High hydrostatic pressure processing of fruit juices and smoothies: research and commercial application
F. Sampedro and X. Fan, United States Department of Agriculture, Agricultural Research Service (USDA-ARS), USA
Abstract:
Several world-wide health organizations have pointed out the importance of increasing the intake of fruits and vegetables in the diet. Consumers are also increasing the demand for more convenient, nutritious, fresh and price-reasonable products. Although thermal pasteurization has been the processing technology of choice to preserve fruit juices, the thermal process damages the nutritional and sensory properties of products. As a result of scientific studies demonstrating the benefits of high hydrostatic pressure (HHP) technology and the advances in the design of process equipment, high quality fruit juices and related products treated by HHP are appearing in food markets around the world.
3.1 Introduction
Fruit and vegetable juices and their derivatives, are major world commodities and part of the economic lifeblood of many countries, particularly in the developing world. The perception of the healthy nature of these products is one of the major reasons for their consumption. Owing to their perishable characteristics, however, it is necessary to process them to extend their shelf-lives. To prolong shelf-life, thermal pasteurization is most commonly employed, but losses of representative flavor compounds, color, and vitamins occur (Yeom et al, 2000).
In recent years, consumers have increasingly sought so-called ‘fresh’ products stored under refrigeration. The trend of increasing consumption of these products is partly due to the application of emerging non-thermal technologies, such as high hydrostatic pressure (HHP). This is an interesting field for application in fruit juices, because HHP employs cold pasteurization, which preserves the nutritional quality and characteristic flavor of the products (Min and Zhang, 2003; Rivas et al., 2006; Sampedro et al., 2009a,b; Sanchez-Moreno et al, 2006).
Large amounts of scientific data have shown the advantages and benefits of HHP processing versus thermal pasteurization in the processing of fruit juices. High microbial reduction of spoilage and acid-resistant pathogens, enzymatic stabilization, preservation of bioactive compounds, and positive consumer attitudes toward this technology have been demonstrated in numerous studies (Balasubramaniam and Farkas, 2008; Oey et al., 2008a, 2008b; Rastogi et al., 2007; San Martin et al., 2002; Wright et al., 2007). As a result of the research efforts showing the benefits of HHP technology and the advances in the design of process equipments that satisfy the industrial production and cost requirements, fruit-based products treated by HHP with a competitive price and high nutritional quality are gaining an increasing share in markets around the world, especially in Australia and Europe.
3.2 Fruit composition, high hydrostatic pressure (HHP) treatment and recommended fruit intake
3.2.1 Fruit composition
Fruits that contain a wide range of different compounds and show considerable variation in composition and structure play a very significant role in human nutrition. The most important components in fruit and its derivatives can be grouped as follows: water, proteins, carbohydrates, fats, minerals and vitamins. Most of these components are essential nutrients that are needed by the human body.
Water is the most abundant component (more than 80%) in fruit, ranging from 82% in grapes to 90% in strawberries (Fourie, 1996). However, the maximum water content varies between stages of maturity and even between individual fruits of the same kind because of structural differences. Proteins usually contribute less than 1% of the fresh weight of fruit. Carbohydrates consist of polysaccharides such as starch, cellulose, hemicellulose and pectic material, and also disaccharides and monosaccharides such as sucrose, fructose, and glucose. The total carbohydrate value varies from 3% in lemons to about 15% in grapes (Fourie, 1996). Dietary fiber makes up a unique component within the total carbohydrate content of fruits and vegetables. Fiber is the structural material of plant cells that are resistant to the digestive enzymes of the human stomach and is essential for human intestinal function.
Lipid content of fruit and vegetables is generally below 1%, and they are therefore not a good source of fats. Fruits also contain a variety of essential mineral elements, among which potassium is the most abundant and occurs mainly in combination with various organic acids. Calcium is always present in the pectic material in the cell walls of the fruit and magnesium in the chlorophyll molecules. Phosphorous can play an important part in carbohydrate metabolism.
As far as vitamin content, considerable differences are reported between fruit species and varieties, as well as between the same varieties grown under different environmental conditions. Fruits and vegetables are specially known as a source of ascorbic acid. Vitamin A is fat soluble and does not occur as such in fruit, although certain fruit carotenoids can be converted to vitamin A in the body. On the other hand, fruit is a moderate to poor source of the members of the vitamin B and E group. Several components with antioxidant activity naturally occur in fruit. These components include ascorbic acid, tocopherols, beta-carotene and other flavonoid components. Fruits are also rich sources of phytochemicals such as phenolics and flavonoids which may reduce the risk of cardiovascular disease, cancer, and other chronic diseases.
3.2.2 Juices, smoothies, and pulps with the potential to be treated by HHP
Fruit juices are made from fresh fruit by mechanical squeezing (premium juices), or also from fruit juice concentrates by diluting with water. Premium or direct juices are considered the best candidates for HHP processing due to their high quality requirement for commercial appeal.
Smoothies are blended cold drinks consisting of a number of ingredients including fruit (and sometimes vegetables) and fruit juice. Depending on the type of smoothie, crushed ice, sugar or honey, some types of thickener such as milk, soymilk, or yogurt, or other flavor enhancers and stabilizers can be added to create a complex composition. Smoothies have milkshake-like consistencies which are thicker than slush drinks. They are usually sold as a drink, snack or meal alternative, they are available either ready-made or made-to-order, and they are becoming an increasingly popular way of consuming dietary fruits. Often marketed to health-conscious people, smoothies are commonly fortified with ‘boosts’ or ‘enhancers’ (additional vitamins, minerals, herbs amino acids or other nutrients).
The fruit pulp is an intermediate product made from fresh fruit, not intended for consumption as such, which also includes whole and large portions of fruit. It can be used as raw material for yogurt and dessert preparations or diluted for consumption in juice.
3.2.3 Fruit intake recommendation
Low fruit and vegetable intake is among the top 10 risk factors contributing to attributable mortality (WHO, 2003). Fruits and vegetables as part of the daily diet could help prevent major noncommunicable diseases (NCD) such as cardiovascular diseases and certain cancers. Eating a variety of vegetables and fruits clearly ensures an adequate intake of most micronutrients, dietary fibers and a host of essential non-nutrient substances. Increased fruit and vegetable consumption can also help displace foods high in saturated fats, sugar or salt. A published report of a Joint FAO/WHO Expert Consultation on Diet, Nutrition and the Prevention of Chronic Diseases recommends the intake of a minimum of 400 g of fruits and vegetables per day (excluding starchy tubers such as potatoes) for the prevention of chronic diseases including heart disease, cancer, type 2 diabetes, and obesity (WHO, 2003).
Presently, the estimated intake levels of fruits and vegetables varies considerably around the world ranging from less than 100 g/day in less developed countries, to about 450 g/day in Western Europe. However, the intake increases if consumption of fresh or canned juice is taken into account, and it is important, therefore, to achieve high quality beverages and to ascertain their nutritional value. In a systematic review, Ruxton et al. (2006) found that pure fruit and vegetable juices appeared to offer similar health benefits to whole fruits and vegetables, probably because of similarities in antioxidant and/or polyphenol content. Therefore, the nutritional quality of fruit juices is extremely important in order to provide vitamins, minerals and fiber to satisfy dietary recommendations.
3.3 Basic research on high hydrostatic pressure (HHP) processing of fruit juices and derivatives
3.3.1 Aspects related to food safety
Among the spoilage microflora encountered in fruit juices, the more common ones include lactic acid bacteria (Lactobacillus and Leuconostoc species), fermentative yeasts (Saccharomyces cerevisiae) and spore-forming molds due to their capability of growing at low pH values (< 4.0). Lactic acid bacteria produce characteristic off-flavors due to the production of diacetyl as a metabolic end product. Yeasts frequently cause spoilage due to the ethanolic fermentation. Spoilage of fruit juices can also result in an increase of the viscosity and the production of hydrogen sulfide and other off-odors (Basak et al., 2002).
It is recognized that fruit juices and fruit based beverages are micro-biologically safe because of their low pH. However, some strains of E. coli O157, Salmonella and Shigella species are acid-resistant and can survive for long periods in acidic environments at low temperatures (Miller and Kaspar, 1994). These microorganisms can be present in the final product in different ways. Post-processing recontamination, high raw material contamination, or high processing resistance could be different ways of introducing contamination, leading to a small proportion of cells surviving, and causing a food safety concern due to their low infective dose.
