Chapter 9. Isotope Separators
All of our technology is based on materials in various forms—elements, compounds, alloys, and mixtures. Ordinary chemical and mechanical processes can be used to separate many materials into components. In the nuclear field, however, individual isotopes such as uranium-235 and hydrogen-2 (deuterium) are required. Because isotopes of a given element have the same atomic number Z, they are essentially identical chemically, and thus a physical method must be found that distinguishes among particles on the basis of mass number A. In this chapter we will describe several methods by which isotopes of uranium and other elements are separated. Four methods that depend on differences in A are: (a) ion motion in a magnetic field, (b) diffusion of particles through a membrane, (c) motion with centrifugal force, and (d) atomic response to a laser beam. Calculations on the amounts of material that must be processed to obtain nuclear fuel will be presented, and estimates of costs will be given.
9.1. Mass Spectrograph
We recall from Chapter 8 that a particle of mass m, charge q, and speed υ will move in a circular path of radius r if injected perpendicular to a magnetic field of strength B, according to the relation r = mυ/qB. In the mass spectrograph (Figure 9.1), ions of the element whose isotopes are to be separated are produced in an electrical discharge and accelerated through a potential difference V to provide a kinetic energy ½ mυ 2 = qV. The charges move freely in a chamber maintained at very low gas pressure, guided in semicircular paths by the magnetic field. The heavier ions have a larger radius of motion than the light ions, and the two may be collected separately. It is found (see Exercise 9.1) that the distance between the points at which ions are collected is proportional to the difference in the square roots of the masses. The spectrograph can be used to measure masses with some accuracy, to determine the relative abundance of isotopes in a sample, or to enrich an element in a certain desired isotope.
Figure 9.1. Mass spectrograph.

The electromagnetic process was used on uranium during World War II to obtain weapons material, using the “calutron” (named after the University of California at Berkeley, where it was developed). A total of 1152 units in the “Alpha” and “Beta” processes were operated at the Y-12 Plant at Oak Ridge, producing the enriched uranium for one atomic bomb by 1945. Because the cost of electrical power for the process is large, alternative processes such as gaseous diffusion and centrifuge are used to produce reactor fuels. However, for more than 50 years a few calutrons were maintained at Oak Ridge. These separated light-stable isotopes in small quantities needed for research and for targets for accelerator-produced radioisotopes. The system was shut down permanently in 1999 (see References). It has been reported that Iraq was developing its own electromagnetic process before the Gulf War (see References).
9.2. Gaseous Diffusion Separator
The principle of this process can be illustrated by a simple experiment (Figure 9.2). A container is divided into two parts by a porous membrane, and air is introduced on both sides. Recall that air is a mixture
of 80% nitrogen, A = 14, and 20% oxygen, A = 16. If the pressure on one side is raised, the relative proportion of nitrogen on the other side increases. The separation
effect can be explained on the basis of particle speeds. The average kinetic energies of the heavy (H) and light (L) molecules in the gas mixture are the same, EH = EL, but because the masses are different, the typical particle speeds bear a ratio.![]()
Figure 9.2. Gaseous diffusion separation of nitrogen and oxygen.

The number of molecules of a given type that hit the membrane each second is proportional to nυ in analogy to neutron motion discussed in Chapter 4. Those with higher speed thus have a higher probability of passing through the holes in the porous membrane, called the “barrier.”
The physical arrangement of one processing unit of a gaseous diffusion plant for the separation of uranium isotopes U-235 and U-238 is shown in Figure 9.3. A thin nickel alloy serves as the barrier material. In this “stage,” gas in the form of the compound uranium hexafluoride (UF6) is pumped in as feed and removed as two streams. One is enriched and one depleted in the compound 235UF6, with corresponding changes in 238UF6. Because of the very small mass difference of particles of molecular weight 349 and 352, the amount of separation is small, and many stages in series are required in what is called a cascade.
Figure 9.3. Gaseous diffusion stage.

Natural uranium has a small U-234 component, atom fraction 0.000055. For simplicity, we will ignore its effect except for Exercise 9.11.
Any isotope separation process causes a change in the relative numbers of molecules of the two species. Let nH and nL be the number of molecules in a sample of gas. Their abundance ratio is defined as![]()
For example, in ordinary air R = 80/20 = 4.
