Chapter 10. Radiation Detectors[†]
† Suggestions by Glenn F. Knoll are recognized with appreciation.
Measurement of radiation is required in all facets of nuclear energy—in scientific studies, in the operation of reactors for the production of electric power, and for protection from radiation hazard. Detectors are used to identify the radioactive products of nuclear reactions and to measure neutron flux. They determine the amount of radioisotopes in the air we breathe and the water we drink, or the uptake of a sample of radioactive material administered to the human body for diagnosis. In recent years detectors have become important in frustrating terrorism.
The kind of detector used depends on the particles to be observed—electrons, gamma rays, neutrons, ions such as fission fragments, or combinations. It depends on the energy of the particles. It also depends on the radiation environment in which the detector is to be used—at one end of the scale is a minute trace of a radioactive material and at the other a source of large radiation exposure. The type of measuring device, as in all applications, is chosen for the intended purpose and the accuracy desired.
The demands on the detector are related to what it is we wish to know: (a) whether there is a radiation field present; (b) the number of nuclear particles that strike a surface per second or some other specified period of time; (c) the type of particles present, and if there are several types, the relative number of each; (d) the energy of the individual particles; and (e) the instant a particle arrives at the detector. From the measurement of radiation we can deduce properties of the radiation such as ability to penetrate matter and to produce ionization. We can also determine properties of a radioactive source, including disintegration rate, half-life, and amount of material.
In this chapter we describe the important features of a few popular types of detectors. Most of them are based on the ionization produced by incoming radiation. The detector may operate in one of two modes: (a) current, in which an average electrical flow is measured, as with an ammeter; and (b) pulse, in which the electrical signals produced by individual particles or rays are amplified and counted. A detector operating in this mode is known as a counter.
Because none of the five human senses will measure nuclear radiation, a detector serves us as a “sixth sense.” A detector also makes it possible to reveal the existence of amounts of material much smaller than can be found by ordinary chemical tests.
10.1. Gas Counters
Picture a gas-filled chamber with a central electrode (anode, electrically positive) and a conducting wall (cathode, negative). They are maintained at different potential, as shown in Figure 10.1. If a charged particle or gamma ray is allowed to enter the chamber, it may produce a certain amount of ionization in the gas. The resultant positive ions and electrons are attracted toward the negative and positive surfaces, respectively. A charge moves in the local field E with a drift velocity υD = μ E, where the mobility μ depends on the time between collisions and the mean free path (see Sections 4.4 and 4.6). If a magnetic field is present, charges tend to execute circular paths interrupted by collisions. When the voltage across the tube is low, the charges merely migrate through the gas, they are collected, and a current of short duration (a pulse) passes through the resistor and the meter. More generally, amplifying circuits are required. The number of current pulses is a measure of the number of incident particles that enter the detector, which is designated as an ionization chamber when operated in this mode.
Figure 10.1. Basic detector.

If the voltage is then increased sufficiently, electrons produced by the incident radiation through ionization are able to gain enough speed to cause further ionization in the gas. Most of this action occurs near the central electrode, where the electric field is highest. The current pulses are much larger than in the ionization chamber because of the amplification effect. The current is proportional to the original number of electrons produced by the incoming radiation, and the detector is now called a proportional counter. One may distinguish between the passage of beta particles and alpha particles, which have a widely different ability to ionize. The time for collection is very short, of the order of microseconds.
If the voltage on the tube is raised still higher, a particle or ray of any energy will set off a discharge, in which the secondary charges are so great in number that they dominate the process. The discharge stops of its own accord because of the generation near the anode of positive ions, which reduce the electric field there to such an extent that electrons are not able to cause further ionization. The current pulses are then of the same size, regardless of the event that initiated them. In this mode of operation, the detector is called a Geiger-Mueller (GM) counter. Unlike the proportional counter, the magnitude of the pulses produced by a GM counter is independent of the original number of electrons released by the ionizing radiation. Therefore the counter provides no information about the type or energy of the radiation. There is a short period, the dead time, in which the detector will not count other incoming radiation. If the radiation level is very high, a correction of the observed counts to yield the “true” counts must be made to account for the dead time. In some gases, such as argon, there is a tendency for the electric discharge to be sustained, and it is necessary to include a small amount of foreign gas or vapor (e.g., alcohol) to “quench” the discharge. The added molecules affect the production of photons and resultant ionization by them.