Conventional thermal pasteurization processes reduce the initial counts of spoilage and pathogenic microorganisms to a safe level, but cause detrimental effects on the overall food quality. High hydrostatic pressure (HHP) technology can be an alternative process to achieve an optimal safety level without affecting the natural properties of food. In the following section, scientific data is provided regarding the effects of HHP technology on the food safety of fruit juice products.
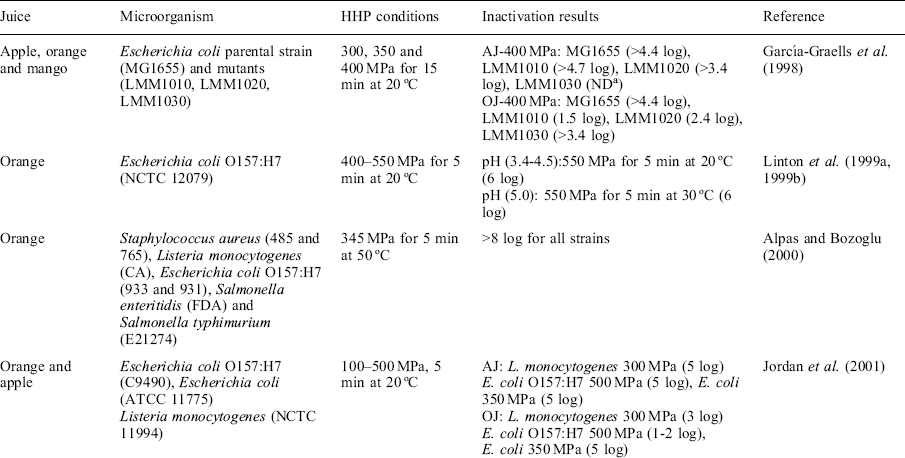
Bacteria
The genus Listeria consists of small, non-spore forming, motile and Gram-positive rods. Listeria monocytogenes is the primary pathogenic species. L. monocytogenes is a soil reservoir, moderately heat-resistant microorganism that is able to grow under refrigeration conditions with or without the presence of oxygen in a wide range of ready-to-eat products with extended shelf-lives (Doğan and Erkmen, 2004). Although Listeria is not known to have caused outbreaks through the consumption of unpasteurized fruit juices, it has been isolated from unpasteurized apple juice (Sado et al., 1998). Table 3.1 shows the main results of the inactivation of Listeria species (L. monocytogenes and L. innocua) achieved by HHP technology in fruit juices. A high pressure treatment of 600 MPa for 5 min at room temperature is typically sufficient to obtain a 5 log reduction of L. monocytogenes in fruit juices. However, the media used to conduct the studies seems to influence the effectiveness of the technology. When comparing the baroresistance of L. monocytogenes inoculated in different substrates (buffer, whole milk, and fruit juices), HHP treatment in milk was not as effective as in other food systems. Fat and protein content in milk seems to protect the microorganism against pressure, whereas the low pH of fruit juices can be an additional inhibitory factor enhancing the effectiveness of HHP technology (Doğan and Erkmen, 2004).
Temperature plays an important role when combined with pressure enhancing the overall process effectiveness. In addition, refrigeration conditions will keep the survival fraction of microorganisms under control. However, due to the psychrotrophic nature of Listeria, the microorganism could survive in the product during the shelf-life period. Fortunately, in most fruit juices, the acidic environment will prevent recovery of injured cells and reduce the surviving Listeria cells to safe levels. This further reduction can be explained by the fact that most cells injured by the pressure become more sensitive to the acidic environment and are not able to survive during the storage period.
Escherichia coli belongs to the family Enterobacteriaceae (enteric bacteria), which are Gram-negative, rod-shaped, motile and facultative anaerobes that live in the intestinal tracts of animals (Ramaswamy et al., 2003). The baroresistance of E. coli in apple juice (AJ) and orange juice (OJ) has been studied by several authors (Table 3.1). Differences in pressure-resistance are known to occur depending on the media and strain used in the study. Ramaswamy et al. (2003) found that a single pressure pulse at 400 MPa and 25 °C was enough to inactivate initial counts of E. coli by 8 log cycles in AJ (pH 3.5). The combination of pressure and mild heat composes a good strategy to improve the overall effectiveness of the HHP technology without compromising food quality. Muñoz et al. (2007) found an optimal combination of moderate levels of high pressure (200–250 MPa) and mild temperature (57–60 °C) for E. coli inactivation (6 log cycles) in OJ and AJ. At these treatment conditions, all natural flora present in the juices were reduced to almost undetectable levels.
In a selective environment of a high-pressure fruit juice processing plant, the occurrence of naturally E. coli pressure-resistant strains cannot be ruled out. In this regard, Garcia-Graells et al. (1998) isolated several mutants that were pressure-resistant (800 MPa at 10–40 °C) from a pressure-sensitive E. coli. The non-treated E. coli mutants were able to survive in acidic substrates (AJ and mango juice pH 3.3–4.0) at refrigeration conditions (8 °C) for at least 30 days. The survival at refrigeration conditions even in acidic conditions could be explained by the fact of a reduced permeability of cell membrane to protons or reduced metabolic activity at reduced temperatures. However, after pressure treatment (300–500 MPa) and further storage (5 days of storage at 8 °C) the number of survivors was below the detection limit (high numbers of cells were injured during the treatment, resulting in a reduced resistance to the low pH during storage). This fact illustrates that sublethal injury is a crucial parameter which should be monitored after HHP treatment to detect any recovery of injured cells, particularly over prolonged storage periods.
It is known that E. coli O157:H7 strains have been implicated in several outbreaks related to unpasteurized fruit juices due to its high resistance to acidic environment and low infective dose. Several studies have been performed using this strain and HHP treatment in different fruit juices (Table 3.1). In a first study, Linton et al. (1999a, 1999b) studied the survival of E. coli O157:H7 in OJ at different pH levels (3.4 to 5.0). The survival of E. coli in OJ was pH dependent. After 20 and 25 days at 3 °C at pH 3.4 and 3.6, respectively, no cells were detected. After 25 d at higher pH levels (3.9, 4.5, and 5.0) the reduction was 4.5, 1.3, and 0.6 log units. This fact could risk the occurrence of food poisoning, if OJ became contaminated with E. coli O157:H7. This is particularly true since its survival is longer than the length of time required for juice spoilage to occur. The authors found an optimal treatment (6 log reduction) of 550 MPa for 5 min at 20 °C at pH levels of 3.4 to 4.5 and 550 MPa at 30 °C at pH 5.0. Other studies have also shown the higher pressure resistance of the E. coli O157:H7 strain. Jordan et al. (2001) used two E. coli strains (type-strain and O157:H7) in AJ and OJ substrates. Both strains were more resistant to the pressure treatment in OJ than in AJ. Slight pH differences (higher pH in OJ), viscosity or other physical-chemical characteristics seemed to affect the microorganism resistance. A reduction of 1 log in E. coli O157:H7 was achieved in OJ, whereas 5 log was reduced in AJ after a treatment of 500 MPa for 5 min. The type-strain of E. coli was much more pressure-sensitive and after 350 MPa, a 5 log reduction was achieved in both substrates. After storage at 4, 25, and 37 °C, a further 3.3 and 7 log reduction at 4 and 25–37 °C temperatures, respectively, was achieved in O157:H7 strains in OJ. This fact also corroborates that refrigeration temperatures seem to protect the pressurized E. coli cells against the acidic environment.
