There are four forces in nature: gravity, two types of nuclear forces, the strong and weak nuclear forces, and electricity and magnetism. Electricity and magnetism may seem like two distinct forces; however, they are intimately related. The electric force is what holds electrons in their orbits around the nucleus. In this chapter, we will look at where the electrical force comes from and some applications of it in technology.
12.1 CHARGE
Consider an atom. At its center there are particles called neutrons and protons, and surrounding it are particles called electrons (Figure 12.1).
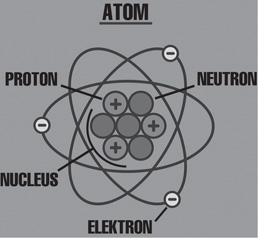
FIGURE 12.1 The atom consists of protons, electrons, and neutrons.
The protons and electrons have a property known as charge. There are two types of charges, positive and negative. The charge on an electron is negative and that of the proton is positive.
The unit of charge is the coulomb.
One coulomb of charge is equivalent to 6.25 × 1018 electrons bunched together.
One electron, e−, therefore has a charge of 1/(6.25 × 1018) = − 1.6 × 10−19 coulombs.
One proton has a charge of + 1.6 × 10−19 coulombs.
Particles that have different charges are attracted to each other, and particles with like charges are repelled by each other. This is similar to the way people behave, “opposites attract, likes repel.” The charge of a particle is therefore the origin of the electrical force. If we place two charges near each other at rest and let go, they will begin to move. This change in motion is called an acceleration. According to Newton, if something is accelerating there must be a force on it. It’s been determined by experiments that force between two charges depends on the amount of charge on each object and is inversely related to the distance squared between the objects (Figure 12.2).
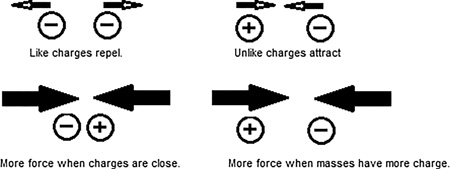
FIGURE 12.2 Like charges repel each other and unlike charges attract each other. The closer the charges are to each other, the stronger the force between them. The larger the charge on a mass, the larger the force.
Coulomb’s law expresses the force between charges:
The constant and is also equal to where ε0 is called the permittivity, and is a constant equal to . After plugging in for ε0 it follows, .
Example 12.1
Two masses are charged and placed 0.1 m apart. For mass 1 assume q1 = m1 C and m1 = 1 kg, and mass 2 has q2 = −m2 C and m2 = 2 kg. Find the force on each mass and their accelerations (Figure 12.3).
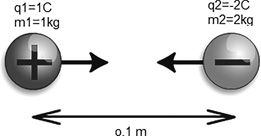
FIGURE 12.3 Find the force on each mass and their acceleration.
Finding the force on each mass:
Finding the magnitude of acceleration of each mass we use F = m ⋅ a.
12.2 THE ELECTRIC FIELD
To explain how charges can influence each other at a distance, the concept of the electric field was invented. An electric field is something that surrounds the space around the charge in which another charge reacts to. This is similar to the gravitational field, where one body can influence the other at a distance (Figure 12.4).

FIGURE 12.4 Electric field lines of positive and negative charges and between charges.
The electric field E has a magnitude and direction and is therefore vector. The force on a charge will be in the same direction as the electric field if the charge is positive and in the opposite direction if the charge in negative (Figure 12.5).
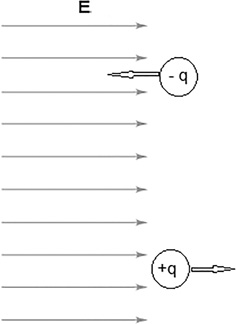
FIGURE 12.5 The force on a charge in an electric field E.
The strength of an electric field is measured in Newton/Coulomb. The force on a charge q due to the electric field E is given by:
Example 12.2
An electron is placed in an electric field of strength 2 N/C pointing in the positive x direction. What is the force on it? (Figure 12.6)

