The design phase of the program improvement model
Abstract:
This chapter will begin the discussion of the role of training in ensuring compliance with current manufacturing practices, and in particular, current good manufacturing practices (cGMPs). It is convenient to present the role of training in terms of the program improvement model. This model provides guidance at a fairly high level for program developers, instructional designers, software engineers, etc. as they author and revise their products. There are several application values of the program improvement model. First, the model clarifies and standardizes the process of addressing performance gaps in an organization, allowing best practices to be identified and implemented. Second, this model is widely utilized in various forms in the pharmaceutical industry, which facilitates benchmarking of program development initiatives between organizations.
5.1 The ADDIE model and the program improvement model
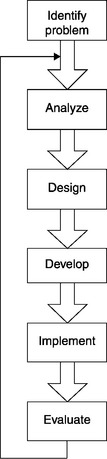
The program improvement model is ultimately derived from the familiar ADDIE model. The phases of the ADDIE model are Analyze, Design, Develop, Implement, and Evaluate. These phases are sequential – each depends upon the successful completion of the preceding phase. Moreover, the ADDIE model is an iterative feedback model, which means that the results of the Evaluation phase are fed back, closing the loop, facilitating further refinement of the program. If the evaluation shows that a training module has shortcomings – for example, that the objectives of the program do not align with organizational objectives – those shortcomings are fed back to be analyzed again. Further design and development efforts follow, until the program meets organizational needs (Figure 5.1).
The ADDIE model is scalable to all size pharmaceutical, biopharm, and medical device companies. The model can be scaled to various size organizations, and can be fitted to the particular needs of a specific department within the organization on a case-by-case basis, or by an overall decision. As an example of a particular case, once a problem has been identified, investigated, and subjected to a CAPA plan, the decision may be made in the Analysis phase of the ADDIE model to forego the needs analysis of employees’ skills and dispositions – these attributes may be well-known and documented, requiring no further analysis. Thus management makes the decision to limit the Analysis phase to a task analysis, that is, to the tasks that have been revised and must be integrated into the learning plans of the affected personnel.
As another example, management may make the overall decision to forego Pilot Implementation – and the associated Formative Evaluation – and roll out the program directly. In this instance, the Implementation phase is followed by Summative Evaluation. In both examples, it is a management decision to save costs by limiting the ADDIE model.1
The Analysis phase of the ADDIE model identifies a training issue such as a performance gap, a discrepancy between a standard stipulated in a standard operating procedure (SOP) and some employee performance. The performance gap can be addressed by a training program (i.e., a set of training and assessment materials, a qualified trainer, and a training audience).
This is followed by the Design phase of the ADDIE model, where a carefully planned approach to addressing the performance gap is outlined and approved. This planned approach has three components:
1. fitting the proposed training program (or module) into the larger training curriculum;
2. outlining the proposed training module; and
3. securing management approval of the outlined training program.
If management approves the proposed design, the Development phase of the ADDIE model comes next, where the training program – the training materials and the assessment materials – is developed to address the performance gap.
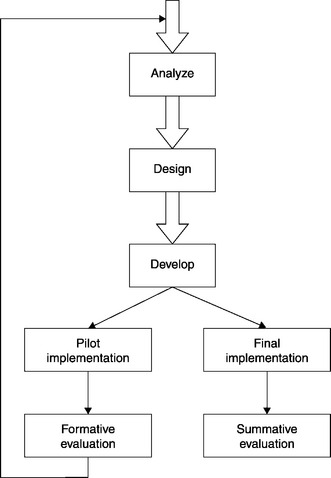
To anticipate, this is the point where the program improvement model and the ADDIE model diverge from one another. While the Development phase is followed by the Implementation phase in the ADDIE model, there are two paths out of the Development phase in the program improvement model. One leads to Pilot Implementation; the other leads to Final Implementation. In turn, Pilot Implementation leads to Formative Evaluation, while Final Implementation leads to Summative Evaluation (Figure 5.2).
This chapter will examine the three components of the Design phase in turn, focusing attention on an illustrative example of a training module.
5.2 The training module in the larger curriculum
Fitting the proposed training module into the larger curriculum ensures the articulation of this module with all other training modules, and the alignment of this module with organizational goals. There are four aspects to this “fit:” the structure of modules; the relationship between the training module and the associated SOP; reducing training modules by consolidation of SOPs; and the relationship between training modules and various regulatory requirements (e.g., FDA, OSHA, EPA, DEA, etc).
