Biodegradable and bioactive polymer/inorganic phase nanocomposites for bone tissue engineering (BTE)
V. Miguez-Pacheco, University of Erlangen-Nuremberg, Germany
S.K. Misra, University of Birmingham, UK
A.R. Boccaccini, University of Erlangen-Nuremberg, Germany
Abstract:
Scaffolds for bone tissue engineering (BTE) must be mechanically competent and chemically compatible with bone tissue to repair defects. Polymeric materials exhibit suitable mechanical and processing abilities but lack the bioactivity of inorganic ceramics. Composites of these materials are being developed to combine and improve their properties. It has become evident that cells respond to the topography of the surrounding extracellular matrix, specifically to nano-sized features; and reproducing these physical characteristics on the surface of scaffolds improves cell spread and attachment. Electrospinning can produce thin fibres with wide-ranging diameters and offers the possibility of fabricating nanocomposite fibrous scaffolds. This chapter focuses on ascertaining the impact of an inorganic phase on the mechanical properties, bioactivity and cell behaviour of electrospun composite structures.
Key words
nanocomposites; electrospinning; bone scaffolds; bioactivity
4.1 Introduction
A possible medical solution to treat critical size bone defects that occur due to trauma or disease could be based on tissue engineering approaches involving the use of engineered porous structures known as scaffolds. Scaffolds for bone tissue engineering (BTE) are made of suitable (bioactive and biodegradable) bioceramics, polymers or combinations of them, e.g. forming composites (Rezwan et al., 2006). In BTE approaches, the scaffolds, in combination with relevant cells and signalling molecules, should promote the regeneration of new bone tissue. Inorganic bioactive materials for bone tissue engineering exhibit high surface reactivity in contact with physiological fluids, which leads to the formation of strong bonds to bone through suitable biological interactions (Hench and Polak, 2002). The group of inorganic bioactive materials includes silicate bioactive glasses and glass-ceramics, as well as titania, silica, hydroxyapatite (HA) and related amorphous or crystalline calcium phosphates (Hench, 1998). A well-known disadvantage of bioactive ceramics and glasses is their high brittleness. A convenient approach to tackle this disadvantage is to develop composites combining bioactive ceramics with biodegradable polymers, forming biodegradable and bioactive composites (Rezwan et al., 2006).
Our chapter in the previous edition of this volume summarised developments in this field before 2007 (Misra and Boccaccini, 2007). Indeed research in the field of composites based on biodegradable polymers and bioactive inorganic phases for bone tissue engineering started more than 10 years ago and suitable chapters and review papers are available (Holzwarth and Ma, 2011; Gentile et al., 2012; Li et al., 2012).
In recent years, attention of the research efforts has shifted to consider nanocomposites, e.g. composites in which at least one dimension, usually the size of one of the dimensions of the filler particulates, is under 100 nm. Therefore, as an update of our previous chapter on composites for bone tissue engineering (Misra and Boccaccini, 2007), this chapter will discuss specifically the development of combinations of biodegradable polymers and bioactive inorganic particles, added as filler or coating to the polymer matrix, with focus on nanocomposites. The combination of degradable polymers and nanoscale inorganic bioactive particles represents an attractive approach to BTE scaffolds in terms of achievable mechanical and improved biological performance in comparison to conventional composites (without nanoscale features) (Liu and Webster, 2007; Boccaccini et al., 2010).
The chapter will thus discuss the composite material strategy for developing nanostructured scaffolds, including basic aspects of composite materials development such as achieving adequate mechanical properties that closely match those of natural bone tissue, controlled degradation rates and increased bioactivity (Section 4.2). In Section 4.3, the properties of nanocomposites in relation to conventional composites will be discussed, followed by dedicated sections on nanocomposites made by electrospinning, an advantageous technique of choice for the fabrication of suitable nanocomposites for bone tissue engineering (Sections 4.4 to 4.7). Finally, Section 4.8 presents a summary, conclusions and the scope for future developments in the field.
4.2 Composite materials for bone tissue engineering
Composite materials are composed of two or more chemically different phases, e.g. coming from the main materials groups such as metals, ceramics and polymers, which are separated by a well-defined interface. This interface has its own chemical composition and properties. Composite materials are classified based on the type of matrix materials (metals, ceramics or polymers) or on the reinforcement dimensions and morphology (particulates, short fibres, continuous fibres, nanofillers) (Matthews and Rawlings, 1999). The demanding properties of composites required for bone tissue engineering have been discussed in the literature (Rezwan et al., 2006; Misra and Boccaccini, 2007; Boccaccini et al., 2010; Guarino et al., 2012) and relevant aspects will be briefly considered in this section.
Biodegradable composites for BTE applications must exhibit specific properties such as suitable structural integrity, initial competent compressive strength and adequate initial elastic modulus (stiffness) which must be similar to the elastic modulus of bone. Scaffolds must be developed from materials that enable the controlled variation of mechanical strength and elastic modulus during in vivo application so that the necessary support for cell attachment and proliferation is provided while bone repair and regeneration take place. Several reasons have been widely discussed in the literature (Lu et al., 2003; Maquet et al., 2003; Rezwan et al., 2006; He et al., 2010; Xu et al., 2011) to support the combination of biodegradable polymers and bioactive ceramics and glasses for bone tissue engineering applications. On one hand, the polymer–inorganic phase combination usually leads to composites with improved mechanical properties (in comparison to the unreinforced polymer matrix) due to the intrinsic higher stiffness and strength of the bioceramic. Secondly, addition of bioactive phases such as HA, bioactive glasses (BG) or tricalcium phosphate (TCP) to bioresorbable polymers imparts the composite ‘inorganic bioactivity’, i.e. the composite can interact with the surrounding bone tissue by forming a strong bond via the growth of a carbonated HA layer, as mentioned above and discussed in detail by Hench (1998). Moreover, the incorporation of inorganic phases in biodegradable polymers can alter the polymer degradation behaviour because of a pH buffering effect of the fluid around the material and hence the fast acidic degradation of the polymer, in particular in case of poly(lactic acid) (PLA), can be controlled or reduced. Indeed, inorganic filler materials have been shown to influence the degradation mechanism of biopolymers by altering the autocatalytic effect of the acidic end groups resulting from hydrolysis of the polymer chains (Rezwan et al., 2006). The presence of internal interfaces in the composite structure also contributes to increased water absorption given the presence of the more hydrophilic inorganic phases. This effect should be considered when controlling the degradation kinetics of composite scaffolds (Blaker et al., 2003; Kim et al., 2005; Blaker et al., 2011).
The above considerations explain the increasing interest in developing composite materials for tissue engineering. Indeed by varying the volume fraction, shape, orientation and arrangement of the inorganic phase, the relevant properties of the scaffolds can be engineered to match the mechanical and physiological requirements of the tissue to be regenerated (Rezwan et al., 2006; Guarino et al., 2012).
In general two morphologies are usually considered for the inclusion (inorganic) phase in composites: fibres and particulates (Ramakrishna et al., 2001). Moreover the fillers can be ‘conventional’, e.g. of micrometric size or nanoscaled (Boccaccini et al., 2010). It has been shown that increased volume fraction and higher surface area to volume ratio of inclusions favour bioactivity (Rezwan et al., 2006); thus nanofillers could be preferred to enhance bioactivity at the same volume fraction (Misra et al., 2008). On the other hand, for certain applications, the incorporation of fibres is preferred (Dong et al., 2012) as fibres can act as a more effective mechanical reinforcement. Indeed, the mechanical properties are influenced by the reinforcement shape, orientation and size as well as by the distribution of the reinforcement in the matrix and the bonding at the interface between matrix and reinforcement. In the case of scaffolds, which are highly porous structures, the mechanical properties are strongly affected by the porosity (pore volume fraction) and by pore characteristics such size, shape, orientation and connectivity of pores.
4.3 Nanocomposites for tissue engineering
Nanoscale materials, also in the context of composites, are being increasingly considered for tissue engineering (Saiz et al., 2012) given the unique properties acquired by matter when its dimensions are at the nanoscale (1–100 nm). The main differences between the behaviour of nanomaterials and that of the bulk material arise from their smaller size, their enhanced surface effects and their quantum effects (Roduner, 2006), characteristics which play a significant role in the specific chemical reactivity, mechanical, optical, electrical and magnetic properties of nanomaterials. Compared with microparticles, particles in the nanosize range have a very large specific surface area, high particle number per unit mass, a higher proportion of their atoms at the surface and a lower binding energy per atom (Roduner, 2006), which significantly enhances the reactivity of these nanoparticles. This behaviour further leads to changes in their dissolution behaviour, mineralisation, protein adsorption, cell–biomaterial interaction and various other properties of relevance in the biomedical field, as extensively considered by Webster and colleagues (Webster et al., 1999, 2001; Liu et al., 2005; Liu and Webster, 2007). Due to the pivotal position of materials science in the field of tissue engineering, the advent of nanomaterials has had a significant effect in the development of advanced 3D scaffold systems mimicking the nanoscale of natural tissues (Meng et al., 2010). Not only are novel applications involving nanocomposites being increasingly considered, but also the performance and properties of existing composite materials are enhanced by using nanoscale fillers.