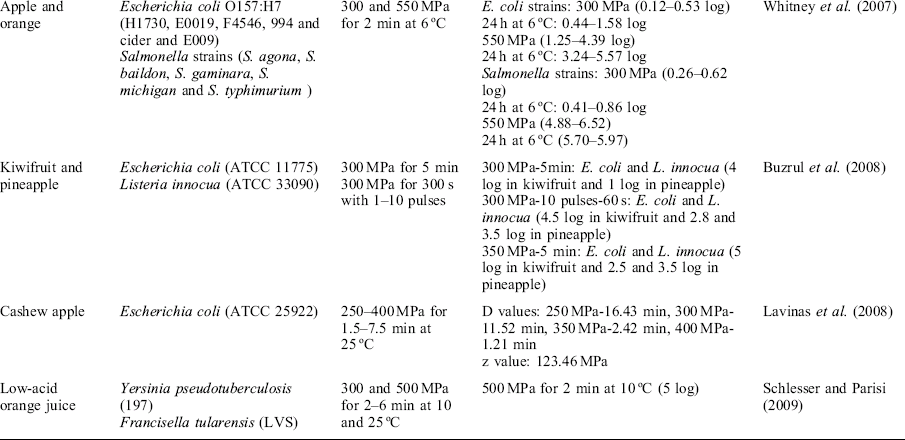
In some cases, more than one bacterial strain can be present in the fruit juice and it is interesting to study the pressure resistance of E. coli strains composed of a cocktail. In this regard, Teo et al. (2001) and Whitney et al. (2007) used a cocktail of several E. coli O157:H7 strains related to different outbreaks to inoculate different juices (OJ, AJ, grapefruit (GJ), and carrot (CJ)). Treatment at 615 MPa for 2 min at 15 °C was able to inactivate more than 5 log cycles in CJ and GJ samples but not in the rest of the substrates. After storage for 24 h, further inactivation was observed (3.2–5.6 log reductions). It seemed that acid-resistant strains were also more resistant to pressurization. These differences among strains could be due to differences in their membrane composition and ability to repair membrane damage in acid environments after pressurization. In addition, differences in gene expression related to stress response could also contribute to increased resistance to pressure.
Some strains of Salmonella are able to survive acidic conditions and therefore are present in the fruit-based products, if low hygienic conditions are present, raw material is highly contaminated, or the product is recontaminated after processing. Several studies have been conducted to assess the pressure sensivity of these acid-resistant strains (Table 3.1). Teo et al. (2001) and Whitney et al. (2007) have shown an optimal treatment of 2 min at 550–615 MPa and 15 °C in the inactivation of several strains of Salmonella (S. hartford, S. muenchen, S. agona, S. enteritidis and S. typhimurium) in several fruit juices (AJ, OJ, GJ and CJ) achieving more than 5 log cycles in all samples.
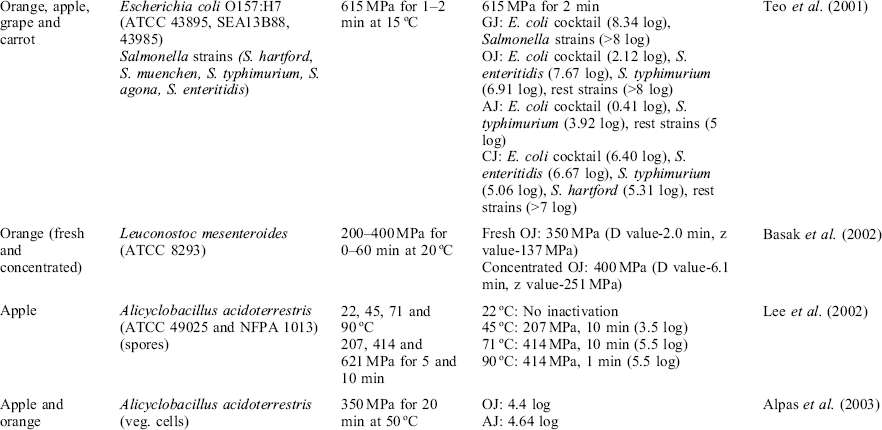
Several lactic acid bacteria species can spoil fruit juices in optimal conditions for their growth. Leuconostoc mesenteroides is one important species among them. L. mesenteroides inactivation was studied by Basak et al. (2002) in fresh and concentrated OJ (42°Brix) after high pressure treatment. The survivor curves showed a biphasic phenomenon. The pressure instantaneously reduced the counts of the microorganism (4.4 log cycles at 400 MPa and 20 °C), then the survivor curves followed first-order rate destruction. The study also showed that the effectiveness was significantly reduced in the concentrated OJ where both the lower aw and higher soluble solids content protected the microorganism from pressure.
Most spore-forming pathogenic bacteria will not germinate or grow in an acidic environment. However, some spoilage spore-forming bacteria such as Alicyclobacillus acidoterrestris have been implicated in acidic beverage and fruit juice spoilage (Lee et al., 2006). A. acidoterrestris is a soilborne and thermoacidophilic microorganism, and can be present in the final product through soil adhering to the surface of fruits during harvest or by the water used during juice processing. Their spores can germinate, grow and cause spoilage in a pH range of 2.5 to 6.0, a level below the typical range for spore-forming bacteria. The germination and growth has been observed in OJ incubated at 44 °C for 24 h and can occur even at higher temperatures (80 °C) (Pettipher et al., 1997). Their spores are resistant to the normal pasteurization conditions normally applied to acidic fruit products and can germinate and grow during storage of retail products where spoilage can occur (Jensen, 1999). This exceptional fact is possibly attributable to its unique cellular membrane composition containing ring structures (cyclohexane fatty acids) closely packed leading to a high stabilization of the membrane and retaining more divalent cations than other bacterial spores, explaining their high resistance to demineralization (Lee et al., 2006). The typical spoilage effects are organoleptic taint due the production of guaiacol leading to a ‘medicinal’ or ‘phenolic’ off-flavor and light cloudiness (Lee et al., 2002).
Some studies have investigated the pressure resistance of A. acidoterrestris spores to high pressure (Table 3.1). It seems that the spores are also baro-resistant, primarily due to the dehydrated state of the core. The inactivation of bacterial spores seems to occur in two steps: high pressure germination and inactivation of germinated spores (350 MPa for 20 min at 50 °C) (Alphas et al., 2003; Lee et al., 2006). Again, the aw of the fruit juice influence the degree of inactivation; HHP is less effective in concentrated juices. The contamination of concentrated fruit juices with A. acidoterrestris and the dilution to produce commercial juice will rapidly spread the microorganism.
Molds and yeasts
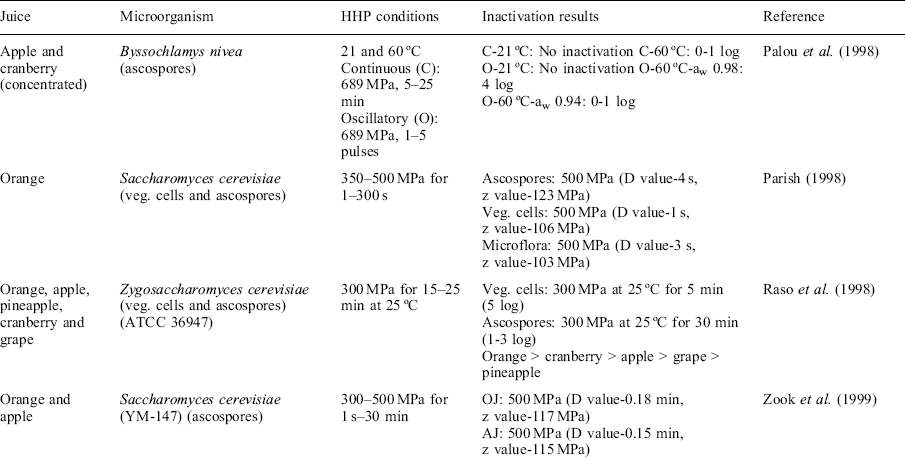
Yeasts such as fermentative Saccharomyces cerevisiae and Zygosaccharomyces bailii can spoil fruit juices. Both yeasts have the ability to produce ascospores. The ascospore protoplast has a structure similar to vegetative cells, but the wall consists of an outer and inner coat (Raso et al., 1998). Spore formation can be induced on fruit surfaces where low concentrations of sugars and ethanol are present. During juice extraction, ascospores may contaminate the juice, eventually causing spoilage. Ascospores are known to be more resistant than vegetative cells to different food processing and chemical and physical agents (Zook et al, 1999).
Vegetative cells are easily inactivated by pressure and usually a treatment of 350 MPa at room temperature is enough to instantaneously reduce the initial yeast load in a fruit juice (Table 3.2) (Bang and Swanson, 2008). When studying the microflora of OJ, Parish (1998) found that the inactivation kinetics parameters of juice microbial flora were similar to the ascospores, meaning that a high percentage of the yeasts present in the juice were in the form of spores. This fact points out the need for validating high pressure treatment in a juice with high populations of ascospores.
