This chapter describes the properties of fluids. Anything that flows can be considered a fluid. Even gases, such as air, can be treated as fluids. The principles developed in this chapter and the next will help you understand cooling electronics, working with hydraulics, aeronautics, buoyancy, pumps, compressors, and heating and ventilation systems (Figure 8.1).

FIGURE 8.1 Microfluidics is a technology of precisely controlling fluids for applications such as the “lab on a chip”, where medical diagnostic tests can be performed in the field.
8.1 PROPERTIES OF FLUIDS
A fluid is a material without a fixed shape. Place it in a container, however, and it will take that shape. If you squeeze some fluid out of an eyedropper, it takes a spherical shape. A droplet takes this shape because of cohesion, which is the attractive force between like molecules (Figure 8.2).
Surface tension in a fluid is responsible for supporting small objects, like a water bug, on the surface of water (Figure 8.3).
Surface tension results from cohesion. It reduces water’s ability to flow into small places, such as fabrics. Surface tension goes down when temperature goes up. Detergents also reduce the surface tension of water. Washing your clothes in warm water with detergent reduces this tension, facilitating the water’s ability to carry away the dirt.
Adhesion is the attractive force between unlike molecules. Glue and tape are considered as adhesives. Whether a fluid beads up on a surface or disperses depends on the relative strength of the cohesion between the molecules of the fluid compared with the adhesion between the fluid and the surface (Figures 8.4 and 8.5).

FIGURE 8.2 Cohesion pulls the fluid into a spherical shape.

FIGURE 8.3 Surface tension in a fluid supports small objects.
8.2 CAPILLARY ACTION
Capillary action is the action of fluids rising up through narrow tubes and is the reason why some paper towels are so absorbent. Capillary action arises from the adhesion between the fluid and the wall of the tube. Fluid will rise upward against the force of gravity until a balance is struck between adhesion, cohesion, and gravity (Figure 8.6).

FIGURE 8.4 Oil does not mix with water because the cohesion of the oil molecules is greater than the adhesion between the oil and water as illustrated with this spill from an oil tanker.
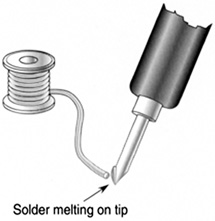
FIGURE 8.5 “Wetting” a soldering iron tip. A hot iron reduces the cohesion of the molten solder until it flows over the tip.
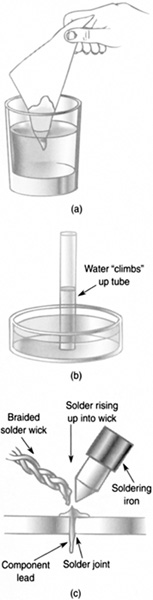
FIGURE 8.6 Capillary action.
8.3 VISCOSITY
The viscosity of a fluid is a measure of how easily it flows under its own weight (Figure 8.7).
The viscosity of a fluid can be reduced with increasing temperature. Motor oils have a viscosity rating as shown in the SAE (Society of Automotive Engineers) number. Oil with a low SAE rating of flows more easily than that with higher SAE rating. In cold weather, oils with low SAE numbers are used, otherwise the engine would not turn over. In warm weather, high-viscosity oils are used to prevent the oil from becoming too thin and leaking from the seals in the engine.
8.4 SPECIFIC GRAVITY
The specific gravity of a substance is its density divided by the density of water.
A substance will sink in water if its specific gravity is greater than that of water.
A substance will rise in water if its specific gravity is less than that of water.
A substance will remain still in water if its specific gravity is equal to that of water.
Specific gravity has no unit; it is just a number. The density of water is 1 g/cm3. The density of lead is 11.4 g/cm3. Therefore, the specific gravity of lead is 11.4. This is much higher than that of water, and therefore lead will sink in water.