As described in previous sections, the development of conventional composites involve the incorporation of bioactive ceramics (particles, fibres) of micrometric size into a biopolymer matrix. This incorporation can be in the form of fillers, coatings or both. The increasing availability of nanoscale bioactive ceramics is contributing to the rapid development of nanocomposites.
Bioactive nanoceramics can be prepared using various methods. The sol–gel technique is one of the most commonly used methods to prepare inorganic bioactive nanoparticles. The shape and morphology of bioactive glass nanoparticles can be controlled by varying the synthesis parameters (Couto et al., 2009; Hong et al., 2009), with microemulsion and gas phase amongst the most commonly used techniques for their synthesis. For the production of inorganic nanofibres, laser spinning can be used, in particular to produce bioactive glass nanofibres (Quintero et al., 2007).
A wide range of nanoscale bioactive inorganic particles such as HA (Kothapalli et al., 2005; Pramanik et al., 2006), TCP (Loher et al., 2006), bioactive glass (Waltimo et al., 2007; Misra et al., 2008) and titanium oxide (Liu et al., 2005) have been used as reinforcement materials in biopolymer matrix composites. The enhanced material properties due to the addition of nanoscale bioactive ceramics have been demonstrated by several authors (Kothapalli et al., 2005; Pramanik et al., 2006; Misra et al., 2008). In the case of polymer composites containing bioactive glass filler particles, a direct comparison between micron-scale bioactive glass and nanoscale bioactive glass composites has been carried out using P(3HB) as the biopolymer matrix (Misra et al., 2008). It was shown that addition of bioactive glass nanoparticles significantly improved the mechanical properties, hydrophilicity, protein adsorption as well as induced a nanotopography suitable for enhanced cell growth. The P(3HB)/bioactive glass nanocomposites also showed cytocompatibility towards MG-63 osteoblast-like cells. A summary of results is presented in Fig.4.1 (Misra et al., 2008).
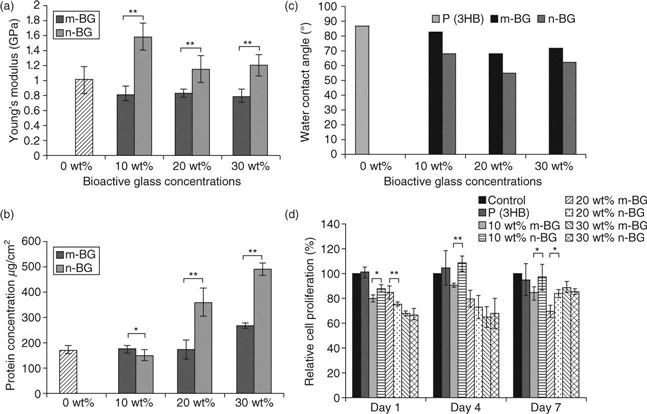
Pramanik et al. (2006) have shown that the addition of HA nanoparticles to poly(ethylene-co-acrylic acid) results in a significant increase in strength and modulus when compared with HA microparticles. In terms of microstructure of nanocomposites, Wei and Ma (2004) showed that nano-HA particle reinforced poly(L-lactic acid) (PLLA) composite scaffold exhibited a better dispersion of HA, which led to superior mechanical properties of the scaffolds compared to those with similar concentration of micron-sized HA particles. It is recognised that for a given mass of material there are significantly greater numbers of nano-sized particles than micronsized particles, and therefore, even at low particulate concentrations, the properties of nanomaterials are highly affected by the presence of the nanoscale phase. For example, 10 wt% bioactive glass nanoparticles can provide a significantly greater number of sites for HA nucleation compared to 10 wt% micron-sized bioactive glass particles. Loher et al. (2006) demonstrated that the enhanced specific surface area of nanoparticles can also improve the degradation and bioactivity for TCP nanoparticles compared to micron-sized particles. Thus the smaller size and greater surface area of nanomaterials further affect the surface reactivity and topography influencing the interaction with biological molecules. In the context of bone tissue engineering, nanocomposites mimic more closely the structure of natural bone, which can be represented as a highly hierarchical nanocomposite involving nanoscale HA crystallites and collagen (Fratzl et al., 2004).
Although the use of nanoparticles in biocomposites has several advantages, as outlined above, agglomeration of nanoparticles during composite processing remains an important issue to address. A poor dispersion of nanoparticles within the polymer solution or melt leading to agglomeration will negatively affect the final distribution of the nanoparticles in the matrix leading to materials with low mechanical properties. Surface modified ceramic nanoparticles are proposed to enable a more homogeneous dispersion of the nanoparticles. Extensive research is being devoted to the development of suitable functionalisation methods for nanoparticles for application in biomedical composites (Horch et al., 2004; Balasundaram et al., 2006; Yoo et al., 2009; Saiz et al., 2012).
Specific nanoparticles can be also used in biocomposites to impart extra functionalities. For example, carbon nanotubes have become a popular component for designing composite materials due to their attractive physical and mechanical properties and also because they can be used to induce nanostructured surface topographies (Misra et al., 2010; Sahithi et al., 2010; Mattioli-Belmonte et al., 2012; Pan et al., 2012). Silver nanoparticles represent another example of nanoparticles used in polymer/ceramic composites due to their proven antimicrobial properties. A recent study (Koizhaiganova et al., 2011) showed that incorporation of 20 nm silver nanoparticles in gelatin/HA nanocomposite films can render strong bactericidal properties against various Gram-positive and Gram-negative bacteria. Core–shell nanoscale structures are also novel nanomaterials for use in BTE. Usually the core of such particles can be composed of silica, silver, iron, etc. and the shell is made of the bioactive component such as HA (Neumeier et al., 2011) or calcium phosphates (Dembski et al., 2011). Such multi-component composite systems impart multifunctionality (optical, magnetic, drug carrier, etc.) to the biocomposites and represent interesting emerging biomaterials that can have a huge impact in the field of tissue engineering. One attractive processing technique to incorporate nanoscale features in BTE scaffolds is electrospinning, which will be discussed in detail in the next section.
4.4 Electrospinning
Electrospinning is now recognised as a practical, cost-effective, reliable and fast fabrication method that produces fibres with a controlled range of diameters in the micro- and nanoscale and can be used to make constructs with morphologies and mechanical properties similar to the extracellular matrix (ECM) of bone, exhibiting a large surface area to volume ratio and high porosity (Su et al., 2007; Jang et al., 2009). This method relies on an electrostatic force to draw out fibres with a very small diameter, ranging from the micro- to nanoscale, from a needle connected to a voltage source. The electrospinning solution is fed to this needle via a syringe, and the flow rate is controlled by means of a pump. The electrospun fibres are deposited onto a grounded collector, and their morphology and diameter are controlled by several parameters including the viscosity of the electro-spinning solution, flow rate, voltage applied and distance between the needle and collector (Fujihara et al., 2005).
Cells are known to react to the topography of the surfaces with which they come into contact. As mentioned above, it is now accepted that nanoscale features have a high impact on cell behaviour. This has been evidenced by increased cell attachment and proliferation on different nanostructures (Webster et al., 2001; Liu et al., 2005; Liu and Webster, 2007), thus electrospun fibres mimic the native ECM and provide adequate feature size for cells. Many different combinations of natural and synthetic polymers with inorganic nanoparticles have been explored to produce composite scaffolds by electrospinning. The following sections cover this emerging family of nanocomposite scaffolds based on electrospun fibres, discussing also the properties of a selection of nanofibrous scaffolds in comparison to purely polymeric or inorganic nanofibres.
4.5 Electrospun composite scaffolds based on natural polymers
4.5.1 Chitosan (CTS)–hydroxyapatite (HA) composite nanofibres
Chitosan (CTS) is a natural polymer with excellent biocompatibility, biodegradability and mechanical properties, but which presents some difficulties for processing by electrospinning given that it can only be dissolved in a few solvents, and that solutions prepared at low concentrations exhibit a high viscosity. Zhang et al. (2007, 2008) produced composite nanofibres of HA–CTS (30:70 mass ratio) dissolved in a 10 wt% aq. acetic acid and dimethyl sulfoxide together with a small amount (10 wt%) of ultrahigh molecular weight poly(ethylene oxide) as a fibre-forming facilitating agent. An X-ray diffraction (XRD) pattern of the electrospun composite fibres showed that the HA nanoparticles were demineralised due to the acidic conditions needed to achieve a suitable electrospinning solution. This demineralisation caused a decrease in the crystallinity of the HA nanoparticles and, thus, a decrease in particle size. Following an immersion in an ethanol buffered mild alkaline solution, the HA nanoparticle size was found to return to its original size, whilst the nanofibre morphology remained intact. The nanofibres produced were seeded with human foetal osteoblasts (hFOB) and cultured for 15 days. The composite nanofibres showed improved bone formation compared to pure electrospun CTS scaffolds as measured by cell proliferation, morphology and mineralisation.
