7
MEMS, MOEMS, Nano, and Bionanotechnologies
7.1 Introduction
The simple distinction between the terms micro-electro-mechanical systems (MEMSs) and nanotechnology is the size of the devices: MEMSs typically range between millimeters down to microns and nano devices are on the nanometer scale. Quoting an excellent set of definitions from SmallTech Consulting [1]:
MEMS is the integration of a number of microcomponents on a single chip which allows the microsystem to both sense and control the environment. The components typically include microelectronic integrated circuits (the “brains”), sensors (the “senses” and “nervous system”), and actuators (the “hands” and “arms”). The components are typically integrated on a single chip using microfabrication technologies similar to those used for integrated circuits.
Nanotechnology takes advantage of the observation that at the nanoscale, properties of materials change. Nanotechnology is that array of technologies that use properties of materials that are unique to structures at the nanoscale.
A Richard Feynman presentation in 1959 discussed material fabrication “from the atom up,” leading to the debate regarding the concept of molecular manufacturing and molecular nanotechnology. The classic Drexler–Smalley debate focused on the feasibility of constructing molecular assemblers [2]. Multidisciplinary efforts continue to pace the complementary fields of MEMS, MOEMS, nano, and bionanotechnology, with exponential growth upon exponential growth, advancing toward Ray Kurzweil’s projected “singularity” around the year 2040. Nanomaterials for biology now include quantum rods and dots (CdSe, CdTe, and CdHgTe), non-heavy-metal-based quantum dots (InP, CuInS2, silicon, magnetic nanoclinics, silica nanoparticles, metallic [gold and silver] particles and rods, and rare-earth-doped nanophosphors [Er and Tm:NaYF4]) [3].
A significant segment of the MEMS market is that of MOEMS— optical MEMS—partly a result of the fusion of computing and signal processing with photonics and communications—including the marriage of optics and semiconductor micromachining and devices—playing heavily in the lab on a chip concepts for biomedicine. Certainly, the time is ripe for the convergence of MEMS, nanotechnology, and biotechnology. Perhaps, MOEMS devices interacting with biological systems will manipulate nanoparticles in applications for drug delivery, gene therapy, and commercial labs on a chip.
MOEMS advanced rapidly in part due to the telecommunication market’s demand for devices such as add-drop mulitplexers, optical cross connect switches, variable optical attenuators, modulators, dense wavelength division multiplexing, wavelength converters, dynamic gain equalizing filters, tunable filters, and advanced packaging technologies. More than 50 start-up ventures in the late 1990s complemented the efforts of the large telecommunication manufacturers in the acceleration of such market applications.
Significant applications are emerging in the related field of biomimetics, involving biologically inspired synthetic materials. These point to the construction of genetic networks from and within cells, along with intelligent implantable sensors. Microfluidic MEMS constructs will provide processing of antibodies, peptides, metabolic markers, and monitors. DNA may even provide the backbone for computer logic chips. Biosensors mimicking mammalian physiological behavior, fabricated from completely synthetic abiotic materials, can be programmed to sense, respond, and adapt to requirements of living systems, along with providing the basis for a range of chemical and biological sensing systems [4].
With the emergence of multidisciplinary fields such as bioinformatics, mathematical biology, computational biological modeling, biophysics, etc., we should continue to see the cataloging and relational systems approach to biology and the sciences as part of the exponential “Kurzweilian” growth characteristic of technological progress. Crystal growth techniques described in earlier chapters for the III–V GaAs semiconductor material system are analogous in many respects to growth techniques used to nucleate the precipitation of protein crystalline structure from aqueous solution.
Quoting D. A. LaVan and R. Langer, MIT, from the NSF Symposium in 2001:
While some may dream of nanorobots circulating in the blood, the immediate applications in medicine will occur at the interfaces among… nanotechnology, micro-electronics, microelectromechanical systems (MEMS) and microopticalelectro-mechanical systems (MOEMS)…The bounty will not be realized until those trained in these new paradigms begin to ….address basic medical and scientific questions.
7.2 MEMS and Nanotechnology*
7.2.1 Introduction
The section provides a discussion of MEMS and nanotechnology.
*Courtesy of Dr. Michael Huff of the MEMS and Nanotechnology Exchange (see http://memsexchange.org) at the Corporation for National Research Initiatives [5].
MEMS is the integration of mechanical elements, sensors, actuators, and electronics on a common silicon substrate through microfabrication technology. While the electronics are fabricated using IC process sequences (e.g., CMOS, Bipolar, or BICMOS), the micromechanical components are fabricated using compatible “micromachining” processes that selectively etch away parts of the silicon wafer or add new structural layers to form the mechanical and electromechanical devices (see Figure 7.1).
MEMS promises to revolutionize nearly every product category by bringing together silicon-based microelectronics with micromachining technology, making possible the realization of complete systems-on-a-chip. MEMS is an enabling technology allowing the development of smart products, augmenting the computational ability of microelectronics with the perception and control capabilities of microsensors and microactuators, and expanding the space of possible designs and applications.