FIGURE 12.6 What is the force on the electron?
12.3 THE ELECTRIC FIELD OF A POINT CHARGE
We can figure out the electric field of a point charge, such as an electron, by way of comparison.
Let’s compare the electric force law to Coulomb’s law.
Equating the two, we see that the electric field due to a point charge is:
Example 12.3
What is the strength of the electric field 0.1 m away from a 1 mC charge? (Figure 12.7)
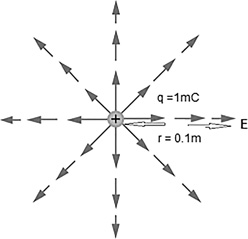
FIGURE 12.7 What is the strength of the electric field 0.1 m away from a 1 mC charge?
12.4 WORK AND THE ELECTRIC FIELD
We learned in a previous chapter that the change in energy in a body is equal to the work done on it. (when the force is in the same direction as motion).
If we substitute the electrical force into W we get:
Example 12.4
A proton moves a distance of 0.01 m in the positive x direction through an electric field of strength 0.1 N/C pointing in the positive x direction. What is the work done on this charge? (Figure 12.8)

FIGURE 12.8 What is the work done on this charge?
12.5 GENERATING AN ELECTRIC FIELD
A uniform electric field can be generated between two metal parallel plates by placing an equal amount of charge on each plate but of opposite signs. So, from where do we get the charge to place on the plates? We can get this charge from a battery. A simple battery consists of two halves, one half is full of negative charges and the other has a deficit of negative charges (Figure 12.9).

FIGURE 12.9 A simple battery consists of two halves, one half is full of negative charge and the other has a deficit of negative charge, courtesy of sparkfun.com.
Consider two parallel plates connected to a battery. The electrons stored in the negative side of the battery are repelled by each other, so if a wire is connected to the negative terminal of a battery, the electrons will “crawl” up the wire to get away from the other electrons in the battery. These electrons will begin to fill the negative plate with charge. Meanwhile, on the other plate the “free” electrons on it will start to leave this plate to get away from the negative charge they “feel” on the negative plate. This exodus of negative charge has only one place to go, into the positive side of the battery. This will result in this plate to be effectively charged positive, that is, if we take away negative charge from a charge neutral region, that region is effectively charged positive (Figure 12.10).

FIGURE 12.10 A uniform electric field can be generated between two metal parallel plates by placing an equal amount of charge on each plate but of opposite signs by using a battery.
Example 12.5
Suppose we place a negative charge next to the positive plate, and move it toward the negative plate. Calculate the work done on an electron by pushing it a distance d through an electric field to the negative plate, and find its increase in potential energy (Figure 12.11).
Assume:
E = 12,000 N/C pointing in the positive x direction and x = 0 at the +Q plate.
d = 0.001 m
q = −1.6 × 10−19 C
Recall from the chapter on energy that the change in the change in Potential Energy = − Work performed, therefore:

FIGURE 12.11 Calculate the work done and the increase in potential energy of the charge.
12.5.1 Electrical Potential Energy and Voltage
The electric field is related to something we are more familiar with, that is, voltage. Voltage is effectively the “electrical pressure” that drives the charges to move in a circuit. A battery at a certain voltage, connected to a circuit, results in charges moving through the circuit, or current. The charges, electrons, stored in the battery are repelled by each other through the electric field generated by each charge. A battery connected up to a circuit, therefore, sets up an electric field in the wires of the circuit, which in turn delivers a force on the electrons to push them through the wires. The battery therefore acts as a source of charge and a type of “electrical pressure” to drive the charge through the circuit. A battery is a store of electrical energy. The electrical potential energy stored in a battery depends on how much charge is stored in it as well as what the voltage of the battery is.
Example 12.6
A fully charged AA battery has a voltage of 1.5 V and 104 coulombs of charge in it. How much electrical potential energy is stored in it? (Figure 12.12)

FIGURE 12.12 AA battery contains ~ 104 J of stored energy.
Compare this to the energy stored in one cup of gasoline at about 8 × 106 J.
12.6 Electric Field and Voltage
Let’s see how the electric field and the voltage are related.
Plugging in for W and U gives:
Solving for E gives:
The electric field can have units of N/C or V/m.
Example 12.7
Consider the previous example where two parallel plates had an electric field E = 12,000 N/C pointing in the positive x direction and x = 0 at the +Q plate. Find the voltage difference between the plates (Figure 12.13).