The fact than ascospores are more pressure-resistant than vegetative cells in fruit juices has been corroborated in several studies (Raso et al., 1998; Parish, 1998). In ascospore survivor curves, a shouldering effect, mainly at low pressure values (300 MPa), indicates that at lower pressures, a time threshold was required before substantial inactivation was observed. The pH of fruit juice (3.5–5) seems not to affect ascospores inactivation by HHP. In addition, no differences are observed among different juices and model systems indicating no protective effects (Zook et al., 1999). This is different than the situation with bacteria, where inactivation increases at pH lower than 4.5. This phenomenon corroborated the ability of yeasts to grow at low pH. Comparing the inactivation data of several studies on yeasts with those obtained by other authors on bacteria species confirmed that yeasts are more sensitive to pressure than non-sporulating bacteria. In addition, a lower pressure-resistance of yeast ascospores is observed in comparison with bacterial spores (Reyns et al., 2000). The ascospores are larger and higher in lipid and carbohydrate content, and do not have dipicolinic acid and a cortex, which is considered very important in the resistance of the spore due to its ability in maintaining the state of osmotic dehydration of the protoplast. However, when using a concentrated juice (42°Brix) the aw can affect the S. cerevisiae inactivation. The inactivation rate significantly decreased in the concentrated OJ. The high content of soluble solids or low aw seems to be the main reason for the low inactivation rate (Basak et al., 2002; Donsí et al., 2007).
Fruits used for juice processing can be contaminated with molds. Most fungi are heat sensitive and usual pasteurization processes in fruit juices are adequate to inactivate them. However, several molds, especially ascospores producing molds, have high heat-resistance even at elevated temperatures of 90 °C (Palou et al., 1998). Ascospores of several Ascomycetes such as Byssochlamys nivea have been found to contaminate commercial fruit juice concentrate due to its wide pH growth range (2.0–9.0). Other molds such as Talaromyces avellanus can also be found as natural contamination in fruit products (Voldøich et al., 2004). Regarding the effect of physico-chemical characteristics of substrates on efficacy of HHP technology, the water activity or soluble solids content plays an important role (Table 3.2). This is especially true when HHP treatment is considered for the pasteurization or decontamination of concentrated fruit juices where severe treatment conditions are necessary.
3.3.2 Aspects related to food quality
Enzymes
Enzymes are a special type of protein with enormous catalytic power and great specificity. Their biological activity arises from active sites brought together by a three-dimensional configuration. They have two important regions; one that recognizes the substrate and the other that catalyzes the reaction once the substrate has been bound (Hendrickx et al., 1998). These two are called the active site and take place in a small part of the enzyme total volume. Changes in the active site interfering in the enzyme-substrate union or protein denaturation can produce an activity loss or functionality variations (Tsou, 1986). In general, covalent bonds are not affected by HHP treatment because the primary structure of the enzyme will not be damaged. The hydrogen bonds are also relatively baroresistant and the secondary structure will not be affected up to pressure values around 700 MPa. However, HHP treatment affects electrostatic and hydrophobic interactions that maintain the tertiary and quaternary structures stability (Ludikhuyze et al., 2002).
Within the food quality related enzymes, the most important in fruit juices are the following:
• Polyphenoloxidase (PPO) which is responsible for enzymatic browning
• Pectinmethylesterase (PME) which is responsible for cloud loss and consistency changes
• Peroxidase (POD) which increases the production of undesirable flavors.
In fruit juices, enzyme baroresistance is generally higher than the majority of naturally found microorganisms. For that reason, fruit juice preservation treatment is based on the inactivation of the enzymes responsible for its quality deterioration (PME in citrus juices, PPO in apple juice, among others). However, in some cases, no relation is observed between enzyme baroresistance and thermoresistance.
Enzyme baroresistance also depends greatly on:
• the type of enzyme – enzymes with different three-dimensional structures containing different percentage of α-helix, β-sheet, β-turn and random coil;
• the source of the enzyme – from native enzyme to purified form extracted from different parts of the plant;
• the nature of the system – from buffer to real food with more complex composition where different interactions can be produced, different physico-chemical characteristics such as pH, sugar content, aw and pulp concentration, different fruits, varieties and harvest season will lead to different enzyme structure; and
• the process conditions – combinations of pressure, temperature and time.
A primary purpose of HHP processing is food preservation by maintaining the quality of fresh product and thus inactivation of quality deteriorative enzymes. Among these enzymes, PME has been extensively studied by HHP technology in order to find the optimal inactivation conditions. PME is a texture-related enzyme mainly associated with the pulp content of citrus juices. It destabilizes the suspension formed by pectin mycelia (cloud loss) leading to a clarified product with low commercial value. Different studies have demonstrated the baroresistance of the enzyme to elevated pressures in OJ. Treatments below 500 MPa at room temperature seem not to affect the native PME activity in OJ (Cano et al., 1997; Nienaber and Shellhammer, 2001). Processing conditions of 600–700 MPa for 1–3 min combined with mild temperatures (50–60 °C) seems to be effective in inactivating the native PME (Nienaber and Shell-hammer, 2001; Polydera, et al., 2004, Sampedro et al., 2008), which stabilizes the OJ between 90 d and 16 wks at 4 °C (Parish, 1998; Goodner et al, 1999). Low pH seems to enhance the PME inactivation while increasing soluble solids content (concentrated OJ) seems to decrease the effectiveness of HPP showing a protective effect (Basak and Ramaswamy, 1996). Regarding the application of HHP technology to smoothies, Sampedro et al. (2008) studied the influence of HHP processing in a beverage based on a mixture of orange juice and milk (50% of OJ, 20% of milk, 30% of water, 0.3% of pectin and 7.5% of sugar). The authors found optimum conditions for PME inactivation at 90 0C for 1 min or 700 MPa at 55 °C for 2 min showing the protective effect of the orange-milk media. In addition, PME was more thermostable and baroresistant in the OJ-milk based beverage system than in OJ.
POD is an oxidoreductase related to the oxidation of a wide range of natural substances present in fruits, especially those containing aromatic groups. The mode of action involves the generation of free radicals able to abstract hydrogen from such substrates. Usually, hydrogen peroxide or oxygen act as oxidizing agents. POD contributes to phenolic oxidation leading to deteriorative changes in flavor, texture, color and nutrition. In OJ, POD is involved in the loss of flavor quality (Elez et al., 2006). Several studies have shown the high baroresistance of POD in different substrates. In one study, Cano et al. (1997) achieved only a 25 and 50% inactivation of POD in strawberry (230 MPa for 15 min at 43 °C) and OJ (400 MPa for 15 min at 32 °C). Below or above these conditions, higher enzyme activity was observed. It seemed that the activation was pH dependent and at higher pH, the activation was strong as in the case of strawberry. In this sense, Garcia-Palazon et al. (2004) only observed a 35% inactivation of POD in strawberry after 600 MPa for 15 min at 20 °C and no more inactivation was achieved with pressures increasing up to 800 MPa, whereas Fang et al. (2008) observed a residual activity of 30% after 600 MPa for 30 min at 50 °C.
PPO is an oxidative enzyme responsible for undesirable color changes, undesirable flavors and nutritional losses. It is mainly related to the browning reactions, catalyzing the hydroxylation of mono-phenols, leading to the formation of di-phenols and the following oxidation of di-phenols to form quinones in the presence of oxygen. Next, the condensation of quinones generates dark substances (melanines) which negatively influence the quality and marketability of commercial fruit juices (Giner et al., 2002). Cano et al. (1997) studied the effects of HHP processing on PPO in strawberry puree. A maximum of 60% of inactivation was achieved combining 285 MPa at room temperature. At higher pressures or temperature levels, higher enzyme activity was observed (enzyme activation). The low pressure and temperature conditions applied in this study could explain the low treatment effectiveness and the activation phenomenon. Palou et al. (1999) studied PPO inactivation in a banana purée. Pressure treatment alone (689 MPa for 10 min at room temperature) was able to reduce enzyme activity by only 20%. Only after a blanching treatment (saturated steam for 7 min) followed by pressure treatment (689 MPa) they were able to reduce the initial activity by more than 95%. In a later study, García-Palazón et al. (2004) showed a high baroresistance of PPO in red raspberries, resulting in a remaining activity of 70% after 800 MPa for 15 min. In contrast, PPO in strawberries was more sensitive to the pressure treatment and 600 MPa for 15 min or 800 MPa for 10 min was enough for a complete inactivation. The authors linked the enzyme stability in raspberries and strawberries with the stability of their anthocyanins and the consequent color loss. Higher PPO activity decreased the stability of anthocyanins. Because raspberries retain high levels of activity of PPO after HPP treatment, anthocyanins in raspberries were more susceptible to HHP treatment.