FIGURE 8.7 Syrup is a very viscous fluid compared with water.
8.5 DETERMINING CONCENTRATIONS OF SOLUTIONS
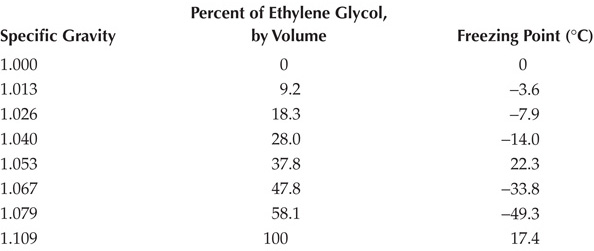
How can you tell if you have enough antifreeze in your radiator? If the concentration is not high enough, the water may freeze! We can use the specific gravity of substances to determine it.
8.6 HYDROMETER
A hydrometer measures the specific gravity of a substance (Figure 8.8). The hydrometer contains a vial sealed with air and a weight, and the combination has the same density as water. As the solution is drawn into the hydrometer, the vial will float to some level where the buoyancy force balances out the weight of the vial. This level is dependent on the density of the fluid. The specific gravity is determined from a scale on the side of the hydrometer.
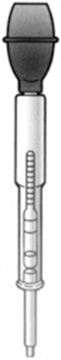
FIGURE 8.8 A hydrometer.
TABLE 8.1
Freezing Points for Various Concentrations of Antifreeze
A car battery contains a solution of sulfuric acid and water. If the battery is fully charged, the solution is about 38% sulfuric acid. When the battery is dead, the solution is about 26% sulfuric acid. The specific gravity changes from 1.28 fully charged to about 1.18 discharged. By measuring the specific gravity of the battery’s solution, the condition of the battery can be determined.
8.7 BUOYANCY
What makes a helium balloon float in the air or a piece of wood float in the water (Figure 8.9)?
Consider a bottle floating in water. The part of the bottle under water is displacing some volume of water. Before the bottle displaced the water, that volume of water was supported by a force equal to its weight. Otherwise, it would have sunk. This fact leads to Archimedes’ principle (Figure 8.10).
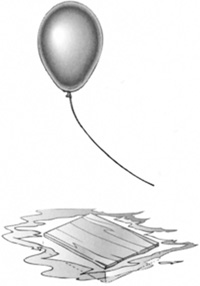
FIGURE 8.9 Archimedes’ principle describes why objects float in air and water.
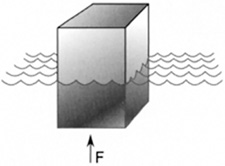
FIGURE 8.10 A buoyant force F on a partially submerged object.
8.8 ARCHIMEDES’ PRINCIPLE
The Archimedes’ principle states that the upward force on a body is equal to the weight of the volume of fluid displaced by the object.
The upward force is called buoyant force.
In the English system of units, density is often given in terms of lb/ft3, in this case use:
If you lift someone in a swimming pool, they feel much lighter because of Archimedes’ principle. (Figure 8.11)
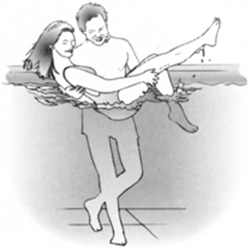
FIGURE 8.11 A person feels lighter in water because of the buoyant force.
Example 8.1
Suppose a 180 lb person is partially submerged in a swimming pool. Suppose the volume of the submerged part equals 1.5 ft3. What is the buoyant force, and how much does the person now weigh? (Figure 8.12)
Weight = 180 lb
V = 1.5 ft3
ρwater = 62.4 lb/ft3
The person now weighs,
No wonder we can pick up someone much bigger than us in a swimming pool!
8.9 PRESSURE
Pressure is a measure of how much force is applied on a given area. Consider placing a 5 lb weight on your arm. That might not feel like much, but what if you apply 5 lb on top of a needle onto your arm. Ouch! That same force, applied on a small area, creates a lot of pressure. Mathematically, pressure is determined by the following equation:
The unit Pa is pronounced as pascal. 1 Pa = 1 N/m2.
In the English system of units, pressure is often given in terms of psi or lb/in.2.
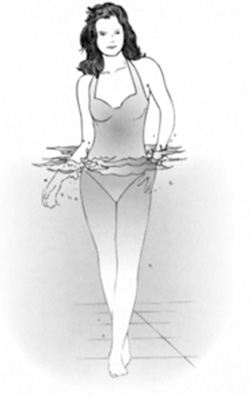
FIGURE 8.12 What is the buoyant force, and how much does the person now weigh?
Example 8.2
A 30.0 lb weight pushes down on a fluid through a piston of area 2.0 in.2. What pressure is the piston applying on the fluid? (Figure 8.13)
One of the differences between a fluid and a solid is that you cannot localize a pressure on part of a fluid (Figure 8.14). The liquid squishes out of the way. Pressure applied on a volume of fluid at the same depth has the same pressure throughout (Figure 8.15).