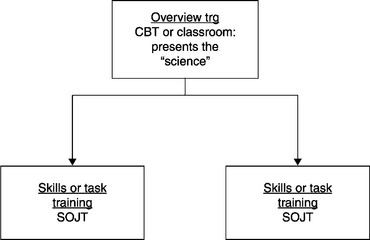
The structure of modules: The larger curriculum is comprised of a set of modules that focus the training effort on accomplishing organizational goals. The Design phase is where the fit between the proposed training module and the larger curriculum is delineated. This means outlining the structure wherein the training module will fit. Each module includes two training elements, an Overview Training element and one or more associated Skills Training elements.2 A module is displayed in Figure 5.3.
In the Design phase, the precise location of the training element – as an Overview Training element or a Skills Training element – is determined. We will briefly review the difference between these two types of elements. Overview Training will be more conceptually focused, while Skills Training will be more task or performance oriented. Concepts tell what a thing is, why it is important; tasks describe how to do something. Concepts provide the “science” for task performance. For example, the tasks involved in sanitizing equipment might be conceptualized as “Reducing the levels of microorganisms and particulates to acceptable limits,” thereby minimizing the risk of product contamination from the equipment.
The Overview Training element will typically be delivered by an instructor in a classroom; if a full-featured Learning Management System (LMS) is available, it may be delivered electronically. There will be an SOP for this Overview Training event. The Skills Training elements will usually be delivered one-on-one by a subject matter expert (SME) who is also a trainer, on the shop floor as a structured on-the-job training (SOJT) event 3; there will be an SOP for each of the SOJTs in the module.
The Overview Training element includes an assessment of training effectiveness – a Knowledge Transfer Assessment (KTA), for example. The training event is documented in a Training Record where the trainer and trainee concur that the trainee has, or has not, successfully concluded the event. In the case of classroom instruction, this training record is entered into the training tracking system and the entry is verified. In the case of a validated LMS, the training record will be an integral part of the training module and will be electronically entered into the trainee’s training history.
Once the Overview Training event is successfully concluded, the trainee goes on to the SOJT events. The several SOJTs are documented in Skill Demonstration Assessments (SDAs), where the trainee’s ability independently to perform the task is documented. The SDA is then entered into the training tracking system, and the entry is verified. After all the relevant SDAs are successfully completed, the trainee is qualified, meaning the trainee is ready to perform that module’s tasks independently.
Let us consider several examples, displayed in Table 5.1.
Table 5.1
| Module | Overview element | SOJT element |
| Central Weigh Module | Material Management | |
| 1st Cleaning Module | Cleaning and Sanitizing I | |
| Preparation of Solutions and Buffers | Media and Buffer Preparation | |
| 2nd Cleaning Module | Cleaning and Sanitizing II |
The precise fit of each of these modules into the larger curriculum is determined in the Design phase.
A second aspect of the fit between training modules and the larger training curriculum is the relationship between the training module and the associated procedure. That too will be delineated in the Design phase.
5.2.1 How training modules relate to SOPs
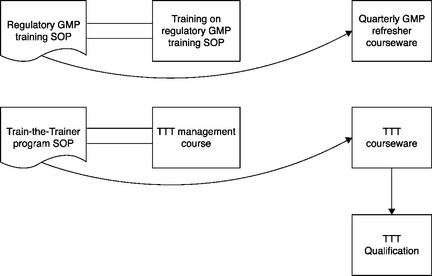
There are two ways that a training module can be related to a procedure. The first is directly, where the module trains to the procedure; this is sometimes called “document based training.” The second is indirectly, where the training module is mandated in the SOP, but the module does not train to the procedure; this is called “non-document based training.” An example of the latter is training in current GMPs, an FDA requirement. The FDA requires that this training be both “current” and “conducted on a continuing basis.”4 These requirements are typically met by training on courseware that is repeatedly offered, say on a quarterly basis, and is also frequently revised to ensure currency. The SOP that provides guidance to the GMP regulatory training is, by contrast, relatively fixed.