The effectiveness of an isotope separation process depends on a quantity called the separation factor r. If we supply gas at one abundance ratio R, the ratio R′ on the low-pressure side of the barrier is given by![]()
If only a very small amount of gas is allowed to diffuse through the barrier, the separation factor is given by ![]() , which for UF6 is 1.0043. However, for a more practical case, in which half the gas goes through, the separation factor is smaller, 1.0030
(see Exercise 9.2). Let us calculate the effect of one stage on natural uranium, 0.711% by weight, corresponding to a U-235 atom fraction of
0.00720, and an abundance ratio of 0.00725. Now
, which for UF6 is 1.0043. However, for a more practical case, in which half the gas goes through, the separation factor is smaller, 1.0030
(see Exercise 9.2). Let us calculate the effect of one stage on natural uranium, 0.711% by weight, corresponding to a U-235 atom fraction of
0.00720, and an abundance ratio of 0.00725. Now![]()
The amount of enrichment is very small. By processing the gas in a series of s stages, each one of which provides a factor r, the abundance ratio is increased by a factor rs. If Rf and Rp refer to feed and product, respectively, Rp= rsRf. For r = 1.0030 we can easily show that 2375 enriching stages are needed to go from Rf = 0.00725 to highly enriched 90% U-235 (i.e., Rp = 0.9/(1 − 0.9) = 9). Figure 9.4 shows the arrangement of several stages in an elementary cascade, and indicates the value of R at various points. The feed is natural uranium, the product is enriched in U-235, and the waste is depleted in U-235.
Figure 9.4. Gaseous diffusion cascade.

Figure 9.5 shows a gaseous diffusion uranium isotope separation plant. Such a facility is very expensive, of the order of a billion dollars, because of the size and number of components such as separators, pumps, valves, and controls, but the process is basically simple. The plant runs continuously with a small number of operating personnel. The principal operating cost is for the electrical power to provide the pressure differences and to perform work on the gas. The Paducah, Ohio, plant is managed by the United States Enrichment Corporation (USEC), which is a business created by privatization of government-owned facilities (see References). That plant provides more than half of the United States market. USEC is participating in a program with Russia called “Megatons to Megawatts” involving dilution of highly enriched uranium to levels used in reactors.
Figure 9.5. Gaseous diffusion plant

(Courtesy United States Enrichment Corporation).
The flow of UF6 and thus uranium through individual stages or the whole plant can be analyzed by the use of material balances. One could
keep track of number of particles, moles, or kilograms, because the flow is continuous. It will be convenient to use kilograms
per day as the unit of uranium flow for three streams: feed (F), product (P), and waste (W), also called “tails.” Then,![]()
Letting x stand for the U-235 weight fractions in the flows, the balance for the light isotope is![]()
(A similar equation could be written for U-238, but it would contain no additional information.) The two equations can be
solved to obtain the ratio of feed and product mass rates. Eliminating W,![]()
For example, let us find the required feed of natural uranium to obtain 1 kg/day of product containing 3% U-235 by weight.
The abbreviation w/o is typically used for weight percent. Assume that the waste is at 0.3 w/o. Now![]() and thus the feed is 6.57 kg/day. We note that W is 5.57 kg/day, which shows that large amounts of depleted uranium tails must be stored for each kilogram of U-235 produced.
Depleted uranium is used for tank bullets. The U-235 content of the tails is too low for use in conventional reactors, but
the breeder reactor can convert the U-238 into plutonium, as will be discussed in Chapter 13.
and thus the feed is 6.57 kg/day. We note that W is 5.57 kg/day, which shows that large amounts of depleted uranium tails must be stored for each kilogram of U-235 produced.
Depleted uranium is used for tank bullets. The U-235 content of the tails is too low for use in conventional reactors, but
the breeder reactor can convert the U-238 into plutonium, as will be discussed in Chapter 13.
The cost of enrichment depends in part on the energy expended, which is measured in “separative work units” (SWU, pronounced “swoo”) with units in kilograms. The method of calculating SWU is reserved for Computer Exercise 9.A. By use of a program called ENRICH3, Table 9.1 was developed. The feed w/o was taken as 0.711, corresponding to an atom percent of 0.720.