Following these results, Peng et al. (2012) fabricated HA/CTS nanofibrous scaffolds to assess whether they supported the osteogenic differentiation of murine mesenchymal stem cells (mMSCs) compared to pure CTS scaffolds. mMSC cells were cultivated on the nanofibrous meshes in the presence and absence of osteogenic medium for 7 days and the changes in morphology, population growth, osteogenic gene expression and protein expression of the seeded cells were observed. Scanning electron microscopy (SEM) images of the scaffolds at 1 and 3 days of culture showed that the mMSC cells adhered and spread significantly more on the HA/CTS scaffolds than on the pure CTS scaffolds, and methylthiazol tetrazolium (MTT) assays of the cell population at 1, 4 and 7 days demonstrated that the cells proliferated on all nanofibrous constructs, although there was a more evident increase in cell population on the composite scaffolds. Crucially, the cells seeded onto the nanocomposite scaffolds that were cultured in the absence of osteogenic medium were found to have an increased transcription and expression of genes thought to be involved in osteoblast maturation (COL I, Runx2, ALP and OCN) as well as exhibiting bone marker proteins characteristic of osteoblastic differentiation (ALP and Collagen I) compared with cells seeded onto pure CTS scaffolds cultured under the same conditions. When the seeded nanofibrous scaffolds were placed in osteogenic medium, the osteogenic differentiation of mMSC cells was potentiated by the osteogenic supplementation and thus these cells exhibited an earlier and higher expression of the aforementioned genes and bone marker proteins.
These results show that the HA/CTS nanocomposite scaffolds are able to support mMSC cell differentiation into osteogenic lineages without osteogenic supplementation and outperformed pure CTS scaffolds in terms of cell growth, proliferation, attachment and differentiation. Thus the nanocomposite scaffolds were proven to be osteoinductive and osteoconductive, making them very attractive options for BTE, although further investigation including in vivo testing is needed.
4.5.2 Gelatin–HA composite nanofibres
Gelatin is a natural biopolymer derived from collagen which is widely used in the medical field owing to its excellent biocompatibility, ease of processing, commercial availability and low cost (Kim et al., 2005; Francis et al., 2010).
In order to achieve a bone-like structure at the nanoscale, Kim et al. (2005) exploited a processing route that involved the preparation of a gelatin–HA nanocomposite solution by biomimetic precipitation to ensure a uniform distribution of the HA nanoparticles within the polymer matrix. This nanocomposite was freeze dried and dissolved in an organic solvent (1,1,1,3,3,3-hexafluoro-2-propanol) at 20%, 40% and 60% HA. The solution was then electrospun and the nanofibres obtained were cross-linked to increase their chemical and structural stability. XRD analysis and Fourier-transform infrared (FTIR) spectra of the samples demonstrated the presence of apatite in the gelatin–HA nanocomposite, and this structural and chemical data was found to be equivalent to that of collagen–HA nanocomposites and natural bone matrix. SEM images of the 20% and 40% HA-containing nanofibres showed that they possessed uniform and continuous fibre morphology with diameters in the range 200–400 nm. However the 60% HA nanofibres showed extensive bead formation and non-continuous fibres (Fig. 4.2). Transmission electron microscopy (TEM) analysis of the samples showed a uniform distribution of the HA nanocrystals within the electrospun fibres (Fig. 4.3). Tensile strength tests revealed that the addition of HA nanocrystals to the gelatin matrix increased the stiffness, preserved the mechanical strength and decreased the strain at failure of the composite nanofibres compared to pure gelatin nanofibres.
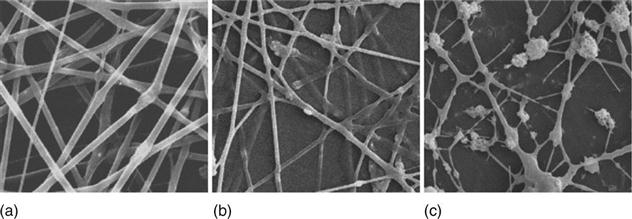
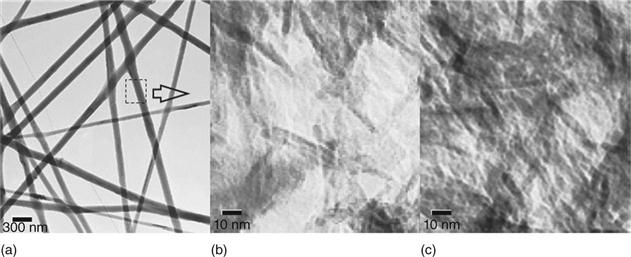
To assess the biocompatibility of the nanocomposite fibres, bone-derived cells (MG63) were seeded onto them and cultured for 7 days. The cells exhibited a normal morphology and intimate contact with the nanofibres. Measurement of alkaline phosphatase (ALP) activity expressed by the cells revealed a significantly higher bone-forming activity of cells seeded on the HA–gelatin nanocomposites compared to the pure gelatin nanofibres, thus demonstrating an improvement of the osteoblastic activity on the nanocomposite fibres.
Another approach to produce a gelatin–HA biocomposite was exploited by Francis et al. (2010) by the simultaneous electrospinning of a pure gelatin solution and electrospraying of a HA and gelatin solution onto a rotating drum. Also, HA–gelatin blend solutions in proportions of gelatin to HA of 4:1 and 2:1 by weight ratio were electrospun. The composite nanofibres were cross-linked by immersing them in a 50% glutaraldehyde solution. SEM micrographs of the electrospun nanofibres revealed the gelatin–HA blend nanofibres to have similar diameters, which were also significantly smaller than those of the pure gelatin nanofibres (around 440 and 590 nm respectively). The porosity of the electrospun nanofibres was found to be in the range 83–88% with a pore size between 0.69 and 1.26 μm. Tensile strength tests were used to analyse the mechanical strength and strain at break of the nanofibrous scaffolds. As expected, cross-linked samples exhibited greater tensile strength than non-cross-linked samples and the gelatin–HA biocomposites showed increased stiffness and decreased strain at break compared to pure gelatin scaffolds.
hFoB cells were seeded onto the electrospun scaffolds to analyse their interaction for up to 15 days. Cell proliferation was found to be significantly higher on the spin-sprayed gelatin–HA scaffolds than on their blended counterparts, and it was comparatively lower on pure gelatin scaffolds. ALP activity of hFoB cells on the nanocomposite scaffolds was considerably higher than on pure gelatin scaffolds, and within the nanocomposites, spinspray scaffolds showed the highest ALP activity. Mineralisation was quantified by Alizarin Red (ARS) studies which revealed that the gelatin–HA spin-spray scaffolds elicited a significantly higher degree of mineralisation than all other materials investigated.
4.5.3 Silk-HA composite fibres
Silk is a natural protein fibre that possesses excellent mechanical properties and high biocompatibility, and thus has considerable potential in the field of BTE (Burdick and Mauck, 2011). Li et al. (2006) produced silk fibroin scaffolds containing bone morphogenic protein 2 (BMP-2) and/or hydroxyapatite nanoparticles (nHA) by electrospinning. The scaffolds were divided in five different groups: silk/polyethylene oxide (silk/PEO) scaffolds (group I), silk/PEO scaffolds where the PEO was extracted (group II), silk/PEO/BMP-2 scaffolds (group III), silk/PEO/nHA scaffolds (group IV) and silk/PEO/nHA/BMP-2 scaffolds (group V), where groups I and II were used as controls. All scaffolds were treated with methanol to eliminate their solubility in water and thus ensure their integrity during cell culture experiments. The same silk/PEO base solution was used to electrospin all scaffolds and was made to a ratio of 5 parts aqueous silk fibroin solution (9 wt%) and 2 parts aqueous PEO solution (5 wt%).
SEM micrographs of the electrospun samples revealed that scaffolds from all groups were composed of uniform and randomly aligned fibres with a fibre diameter of about 550 nm on average. Samples from all groups were analysed using attenuated total reflectance-Fourier transform infrared spectroscopy (ATR-FTIR) to confirm the presence of nHA. The IR spectra belonging to nanofibres containing nHA showed different features in the range of 900–1200 cm−1 compared to fibres void of nHA, mostly related to phosphate peaks, thus confirming the presence of nHA within the silk nanofibres. TEM micrographs of the samples confirmed the presence of nHA, but although the electrospinning solutions were sonicated and stirred prior to scaffold production to ensure an even distribution of nanoparticles within the fibres, aggregates of nHA particles were identified.