Color
The color observed by human beings is the perception of the wavelengths coming from the surface of the object on the retina of the eyes (Tijsken et al., 2001). Food appearance can change depending on the amount of light, the light source, the observer’s angle of view, size and background differences. However, standardized instrumental color measurements used in the food industry such as the Hunter color (L*, a* and b*). L* is a measure of brightness/whiteness that ranges from 0 to 100 (white if L* = 100, black if L* = 0), a* is an indicator of redness that varies from –a* to + a* (–a* = green, + a* = red) and b* is a measure of yellowness that varies from –b* to + b* (–b* = blue, + b* = yellow). The CIELAB system is used as a quality index in fruit juices to assess the conformity to specifications or measure the changes as a result of food processing or storage (Giese, 2000). Maintaining the natural color of fruit juices is a major challenge to the application of HHP technology, because color is the first characteristic that is noticed in food and predetermines consumer perceptions of freshness and expectations of both flavor and quality (Rodrigo et al., 2007). The changes in the natural color of fruit juices are based on the degradation of pigments by enzymatic and non-enzymatic reactions. Compounds such as anthocyanins are responsible for the color in some fruits. Rodrigo et al. (2007) studied the degradation kinetics of strawberry juice color after HHP processing and concluded that the combination of L*, a* and b* parameters in the form of L*x a*/b* was the most accurate way to describe the color degradation in strawberry juice. No differences were found between treated and control samples up to 700 MPa for 60 min at 65 0C. However, the effect of pH was found to be significant in the strawberry samples. The a* parameter after HHP processing increased as the pH increased from 3.7 to 5. It seems that pelargonidin-3-glucoside anthocyanin is the main compound responsible for the red color in strawberries and it is not stable in a pH range of 5–7.
Sensory and consumer studies
Some sensory studies have been conducted in fruit juices in order to demonstrate the advantages of HHP processing versus thermal pasteurization on preserving the natural sensory properties. Some studies have used trained sensory panelists in order to compare the freshness and acceptability of samples treated by HHP and traditional thermal pasteurization. However, due to their training, sensory panelists may not be representative of the typical consumers of fruit juices. In these cases, consumer acceptance studies are necessary. In addition, the analytical profile of volatile compounds related to the fruit juices aroma is performed, to try to connect the unique flavor of fruit juices with specific chemical compounds.
Baxter et al. (2005) studied changes in the sensory properties and flavor compounds during a 12-wk shelf-life storage of OJ processed by HHP and thermal pasteurization at 4 and 10 °C. Ten trained sensory panel members were used for the descriptive sensory analysis of samples and 30–40 regular consumers of OJ participated in a consumer acceptability study. Regarding the color, the trained panel did not observe differences among the different samples during the overall period. Increasing the duration of the storage period led to a decrease in the sweetness and strength of orange odors and an increase of aged, artificial and fermented odors. At the end of the storage period, consumers did not differentiate between control, thermally and pressure-treated samples at 4 °C. However, scores were lower in the HHP for 10 °C samples and were unacceptable for the thermally processed samples at 10 °C. Twenty volatile compounds were analyzed in the storage of OJ at 4 and 10 °C. Considerable reductions were found for most compounds in both HHP and thermally processed juices compared with the control sample at –20 °C. The compounds showing the greatest reductions were octanal, citral, ethyl butanoate and limonene with the final concentration of compounds 6–38% lower than the initial level. The decrease in the volatile compounds concentration during the storage was produced by a combination of factors. PET bottles used for the OJ storage seemed to absorb some volatile compounds. In addition, oxidation, hydrolysis and acid-catalyzed reactions were responsible for the degradation of volatile compounds.
Working with an OJ-milk beverage, Sampedro et al. (2009a,b) studied the volatiles profile after HHP treatment. After HHP treatment (650 MPa for 15 min at 30 °C), some volatile compounds increased. The authors argued that in a complex matrix such as OJ, with the presence of suspended solids, a portion of analytes could be entrapped in the pulp. HHP treatment could increase the membrane permeabilization and facilitate the release of several compounds from the suspended solids to the liquid phase, facilitating its extraction into the headspace. The average loss of volatile compounds concentration was between – 14.2 and 7.5% at 30 °C and 22.9 and 42.3% at 50 °C.
As for tropical fruit juices, Laboissiere et al. (2007) conducted a study on the effects of HHP processing on the sensory characteristics of a Brazilian yellow passion fruit juice. They found very similar patterns for sensory properties between fresh passion fruit juice and HHP processed juice. The only parameter that differentiated both samples was color. In addition, a panel could distinguish between fresh and HHP treated samples and commercially pasteurized samples. The main sensory attributes that differentiated those samples were the presence of suspended particles, phase separation, natural aroma and flavor, artificial aroma and flavor, cooked aroma and flavor and fermented flavor. Most of these sensory attributes considered as sensory defects possibly resulted from the heat pasteurization, addition of artificial aromas, flavor compounds and stabilizers.
Consumers are becoming more conscious about the potentially negative impact of food processing on their health and the environment. Healthy and natural foods are the most important area of research by the majority of food companies (Katz, 2000). ‘Fresh’ remains the most desirable food label claim. Other aspects such as country-of-origin, organic food, local foods and environmental concerns, have continued to rank high in public attention (Deliza et al., 2005). A positive consumer attitude towards the use of HHP technology is necessary to guarantee the success of the product in today’s competitive global market, where new food product innovation is required for survival. That means that the role of the consumer in the technology validation process must be taken into account.
In this sense, Deliza et al. (2003 and 2005) conducted two studies concerning the Brazilian consumer attitudes toward to the use of high pressure processing. They chose four consumer groups consisting mainly of women and fruit juice consumers. The product chosen for the study was pineapple juice processed by HHP with three different information labels (low, medium and high information). The first statement, pointed out by the majority, was the price as an important attribute during their decision-making process. The study also revealed that most consumers inferred the product taste based on the label information which affected the product expectation and perception. When the information about the technology was presented, three of the four groups perceived the product as having higher quality. The information about the technology had a significant impact on the intention of purchase. However, information about the technology without further explanation led to a negative impact on the consumer intention of purchase. These results lead to important information for the food producers. The information about the technology in the product label is essential for the product to be perceived by consumers as higher quality. In addition, factors influencing fruit juice purchasing include convenience, taste and cost.
In a later study, Nielsen et al. (2009) conducted a consumer study using focus groups in six European countries (two from northern Europe and four from eastern Europe). A baby food and fruit juice were used as selected products for focus group discussions. Participants were positive towards HHP products for naturalness, improved taste, and high nutritional value (high vitamin content). Longer shelf-life in comparison to fresh squeezed juice products, high prices and lack of information were seen as negative toward the technology. Environmentally friendly and natural products (no preservatives) were positive towards the technology. Differences were observed among the different cultures. Participants from northern countries were more skeptical about the new technologies and were more worried about the impact on the environment, whereas eastern countries saw the higher price solely as negative. Differences were also seen among the products. Longer shelf-life and higher prices were seen negatively in fruit juices, since consumers are more accustomed to fresh squeezed juices. In contrast, participants saw higher price and longer shelf-life as positives for baby food; however, some participants were negative to HHP baby food, since it is not homemade. In a potential buying situation, quality and especially taste play a critical role in accepting and maintaining the commercial marketability of these novel products.
Bioactive compounds
Daily intake of fruits and vegetables has been related with the prevention of degenerative processes such as cardiovascular disease and certain cancers. This protective action has been attributed to their bioactive compounds, which have antioxidant properties. HHP treatment is expected to be less detrimental than thermal treatment to low molecular weight food compounds such as flavoring agents, pigments and vitamins as covalent bonds are generally not affected by pressure (Butz et al., 2004). Vitamin C is the most important water-soluble nutrient and is related to the antioxidant capacity of the OJ (Cortés et al., 2008).