Table 9.1. Nuclear Fuel Data
| Weight Percent U-235 | Ratio of Feed to Product | Separative Work Units (SWU) |
|---|---|---|
| 0.711 | 1.000 | 0 |
| 0.8 | 1.217 | 0.070 |
| 1.0 | 1.703 | 0.269 |
| 2.0 | 4.136 | 1.697 |
| 3.0 | 6.569 | 3.425 |
| 5.0 | 11.436 | 7.198 |
| 10.0 | 23.601 | 17.284 |
| 20.0 | 47.932 | 38.315 |
| 90.0 | 218.248 | 192.938 |
Let us use the table to find the amount of fuel needed and its cost to a utility. Assume that the fuel is to be enriched to 3 w/o. Thus each kg of fuel contains 30 grams of U-235 and 970 grams of U-238. The natural uranium feed required for the isotope separation process is 6.569 kg or 14.48 lb. It is easy to show (Exercise 9.8) that the U weight fraction in the U3O8 that would contain it is 0.848. Thus our feed becomes 6.569/0.848 = 7.75 kg of the oxide, or 17.1 lb. The price of uranium oxide varies, but we assume $20/lb, giving $342 as the cost of U.
There is a cost for conversion of U3O8 into UF6 for use in the enrichment process. Assuming $10/kg U, this amounts to $65.69. In column 3 of the table is found the SWU value of 3.425, and by use of a reasonable enrichment charge of $100/SWU, the cost is $342.50. A fuel element fabrication cost of say $275/kg adds for the 1 kg/day a sum of $275. Excluding transportation cost, the total of the preceding numbers is approximately $1025/kg. To fuel a nuclear reactor rated at 1000 MWe, an electric utility may need approximately 60,000 lb/y or 27,200 kg/y giving an annual fuel cost of $27.88 million. However, it can typically produce an average of 850 MW of electrical power over the 8760 hours per year, a total of 7.45 × 109 kWh. The basic fuel cost is thus 0.37 cents or 3.7 mills per kilowatt-hour.
The world picture on uranium enrichment has been changing in recent years, as more suppliers have appeared and United States utilities have diversified their sources. A large fraction of the natural uranium used in the United States comes from other countries such as Canada, Russia, and Australia. Approximately half of the enrichment services are provided by USEC, with the remainder from abroad (e.g., Eurodif, Urenco, and Tenex). A factor that renders the future situation uncertain is the amount and speed of reduction in weapons-grade uranium in the stockpiles of the United States and the Commonwealth of Independent States (CIS). Conversion of highly enriched uranium (HEU) into fuel suitable for reactor use as low-enrichment uranium (LEU) affects the supply situation significantly, including the mining and refining industries and the isotope separation process.
9.3. Gas Centrifuge
This device for separating isotopes, also called the ultracentrifuge because of the very high speeds involved, has been known since the 1940s. It was tested and abandoned during World War II because materials that would withstand high rotation speeds were not available and existing bearings gave large power losses. Developments since have made centrifuges practical and economical. The centrifuge consists of a cylindrical chamber—the rotor—turning at very high speed in a vacuum as sketched in Figure 9.6(A).
Figure 9.6. (A) Gas centrifuge. (B) Gas streams in centrifuge.


The rotor is driven and supported magnetically. Gas is supplied and centrifugal force tends to compress it in the outer region, but thermal agitation tends to redistribute the gas molecules throughout the whole volume. Light molecules are favored in this effect, and their concentration is higher near the center axis. By various means a countercurrent flow of UF6 gas is established that tends to carry the heavy and light isotopes to opposite ends of the rotor. Depleted and enriched streams of gas are withdrawn by scoop pipes, as shown schematically in Figure 9.6(B). More detailed diagrams are found in the References.
The theory of separation by centrifugal force starts with the formula for the gas density distribution in a gravitational
field,![]() where the potential energy is mgh. Adapt the expression to a rotating gas, with kinetic energy at radius r being ½ mυ2 = ½ mω2r2, where ω is the angular velocity, υ/r. Apply to two gases of masses mH and mL to obtain the abundance ratio as a function of distance
where the potential energy is mgh. Adapt the expression to a rotating gas, with kinetic energy at radius r being ½ mυ2 = ½ mω2r2, where ω is the angular velocity, υ/r. Apply to two gases of masses mH and mL to obtain the abundance ratio as a function of distance![]()
Note that separation depends on the difference in masses rather than on their square roots as for gaseous diffusion.
Separation factors of 1.1 or better were obtained with centrifuges approximately 30 cm long, rotating at a rate such that the rotor surface speed is 350 m/s. The flow rate per stage of a centrifuge is much lower than that of gaseous diffusion, requiring large numbers of units in parallel.