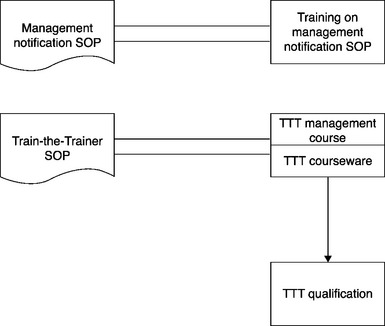
In Figure 5.4 the procedure is on the left and the training module is on the right. In the case of a procedure like Management Notification, which identifies the routine as well as exceptional situations where employees must notify their management, the module trains directly to the procedure.
In the case of a procedure like Train-the-Trainer (TTT), by contrast, there are several training modules: one trains to the management of the TTT program; another is the courseware for the TTT classroom sessions; and a third is the courseware for the subsequent TTT qualification session. These training modules have different training audiences: the first module – the program management module – has the organization’s training unit as its audience; the second and third modules have the prospective qualified trainers as their audience.
Document-based training and non-document-based training must carefully be distinguished in the Design phase; if not, there is the possibility that all the training modules in non-document-based training will be named after the same procedure. We have seen instances where a several-hour classroom session of mandated training had the same name and course number as a one-hour program management course, causing confusion in the training tracking system and among the several training audiences.
It is better to delineate clearly the more complex relationship between training modules and the associated procedure. Figure 5.5 more clearly displays the non-document-based training structure; it is now viewed as similar to a GMP regulatory procedure, where there is training to the procedure and also (a different thing) training on courseware that is mandated by the procedure.
Now the one-hour training on the management of the TTT program will have its own name in the training tracking system, and the several-hour-long TTT classroom course will have a different name, as will the subsequent TTT qualification session. The two different training audiences can clearly recognize the relevant training modules.
The clear statement of the relation between the training module(s) and the associated procedure should take place during the Design phase of the program improvement model, and will be an important contribution to the ultimate success of the training module.
5.2.2 Consolidate SOPs, reduce the number of training modules
Several training modules can be associated, directly or indirectly, with a single procedure. This suggests that a straightforward means of reducing training time within a training system might be to consolidate SOPs, thereby reducing the number of training modules. However, consolidation (or “streamlining”) of SOPs needs to be logical and to eliminate redundancies, not simply reduce the number of SOPs. We will clarify this point. Consider four examples that illustrate the issue:
1. The FDA requires gowning procedures.5 Department A has a gowning procedure. Department B has a different gowning procedure. Consolidation of SOPs would remove the redundancies here; Departments A and B would work together toward a single gowning procedure.
2. Department C has a protocol on the use of Equipment-Specific Instructions (ESIs), say involving equipment maintenance manuals. Department D has a different protocol on the same kind of ESIs. Again, streamlining procedures would remove the redundancies; Departments C and D would work together toward a single protocol on the use of ESIs.
3. Department E has an SOP for operating an autoclave, and another SOP for operating a capping machine. There is no redundancy here; it would be counterproductive to consolidate the two procedures, since they deal with “apples and oranges”.
4. Department F has three SOPs and three packaging lines, one procedure for the operation of each line; each includes a brief section on equipment maintenance. There is redundancy here, but not like that in Examples 1 and 2. The redundancy here is in the sections on maintenance. Consolidation of the procedures would remove the sections on maintenance and put them in a maintenance procedure of its own. We will return to this issue in the next section.
Consolidation of SOPs is essentially an issue of correctly writing procedures. Very briefly, procedure writing has six steps, all but the last involving the collaboration of a procedure author (usually a technical writer) and one or more SMEs. First, the SME(s) and the author identify the process to be captured in this SOP. Second, they identify the audience for this SOP. Third, they develop the process map for this process. The process map breaks down the process into its elements, and displays the logical interconnections between the elements. Fourth, the SME(s) and the author “chunk” the process. The chunks are developed from the process map, putting like elements together, and putting unlike elements apart. Fifth, the text of the SOP will be written from the chunks. The author writes this up and the SME(s) reviews the text in light of the intended audience. Finally, the text will be revised by the author of the procedure into the standard format of SOPs.
In conclusion, if procedures are correctly written, they will need little streamlining in the future, and will facilitate consolidation whenever new processes come on line and need to be captured in a procedure. Of course, if procedures have been poorly written, poorly chunked, or if there is a great deal of redundancy, then they must be revised along the lines sketched out above.
A fourth aspect of the fit between training modules and the larger curriculum is the relationship between training modules and the various regulatory requirements. That aspect will also be delineated in the Design phase.