FIGURE 8.13 What pressure is the piston applying to the fluid?

FIGURE 8.14 You cannot localize a pressure on part of a fluid.

FIGURE 8.15 Pressure applied on a volume of fluid at the same depth has the same pressure throughout.
8.10 MEASURING PRESSURE
When you measure your tire pressure, what is it exactly that the pressure gauge is measuring?
Inside a tire there is a lot of air, which is confined to a small place. The air is composed of molecules moving around, with some striking the inside of the inner tube. The tube bulges out because it is being struck by the air molecules from the inside, forcing it outward (Figure 8.16). If you step on the tube, the rest of the tube bulges out more because the same amount of air is confined to a smaller place, resulting in more molecules pushing or applying force on a smaller area. A pressure gauge measures how much force is applied on a given area (Figure 8.17).
An electronic pressure gauge typically uses a strain gauge, a device that, when strained or made to change shape, will change its electrical properties (Figure 8.18). In a pressure gauge, the strain gauge is mounted in some type of chamber that can be attached to the liquid or gas volume being measured. As the liquid or gas molecules strike the gauge, they deliver a force to the resistive pattern the sensor is made of. This will cause it to deform, resulting in a change in resistance.
A differential pressure gauge measures the difference in pressure between a fixed pressure and the atmosphere. Most pressure gauges measure relative to the atmospheric pressure. These relative pressures are called gauge pressures and are designated as psig, which stands for pounds per square inch relative. psia means pounds per square inch absolute. This is a scale relative to a perfect vacuum. See Figure 8.19.

FIGURE 8.16 Air moving around inside a tire pushes the walls of the tire outward.

FIGURE 8.17 A typical tire gauge consists of a piston contained in a cylinder constrained by a spring. The piston is connected to a calibrated rod from which the pressure is read.

FIGURE 8.18 A strain gauge used to measure pressure.
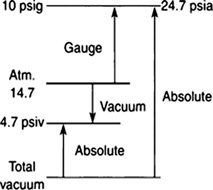
FIGURE 8.19 Chart comparing psia and psig.
If a tire has a pressure of 32 psi, this refers to psig or gauge pressure.
To convert to absolute pressure, you must add the atmospheric pressure to this number.
At the bottom of a swimming pool, the pressure is higher than near the top. This is because at the bottom there is a lot of water above you that is pushing down on you. The atmosphere itself exerts a pressure on us. There are miles of atmosphere above us, weighing us down (Figure 8.20). Air is very light, but enough of it can weigh a substantial amount.
8.11 BAROMETER
A barometer measures the pressure of the atmosphere. In a simple barometer, the height of the liquid depends on the weight of the liquid versus the force on the liquid from the atmosphere (Figure 8.21). An electronic barometer is shown in Figure 8.22.
8.12 PRESSURE VERSUS DEPTH
Pressure in a fluid increases with depth. The pressure at the bottom of a swimming pool is higher than near the surface. The pressure does not depend on the shape of the container, but only on the depth and weight density and is determined by:
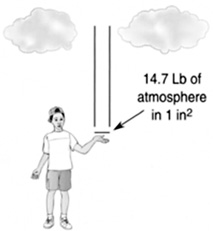
FIGURE 8.20 You are essentially supporting the atmosphere above you. If you hold out your hand, 1 in.2 on your palm is supporting 14.7 lb of air above you.

FIGURE 8.21 A simple barometer.
FIGURE 8.22 An electronic barometer, courtesy of adafruit.com.
In the English system of units, density is often given in terms of lb/ft3, in this case use:
This equation says that the pressure in a fluid increases with the depth below the surface.
Example 8.3
A swimming pool is 6 ft deep. What is the pressure at the bottom in psia and psig?
The pressure at the bottom of a pool is a combination of the pressure due to the water plus atmospheric pressure (Figure 8.23).
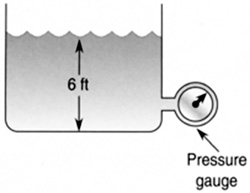
FIGURE 8.23 The pressure at the bottom of a pool is a combination of the pressure due to the water plus atmospheric pressure.
Dams are built with a much wider base than a top because of this increasing water pressure with depth (see Figure 8.24). A device to measure depth (Figure 8.25).
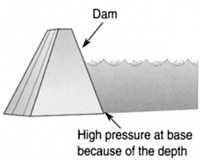
FIGURE 8.24 Pressure at the bottom of a dam is much greater than at the top. Therefore, the dam is built much wider near the bottom to support the extra pressure.