A qualitative distinction between the preceding three types of counters is displayed graphically in Figure 10.2, which is a semilog plot of the charge collected as a function of voltage. We note that the current varies over several orders of magnitude.
Figure 10.2. Collection of charge in counters.

10.2. Neutron Detectors
To detect neutrons, which do not create ionization directly, it is necessary to provide a means for generating the charges
that can ionize a gas. Advantage is taken of the nuclear reaction involving neutron absorption in boron![]() where the helium and lithium atoms are released as ions. One form of boron counter is filled with the gas boron trifluoride (BF3) and operated as an ionization chamber or a proportional counter. It is especially useful for the detection of thermal neutrons
because the cross section of boron-10 at 0.0253 eV is large, 3840 barns, as noted in Chapter 4. Most of the 2.8 MeV energy release goes to the kinetic energy of the product nuclei. The reaction rate of neutrons with
the boron in BF3 gas is independent of the neutron speed, as can be seen by forming the product R = nυNσa, where σa varies as 1/υ. The detector thus measures the number density n of an incident neutron beam rather than the flux. Alternately, the metal electrodes of a counter may be coated with a layer
of boron that is thin enough to allow the alpha particles to escape into the gas. The counting rate in a boron-lined chamber depends on the surface
area exposed to the neutron flux.
where the helium and lithium atoms are released as ions. One form of boron counter is filled with the gas boron trifluoride (BF3) and operated as an ionization chamber or a proportional counter. It is especially useful for the detection of thermal neutrons
because the cross section of boron-10 at 0.0253 eV is large, 3840 barns, as noted in Chapter 4. Most of the 2.8 MeV energy release goes to the kinetic energy of the product nuclei. The reaction rate of neutrons with
the boron in BF3 gas is independent of the neutron speed, as can be seen by forming the product R = nυNσa, where σa varies as 1/υ. The detector thus measures the number density n of an incident neutron beam rather than the flux. Alternately, the metal electrodes of a counter may be coated with a layer
of boron that is thin enough to allow the alpha particles to escape into the gas. The counting rate in a boron-lined chamber depends on the surface
area exposed to the neutron flux.
The fission chamber is often used for slow neutron detection. A thin layer of U-235, with high thermal neutron cross section, 681 barns, is deposited on the cathode of the chamber. Energetic fission fragments produced by neutron absorption traverse the detector and give the necessary ionization. Uranium-238 is avoided because it is not fissile with slow neutrons and because of its stopping effect on fragments from U-235 fission.
Neutrons in the thermal range can be detected by the radioactivity induced in a substance in the form of small foil or thin
wire. Examples are manganese ![]() , with a 13.3 barn cross section at 2200 m/s, which becomes
, with a 13.3 barn cross section at 2200 m/s, which becomes ![]() with half-life 2.58 h; and dysprosium
with half-life 2.58 h; and dysprosium ![]() , 1.7 × 103 barns, becoming
, 1.7 × 103 barns, becoming ![]() , half-life 2.33 h. For detection of neutrons slightly above thermal energy, materials with a high resonance cross section
are used (e.g., indium) with a peak at 1.45 eV. To separate the effects of thermal neutron capture and resonance capture, comparisons are made between measurements made with thin foils
of indium and those of indium covered with cadmium. The latter screens out low-energy neutrons (below 0.5 eV) and passes those
of higher energy.