FIGURE 12.13 Find the voltage difference between the plates.
Assume:
d = 0.001m
E = 12,000 N/C or we can write E = 12,000 V/m
12.6.1 Armed and Dangerous, Building an Electron Gun
An electron gun can be built by accelerating electrons from one plate of a capacitor to another plate with a hole in it, Figure 12.14. The plates have a battery connected to them to create an electric field between them that in turn accelerates the electrons. The source of the electrons to be accelerated will come from a heated wire, the filament. The filament will heat up by running electricity through it from a battery. Once the filament becomes hot enough it will begin to “boil” off electrons. These electrons will pass through the negative plate, which is made from a metal screen. The electrons that make it through the screen will enter the region where the electric field exits. These electrons are accelerated toward the positive plate and some will pass through the hole in the plate at a high velocity. Now, let’s do the numbers and calculate this velocity.

FIGURE 12.14 An electron gun.
Example 12.8
Calculate the speed of the electrons exiting an electron gun, given the following:
V = 12 V (voltage between the pates)
d = 0.02 m (distance between the plates)
q = −1.6 × 10−19 C (charge of an electron)
m = 9.11 × 10−31 kg (mass of an electron)
We will assume that the electrons start with an initial velocity of zero to simplify the calculation. We will also assume that the system sits in a vacuum so that the electrons do not “bump” into air molecules. We can use energy conservation to determine the electrons’ exit velocity,
The Large Hadron Collider (LHC) is currently the world’s largest and most powerful particle accelerator. The LHC consists of a 27 km ring of magnets wrapped around two pipes under vacuum that the accelerated particles travel through in opposite directions. Situated throughout the ring are a number of accelerating devices to boost the energy of the particles as they move through this beamline. The acceleration of these particles is done using electric fields that are oscillating at just the right frequency and phase to boost the particles to move at near the velocity of light. It is analogous to pushing someone on a swing; if you push at just the right time they will move higher and higher. When the particles reach a particular energy, the magnets steer the two beams of particles that are moving in opposite directions into each other. The result of these colliding particles is recorded with large detectors surrounding the collision zone. The 2015 Nobel Prize in Physics was awarded based on a particle called the Higgs Boson discovered at the LHC (Figure 12.15).

FIGURE 12.15 The largest particle accelerator in the world called the LHC, Large Hadron Collider, located in Geneva, Switzerland.
12.7 CAPACITANCE
The ability to store charge is very useful and used throughout modern technology. The capacity to store charge is known as capacitance, and a device that is designed to store charge is called a capacitor. Capacitors are used for regenerative breaking in electric cars, memories in computers, microphones, computer touch screens, accelerometer sensors in smartphones, and the list goes on. The schematic symbol for a capacitor in electronics is drawn as a set of parallel plates. Figure 12.16 shows some types of capacitors used in electronics.

FIGURE 12.16 Capacitors used in electronics.
The charge a body can store depends on its capacitance, which depends on its geometry and its voltage.
The unit of capacitance is the farad. One farad is a very large unit and normally the capacitance of something is declared in units such as:
microfarad (1 μ F) = 10–6 F
picofarad (1 pF) = 10–12 F
Example 12.9
How much charge can a 10 μF capacitor store if it has a voltage of 5 V across it?
Parallel plate capacitors consist of two parallel conductive plates separated by a small distance with air or a nonconductive material, called a dielectric, separating the plates. (Figure 12.17)

