Bioactive ceramics and glasses for tissue engineering
M.N. Rahaman, Missouri University of Science and Technology, USA
Abstract:
The main bioactive ceramics and glasses used or under development for tissue engineering applications are reviewed. Applications of these biomaterials are mainly in bone tissue engineering but recent studies are also showing promising results for the use of bioactive glasses in healing soft tissue wounds. Hydroxyapatite, beta-tricalcium phosphate, and biphasic calcium phosphate are the most widely used bioactive ceramics. Silicate-based glasses such as the composition designated 45S5 and more recently developed borate and phosphate compositions are the main types of bioactive glass. Bioactive composites and inorganic–organic hybrids are also of interest. The properties of these bioactive ceramics and glasses and their performance in vitro and in vivo are reviewed.
Key words
bioactive ceramics; bioactive glass; tissue engineering; bone regeneration; soft tissue repair; growth factor delivery; bioinorganics
3.1 Introduction
Bioactive ceramics and glasses have been investigated mainly for the repair and regeneration of bone. Recently, bioactive glasses have also been attracting interest for healing soft tissue wounds. This chapter will review the main types of bioactive ceramics and glasses being used or under investigation for tissue engineering applications. Bioactive composites and inorganic–organic hybrids currently under development are also reviewed. The applications will focus on the use of these materials in bone regeneration, but recent studies on the use of bioactive glasses for soft tissue repair will also be discussed. The structure and properties of these bioactive ceramics and glasses relevant to these tissue engineering applications, their formation into porous scaffolds with the requisite architecture, and the performance of the scaffolds in vitro and in vivo will be reviewed.
Millions of patients suffer each year from considerable bone loss resulting from trauma, malignancy, and congenital diseases. Approximately 500 000 bone graft procedures are performed annually in the United States and 2.3 million worldwide to repair defects in orthopedics, dentistry, and neurosurgery [Giannoudis et al., 2005]. These numbers are expected to increase substantially in the next 10 to 15 years with the doubling of the US population over the age of 65 years [US Census Bureau, 2006], and the growing need for healthcare worldwide.
Clinically, bone defects can be reconstructed through the use of various bone grafts, such as autogenous bone grafts (autografts), allografts, and biocompatible synthetic materials [McAuliffe, 2003; Giannoudis et al., 2005; Laurencin et al., 2006]. Autogenous bone grafts, or bone from within the individual’s body, provide positive results in healing bone defects as they contain osteoinductive growth factors, osteogenic cells, and provide a structural scaffold. However, they suffer from problems such as limited tissue availability, increased surgery time, additional pain, and cosmetic imperfection at the donor site [Fernyhough et al., 1992]. Many of these factors can increase the cost of the procedure for the patient [Dahlin and Johannson, 2011]. An alternative to autogenous bone is an allogenic bone (allograft), bone originating from a donor or cadaver. Allografts are the most widely used bone graft but they are costly, have unpredictable healing to bone due to donor variance and adverse immune reactions, and increase the risk of disease transference [Wolfe, 1982; Giannoudis et al., 2005; Laurencin et al., 2006]. Because of these disadvantages, the need for synthetic bone grafts continues to increase.
There is no general consensus on the definition of the term ‘bioactive’. A bioactive material has been defined generally as a material designed to induce a specific biological activity [Williams, 1987]. In a more functional and measurable sense, bioactivity has been defined as the characteristic of an implant material which allows it to form a bond with living tissues [Hench and Wilson, 1984]. In this case, materials that are not bioactive lead to the formation of a non-adherent layer of tissue at the implant interface. A bioactive material has been defined more narrowly as a material that undergoes specific surface reactions, when implanted in the body, leading to the formation of a hydroxyapatite (HA)-like layer that is responsible for the formation of a firm bond with hard and soft tissues [Kokubo and Takadama, 2006]. The ability of a material to form a hydroxyapatite surface layer when immersed in simulated body fluid (SBF) in vitro is often taken as an indication of its bioactivity [Ducheyne, 1987; Kokubo et al., 1990]. However, this narrow definition of bioactivity has been called into question recently [Bohner and Lemaître, 2009]. Dicalcium phosphate dihydrate, for example, shows the formation of an HA surface layer when immersed in SBF in vitro, but no direct bone bonding in vivo [Ohura et al., 1996; Apelt et al., 2004; Theiss et al., 2005]. Furthermore, beta-tricalcium phosphate does not always lead to the formation of an HA surface layer in SBF despite its extensive bonding to bone [Kotani et al., 1991].
Certain compositions of ceramics, glasses, and glass–ceramics have been shown to be bioactive [Yamamuro et al., 1990a, 1990b], whether the term is used in a general or narrow sense. Bioactive ceramics typically have a calcium phosphate-based composition and a crystalline structure (such as HA), but they can exist as an amorphous phase (amorphous calcium phosphate) prior to crystallization [Hench, 1998]. Bioactive glasses are amorphous, and they are typically characterized by a three-dimensional (3D) network structure composed of a glass-forming oxide such as SiO2 (e.g., a silicate bioactive glass such as the glass designated 45S5), B2O3 (borate glass), or P2O5 (phosphate glass). A glass–ceramic is a material formed typically by the controlled crystallization of a glass; it consists commonly of a two-phase mixture of crystals in a glass matrix. Because bioactive ceramics and glasses (and glass–ceramics) are commonly brittle, the formation of composites composed of a bioactive phase and a ductile or softer organic phase, typically a synthetic or natural biodegradable polymer, has also been investigated for tissue engineering applications [Rezwan et al., 2006; Russias et al., 2007; Jones, 2012].
In the common tissue engineering strategies, bioactive ceramics and glasses are often used in the form of particles, granules, fibers, or porous 3D scaffolds, and they are designed to provide the requisite combination of properties, such as degradation or resorption rate, mechanical properties, and the capacity to support tissue regeneration [Hutmacher, 2000; Hollister, 2005; Gerhardt and Boccaccini, 2010]. Bioactive ceramics and glasses are osteoconductive (capacity to support bone formation in osseous defect sites) but they are generally not osteoinductive (i.e., they do not have ability to induce de novo bone formation without the presence of osteogenic factors). Consequently, some tissue engineering applications may require the use of bioactive ceramics and glasses in combination with osteogenic inductive factors or cells into order to stimulate tissue regeneration, particularly in large defects. Another development that has been receiving heightened interest recently is the use of bioactive ceramics, glasses, and glass–ceramics as delivery systems for inorganic ions known to have a positive effect on tissue regeneration and angiogenesis [Hoppe et al., 2011; Habibovic and Barralet, 2011; Lakhkar et al., 2013]. As the biomaterials degrade in vivo, the ions are released at a therapeutically desirable rate.
3.2 Scaffolds for tissue engineering
Bioactive ceramics and glasses in the form of particles, granules, or aggregates have been used to fill contained bone defects [Hench, 1998]. However, bioactive ceramics and glasses investigated for the regeneration of large bone defects are often designed to act as 3D scaffolds or templates to guide tissue regeneration [Hutmacher, 2000]. Ideally, scaffolds for bone regeneration should have a combination of properties. They should be biocompatible, osteoconductive, and should have an interconnected porosity for tissue ingrowth. Interconnected pores with a size between adjacent pores of ~100 μm are generally considered to be the minimum requirements to permit tissue ingrowth and function in porous scaffolds [Hulbert et al., 1970], but pores of size >300 μm may be required for enhanced bone ingrowth and formation of capillaries [Karageorgiou and Kaplan, 2005].
Scaffolds for bone regeneration should also be bioactive, with the ability to form a strong bond with bone and soft tissues. In addition, the scaffold should degrade or resorb at a controllable rate, comparable to the rate of bone regeneration, producing non-toxic products that are resorbed or excreted easily by the body. For the regeneration of large bone defects, which can also be defects in load-bearing bones, the target mechanical properties of the scaffolds are not well established, but an often-used guideline is that the mechanical properties of the scaffold should match those of the host bone. In addition, the strength and stability of the implant–bone interface should be maintained during degradation or resorption of the scaffold. Other criteria for scaffolds intended for bone regeneration include the ability to be formed into anatomically relevant shapes by commercial methods and to be sterilized according to international standards for clinical use.
A variety of methods have been used to produce porous 3D scaffolds of bioactive ceramics, glasses, and glass–ceramics for bone regeneration, including thermal bonding of particles or short fibers [Pirhonen et al., 2003; Fu et al., 2007; Jung, 2007], consolidation of particles with a pore-producing fugitive phase such as starch or PVA [Rodríguez-Lorenzo et al., 2002; Li et al., 2002], use of foaming agents [Sepulveda et al., 2000; Tamai et al., 2002], sol–gel processing [Sepulveda et al., 2002]; polymer foam replication [Chen et al., 2006; Fu et al., 2008b], freezing of suspensions [Deville et al., 2006; Q. Fu et al., 2008a, 2010a], and solid freeform fabrication [Sachlos and Czernuszka, 2003; Hollister, 2005; Miranda et al., 2008; Q. Fu et al., 2011c]. Figure 3.1 shows the architectures of 3D scaffolds prepared by a variety of methods.
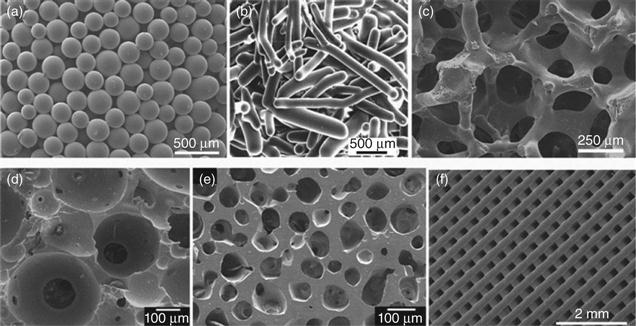
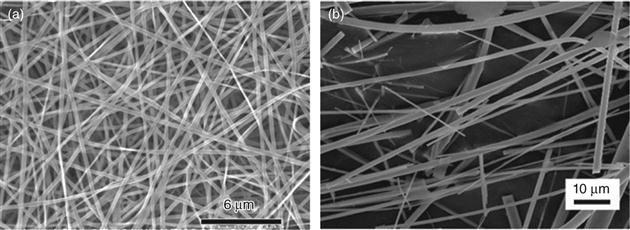
Recent work has shown the ability to create microfibrous or nanofibrous bioactive glasses and ceramics composed of fibers with diameters smaller than a few microns [Kim et al., 2006; Lu et al., 2009]. The architecture of these fibrous materials has a structural similarity to the extracellular matrix (ECM), and they are typically soft and pliable, with the feel of cotton wool. Because of their attractive properties such as high surface area, rapid degradation and conversion to HA, easy handling, and shape flexibility, microfibrous and nanofibrous bioactive glasses have been attracting growing interest for applications in hard and soft tissue repair [Wray, 2011]. Microfibrous and nanofibrous bioactive glasses have been prepared in the laboratory by electrospinning of sol–gel precursor solutions [Kim et al., 2006; Xia et al., 2007; Gao et al., 2011], and by laser spinning of a melt-derived glass [Quintero et al., 2009]. Figure 3.2 shows the fibrous architectures of bioactive glass prepared by electrospinning of a sol–gel precursor solution and from a melt-derived glass.
3.3 Bioactive ceramics
Bioactive ceramics in clinical applications typically have a calcium phosphate-based composition. The rationale for the interest and development of calcium phosphate bioceramics for bone regeneration is their similarity in composition with the main mineral constituent of bone and similarities in some properties. Composed of the same ions as bone, those bioactive ceramics are biocompatible, bond directly to bone, and they produce no systemic toxicity or immunological reactions. Calcium phosphate-based bone substitutes have been shown to be biocompatible and osteoconductive when implanted in bone defects [Jarcho, 1986; De Groot, 1986].