Microelectronic integrated circuits (ICs) can be thought of as the “brains” of a system, and MEMS augments this decision-making capability with “eyes” and “arms” to allow microsystems to sense and control the environment. Sensors gather information from the environment through measuring mechanical, thermal, biological, chemical, optical, and magnetic phenomena. The electronics then process the information derived from the sensors and through some decision-making capability direct the actuators to respond by moving, positioning, regulating, pumping, and filtering, thereby controlling the environment for some desired outcome or purpose. Because MEMS devices are manufactured using batch fabrication techniques similar to those used for ICs, unprecedented levels of functionality, reliability, and sophistication can be placed on a small silicon chip at a relatively low cost.
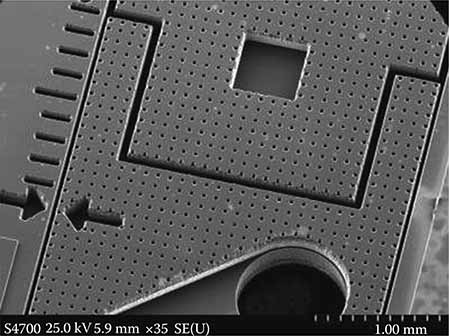
FIGURE 7.1
MEMS structure. (Courtesy of the MEMS and Nanotechnology Exchange.)
There are numerous possible applications for MEMS and nanotechnology. As a breakthrough technology, allowing unparalleled synergy between previously unrelated fields such as biology and microelectronics, many new MEMS and nanotechnology applications will emerge, expanding beyond that which is currently identified or known. Following are a few applications of current interest.
7.2.2 Biotechnology
MEMS and nanotechnology are enabling new discoveries in science and engineering such as the polymerase chain reaction (PCR) microsystems for DNA amplification and identification, micromachined scanning tunneling microscopes, biochips for detection of hazardous chemical and biological agents, and microsystems for high-throughput drug screening and selection.
7.2.3 Communications
High-frequency circuits will benefit considerably from the advent of the RF-MEMS technology. Electrical components such as inductors and tunable capacitors can be improved significantly compared to their integrated counterparts if they are made using MEMS and nanotechnology. With the integration of such components, the performance of communication circuits will improve, while the total circuit area, power consumption, and cost will be reduced. In addition, the mechanical switch, as developed by several research groups, is a key component with huge potential in various microwave circuits. The demonstrated samples of mechanical switches have quality factors much higher than anything previously available.
Reliability and packaging of RF-MEMS components seem to be the two critical issues that need to be solved before they receive wider acceptance by the market.
7.2.4 Accelerometers
MEMS accelerometers are quickly replacing conventional accelerometers for crash air-bag deployment systems in automobiles. The conventional approach uses several bulky accelerometers made of discrete components mounted in the front of the car with separate electronics near the air bag; this approach costs over $50 per automobile. MEMS and nanotechnology have made it possible to integrate the accelerometer and electronics onto a single silicon chip at a cost between $5 and $10. These MEMS accelerometers are much smaller, more functional, lighter, and more reliable and are produced for a fraction of the cost of the conventional macroscale accelerometer elements.

FIGURE 7.2
MEMS structure. (Courtesy of the MEMS and Nanotechnology Exchange.)
MEMS and nano devices are extremely small, for example, MEMS and nanotechnology have made possible electrically driven motors smaller than the diameter of a human hair (see Figure 7.2), but MEMS and nanotechnology are not primarily about size. MEMS and nanotechnology are also not about making things out of silicon, even though silicon possesses excellent materials properties, which make it an attractive choice for many high-performance mechanical applications; for example, the strength-to-weight ratio for silicon is higher than many other engineering materials, which allows very high bandwidth mechanical devices to be realized. Instead, the key importance of MEMS and nano is as a new manufacturing technology—a way of making complex electromechanical systems using batch fabrication techniques similar to those used for ICs and uniting these electromechanical elements together with electronics.
7.2.5 Advantages of MEMS and Nano Manufacturing
First, MEMS and nanotechnology are extremely diverse technologies that could significantly affect every category of commercial and military product. MEMS and nanotechnology are already used for tasks ranging from in-dwelling blood pressure monitoring to active suspension systems for automobiles. The nature of MEMS and nanotechnology and its diversity of useful applications make it potentially a far more pervasive technology than even IC microchips.
Second, MEMS and nanotechnology blur the distinction between complex mechanical systems and IC electronics. Historically, sensors and actuators are the most costly and unreliable part of a macroscale sensor-actuator-electronics system. MEMS and nanotechnology allow these complex electromechanical systems to be manufactured using batch fabrication techniques, decreasing the cost, and increasing the reliability of the sensors and actuators to equal those of ICs. Yet, even though the performance of MEMS and nano devices is expected to be superior to macroscale components and systems, the price is predicted to be much lower.
MEMS technology is based on a number of tools and methodologies, which are used to form small structures with dimensions in the micrometer scale (one millionth of a meter). Significant parts of the technology have been adopted from IC technology. For instance, almost all devices are built on wafers of silicon, like ICs. The structures are realized in thin films of materials, like ICs. They are patterned using photolithographic methods, like ICs. There are however several processes that are not derived from IC technology, and as the technology continues to grow the gap with IC technology also grows.