FIGURE 12.17 A parallel plate capacitor.
The capacitance of these parallel plates, or the ability to hold charge depends on the area A of the plates, the separation distance of the plates d, and the permittivity ε of the dielectric, i.e., the material between the plates. The permittivity is the material’s ability to “resist” an electric field. This is all summarized by the following equation:
The permittivity of a material is given by ε = εr ∙ ε0 where εr is called the relative permittivity and .
Example 12.10
A capacitor consists of two parallel plates of area A = 0.01 m2 separated by a distance d = 10−4 m with Mylar, which has a relative permittivity ε = 3.2. Find its capacitance.
12.7.1 Energy Storage
The energy storage capability of capacitors continues to rise with advances in technology. Electric cars, trains, backup power for modern electronics are some of the uses of modern capacitors. The energy storage capability of a capacitor is given by:
Example 12.11
A 10 F capacitor is charged to a voltage of 5 V. How much energy is stored in it? How does this energy stored compare to a AA battery?
Compare this stored energy to 104 J of energy stored in a AA battery. The AA battery has about 100 times more stored energy than this capacitor!
Capacitors are not going to replace batteries yet for large, long-term energy storage, such as in an electric car. Their energy density, that is, how much energy thay can store per weight is much smaller than that for batteries (Figure 12.18).

FIGURE 12.18 Graph of the energy densities of batteries and capacitors along with their charging times, courtesy of the US Defense Logistics Agency.
12.7.2 Don’t Be Shocked by the Following
In the winter, when the air is dry, walking across a rug sometimes results in you receiving an electrical shock; this is called static electricity. What is happening here is your feet are rubbing electrons off of the carpet. The electrons lost by the carpet have now accumulated on your body, which now has a negative charge. It is possible to reach a voltage of tens of thousands of volts relative to your surroundings. Now if you touch, say a door knob, the excess electrons on your body will discharge to the knob.
Example 12.12
Suppose you walk across the rug accumulating charge as you walk. When reaching for the doorknob you observe a spark 0.5 cm long leaving your hand. Being the curious person you are you look up the breakdown voltage of air, that is, the maximum voltage difference between two objects separated by some distance in air before an arc occurs. A quick Google search comes up with a breakdown voltage of air 3 kV/mm and that the human body roughly has a capacitance of 100–200 pF; let’s call it 150 pF. What was the accumulated charge on your body?
Is this a lot of charge? Let’s see how many electrons this would be. The charge of one electron is −1.6 × 10−19 C so that the number of these extra electrons on your body is:
12.7.3 Van de Graaff Generator
Imagine if you could automate the collecting of charge as in the case of walking across a rug in the winter. A Van de Graaff generator is a device that does just this. It consists of a metal sphere acting as a capacitor and a moving belt between two rollers, see Figure 12.19. The lower roller has a motor connected to it to drive the belt. There is a metal “comb” that rubs the belt, as in the case of you walking across the rug in the winter, and becomes charged negative, and the belt becomes positive. This positive charge is transported upward to a metal sphere. The sphere is connected to another comb rubbing the belt. The electrons on the sphere are attracted to the positive belt. The belt removes electrons from a metal sphere by transferring this charge off of the sphere through the comb to the belt. The removal of negative charge results in the sphere becoming positively charged. Tabletop Van de Graaff generators can typically have voltages of hundreds of thousands of volts.

FIGURE 12.19 A Van de Graaff generator.
12.7.4 Catching a Lightning Bolt (Don’t Try This at Home!)
Capacitors are able to get charged and discharged much more quickly as compared to batteries. Could a capacitor be used to collect energy from lightning? Lightning strikes, over the entire earth, roughly 44 times/s. One bolt of lightning contains roughly a billion joules of energy delivered in several microseconds. Recall that power P = E/t, therefore we can approximate the power in a single lightning bolt to be:
P = 1015 watts (This is only an estimate sincebolts vary according to voltage, current, and duration.)
Wow! Looks like free energy if we could just capture and store it. However, harvesting energy from lighting is not easy. It is sporadic, and varies in location and intensity. Methods to capture this energy range from indirect energy conversion, such as capturing the hydrogen that disassociates from rapidly heated water by the lightning bolt, to direct conversion using lightning arrestors to capture part of the strike and store the energy in a capacitor. So far, no company has developed a practical commercial system (Figure 12.20).