Several calcium phosphates have been identified as constituents of calcified tissues, and they have been studied or used for tissue engineering applications (Table 3.1). HA, identified by the idealized formula Ca10(PO4)6(OH)2, beta-tricalcium phosphate (β-TCP), Ca3(PO4)2, and composites of HA and β-TCP with a range of compositions (ratio of HA to β-TCP), generally referred to as ‘biphasic calcium phosphate’ (BCP), are the synthetic calcium phosphate bioceramics that have received most attention. HA, the main inorganic constituent of bones and teeth, is the most abundant mineral in human tissues [Kay et al., 1964]. Biological HAs contain minor and trace elements and are therefore not pure HA. The most important minor substituents are carbonate (CO3), magnesium (Mg), and sodium (Na). Systematic studies on biological HA and carbonate-substituted synthetic HA led to the general conclusion that biological HA should be considered as a carbonate-substituted HA, with a formula approximating to (Ca,X)10(PO4,HPO4,CO3)6(OH,Y)2, where X can be cations, such as Mg and Na, that can substitute for Ca, and Y can be anions, such as F or Cl, that can substitute for OH [LeGeros and LeGeros, 1984; Elliott 1994].
Table 3.1
Properties of some calcium phosphate materials used or under investigation for tissue engineering applications
| Material | Abbreviation | Chemical formula | Ca/P atomic ratio | Solubility at 25 °C −log Ksp |
| Dicalcium phosphate dihydratea | DCPD | CaHPO4·2H2O | 1.0 | 6.6 |
| Dicalcium phosphate anhydrousb | DCPA (or DCP) | CaHPO4 | 1.0 | 6.9 |
| Octacalcium phosphate | OCP | Ca8H2(PO4)6·5H2O | 1.33 | 47 |
| α-Tricalcium phosphate | α-TCP | Ca3(PO4)2 | 1.5 | 25.5 |
| β-Tricalcium phosphate | β-TCP | Ca3(PO4)2 | 1.5 | 29 |
| Amorphous calcium phosphate | ACP | CaxHy(PO4)z·nH2O | 1.2–2.2 | c |
| Calcium-deficient hydroxyapatite | CDHA | Ca10–x(HPO4)x(PO4)6–x(OH)2–x; (0 < x < 1) | 1.5–1.67 | ~85 |
| Hydroxyapatite | HA | Ca10(PO4)6(OH)2 | 1.67 | 117 |
| Fluorapatite | FA | Ca10(PO4)6F2 | 1.67 | 120 |
| Tetracalcium phosphate | TTCP | Ca4(PO4)2O | 2.0 | 38–44 |
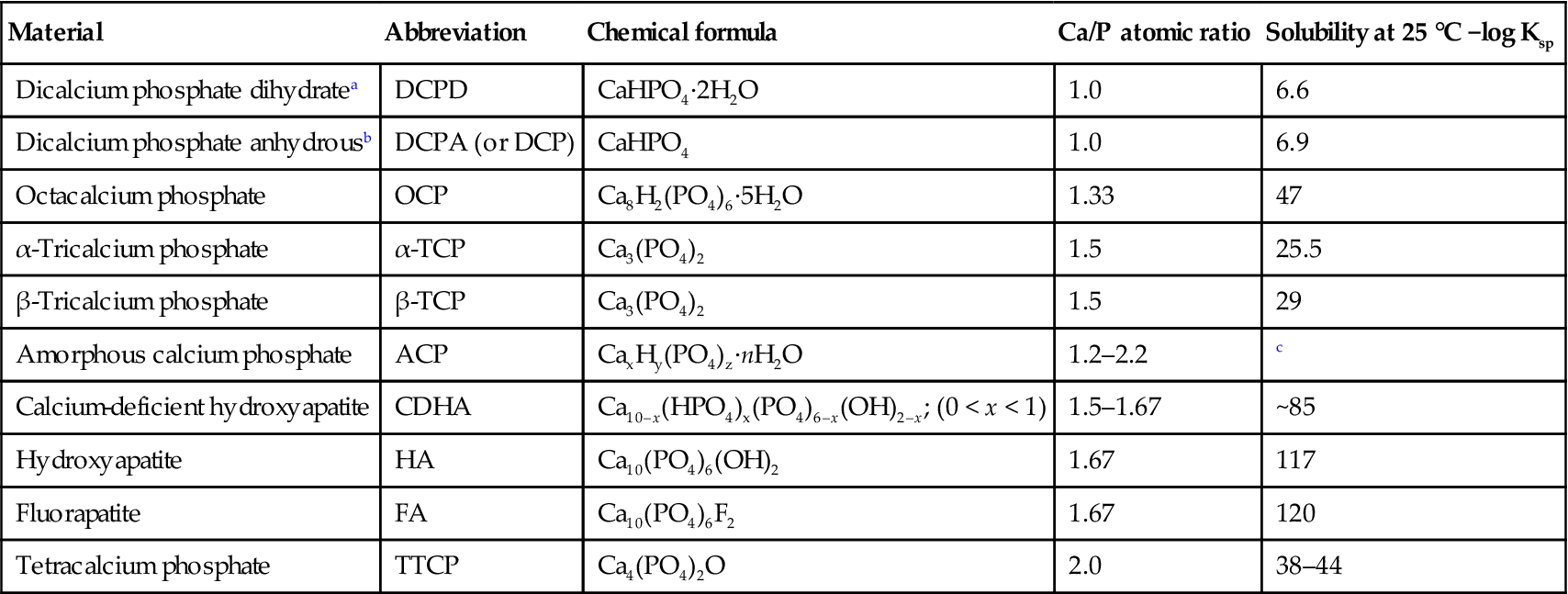
Compiled from: LeGeros (1993); Dorozhkin (2011b).
aMineral name: brushite;
bmineral name: monetite;
cvariable depending on the composition.
The composition and structure of calcium phosphate bioceramics have been reviewed in several publications [De Groot, 1983; LeGeros, 1991; Ravaglioli and Krajewski, 1992; Elliott, 1994; Dorozhkin, 2011b]. There are only two calcium phosphate bioceramics that are stable in aqueous media near room temperature, such as the body fluids, and it is the pH of the aqueous media that determines the most stable phase. At pH values less than ~4.2, dicalcium phosphate dihydrate (DCPD) is the most stable phase, while at higher pH, HA is the most stable phase. Consequently, in the body fluids that have a pH of ~7.4, HA is the most stable phase. However, at higher temperatures (typically under the exclusion of water) and in solidstate reactions, other calcium phosphate phases can be formed which can remain stable on cooling to room temperature.
The most important properties of the calcium phosphate bioceramics are their Ca/P atomic ratio, acidity/basicity, and solubility, all of which correlate strongly with the pH. The lower the Ca/P ratio, the more acidic and watersoluble the calcium phosphate material is. The order of the relative solubility of some calcium phosphate-based bioceramics in an acidic buffer has been reported as [LeGeros, 1993; Dorozhkin, 2011b]:
One of the most emphasized differences in properties between HA and β-TCP is their relative degradation rates. Synthetic HA is generally considered to be almost non-degradable, while β-TCP degrades far faster [De Groot, 1983; LeGeros, 1993]. However, the mechanical properties of HA are superior to those of β-TCP [Wagoner Johnson and Herschler, 2011]. The formation of BCP, composed of varying ratios of HA to β-TCP, has been used effectively as an approach to optimize the degradation rate and mechanical strength of HA [LeGeros, 1993].
Calcium phosphate bioceramics for tissue engineering applications are generally osteoconductive but not osteoinductive [LeGeros, 2002, 2008]. However, several calcium phosphate bioceramics, including HA, β-TCP, and biphasic calcium phosphate (BCP), have been reported to have the ability to form bone in non-bony sites without the addition osteogenic factors [Yamasaki and Sakai, 1992; Ripamonti, 1996; Yuan et al., 1999, 2001a]. Since the osteoinductivity was observed in some calcium phosphate bioceramics but not in others, these materials were described as having ‘intrinsic’ osteoinductivity [LeGeros, 2008]. This osteoinductive property has been attributed to a variety of factors such as the chemical composition, surface topography, geometry, and pore characteristics of the biomaterial [De Groot 1998; Jin et al., 2000; Reddi, 2000; Ripamonti, 2000; Yuan et al., 2010]. Macroporosity, mesoporosity, and concavity of the macropore surface appear to be important characteristics because they allow entrapment and concentration of circulating osteogenic growth factors (such as bone morphogenetic proteins, BMPs) and osteoprogenitor cells already present in the defect site in vivo [De Groot 1998; Jin et al., 2000; Reddi, 2000; Ripamonti, 2000].
3.3.1 HA
HA of natural origin is commonly derived from special species of corals or from bovine bone [LeGeros, 2002]. HA derived from corals (referred to as coralline HA) is prepared by converting coral, mainly calcite (CaCO3), in an ammonium phosphate solution under hydrothermal conditions (~250 °C and 100 MPa pressure). HA derived from bovine bone can consist of different forms, depending on whether the organic matrix is removed from the bone, and whether the resulting inorganic apatitic phase is sintered or not. These HAs of natural origin are not pure; they contain some of the minor and trace elements originally present in the coral or bone, such as Mg, Sr, CO3, and F in coralline HA, and Na, Mg, CO3, and other trace elements in bovine-derived HA. However, they retain the interconnected macroporosity, similar to that of human trabecular bone, from the original coral or the apatitic phase of bone.
HA and other apatitic materials crystallize in the hexagonal system [Elliott, 1994]. A characteristic feature of the apatitic structure is the capacity for substitution of ions in the lattice by a variety of ions. Substitution of various ions or atomic clusters is a widely known approach for modifying the physico-chemical, mechanical, and biological properties of synthetic HA. Ionic substitutions that have been investigated include Si [Pietak et al., 2007; Bohner, 2009; Bandyopadhyay et al., 2006], K [Xie et al., 2012], F [Okazaki et al., 1981], and CO3 [Gibson and Bonfield, 2002; Rupani et al., 2012]. Another group of substituting ions, such as Sr [Landi et al., 2007], Zn [Fujii et al., 2006; Matsunaga et al., 2010], and Cu [Sahithi et al., 2010; Li et al., 2011], are reported to promote osteogenesis and/or angiogenesis. Ionic substitution of the native ions of the HA lattice occurs at the atomic level. However, in some cases, nanosize clusters of atoms can form in the HA matrix and exist as discrete phases. The clusters are typically composed of the original metal or the metal oxide.
The composition of the dopant ions or atoms can have a marked influence on the physico-chemical and biological properties of HA implants. Si, one of the most studied substitutions, is found in native bone. It appears that trace amounts of Si play a unique role in the early stage of mineralization in osteoid calcification. A silicon-deficient diet can disturb healthy bone growth and homeostasis, which can induce osteoporosis [Kim et al., 2009; Arens et al., 2011; Price et al., 2012]. Si can be incorporated into the HA crystal lattice and alter the dissolution rate of HA. Si-substituted HA has been shown to have enhanced bioactivity [Balas et al., 2003; Thian et al., 2005; Zou et al., 2009], while the release of Si from Si-substituted HA is believed to stimulate osteogenic cells [Zou et al., 2009; Lopez-Alvarez et al., 2009; Gomes et al., 2010].
3D scaffolds of synthetic HA are commonly created from powders that are either prepared in the laboratory or obtained commercially. The powder is formed into the desired shape and architecture by one of the methods described previously for forming 3D scaffolds; then the porous construct is heated (sintered) typically at temperatures above ~1000 °C to bond the particles into a strong network. Porous HA granules (a few hundred microns to a few millimeters in diameter) have been formed using a variety of methods, such as tumbling or vibrating the powder with a liquid granulating agent or freezing droplets of a suspension of the fine powder. The granulated powder is then heated at temperatures above ~1000 °C to bond the particles together into a strong network while providing the requisite pore characteristics (Fabbri et al., 1995; Liu, 1996; Komleva et al., 2002; Gonda et al., 2009).