Regarding the effects of HHP treatment on the stability of vitamin C, ascorbic acid (L-AA) and total antioxidant capacity in OJ were studied. Specifically, (Sánchez-Moreno et al. 2003b, 2005) and Plaza et al. (2006) studied the effects of different high pressure (100–400 MPa for 1–5 min at 30–60 0C) and thermal treatments (70 °C, 30 s and 90 °C, 1 min) in an OJ stored at 4 °C for 40 d. Around 10% of the vitamin C content was lost after the combined treatment (400 MPa for 1 min at 40 °C or 100 MPa for 5 min at 60 °C) but no loss was found at 30 °C. They argued that the high contents after processing could be attributed to a partial elimination of enzymes (POD and ascorbate oxidase) responsible for L-AA and vitamin C oxidative degradation. At higher temperatures, greater decreases in vitamin C content were observed due to possible thermal degradation. During storage, the losses reached 24 and 32% after thermal and HHP treatments, respectively. The untreated sample only lost 10% of the initial content. These differences were attributed to the different levels of enzyme inactivation (POD and ascorbate oxidase) achieved by the treatments that could degrade the L-AA during storage by an oxidative process. The antioxidant capacity was unaffected by HHP and low pasteurization processes, whereas high pasteurization processing (90 °C for 1 min) reduced the antioxidant capacity by 6.5%. The authors found a correlation between L-AA, vitamin C and antioxidant capacity, thus the high stability of both compounds after the different treatments also stabilized the antioxidant capacity. On the other hand, no correlation was found between total carotenoids and flavonones and antioxidant capacity, indicating the lack of relevant effect of these compounds on the total OJ antioxidant capacity.
The type of packaging used in the storage of the fruit juice after processing can influence the stability of the antioxidant capacity of OJ. Polydera et al. (2003) studied the degradation kinetics of ascorbic acid after a HHP treatment (500 MPa at 35 °C for 5 min) and thermal treatment (80 °C for 20 s) using two different packaging materials, an intermediate oxygen barrier (polypropylene bottles) and a high oxygen barrier (polyethylene, aluminum and cellophane) during 1–2 months storage of an OJ at 0, 5, 10, and 15 °C. When polypropylene bottles were used, the degradation kinetics of L-AA during the storage period seemed to follow a first-order reaction. The rate parameter (k) was lower in the pressure-treated sample than in the thermally-treated sample, indicating HPP juice had a lower degradation rate in L-AA than thermally processed juice during storage. The degradation rate of L-AA increased in both samples as storage temperatures increased. When flexible pouches were used, the degradation rates seemed to have two stages. The first part followed first-order kinetics and the second part followed zero-order kinetics. The more rapid decrease of L-AA at the beginning of storage can be attributed to autoxidation, the reaction of L-AA with dissolved oxygen, and then the lower rates could be controlled by the low diffusion of oxygen of the material or by an anaerobic decomposition. Inactivation rates of anaerobic decomposition are usually 2–3 orders of magnitude lower than the oxidative degradation (Gregory, 1996). For these reasons, the inactivation rates were significantly higher in the propylene bottles at both treatments. The authors estimated the shelf-life of OJ based on the vitamin C content (> 20 mg/100 mL of vitamin C in the OJ at the expiration date) regulated by the Association of the Industry of Juices and Nectars from Fruits and Vegetables of the European Union. Using polypropylene bottles, the shelf-life at 5 °C was estimated to be 50 and 34 d for the HHP and thermal samples, respectively. When the storage temperature was increased to 15 °C, the shelf-life period decreased to 20 and 18 d, respectively. On the other hand, using the flexible pouches, the shelf-life was estimated to be 90 and 62 d after HHP and thermal treatment at 5 °C and 62 and 50 d at 15 °C, respectively. The lower degradation rates of vitamin C in the HHP-treated samples extended the shelf-life compared to thermal pasteurization. Owing to the high oxygen barrier of the flexible pouches, the shelf-life was increased with respect to the polypropylene bottles. The sensory evaluation during the shelf-life indicated higher scores for the pressure-treated samples when comparing with the thermal ones.
The degradation of water soluble vitamins (vitamin C, B1 and B6) after high pressure treatments were studied by Sancho et al. (1999) using a model system and strawberry smoothie to check the effects of HHP treatment as well as thermal pasteurization (76C for 20 s) and sterilization (120C for 20 min) on water soluble vitamins. In the model system, the vitamin C content varied from 10 to 12% after 200–600 MPa for 30 min at room temperature. Changes in B1 and B6 vitamins after the pressure treatment were insignificant. In the strawberry system, changes in vitamin C content were not significant after the HHP processing and thermal pasteurization. However, a 33% loss was observed after the sterilization process.
Carotenoids are among the most abundant bioactive compounds in fruits and have diverse biological functions and actions. Provitamin A activity carotenoids and xantophylls are known to provide protection against macular degeneration. They also are potent antioxidant and free-radical scavengers and modulate the pathogenesis of cancers and coronary heart disease (Torregrosa et al., 2005).
Studies conducted by de Ancos et al. (2002) and Sánchez-Moreno et al. (2003a) showed an increase in the total carotenoid content by 23 and 43% after a pressure treatment at 100 and 350 MPa, respectively. Regarding the individual carotenoids, β-carotene increased by 50%, a-carotene by 60%, α-cryptoxanthin by 42% and β-cryptoxanthin by 63% after 350 MPa for 5 min at 30 °C. Differences in the oxygen and hydrocarbon carotenoids in the intracellular locations of juice vesicles could lead to variability in the release of carotenoids after HHP treatment. The higher carotenoid content could be explained by the release of carotenoids from the food matrix (orange cloud) after denaturation of protein-carotenoid complexes induced by pressure. The carotenoids extraction was pressure-dependent and increased at higher pressure levels. These changes could increase the amount of antioxidant carotenoids available, improving the bioavailability and absorption in HHP treated juices. The effect of treatment time was checked at 350 MPa at 30 °C. The carotenoids content was increased by 43% after 5 min, but increasing the treatment time to 15 min did not show any further improvement. The effect of temperature (30 and 60 °C) was studied at 100 MPa. Results showed that an increase in the temperature did not exhibit any improvement in the carotenoid content. The carotenoid degradation is mainly due to oxidation and geometric isomerization. The isomerization of carotenoids goes through covalent bonding rupture and it seems that pressure does not significantly affect covalent bonding. During the refrigerated storage of OJ after different treatments, no losses were found after 15 d at 4 °C. At the end of storage (30 d) losses in the 50 MPa and control samples were 17 and 42%, respectively, whereas the 350 MPa sample had 72% higher content than the fresh one. Long treatment times increased carotenoid content after storage (30 d) with 68, 72 and 100% increasing after 2.5, 5 and 15 min, respectively. The sample at the higher treatment temperature (60 °C) showed higher losses during the storage (28%) with respect to the sample at 30 °C. Vit-A carotenoid content increased (52 and 45%) after treatments at 100 and 300 MPa. Neither increasing the time nor temperature increased the carotenoid content. During the storage, Vit-A carotenoid content increased in the 200 and 350 MPa samples (36 and 63%) but decreased in the control, 50 and 100 MPa samples (9, 42 and 24%). It seemed that at lower pressure, Vit-A carotenoid losses were higher possibly due to the residual POD activity that survived the lower pressure treatments.
Flavonoids belong to a group of natural substances with variable phenolic structures and are found in different quantities in fruit juices. They possess anti-inflamatory, antiallergic, anti-viral, hypocholesterolemic, and anticarcinogenic properties (Sánchez-Moreno et al., 2003a). Orange juice is a dietary source of flavonoids, mainly flavanones. (Sánchez-Moreno et al. 2003a, 2005) studied the effects of HHP treatment at different temperatures and thermal pasteurization (70 °C for 30 s and 90 °C for 1 min) on the main flavanones (hesperidin and naringenin) in OJ. Just after pressure treatments at 350 and 400 MPa, the naringenin content increased by 13 and 12%, respectively, and by 34 and 22%, respectively, for hesperidin content. They argued that some structural changes and permeabilization of cell walls of OJ sacs could release phenols from proteins and increase the extraction of flavanones. Pasteurization processes led to diminishing naringenin content (16.0%) but hesperidin content was unaffected. During the storage of pressure treated samples, the naringenin content increased around 11% for 350 and 400 MPa samples and no differences were observed between the 100 MPa samples and the control. Regarding hesperidin content, an increase was observed for 350 and 400 MPa samples (19 and 21%) with no differences among the 100 MPa sample and the control. The authors argued that the remaining activities of POD and PPO suggested for the degradation of polyphenols in the 100 MPa sample could explain the lower content of flavanones at that processing condition. Flavanones tend to precipitate at low pH from the soluble fraction to the cloud in OJ, leading to an increase in the proportion of flavanones in the cloud after processing. For that reason, a lower release of naringenin after processing during extraction could occur.