Scaffold samples from all groups were seeded with human mesenchymal stem cells (hMSC) and placed in culture, and after 14 days it was observed that the surfaces of the nanofibrous membranes were almost completely covered by cells and/or extracellular matrix. After 31 days in culture, the calcium and total DNA contents of samples from all groups were measured, and it was found that group I scaffolds exhibited the lowest calcium and the highest DNA content, while hMSCs had a lower level of differentiation. All other groups contained similar DNA amounts. Groups III, IV and V showed a significant increase in calcium levels but, notably, group V samples had the highest calcium deposition. The expression of bone-related markers by the cells cultured for 14 days, specifically BMP-2, bone sialoprotein (BSP) and collagen type I, was measured by real-time reverse transcription polymerase chain reaction. Groups III and V showed the highest levels of BMP-2 and BSP expression, although collagen type I expression was significantly higher in group III than in the other groups.
These results showed that the incorporation of BMP-2 and nHA into silk fibroin scaffolds improved bone formation in vitro, and demonstrated that silk-HA fibres can be employed as a carrier for BMP-2 to enable its sustained delivery within the culture medium.
4.6 Electrospun composite scaffolds based on synthetic polymers
4.6.1 Poly(ε-caprolactone)-silica nanoparticle composite fibres (PCL/nSiO2)
Poly(ε-caprolactone) (PCL) is a polymer extensively used in tissue engineering applications because of its excellent biocompatibility although for BTE it has inadequate bioactivity. Silica is an important component for bone formation that, when added to polymeric matrices, improves the mechanical behaviour of scaffolds and stimulates the osteogenic behaviour of pre-osteoblastic cells (Kang et al., 2009).
Ganesh et al. (2012) added 0.5 and 1 wt% silica nanoparticles (nSiO2) of 10 nm average particle size to a 14 wt% PCL solution, which was electrospun into nanofibrous composite scaffolds to improve their mechanical properties and increase their bioactivity.
The mechanical properties of the electrospun scaffolds were examined by uniaxial tensile testing of pre-cut samples, which showed that the nanocomposite scaffolds were superior to pure PCL samples in terms of strength. TEM images showed that the silica nanoparticles were both partially and fully embedded within the polymeric nanofibres and SEM images showed uniform fibres that were randomly oriented with diameters ranging from 150 to 350 nm. FTIR analysis of the electrospun composite scaffolds confirmed the presence of the silica nanoparticles in the scaffolds, which after immersion in simulated body fluid (SBF) solution for 21 days showed deposition of HA proportional to the amount of nSiO2 contained in the scaffolds, confirmed by the appearance of phosphate peaks, whereas pure PCL samples did not exhibit such changes. This increase in mineralisation can be attributed to the greater surface area covered by silica nanoparticles as their ratio in the polymeric matrix increases.
Nanocomposite and pure PCL samples were seeded with hMSCs to investigate the effect of silica nanoparticles on cell behaviour and, in parallel, hMSCs were cultured in media containing the same silica nanopowder ratios as in the electrospun fibres. It was found that although the nanoparticles had no effect on the proliferation rate of the cells, free-state silica nanoparticles were ingested, causing cell death even at the lowest concentration. On the other hand, fibres containing the same concentrations of silica nanoparticles showed similar cell viability compared to the positive control (pure PCL scaffolds) as the nanoparticles could not be taken up by the cells. The seeded scaffolds were cultured with normal and osteogenic media and the ALP activity of the cells was measured at 1, 7, 14 and 21 days. Results showed that the cell population proliferated in the first 7 days and the ALP activity remained relatively constant for all samples. However, after day 7 the registered ALP activity of cells cultured on scaffolds containing 1% silica nanoparticles and in osteogenic medium was the highest, suggesting that the combination of the inorganic particles and culture medium gave the maximum differentiation potential. Thus, it can be concluded that the addition of silica nanoparticles to PCL fibres enhances the mechanical and biological properties of the electrospun scaffolds, making them interesting for BTE strategies.
4.6.2 PCL and calcium carbonate (CaCO3) composite fibres
Calcium is indispensable in the formation of new bone and a BTE scaffold should provide a sufficient amount of this mineral to provide cells with an ideal osteogenic environment. Calcium carbonate (CaCO3) has been proven as an osteoconductive bone filling material which can provide a calcium rich environment under culture conditions. Fujihara et al. (2005) produced guided bone regeneration membranes aimed to cover exposed bone implants or material filled bone defects in order to avoid scar-tissue formation.
Two different ratios of PCL : CaCO3 were used, 75:25 (A) and 25:75 (B) wt%, and dissolved in a mixture of 75 wt% chloroform and 25 wt% methanol at 3, 5 and 7.5 wt%. These solutions were electrospun onto supporting PCL nanofibrous membranes used as a mechanical support layer, and the resulting membranes underwent plasma treatment to improve surface wettability. Only the combination of solution with ratio (A) containing 7.5 wt% PCL and at ratio (B) containing 5% PCL produced continuous fibres without beads, although the latter exhibited a granulated surface when examined by SEM.
Plasma treatment of the surface of the composite nanofibres for 10 minutes dramatically increased their hydrophilicity, considered to be crucial for cell attachment and proliferation. Tensile testing of the samples showed that the addition of CaCO3 decreased the strain at failure of the nanocomposite meshes compared to pure PCL nanofibres. Membrane (B) (PCL:CaCO3 = 25:75 wt% + PCL) could be stretched to around 200% strain without breaking, which is considered to be sufficient for membranes used in surgical applications. In any case, the mechanical properties of the nanofibrous membranes could be adjusted by changing the thickness of the PCL mechanical support layer.
The interaction of human foetal osteoblast cells (hFOB1) with the nanocomposite meshes in culture for 5 days was investigated and it was found that cell proliferation rates on membrane (A) (PCL : CaCO3 = 75:25 wt% + PCL) were not significantly different from those of cells seeded on tissue culture polystyrene (TPCS), whereas the proliferation rates on membrane (B) (PCL : CaCO3 = 25:75 wt% + PCL) were significantly lower. This difference is thought to arise from increased cell differentiation on membrane (B), given that a high amount of granulates consistent with mineralisation were observed on its surface. SEM micrographs of the seeded membrane showed intimate contact between the cell membrane and the nanofibres, which indicates good cell attachment.
4.6.3 PCL and β-tricalcium phosphate (βTCP) composite fibres
The natural microstructure of bone tissue shows spatial differences and varying concentrations in its two-phase composition. This suggests that the engineering of scaffolds for tissue replacement should focus on producing graded scaffolds to more closely match the in vivo characteristics of tissue. Erisken et al. (2008a, 2008b) investigated the use of twin screw extrusion-electrospinning (TSEE) process, which allows a time-dependent feeding of different solid or liquid phases and their thermal processing and mixing within a twin-screw extruding chamber equipped with intermeshing and co-rotating screws. This chamber was connected to a split die that allowed electrospinning of multiple fibres. A schematic representation of this set-up is shown in Fig. 4.4. In a preliminary study, and as a demonstration of the capability of TSEE to produce electrospun nanofibres with a uniform distribution of incorporated nanoparticles, Erisken et al. (2008a) produced PCL–βTCP composite nanofibres by TSEE and found that the integrated technique successfully generated such nanofibres. Mechanical testing of the fibrous meshes demonstrated that the addition and uniform distribution of βTCP nanoparticles increased their tensile strength and water contact angle measurements, showing that the hydrophilicity of the nanofibres increased as the concentration of the βTCP nanoparticles within the polymer increased. The TSEE process also allowed for the production of non-woven and randomly oriented nanofibres and fibres with diameters in the range 200–2000 nm and with a concentration of βTCP ranging between 0 and 15% wt (Erisken et al., 2008b), in other words, fibrous meshes with a βTCP concentration gradient. The resulting scaffolds showed well-interconnected pores with pore size ranging between 5 and 50 μm. Thermo-gravimetric analysis of a cross-section of the electrospun scaffold showed that the concentration of βTCP particles increased from 0 at the bottom to 15% wt at the top end of the scaffold, while von Kossa staining further revealed the gradual increase of βTCP nanoparticle concentration from the bottom to the top of the scaffold. The mechanical properties of specific sections of the graded meshes containing different concentrations of βTCP (0, 6 and 12% wt βTCP) nanoparticles were investigated by loading said sections in tension. Results obtained showed that as the amount of βTCP increased, the stress at break and elastic modulus of the samples increased from 810 to 1080 kPa and from 18.5 to 27.5 kPa respectively, whereas the elongation decreased from 259 to 171%.