HA microspheres, porous or hollow, and 3D scaffolds composed of HA microspheres were formed recently using a novel glass conversion process near room temperature [Day and Conzone, 2002; Day et al., 2003; Conzone and Day, 2009]. The method is based on the observation that borate glass with special compositions can be converted rapidly and completely to HA when immersed in an aqueous phosphate solution near room temperature. The HA microspheres and 3D scaffolds have potential applications in the regeneration of non-loaded bone. HA microspheres are formed by reacting borate glass microspheres with the requisite diameter in the phosphate solution. Since the conversion to HA is pseudomorphic and the amount of HA formed depends on the CaO content of the starting borate glass, hollow or porous HA microspheres with the required diameter can be produced (Plate Va, between pages 354 and 355) [H. Fu et al., 2010]. Macroporous 3D scaffolds composed of hollow or porous HA microspheres are formed by first sintering borate glass microspheres in a shaped mold to bond the microspheres at their contact points, and then converting the glass to HA [H. Fu et al., 2011]. As prepared, the HA phase in the individual microspheres or 3D scaffolds has a mesoporous structure (pore size = 10–20 nm) of nanosize particles with a high surface area (>100 m2/g) (Plates Vb, c). The 3D scaffolds consist of an interconnected hierarchical structure of macropores between the hollow HA microspheres and mesopores in the shell wall. These hollow HA microspheres and 3D scaffolds are being evaluated as an osteoconductive carrier for growth factors in bone regeneration [Fu et al., 2013b; Xiao et al., 2013].
3.3.2 β-TCP
TCP has three polymorphs at atmospheric pressure [Elliott, 1994]. The β-phase is stable at room temperature, and transforms to the α-phase at ~1125 °C, which can be retained on cooling to room temperature. The α'-phase, stable at temperatures above ~1430 °C, is often of little interest because it transforms rapidly to the α-phase on cooling below the transition temperature. Of the two phases with practical interest for tissue engineering applications, β-TCP has seen much wider use in clinical applications, as a biodegradable calcium phosphate or as a component in BCP, in the form of dense or macroporous granules or scaffolds. In comparison, the more soluble and reactive α-TCP is often used as a fine powder in the preparation of calcium phosphate cements [Carrodegaus and De Aza, 2011]. As bone cements are not a focus of this chapter, only β-TCP will be considered.
The preparation of β-TCP follows procedures similar to those for HA. Granules and 3D scaffolds are commonly prepared from powders, obtained commercially or prepared in the laboratory by solid-state reaction, precipitation from solution, or other methods [Metzger et al., 1982; Tas et al., 1997; Destainville et al., 2003]. The powders are formed into the desired architecture by a variety of methods described earlier and sintered below ~1200 °C to form porous granules or 3D scaffolds [Bohner et al., 2005; Miranda et al., 2008]. Sintering above that temperature can lead to the formation of α-TCP, which is commonly undesirable. The use of dopants (e.g., Mg) can lead to greater stability of the β-phase, allowing the use of higher sintering temperatures [Bandyopadhyay et al., 2006].
Although less widely studied than in the case of HA, substitution of ions or ion clusters is also used to modify the physico-chemical, mechanical, and biological properties of β-TCP. Ionic substitutions that have been studied include Mg, Zn, Si, and Sr, either as single dopants or as binary combinations such as Mg + Sr and Mg + Zn [Bandyopadhyay et al., 2006; Xue et al., 2008; Banergee et al., 2010; Bose et al., 2011]. The substituted ions have been shown to alter the sintering charateristics of β-TCP powders, as well as the phase stability and degradation rate of β-TCP. Mg and Sr have been found to be particularly effective for modifying the degradation of β-TCP and its response to cells [Bose et al., 2011; Habibovic and Barralet, 2011].
3.3.3 BCP
BCP consists of an intimate mixture of HA and β-TCP of varying HA/β-TCP ratios [LeGeros et al., 2003]. As a result of the preferential dissolution of the β-TCP component, the degradation rate of BCP depends inversely on the HA/β-TCP ratio. BCP powders can be formed by a variety of methods, such as heating calcium-deficient HA to temperatures above ~700 °C [LeGeros, 1991], hydrolysis of non-apatitic calcium phosphates such as DCPD [Bouler et al., 2000], or by direct precipitation from a solution of calcium nitrate and diammonium hydrogen phosphate [Kivrak and Tas, 1998]. Three-dimensional macroporous scaffolds of BCP for tissue engineering applications are commonly prepared from powders using the methods described earlier.
3.4 Properties of bioactive ceramics
Biomaterials intended for bone tissue engineering applications should have the requisite degradation rate (resorption rate or solubility) to participate in bone remodeling and become fully integrated into the host bone. Ideally, degradation of the biomaterial should occur in conjunction with new bone formation. Consequently, the degradation rate of bioactive ceramics is relevant. The degradation of HA and other calcium phosphate bioceramics can occur by the combined action of two processes: (1) solution-mediated physico-chemical dissolution and (2) cell-mediated degradation resulting from the activities of resorptive (osteoclastic) cells. While the limitations of these in vitro tests for predicting the in vivo behavior are well recognized, they can be valuable for rapidly comparing the degradation rate of different calcium phosphate bioceramics.
The solution-mediated degradation of HA is strongly dependent on its physico-chemical properties [LeGeros et al., 1988; LeGeros, 1993]. Pure, well-crystallized synthetic HA is almost non-degradable. However, ionic substitution by (CO3)2−, Mg2 +, or Sr2 +, fine particle size, high surface area and high porosity have been shown to enhance its degradation rate [Klein et al., 1983; LeGeros, 1993, 2002; Doi et al., 1998]. Another approach is the combination of HA with β-TCP to form BCP which has a higher degradation rate than HA [LeGeros et al., 2003].
Several studies have evaluated the degradation of calcium phosphate bioceramics by osteoclastic cells in vitro. In one study using sintered discs of various calcium phosphate bioceramics (HA, carbonate-substituted HA, α-TCP, β-TCP, TTCP, OCP, and DCPD), apparently only the carbonate-substituted HA was resorbed by osteoclastic cells [Doi et al., 1999]. The OCP discs were degraded but only after pretreatment in a cell-free medium. Amorphous plasma-sprayed HA coatings were resorbed by osteoclasts whereas crystalline plasma-sprayed coatings of HA were not [de Bruijn et al., 1994]. Fine-grained coatings of carbonate-substituted HA and OCP, deposited on Ti-6Al-4 V substrates by precipitation from solution, were also found to be resorbed by osteoclastic cells [Leeuwenburgh et al., 2001]. The results of those studies indicate that the osteoclastic resorption of calcium phosphate bioceramics may be related to their composition and grain (or crystal) size.
As in the case of HA, ionic substitution influences the degradation of β-TCP [LeGeros, 1993]. Substitution by Al3+ (for Ca2+ in β-TCP) leads to an increase in the dissolution-mediated degradation rate, whereas substitution by Mg2+ leads to a decrease. Osteoclastic cell-mediated degradation was observed for β-TCP and Sr-substituted β-TCP, but the extent of degradation was significantly reduced for Mg-substituted β-TCP [Roy and Bose, 2012].
The mechanical properties (strength; elastic modulus) of HA, β-TCP, and BCP in compression and flexure have been reviewed recently [Wagoner Johnson and Herschler, 2011]. For each material, the strength and modulus cover a wide range, depending on the porosity, and the fabrication method. For reference, Table 3.2 gives the mechanical properties of dense HA, dense β-TCP, and bone. As observed for other porous ceramics, the porosity, as well as the size, geometry, and distribution, of the pores influence the strength of HA and β-TCP. Commonly, an exponential dependence of strength σ on porosity P is often observed for porous ceramics:
[3.1]
Table 3.2
Mechanical properties of human bone, dense HA, and dense β-TCP
| Property | Cortical bone | Trabecular bone | Dense HA | Dense β-TCP |
| Compressive strength (MPa)* | 100–150 | 2–12 | 500–1000 | 460–687 |
| Flexural strength (MPa) | 135–190 | 10–20 | 115–200 | 140–154 |
| Tensile strength (MPa)* | 50–150 | 1–5 | 79–196 | – |
| Elastic modulus (GPa) | 10–20 | 0.1–5 | 80–110 | 33–90 |
| Fracture toughness (MPa · m1/2) | 2–12 | 0.1–0.8 | 1.0 | – |
| Porosity (%) | 5–10 | 50–90 | – | – |
| Density (g/cm3) | – | – | 3.16 | 3.07 |
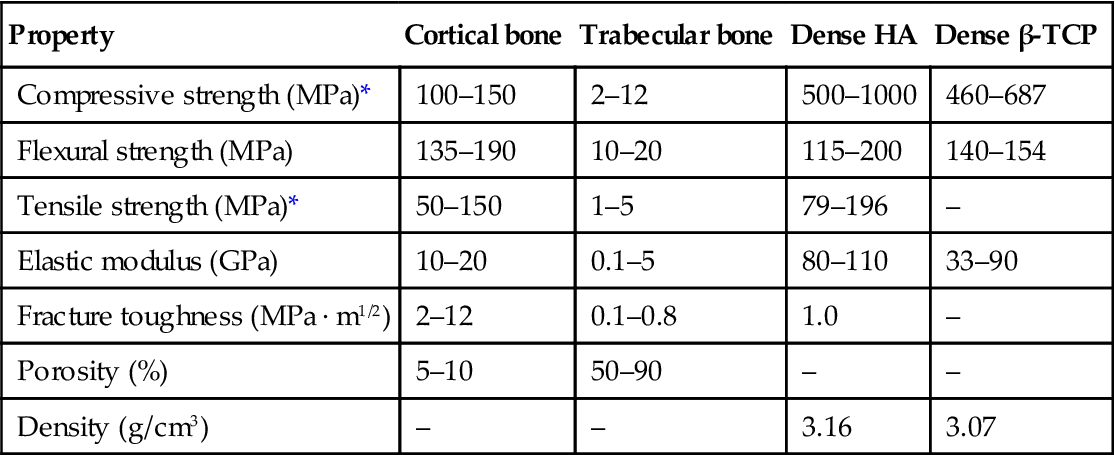
*Tested in the long direction. Compiled from Q. Fu et al. (2011a); Hench (1998).
where σo is the strength of the fully dense material, and b is a constant determined experimentally. An exponential dependence of the elastic modulus on porosity is also commonly observed.
Generally, for scaffolds prepared by the same method and with the same porosity, the strengths reported for HA are higher than those for β-TCP [Wagoner Johnson and Herschler, 2011]. For example, HA scaffolds prepared by robocasting (porosity = 40%) had a mean compressive strength of ~50 MPa and an elastic modulus of ~7 GPa, compared to mean strength and elastic modulus values of ~15 MPa and 2 GPa, respectively for β-TCP scaffolds [Miranda et al., 2008]. For the same calcium phosphate bioceramic and porosity, scaffolds prepared by solid freeform fabrication techniques often have mechanical properties that are superior to those prepared by more conventional methods, presumably because of the greater capacity to control the microstructure. It is also found that the mechanical properties of BCP scaffolds generally decrease with increasing amount of β-TCP [Wagoner Johnson and Herschler, 2011].