There are three basic building blocks in MEMS technology, which are the ability to deposit thin films of material on a substrate, to apply a patterned mask on top of the films by photolithograpic imaging, and to etch the films selectively to the mask. A MEMS process is usually a structured sequence of these operations to form actual devices.
7.2.6 Developments Needed
MEMS and nanotechnology are currently used in low- or medium-volume applications.
Some of the obstacles preventing its wider adoption are the following.
7.2.6.1 Limited Options
Most companies who explore the potential of MEMS and nanotechnology have very limited options for prototyping or manufacturing devices and have no capability or expertise in microfabrication technology. Few companies will build their own fabrication facilities because of the high cost. A mechanism giving smaller organizations responsive and affordable access to MEMS and Nano fabrication is essential.
7.2.6.2 Packaging
The packaging of MEMS devices and systems needs to improve considerably from its current primitive state. MEMS packaging is more challenging than IC packaging due to the diversity of MEMS devices and the requirement that many of these devices be in contact with their environment. Currently, almost all MEMS and nano development efforts must develop a new and specialized package for each new device. Most companies find that packaging is the single most expensive and time-consuming task in their overall product development program. As for the components themselves, numerical modeling and simulation tools for MEMS packaging are virtually nonexistent. Approaches that allow designers to select from a catalog of existing standardized packages for a new MEMS device without compromising performance would be beneficial.
7.2.6.3 Fabrication Knowledge Required
Currently, the designer of a MEMS device requires a high level of fabrication knowledge in order to create a successful design. Often, the development of even the most mundane MEMS device requires a dedicated research effort to find a suitable process sequence for fabrication. MEMS device design needs to be separated from the complexities of the process sequence.
7.3 Nanotechnology Applications
Figure 7.3 from the Foresight Nanotech Institute [6] represents the technology roadmap for the key application areas of nanotechnology. This chart illustrates the technology convergence and rapid progression to a host of diverse applications and implementations. As the theme of this text and the cover art suggest, progress in optical bionanotechnology will follow cross-disciplinary endeavors in achieving some of the most significant breakthroughs in science.
Applications in biotechnology include bioimaging, biosensors, flow cytometry, photodynamic therapy, tissue engineering, and bionanophotonics. Breakthroughs in biological signaling, genomics, and biosystems engineering will follow as well.
7.4 V-Groove Coupler Geometry and Design Considerations
Bulk micromachining of silicon and other semiconductor materials represents the early work in the MEMS arena. A very early application of anisotropically etched silicon was the V-groove coupler. Figure 7.4 illustrates a round fiber in a V-groove, providing a very precise means of aligning a fiber to another fiber or for coupling to waveguides, detector elements, or other optical components. We consider here the geometry and design considerations for such a structure.
It is found that the problem of finding b, the location of the center of the fiber with respect to the V-groove, is dependent upon whether the point of contact is at the top corner of the groove or on the side wall. The point of contact is described by Ψ, c′, and d′. Then, c′/r = cos Ψ.
The value of b sat this point is defined as
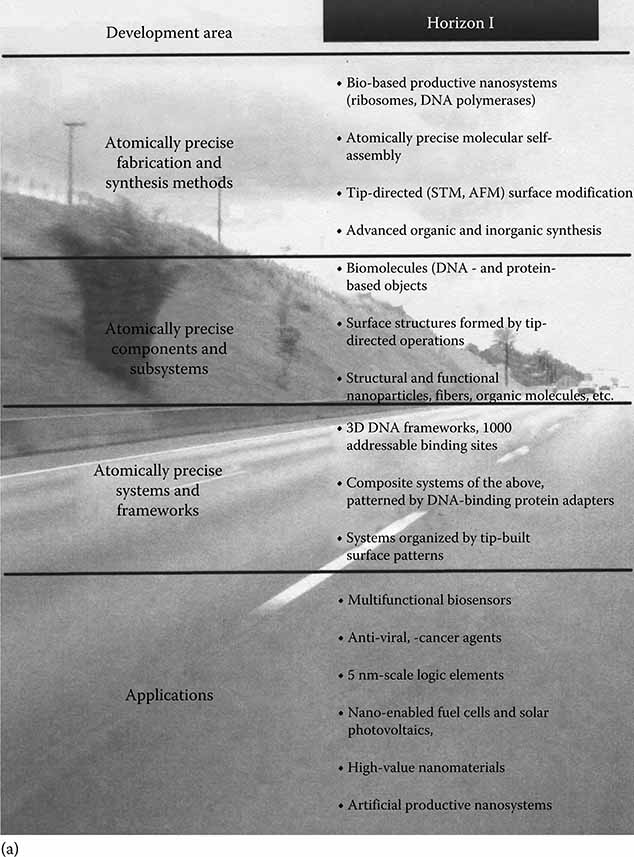
FIGURE 7.3
Nanotechnology roadmap. (Courtesy of the Foresight Nanotech Institute, Palo Alto, CA.)