5.2.3 How training modules relate to regulatory requirements
There are a number of regulatory regimes that impact on the training environment. These regimes include such agencies as FDA, OSHA, EPA, DOT, DEA, and others, each with its own set of regulations.6
On the one hand, the number of regimes means that there are simply more regulations to be taken into account. However, the various regimes can present the problem of regulatory overlap, where different agencies have differing regulations covering the same situation.7 We will consider how this impacts the design of the training module.
First, it overlooks the very abnormality of the abend. After all, this is called an abend because, in important ways, it is abnormal. Second, it overlooks the distinct division of labor between operators who enact the routine steps of the manufacturing cycle and the mechanics who address the abnormal events. This has substantial training implications; the procedures tend to be much longer, and both groups must train on the whole GMP procedure. Third, it confounds the “operational” level of detail in the routine situations with the much more fine-grained level of detail in the abnormal situations, a level of detail that can only be addressed by reference to technical manuals. Fourth, it blurs regulatory requirements that differ between normal situations and exceptional situations, for example, OSHA safety regulations.
For these reasons, among others, it seems more appropriate to deal with abends by having separate procedures; an illustration will clarify this.
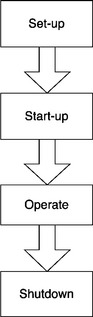
If we represent the manufacturing cycle in a vertical process map, displayed in Figure 5.6, consisting of the Set-up Period, followed by the Start-up Period, then the Operate Period, and finally the Shutdown Period, then abnormal events can be represented in a horizontal process map that intersects the manufacturing cycle at the point of the disruption. This horizontal map lays out the process of trouble-shooting, reviewing service or maintenance instructions that are specific to the equipment, implementing a set of corrective and preventive actions, conducting follow-up monitoring, as well as addressing safety or other relevant regulatory concerns (Figure 5.7).
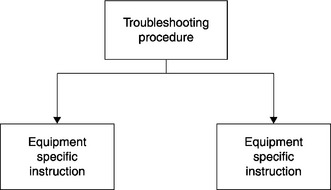
At the point of an abnormal event, the GMP requirements of the manufacturing cycle are suspended, temporarily, by an OSHA-mandated Lockout/Tagout (LOTO).8 That is where the mechanics or engineers (in OSHA terms, the “LOTO Authorized employees”) intervene with their Troubleshooting SOP and associated ESI to troubleshoot and maintain/repair the equipment.
These troubleshooting procedures and ESI protocols make up the horizontal process map. Its training modules would look much the same as the template given above, where the Troubleshooting SOP would be delivered by an instructor in a classroom, or electronically; the ESI protocols would be SOJTs. These would appear on the curriculum of the mechanics, as shown in Figure 5.8.
After the abnormal event is under control, the LOTO is removed and the LOTO-affected employees (the operators) resume the manufacturing process again, under the guidance of the GMP procedures.
Back in the GMP realm again, and depending on the specifics of the abnormal event – for instance, the impact on the product – a Management Notification is prepared (a Notification of Event, NoE) that could lead to an investigation, corrective action and preventive action, and follow-up monitoring.9
By keeping these processes separated (vertical and horizontal), the operators would have training on the GMP procedures on their curricula, and would qualify on these modules. The mechanics would have training on the troubleshooting SOPs on their curricula, and would qualify on these modules. Thus the operators would not need to train on the troubleshooting modules and the mechanics would not need to train on the operational modules. This would of course require that the SOPs would be written in a more focused fashion.
We have seen how the proposed training module is fitted into the larger curriculum in the Design phase of the program improvement model. The training module thereby aligns with other training module, and with organizational goals. We reviewed four aspects to this “fit:” the structure of training modules; the relationship between training module and SOP; how to reduce training time by consolidating SOPs; and the relationship between training module and various regulatory requirements.
Now we will consider how the proposed training module is outlined in the Design phase.
5.3 Outlining the proposed training module
Outlining the proposed training module will usually consist of completing a Training Outline template; the content of this is illustrated in Table 5.2. We will first display an illustrative template, with 12 fields and instructions for completing each field. This will be followed by comments on several of the fields.