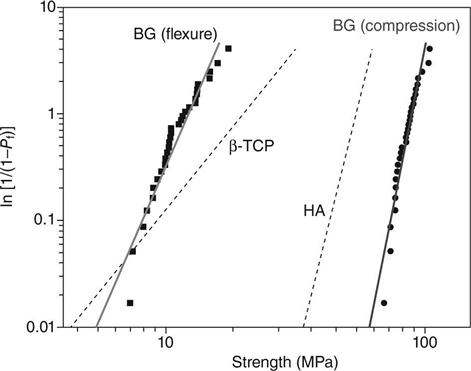
The flexural strength, mechanical reliability (Weibull modulus), fatigue resistance, and fracture toughness of scaffolds are also relevant for bone repair applications, but these properties have been rarely characterized. A few studies have reported Weibull modulus values in the range 3–10 for porous HA and β-TCP scaffolds tested in compression [Miranda et al., 2008; Martinez-Vásquez et al., 2010]. In comparison, the Weibull modulus of dense ceramics has been reported in the range 3–20. Figure 3.3 shows Weibull plots for HA and β-TCP scaffolds fabricated by robocasting and tested in compression. Under the same allowable failure probabilities, the HA scaffolds (Weibull modulus = 9) showed a higher compressive failure strength than the β-TCP scaffolds (Weibull modulus = 3). The fracture toughness of dense HA and β-TCP has been reported in the range 0.9–1.3 MPa m1/2 [Chevalier and Gremillard, 2009], while the fracture toughness of porous HA with a porosity of ~40% was ~0.3 MPa m1/2 [Zhang et al., 2006]. These fracture toughness values are in the range reported for trabecular bone but are lower than the lowest values reported for cortical bone (Table 3.2).
3.5 Tissue engineering applications of bioactive ceramics
The calcium phosphate bioceramics HA, β-TCP, and BCP have been considered for clinical applications since the 1920s, and they have been used in dentistry and medicine for bone repair and augmentation since the 1980s [Jarco, 1981; Metzger et al., 1982; De Groot, 1983; Aoki, 1991; LeGeros, 1991; Bohner, 2000; Dorozhkin, 2011a]. HA and β-TCP, in the form of putties, pastes, granules, or porous scaffolds, make up the majority of the synthetic bone graft substitutes available commercially for dental and orthopedic applications [Laurencin et al., 2006]. They have been used as bone cements for the repair of craniofacial defects, for maxillary floor augmentation, and as coatings for the femoral stem in hip implants [LeGeros, 2002; LeGeros et al., 2003]. However, the use of porous scaffolds of these bioceramics, in particular β-TCP, is not recommended in the regeneration of large defects in load-bearing sites because of their inadequate strength (particularly in flexural loading), limited mechanical reliability, and brittle mechanical response.
Calcium phosphate bioceramics have a high affinity for binding and concentrating proteins which make them ideal carriers for growth factors such as bone morphogenetic-2 (BMP-2), bioactive peptides, and mesenchymal stem cells [Reddi, 2000; LeGeros, 2002; Bose and Tarafder, 2012]. Several studies have shown that the combination of porous particles, granules, or scaffolds of calcium phosphate bioceramics with BMP enhanced osteogenesis in animal models when compared to the calcium phosphate bioceramics alone [Ripamonti et al., 1992; Laffargue et al., 1999; Alam et al., 2001; Yuan et al., 2001b; Ruhé et al., 2004; Gonda et al., 2009; Tazaki et al., 2009; Notodihardjo et al., 2012]. It has been shown that the architecture of the calcium phosphate carrier, such as porosity, pore size, and pore interconnectivity, and the presence of microporosity can have a marked effect on osteogenesis [Kuboki et al., 1998; LeGeros, 2002; Sohier et al., 2010; Lan Levengood et al., 2010; Polak et al., 2011].
Hollow HA microspheres and 3D scaffolds composed of hollow HA microspheres bonded at their contact points (Plate V, between pages 354 and 355) are bioactive and osteoconductive. They can also serve as a carrier for controlled local delivery of proteins such as growth factors [Fu et al., 2013a, 2013b]. When loaded with BMP-2 (1 μg/defect) and implanted for 3 or 6 weeks in rat calvarial defects, the hollow HA microspheres (106–150 μm) showed an excellent ability to regenerate bone (Plates Vd, e) [Xiao et al., 2013].
Table 3.3 gives a comparison of the amount of new bone formed in rat calvarial defects implanted with a variety of bioactive ceramics and glasses. This list is not meant to be exhaustive; instead it provides data for a few selected biomaterials. Bone regeneration in defects implanted with BMP2-loaded HA microspheres was ~3 times higher than in similar HA implants without BMP-2, and 4–5 times higher than in implants composed of 45S5 particles (150–250 μm), the gold standard for bioactive glasses. The data also show that hollow HA microspheres loaded with BMP-2 had a significantly greater capacity to regenerate bone when compared to borate 13-93B3 bioactive glass scaffolds.
Table 3.3
Comparison of the percent new bone (mean ± standard deviation) formed in rat calvarial defects implanted with a variety of bioactive ceramics and glasses (with or without growth factor)
| Implant | Growth factor | Implantation time (weeks) | % new bone | Reference |
| Hollow HA microspheres(106–150 μm) | – | 6 | 17 ± 10 | Xiao et al. (2013) |
| Hollow HA microspheres (106–150 μm) | BMP-2(1 μg/defect) | 6 | 43 ± 6 | Xiao et al. (2013) |
| 45S5 particles (150–300 μm) | – | 12 | 19 ± 3 | Bi et al. (2012) |
| 13-93B3 scaffold (fibrous microstructure: porosity = 50%; pore size = 50–500 μm) | – | 12 | 15 ± 3 | Bi et al.(2012) |
| CDHA scaffold (porosity = 75%; pore size = 400–500 μm) | – | 8 | 10 ± 3 | Zhao et al.(2012) |
| CDHA scaffold (porosity = 75%; pore size = 400–500 μm) | BMP-2 (2 μg/defect) | 8 | 27 ± 4 | Zhao et al.(2012) |
| BCP scaffold (porosity = 70%; pore size = 300–600 μm and <10 μm)a | – | 8 | 23 ± 5 | Jang et al.(2011) |
| BCP scaffold (porosity = 70%; pore size = 300–600 μm and <10 μm)a | BMP-2(2.5 μg/defect) | 8 | 55 ± 8 | Jang et al.(2011) |
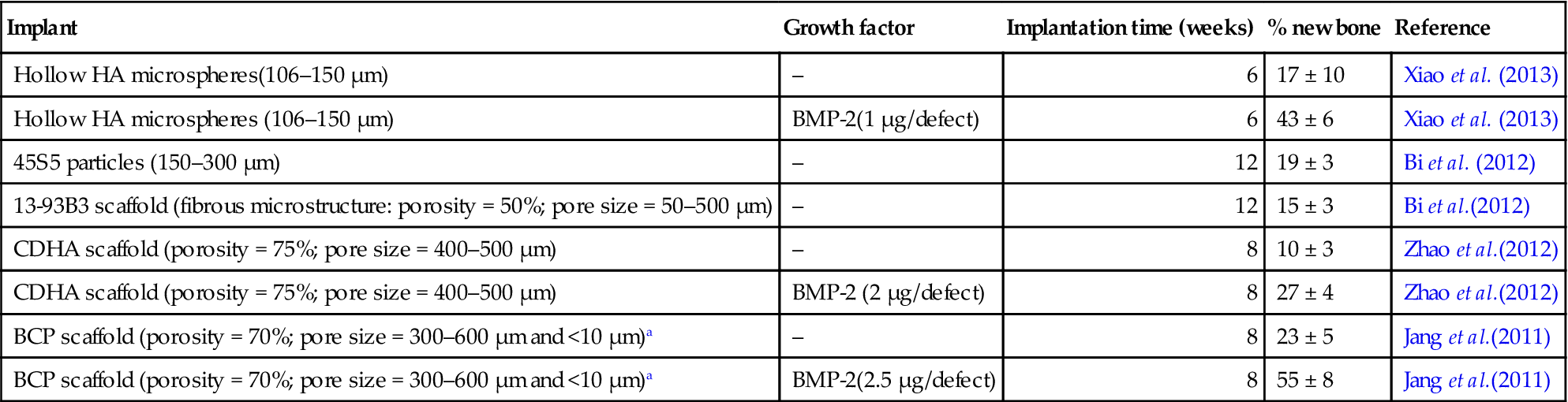
aHA: β-TCP = 20:80; two-thirds of the porosity in the range 300–600 μm; one-third of the porosity <10 μm.
Implants composed of hollow HA microspheres also showed a better capacity to regenerate bone at 6 weeks when compared to highly porous CDHA scaffolds implanted for 8 weeks. When loaded with BMP-2, the HA microspheres also showed a better capacity to regenerate bone than the CDHA scaffolds loaded with twice the amount of BMP-2. The hollow HA microspheres (without BMP-2) showed a similar capacity to regenerate bone at 6 weeks when compared to highly porous commercial BCP scaffolds at 8 weeks. Bone regeneration in defects implanted with BMP2-loaded BCP scaffolds was higher than in defects implanted with BMP2-loaded HA microspheres but the implantation time was 2 weeks longer and the BMP-2 dose was 2.5 times higher. Based on the data in Table 3.3, implants composed of hollow HA microspheres could provide a novel osteoconductive carrier for BMP-2 which is applicable in different bone graft procedures.
The combination of cells with osteoconductive scaffolds is also recognized as a key approach in the repair of bone defects [Ohgushi and Caplan, 1999]. Mesenchymal stem cells (MSCs) derived from bone marrow have been cultured in porous calcium phosphate bioceramics in vitro, after which the constructs were implanted as tissue-engineered scaffolds to regenerate bone. Several reports have shown a greater capacity of MSC-loaded HA and BCP scaffolds to support new bone formation and healing to bone when compared to the cell-free scaffolds [Kadiyala et al., 1997; Bruder et al., 1998; Touquet et al., 1999]. The microstructure of the scaffold can markedly affect its ability to support bone formation. In a recent study [Mankani et al., 2011], BCP scaffolds with a grid-like microstructure were fabricated with different pore widths (50–1000 μm), seeded with cultured human bone marrow stromal cells, and implanted in subcutaneous sites of immunodeficient mice. Scaffolds with a pore width of 500 μm were found to support the largest amount of new bone formation.
Trace quantities of various elements such as Sr, Zn, and Mg are present in human bone, and they have been shown to play a positive role in bone formation [Beattie and Avenell, 1992]. Because they are very amenable to ionic substitution, the calcium phosphate bioceramics have been studied as delivery systems for ions of those elements in bone regeneration [Habibovic and Barralet, 2011]. However, results showing enhancement of bone regeneration are limited and, in some cases, controversial. A positive effect on bone formation has been reported for calcium phosphate bioceramics substituted with Zn and Mg [Otsuka et al., 2008], Mg-substituted HA [Landi et al., 2008], and for β-TCP substituted with Mg and Sr [Bose et al., 2011]. In comparison, while Si-substituted HA has been reported to enhance bone regeneration compared with HA (without Si) [Patel et al., 2002; Hing et al., 2006], a review of the literature showed no direct evidence that it was attributable to Si release from the substituted HA [Bohner, 2009].
