidelines Series – No. 2. United Nations Children’s Fund, New York, 1999. Available at. http://www.scribd.com/doc/34473748/A-Water-Handbook
UNICEF. Promotion of household water treatment and safe storage in UNICEF wash programmes. http://www.unicef.org/wash/files/ Scaling_up_HWTS_Jan_25th_with_comments.pdf. [Available at:].
Uyeda, R. Studies of ultrafine particles in Japan: crystallography, methods of preparation and technological applications. Prog Mater Sci. 1991; 35:1–96.
Wang, F., Gao, F., Lan, M., Yuan, H., Huang, H., Liu, J. Oxidative stress contributes to silica nanoparticle-induced cytotoxicity in human embryonic kidney cells. Toxicol InVitro. 2009; 23:808–815.
Wang, H., Xie, C., Zhang, W., Cai, S., Yang, Z., Gui, Y. Comparison of dye degradation efficiency using ZnO powders with various size scales. J Hazard Mater. 2007; 141:645–652.
Wang, J., Teng, X., Wang, H., Ban, H. Characterizing the metal adsorption capability of a class F coal fly ash. Environ Sci Technol. 2004; 38:6710–6715.
Wang, J., Ma, T., Zhang, Z., Zhang, X., Jiang, Y., Dong, D., Zhang, P., Li, Y. Investigation on the sonocatalytic degradation of parathion in the presence of nanometer rutile titanium dioxide (TiO2) catalyst. J Hazard Mater. 2006; 137:972–980.
Wani, M.Y., Hashim, M.A., Nabi, F., Malik, M.A. Nanotoxicity: dimensional and morphological concerns. Adv Phys Chem. 2011; 2011:450912.
Warheit, D.B., Laurence, B.R., Reed, K.L., Roach, D.H., Reynolds, G.A.M., Webb, T.R. Comparative pulmonary toxicity assessment of single-wall carbon nanotubes in rats. Toxicol Sci. 2004; 77:117–125.
Warheit, D.B., Brock, W.J., Lee, W.J., Webb, K.P., Reed, K.L. Comparative pulmonary toxicity inhalation and instillation studies with different TiO2 particle formulations: impact of surface treatments on particle toxicity. Toxicol Sci. 2005; 88:514–524.
Warheit, D.B., Webb, T.R., Sayes, C.M., Colvin, V.L., Reed, K.L. Pulmonary instillation studies with nanoscale TiO2 rods and dots in rats: toxicity is not dependent upon particle size and surface area. Toxicol Sci. 2006; 91:227–236.
Watlington, K. Emerging nanotechnologies for site remediation and waste-water treatment. Available at: http://nepis.epa.gov/Adobe/PDF/P1003FG4.pdf, 2005.
Whitby, M., Cagnon, L., Thanou, M., Quirke, N. Enhanced fluid flow through nanoscale carbon pipes. Nano Lett. 2008; 8:2632–2637.
WHO Guidelines for Drinking Water Quality, Surveillance and Control of Community Supplies. 2nd edn, Geneva: WHO; 1997. Available at:, Vol. 3. www.who.int/water_sanitation_health/dwq/2edaddvol2a.pdf
WHO, Combating waterborne disease at the household level. The International Network to Promote Household Water Treatment and Safe Storage. 2007 Report, Available at: www.who.int/water_sanitation_health/publications/combating_diseasepart1lowres.pdf
World Bank. World Development Indicators. Available at: http://data.world-bank.org/products/data-books/WDI-2005, 2005.
Xiong, Z., Zhao, D., Pan, G. Rapid and complete destruction of perchlorate in water and ion-exchange brine using stabilized zero-valent iron nanoparticles. Water Res. 2007; 41:3497–3505.
Xu, Y., Zhao, D. Reductive immobilization of chromate in water and soil using stabilized iron nanoparticles. Water Res. 2007; 41:2101–2108.
Yang, H., Liu, C., Yang, D., Zhang, H., Xi, Z. Comparative study of cytotoxicity, oxidative stress and genotoxicity induced by four typical nanomaterials: the role of particle size, shape and composition. J Appl Toxicol. 2009; 29(1):69–78.
Yang, Y., Ma, J., Qin, Q., Zhai, X. Degradation of nitrobenzene by nano-TiO2 catalyzed ozonation. J Molec Catal A: Chem. 2007; 267:41–48.
Zang, L., Lange, C., Abraham, I., Storck, S., Meier, W.H., Kisch, H. Amorphous microporous titania modified with platinum(IV) chloride: a new type of hybrid photocatalyst for visible light detoxification. J Phys Chem B. 1998; 102:10765–10771.
Zvyagin, A.V., Zhao, X., Gierden, A., Sanchez, W., Ross, J.A., Roberts, M.S. Imaging of zinc oxide nanoparticle penetration in human skin in vitro and in vivo. J Biomed Opt. 2008; 13:064031.