Folates are hematopoietic vitamins with special importance during pregnancy. Regarding the nutritional needs of humans, folate deficiency occurs frequently, probably due to poor diet selection and losses during food processing (Sauberlich et al., 1987). Fruits are an important source of folate. For example, a 200 g portion of fresh oranges contains nearly 50% of the recommended daily intake (Butz et al., 2004). Very little data has been published in relation to the effects of HHP treatment and folate stability. One study conducted by Butz et al. (2004) studied the stability of three main types of folates found in OJ (tetrahydrofolate, 5-methyltetrahydrofolate and 5-formyltetrahydrofolate) after HHP treatment (600 MPa at 25 and 80 °C). An orange juice model containing ascorbic acid was used to check its protective effect against pressure in the folates content. At 25 °C, the losses ranged from 10 to 40% and at 80 °C from 25 to 95% after a 24 min treatment. The pressure sensivity was as follows: 5-methylfolate > 5-formylfolate > tetrahydrofolate. To check the thermal effects on the folates content, the authors performed a thermal treatment at 80 °C, observing that heat alone decreases folates content by 20, 50 and 80% in 5-methylfolate, 5-formylfolate and tetrahydrofolate, respectively. Combinations of HHP and thermal treatment did not increase the losses in 5-methylfolate but increased the losses in 5-formylfolate and tetrahydrofolate by 30 and 20% respectively. This indicates that small molecules such as vitamins are stable to HHP treatment and do not undergo cleavage of covalent bonds, certain reactions are accelerated by pressure. This is the case with 5-formylfolate where the formation of a 5, 10-methenylfolate derivative is accelerated by pressure. When comparing the model juice with fresh squeezed orange juice, the authors observed that natural folates in OJ were more stable than in the model system after the high pressure treatment. As the model and fresh juice had the same L-AA, the authors suggested that the presence of other substances such as vitamin C and flavonoids in the natural juice protected folate.
Anthocyanins play an important role in the antioxidant and antiradical capacities of fruits. Twenty anthocyanins are known, but only six (pelargonidin, cyanidin, peonidin, delphinidin, petunidin and malvidin) are important in food (Zabetakis et al., 2000). They are known for their brilliant red and purple colors and products containing anthocyanins are more susceptible to color changes during processing and storage. The degradation of anthocyanins can be influenced by different parameters such as temperature, enzymes, oxygen, and sugar content.
Grape juice is known for its high content of polyphenolics and anthocyanins. Talcott et al. (2003) and del Pozo-Insfran et al. (2007) studied the effect of HHP (600 MPa for 15 min) and thermal (90 °C for 15 min) treatments on anthocyanin content of a grape juice. The fortification of L-AA is a common practice in the food industry to protect against oxidation, but its combination with anthocyanins may be mutually destructive in the presence of oxygen. Treatment at 400 MPa caused the highest loss (70%) of anthocyanin content due to the activation of PPO activity, whereas at 600 MPa or thermal treatment achieved low losses (3–5%). The presence of L-AA enhanced the losses (12.4 and 18.1%) after thermal and HHP treatments, respectively. In addition, the formation of hydrogen peroxides from the oxidation of L-AA may contribute to the degradation of anthocyanin. Furthermore, these peroxides may activate residual POD, which further degrades L-AA and anthocyanins. After 21 d of storage at 25 °C, the anthocyanins content were reduced by 28–34% for all the samples. By-products from the degradation of L-AA and/or monosaccharides, such as furfuraldehydes, could contribute to anthocyanin degradation during storage. The individual anthocyanins differed in their resistance to the HHP treatment. The anthocyanins containing ï-diphenolic groups were less stable to HHP processing, possibly because they were more susceptible to the enzymatic oxidation.
Strawberry juice is also a medium in which thermal treatment can damage the natural color by the degradation of anthocyanins, thus the use of HHP technology can be a challenge. Zabetakis et al. (2000) focused on the pelargonidin derivatives, 3-glucoside and 3-rutinoside, which are the main anthocyanins in strawberry. At 4 °C, the losses the of 3-glucoside derivative were similar for all pressure treatments (400–600 MPa at 22 °C) and the control sample lost 20% at the end of 9d of storage. However, at 400 MPa the losses were higher and reached 40% after 9 d. The behavior of 3-rutinoside was similar, with higher losses after 400 MPa (50% after 9d) and similar losses after 600 MPa and control sample (25%). However, at 200 and 800 MPa, the losses were lower (10%). At 20 °C, there were no differences in the losses of 3-glucoside of all samples at the end of storage (50%), whereas 3-rutinoside treated at 200 and 800 MPa had a higher anthocyanin content (25% losses). At 30 °C, there were no differences in 3-glucoside in all samples (70–80% losses after 9d), whereas no differences (60–80%) in 3-rutinoside were found except for 200 and 800 MPa with losses of 42%. The authors explained this behavior by the residual enzyme activity after the different pressure treatments. Three main enzymes are involved in the oxidation of the anthocyanins: POD, PPO, and β-glucosidase. These enzymes have lower activity at lower temperatures, thus the losses of antho-cyanins were lower at 4 °C in comparison with 20 and 30 °C. In addition, β-glucosidase seems to be activated at pressures around 400 MPa, which could explain the higher losses at that pressure. At higher pressures (800 MPa) the activity of these enzymes is irreversibly reduced, corresponding to lower losses in anthocyanin content. The differences in the degradation of both derivatives could be explained by the differences in substrate specificity of β-glucosidase which was higher for 3-glucoside than 3-rutinoside, and the breakdown would therefore be more extensive and losses higher for 3-glucoside.
Blackcurrants contain high levels of flavonoids with anthocyanins as the most important group. They are present in the skin of the berries and are responsible for the characteristic aroma and color. Kounaki et al. (2004) studied the effects of pressure (200–800 MPa for 15 min at room temperature) on two important anthocyanins in black currant, delphinidin-3-rutinoside and cyanidin-3-rutinoside during the storage at 5, 20 and 30 °C for 7 d. At 5 °C, the losses of anthocyanins were the lowest because they were mediated by enzymatic action (PPO activity) and the enzyme activity would be low at that temperature. Losses of delphinin-3-rutinoside and cyanidin-3-rutinoside contents reached 58 and 40%, respectively, after 7 d of storage at 4 °C. Treatment at 600 MPa seemed to retain higher levels of both anthocyanins. At 20 °C, the losses reached 58% for both anthocyanins. The sample treated at 200 MPa counted for the highest losses (55-60%). At 30 °C, the losses were around 70–75%, and the samples treated at 200 MPa had the lowest anthocyanin content with less than 20%. The authors found a relation between L-AA content and anthocyanin degradation, where the rapid loss of L-AA appeared to contribute to the lower rate of anthocyanin loss. Also, the higher loss after 200 MPa could respond to an oxidative enzyme activation.
3.4 Commercialization of juices treated by high hydrostatic pressure (HHP)
3.4.1 Key drivers to employ novel rather than conventional processing technologies
High hydrostatic pressure processing provides a unique opportunity for food processors to develop generations of new, value-added food products having a superior quality than those produced by conventional thermal methods. These processes can help meet the challenges of producing innovative products from natural sources without compromising biologically active compounds, while ensuring foods with low microbial counts of spoilage organisms and safe from pathogens. Further, HHP can preserve food products without heat or chemical preservatives to significantly extend refrigerated shelf-life has opened new market opportunities, particularly in the area of ‘natural’ preservative-free, wholesome products.
Tropical fruit juices are another area of interesting consideration with regard to HHP processing. Tropical fruits have gained popularity in the last several years due to their unique flavors, aromas, and colors and their annual production is unlimited by seasonality. Some Latin American countries such as Brazil, Colombia, Ecuador, Mexico and Costa Rica are significant producers of tropical fruits. However, in some cases, the production of these local fruits is lost due to the lack of a means of commercialization. HHP technology could be a viable alternative to process these fruits in the form of juice and pulps, to retain the original sensory properties while extending their shelf-lives. This could improve trade exportation to demanding markets such as the EU and USA.
The Food and Drug Administration (FDA) has recently accepted pressure-assisted thermal-sterilization (PATS) as a process for commercial application in low acid foods. It combines heat with high pressure to produce commercially sterile, low acid food, which improves the quality compared to thermally processed foods, while simultaneously eliminating the food safety risks associated with pathogenic bacterial spores such as C. botulinum and its toxins. Although most juices have a pH below 4.5 and are considered as ‘acidic foods’, the pH varies in relation to juice composition. Undoubtedly, this new regulation will help increase the number of commercial HHP applications available.