3.6 Bioactive glasses
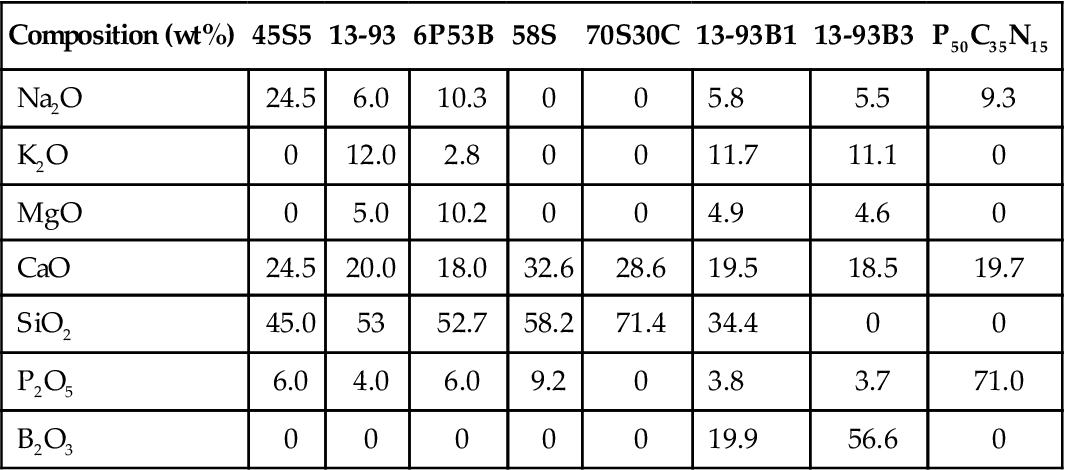
Since the report of its bone-bonding properties nearly 40 years ago [Hench et al., 1971], the bioactive glass designated 45S5, sometimes referred to by its commercial name Bioglass®, has been the most widely researched glass for biomedical applications [Hench, 2006; Jones, 2012]. This glass is a silicate glass based on the three-dimensional (3D) glass-forming SiO2 network. Since that time, other silicate-based glasses and glass–ceramics have been shown to be bioactive [Hench, 1998]. The majority of the earlier compositions were based on the 45S5 composition, but they contained additional glass modifiers (such as K2O and MgO) to control the processing and properties of the glass. More recent work has shown that certain compositions in other glass-forming systems, such as borate glass and phosphate glass, are also bioactive [Rahaman et al., 2011]. Examples of bioactive glass compositions are given in Table 3.4.
Table 3.4
Compositions of some bioactive glasses
| Composition (wt%) | 45S5 | 13-93 | 6P53B | 58S | 70S30C | 13-93B1 | 13-93B3 | P50C35N15 |
| Na2O | 24.5 | 6.0 | 10.3 | 0 | 0 | 5.8 | 5.5 | 9.3 |
| K2O | 0 | 12.0 | 2.8 | 0 | 0 | 11.7 | 11.1 | 0 |
| MgO | 0 | 5.0 | 10.2 | 0 | 0 | 4.9 | 4.6 | 0 |
| CaO | 24.5 | 20.0 | 18.0 | 32.6 | 28.6 | 19.5 | 18.5 | 19.7 |
| SiO2 | 45.0 | 53 | 52.7 | 58.2 | 71.4 | 34.4 | 0 | 0 |
| P2O5 | 6.0 | 4.0 | 6.0 | 9.2 | 0 | 3.8 | 3.7 | 71.0 |
| B2O3 | 0 | 0 | 0 | 0 | 0 | 19.9 | 56.6 | 0 |
3.6.1 Silicate bioactive glasses
As described above, 45S5 glass has been the most widely researched bioactive glass for biomedical applications. The key compositional features that are responsible for its bioactivity are its low SiO2 content (compared with more chemically durable silicate glasses), high Na2O and CaO content (glass network modifiers), and high CaO/P2O5 ratio. The mechanisms of bioactivity and bone bonding of 45S5 glass have been widely studied, and they are described in detail elsewhere [Hench, 1998; Rahaman et al., 2011]. Based on those studies, the bonding of 45S5 glass to bone has been attributed to the formation of a carbonate-substituted HA layer on the glass surface in contact with the body fluid. Because this carbonate-substituted HA layer is similar to the mineral constituent of bone, it bonds firmly with living bone and soft tissues.
With the initial formation of a carbonate-substituted HA layer, the biological mechanisms of bonding to bone are believed to involve adsorption of growth factors, followed by attachment, proliferation, and differentiation of osteoprogenitor cells [Hench and Polak, 2002]. Osteoblasts (bone-forming cells) create ECM (collagen), which mineralizes to form a nanocrystalline mineral and collagen on the surface of the glass implant while the degradation and conversion of the glass continues over time [Ducheyne and Qiu, 1999]. The biocompatibility of 45S5 glass has long been established [Wilson et al., 1981]. Degradation of 45S5 bioactive glass and, conversion to HA leads to the release of ions such as Na+ and Ca2+, and Si, presumably in the form of silicic acid, Si(OH)4. A study of Si release from 45S5 granules implanted in the muscle and tibiae of rabbits indicated that Si was harmlessly excreted in soluble form through the urine [Lai et al., 2002].
45S5 glass remains the gold standard for bioactive glasses but, as a scaffold material, it has a few limitations. One limitation is the difficulty of processing 45S5 glass into porous 3D scaffolds by sintering constructs composed of particles. Because of the limited ability of 45S5 glass to sinter by viscous flow above its glass transition temperature (Tg), and the narrow window between Tg and the onset of crystallization, difficulties are encountered in sintering the particles into a dense network. Consequently the scaffold often has low strength [Chen et al., 2006]. Commonly, the glass devitrifies during sintering to form a predominantly combeite crystalline phase (Na2O–2CaO–3SiO2), resulting in the formation of a glass–ceramic. While devitrification does not inhibit the ability of 45S5 glass to form an HA surface layer, it has the effect of reducing the rate of conversion to HA [Filho et al., 1996]. Another limitation of 45S5 glass is that the glass–ceramic scaffold formed by sintering degrades slowly and converts incompletely to HA [Fu et al., 2012], making it difficult to match the degradation rate of the scaffold to the rate of new bone formation.
A silicate bioactive glass designated 13-93 [Brink, 1997; Brink et al., 1997] is based on the 45S5 composition, but it has a comparatively higher SiO2 content and additional network modifiers, such as K2O and MgO, when compared to 45S5 (Table 3.4). Because 13-93 has better processing characteristics by viscous flow sintering (larger window between Tg and the onset of crystallization), the glass phase in porous 3D scaffolds can be sintered to high density without crystallization. In vitro cell culture showed no marked difference in the proliferation and differentiated function of osteoblastic MC3T3-E1 or MLO-A5 cells between dense disks of 45S5 and 13-93 glass [Brown et al., 2008]. However, 13-93 glass degrades and converts to HA more slowly than 45S5 glass.
3.6.2 Borate bioactive glasses
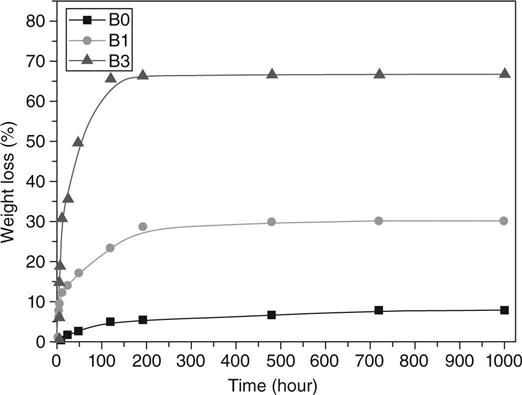
Certain compositions of borate glass have also been shown to be bioactive [Day et al., 2003; Han and Day, 2007; Zhao et al., 2009; Pan et al., 2010]. An example is the borate glass designated 13-93B3 (Table 3.4), obtained by replacing the molar concentration of SiO2 in silicate 13-93 glass with B2O3. Because of their lower chemical durability, some borate bioactive glasses (such as 13-93B3) degrade faster and convert more completely to HA compared with silicate 45S5 or 13-93 glass [Huang et al., 2006; Yao et al., 2007; Q. Fu et al., 2010b].
A concern associated with borate bioactive glasses is the toxicity resulting from high levels of boron released into the medium as borate ions, (BO3)3−. In conventional ‘static’ in vitro culture conditions, some borate glasses were observed to be toxic to cells, but the toxicity was diminished in ‘dynamic’ culture conditions [Brown et al., 2009]. Scaffolds of borate bioactive glass (13-93B3), were found to be toxic to murine MLO-A5 osteogeneic cells in vitro [Q. Fu et al., 2010b]. However, the same scaffolds did not show toxicity to cells in vivo and supported new tissue infiltration when implanted subcutaneously in rats [Q. Fu et al., 2010b]. Histological analyses of the kidney and liver showed no adverse effects resulting from subcutaneous implantation of borate bioactive glass (13-93B3) scaffolds in rats [Jung, 2012]. Borate glass pellets implanted in rabbit tibiae produced a boron concentration in the blood which was far lower than the toxic level [Zhang et al., 2010].
Recent work has shown the ability to control the degradation rate of bioactive glass by manipulating its composition. For example, by partially replacing the SiO2 in silicate 45S5 or 13-93 glass with B2O3 (yielding a borosilicate bioactive glass), or fully replacing the SiO2 with B2O3 (producing a borate bioactive glass), the degradation rate can be varied over a wide range [Huang et al., 2006; Yao et al., 2007; Q. Fu et al., 2010b]. The ease of manufacture and the ability to control the degradation rate of these borate-based glasses make them particularly useful for applications in bone regeneration. By controlling the glass composition, it should be possible to match the degradation rate of borate-based bioactive glass with the bone regeneration rate. The compositional flexibility of glass has also been exploited so that it also can serve as a source of many of the minor elements known to favor bone growth or angiogenesis, such as Zn, Cu, F, Mn, Sr, and B [Hoppe et al., 2011]. As the glass degrades in vivo, these elements are released at a biologically acceptable rate. For example, borate 13-93B3 bioactive glass doped with 0.4 wt% CuO has been found to enhance angiogenesis upon implantation in rat subcutaneous sites [Jung, 2012].
3.6.3 Phosphate bioactive glasses
Phosphate glasses, based on the P2O5 glass-forming network and CaO and Na2O as modifiers, have also been developed for biomedical use [Franks et al., 2000; Salih et al., 2000; Knowles, 2003; Ahmed et al., 2004a, 2004b]. As their constituent ions are present in the organic mineral phase of bone (Table 3.4), these glasses have a chemical affinity with bone. Alteration of the glass composition allows control of the degradation rate over several orders of magnitude [Knowles, 2003]. Consequently, these glasses may have additional clinical potential as resorbable materials.
The weight loss of the glass as a function of time and the concurrent release of ions are reported to be approximately linear over a wide range of compositions, providing a sustained release of the ions over the lifetime of the glass. This linear release of ions is claimed to be a key difference between phosphate and silicate bioactive glasses. For comparison, the release of ions from silicate bioactive glasses can vary markedly over time, often showing a rapid rate initially, followed by a slow rate thereafter. However, some phosphate glass compositions, such as ternary CaO–Na2O–P2O5 glasses, show significant pH changes with time [Salih et al., 2000; Ahmed et al., 2004a]. Compositional modification of the glass has been used to control the degradation rate and, therefore, to alleviate large pH changes [Abou Neel et al., 2008].
3.7 Preparation and properties of bioactive glasses
Bioactive glasses are commonly prepared by the conventional melting and casting route. In the creation of scaffolds, the glass is ground into particles, then formed into the desired shape using one of the methods described earlier, and thermally treated (sintered) to form a dense glass phase with an interconnected network of macropores (see Fig. 3.1).
Another approach, used far less frequently, is sol–gel processing. Silicate bioactive glass with simple binary and ternary compositions such as 58S and 70S30C (Table 3.4) have been prepared using this route. The silica network is formed by hydrolysis, condensation, and polymerization reactions at or near room temperature. The addition of a foaming step leads to the production of porous scaffolds that, after thermal treatment, have an overall microstructure similar to that of dry human trabecular bone [Sepulveda et al., 2002]. The pore structure is hierarchical, consisting of interconnected macropores (>100 μm) resulting from the foaming process and mesopores (less than several tens of nanometers) that are inherent to the sol–gel process [Jones et al., 2007].