, half-life 2.33 h. For detection of neutrons slightly above thermal energy, materials with a high resonance cross section
are used (e.g., indium) with a peak at 1.45 eV. To separate the effects of thermal neutron capture and resonance capture, comparisons are made between measurements made with thin foils
of indium and those of indium covered with cadmium. The latter screens out low-energy neutrons (below 0.5 eV) and passes those
of higher energy.
For the detection of fast neutrons, up in the MeV range, the proton recoil method is used. We recall from Chapter 4 that the scattering of a neutron by hydrogen results in an energy loss, which is an energy gain for the proton. Thus a hydrogenous
material such as methane (CH4) or H2 itself may serve as the counter gas. The energetic protons play the same role as α particles and fission fragments in the
counters discussed previously. Nuclear reactions such as ![]() can also be used to obtain detectable charged particles.
can also be used to obtain detectable charged particles.
10.3. Scintillation Counters
The name of this detector comes from the fact that the interaction of a particle with some materials gives rise to a scintillation or flash of light. The basic phenomenon is familiar—many substances can be stimulated to glow visibly on exposure to ultraviolet light, and the images on a color television screen are the result of electron bombardment. Molecules of materials classed as phosphors are excited by radiation such as charged particles and subsequently emit pulses of light. The substances used in the scintillation detector are inorganic (e.g., sodium iodide or lithium iodide) or organic, in one of various forms—crystalline, plastic, liquid, or gas.
The amount of light released when a particle strikes a phosphor is often proportional to the energy deposited, and thus makes the detector especially useful for the determination of particle energies. Because charged particles have a short range, most of their energy appears in the substance. Gamma rays also give rise to an energy deposition through electron recoil in both the photoelectric effect and Compton scattering, and through the pair production-annihilation process. A schematic diagram of a detector system is shown in Figure 10.3. Some of the light released in the phosphor is collected in the photomultiplier tube, which consists of a set of electrodes with photosensitive surfaces. When a photon strikes the surface, an electron is emitted by the photoelectric effect, it is accelerated to the next surface where it dislodges more electrons, and so on, and a multiplication of current is achieved. An amplifier then increases the electrical signal to a level convenient for counting or recording.
Figure 10.3. Scintillation detection system.

Radiation workers are required to wear personal detectors called dosimeters to determine the amount of exposure to X- or gamma rays or neutrons. Among the most reliable and accurate types is the thermoluminescent dosimeter (TLD), which measures the energy of radiation absorbed. It contains crystalline materials such as CaF2 or LiF that store energy in excited states of the lattice called traps. When the substance is heated, it releases light in a typical glow curve as shown in Figure 10.4. The dosimeter reader consists of a small vacuum tube with a coated cylinder that can be heated by a built-in filament when the tube is plugged into a voltage supply. A photomultiplier reads the peak of the glow curve and gives values of the accumulated energy absorbed (i.e., the dose). The device is linear in its response over a very wide range of exposures; it can be used over and over with little change in behavior.
Figure 10.4. Glow curve of the phosphor CaF2.

10.4. Solid State Detectors
The use of a solid rather than a gas in a detector has the advantage of compactness, because of the short range of charged particles. Also, when the solid is a semiconductor, great accuracy in measurement of energy and arrival time is possible. The mechanism of ion motion in a solid detector is unique. Visualize a crystal semiconductor, such as silicon or germanium, as a regular array of fixed atoms with their complement of electrons. An incident charged particle can dislodge an electron and cause it to leave the vicinity, which leaves a vacancy or “hole” that acts effectively as a positive charge. The electron-hole pair produced is analogous to negative and positive ions in a gas. Electrons can migrate through the material or be carried along by an electric field, while the holes “move” as electrons are successively exchanged with neighboring atoms. Thus, electrons and holes are negative and positive charge carriers, respectively.