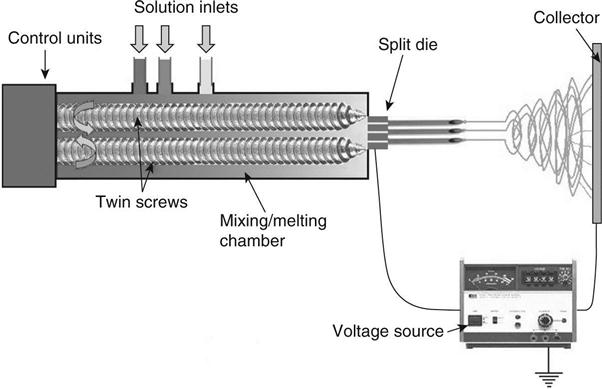
MC3T3-E1 cells were seeded onto the surface of a tissue culture plate, pure PCL and PCL/βTCP fibres with 15% wt βTCP. After 24 h in culture, the pure PCL scaffolds showed moderately good cell adhesion, whereas on the PCL/βTCP scaffolds the cell attachment was relatively poor compared to that on the surface of the tissue culture plate. Similarly, the proliferation rate of MC3T3-E1 cells was lower on pure PCL scaffolds compared to the tissue culture plate and even lower on the PCL/βTCP scaffolds after 72 h in culture. The behaviour of the cells is thought to arise from the increase in surface irregularities in the PCL/βTCP scaffolds, which impair the formation of mature and stable focal adhesions and hinder their proliferation. Another cause for the limited proliferation observed on the βTCP containing scaffolds may be related to an accelerated differentiation process, triggered by the βTCP nanoparticles, which decreases the proliferation rate of a cell population. Regardless, graded PCL/βTCP scaffolds seeded with pre-osteoblastic MC3T3-E1 cells and kept in culture for 4 weeks showed complete cell infiltration and near complete differentiation of the cell population into osteoblasts. This was confirmed by histological examination of the scaffolds after culture, which found the formation of ECM, mineralisation and deposition of type I collagen onto the scaffold.
4.6.4 PLC and HA composite fibres
Li et al. (2012) produced PCL/nHA nanofibrous scaffolds by electrospinning a solution of nHA pre-treated with γ-glycioxypropyltrimethosilane (A-187) mixed with untreated nHA and PCL dissolved in N,N-dimethyl formamide (MC/DMF) to a concentration of 8% wt nHA/PCL. The HA nanoparticles were treated with A-187 to improve their dispersion in the PCL solution to ensure an even distribution. Mechanical testing of the nanofibrous mats showed that the addition of A187 to nHA/PCL nanofibres resulted in a significantly higher tensile strength compared to nHA/PCL and pure PCL scaffolds, whilst also allowing enough deformation to qualify as a guided bone regeneration scaffold. This enhancement is thought to arise due to the uniform distribution of nHA in PCL achieved through the addition of A187. The prepared scaffolds were immersed in concentrated SBF (1.5 × SBF) for up to 7 days to assess their in vitro bioactivity, and the atomic concentrations of calcium and phosphate ions in the soaking solutions were measured daily. For the nHA containing composites, these concentrations were found to decrease with soaking time whereas for pure PCL nanofibres the concentrations of Ca and P remained unchanged. This decrease in concentration of calcium and phosphate ions in solution is indicative of apatite formation on the surface of the nanofibres, thus proving their bioactivity. There was no significant difference between the consumption rates of calcium and phosphate ions between the nHA/PCL and A187–nHA/PCL nanocomposites.
MG63 cells seeded onto the nanocomposite fibrous scaffolds were found to proliferate at similar rates to the control group and on PCL fibrous scaffolds. This result suggests that the addition of A187-nHA to the PCL matrix did not affect cell viability and proliferation.
4.6.5 PCL–bioactive glass (BG) composite fibres
Jo et al. (2009) produced two different composite electrospun nanofibres of silica-based bioactive glass (BG) and PCL: in one case the BG was in powder form (BGP) and in the other, BG nanofibres (BGNF) were produced by electrospinning and incorporated into the PCL organic matrix. Silica-based BG was chosen because of its excellent bioactivity and was prepared in a composition (in mol %) of: 60% SiO2, 36% CaO and 4% P2O5.
The BG : PCL ratio used in both cases was 20:80, since preliminary in vitro cell tests with a pre-osteoblast cell line showed that this ratio provided the highest cell attachment and metabolic activity, indicating higher bioactivity than pure PCL membranes and other ratios of BG and PCL. These preliminary tests involved measuring the osteoblastic activity, i.e. viability and proliferation. The BGNF composite showed a higher bioactivity and significantly higher elastic modulus than pure PCL membranes and the BGP composite because the BGNFs were shown to be uniformly distributed in the PCL matrix whereas the BGP particles were agglomerated, as shown by SEM. Cranial bone defects in rats were covered with PCL and BGNF composite membranes and new bone formation was observed and compared with empty defects used as controls. The rate of bone regeneration was shown to be significantly higher in the case of the BGNF composite treated defects than in pure PCL membrane and empty defects.
In a study involving the same organic and inorganic phases, Allo et al. (2012) produced hybrid PCL/BG nanofibres by electrospinning a solution produced by sol–gel that allowed the introduction of the polymer phase within the inorganic network as it was being formed. This was done by adding BG precursors to a PCL solution under constant vigorous stirring at 35 °C for 72 h until a homogeneous solution was obtained. This solution was then placed in a furnace at 50 °C for another 72 h for polycondensation and network formation. By using calcium chloride dihydrate (CaCl2 · 2H2O) as a calcium precursor instead of calcium nitrate, there was no need to treat the electrospun fibres at 600 °C at least to remove the toxic nitrate residues from the matrix, thus also conserving the PCL matrix intact in a less labour-intensive manner.
In order to gauge the homogeneity of the hybrid system, the electrospun samples were analysed using SEM and energy dispersive X-ray spectroscopy (EDX), which showed that the fibres had a smooth surface with a homogenous distribution of carbon, calcium, phosphorus and silicon atoms. FTIR analysis indicated that the inorganic and organic phases within the hybrid nanofibres interacted via hydrogen bonding of the carbonyl group of PCL and silanol hydroxyl groups of the silica network, whereas XRD analysis further demonstrated the hybrid nature of the fibres by showing that the material was amorphous, indicating that the semi-crystalline structure of the PCL interacted with the amorphous BG nanoparticles.
4.6.6 PCL and aluminium oxide (Al2O3) composite fibres
Aluminium oxide (Al2O3) is a bioceramic widely used in various medical applications and which is also cost-effective, biocompatible and has good mechanical properties including high corrosion and wear resistance. Dong et al. (2012) produced PCL and Al2O3 composite fibre membranes by using a 10 wt% PCL sample in a dichloromethane (DCM) and methanol solvent mixture at a 4:1 ratio. A 20 wt% suspension of Al2O3 whiskers of between 2 and 4 nm in diameter and Al2O3 nanoparticles in ethanol was added to produce samples containing 1, 10 and 20% Al2O3 whiskers/PCL and 20% Al2O3 nanoparticles/PCL. Al2O3 whiskers were used because they showed a strong interface adhesion with the polymer matrix and also they would be oriented in parallel to the electrospun nanofibres. To verify that the Al2O3 whiskers had been properly incorporated into the polymer membrane, XRD analyses were carried out and compared with the pattern of pure Al2O3 whiskers. SEM micrographs indicated that there was a uniform distribution of nanofibres within the electrospun membranes, which also exhibited interconnected pores. Pure PCL nanofibres were found to have a smooth surface, whereas the addition of Al2O3 whiskers caused a roughening of the surface in the composite nanofibres, increasing the surface area of the composite membranes which more closely resembled the natural bone ECM. TEM micrographs of the composite fibres showed that the Al2O3 whiskers were evenly distributed within the PCL matrix and that they were aligned almost parallel to the direction of the fibre. The tensile strength of the composite membranes was tested and compared with that of pure PCL membranes. It was found that an increase in the Al2O3 whisker content caused an increase in the ultimate tensile strength of the nanocomposites up to 10 wt%, above which the mechanical integrity of the nanocomposites decreased. The elastic modulus of the samples increased significantly with an increase in the Al2O3 whisker content.
To assess cell adhesion and proliferation on the electrospun membranes, pure PCL membranes used as controls and 10 wt% Al2O3 whisker/PCL composite membranes were seeded with human bone marrow stromal cells (hBMSC5077-GFP) for up to 48 h and examined at 6, 24 and 48 h. These tests showed that there was no significant difference between the initial and final cell numbers in the samples, although there was a considerable increase in the cell population between the two time points. The cells seeded on both nanofibrous membranes exhibited a spindle-like morphology. These results indicated that the addition of Al2O3 whiskers did not negatively affect the cell proliferation and adhesion compared to pure PCL membranes and thus Al2O3 whisker/PCL composite membranes are a promising alternative for tissue engineering scaffolds. Aspects related to the in vitro and in vivo degradation behaviour of the scaffolds and the persistency of the alumina whiskers remain to be investigated.