The hierarchical pore structure of the scaffold formed by the foaming of a sol–gel material is beneficial for stimulating interaction with cells as it mimics the hierarchical structure of natural tissues and more closely simulates the physiological environment. Because of the nanosize pores in the glass, sol–gel derived scaffolds have high surface area (100–200 m2/g). As a result, these scaffolds degrade and convert faster to HA than scaffolds of melt-derived glass with the same composition. However, these sol–gel derived scaffolds have low strength (2–3 MPa) [Jones et al., 2006], and consequently they are suitable for substituting defects in low-load bone sites only.
3.7.1 Degradation of bioactive glasses and conversion to HA
Typically, the kinetics of degradation of bioactive glasses and their conversion to HA in vitro have been evaluated by immersing the glass (in the form of particles, discs, or porous scaffolds) in an aqueous phosphate solution such as SBF at 37 °C, and measuring the weight loss of the glass as a function of time [Huang et al., 2006; Q. Fu et al., 2010c] (Fig. 3.4). In addition, the conversion product has been characterized using structural, chemical, and microchemical techniques. The degradation of the glass is accompanied by the dissolution of ions and soluble species into the solution, (such as Na+, K+, (BO3)3−, and Si(OH)4 depending on the glass composition),so there is also a change in the pH and the ionic concentration of the solution as a function of time [Q. Fu et al., 2010c].
If the reaction time is sufficiently long for the product to crystallize, the X-ray diffraction pattern of the converted materials often shows peaks that correspond to a reference HA, Ca10(PO4)6(OH)2 [Huang et al., 2006; Q. Fu et al., 2010c]. However, microchemical analysis by energy-dispersive X-ray (EDS) methods, for example, often shows a calcium-deficient HA in which the Ca/P atomic ratio is lower than 1.67, the value for a pure or stoichiometric HA. Furthermore the Ca/P ratio of the converted material often varies from the surface of the reacted glass to the interior. Chemically, Fourier transform infrared (FTIR) spectra of the reacted material often show resonances attributable to carbonate (CO3)2− groups. This has been generally interpreted as indicating the formation of a carbonate-substituted HA, in which some of the (PO4)3− ions in the HA are substituted by (CO3)2−, as a result of dissolved CO2 in the aqueous phosphate solution.
3.7.2 Mechanical properties of bioactive glass scaffolds
The mechanical properties of bioactive glass scaffolds depend on the composition of the glass, the microstructure, and the fabrication method [Q. Fu et al., 2011a]. Table 3.5 gives the compressive strength of bioactive glass scaffolds prepared by a variety of methods. This summary is not meant to be exhaustive, but rather to indicate representative examples. Bioactive glass scaffolds prepared by methods such as polymer foam replication, gelcasting, and sintering of particles or short fibers typically have strengths comparable to those reported for human trabecular bone. Because of their low strength, they are unsuitable for the regeneration of large defects in loaded bone.
Table 3.5
Mechanical properties of bioactive glass scaffolds prepared by a variety of methods
| Method | Glass | Porosity (%) | Pore sizea (μm) | Strengthb (MPa) | Reference |
| Thermal bonding: | |||||
| Particles | 13-93 | 40–45 | 100–300 | 22 ± 1 | Fu et al. (2007) |
| Short fibers | 13-93 | 45–50 | > 100 | 5 | Jung (2007) |
| Polymer foam replication | 45S5 | 89–92 | 510–720 | 0.4 ± 0.1 | Chen et al. (2006) |
| 13-93 | 75–85 | 100–500 | 11 ± 1 | Fu et al. (2008c) | |
| 13-93B3 | 80–85 | 100–500 | 5 ± 0.5 | Q. Fu et al. (2010c) | |
| Sol–gel foam | 70S30C | 82 | 500 (100)c | 2.4 | Jones et al.(2006) |
| Unidirectional | 13-93 | 53–57 | 90–110 | 25 ± 3d | Q. Fu et al. (2010a) |
| freezing of suspensions | 13-93 | 50 | 50–150 | 47 ± 5d | Liu et al. (2012) |
| Solid freeform fabrication: | |||||
| Freeze extrusion fabrication | 13-93 | 50 | 300 | 140 ± 70 | Huang et al.(2011) |
| Robocasting | 6P53B | 60 | 500 | 136 ± 22 | Q. Fu et al.(2011c) |
| 13-93B3 | 50 | 420 | 65 ± 11 | Deliormanli and Rahaman (2012) | |
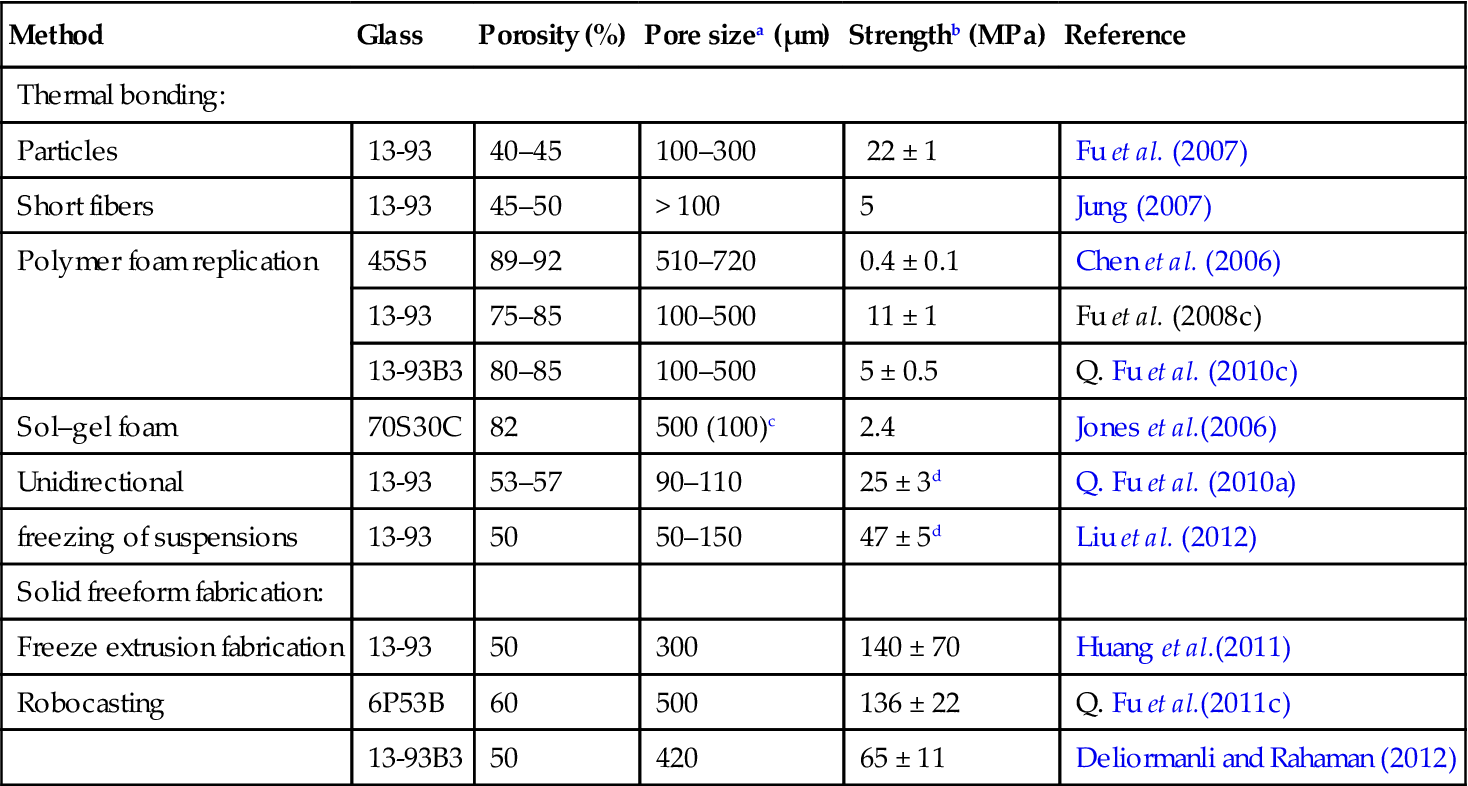
aDiameter or width;
btested in compression;
cmacropore diameter = 500 μm; interconnected pore diameter = 100 μm;
dtested in the orientation direction.
Attempts have been made to prepare glass or glass–ceramic scaffolds with higher strength using methods such as sintering particles that were compacted with a pore-forming phase [Baino et al., 2009] and unidirectional freezing of suspensions [Liu et al., 2012]. However, the range of pore sizes and the interconnectivity of the pores are difficult to control by those methods, which could limit the capacity of the scaffolds to support bone infiltration. Recent studies have shown that silicate 13-93 and 6P53B glass scaffolds fabricated by solid freeform fabrication methods such as freeze extrusion fabrication (FEF) and robocasting have compressive strengths comparable to those of human cortical bone, as well as a highly interconnected porous microstructure that is known to be favorable for supporting tissue infiltration [Huang et al., 2011; Q. Fu et al., 2011c, 2011d; Deliormanli and Rahaman, 2012].
The mechanical properties of bioactive glass scaffolds have been widely reported in the literature, but most studies provide data for the strength and elastic modulus in compression of the as-fabricated scaffolds or scaffolds that were immersed in an aqueous phosphate solution such as SBF [Q. Fu et al., 2011a]. As described earlier, other mechanical properties such as strength and elastic modulus in flexure, mechanical reliability (Weibull modulus), fatigue resistance, and fracture toughness are relevant because load-bearing bones, such as long limb bones, are subjected to multiple loading modes as well as cyclic loading. As the bioactive glass converts to HA, its properties change with time. Data for the time-dependent mechanical response in vitro and in vivo are also critically important for the design of bioactive glass scaffolds for loaded bone repair.
Silicate 13-93 bioactive glass scaffolds with a grid-like microstructure (porosity ~50%; pore width ~300 μm), prepared by robocasting, showed a compressive strength and elastic modulus comparable to the values for human cortical bone, and a Weibull modulus of 12 in compression, which was higher than the values (3–10) reported for porous HA and β-TCP scaffolds with a similar microstructure [Liu et al., 2013a]. Under the same allowable failure probabilities, the bioactive glass scaffolds showed a higher compressive failure strength than the HA scaffolds, which was also far higher than that for the β-TCP scaffolds (Fig. 3.3). The bioactive glass scaffolds also showed excellent fatigue resistance (~106 cycles) under cyclic stresses (2–20 MPa) higher than those in normal physiological loading. However, the flexural strength of the 13-93 bioactive glass scaffolds (11 ± 3 MPa) was far lower that the compressive strength, and also far lower than the flexural strength of human cortical bone.
For the same microstructure, as-fabricated scaffolds of silicate 13-93 glass have higher strength than borate 13-93B3 scaffolds (Fig. 3.5) [Deliormanli and Rahaman, 2012]. This has been explained in terms of the stronger four-fold coordinated silicate glass network when compared to the lower coordinated borate glass network. As described earlier, when immersed in an aqueous phosphate solution in vitro (such as SBF) or implanted in vivo, bioactive glasses degrade and convert to HA. This conversion also results in a degradation of the mechanical properties of the bioactive glass scaffolds. For the same scaffold microstructure, the degradation of the mechanical properties depends on the composition of the bioactive glass. Borate 13-93B3 glass converts to HA faster than silicate 13-93 glass and, consequently, its strength deceases faster as a function of time in SBF (Fig. 3.5).