The problem is now dependent upon the value of c/r. For values of c/r such that c/r ≤ cos Ψ, we term the value of b as b<:
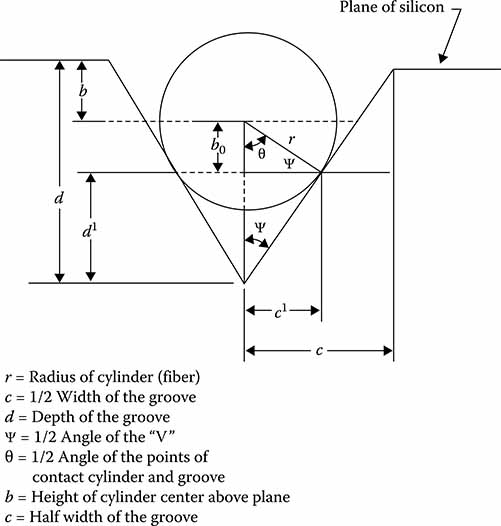
FIGURE 7.4
V-groove geometry.
Assuming Ψ constant, we look for variations in b< caused by variations in c.
For values of c/r, such that c/r > cos ψ, we term the value of b as b>:
The values of d and d′ are
Substitution yields
It is found that the variation of b> with changes in c is constant:
Figure 7.5 illustrates the form of Equations 7.3 and 7.6 for an arbitrary value of ψ. This analysis shows that the vertical alignment improves for decreasing values of c/r. On the other hand, it is found that the percent of exposed fiber, expressed for convenience as the percent of the fiber diameter above the surface of the chuck, decreases for increasing values of c/r. This percentage is a parameter that can be indicative of the quality of the horizontal stability of the fiber alignment, with lower values indicating better stability. It is seen that better vertical alignment is obtained to some degree at the expense of this horizontal stability.
As a numerical example, we use the value of ψ = 36°. This is the value for the anisotropic etching angle in silicon for coupling chucks. The table at Figure 7.6 provides results for several values of c/r. Point 1 shows that perfect vertical alignment is only obtained with an infinite horizontal uncertainty as there is of course no groove (c = 0 = c/r). At point 2, there is still a great deal of the fiber above the plane of the chuck. Point 3 shows that, even when there can be no further increase in the quality of vertical alignment, there is still 80% exposure. It therefore appears necessary to sacrifice some vertical accuracy in order to obtain a reasonable amount of horizontal stability. It appears that the most reasonable value of c/r to use is in the vicinity of point 4 where the fiber core is aligned near the surface of the chuck.
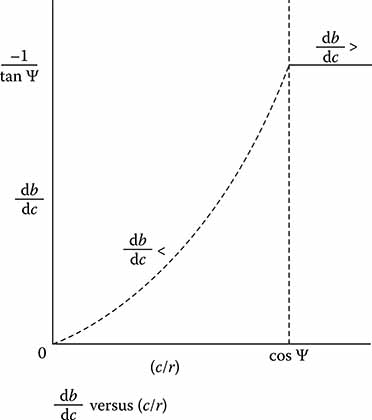
FIGURE 7.5
Alignment error as a function of groove dimension.
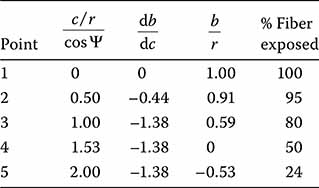
FIGURE 7.6
Vertical misalignment (db/dc), height of fiber core above chuck plane (b/r), and percent of exposed fiber as functions of groove width to fiber diameter ratio (c/r).
To obtain an idea of the actual sizes of the misalignment, we consider a total groove width error of +0.5 μm. Then, the center of the fiber will be lower than expected by −0.69 μm. This would be an important consideration for direct integration of grooves and waveguides, considering a single-mode core diameter of perhaps 4 μm; however, many efforts are directed toward separately aligning the chuck and guides, so the real interest is in keeping a consistent groove width among multiple grooves. One might expect a plus or minus 0.1 μm variance between three grooves, and, therefore, the three fiber cores would be aligned to ±0.14 μm.
Consider two fiber diameters of 80 and 125 μm. By aligning the core of the smaller fiber with the chuck surface,
Using the values r = 40 μm and ψ = 36°,
The critical values for both fibers are summarized in Figure 7.7.
Some representative V-groove chuck dimensions are shown at Figures 7.8 and 7.9. The groove width would be the critical dimension and would determine the groove depth by the crystal geometry. The groove width may be 96–100 μm as long as each of the three grooves is the same. The 200.6 μm groove separation is also very important. This basic approach represents the basis for a range of early silicon-bench optical-packaging devices.
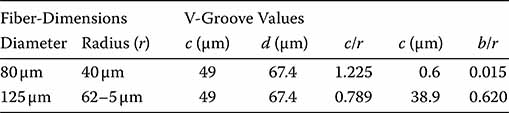
FIGURE 7.7
Critical values for 80 and 125 μm diameter fibers.