4.6.7 PLLA–HA hybrid membranes
Sui et al. (2007) fabricated PLLA/HA hybrid membranes by electrospinning a solution composed of PLLA dissolved in chloromethane and HA nanoparticles suspended in 1,4-dioxane. These hybrid membranes were compared with pure PLLA membranes in terms of mechanical and structural properties, in vitro degradation and MG-63 osteoblast cell growth and adhesion. Structurally, specifically in terms of specific surface area and cumulative pore volume, PLLA/HA composites showed significantly higher values than pure PLLA membranes indicating that the hybrid membranes would allow better cell penetration and growth. Additionally, the larger specific surface area indicates a rough nanofibre surface, which may have contributed to increased cell attachment and spread. FTIR analysis of the samples revealed that the distribution of HA particles within the polymeric matrix of PLLA/HA nanocomposites was uniform and, furthermore, HA nanoparticles were found to have reacted with PLLA, resulting in strong surface bonding. The addition of HA nanoparticles to the polymer matrix resulted in increased elastic modulus and lower strain at break of the PLLA/HA hybrid membranes compared with pure PLLA membranes. In vitro degradation tests showed a significantly faster mass loss and higher water uptake by the pure PLLA membranes, resulting in increased nanofibre breakage and a decrease in diameter. This effect was supported by the greater decrease in the molecular weight of pure PLLA membranes compared with that in PLLA/HA composite membranes after 8 weeks of immersion in phosphate buffered solution (PBS). Analysis of the pH of this solution throughout this timespan showed that the degradation of pure PLLA produces a steady decrease in pH, whereas the PLLA/HA hybrid membranes exhibited a relatively stable neutral pH. Osteoblast cell culture with degradation medium was carried out, and the cultures containing PLLA/HA membrane medium were found to exhibit greater cell viability and proliferation than those cultured in pure PLLA membrane medium. Similarly, cell adhesion was significantly higher on PLLA/HA hybrid membranes.
4.6.8 PLLA, PCL and HA composite fibres
Liao et al. (2012) produced electrospun nanofibrous scaffolds from a combination of PLLA, PCL and HA for BTE strategies. PCL was added to the composite to counteract the relative brittleness of PLLA and HA nanoparticles were included to increase the bioactivity and mechanical properties of the electrospun scaffolds. The electrospinning solution was made by dissolving PCL and PLLA at a weight ratio of 1:1 in a solvent mixture of methanol and chloroform at 1:3 volume ratio under constant stirring and subsequently adding HA nanopowder. Ultrasonication was used to disperse the HA nanoparticles. This solution was then electrospun onto a grounded drum rotating at 3000 rpm to produce three different scaffolds, i.e. PLLA/PCL with 0, 10 and 20 wt% HA. TEM and SEM analyses on the scaffolds showed aligned non-woven fibres with diameters in the nanoscale. The pure PLLA/PCL samples had smooth surfaces, whereas the fibres that contained HA were rougher, owing to the fact that the HA nanoparticles agglomerated within the fibres, thus causing beading. This behaviour caused an increase in the diameter of the electrospun fibres as the HA fraction increased, and as a consequence, the porosity of the scaffolds also increased. Energy dispersive X-ray spectroscopy (EDX) of the PLLA/PCL/HA composite samples confirmed the presence of HA due to sharp calcium and phosphate peaks in the sample spectrum. The water contact angle measurement of the samples showed that the addition of HA nanoparticles increased the hydrophilicity of the samples, although they remained hydrophobic overall.
Degradation investigation of the scaffolds in PBS showed that the samples containing higher percentages of HA exhibited greater weight loss and marked thinning of fibres. This can be explained by the hydrophilicity of HA nanoparticles which allows diffusion of water molecules into the fibres and thus facilitates hydrolysis of the polymer chains. It can be concluded that the increased hydrophilicity and porosity imparted to the fibrous scaffolds by the HA nanoparticles improved their biodegradation and, by varying the composition of the nanocomposites, scaffolds with controllable degradation profiles suitable for TE can be produced.
4.6.9 PLA, HA and graphene oxide (GO) composite fibres
PLA is a synthetic polymer with mechanical properties suitable for tissue engineering and is also biodegradable and biocompatible (Rezwan et al., 2006). Ma et al. (2012) produced composite fibrous membranes of PLA with added HA and graphene oxide (GO) nanoparticles. GO has excellent mechanical properties and a high hydrophilicity owing to the high density of oxygen containing groups in its structure. A precursor solution was made with a suspension of HA and GO powders in a solution of dimethylformamide (DMF) and dimethyl carbonate (volume ratio 2:3) to which PLA was added at a concentration of 12 wt%. This precursor solution, along with pure PLA and PLA/HA solutions, was electrospun producing nanofibrous membranes that were seeded with MC3T3-E1 pre-osteoblast cells.
Full chemical and morphological characterisation of the nanocomposite membranes was carried out using various techniques. SEM images of the electrospun fibres showed that the addition of the nanoparticles caused an increase in the surface roughness of the fibres, which otherwise appeared to have uniform diameters and 3D structures. XRD analysis of the nanocomposite samples showed that the crystallisation of the PLA phase in the composite nanofibres had been disrupted due to the addition of the inorganic powders but, more importantly, HA peaks were significantly weakened and GO peaks were altogether absent, demonstrating a good dispersion of these inorganic phases within the polymeric matrix. FTIR and Raman analyses of the samples demonstrated that PLA/HA/GO nanofibres had a very similar spectra to PLA nanofibres due to the overlap of HA and GO peaks with those of pure PLA and the successful incorporation of GO into the nanofibres. Thermogravimetric analysis showed that the addition of the inorganic phases increased the thermal stability of the nanocomposite fibres, demonstrated by an increase in degradation temperature compared to PLA nanofibres.
Cells seeded on the fibrous membranes were checked at 24 and 48 h and all samples appeared to be covered with cells and ECM components, thus demonstrating that the addition of GO does not adversely affect cell viability. Furthermore, the amount of MC 3 T3-E1 cells found in PLA/HA/GO scaffolds was greater than that on pure PLA scaffolds presumably due to the biocompatibility and hydrophilicity of GO nanoparticles. These results demonstrate that the nanocomposite scaffolds had superior biocompatibility whilst preserving the 3D structure of pure PLA scaffolds.
4.6.10 PLA and BG composite fibres
Noh et al. (2010) fabricated nanofiller particles by milling electrospun BG nanofibres in ethanol. These nanoparticles were combined with the biopolymer PLA solution at 10% w/w and homogenised to produce a composite solution that remained stable for several days and showed no agglomeration of the nanofiller particles or phase separation. This solution was electrospun, producing thin nanofibrous meshes. After immersion of pure PLA and composite meshes in SBF for 14 days, the latter showed the formation of a crystallite layer on the surface of the nanofibres whereas the pure polymer nanofibres did not. This proved that the BG nanoparticles stimulate mineral formation on the surface of the nanofibres. The composite nanofibre constructs were seeded with murine preosteoblast cells (MC3T3-E1) and cultured for up to 7 days together with a cell culture plate that was used as a positive control. After 3 days in culture, cells on the composite nanofibre constructs showed normal morphology (elongated and with active cytoskeleton processes) as well as good adhesion. When the cells had reached 7 days in culture, cell viability in the composite mesh was assessed and found to be similar to that of the positive control. Similarly, the cell adhesion and proliferation rate in the nanocomposite constructs was comparable to that in the cell culture plate.
4.6.11 PLA–HA composite fibres
Nanocomposite fibres of PLA and HA were developed by Kim et al. (2006) by electrospinning a solution into which they incorporated a surfactant, hydroxysteric acid (HSA), at around 0.1 wt% together with the chloroform-dissolved PLA (20% w/v) and HA nanoparticles (12.5 wt%). The surfactant was added in order to avoid agglomeration and thus ensure a uniform distribution of the bioceramic in the polymeric matrix. Some of this solution was concentrated to twice the original concentration of HA–PLA to 25%. This concentration produced fibres with homogeneous diameters and showed no bead formation and uniform distribution of the HA nanoparticles. In vitro tests to assess biocompatibility of the composite nanofibres were carried out by seeding MG63 human osteoblast cells onto HA-PLA and pure PLA control specimens. The cells were kept in culture for up to 7 days and cell viability, confluence, morphology and ALP expression levels were assessed. ALP activity is regarded as a crucial characteristic of metabolically active bone-forming cells. Electron micrographs of cells seeded on the HA–PLA fibrous meshes showed intimate contact between the cells and fibres and also a good cell spread, suggesting good cell viability. MTT and ALP assays showed significantly higher initial cell attachment (6 h) and proliferation (2 days) as well as bone-forming potential of the cells seeded on HA–PLA composites compared with those seeded on pure PLA nanofibres and culture dishes. These results show that the HA–PLA nanocomposite fibres are able to sustain osteoblastic cells and stimulate an osteoblastic behaviour.