FIGURE 12.20 Harvesting energy from lighting is not easy.
12.8 CHAPTER SUMMARY
Symbols used in this chapter:

Coulomb’s law describes the force between two charges.
The electric field is the medium through which charges interact with each other. The force on a charge q due to the electric field E is given by:
Work and the Electric Field
The electrical potential energy stored in a battery depends on how much charge is stored in it as well as what the voltage of the battery is.
Electric Field and Voltage
Capacitance is a measure of how much charge a body can store.
Parallel plate capacitors consist of two parallel conductive plates separated by a small distance with air or a nonconductive material, called a dielectric, separating the plates.
The capacitance of these plates or the ability to hold charge is given by the following:
The permittivity of a material is given by ε= εr ∙ ε0 where εr is called the relative permittivity and.
The energy storage capability of a capacitor is given by:
PROBLEMS
What is the force between an electron and a proton separated by 10−10 m in a hydrogen atom?
A hydrogen atom at first seems like an unstable system in the sense that the electron should “crash” into the proton because they are electrically attracted to each other. Why does this not happen?
Suppose you build a hover board by charging a board you stand on and a platform below it with an equal charge. What would the charge have to be to support the weight of a 712 N person, 0.001 m above the ground?
Two electrons separated by 1 m will accelerate away from each other at what rate?
What is the electric field generated by a proton at a distance of 0.01 m?
What is the force on an electron placed 0.01 m from a proton?
A charge of + 2 C is placed in an electric field of strength 2 N/C, pointing in the positive x direction. What is the force on it?
An evil scientist charges the earth and the moon equally with a charge in hopes of separating them even more, just to see what would happen. There would now be both an electrical force and a gravitational force on them. Find the charge so that the net force between them, FNet =FElectric −FGravitation = 0. Use mearth = 5.972 × 1024 kg, mmoon = 7.35 × 1022 kg.
What is the electric field between the parallel plates of a capacitor if the plates are separated by 1 mm and have a voltage of 100 V across them?
A hydrogen atom is placed between the plates separated by 0.001 m of a capacitor. What voltage would have to be placed across the plates to separate the electron from the proton in the hydrogen atom?
How fast will a charge of 0.1 C and mass 0.01 kg be moving if released from rest in an electric field of 0.1 N/C after 1 s?
Let’s build a particle collider by accelerating charges at each other between the parallel plates of a capacitor. How many times faster will the electron be moving relative to the proton when they collide?
How much work is done on a charge of + 2 C moved 1cm in the positive x direction through an electric field of 10 N/C pointing in the negative x direction? What is the change in the charge’s potential energy?
What is the permeability of a dielectric with a relative permeability = 5?
What is the energy stored in a AA battery holding a charge of 3,600 C?
Compare the energy content stored in the following batteries, D-cell, AA, 9 V. Assume a AA battery has 3,600 C of charge, a D-cell has 20 times the of charge of a AA battery, and a 9 V battery has half the of charge of a AA battery.
How much charge can a capacitor of 10 µ F store if it has 5 V across it?
How does the capacitance change if the plate separation increases?
How does the capacitance change if the dielectric permeability increases?
What is the voltage on a capacitor of 10 µ F if it has 0.1 µ C of charge on it?
What is the capacitance of a parallel plate capacitor with plates of area 0.001 m2, plate separation of 1 mm, and a dielectric of relative permittivity ε = 2.6?
If the plates of a capacitor are doubled in area, how does this change the capacitance?
How much energy can a capacitor of 10 F store if charged to 3.3 V?
A 2 F capacitor stores 25 J of energy. What is the voltage across it?
How many capacitors with a capacitance of 2 F and a voltage of 5 V would it take to equal the energy contained in a stick of dynamite at 106 J/stick?
A defibrillator delivers a 250 J of energy to a patient at 500 V. How much charge is delivered?
What would happen if a capacitor has too much voltage applied across it?
Suppose an electric car is powered by a 12 V battery containing 4.1 MJ of energy. If it takes 150 lb or 668 N of force to move the car at a constant rate, how far can the car travel? Assume an efficiency of 70%.
Suppose a cloud in a thunderstorm has a voltage relative to the earth of 4 × 105 V and is 4 × 103 m above the earth. What is the electric field in the atmosphere?
How many AA batteries holding 3,600 C of charge would equal the energy in a bolt of lightning at 109 J?