FIGURE 8.25 An eTape Liquid Level Sensor has a resistive sensor that varies with the level of the fluid, similar to a resistive strain gauge. The pressure of the fluid in which it is immersed causes a change in resistance related to the distance from the top of the sensor to the surface of the fluid, courtesy of adafruit.com.
8.13 HYDRAULICS
Hydraulics is about transferring pressure from one region to another to obtain some mechanical advantage. A hydraulic jack can generate a force much greater than an applied force on the jack handle (Figure 8.26). Inside the jack there are two chambers with pistons. The jack handle is connected to a small piston in the small chamber that pressurizes the fluid. When the jack handle is pushed down with some force, a pressure in the fluid builds up and is transferred to the big piston, creating a much greater force upward than the applied force. One-way valves prevent the fluid from moving in the wrong direction.
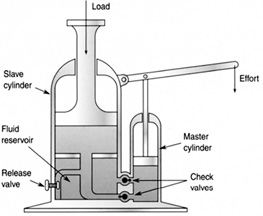
FIGURE 8.26 A hydraulic jack.
In a hydraulic jack, the pressures inside the chambers are equal. The pressure is generated by the small piston and transferred through the fluid to the big piston. Because the pressures are equal:
Therefore,
This equation says that the force going in can be increased if the area of the out-piston is bigger than that of the in-piston.
A hydraulic jack is a machine and therefore obeys the law of machines:
It therefore follows that
This equation says that the small piston will travel through a greater distance than the big piston if the output force is larger than the input force. In other words, you have to pump the jack handle up and down many times to lift a car just a little.
Example 8.4
A hydraulic press contains a small and a large piston of areas 3 and 30 in.2, respectively. The handle of the jack to the small piston delivers 50 lb of force. How much force is generated? (Figure 8.27)
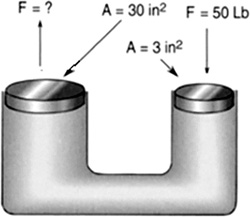
FIGURE 8.27 How much force is generated?
8.14 BRAKING SYSTEMS
Automotive brakes work on a hydraulic system (Figure 8.28). They consist of a master cylinder, which is a reservoir of fluid, and a brake cylinder at each wheel. The master cylinder contains two pistons, one for the front and one for the back brakes. When the brake pedal is pressed, the pressure generated by the master cylinder is transmitted to each wheel cylinder, causing the brake shoes or the calipers to apply a mechanical pressure on the drums or calipers to slow the vehicle down.

FIGURE 8.28 A hydraulic brake system.
Hydraulic systems are also used in heavy equipment. For this use, a pump generates the pressure in the master cylinder.
8.15 PNEUMATICS
Pneumatics is the fluid mechanics of air. Air is considered a low-viscosity fluid. One application of pneumatics is the air hammer, which consists of a heavy piston that oscillates up and down with air pressure controlled by a rocker valve (Figure 8.29).