The electrical conductivity of a semiconductor is very sensitive to the presence of certain impurities. Consider silicon, chemical valence 4 (with 4 electrons in the outer shell). Introduction of a small amount of valence 5 material such as phosphorus or arsenic gives an excess of negative carriers, and the new material is called n-type silicon. If instead a valence 3 material such as boron or gallium is added, there is an excess of positive carriers, and the substance is called p-type silicon. When two layers of n-type and p-type materials are put in contact and a voltage is applied, as in Figure 10.5, electrons are drawn one way and holes the other, leaving a neutral or “depleted” region. Most of the voltage drop occurs over that zone, which is very sensitive to radiation. An incident particle creates electron-hole pairs that are swept out by the internal electric field to register as a current pulse. High accuracy in measurement by an n-p junction comes from the fact that a low energy is needed to create an electron-hole pair (only 3 eV versus 32 eV for an ion pair in a gas). Thus a 100 keV photon creates a very large number of pairs, giving high statistical accuracy. The collection time is very short, approximately a billionth of a second, allowing precise time measurements.
Figure 10.5. Solid-state n-p junction detector.

One way to produce a semiconductor detector with a large active volume is to introduce lithium on one surface of a heated crystal and apply an electric field. This “drifts” the Li through the volume that compensates residual p-type impurities. This detector must be kept permanently at liquid nitrogen temperature (−195.8 °C) to prevent redistribution of the lithium. A preferable detector for many applications is made of an ultrahigh-purity germanium, with impurity atoms reduced to 1 in approximately 1012. A simple diode arrangement gives depletion depths of several centimeters. Such detectors still require liquid N2 for operation, but they can be stored at room temperature.
10.5. Statistics of Counting
The measurement of radiation has some degree of uncertainty because the basic processes, such as radioactive decay, are random
in nature. From the radioactive decay law, Section 3.2, we can say that on the average in a time interval t a given atom in a large collection of atoms has a chance exp(−λt) of not decaying, and thus it has a chance 1 − exp(−λt)
of decaying. Because of the statistical nature of radioactivity, however, more or less than these numbers will actually be
observed in a certain time interval. There is actually a small probability that either none or all would decay. In a series
of identical measurements there will be a spread in the number of counts. Statistical methods may be applied to the data to
estimate the degree of uncertainty or “error.” The laws of probability may be applied. As discussed in texts on statistics
and radiation detection (see References), the most rigorous expression is the binomial distribution (see Exercise 10.6), which must be used to interpret the decay of isotopes of very short half-life. A simple approximation to it is the Poisson
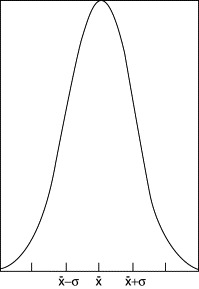
distribution (see Exercise 10.7) required for the study of low-level environmental radioactivity. A further approximation is the widely used normal or Gaussian
distribution, shown in Figure 10.6. Measured values of the number of counts x in repeated trials tend to fit the formula,![]() where P(x) is the probability of being in a unit range at x and
where P(x) is the probability of being in a unit range at x and ![]() is the mean value of the counts. A measure of the width of the curve is the standard deviation, σ. For this function[†],
is the mean value of the counts. A measure of the width of the curve is the standard deviation, σ. For this function[†], ![]() . The area under the curve between
. The area under the curve between ![]() and
and ![]() is 68% of the total, which indicates that the chance is 0.68 that a given measurement will lie in that range. The figure
for 95% is ±2σ. It can be shown that the fractional error in count rate is inversely proportional to the square root of the
total number of counts.
is 68% of the total, which indicates that the chance is 0.68 that a given measurement will lie in that range. The figure
for 95% is ±2σ. It can be shown that the fractional error in count rate is inversely proportional to the square root of the
total number of counts.
† In general, for a series of trials, 1,2, 3,…, N, if count rates are xi and the average is ![]() , the standard deviation is
, the standard deviation is
Figure 10.6. Gaussian distribution. The area between the limits x ± σ is 68% of the total.
