Thermodynamics is the physics of heat. Practical applications of thermodynamics include temperature regulation of electronics, heating and air conditioning, automobile engine design, weather prediction, and anywhere else where heat is exchanged.
Why doesn’t there exist a car that gets over a 100 miles/gallon? Have the big oil companies been suppressing the patents for this technology in order to suppress the building of such efficient engines? Let’s look at the physics behind these ideas (Figure 11.1).

FIGURE 11.1 A cooling tower exchanging heat with the environment.
11.1 HEAT ENGINES
A heat engine is any device that borrows heat energy from some reservoir of heat at a high temperature and converts some of it into work plus some wasted heat at a lower temperature (Figure 11.2).
Examples of heat engines are gas, diesel, jet, steam, and rocket engines (Figure 11.3). They all take heat energy from fuel and convert it into some type of work to move a vehicle.
11.1.1 Fuels
Fuels store chemical potential energy. A molecule of gasoline can be thought of as a bunch of atoms separated by compressed springs. The springs are the interatomic forces between atoms. With the addition of heat, the springs will be released from compression, hurling the atoms outward. This is an explosion of the gas molecules, turning the chemical potential into kinetic energy (Figure 11.4). Table 11.1 lists the amount of energies released when these fuels are burned.
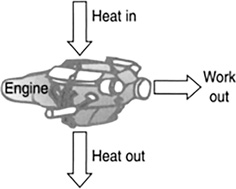
FIGURE 11.2 A heat engine.
TABLE 11.1
Btu of Heat Produced by the Combustion per Amount of Fuela
FIGURE 11.3 Examples of heat engines.
11.1.2 Gas Mileage
The gas mileage of a car is limited by how much energy an engine can extract from a gallon of gas and use it to move the car. We can increase gas mileage by making the car lighter or more aerodynamic or by making a host of other mechanical engine improvements, but there is a limit to how far we can go. A gallon of gas contains about 0.1 million Btu of heat energy. Not all of this energy is used to move the car. The limits are constrained by the laws of thermodynamics.
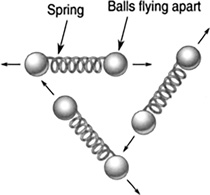
FIGURE 11.4 A chemical reaction releasing heat.
11.1.3 Laws of Thermodynamics
The first law of thermodynamics is about energy conservation (Figure 11.5). You will recall that energy conservation means that the total energy of an isolated system is a constant. Energy is never created or destroyed; it just changes form. Heat is energy. Part of its input into a heat engine goes into performing some useful work, part goes into just heating up the engine, and the rest is just wasted in terms of the exhaust. A more general definition of the first law is:

FIGURE 11.5 The first law of thermodynamics.
First law of thermodynamics: Energy is conserved as it moves through a thermodynamic system.
The first law applied to heat engines implies that the energy gained from a heat source equals the work done plus the heat wasted.
The first law is a statement about the conservation of energy. The energy gained from some fuel source is used to do work by a heat engine plus that wasted in just heating up the environment.
The second law of thermodynamics is related to your experience when making chocolate milk (Figure 11.6). You start off with just milk and chocolate syrup. When you mix the two, you get chocolate milk. Now try to separate the chocolate milk into just chocolate syrup and milk. Impossible, you say! This is the second law at work. The second law says it is easier to create a disordered mixture than to try to bring order back into the mixture. A more general way of saying this is:
Second law of thermodynamics: Systems in the universe normally evolve from order to disorder.