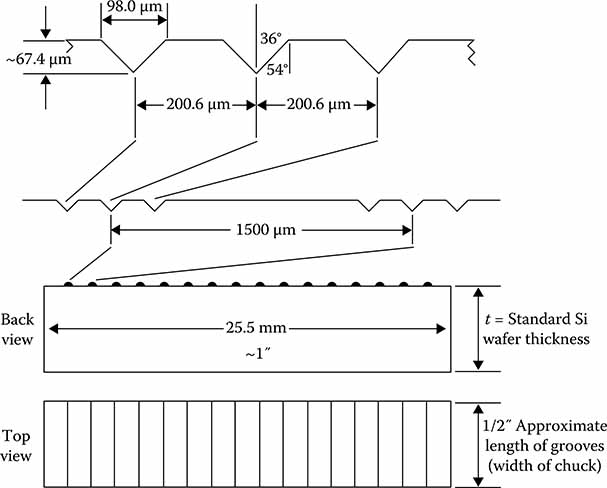
FIGURE 7.8
V-groove chuck for 80 and 125 μm diameter fibers.
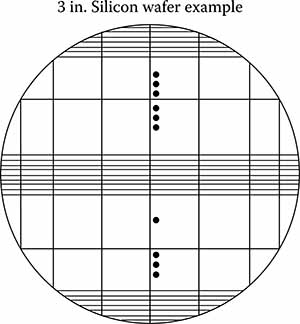
FIGURE 7.9
Cleavage lines yielding about 14 useful chucks, set of 3 grooves, set spacing, 1.5 mm indicate continued grooving.
7.5 Bionanotechnology
7.5.1 Introduction
Nanoscale scaffolds may ultimately be utilized for molecular structuring (ala the classic Drexler versus Smalley debate regarding molecular building blocks and how they might be manipulated) as well as for regulating information transfer. Ron Weiss’s page at Princeton [7] describes building biologic circuits. SPICE electrical circuit simulations are now complemented with bioSPICE for modeling genetic circuits [8]. Biological system simulation programs are available as open source code.
Molecular recognition is a key objective for a host of multiplexed biosensors technologies in development. These include biomedical MEMS cantilever sensors, surface effects on nanocantilevers, and cancer cantilever sensors. Surface acoustic wave (SAW) sensors prove quite sensitive to adsorbed materials on the device surface, and a wide range of electrochemical sensors detects free radicals, glucose, and nitric oxide in tissues.
Photonic nanosensors monitor biochemical reactions through conversion of chemical energy to light signals. This is now accomplished for antibody detection inside a single cell [9]. Charge density waves resulting from light impinging on the interface between a thin film and a second medium, called surface plasmons, are utilized to study the formation of self-assembled layers and molecular structures, including DNA and proteins.
7.5.2 Biology Labs on a Chip
Integrated biochips are now commercially available, integrating biotechnology with MEMS, optics and electronics, imaging, and processing in a miniaturized hybrid system [10]. The term biochip is synonymous in this context with the IUPAC definition of biosensor: “a self-contained integrated device, which is capable of providing specific quantitative or semiquantitative analytical information using a biological recognition element (biochemical receptor), which is retained in direct spatial contact with a transduction element.”
The general concept for analysis of a sample (analyte) is for the bioreceptor to provide molecular recognition of the DNA, RNA, or protein molecule and to affect a signal through a transducer that provides a measurement or characterization output. Biochips are distinguished from microarray assay systems, in that the biochip contains both the microarray and the detector array transduction system. While array plates provide the ability to identify analytes with very high volume, speed, and throughput, biochips afford portability, lower cost for small batch processing, and automated analysis algorithms. This is more convenient for applications such as field monitoring or physician’s office testing.
Multifunction biochips provide the ability to detect DNA, RNA, protein, and specific characteristics unique to the biological probe composition, such as specific enzymes, antibodies, antigens, and cellular structures [11]. Transducers vary widely, such as surface plasmon resonance devices, optical property measurement devices (absorption, luminescence), electrochemical, SAW, and other mass measurement devices. Progress in medicine will rely heavily upon the biochip systems currently in development [12].
7.5.3 Applications in Bioorganic Chemistry
7.5.3.1 Smell
Carvone exists as a pair of enantiomers. (R)-(−)-carvone smells like spearment, whereas (S)-(+)-carvone smells like caraway. Why do these enantiomers have different smells (i.e., different biological activity)? Olfactory receptors in the cell membranes of humans apparently possess the ability to respond differently to the two carvone enantiomers. Considering each cell as an analog computer, processing fuzzy logic [13], the reaction of the receptor proteins in the olfactory complex has the ability to regulate ion transport through the cell membrane differentially for the two carvone enantiomers, thereby producing a different electrochemical signal to the brain. Perhaps, the selection processes in mitochondrial evolution resulted in certain specific cellular logic functions for smell, and specific abilities of species to distinguish and differentiate enantiomers such as carvone [14]. The two enantiomers “present themselves” differently to the olfactory receptors, resulting in a differential response. It is also possible that the two enantiomers present a different fit to the hydrogen-bonded water networks that play such an important role in “presenting” molecules to one another in biological solution. Complementary shapes are thus selected for a specific reaction.
7.5.3.2 Protein Structure and Folding and the Influence of the Aqueous Environment
Analysis and characterization of proteins represent an important branch of biotechnology. The theories of hydrogen bonding are proving of great importance to the understanding of protein behavior. Martin Chapman’s site [15] describes extended hydrogen-bonded water molecules, such as icosahedrals, which should be visible under a 1000× or 2000× microscope, if indeed they are stable and exist for more than the expected femtosecond duration.