Because the calculation for plotting of the preceding statistical distribution is quite tedious, we have provided the computer program STAT, the use of which is described in Computer Exercises 10.A through 10.D. Also, program EXPOIS generates simulated counting data for study by use of the Poisson distribution.
10.6. Pulse Height Analysis
The determination of the energy distribution of nuclear particles and rays is important for identifying radioactive species. If an incoming particle deposits all of its energy in the detector, the resulting voltage signal in the external electric circuit of Figure 10.7(A) can be used as a measure of particle energy. The particle ionizes the medium, a charge Q is produced, and a current flows, giving a time-varying voltage. If the time constant τ = RC of the circuit is short compared with the collection time, the voltage rises and drops to zero quickly, as in Figure 10.7(B). If τ is large, however, the voltage rises to a peak value Vm = Q/C, where C is the capacitance, and then because of the circuit characteristics declines slowly, as in Figure 10.7(C). The particle energy, proportional to charge, is thus obtained by a voltage measurement.
Figure 10.7. Effect of circuit on pulse (after Knoll, see References).

Suppose that there are two types of particles entering the detector, say alpha particles of 4 MeV and beta particles of 1 MeV. By application of a voltage bias, the pulses caused by beta particles can be eliminated, and the remaining counts represent the number of alpha particles. The circuit that performs that separation is called a discriminator.
The radiation from a given source will have some variation in particle energy and thus a series of pulses caused by successive particles will have a variety of heights. To find the energy distribution, a single-channel analyzer can be used. This consists of two adjustable discriminators and a circuit that passes pulses within a range of energy. The multichannel analyzer is a much more efficient and accurate device for evaluating an entire energy spectrum in a short time. Successive pulses are manipulated electronically and the signals are stored in computer memory according to energy. The data are displayed on an oscilloscope screen or are printed out.
10.7. Advanced Detectors
A number of specialized instruments have been developed in addition to basic detectors. They are used for precise measurements of the products of high-energy nuclear collisions. Examples are:
- Nuclear emulsion track detectors, originally used for cosmic ray studies. By application of the energy loss formula (see Section 5.2) information is obtained on particle energy, mass, and charge. Special etching techniques are used and the counting of tracks with a microscope is automated.
- Cerenkov counters, which measure the light produced when a particle has a speed higher than that of light in the medium. Cerenkov radiation gives the “blue glow” seen near a pool reactor core.
- Hadron calorimeters, which measure showers of hadrons (mesons and nucleons), protons, and neutrons produced by bombardment of materials of particles in the GeV range.
- Neutrino detectors, consisting of large volumes of liquid or metal in which the rare collisions resulting in a scintillation occur.
These specialized devices are discussed in the book by Kleinknecht (see References).
10.8. Detectors and Counterterrorism
Radiation detectors play a vital role in protecting against terrorist action. Of greatest importance is the screening of shipments at their point of origin and on their arrival at an United States port. X-rays are helpful in finding well-shielded items in large shipping containers, prompting further inspection. Portable X-ray generators powered with batteries are available, giving 150 keV rays that can penetrate half an inch of steel.
To check for the presence of fissionable materials—enriched uranium or plutonium—several techniques are available. One involves an intense beam of 14 MeV neutrons from a D-T generator. Pulses of very short duration are introduced to cause fission and neutron release. Silicon carbide (SiC) semiconductor detectors measure the fission neutrons very quickly. Devices used in research are considered suitable for commercial production.
An alternative method to detect fissionable materials uses a pulsed photonuclear neutron detector. It consists of a portable accelerator that produces energetic photons. These cause fission, which releases neutrons that are detected externally. In tests, a sample vial shielded by wood, polyethylene, and lead was quickly and readily identified. By use of different photon energies, distinctions between enrichments of uranium or fissionable elements were achieved.