FIGURE 11.6 The second law of thermodynamics. The universe normally evolves from order, milk and chocolate separated, to disorder, milk and chocolate mixed.
When a heat engine burns fuel, it converts the internal energy of the fuel, which, when burned, produces molecules moving about randomly at high speeds into an ordered motion, moving a piston or wheel. All systems in the universe normally evolve from order to disorder. We are asking a heat engine to go against this, to turn randomness into order. The second law implies that it is impossible to build a heat engine that converts all the energy in the fuel into work. Some of the energy will be converted into the orderly motion of a piston, and the rest will go on to just heating the environment, into more disorder. In case of an automobile, most of the energy released from the fuel goes into heating up the cooling water and the exhaust.
11.1.4 Efficiency
The efficiency of an engine is a measure of how much work it can do versus how much heat energy is put in:
The most efficient engine would be one where all of the fuel’s energy is extracted out, performing some useful work. Looking at the first law:
Solving for W gives:
Therefore,
To maximize the efficiency, we want to maximize this difference in the numerator. Heat and temperature are related to each other, and it can be shown that efficiency depends mainly on the difference between the input and exhaust temperatures. The larger the temperature difference, the more efficient the heat engine. The Carnot engine, named after French scientist N.L. Sadi Carnot, who first proposed it, is ideally the most efficient heat engine possible.
The efficiency of a perfect heat engine is given by:
This equation says that if the temperature of the heat source of the engine Tin is high and the wasted heat that is exhausted into an environment at temperature Tout is low, then the efficiency will be high. Fuel burned in an automobile engine raises the temperature inside the combustion chamber to thousands of degrees. The most efficient engine of this type is one where the combustion chamber temperature is very high and the exhaust temperature is very low (Figure 11.7).

FIGURE 11.7 What is the maximum efficiency of this engine?
Example 11.1
When gas is burned in a car engine, it reaches temperatures of 2,200°C and puts out exhaust at 1,200°C. What is the maximum efficiency of this engine?
The temperatures must be converted to kelvin.
From this example, we can see that even a perfect heat engine that has no losses due to practical considerations such as friction will not be 100% efficient. There will still be some heat wasted to the environment (Figure 11.8).
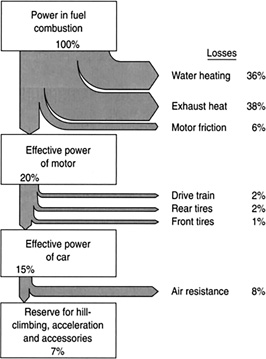
FIGURE 11.8 Most automobile engines have an efficiency of 20%.
Because the efficiency of any type of heat engine is always less than 100%, it follows that no one will ever build a perpetual motion machine because there will always be a need for some additional energy input without which the machine will eventually slow down and stop.
11.1.5 External Combustion Engine
A steam engine is an external combustion engine. External to the engine, fuel is burned to boil the water to generate steam, which drives the engine. An example of a steam engine with a piston is shown in Figure 11.9. An example of a steam turbine is shown in Figure 11.10.
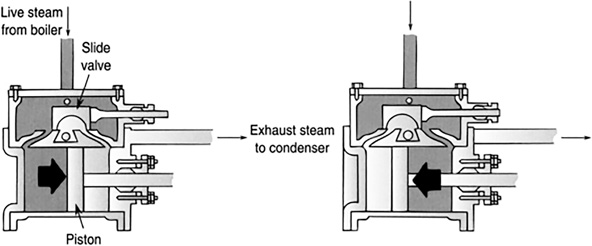
FIGURE 11.9 A steam engine. Valves open and close, alternately pushing the piston back and forth.

FIGURE 11.10 A steam turbine. The fins on a turbine are designed to extract as much thermal energy as possible from the steam and turn it into rotary motion. After exiting the turbine, the steam is cooled and returns to the boiler to become steam again and repeat the process.
11.1.6 Internal Combustion Engine
Fuel is burned inside the internal combustion engine. Four-stroke engines are illustrated in Figures 11.11 and 11.12.
These engines are called four-stroke because of the four-stroke cycle the engine is taken through.
Intake: The piston pulls the gas-air mixture into the cylinder.
Compression: The piston rises, compressing the fuel mixture.
Power: The fuel ignites, driving the piston down.
Exhaust: The piston pushes the burned fuel out.
The cycle then repeats itself, beginning with stroke 1.
The differences between diesel and gasoline engines are as follows:
■ Gasoline engines use spark plugs to ignite the fuel. Diesel engines operate under much higher pressures, which ignite the fuel. Compressing any fuel enough will cause it to ignite.
■ Diesel engines operate under much higher pressures, 20:1 compression ratio, compared with 8:1 for gasoline. They must be made stronger and therefore heavier than gas engines.
■ Diesel engines are more efficient, about 30% efficient, compared with 20% for gas engines.