Table 5.2
| FIELDS | INSTRUCTIONS |
| 1. COURSE TITLE: | Enter the title of the document or course |
| 2. COURSE NUMBER and VERSION: | Enter the number and version of the procedure, protocol, or document |
| 3. TRAINING AUDIENCE | Those required job positions included in the scope of the training module. Identify the areas, departments, and positions. For example, a training audience may consist of: |
| 4. CURRICULUM FIT | Identify the training module; other associated courses |
| 5. PREREQUISITE COURSES/ REQUIRED SKILLS | List any prerequisite courses; any required skills |
| 6. TRAINERS: | All qualified trainers who have been identified as SMEs on the course, including the Originator and Business Owner, if they are qualified trainers |
| 7. BEHAVIORAL OBJECTIVES: | Specify the observable competencies that trainees will demonstrate upon completing the training. For example, “At the end of this training session, the trainee will be able to demonstrate the following skills or perform the following tasks . . .” |
| 8. TRAINING DELIVERY METHOD: | Check as appropriate: |
| 9. COURSE LENGTH: | Enter the approximate time required to deliver the training session. This information is for planning purposes only. |
| 10. SPECIAL INSTRUCTIONS: | Instructions to facilitate the preparation and execution of the event (e.g., safety issues, logistical requirements, pre-work, handouts, etc.) |
| 11. MEASURES of EFFECTIVENESS: | The KTA (and answer sheet) or SDA should be attached. The content of the KTA or SDA is derived from the Behavioral Objectives. |
| 12. APPROVAL: | Includes dated signatures from: |
5.3.1 Training audience
The personnel included in the module’s training audience must be negotiated. Many times a training module will impact not only the business unit of the business owner of the SOP, but other units as well. Personnel in those impacted units will be listed on the Scope Statement of the SOP, and also in the list of Task Responsibilities within the SOP itself. Unfortunately, these two lists of personnel do not always coincide.
Precisely defining the training audience becomes critical because those are the personnel who must be trained on the training module associated with the new or revised SOP. After a new or revised SOP has been approved, there is a “training window” before the procedure goes into effect, within which the impacted personnel can be trained on the SOP. This window is typically a week or two in length. It is critical that the training audience be defined before that window opens – before the SOP is approved – so that all the training will be completed before the effective date. Thus the risk of untrained personnel “touching” the regulated product will be minimized.
When the training module is in the Design phase, the author of the module can provisionally prepare a Target Audience List based on a review of the SOP Scope Statement as well as the Task Responsibilities. When the Training Outline is circulated for approval, the Target Audience List can be circulated as well. Management of each impacted unit reviews the list and recommends limiting it or expanding it, based on their direct responsibility for the task assignments of the impacted personnel. The author of the training module can then take those recommendations into account as the module is finalized. Moreover, management in the impacted areas is alerted for the approval and implementation dates of the SOP, and can accordingly schedule personnel for necessary training. This topic will be discussed further in Chapter 13.
As an additional comment, it is important to recognize the different kinds of personnel that may be included in the training audience for a given SOP: (a) employees (in the strict sense), (b) independent contractors, (c) contract company (third-party) workers, and (d) temporary agency workers.10 These four kinds are cross-cut by several kinds of ranks: (α) subordinates, (β) supervisors (i.e., managers, directors, etc.), and (χ) executives. The finalized Target Audience List must identify impacted (and non-impacted) personnel from each of these groups.
5.3.2 Behavioral objectives
There is a strong case to be made for behavioral objectives, sometimes called S.M.A.R.T. objectives, in training.11 Moreover, behavioral objectives permit the alignment of the intended training outcomes with organizational objectives. Anyone who advocates cognitive (i.e., non-behavioral) objectives for training must be prepared to explain how these objectives are to be aligned with those of the organization. Also, behavioral objectives permit the trainee to have clear expectations of the trainer’s (and the organization’s) intended training outcomes.12 These clear expectations play a critical role in effective adult learning.