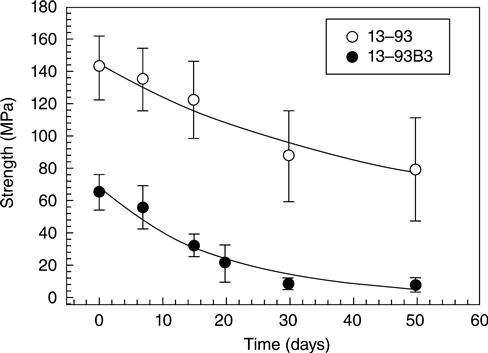
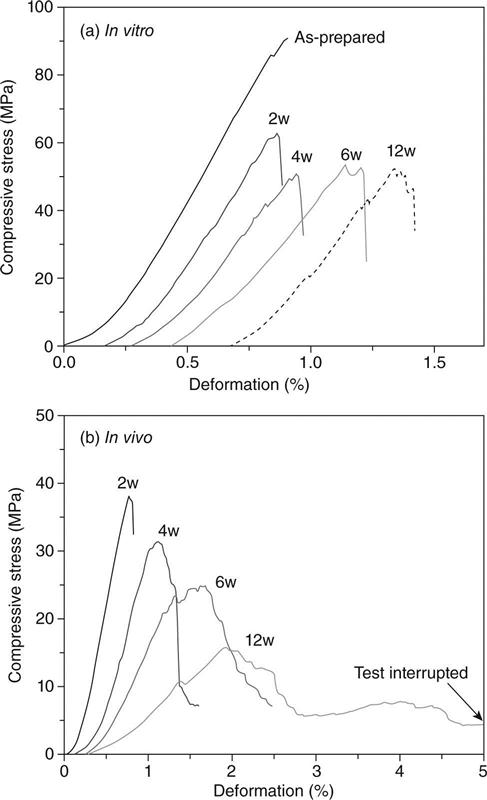
For the same bioactive glass and scaffold microstructure, the strength degrades faster in vivo than in vitro because of the faster conversion in vivo [Liu et al., 2013a]. In SBF in vitro, the strength of silicate 13-93 bioactive glass scaffolds degrades rapidly in the first two weeks and more slowly thereafter. However, in vivo (rat subcutaneous implantation model), after the rapid decrease in the first two weeks, the strength continues to degrade with implantation time. There is also a marked difference between the mechanical response of bioactive glass scaffolds in vitro and in vivo. As fabricated or after immersion in SBF in vitro, bioactive glass scaffolds show a brittle mechanical response (Fig. 3.6a). In comparison, the mechanical response of 13-93 bioactive glass scaffolds gradually changes to elastoplastic after implantation for a few weeks in vivo (Fig. 3.6b). This is because tissue infiltration of the scaffolds produces a composite composed of a brittle bioactive glass phase in a deformable (ductile) tissue matrix. When silicate 13-93 bioactive glass scaffolds with a grid-like microstructure (porosity ~50%; filament diameter ~300 μm; pore width ~300 μm) are implanted in rat subcutaneous sites, the time taken for the change-over from brittle to elasto-plastic response is ~3–4 weeks.
3.8 Bioactive glasses in tissue engineering
Since the discovery of 45S5 glass by Hench et al. in 1971, there has been a growing research and development interest in bioactive glasses for tissue engineering applications, particularly for bone repair and regeneration. However, although 45S5 glass has been reported to be capable of supporting more bone regeneration than HA [Oonishi et al., 1999] and to bond more rapidly to bone than the calcium phosphate bioceramics [Hench, 1998], clinical applications of bioactive glasses are far fewer than the calcium phosphate bioceramics such as HA and β-TCP. Actual clinical applications of bioactive glasses are few; the main applications use particles or granules in bone graft substitutes for healing defects in non-loaded bone, such as the periodontium and the jaw [Hench, 1998; Jones, 2012]. However, there is a growing interest in the use bioactive glasses for other tissue engineering applications.
3.8.1 Bioactive glasses for bone tissue engineering
Particles and granules of silicate 45S5 bioactive glass have been shown to enhance new bone formation in vivo [Wheeler et al., 1997, 1998; Oonishi et al., 1999]. The effect of bioactive glass composition on the capacity of 3D scaffolds to support bone regeneration has been evaluated recently [Bi et al., 2012]. Scaffolds with the same ‘fibrous’ microstructure (see Fig. 3.1b) but with three different compositions, silicate 13-93, borosilicate 13-93B1, and borate 13-93B3 (Table 3.4) were implanted for 12 weeks in rat calvarial defects. Defects filled with particles of silicate 45S5 bioactive glass (150–300 μm) and empty defects were used as positive and negative controls, respectively. The results showed that new bone formation was markedly dependent on the composition of the bioactive glass scaffolds. While all three scaffolds integrated well with the surrounding bone, a greater amount of the borate 13-93B3 scaffolds was converted to HA and a larger amount of new bone was formed in the defects implanted with the borate 13-93B3 scaffolds. The borate 13-93B3 scaffolds were completely converted to HA, and some fibers developed a hollow core in which new tissue had infiltrated. While the amount of new bone formed in the borate 13-93B3 scaffolds (~15%) after the 12-week implantation was small, it was almost twice that for the silicate 13-93 scaffolds.
In addition to the bioactive glass composition, the microstructure can also have an effect on the ability of the scaffold to support new bone formation [Liu et al., 2013b; Bi et al., 2013]. In a recent study, bone regeneration in silicate 13-93 bioactive glass scaffolds with two different microstructures was evaluated in a rat calvarial defect model [Liu et al., 2013b]. Scaffolds with an oriented microstructure of columnar pores (porosity = 50%; pore diameter = 50–150 μm) (see Fig. 3.1e) showed mostly osteoconductive bone regeneration, and new bone formation, normalized to the available pore area (volume) of the scaffolds, increased from 37% at 12 weeks to 55% at 24 weeks. In comparison, bone regeneration in scaffolds of the same glass with a trabecular microstructure (porosity = 80%; pore width = 100–500 μm) (see Fig. 3.1c) was 25% and 46% at 12 and 24 weeks, respectively.
As described earlier, 13-93 bioactive glass scaffolds with a grid-like microstructure (porosity ~50%; pore width = 300 μm), prepared by robocasting, have a compressive strength and elastic modulus comparable to those of human cortical bone, good mechanical reliability (Weibull modulus), and excellent fatigue resistance. When treated in a phosphate solution to convert a thin surface layer to HA or loaded with BMP-2 (1 μg/defect), those scaffolds showed a significantly greater capacity to support new bone formation in rat calvarial defects when compared to similar scaffolds without the surface treatment or BMP-2 loading (Plate VI, between pages 354 and 355) [Liu et al., 2013c]. The scaffolds also showed a far greater capacity to support new bone formation when compared to silicate 13-93 or borate 13-93B3 scaffolds with a fibrous, trabecular, or oriented microstructure.
3.8.2 Bioactive glasses for the treatment of bone infection (osteomyelitis)
The treatment of chronic osteomyelitis (bone infection) remains a clinical challenge. Bioactive glasses form one of the most recent carrier systems that have been investigated for treating bone infection. When incorporated into a biodegradable matrix loaded with antibiotics, borate 13-93B3 bioactive glass has shown promising results in simultaneously curing bone infection and regenerating bone in animal models. The ability of borate bioactive glass carriers to regenerate bone in the infected sites could eliminate the need for subsequent bone reconstruction.
The capacity of composite pellets composed of borate bioactive glass particles and vancomycin (8 wt%) in a phosphate cement to eradicate osteomyelitis in a rabbit tibial model has been studied recently [Xie et al., 2009]. After methicillin-resistant Staphylococcus aureus (MRSA)-induced osteomyelitis, debridement, and implantation for 8 weeks in rabbit tibiae, the vancomycin-loaded composites eradicated osteomyelitis in 81% of the rabbits. In comparison, osteomyelitis was eradicated in 73% of the rabbits treated with vancomycin-loaded CaSO4 pellets prepared from commercial OsteoSet beads, but the difference was not significant. In another study [Zhang et al., 2010], a mixture of borate 13-93B3 bioactive glass particles and teicoplanin powder was incorporated in a chitosan matrix to form a composite. When implanted for 12 weeks in rabbit tibiae infected with MRSA-induced osteomyelitis, the teicoplanin-loaded borate bioactive glass–chitosan composites eradicated the infection in 86% of the rabbits. In comparison, the osteomyelitis was eradicated in only 43% of the rabbits treated intravenously with teicoplanin once a day for 4 weeks.
In addition to being effective in eradicating osteomyelitis in a rabbit tibial model, antibiotic-loaded borate bioactive glass composites have also shown clear benefits over CaSO4 in promoting new bone formation and filling the defect. In one study [Q. Fu et al., 2011b], borate bioactive glass–chitosan composites or CaSO4 pellets loaded with teicoplanin were implanted for 12 weeks in rabbit tibiae infected with MRSA-induced osteomyelitis. Histology and synchrotron X-ray microcomputerized tomography showed that the teicoplanin-loaded borate bioactive glass implants converted to HA, promoted new bone formation, and integrated with the host bone after the 12-week implantation. In comparison, CaSO4 beads degraded completely, and no new bone formation was found in the defects implanted with the teicoplanin-loaded CaSO4 beads or in the untreated defects (control).
3.8.3 Bioactive glasses in angiogenesis and soft tissue repair
While bioactive glasses have been extensively investigated for bone repair and regeneration, there has been relatively little research on the application of bioactive glasses to the repair or regeneration of soft tissues. However, recent work has shown the ability of bioactive glasses to promote angiogenesis (formation of blood vessels), which is critical to numerous applications in tissue regeneration and the healing of soft tissue wounds.
The beneficial effects of small concentrations of 45S5 bioactive glass for stimulating angiogenesis has been shown in several recent studies, as reviewed recently [Gorustovich et al.,2010; Rahaman et al.,2011]. Polystyrene surfaces coated with a low concentration (0.01–0.2 wt%) of silicate 45S5 bioactive glass particles (<5 μm) were found to enhance the proliferation of fibroblast 208 F cells during in vitro culture for 24 h, when compared to the uncoated surfaces [Day et al., 2004]. Culture media collected from fibroblasts grown for 24 h on surfaces coated with 0.01 wt% 45S5 glass contained significantly higher concentrations of vascular endothelial growth factor (VEGF). Furthermore, coating poly(glycolic acid) meshes with 45S5 glass was found to enhance neovascularization after subcutaneous implantation of the scaffolds in rats for 28 and 42 days.
Human fibroblasts encapsulated in alginate beads with specific quantities (0–1 wt%) of silicate 45S5 bioactive glass particles (average size = 4 μm) secreted larger quantities of VEGF for bioactive glass concentrations of 0.01 and 0.1 wt%, but not for 1.0 wt%, indicating a dose-dependent curve for VEGF production which was dependent on the concentration of loaded material [Keshaw et al., 2005]. Furthermore, conditioned media collected from the cell-seeded alginate beads containing 0.1 wt% 45S5 glass particles increased endothelial cell proliferation. Human fibroblasts cultured on tissue culture surfaces coated with 45S5 glass particles (<5 μm) secreted larger amounts of VEGF and basic fibroblast growth factor (bFGF), while conditioned media from the stimulated fibroblasts increased endothelial cell proliferation and tubule formation [Day, 2005].
Coating a VEGF-releasing biodegradable poly(lactic co-glycolic acid), PLGA, scaffold with 45S5 bioactive glass particles resulted in an increase in endothelial cell proliferation in vitro, as well as greater neovascularization in vivo when implanted for 2 weeks in critical size defects in the cranium of Lewis rats [Leach et al., 2006]. By loading varying amounts of 45S5 bioactive glass into absorbable collagen sponges, the proangiogenic potential of 45S5 bioactive glass was shown to be related to the soluble products of the glass dissolution and the subsequent secretion of at least one angiogenic induction factor (VEGF) by the stimulated cells [Leu and Leach, 2008]. In vivo, collagen sponges loaded with the optimum amount of 45S5 bioactive glass were found to enhance neovascularization 2 weeks post-implantation in irradiated calvarial defects of Sprague-Dawley rats, compared with the collagen sponge without bioactive glass (control) [Leu et al., 2008]. This neovascularization induced by the local delivery of bioactive glass was believed to be responsible for the significantly greater new bone formation observed in the collagen/bioactive glass scaffolds 4 and 12 weeks post-implantation.