FIGURE 11.11 Four-stroke gasoline engine.
To obtain maximum efficiency/power:
■ Increasing the volume of the cylinders can increase the power of an engine; this will allow for more fuel/air mixture to burn. Increasing the amount of fuel/air mixture in the cylinder can also be done by pumping the mixture into the cylinder. This is known as supercharging.
■ Increasing the fuel/air pressure in a cylinder by reducing the clearance between the piston and the valves can increase the power. There is a limit, however, based on the strength of the material the engine is made of.
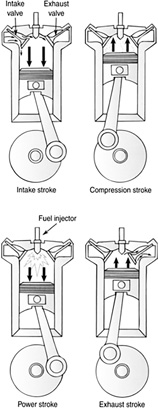
FIGURE 11.12 Four-stroke diesel engine.
11.1.7 The Efficiency of a Human Being
Food is a form of fuel. Its unit of energy is the Calorie. One food Calorie equals 1,000 physics calories (1 C = 1,000 c). One food Calorie equals 4,186 J. The human body is about 25% efficient, meaning that only 25% of the energy we get from food is turned into muscular energy. This is about the same efficiency as that of a gasoline automobile engine. Let’s analyze the energy needed over the period of one day. A man weighing approximately 155 lb will burn 100 Cal/h just to maintain life (breathing, circulating blood, etc.) in normal waking conditions, and 60 Cal/h while sleeping (Figure 11.13).
Examples of Calories burned in a 24 h period:
Cal/h for 16 waking hours equals 1,600 Cal.
Sleeping for 8 h at 60 Cal/h equals 540 Cal.
Walking slowly, at 2.5 mph, burns 220 Cal/h. If we approximate the day’s activities to walking 5 miles, that would total 440 Cal.
Total Calories burned = 2,580 Cal
Therefore, just to maintain this man’s weight, he must consume,
Qin= W + Qout = 2,580 Cal every day just to maintain weight.
Compare 440 Cal burned by walking 5 miles with eating a Big Mac hamburger at 530 Cal. It is clear that if you want to lose weight, reducing your food intake will benefit you a lot more than exercising.
FIGURE 11.13 The human body is about 25% efficient meaning, that only 25% of the energy we get from food is turned into muscular energy.
11.1.8 Artificial Leaf
A leaf harnesses energy from the sun, by taking in CO2 from the atmosphere and sunlight to generate glucose that feed the plant. Plants use only 1% of the energy in the sunlight falling on them to accomplish this. An artificial leaf generates fuel by separating water, H2O, into hydrogen and oxygen. The hydrogen can be used directly as fuel or the hydrogen can be fed to engineered organisms, which then excretes a wide variety of fuels. Artificial leaves are achieving an efficiency of roughly 10%, that is ten times better than a real leaf! (Figure 11.14)

FIGURE 11.14 An artificial leaf consists of a photovoltaic material (the artificial leaf) that creates an electric current when exposed to light that separates the hydrogen from oxygen in water. The collected hydrogen can then be used as fuel.
11.2 HEAT PUMPS
Heat pumps pump heat from a low-temperature region to a high-temperature region (Figure 11.15).
When a gas expands and evaporates, it cools. Anything coming in contact with this gas will cool down as well. This is the principle behind a refrigerator, which is an example of a heat pump (Figure 11.16).