Many academics reject the role of behavioral objectives in the university classroom; this highlights the difference between training in industry, on the one hand, and higher education on the other. In higher education, accredited institutions award diplomas to students, on the basis of a series of learning experiences over an extended period of time. The organizational objectives include (a) awarding the diplomas, and (b) maintaining the accreditation. This has very little to do with training in industry, where the organizational objectives include (a) improving employees’ task performance on-the-job, and (b) addressing the requirements of various regulatory regimes.13
5.3.3 Training effectiveness
Assessment of training effectiveness must be distinguished from evaluation of training programs. There is a difference in kind – trainees are human individuals, training programs are organizational entities. Of course trainees participate in training programs, but the difference in kind means that the measures are different. For instance, trainee reactions (Donald Kirkpatrick’s Level One)14 are perhaps useful in evaluating training programs – favorable trainee reactions may weigh in decisions about program continuity. Trainee reactions are much less useful in assessing training effectiveness, which involves assessing performance improvement that will impact on-the-job – a supervisor’s reactions are much more relevant.15
In the present context, training effectiveness is assessed by one of two types of measures – a KTA or a SDA. The KTA in particular need not be validated in terms of the task(s) at hand. If the KTA is validated, then performance improvement on-the-job can be predicted from trainee performance on the KTA. If the KTA has not been validated, the measure can still be included in the training module, as an interactive element of the courseware, and as a promissory note of future validation. The training event will be concluded in this case by the trainee (and trainer) concurrence that the trainee was trained on this courseware, and thereby on the SOP.
An SDA, by contrast, directly and validly documents the trainee’s ability independently to perform the task(s). Furthermore, once the relevant SDAs for a process are completed, the trainee is qualified, able independently to perform the tasks in that process. These matters are discussed further in Chapter 9.
Once the template is completed by the author, it is ready for management signoff, which concludes the Design phase of the program improvement model.
5.3.4 Securing management approval of the outlined training module
The final component of the Design phase is management approval of the proposed training module. This approval is important for at least three reasons. First, this ensures that resources allocated to the subsequent phases, Development, Implementation, and Evaluation, have approval at the appropriate organizational level. The investment of resources – particularly in the Development phase – will be substantial, and knowledge workers, be they program developers, instructional designers, software engineers, or whomever, are in no position to make the management decision about resource allocation. Second, Quality Unit approval ensures that the proposed training module meets the organization’s quality criteria. Finally, there are a number of points where training implications of the proposed module – the training audience, the course length, etc. – can have a profound impact on business lines, and again, this impact must have managerial approval. The signatures on the Training Outline satisfy these needs.
5.4 Conclusion
The Design phase of the program improvement model is the occasion for a carefully planned approach to addressing a problem or performance gap identified in the Analysis phase. This planned approach includes fitting the proposed program into the larger programmatic framework; it involves outlining the program in terms of a systematic template, the Training Program Outline; and it includes the need for securing management approval of the outlined program.
When the proposed program has moved through the Design phase, it is ready for the Development phase where training materials and assessment materials are developed to address the problem or performance gap.
5.6 References
Bender, A., Shannon, N., Braun-Davis, J. Orchestrating compliance. Pharma, Exec.. 4, 2005. [October.].
Bierlein, L. Who has responsibility for Employee Hazmat Training? Transp. Distrib.. 1998; 39(11):123.
Bierlein, L. Conduct a Hazmat Review before the DOT comes calling. Log. Today.. 2005; 46(7):16.
Cochran, C. Creating and meeting objectives. Qua. Dig.. 2004; 24(9):56.
Dijkstra, S., Leemkuil, H., Developments in the design of instructionIfenthaler D., Pirnay-Dummer P., Spector J.M., eds. Understanding Models for Learning and Instruction. Springer: New York, 2008:197–199 Chapter 10
Ely, D.D. Training by objectives, a systems approach to instruction. Train. Dev. J.. 1975; 29(6):23–24.
Eudy, J. Clean manufacturing. Control. Envir. 7(3), 2004. [March.].
Garen, J. Use of employees and alternative work arrangements in the United States. Lab. Econ.. 2006; 13(1):107. [ff].
Hahn, R.W. Government analysis of the benefits and costs of regulation. J. Econ. Pers.. 1998; 12(4):201–210.
Hall, B., LeCavalier, J. Learning across the Enterprise: The Benchmarking Study of Best Practices. Sunnyvale, CA: BrandonHall.com; 2000. [p. 6].
Jacobs, R.L. Structured On-The-Job Training. San Francisco: Berrett-Koehler Publishers; 2003.
Jamil, S., Floyd, H.L., Pace, D. Implementing electrical safety regulations and standards. IEEE Ind. Applic. Mag.. 1999; 5(1):16–21.
Kirkpatrick, D.L. Evaluating Training Programs. San Francisco: Berrett-Koehler Publishers; 1994.