The electrical power consumption for a given capacity is lower, however, by a factor of 6 to 10, giving a lower operating cost. In addition, the capital cost of a centrifuge plant is lower than that of a gaseous diffusion plant. European countries have taken advantage of the lower costs of centrifuge separation to challenge the former United States monopoly on enrichment services. In fact, several American utilities buy fuel from Europe. Examples of facilities are the French Eurodif operated by Cogema and the three plants of Urenco, Ltd. at Capenhurst in the United Kingdom, at Almelo in The Netherlands (see Figure 9.7), and at Gronau in West Germany.
Figure 9.7. One of the centrifuge enrichment halls of the plant at Almelo, The Netherlands.

(Courtesy Urenco, Ltd.).
A centrifuge-based uranium enrichment plant is planned for Lea County, New Mexico, by Louisiana Energy Services (a project in Louisiana was abandoned). The partnership of four companies (see References) will install the latest and best equipment developed by Urenco's technology as used in Europe. Full capacity is expected to be 3 million SWU per year, available around 2010.
9.4. Laser Isotope Separation
A different technique for separating uranium isotopes uses laser light (see Section 2.4) to selectively ionize uranium-235 atoms, which can be drawn away from uranium-238 atoms. Research and development on the process, called atomic vapor laser isotope separation (AVLIS), was done in a cooperative program between Lawrence Livermore National Laboratory and Oak Ridge National Laboratory.
An element such as uranium has a well-defined set of electron orbits, similar to those described in Section 2.3, but much more complex because there are 92 electrons. The difference in masses of the nuclei of uranium-235 and uranium-238 results in subtle differences in the electronic orbit structure and corresponding energies required to excite or to ionize the two isotopes.
A laser can supply intense light of precise frequencies, and a fine-tuned laser beam can provide photons that ionize the U-235 and leave the U-238 unchanged. The ionization potential for U-235 is 6.1 volts. The method performs resonance stepwise excitation of an atom. In the AVLIS technique, three photons of approximately 2 eV achieve the ionization.
The virtue of the method is the almost perfect selection of the desired isotope. Of 100,000 atoms ionized by a laser beam, all but 1 are U-235. This permits enrichment from 0.7% to 3% in a single stage rather than thousands as with gaseous diffusion. One kilogram of enriched product comes from 6 kg of natural uranium. The metallic uranium is vaporized by a stream of electrons as in Figure 9.8. A yellow-green laser energizes (“pumps”) a second orange-red laser. This irradiates the uranium vapor, with selective ionization of uranium-235 atoms. An electric field draws those ions off to condense on product collector plates. U-238 atoms pass through the laser beam and condense on the walls of the chamber to be removed as tails.
Figure 9.8. Atomic vapor laser isotopic separation.
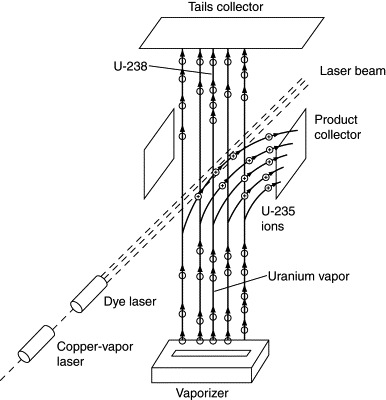
AVLIS was regarded as promising, but the USEC determined that is was less economic than gaseous diffusion or centrifuge separators. The R&D program was terminated in 1999. Some support was given to the Silex process (see References), but in 2003 that too was abandoned in favor of the American Centrifuge.
As a postscript, the French carried out tests with SILVA (AVLIS backward), obtaining 200 kg of low-enrichment uranium and a ton of depleted uranium. However, they concluded that the method would be useful only sometime in the future.
Thanks are due to James I. Davis of Lawrence Livermore National Laboratory and N. Haberman of the Department of Energy (DOE) for some of the information in this section.