Copper has been reported to promote angiogenesis [McAuslan and Reilly, 1980; Raju et al., 1982], stimulate endothelial cell proliferation [Hu, 1998], and to promote wound healing in rats [Sen et al., 2002; Frangoulis et al., 2007]. Scaffolds of borate 13-93B3 bioactive glass doped with Cu have shown the ability to enhance angiogenesis when implanted in rat subcutaneous sites [Jung, 2012]. As the glass degrades and converts to HA, the Cu ions are released at a desired rate. The enhancement of angiogenesis by the Cu-doped borate glass was found to be dose-dependent, with 0.4 wt% CuO providing the optimum enhancement.
Microfibrous borate bioactive glass, composed of fibers with diameters in the range of a fraction of a micron to a few microns (Fig. 3.2b), have shown a remarkable ability to heal soft tissue wounds in humans. Because of its fine diameter, the material is very pliable and dissolves rapidly in an aqueous phosphate solution such as SBF [Liu et al., 2013d]. A clinical trial involving 12 patients with venous stasis wounds showed that the bioactive glass fibers had the capacity to heal the wounds without scarring (Plate IV, between pages 354 and 355) [Wray, 2011].
3.9 Bioactive glass–ceramics
Glass–ceramics, as described earlier, are crystallized glasses formed by a controlled heat treatment of the parent glass or as a result of thermal treatment (sintering) during fabrication. With a composite structure composed typically of a fine crystalline phase dispersed in a matrix of glass, the glass–ceramic often has better physical and mechanical properties (e.g., strength; fracture toughness) than the parent glass formed by melting and casting. Consequently, one rationale for the development of bioactive glass ceramics was the potential for achieving better mechanical properties. An early example was the development of apatite/wollastonite (A/W) bioactive glass–ceramics for the replacement of vertebrae [Nakamura et al., 1985; Kokubo et al., 1986]. Since that time, other glass–ceramics and glass–ceramic scaffolds with a variety of composition and architecture have been developed for bone replacement [Wu, 2009; Gerhardt and Boccaccini, 2010].
Because of the tendency of 45S5 glass to crystallize during sintering (which reduces the ability of the glass particles to sinter by viscous flow), glass–ceramic scaffolds of 45S5 glass have low strength, in the range reported for trabecular bone [Chen et al., 2006]. A fluorite-containing glass–ceramic (porosity 23.5–50%), formed by sintering particles with a pore-forming (polyethylene) phase, showed strengths (100–150 MPa) comparable to those of cortical bone. However, a common difficulty with glass–ceramic scaffolds for tissue engineering applications is the slow and unpredictable degradation resulting from the presence of the crystalline phase. Furthermore, the advantage of using bioactive glass–ceramics to create scaffolds with high strength has diminished because of the capacity shown recently to create strong porous scaffolds of silicate bioactive glass [Huang et al., 2011; Q. Fu et al., 2011c).
3.10 Bioactive composites
Composites consisting of an organic matrix, typically a biodegradable polymer, and an inorganic phase, typically particles of a bioactive material such as HA or bioactive glass, have attracted interest for tissue engineering applications [Rezwan et al., 2006; Russias et al., 2007]. Commonly, the objective of creating these composites is to improve the strength of the polymer phase, provide a bioactive function to the polymer phase, or to alleviate concerns about the brittleness and mechanical reliability of HA or bioactive glass. However, as the bioactive phase is contained within the polymer phase, its interaction with cells is limited only to the bioactive phase exposed at the surface of the composite.
Polymer coatings have been applied to porous bioactive glass–ceramic scaffolds using poly(D,L-lactic acid), PDLLA [Chen and Boccaccini, 2006], or poly(3-hydroxybutyrate), PHB [Bretcanu et al., 2007]. The coatings enhanced the work of fracture of the scaffolds, but had little effect on the compressive strength (<0.3 MPa). Another effect of the polymer coating is that, initially, it limits the conversion of the bioactive glass surface to HA and prevents the interaction of cells and tissues with the bioactive glass surface.
Nanocomposites composed of discreet nanoparticles of HA or bioactive glass dispersed in a biodegradable polymer matrix can provide improved interaction between the scaffold and cells/tissues. Bioactive glass nanoparticles were incorporated into a dense collagen matrix to mimic the microstructural, mechanical, and biological properties of native bone [Marelli et al., 2011]. The high surface area and high reactivity of the bioactive glass nanoparticles significantly promoted HA deposition on the composite scaffolds upon immersion in SBF. After immersion for 7 days, the elastic modulus of the scaffold increased by 13-fold. The composite scaffold showed the ability to enhance the metabolic activity and alkaline phosphatase activity of pre-osteoblastic MC3T3-E1 cells in vitro.
Bioactive glass (45S5) nanoparticles were dispersed in a PHB matrix, and the mixture was used to form composite films [Misra et al., 2010]. The nanocomposite showed high bioactivity, as evidenced by the formation of HA on its surface upon immersion in SBF, and the ability to support the attachment, proliferation, and differentiation of human MG-63 osteoblast-like cells in vitro. The bioactive glass nanoparticles significantly enhanced the modulus of the matrix when compared to similar composites containing microparticles. They also produced a nanostructured topography on the surface of the composite whereas the microparticles had little effect on the surface topography. The results indicate that bioactive glass nanoparticles can have a significantly greater effect than microparticles on the mechanical, structural, and protein adsorption properties of a biodegradable polymer [Misra et al., 2008].
A composite composed of HA nanoparticles in a PLGA matrix was fabricated into porous scaffolds with high pore interconnectivity by a thermally induced phase separation technique [Huang et al., 2008]. The technique provided good control of the porosity and pore architecture, coupled with a high fraction of HA particles exposed on the surface of the composite for enhanced bioactivity. The presence of the HA nanoparticles in the PLGA matrix directly influenced the mechanical properties and significantly increased water absorption into the composite. The composite scaffolds showed a greater capacity to support proliferation and alkaline phosphatase activity of MSCs than the control, with the optimum enhancement observed at 10 wt% HA nanoparticles.
A problem with the nanocomposites described above is that the nanoparticles, particularly bioactive glass nanoparticles, are difficult to prepare. Another problem is that the nanoparticles are prone to agglomeration, which makes it difficult to disperse them homogeneously throughout the matrix. An alternative approach that has received interest recently for bone and tissue regeneration applications is the formation of inorganic–organic hybrids [Valliant and Jones, 2011; Jones, 2012]. These inorganic–organic hybrid materials are nanocomposites, commonly formed by a sol–gel method, in which the constituent phases interact at a molecular level. Because of the molecular level interactions, the hybrids behave essentially like a single phase, giving rise to controlled degradation and tailored mechanical properties. It has been suggested that combining the bioactivity of the bioactive component with the plasticity of the organic polymer component can lead to the creation of bioactive materials with high toughness which are better able to fulfill the requisite properties for bone tissue engineering [Valliant and Jones, 2011]. However, the preparation of inorganic–organic hybrids for tissue engineering applications can be difficult.
Inorganic–organic hybrids have been made into macroporous scaffolds using a combined sol–gel and freeze-drying process [Valliant and Jones, 2011; Jones, 2012]. In a recent study [Gao et al., 2013], hybrid 3D scaffolds composed of gelatin and bioactive glass (70SiO2–25CaO–5P2O5; mol %) were prepared with a fibrous microstructure using a combined sol–gel and electrospinning technique (Fig. 3.7). The hybrid scaffold had a tensile strength of 4.3 MPa and an elongation to failure of 168% which were significantly higher than the values (0.5 MPa and 63%, respectively), for the gelatin control. Osteoblastic MC3T3-E1 cells cultured on the hybrid showed significantly higher alkaline phosphatase activity than on the gelatin control. The results indicate that these gelatin–BG hybrid scaffolds might have promising potential for bone tissue engineering applications.
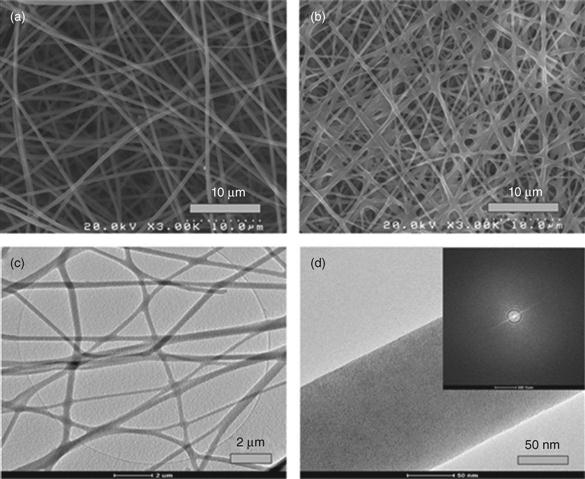
3.11 Conclusions and future trends
Bioactive ceramics, such as the calcium phosphates hydroxyapatite (HA), beta-tricalcium phosphate (β-TCP), and biphasic calcium phosphate (BCP), have been considered for clinical applications since the 1920s, and they have been used in dentistry and medicine for bone repair and augmentation since the 1980s. Despite considerable research since the first bioactive glass (45S5) was reported in 1971, there are few clinical applications of bioactive glass. However, recent developments in the last decade have generated heightened interest in bioactive ceramics and glasses for both bone and soft tissue repair.
The use of more recently developed processing techniques, such as solid freeform fabrication and electrospinning, and the development of new bioactive glass compositions, such as borate bioactive glasses, are showing considerable potential for greater use of bioactive glass in tissue engineering applications. Three-dimensional bioactive glass scaffolds with strengths comparable to human cortical bone are being developed for the repair of large defects in loaded or non-loaded bone. However, in vivo data on the capacity of bioactive glass to regenerate loaded bone, such as segmental defects in the long limb bones, are currently limited.
New bioactive glass compositions, such as borate bioactive glass, and new designs, such as nanofibrous and microfibrous architectures, are showing remarkable results in healing bone infection and soft tissue wounds. Research efforts to understand the role of bioactive glasses in soft tissue healing and to develop optimized bioactive glass treatments for healing soft tissue wounds are expected to grow considerably.
The role of biomaterials in tissue engineering is rapidly changing from a structural role of guiding tissue regeneration to a multifunctional role in which the implant will orchestrate the process of tissue regeneration and provide the body with the capacity to heal itself. The use of bioactive ceramics and glasses to locally deliver inorganic ions that are known to have a beneficial effect on bone regeneration, angiogenesis, treatment of bone infection, and healing of soft tissues is a rapidly developing area. However, little is known about how the ions are effective therapeutically in enhancing bone regeneration and soft tissue healing. While both bioactive ceramics and glasses are being studied, the compositional flexibility of glass makes bioactive glasses particularly attractive as a delivery system for inorganic ions.
Nanocomposites provide considerable potential for the application of bioactive ceramics and glasses in tissue engineering. Conventional nanocomposites composed of hydroxyapatite or bioactive glass nanoparticles dispersed in a biodegradable polymer matrix are showing enhanced biological and mechanical properties when compared to similar composites prepared with microparticles. Inorganic–organic hybrids, composed of interpenetrating networks of inorganic and organic components that interact at the nanoscale, are showing promising potential for providing scaffolds with improved toughness and enhanced interactions with cells and tissues.
Bioactive ceramics and glasses are currently receiving heightened interest as biomaterials for regenerating hard and soft tissues. These efforts are expected to result in the development of implants with improved properties and functionality to better meet clinical needs and for providing costeffective healthcare for a growing world population.