4.6.12 Poly(D,L-lactic acid) (PDLLA), poly(lactic acid co-glycolic acid) (PLGA) and calcium phosphate (Ca-P) composite fibres
Dual-source dual-power electrospinning is a technique used to produce bicomponent scaffolds formed by two different types of nanofibres. The set-up is quite similar to normal electrospinning, but with an additional voltage source that can be connected to one or more syringes. The electrospun fibres are deposited on a rotating collector, forming a non-woven bicomponent scaffold. Using this technique, Wang and Wang (2012) produced single and bicomponent scaffolds with varying amounts of two different fibres: hollow poly(D,L-lactic acid) (PDLLA) polymer fibres with an aqueous recombinant human bone morphogenetic protein (rhBMP-2) core, fabricated using emulsion electrospinning; and nanocomposite poly(lactic acid co-glycolic) acid (PLGA) and calcium phosphate (Ca-P) fibres, obtained by the normal electrospinning route. Ca-P is known to be osteoconductive and rhBMP-2 is an osteogenic growth factor, and they were combined within the electrospun scaffolds to potentiate their effects and accelerate bone healing and growth when implanted in an injury site. The rhBMP-2/PDLLA nanofibres were electrospun from a water-in-oil emulsion of 10 μg rhBMP-2 with 2 mg bovine serum albumin (used as a stabiliser) dissolved in 1 ml de-ionised water, which was dripped into a solution of 10 ml of chloroform, 1.5 g PDLLA and 75 μl Span-80 (used as a surfactant). The Ca-P/PLGA composite nanofibres were electrospun from a solution of 1.25 g of PLGA dissolved in 10 ml of a mixture of chloroform and DMF at a ratio of 4:1, to which 138 mg of Ca-P nanoparticles were added and ultra-sonicated to ensure an even dispersion of the nanoparticles. SEM micrographs showed that the rhBMP-2/PDLLA nanofibres had smooth surfaces and a core–shell structure and TEM images confirmed the presence of the water phase containing rhBMP-2 within the shell of the fibre. Ca-P/PLGA fibres were relatively smooth, suggesting that most of the Ca-P nanoparticles were fully embedded within the polymeric matrix with only a small proportion exposed on the surface of the fibre. TEM images showed that there was a uniform dispersion of the inorganic nanoparticles within the fibre, and EDX spectra of the nanocomposite scaffolds confirmed the presence of these nanoparticles within the fibre, with calcium and phosphorus peaks being the dominant peaks in the EDX spectrum. Water contact angle measurements showed that the bi-component scaffolds were hydrophobic, but as the proportion of Ca-P/PLGA within them increased, they became more hydrophilic.
The bicomponent scaffolds were produced with different ratios of rhBMP-2/PDLLA and Ca-P/PLGA fibres and their in vitro degradation was compared to that of pure PDLLA and PLGA scaffolds when immersed in PBS at 37 °C for 8 weeks. At 4 weeks, all scaffolds had an increase in their fibre diameter due to swelling and those that contained Ca-P/PLGA fibres developed a porous surface and significant degradation, which could be attributed to the initial dissolution of the superficial Ca-P nanoparticle that would allow diffusion of water molecules into the PLGA fibre, which has a much shorter degradation time (1–2 months) than the PDLLA fibres (12–14 months). At 8 weeks, the PDLLA fibres still had smooth surfaces, whereas the scaffolds containing Ca-P/PLGA fibres showed extensive degradation, although the fibres were still continuous.
All rhBMP-2/PDLLA containing scaffolds exhibited a sustained rhBMP-2 release. After an initial burst, the core–shell nanofibres continued to release rhBMP-2 into the solution, with diffusion being the dominant mechanism of release. By varying the mass ratio of rhBMP-2/PDLLA within the scaffolds, the amount of rhBMP-2 released could be increased or decreased as needed, given that while the initial burst in the concentration of the growth factor would increase or decrease accordingly, its release profile would remain unchanged.
4.6.13 Poly(lactide co-glycolyde) (PLG) amorphous tricalcium phosphate (ATCP) composite fibres
Schneider et al. (2007) prepared cotton-wool like nanocomposite scaffolds by electrospinning a solution containing PLG and amorphous tricalcium phosphate (ATCP) nanoparticles at different ratios and compared the in vitro bioactivity and hMSC morphology and proliferation with those of cells in contact with pure PLG scaffolds. The bioactivity of ATCP containing scaffolds was much higher than that of PLG scaffolds, shown by the deposition of a HA layer on the surface of the spun fibres when immersed in SBF. As the proportion of ATCP and immersion time increased, the process of deposition also increased. After assessing cell morphology and proliferation on scaffolds seeded with hMSCs, it was concluded that ATCP nanoparticles (up to 40% by weight) did not cause any cytotoxic effects given that the cell population and ALP activity between pure PLG and PLG/ATCP composites were comparable. In addition to this, the cotton wool-like morphology of the fibres would make it possible to fill irregularly shaped and non-load bearing bone defects.
4.6.14 Polyurethane (PU) and HA composite fibres
Polyurethanes (PU) can be tailored to match different mechanical requirements and also possess a relatively good degree of biocompatibility, being utilised in many medical applications. Combining these properties with nHA results in composite materials with a high bioactivity and adequate mechanical properties.
Khan et al. (2008) prepared such composites using two approaches: one by physical mixing of polyurethane dissolved in 5% (w/v) DMF solvent with 10 wt% nHA and another by chemical mixing, where the nHA were added at the step of polymerisation of PU. Both mixtures were electrospun to produce nanofibrous, non-woven meshes.
FTIR and XRD analyses were carried out and results were compared with pure PU electrospun nanofibres. These analyses showed that there was formation of a covalent bond between nHA and PU in the case of chemically mixed composites, whereas the physically mixed ones did not exhibit such links. SEM images of the composite nanofibres showed uniform nanofibre morphology, unlike those obtained from pure PU. Physically mixed composites exhibited nHA particles on its surface, unlike CM nanofibres where nHA were fully embedded in the polymer matrix. This result confirms the chemical link between the inorganic and polymeric phases of the composite which results in a decreased loss of nHA due to physical abrasion of the nanofibres thus potentially increasing the bioactivity of the nanocomposite.
4.6.15 Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV)–HA composite fibres
Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV) is a copolymer of microbial polyester that is biocompatible, biodegradable and inexpensive. It was used by Ito et al. (2005) to produce composite PHBV–HA nanofibres by electrospinning, using a solution of 2 wt% PHBV dissolved in 2,2,2-trifluoroethanol and soaking the resulting nanofibrous mats in an aqueous solution of 2% calcium hydroxide together with a flat cast PHBV film used as a control. The specimens were then immersed in SBF at 1.5-fold concentration. Pure PHBV nanofibrous and flat cast films were used as controls. SEM observation of the electrospun films revealed a homogeneous structure with an average nanofibre diameter of 185 nm. Similar HA deposition was observed on both the electrospun and flat cast films with no significant difference in HA particle size. The presence of this inorganic phase turned the normally hydrophobic PHBV films into highly hydrophilic ones. Degradation tests were conducted by immersing the flat and electrospun films in solutions containing different concentrations of poly(hydroxybutyreate) (PHB) depolymerase. It was shown that the degradation rate of the films depended on both the concentration of the enzyme, the presence of HA particles and the film morphology. The nanofibrous films that contained HA nanoparticles degraded the fastest because they possessed increased surface area and hydrophilicity, which facilitated permeation of the film by the enzymatic solution and thus underwent a faster breakdown. COS-7 cells obtained from the kidney of an African green monkey were cultured on the prepared films and cell adhesion was observed after 1.5 h. The nanofibrous films exhibited significantly higher cell attachment than the flat films, although the presence of HA did not seem to significantly affect cell attachment. As discussed above, the increased attachment onto nanofibrous films is thought to arise due to the 3D nano-scale topography present on these films.
4.7 Natural and synthetic polymer combinations
4.7.1 Collagen, PLLA and HA composite fibres
Type I collagen is the main component of bone ECM and therefore it has been extensively studied for BTE applications. Collagen provides attachment sites that cells are able to recognise and it has been successfully electrospun to various diameters. As a disadvantage however, it is degraded easily within the body. Prabhakaran et al. (2009) produced nanofibrous scaffolds of PLLA, collagen and HA with a ratio of 40:40:20 (respectively) and performed hFOB cell proliferation, morphology and mineralisation assays on the composites. Results were compared with PLLA–HA composite nanofibres and pure PLLA fibres. The results showed that cell proliferation and mineralisation (measured by calcium deposition) were significantly higher in the PLLA/collagen/HA scaffolds than in both PLLA/HA and PLLA after 20 days in culture. This result was due to the presence of collagen, which in addition to providing attachment sites for cells, thus supporting adhesion, also acts as a chelating agent for the mineralisation of the hFOB cells.