Further experimentation is needed to properly characterize these structures. It may be possible for water to remain in an aggregate represented by one of Chaplin’s “magic numbers” for more than a very short duration. This is a bit controversial in the literature. Some of the abstracts from a recent conference on water [16] indicate that indeed this occurs. If so, perhaps we can control the behavior of protein folding in “conditioned” structured aqueous environments. Another site also discusses water as a “designer fluid” that helps proteins change shape [17].
Protein folding helps determine the functionality of the biomacromolecule. Aspartic acid, for example, is considered acidic hydrophilic [18]. When the protein folds, the side chains can be transferred from a solution-like environment to one determined by the 3-D structure of the folded protein. So, exposure to solvents could be impacted differentially on one side of the protein versus the other. Differential interaction of the aspartic acid side chain with permanent charges and protein dipoles will also affect the pH of the side chain [19]. Exposure to the physiological conditions of the solvent is affected by the amount of shielding by the protein fold—different on one side versus the other. Specific biological function could be impacted and determined by the solvent-determined activity of the side chains. Those side-chain pH’s could be determined by their ability to interact with the solvent.
7.5.3.3 Evolution and the Biochemistry of Life
When formed synthetically, amino acids contain equal amounts of L and D enantiomers:
“Amino acids synthesized in the laboratory are a mixture of the right-and left-handed forms, and thermodynamically the two forms are indistinguishable” [20]. This fact rather disproved the famous Miller experiments of 1953, in which traces of nonliving amino acids were created in a mix of laboratory gases and even represented one of the holes in the evolutionary origin-of-life theories [21].
One theory is that electroweak interactions of electromagnetism and the weak nuclear force, which calculations suggest, will result in a difference in the L and D enantiomers of one part in 10−17, which eventually dominated, although this theory is about as convincing (not very) as the idea that earth’s first biopolymers were delivered to earth by meteorites. The lack of convincing experimental (or fossil) support leaves these as unproven theories [22].
If the amino acids in meteorites are used as the basis to represent those available on the primitive earth, we must account for methylation into a form that is not biologically useful. Ron Breslow and Mindy Levine at Columbia University determined that the very small imbalance in favor of the L enantiomer can be amplified to 90% L through just several cycles of evaporation. Also, the presence of copper in the primordial stew results in a strong initial L bias [23].
Equally intriguing and unproven theories include the delivery of “structured water” via meteorites to the ancient Dogon Nomos in Africa. Legend has it this water delivered the “ancient message” and contained extraterrestrial communications that provided man (through biochemistry) with intelligence. This may be the intelligence in Genesis that separated the robotic, programmed humanoids from the reasoning man with a soul, ala “The Origins of Consciousness in the Breakdown of the Bi-cameral Mind” [24].
It would seem that there is only one unique solution to the biochemistry of life. The 20 amino acids must occur in the correct sequence, in the correct form, for life to exist in the protein structures. Because of the incredibly complex series of interactions needed for the process of life, the wrong orientation would upset the entire logical mesh represented in the human form. While arguments for primordial bias due to volcanic conditions, heat for evaporation, and isolated copper-laden lakes might provide the basis for the L configuration bias and therefore an explanation for the specific L role in biological complexity, it seems that there was ample opportunity for the complementary enantiomer to evolve into life forms as well—if indeed, it provided an eigenvalue for mathematical resolution.
A single error in the amino acid sequence can manifest itself in genetic dysfunction and death. Astounding as it is to comprehend the complexity of life, it would seem that we are dealing with the simultaneous solution to a host of equations, with a single unique solution. It is possible that the first self-replicating protein structure could have started with the D structure, but it apparently did not. Panspermia, regional specificity, meteorites, etc. may, in totality, represent the initial bias, but systems biology and mathematical uniqueness may be the ultimate determinant. Perhaps, even the influence of aggregated water on the deep major and minor grooves has a dominant unique solution. Absence of proof implied proof of lack of a physically realizable solution to an alternate life form. The 1% bias theories are interesting, but probably not necessary for the real solution we have realized in our biochemistry.
7.5.3.4 Biochemical Analysis and Cancer
Jonsson et al. published “A strategy for identifying differences in large series of metabolomic samples analyzed by GC/MS” [25] in which they describe the process for identification of metabolites in a biological system, using standard techniques of organic chemistry including gas chromatography, mass spectrometry, 1H NMR, and data-processing algorithms for rapid analysis of large data sets. Additional organic chemistry analytical methods applied to metabolomics include chromatography, electrophoresis, and mass spectroscopy. Thus, the traditional techniques of organic chemistry are applied to biological systems of metabolites, hormones, and signaling mechanisms in the emergent field of metabolomics.
Metabolmics provides a complete understanding of cell physiology, complemented by the studies of related genomics and proteomics. This approach was first applied to improved understanding of metabolism [26]. It is thought that a systematic cataloging of the human metabolome will provide a baseline for studies of cellular processes and their perturbations.
The Human Metabolome Database database, supported by the University of Alberta [27] and Genome Canada, contains links to chemical, clinical, and biochemical/molecular biological data, with links to protein and DNA sequences. This systems approach to biological studies promises rapid streamlining of previously incredibly tedious processes.