FIGURE 8.29 An air hammer.
8.16 CHAPTER SUMMARY
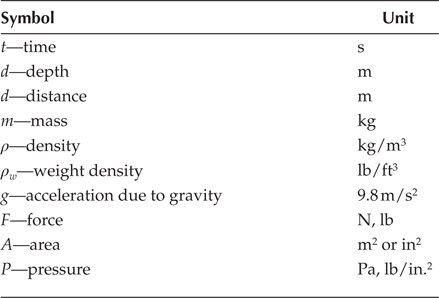
Symbols used in the chapter:
Cohesion is the attractive force between like molecules.
Adhesion is the attractive force between unlike molecules.
Capillary action is the behavior of fluids as they rise up through narrow tubes and the reason why some paper towels are so absorbent.
Viscosity of a fluid is a measure of how easily it flows under its own weight.
The specific gravity of a substance is its density divided by the density of water.
Archimedes’ Principle
The Archimedes’ principle states that the upward force on a body is equal to the weight of the volume of fluid displaced by the object.
The upward force is called buoyant force.
In the English system of units, density is often given in terms of lb/ft3, in this case use:
Pressure
Pressure is a measure of how much force is applied on a given area.
The unit Pa is pronounced as pascal. 1 Pa = 1 N/m2.
In the English system of units, pressure is often given in terms of psi or lb/in.2.
To convert to absolute pressure, you must add the atmospheric pressure to this number.
Pressure in a fluid increases with depth and is determined by:
In the English system of units, density is often given in terms of lb/ft3, in this case use:
For a hydraulic jack:
PROBLEM SOLVING TIPS
■ Be careful to monitor your units, making sure you are in the same system of units throughout each problem.
PROBLEMS
What is the difference between cohesion and adhesion?
Explain capillary action.
Why are some motor oils multi grades?
If pressure is responsible for moving fluids, can you explain why a low barometric pressure reading indicates that a storm is approaching?
What is the difference between psig and psia?
What is the pressure relative to the atmosphere at the bottom of a swimming pool 6 ft deep?
If a ship displaces 25,000 ft3 of water, what is the buoyant force?
Given an air bag and an air compressor, how would you raise a sunken ship?
Design a hydraulic jack that is able to lift 1,500 lb with 50 lb of force. What does the ratio of the pistons have to be?
What is the purpose of the oil in a hydraulic jack?
Why does a hot air balloon float?
If a rectangular piece of wood measuring 2.5ʺ deep × 3.5 ʺ wide × 6.0ʺ long is thrown into water, how much of the wood will be submerged?
If the specific gravity of your radiator fluid is 1.053, what temperature will it freeze at?
How does a solder wick remove solder?
How does a detergent aid in cleaning your clothes?
Suppose a pressure gauge uses a strain gauge that changes resistance with pressure as 125Ω/psi, what is the pressure change if the resistance of the gauge changes by 290 Ω?
If a flow sensor monitors the flow through a pipe and the viscosity of the fluid decreases, what happens to the flow rate?
A pressure sensor outputs 0–25 mV for a change in pressure of 0–60 psig. What is its output when it is measuring 30 psig?
A chamber contains a gas under a pressure of 43 psi. Inside the chamber is a port of area 4.5 in.2. What is the force on the port?
An automobile tire is filled with a pressure of 32 psi. What is the absolute pressure inside the tire?
An air shock consists of a piston that fits into a cylinder containing air. If the piston has an area of 12.2 in.2 and a force of 200 lb is placed onto the piston, what is the pressure inside the cylinder?
A barometer contains a column of mercury 76 cm high. The density of mercury is 13.6 g/cm3. What is the pressure at the bottom of the column?
A sensor measuring the pressure at the bottom of an oil liquid filled tank records a pressure of 122 psi. If the oil has a density of 54.2 lb/ft3, what is the depth of the oil? Be careful of the units!
A man jumps into a swimming pool displacing 1.1 ft3 of water. What is the buoyant force exerted on the man?
A spring scale is measuring the weight of a volume of 2.5 cm3. It is then submerged into a liquid and its weight is re-measured. If the difference in weight in and out of the liquid is 5.0 N, what is the density of the liquid?
A hydraulic jack contains pistons of areas 2.0 and 4.0 in.2. If 25 lb is applied on the little piston, what force does the jack generate?
If the output force is three times as big as the input force on a hydraulic jack, how many times farther does the input piston move relative to the output piston?
Why is the base of a dam wider than the top?
What is the specific gravity of a substance that has a density of 4.6 g/cm3?
If the piston in the master cylinder in a car has an area of 2.1 in.2 and the piston in the wheel cylinder has an area of 4.2 in.2, and 25 lb of force is applied on the master cylinder, what is the force generated at the wheel cylinder?
The metric unit of pressure is the pascal (Pa), 1 Pa = 1 N/m2. If 10.0 N of force is acting upon an area of 2.0 m2, what is the pressure in Pa?
If a barometer is measuring 30.1 in. of mercury, what is the atmospheric pressure in lb/in.2?
What is the cause of surface tension in a fluid?
What is Archimedes’ principle?
Does a tire pressure gauge measure psia or psig?
What is a hydrometer?
A plant uses capillary action to draw water up its stem. In terms of adhesion and cohesion how does it do this?