FIGURE 11.15 Heat pumps pump heat from a cold to a hot region.

FIGURE 11.16 A refrigerator is a heat pump that draws heat from a low-temperature region to a higher-temperature region.
11.2.1 Refrigeration Cycle
A liquid refrigerant, such as ammonia or dichlorodifluoromethane, with a very low boiling point (around—27°F) passes through an expansion valve like the atomizer on a spray gun. As this mist expands and evaporates, it cools (Figure 11.17). This cool gas is run through coils in a refrigerator, cooling the inside.
At the other end of this coil is a compressor. The compressor draws in this gas and outputs it at a higher pressure and temperature (Figure 11.18).
This high-pressure, high-temperature gas passes through a set of coils on the back of the refrigerator. Passing through the coils, it emits this heat to the room and therefore lowers its temperature. As the gas cools, it turns back into a liquid.
The cycle repeats itself.
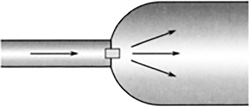
FIGURE 11.17 Expanding gas cools down.
Heat pumps can be used to cool a house, as in the case of an air conditioner (Figure 11.19), or warm a house (see Figure 11.20).
A Peltier device is an electronic refrigerator made from several metal-semiconductor junctions in series with an alternating array of n-type and p-type semiconductors (Figure 11.21). Current flowing through this device results in one side getting hot and the other cold. These devices can be found in electronic coolers.
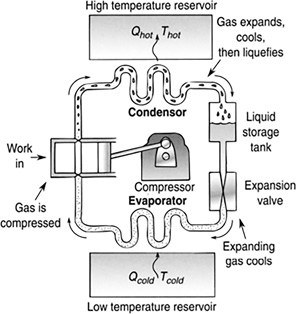
FIGURE 11.18 The gas is compressed, raising its temperature and pressure. The high-pressure, high-temperature gas expands, cools down, and turns back into liquid.
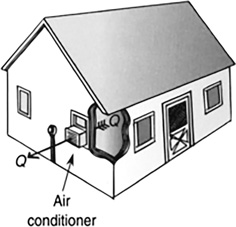
FIGURE 11.19 Air conditioners are basically refrigerators without the insulated box.
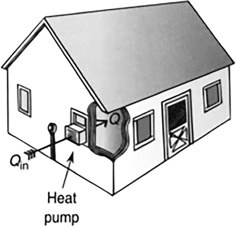
FIGURE 11.20 Heat pumps for heating. A heat pump can absorb heat from the outside during the winter and deliver it to the inside, where it is warmer. It is basically an air conditioner turned around.
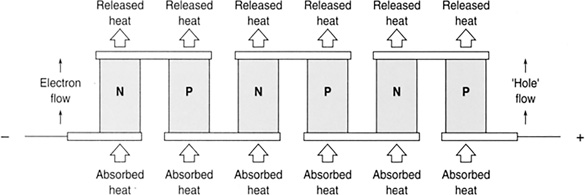
FIGURE 11.21 A Peltier device is an electronic refrigerator made from several metal-semiconductor junctions in series with an alternating array of n-type and p-type semiconductors.
11.3 THERMAL EXPANSION OF GASES, LIQUIDS, AND SOLIDS
Most materials expand when heated. Thermal expansion properties of materials can be exploited to build temperature switches, valves, refrigerators, air conditioners, and a host of other useful devices. Knowledge of how much they expand is important in designing anything that cycles through temperature ranges such as bridges, roads, or even printed circuit boards (Figure 11.22).
FIGURE 11.22 An expansion joint. As the seasons change, the bridge will expand in the heat and shrink in the cold.
11.3.1 Linear Expansion
A change in temperature causes most materials to change length (Figure 11.23). This can be expressed mathematically as:
In the English system:

FIGURE 11.23 Linear thermal expansion.
11.3.2 Area and Volume Expansion
The thermal expansion of an area of a material is twice as big as the linear expansion, and the volume expansion is three times as big (Figure 11.24 and Table 11.2).
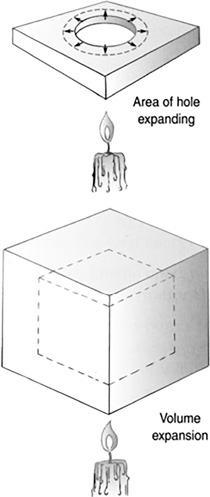
FIGURE 11.24 Area and volume thermal expansion.
TABLE 11.2
Coefficients of Linear Expansion
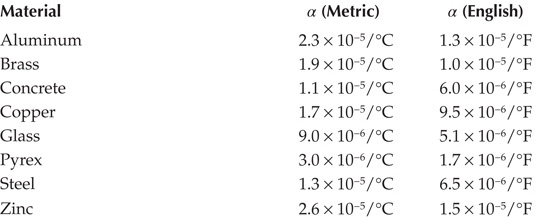
Example 11.2
A steel tape measure is 100 ft at 72°F. The coefficient of linear expansion for steel is 6.5 × 10-6/°F. How much did the tape measure change length at 5°F? (Figure 11.25)
Example 11.3
A brass bushing with an inside diameter of 1.980 in. at 72°F is to be sweat fit onto a shaft of diameter 2.000 in. Sweat fitting is a process of increasing the size of something by heating it. What temperature must the bushing be brought to before it will fit? (Figure 11.26)
FIGURE 11.25 The length will change by as much as half an inch!
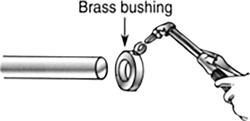
FIGURE 11.26 A thermal switch.