4.7.2 Polyvinyl alcohol (PVA), HA and collagen composite fibres
Polyvinyl alcohol (PVA) is a water-soluble and biodegradable polymer that possesses high biocompatibility but, more interestingly, it is capable of self-cross-linking due to the high density of hydroxyl groups located on its side chains. Conversely, PVA also undergoes fast hydrolysis and is bioinert; thus protein and cell adhesion are limited on the pure material.
Biomimetic electrospun nanofibrous meshes composed of PVA, collagen and HA (PVA–collagen–HA) were developed by Song et al. (2012), to be employed in the surface modification of orthopaedic implants. To obtain these composite nanofibres, solutions of 10% w/v PVA, different ratios of PVA/HA (1/2, 1/4 and 1/9, v/v) and 10% v/v collagen were electrospun, and for comparison purposes, the same solutions minus collagen (PVA–HA) were also produced by electrospinning. The addition of encapsulated HA to the PVA polymer matrix increased Young’s modulus of the nanofibres proportionately, although the addition of collagen did not seem to influence the stiffness of the nanofibres. The nanofibrous meshes were immersed in ultrapure water to assess their hydrolytic degradation, and it was observed that the composites with HA/PVA ratios of 1/4 and 1/2 retained their fibrous structure after 48 h, whereas the ones with a ratio of 1/9 had mostly failed. Murine MC3T3-E1 pre-osteoblasts were seeded on all nanofibre constructs and after 72 h in culture, live–dead staining was used to investigate the viability and morphology of cells in culture. After 8 days in culture, the MTT assay was used to investigate cell proliferation rates. All nanofibrous constructs showed good cell attachment and none demonstrated any evident dead cells. Proliferation rates seemed consistent across samples compared to cells seeded on coverslips (Control), although the collagen-containing nanofibres at 1/2 and 1/4 HA–PVA ratios showed the highest cell proliferation and adhesion as well as outperforming other samples in terms of durability. The incorporation of collagen is thought to positively affect cell attachment by providing anchorage sites and HA provided osteoconductive properties which would further enhance osteoblastic cell attachment and proliferation, as discussed above.
These studies show that there is a vast range of possibilities to produce guided bone regeneration matrices with variable mechanical properties that can be tailored to specific requirements, ranging from load-bearing to dental applications and scaffolds for bone regeneration.
4.8 Conclusions and future trends
Bone is a natural nanocomposite material with excellent mechanical properties, where collagen nanofibres form a matrix reinforced with dispersed mineral particles. The search for suitable biomaterials for bone tissue engineering has advanced greatly, leading to the production of materials that more closely resemble the mechanical properties of bone whilst also providing adequate chemistry for cell attachment and support. This research has moved a long way from the use of monophasic ceramics or polymeric materials to consider suitable composite materials. Biodegradable polymer/inorganic bioactive phase composites are thus an attractive combination of materials for BTE scaffolds.
The challenge in tissue engineering scaffolds is to develop bioactive and bioresorbable 3D structures of tailored porosity and pore structure, which are able to maintain their structure and integrity for predictable times, even under load-bearing conditions similar to those experienced by natural bone. The inclusion of nanoparticles into the biopolymer matrix with the dual objective of improving the mechanical properties as well as incorporating nanotopographic features that mimic the nanostructure of natural bone is a currently area of intensive research (Liu and Webster, 2007; Torres et al., 2007; Ganesh et al., 2012; Gentile et al., 2012; Chen et al., 2013). Also, depending on the location and loading of the bone, the hierarchical disposition of the collagen and mineral phases varies (Fratzl et al., 2004); thus, manipulating the geometrical arrangement of the inorganic nanoscale phase within the polymeric matrix would allow for better control and tailoring of the mechanical properties of the nanocomposite materials. This can be achieved, for example, by using inorganic nanoparticles or fibres and adjusting their volume fraction according to the application. Also, composite nanofibres could be electrospun in predetermined patterns into woven membranes to mimic the natural alignment of collagen along load lines in natural bone, improving the mechanical properties of the scaffolds. In particular, electrospun nanocomposites are especially promising due to their fibrous morphology and increased surface area, as well as the ability to incorporate inorganic nanoparticles within the electrospun polymeric matrix. The review of the relevant literature conducted in this chapter indicates that electrospinning is leading to a broad range of nanostructured composite scaffolds exhibiting increased bioactivity and controllable degradation kinetics that can be tailored by altering the inorganic phase concentration. Furthermore, the embedding of the particles in the nanofibres prevents their uptake by cells which, in the case of loose nanoparticles, could be cytotoxic and cause cell death (Ganesh et al., 2012).
In addition, the tailoring of roughness and topography of fibrous scaffolds is being explored due to the significant effect that surface roughness has on early cell attachment and possibly on subsequent cell adhesion, cytoskeletal organisation and gene expression. The incorporation of biomolecules such as growth factors in the scaffold and their sustained release, with the aim of controlling cell proliferation and upregulating osteogenic genes to ultimately accelerate local bone healing, is promising and currently under extensive research. Moreover, there is significant scope in the application of surface modification, through the addition of amino specific functional groups for protein or antibody attachment or the use of protein adsorption and plasma treatment to provide cues that can influence cell attachment and response, thus making the scaffold more biocompatible.
In order to translate the basic research to clinical applications, more in vitro and in vivo studies are indispensable and there is need for more studies on composite scaffolds in relevant biological systems using, for example, bioreactors. Dedicated research directed at assessing the suitability of bioactive composite scaffolds for enhancing the angiogenesis and vascularisation of tissue/scaffold constructs has recently started (Gerhardt et al., 2011).
Future research will see increased activities related to the application of polymer–inorganic phase composites in the emerging field of interface tissue engineering (Khanarian et al., 2012). Here, the combination of different scaffold structures in a layered or stratified fashion is required for developing complex scaffolds for osteochondral tissue defects (Nooeaid et al., 2012). In the fabrication of these structures the combination of a soft phase (polymer) and a rigid phase (bioceramic) is required to mimic the transitions in mechanical behaviour between cartilage and subchondral bone. Also, many challenges are present in the repair of broken and damaged ligaments given that there is a compositional gradient between the ligament and the bone which allows the efficient transfer of loads between soft and hard tissue. Spalazzi et al. (2006) produced triphasic scaffolds to enable this load transmission by sintering polymeric and composite layers into a porous cylindrical scaffold that supported cell growth, proliferation and production of ECM characteristics for each phase. In addition, incorporation of GO has been shown to dramatically improve the mechanical properties of nanofibrous scaffolds (Ma et al., 2012), GO is a novel material with potential applications in this field, it is of lower cost than carbon nanotubes (the use of which is controversial in tissue engineering due to their toxicity) and as such is a promising route that should be further explored.
As processing techniques improve, it is possible to precisely control the incorporation of the mineral phase in different concentrations within the scaffold (Erisken et al., 2008a), thus offering a wide range of possibilities in terms of the geometrical arrangement of the inorganic nanoparticles and thus the mechanical behaviour of the scaffolds according to the loading conditions in a specific bone region.
All these innovations, combined with novel techniques such as electrospraying, air-jet spinning and other electric field-assisted processing methods, together with the combination of different techniques, is expanding the range of ways in which additional inorganic particles or biochemical cues can be added to the surface of electrospun fibres (Francis et al., 2010; Abdal-Hay et al., 2012).
Recent examples of innovative organic–inorganic composites being explored for tissue engineering strategies on the basis of alternative combined methods include PLA and TiO2 hybrid nanofibrous scaffolds (Gupta et al., 2012), where a hydrolysed titanium precursor was electrosprayed on electrospun PLA nanofibres and treated hydrothermally to convert the precursor into TiO2. Air-jet spinning was utilised by Abdal-Hay et al. (2012) to produce nHA/PLA composites, while Chen et al. (2013) prepared nanofibrous gelatin/bioactive glass composite hydrogels via phase separation to obtain hybrid hydrogels and electrostatic cross-linking with hyaluronic acid and infiltration with chitosan to optimise the balance of charges between shell layers in order to improve microstructural and thermal integrity of the composites. Further progress in processing methods and smart combination of polymers, inorganic phases and bioactive molecules will eventually bring the final aim of producing a biomimetic material that could fulfil all the requirements for BTE, bringing the novel constructs closer to their clinical use.
4.9 Acknowledgement
V.M. P. acknowledges financial support from EU ITN Project BIOBONE.