Kirkpatrick, J. Transferring learning to behavior. 2005 T + D, April, 19–20.
Liggett, D.P. Training and qualifying your employees. Petroleum and Chemical Industry Conference, 2005. Industry Applications Society 52nd Annual Conference, 2005. [September 2005, pp. 327–32.].
Mahapatra, R.K., Lai, V. Evaluating end-user training programs. Comm. ACM. 2005; 48(1):67–70.
Moser, H. The ROI for manufacturing training. Mod. Mach. Shop.. 2005; 77(12):98. [ff].
National Academy of Sciences/Institute of MedicineEnsuring Safe Food: From Production to Consumption. Washington, DC: National Academy Press, 1998.
Rice-Munro, E.J., Munro, R.A. Continual improvement of training. Qual. Dig.. 2004; 24(8):43–53.
Rothwell, W., Kazanas, H.C. Improving On-The-Job Training: How to Establish and Operate a Comprehensive OJT Program. San Francisco: Pfeiffer; 2004.
Singh, H. Building effective blended learning programs. Educ. Tech.. 2003; 43(6):51–54.
Society of the Plastics Industry, Food, Drug, and Cosmetic Packaging Materials Committee Newsletter, 1998. [8 June 1998.].
1See Sanne Dijkstra and Henny Leemkuil (2008).
2See D. Zielinski’s (2005) discussion of “blended learning models.” See also Harvey Singh (2003). As B. Hall and J. LeCavalier (2000) put it: “Across a range of industries, the emerging best practices model is a highly compatible ‘ménage à trois’ uniting online learning for information transfer and procedural skill acquisition (often this constitutes pre-work for the next tier of the model), classroom or other site-based learning for higher order competencies, and SOJT learning, integrated with knowledge management and competency evaluation.”
3On SOJT, see William Rothwell and H C Kazanas (2004); also Ronald L Jacobs (2003).
4See 21 CFR Part ∫211.25, “Personnel qualifications.”
5See 21 CFR Part ∫211.28, “Personnel responsibilities.” Also Jan Eudy (2004).
6See, for example, Lawrence Bierlein (1998); also Bierlein(2005); and Shadid Jamil, H.L. Floyd, and D. Pace (1999). There are also state laws and regulations that must be taken into account; see A. Bender, N. Shannon, and J. Braun-Davis (2005).
7For instance, the chain of custody required by DEA 21 CFR Part ∫ 1301.73, “Physical Security Controls . . .” and the evacuation requirements of OSHA 29 CFR ∫ 1910.38(c), “Emergency Action Plans.” See also National Academy of Sciences/ Institute of Medicine (1998). See Society of the Plastics Industry (1998):
over the years, the food packaging industry has been subjected to an undue burden as a result of the regulatory overlap among FDA, USDA, and the Bureau of Alcohol, Tobacco, and Firearms (BATF);
and “FDA Cancels Part 11 Meeting,” Part 11 Compliance Report (9 June 2004), Vol. 4, No. 12, p. 2, for a discussion of the regulatory overlap between 21 CFR Part 11 and other regulations affecting life science companies, such as HIPAA and Sarbanes-Oxley. For an overview, see Robert W. Hahn (1988).
8See 29 CFR ∫ 1910.147, “Control of hazardous energy.” This standard mandates that each workplace, with few exceptions, must develop a program to “disable machinery or equipment and prevent the release of potentially hazardous energy while maintenance and servicing are being performed.” Hazardous energy includes electrical, mechanical, hydraulic, pneumatic, and other energy sources. The mandated LOTO program will have three components:
1. a set of written procedures for the control of hazardous energy;
2. an employee training program to ensure that the procedures are implemented; and
3. an annual inspection to ensure that the procedures continue to be followed.
See also Federal Register (6 November 1989), Vol. 54, No. 213, p. 46610; and Danny P. Liggett (2005).
9The corrective and preventive actions (CAPA) may, in the event, be the same for the troubleshooting process and the manufacturing process.
10See John Garen (2006).
11See D.D. Ely (1975).
12As Craig Cochran (2004) has stated, “People have trouble contributing to fuzzy, undefined objectives.”
13As Harry Moser (2005) points out, higher education is not incompatible with training in industry – just different. See also E.J. Rice-Munro and R.A. Munro (2004).
14See Donald Kirkpatrick (1994); also James Kirkpatrick (2005).