FIGURE 11.27 A thermal switch consists of two dissimilar pieces of metal that have different coefficients of thermal expansion. When heated, the result is a bending of the strip.
11.3.3 Bimetal Strip Thermostat
A bimetal strip thermostat is essentially a heat switch consisting of two types of metal bonded together, with electrical connections on either end. As the temperature changes, the two metals expand at different rates, causing the strip to bend and thereby closing the switch (Figure 11.27). Varying the spring tension that is holding the switch closed can set the operating temperature of the switch.
11.3.4 Nitinol Wire
Nitinol wire is an alloy of nickel and titanium that is made specifically for actuating mechanical arms. It has a very large negative coefficient of expansion, that is, it contracts when heated. When heated, these wires contract as much as 20%. Running enough current through them will cause them to heat and therefore contract (see Figure 11.28). Turning off the current allows the wire to cool and slowly return to its original length. These wires can be used like motors to move arms or levers.
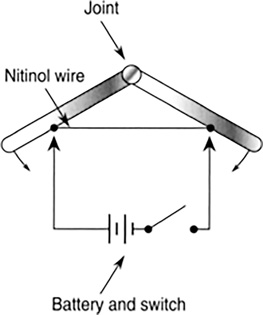
FIGURE 11.28 Nitinol wire contracts when heated, lending itself to actuating arms or levers. Heating takes place when enough current flows through the wire.
11.4 CHAPTER SUMMARY
First law of thermodynamics: Energy is conserved as it moves through a thermodynamic system.
The first law applied to heat engines implies that the energy gained from a heat source equals the work done plus the heat wasted.
Second law of thermodynamics: All systems in the universe normally evolve from order to disorder.
The efficiency of an engine is a measure of how much work it can do compared with how much heat energy is put in.
Ideal efficiency: The efficiency of a perfect heat engine is given by
Linear expansion: A change in temperature causes most materials to change length. This can be expressed mathematically as:
In the English system:
Area and Volume Expansion
Heat pumps: Heat pumps pump heat from a low-temperature region to a high-temperature region.
PROBLEM SOLVING TIPS
■ When calculating ideal efficiency, remember to calculate in degree kelvin.
■ When solving problems dealing with thermal expansion, look at the units of the coefficient of thermal expansion when determining what units the other variables in the equation should be in.
PROBLEMS
If an engine performs 125,000 J of work using a fuel containing 620,000 J of energy, how much energy is wasted? What is the efficiency?
Why can’t a perpetual motion machine ever be invented?
Compare two engines, one with an exhaust temperature of 1,600 ° C and the other with an exhaust temperature of 1,100 ° C. Which engine is more efficient?
Determine your calorie intake for one day and approximate the amount of work you perform. What is your efficiency?
A piece of brass 12.00 in. long is heated and changes temperature by 123 ° C. What is its change in length?
A piece of steel has a hole in it with a diameter of 1.00 in. A pipe is to fit into it with a diameter of 1.05 in. How high must the steel be heated so that the pipe will fit into the hole?
What is the purpose of an expansion joint on a bridge?
How does a thermostat work?
A thermal circuit breaker uses a bimetal strip to open a circuit in the event of an overload. How does this work?
How does an air conditioner work? Provide a diagram.
Give an example of the second law of thermodynamics from everyday life.
Suppose you connect an electric motor to a generator and use the output from the generator to run the motor. Will this work? What do the laws of thermodynamics say about this?
If a heat engine takes in 2,900 J of heat and expels 1,800 J, how much work did it perform? What is its efficiency?
What is the difference between an internal and an external combustion engine?
What does the first law of thermodynamics say?
How could you use an air conditioner to heat your house in the winter?
Gasoline can produce 19,000 Btu of heat/pound of fuel. If an engine burns 2 lb of gasoline and performs 8,500 Btu of work, how much energy was wasted?
What is the efficiency of the engine in the previous problem?
An electric motor consumes 5,000 J of energy producing 4,000 J of work. What is its efficiency?
If the temperature in the combustion chamber of a lawn mower engine reaches 2,000°C and the exhaust temperature is 1,100°C, what is its maximum possible efficiency?
If a human being is 25% efficient and eats a Big Mac hamburger of 540 Cal, how much work can he perform using this burger for fuel?
If we consider a perfect heat engine, a Carnot engine, running at 25% efficiency with an internal temperature at 1,900°C, what would the exhaust temperature be? Remember to convert the temperature into kelvin!
If an aluminum computer case 14 in. long warms up 25° F, what is its change in length?
A thermal switch made from two brass rods is separated by 0.05 cm before heating and close when heated up by 100° C. Assuming both the rods are of the same length and both are heated uniformly, what was their original length before heating? Remember both are expanding!
A square steel plate measuring 25 cm on a side is heated up by 500° C. What is its change in area?
A piece of Nitinol wire contracts when heated; it has a negative coefficient of expansion. If the contraction is 0.012 in./° F, how many inches will a 12 in. piece of wire contract if heated up by 10° F?
From Table 11.1, find out which fuel has the highest heat of combustion.
Where is most of the energy wasted in an automobile?
Cogeneration of electrical energy is the generation of electrical energy by burning fuel in a generator and using the wasted heat to warm a living space. If the generator is 25% efficient and burns 2,500 Btu of energy, how much energy is available for heat?
Your mom yells at you for having a messy room. How would you use the second law of thermodynamics in your defense to explain the condition of your room?
If an automobile loses a lot of its energy in heating up the water in the radiator, why don’t they eliminate the radiator?
Name two ways to increase the power in an internal combustion engine.
A combination of diet and exercise is the best way to lose weight. If you could lower the efficiency of a human being, would this help in their weight loss?
A Peltier device is an electronic refrigerator. If it generates 40 W of cooling power and is 30% efficient, how much electrical power does it consume?
A 100 ft long house has aluminum siding put on it one winter. The summer comes and the house warms up 70° F from the winter. Do you have to worry about the siding falling off the house? Do a calculation.
From Table 11.2, find out which material has the lowest coefficient of expansion.