9.5. Separation of Deuterium
The heavy isotope of hydrogen ![]() , deuterium, has two principal nuclear applications: (a) as low-absorption moderator for reactors, especially those that use
natural uranium, and (b) as a reactant in the fusion process. The differences between the chemical properties of light water
and heavy water are slight, but sufficient to permit separation of
, deuterium, has two principal nuclear applications: (a) as low-absorption moderator for reactors, especially those that use
natural uranium, and (b) as a reactant in the fusion process. The differences between the chemical properties of light water
and heavy water are slight, but sufficient to permit separation of ![]() and
and ![]() by several methods. Among these are electrolysis, in which the H2O tends to be more readily dissociated; fractional distillation, which takes advantage of the fact that D2O has a boiling point approximately 1 °C higher than that of H2O; and catalytic exchange, involving the passage of HD gas through H2O to produce HDO and light hydrogen gas.
by several methods. Among these are electrolysis, in which the H2O tends to be more readily dissociated; fractional distillation, which takes advantage of the fact that D2O has a boiling point approximately 1 °C higher than that of H2O; and catalytic exchange, involving the passage of HD gas through H2O to produce HDO and light hydrogen gas.
9.6. Summary
The separation of isotopes requires a physical process that depends on mass. In the electromagnetic method, as used in a mass spectrograph, ions to be separated travel in circles of different radii. In the gaseous diffusion process, light molecules of a gas diffuse through a membrane more readily than do heavy molecules. The amount of enrichment in gaseous diffusion depends on the square root of the ratio of the masses and is small per stage, requiring a large number of stages. By the use of material balance equations, the amount of feed can be computed, and by the use of tables of separative work, costs of enriching uranium for reactor fuel can be found. An alternative separation device is the gas centrifuge, in which gases diffuse against the centrifugal forces produced by high speeds of rotation. Laser isotope separation involves the selective excitation of uranium atoms by lasers to produce chemical reactions. Several methods of separating deuterium from ordinary hydrogen are available.
9.7. Exercises
- The ideal separation factor for a gaseous diffusion stage is
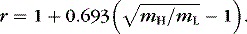 Compute its value for 235UF6 and 238UF6, noting that A = 19 for fluorine.
Compute its value for 235UF6 and 238UF6, noting that A = 19 for fluorine.
- The total fuel loading of a new research reactor is 2000 kg of uranium at 20 w/o U-235. From Table 9.1, find the amount of natural uranium feed and the SWUs required to fuel the reactor, assuming tails of 0.3 w/o.
- A typical reactor that uses product uranium from an isotope separator at 3% enrichment burns 75% of the U-235 and 2.5% of the U-238. What percentage of the mined uranium is actually used for electrical power generation?
- Find the amount of natural uranium feed (0.711% by weight) required to produce 1 kg/day of highly enriched uranium (90% by weight), if the waste concentration is 0.25% by weight. Assume that the uranium is in the form of UF6.
- How many enriching stages are required to produce uranium that is 3% by weight by use of natural UF6 feed? Let the waste be 0.2%.
- By use of the atomic weights of uranium and oxygen in the Appendix, verify that the weight fraction of U in U3O8 is 0.848.
- The number density of molecules as the result of loss through a barrier can be expressed as n = n0 exp(−c υ t) where c is a constant, υ is the particle speed, and n0 and n are values before and after an elapsed time t. If half the heavy isotope is allowed to pass through, find the abundance ratio R′/R = r in the enriched gas as a function of the ratio of molecular masses. Test the derived formula for the separation of uranium isotopes.
- Depleted uranium (0.3% U-235) is processed by laser separation to yield natural uranium (0.711%). If the feed rate is 1 kg/day and all of the U-235 goes into the product, what amounts of product and waste are produced per day?
- By use of natural uranium atom percents of 99.2745 for U-238, 0.7200 for U-235, and 0.0055 for U-234, and atomic masses given in the Appendix, calculate the atomic mass of natural U and the weight percents of each isotope. Suggestion: make a table of numbers.
- A utility plans to increase the enrichment of its nuclear fuel from 3 w/o to 5 w/o, achieving an increase in capacity factor from 0.70 to 0.80 as the result of longer operating cycles. Estimate costs in the two cases and determine whether there is a net financial gain or loss, assuming that electricity is worth approximately 20 mills/kWh.
- A certain country covertly builds production mass spectrographs to separate uranium isotopes. The objective is to obtain 50 kg of highly enriched uranium for a nuclear weapon, in 1 year of continuous operation. (a) Assuming optimistically that separation is perfect, what current of U+ ions would be required? (b) Neglecting power needed for heating and magnets, what amount of electrical power at 50 kV is required? (c) Would the power source be difficult to conceal?