Amounts of weapons material that are significant are 25 kg for uranium and 4 kg for plutonium. The half-lives of U-235 and U-238 are too long for their inherent radiation to be detected. For the radiological dispersal device, the so-called dirty bomb, there is direct detection of their radiation. Isotopes that are likely to be used are cobalt-60, half-life 5.27 y, average 1.25 MeV gammas, and cesium-137, half-life 30.2 y, 0.662 MeV gammas. Other candidate radioisotopes are americium-241, californium-252, iridium-192, and strontium-90. Californium is a neutron emitter with half-life 2.65 y. In the case of Sr-90, the beta particles are readily shielded, but the bremsstrahlung emitted as the electrons slow down in matter can be detected. All of these isotopes have commercial applications, which makes them vulnerable for theft.
Nuclear forensics involves a signature method, the determination of the origin of radioactivity whether it is from a dirty bomb or from the explosion of a nuclear assembly. Search algorithms are developed that correlate the time dependence of isotope decay with reactor type and neutron irradiation history.
Research continues on the problem of finding the radiation dosage to individuals in emergency situations. Plans are being developed for the handling of a large number of people who are irradiated or contaminated.
Tracer studies in Manhattan are used to develop models for the dispersal of radioactivity in a city with streets between skyscrapers.
In methods that use or produce neutrons, consideration must be given to the “neutron ship effect.” When muons in cosmic rays bombard iron as in bridges or ships there is a release of neutrons, which constitutes a competing background.
Much of the R&D on detectors is sponsored and supported by the Department of Homeland Security, formed in the wake of the September 11, 2001, attacks. The agency has a mammoth task in providing radiation detection equipment at the 1205 seaports and airports. The General Accountability Office (GAO) has reported slow progress (see References).
10.9. Summary
The detection of radiation and the measurement of its properties are required in all aspects of the nuclear field. In gas counters, the ionization produced by incoming radiation is collected. Depending on the voltage between electrodes, counters detect all particles or distinguish between types of particles. Neutrons are detected indirectly by the products of nuclear reactions—for slow neutrons by absorption in boron or uranium-235, for fast neutrons by scattering in hydrogen. Scintillation counters release measurable light on bombardment by charged particles or gamma rays. Solid-state detectors generate a signal from the motion of electron-hole pairs created by ionizing radiation. Pulse-height analysis yields energy distributions of particles. Statistical methods are used to estimate the uncertainty in measured counting rates. Advanced specialized detectors are used in high-energy physics research. Detectors of nuclear radiation play a vital role in the United States counterterrorism programs.
10.10. Exercises
- Find the number density of molecules of BF3 in a detector of 2.54 cm diameter to be sure that 90% of the thermal neutrons incident along a diameter are caught (σa for natural boron is 760 barns).
- How does this compare with the number density for the gas at atmospheric pressure, with density 3.0 × 10−3 g/cm3?
- Suggest ways to achieve the high efficiency desired.
- An incident particle ionizes helium to produce an electron and an He++ ion halfway between two parallel plates with potential difference between them. If the gas pressure is very low, estimate the ratio of the times elapsed until the charges are collected. Discuss the effect of collisions on the collection time.
- We collect a sample of gas suspected of containing a small amount of radioiodine, half-life 8 days. If we observe in a period of 1 day a total count of 50,000 in a counter that detects all radiation emitted, how many atoms were initially present?
- In a gas counter, the potential difference at any point r between a central wire of radius r1 and a concentric wall of radius r2 is given by
 where V0 is the voltage across the tube. If r1 = 1 mm and r2 = 1 cm, what fraction of the potential difference exists within a millimeter of the wire?
where V0 is the voltage across the tube. If r1 = 1 mm and r2 = 1 cm, what fraction of the potential difference exists within a millimeter of the wire?
- How many electrodes would be required in a photomultiplier tube to achieve a multiplication of 1 million if one electron releases four electrons?