For instance, how much additional progress would have resulted in the studies of Dr. Judah Folkman on angiogeniesis and its relationship to cancer, had the tools of metabonomics been available a few years ago [28]? Evidence of the growing success of the field is demonstrated in part by growing conferences and attention in the literature to metabolomics, following the first mention of the approach about 10 years ago.
Soon perhaps, a treatment for cancer epigenetics will be created through localized solvent physiology changes that reprogram the cancer cell behaviors. If the triggering mechanism for cancer cell programming is identified and reversed, the basis for a cure or reversal of the condition may be possible. Antiangiogensis agents, epigenetic reprogramming, perhaps even structured water with appropriate solvents changing the ion transport of potassium across cell boundaries, may alter sufficiently the folding of proteins during the cancer cell processes. It seems that recent breakthroughs [29] in the understanding of DNA methylation may provide the epigenetic key for screening, prevention, and cure of many or all cancers.
7.5.4 Bioimaging Applications
Optical imaging techniques include fluorescence microscopy, Raman imaging, interference imaging, optical coherence tomography, total internal reflection imaging, multiphoton microscopy, confocal microscopy, and other developing tools including fluorescence imaging. Fluorescence optical imaging systems include spatial filtering confocal microscopy, spatially resolved localized spectroscopy, polarization and time resolved fluorescence lifetime imaging, and fluorescence resonance energy transfer. Applications include whole body imaging, drug distribution, protein engineering, and identification of structural changes in cells, organelles, and tissues [30].
X-ray crystallography provides another means to characterize the atomic structure of crystalline materials. (Rosalind Franklin used the technique to produce the famous demonstration that DNA is helical.) This characterization includes proteins and nucleic acids. While certain proteins are difficult to crystallize, nearly 50,000 proteins, nucleic acids, and other biological macromolecules have now been measured with x-ray crystallography [31,32].
7.5.5 Biologically Inspired Computing and Signal Processing
Interesting switching behaviors occur in proteins [33]. There are aspects of epigenetic programming that may possibly be “switched” with light. If we express such a protein in a cell, we could control aspects of the protein’s behavior with light. We must determine what controls and expresses the switching information and what activates it. Some work has been accomplished with cutting and pasting proteins to create light-sensitive kinases. Adding and subtracting amino acid residues modulate the activity [34,35].
As discussed in Chapter 1, this is analogous to the progression of (electrical) signal processing using combinations of digital logic gates and application-specific ICs; to optical signal processing using operator transforms, optical index, and nonlinear bistable functions through acousto- and electro-optic effects to perform optical computing; to Dennis Bray’s “Wetware” biologic cell logic; and to the biophilosophical, astounding insights of Nick Lane in the last two chapters of his recent “Life Ascending- the Ten Great Inventions of Evolution” [36], where Lane characterizes the evolutionary development of consciousness and death (through the mitochondria), providing the basis for biological logic and how best to modify and control that inherent logic.
We are now on the cusp of an epoch, where it is possible to design light-activated genetic functionality, to actually regulate and program biological functionality. What if, for instance, we identify/isolate the biological “control mechanism” for the switching on/off of limb regeneration in the salamander, or, with the biophotonic emission associated with cancer cell replication and analogous to the destruction of the cavity resonance in a laser, we reverse the biological resonance and switch or epigenetically reprogram the cellular logic? Soon we will engineer time-sequenced reaction pathways to regulate and affect a huge variety of complex biochemical functionalities [37,38].
The emerging field of optogenetics addresses how specific neuronal cell types contribute to the function of neural circuits, the idea that two-way optogenetic traffic could lead to “human–machine fusions in which the brain truly interacts with the machine rather than only giving or only accepting orders…” [39]. This could be Ray Kurzweil’s “singularity”; he said that it is “near” (about the year 2040) in his documentary “Transcendent Man.” Device physics and electro-optic/electrical engineering, optical MEMS (“MOEMS the word!”), and bionanotech have converged!
Protein scaffolds bind core kinases that successively activate one another in metabolic pathways engineered to provide biological signal processing functions. Envision three-dimensional nanostructures fabricated using laser lithography. Then assemble complex protein structures onto these lattices in a preferred manner, such that introduction of the lattice frames might “seed” the correct protein configuration. We then conceive biocompatible nanostructures for “biologically inspired” computing and signal processing, for biosensors in the military, for example, or for recreating two-way neural processes and repairs.
By spatially recruiting metabolic enzymes, protein scaffolds help regulate synthetic metabolic pathways by balancing proton/electron flux. It seems that this represents a synthesis methodology with advantages over more standard chemical constructions. We used to design sequences of digital optical logic gates to remove the processing bottlenecks of conventional computers. But if we look to producing non-Von Neumann processors with biological constructs, we may truly be at the computing crossroad for the “Kurzweil singularity”!
References
1. Small Tech Consulting. MEMS and nanotechnology. http://www.smalltechconsulting.com/What_are_MEMS_Nanotech.shtml
2. Bueno, O. 2004. The Drexler-Smalley debated on nanotechnology: Incommensurability at work? Hyle-Int. J. Philos. Chem. 10(2):83–98.