- Calculate the centrifugal acceleration a = υ2/r in a centrifuge at radius r = 0.1 m with an angular speed of 5000 radians/second. By what factor is that larger than the acceleration of gravity 9.8 m/s2? (b) Find the ratio R/R0for UF6 of molecular weights 349 and 352 at 330 K, recalling k = 1.38 × 10−23 J/K and the mass of 1 amu = 1.66 × 10−27 kg.
Computer Exercises
- The tails concentration of a gaseous diffusion separation process is typically 0.3 w/o. For a fixed product (e.g., 1 kg of 3 w/o fuel) study the variation of feed plus enrichment cost with the tails concentration, with (a) computer program ENRICH3 and some hand calculations, or (b) by adapting ENRICH3 to calculate costs.
- Adapt computer program ENRICH3 to calculate costs as well as flows and SWU. Then, find the cost per gram of U-235 and cost per kilogram of U in product of 3 w/o, 20 w/o, and 90 w/o. Keep a constant tails assay of 0.3 w/o.
9.8 References
Smyth Report on Separation of Uranium Isotopes 1945 Smyth Report on Separation of Uranium Isotopes 1945
http://nuclearweaponarchive.org/Smyth/index.html http://nuclearweaponarchive.org/Smyth/index.html
Reproduction of Chapters IX-XI Reproduction of Chapters IX-XI.
Stelio and Villani, 1976 Stelio Villani, Isotope Separation 1976 American Nuclear Society La Grange Park, IL A monograph that describes most of the techniques for separating isotopes, including theory, equipment, and data
Willam Willam E. Parkins, The Uranium Bomb, the Calutron, and the Space Charge Problem Physics Today 58 May 200545-
Uranium Enrichment Uranium Enrichment
http://www.uic.com.au/nip33.htm http://www.uic.com.au/nip33.htm
Facts and figures in a briefing paper by Uranium Information Centre Facts and figures in a briefing paper by Uranium Information Centre (Australia).
Calutrons at Oak Ridge Calutrons at Oak Ridge
http://www.ornl.gov/reporter/no1/calutron.html http://www.ornl.gov/reporter/no1/calutron.htm
Final shutdown of machines Final shutdown of machines.
Iraq's calutrons: 1991–2001 Iraq's calutrons: 1991–2001
http://nuclearweaponarchive.org/Iraq/Calutron.html http://nuclearweaponarchive.org/Iraq/Calutron.html
Posting by Andre A. Gsponer, including report ISRI-95-03 Posting by Andre A. Gsponer, including report ISRI-95-03.
United States Enrichment Corporation United States Enrichment Corporation
http://www.usec.com http://www.usec.com
Select Uranium Enrichment or The American Centrifuge Select Uranium Enrichment or The American Centrifuge.
Olander, August 1978 Donald R. Olander, The Gas Centrifuge Scientific American August 197837-
Enrichment Technology Enrichment Technology
http://www.enritec.com http://www.enritec.com
Urenco-Areva. Select Spotlight for gas centrifuge information Urenco-Areva. Select Spotlight for gas centrifuge information.
Louisiana Energy Services (LES) Gas Centrifuge Facility Louisiana Energy Services (LES) Gas Centrifuge Facility
http://www.nrc.gov/materials/fuel-cycle-fac/lesfacility.html http://www.nrc.gov/materials/fuel-cycle-fac/lesfacility.html
Partners: Urenco, Exelon, Duke Power, and Energy Partners: Urenco, Exelon, Duke Power, and Energy.
Zare, February 1977 Richard N. Zare, Laser Separation of Isotopes Scientific American February 197786-
Silex Isotope Separation Silex Isotope Separation
http://www.silex.com.au/s03_about_silex/s30_1_content.html http://www.silex.com.au/s03_about_silex/s30_1_content.html
R&D with General Electric on laser method R&D with General Electric on laser method.
EIA−Nuclear Data EIA−Nuclear Data
http://www.eia.doe.gov/fuelnuclear.htm http://www.eia.doe.gov/fuelnuclear.html
Links to reports with data on nuclear fuel Links to reports with data on nuclear fuel.
Uranium Enrichment Calculator Uranium Enrichment Calculator
http://www.wise-uranium.org/nfcue.html http://www.wise-uranium.org/nfcue.html
Reproduces calculations in Section 9.2 Reproduces calculations in Section 9.2.