- The probability of x successful events in n trials, each of which has a chance p, is given by the binomial distribution formula,

- Apply to flipping a coin l, 2, and 3 times, finding the number of times the result is heads, including zero. Check by simple logic.
- Apply to throwing a single die 1 or 2 times, finding the number of sixes. Check.
- Repeat the preceding calculations with program STAT (see Computer Exercise 10.A).
- For a situation in which the chance of success p is much smaller than 1, the probability of x successes in n trials in the binomial formula of Exercise 10.6 is well approximated by the Poisson formula
 where
where  is the mean value of x. What is the value of p in throwing a single die? Find x for 1 or 2 throws of a die and calculate P(x) for each case.
is the mean value of x. What is the value of p in throwing a single die? Find x for 1 or 2 throws of a die and calculate P(x) for each case.
- Counts are taken for a minute from a microcurie source of cesium-137, half-life 30.2 years. (a) Assuming one count for each 50 disintegrations, find the expected counting rate and the number of counts for the interval. (b) Find the standard deviation in the counting rate. (c) Find the probability of decay of a given atom of cesium in the 1-minute interval.
- A pair of dice is thrown n = 10 times. (a) Verify that the chance on one throw of getting a 7 is p = 1/6. (b) By use of the binomial distribution, find out the chance of getting a 7 exactly x = 2 times out of the 10. (c) Repeat with the Poisson distribution.
- The cross section for absorption for low-energy neutrons of nuclides such as boron-10 varies as l/υ, as discussed in Section 4.5. Formally, we may write
 where σa0 is the cross section at υ0 = 2200 m/s. A boron neutron detector is placed in a neutron speed distribution n(υ), with n0 as the total number of neutrons per cm3 and N as the number of boron nuclei per cm3. Form the total reaction rate per cm3 by integrating over the distribution, as a generalization of the equation in Section 4.3. Discuss the result in terms of what is being measured by the detector.
where σa0 is the cross section at υ0 = 2200 m/s. A boron neutron detector is placed in a neutron speed distribution n(υ), with n0 as the total number of neutrons per cm3 and N as the number of boron nuclei per cm3. Form the total reaction rate per cm3 by integrating over the distribution, as a generalization of the equation in Section 4.3. Discuss the result in terms of what is being measured by the detector.
Computer Exercises
- Program STAT calculates the probability distribution P(x) with a choice of the Binomial, Poisson, or Gauss formulas. (a) What is the value of p for throwing a “six” with a single die? (b) Run the program with n = 1, 2, 5, and 10 and note the probabilities of finding 0, 1, 2, … sixes. (c) Assuming that Binomial is exact, comment on the apparent accuracy of the other two methods.
- An alpha particle detector for surface contamination is counted for 30 1-minute intervals, with a total of 225 counts. What is the value of p? With the Binomial and Poisson distributions in the computer program STAT, calculate P(x) for x = 0, 1, 2 …, 30. How accurate is the Poisson formula?
- (a) What is the chance that any given person's birthday is today? (b) If we select 1000 people at random, with the Poisson distribution in program STAT, what is the probability that none has a birthday today? (c) Calculate P(x) for x = 0 to 10 and plot a bar graph of the results. What is the most likely number that has a birthday today and what is the mean value? (d) Run STAT in Binomial mode for 20 people at a party and show that the chance of two people having the same birthday is almost one-half.
- Computer program EXPOIS calculates “experimental” particle counting data that can be analyzed by Poisson statistics. It uses random numbers with the command RND(N), where N is an assigned set of numbers. Run the program for a typical time from 10 to 30 minutes and compare the results graphically with Poisson data produced by the program STAT (Computer Exercise 10.A).