3. Prasad, P. N. 2003. Introduction to Biophotonics. Hoboken, NJ: John Wiley & Sons.
4. Valdes, J., E. Valdes, and D. Hoffman. 2009. Towards complex abiotic systems for chemical and biological sensing. Final report to Aberdeen Proving Ground, ECBC-TR-720. Approved for public release and unlimited distribution.
5. MEMS and Nanotechnology Exchange. http://www.mems-exchange.org/
6. Foresight Nanatech Institute. 2007. Productive nanasystems, a technology road-map. UT-Battelle, LLC, Publisher.
7. Weiss, R. 2005. Princeton University Department of Electrical Engineering. http://www.ee.princeton.edu/people/Weiss.php
8. Bio-SPICE. Biological simulation program for intra- and inter-cellular evaluation. http://biospice.sourceforge.net/
9. Hornyak, G., J. Moore, H. Tibbals, and J. Dutta. 2009. Fundamentals of Nanotechnology. New York: Taylor & Francis Group.
10. Vo-Dinh, T. 2003. Biomedical Photonics Handbook. Boca Raton, FL: CRC Press.
11. Vo-Dinh, T. and M. Askari. 2001. Micro-arrays and biochips: Applications and potential in genomics and proteomics. Curr. Genomics 2:399.
12. Prasad, P.N. Introduction to Biophotonics. New York: Wiley.
13. Bray, D. 2009. Wetware: A Computer in Every Living Cell. New Haven: Yale University Press.
14. Lane, N. 2006. Power, Sex, Suicide: Mitochondria and the Meaning of Life. Oxford: Oxford University Press.
15. Chaplin, M. Water structure and science http://www1.1sbu.ac.uk/water/models.html. Accessed December 2010.
16. Schedule and speakers. Conference on Physics, Chemistry & Biology of Water. http://www.watercon.org/schedule.html
17. University of Illinois at Urbana-Champaign. 2008. Water is “designer fluid” that helps proteins change shape. Science Daily. http://www.sciencedaily.com/releases/2008/08/080806113314.htm
18. File: L-aspartic acid skeletal.png. http://en.wikipedia.org/wiki/File:L-aspartic-acid-skeletal.png
19. Protein pKa calculations. http://en.wikipedia.org/wiki/Protein_pKa_calculations.
20. Blum, H. 1970. Time’s Arrow and Evolution. Princeton, NJ: Princeton University Press.
21. Dembski, W. and J. Wells. 2007. The Design of Life: Discovering Signs of Intelligence in Biological Systems. Dallas, TX: Foundation for Thought and Ethics.
22. Haynie, D. 2001. Biological Thermodynamics. New York: Cambridge University Press.
23. 2008. The origin of life: Not that sinister. The Economist April 12.
24. Jaynes, J. 1976. The Origin of Consciousness in the Breakdown of the Bicameral Mind. Boston, MA: Houghton Mifflin.
25. National Center for Biotechnical Information. A strategy for identifying differences in large series of metabolomic samples analyzed by GC/MS. U.S. National Library of Medicine. http://www.ncbi.nlm.nih.gov/pubmed/15018577
26. Nicholson, J. K., J. C. Lindon, and E. Holmes. 1999. Metabonomics: Understanding the metabolic responses of living systems to pathophysiological stimuli via multivariate statistical analysis of biological NMR spectroscopic data. Xenobiotica 11:1181–1189.
27. Human metabolome database version 2.5. Genome Alberta. http://www.hmdb.ca/
28. Cooke, R. 2001. Dr. Folkman’s War. New York: Random House.
29. Medical College of Georgia. 2009. Cancer’s distinctive pattern of gene expression could aid early screening and prevention. Science Daily. http://www.sciencedaily.com/releases/2009/07/090727110641.htm
30. Prasad. Introduction to Biophotonics.
31. Scapin, G. 2006. Structural biology and drug discovery. Curr. Pharm. Des. 12(17):2087. doi:10.2174/138161206777585201.PMID 16796557.
32. Lundstrom, K. 2006. Structural genomics for membrane proteins. Cell. Mol. Life. Sci. 63(22):2597. doi:10.1007/s00018-006-6252-y. PMID 17013556
33. Photonics Media. Light reveals neuron function. http://www.photonics.com/Content/ReadArticle.aspx?ArticleID=39843
34. Thompson, E. Professor at Johns Hopkins University. Spring 2010. Private conversation with author.
35. Moglich, A., R. A. Ayers, and K. Moffat. February 2009. Design and signaling mechanism of light-related histidine kinases. J. Mol. Biol. 385(5):1433–1444.
36. Lane. Power, Sex, Suicide.
37. Bashor, C. J., N. C. Helman, S. Yan, and W. A. Lim. 2008. Using engineered scaffold interactions to reshape MAP kinase pathway signaling dynamics. Science 319(5869):1539–1543.
38. Dueber J. E., G. C. Wu, G. R. Malmirchegini, T. S. Moon, C. J. Petzold, A. V. Ullal, K. L. Prather, and J. D. Keasling. 2009. Synthetic protein scaffolds provide modular control over metabolic flux. Nat. Biotechnol. 27(8):753–759.
39. Chorost, M. November 2009. Powered by photons. Wired.