10.11 References
Bevington and Robinson, 1992 Philip R. Bevington, D. Keith Robinson, Data Reduction and Error Analysis for the Physical Sciences 2nd Ed. 1992 McGraw-Hill New York An update of a classic text on statistical methods, illustrated with examples from technology. Includes computer diskette
Ku, 1969 Harry H. Ku, Precision Measurement and Calibration—Statistical Concepts and Procedures Volume 1 1969 United States Government Printing Office Washington, DC National Bureau of Standards Publication 300
Eichholz and Poston, 1979 Geoffrey G. Eichholz, John W. Poston, Principles of Nuclear Radiation Detection 1979 Ann Arbor Science Ann Arbor, MI A laboratory manual is also available
Knoll, 1989 Glenn F Knoll, Radiation Detection and Measurement 2nd Ed. 1989 John Wiley & Sons New York A very comprehensive, modern, and readable text that should be in every nuclear engineer's library
Evans, 1982 Robley D Evans, The Atomic Nucleus 1982 Krieger New York Excellent treatment of statistical distributions. Reprint of 1955 classic book
Tsoulfanidis, 1995 Nicholas Tsoulfanidis, Measurement and Detection of Radiation 2nd Ed. 1995 Taylor & Francis Washington, DC
Kleinknecht, 1999 Konrad Kleinknecht, Detectors for Particle Radiation 2nd Ed. 1999 Cambridge University Press Cambridge, U.K
Fleischer, 1998 Robert L Fleischer, Tracks to Innovation: Nuclear Tracks in Science and Technology 1998 Springer-Verlag New York
Wackerly et al., 2001 Dennis D. Wackerly, William Mendenhall III, Richard L. Schaeffer, Mathematical Statistics with Applications 6th Ed. 2001 Cengage Learning Stanford, CT
L'Annunziata, 2003 Michael F. L'Annunziata, Handbook of Radioactivity Analysis 2nd Ed. 2003 Academic Press San Diego
The Smyth Report online The Smyth Report online
http://nuclearweaponarchive.org/Smyth http://nuclearweaponarchive.org/Smyth
Appendix 1 on detectors Appendix 1 on detectors.
Caltech Senior Physics Laboratory Caltech Senior Physics Laboratory
http://www.pma.caltech.edu/~ph77 http://www.pma.caltech.edu/~ph77
Nuclear measurements that prepare students for research Nuclear measurements that prepare students for research. Select for example Gamma-and X-Ray Spectroscopy.
Commercial suppliers of nuclear radiation detectors Commercial suppliers of nuclear radiation detectors
http://www.orau.gov/DDSC/instrument/instsuppliers.htm http://www.orau.gov/DDSC/instrument/instsuppliers.htm
Companies providing survey instrumentation Companies providing survey instrumentation.
Binomial and Poisson Statistics Functions Binomial and Poisson Statistics Functions
http://www.ciphersbyritter.com/JAVASCRP/BINOMPOI.HTM http://www.ciphersbyritter.com/JAVASCRP/BINOMPOI.HTM
Interactive computation. By Terry Ritter Interactive computation. By Terry Ritter.
http://integrals.wolfram.com http://integrals.wolfram.com
Interactive integration of math functions Interactive integration of math functions. From Mathematica.
Combating Nuclear Smuggling (Report of GAO) Combating Nuclear Smuggling (Report of GAO)
http://www.gao.gov/new.items/d061015.pdf http://www.gao.gov/new.items/d061015.pdf
Challenge of providing protection Challenge of providing protection.
Kiess, 2006 Thomas E. Kiess, Results and Characteristics of the Homeland Security Office of Research and Development Radiological and Nuclear Countermeasures Program Transactions of American Nuclear Society 95 20069-
IEEE 2006, October 2006 IEEE 2006 Nuclear Science Symposium October 2006
Radiation threat Radiation threat
http://www.ready.gov/america/beinformed/radiation.html http://www.ready.gov/america/beinformed/radiation.html
Homeland Security advisory and information source Homeland Security advisory and information source
![Chapter 10. Radiation Detectors[†]](https://imgdetail.ebookreading.net/cover/cover/hardware/EB9780123705471.jpg)