3.4.2 Commercial application
HHP technology has become a commercially implemented technology in fruit juice processing, spreading from its origins in Japan, followed by USA and Europe, and now Australia with worldwide utilization increasing almost exponentially since 2000 (Norton and Sun, 2008). In the US, Genesis Juice Corp. processes eight types of organic juices by HHP including apple, carrot, apple-ginger, apple-strawberry, ginger lemonade, strawberry lemonade, a herbal tea beverage, and apple-and banana-based smoothies. European companies presently employing this technology in fruit juice processing include smoothies by Invo in Spain, orange and grapefruit juices and a mixture of strawberry-orange juice by UltiFruit in France, Fruba§a manufacturing different fruit-based beverages in Portugal, Juicy Line-Fruity Line in Holland, Beskyd Frycovice, a.s manufacturing mixtures of broccoli-apple-lemon and broccoli-orange-lemon in Czech Republic, and ATA S.P.A manufacturing carrot and apple juices in Italy, and Puro commercializing smoothies in the UK (Table 3.3).
Some interesting information and general trends can be drawn from Table 3.3. Most of these companies incorporating HHP technology did so before the establishment of the international standards of GMP (Good Manufacturing Practice) for raw material suppliers and processes. Manufacturers of HPP juices are usually small to medium sized companies comprising less than 100 employees. Significant collaborations with national food technology research bodies have been developed in order to accomplish shelf-life, consumer studies, and to satisfy legal and regulatory requirements. Regarding the processing conditions, treatments are optimized at a pressure level of 600 MPa in combination with moderate heat. In addition, due to the special characteristics of fruit juices, small productions are achieved ranging from 50 to 200 kg/h to satisfy consumer demand. Shelf-lives are estimated at ca. 10–35 d of refrigeration conditions, depending on the type of juice. Products are sold at supermarkets chains, specialty and gourmet stores, and food services providing fruit preparations and dressings. Two main packaging formats are used, a small volume of 250 mL quantity corresponding to a single portion, and a bigger format of 1 L. Market prices are around €3 or $4.5 per 250 mL serving. Marketing is a key instrument used by companies to highlight the benefits of their HHP products compared to the competition, and advertising generally emphasizes that the fruit products are natural, supplemented with vitamins, and can even be considered as sport beverages.
Case study: HHP fruit juice processing in Australia
In the last few years, Australia has become a leader in the developing of HHP-treated juices and derivatives. The Australian Research organization (CSIRO) and their joint venture Food Research Group, Food Science Australia have been developing high pressure processing systems in Australia for over a decade. Several companies are using HHP technology in their processes.
A questionnaire was compiled and sent from the authors of the present chapter to the fruit juice manufacturing companies to obtain the relevant data about the industrial experience of using HHP technology. General trends were drawn from the questionnaire. Companies were small to medium in size (from 30 to 100 employees). Although most of companies have been in operation for over a decade, the introduction of HHP processing occurred more recently. In addition, thermal processing is still used in many of the companies for the production of vegetable and herb products, stabilized fruit preparations for yogurt, ice-creams (ripples) and fruit ices, diced chicken (cooked) and egg and mayonnaise salad for sandwiches.
The main reasons reported by companies for choosing HHP technology were their efforts to provide a product with a clearly perceptible improvement and the ability to achieve a premium price for a premium product. Companies added that HHP products are superior in taste, texture and color to current competitor products, and consumers have to be informed about these differences. In addition, the product must be value-added to be able to support the extra processing costs. Supply chains must be in place or developed for the delivery of high quality raw materials, to ensure that the products are microbiologically safe. Optimum market survival is improved by creating new products that have never been made before. Fresh processed juices, stabilized fruit preparations, chilled packaged salads, whole and value-added egg products and processed diced chicken products for sandwich bars, chilled cooking sauces and curry pastes and guacamole, pomegranate juices and fruit puraes are the main products processed by companies using the HHP technology.
When asked if sensory tests were performed, companies answered that small group sensory tests were conducted based on preference testing to confirm that the product flavor and color benefits could be translated to the consumer’s preference and purchase intent. In addition, changes in the product labeling were introduced after HHP processing, highlighting the benefits of HPP as a cold pasteurization process that produces the most natural, freshest tasting, and most nutritious juice on the market (Fig. 3.1).
The main clients of HHP fruit juice products were supermarket chains, and specialty and deli stores. No special legal approvals were required for the domestic products. Regarding consumer attitudes, no negative attitudes, apart from the higher price, were observed, and mostly there were difficulties explaining the technology and its benefits to the consumer without becoming too technical and either losing their interest or confusing them.
One of the problems mentioned by HHP fruit juice processors was to develop a PET bottle for the unit that was extremely space-efficient to allow for maximum utilization of the cylindrical processing cavity to maximize throughput. A clever bottle design with a local supplier solved this issue by creating an immediately differentiated and individual wedge design.
On average (due to differences in packaging size), the cost comparisons for the actual processing step was approximately 40% more expensive than traditional thermal pasteurization. The recovery of the investment was around 5–10 yr. The packaging materials used in the HHP process included: blow molded PET bottles, standup high barrier laminated spout pouches, high barrier laminated bag in box systems, and high barrier laminated preformed bags, which were thermally sealed.
Changes in the product formulation, such as the performance of hydrocolloid and polysaccharide stabilizers, post HPP treatment, meant that it was possible in some cases to actually reduce the amount of stabilizers required and still achieve the same viscosity, texture and mouthfeel.
3.5 Future trends
Taking into account the limitations of traditional thermal processes and the trends in consumer demand, the future of food preservation is moving towards ala-carte processing, consisting of a specifically designed process treatment for each type of food. There are foods for which traditional technologies are, and will continue to be the most efficient processing option. However, some market niches have appeared for emerging technologies to be viable for producing food products that are healthier, retain more of their fresh-like character, and, most importantly, are safe for the consumer from a microbiological point of view. Thus, there are cases where HHP is the most appropriate technology to meet consumer demand, and the use of HHP for juices and derivative products will likely continue to grow as costs decline and food manufacturers identify new applications where HHP can deliver product quality improvements that consumers demand and appreciate.
3.6 Sources of further information and advice
Avure Technologies Inc. (www.avure.com)
Elmhurst Research, Inc. (www.elmhurstresearch.com)
Engineered Pressure Systems Inc. (www.epsi-highpressure.com)
Epsi Inc. (www.epsi-highpressure.com)
Kobelco (www.kobelco.co.jp)
Mitsubishi Heavy Industries (www.mhi.co.jp)
NC Hyperbaric (www.nchyperbaric.com)
Resato International (www.resato.com)
Stansted Fluid Power Ltd (www.sfp-4-hp.demon.co.uk)
Uhde Hockdrucktechnik (www.uhde-hpt.com)
Invo (Madrid, Spain) (www.invo.es)
Beskyd Frycovice, a.s. (Horni Cerkev, Czech Republic)
(www.beskyd.cz/index.php) Juicy Line-Fruity Line (Ochten, Holland) (www.fruity-line.com) Pressure Fresh Australia Pty Ltd (Bundaberg Qld, Australia) (www.austchilli.com.au/devindexpf.aspx)
Preshafood Ltd. (Victoria, Australia) (www.preshafood.com.au)
Fruba9a (Leiria, Portugal) (www.glsa.pt)
Ulti Fruit (Vigneux-sur-Seine, France)
Puro-www.barefruitproducts.com/about/index.html
Genesis Juice Corporation (Oregon, US) (www.genesisorganicjuice.com)
3.7 Acknowledgements
Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the US Department of Agriculture.
The authors want to express their gratitude to Bradley Wardrop-Brown from BOI Food Tech and Packaging, Andrew Gibb from Preshafood Ltd, Trent DePaoli from Pressure Fresh Australia Pty Ltd and Sheldon Rubin from Toby’s Family Foods, LLC/Genesis Organic Juice for their interest and willingness in participating in this chapter. The authors are also grateful to Carole Tonello from NC Hyperbaric S.A. for providing useful information.
3.8 References
Alpas, H., Bozoglu, F. The combined effect of high hydrostatic pressure, heat and bacteriocins on inactivation of foodborne pathogens in milk and orange juice. World J Microbiol Biotech. 2000; 16:387–392.
Alpas, H., Bozoglu, F. Efficiency of high pressure treatment for destruction of Listeria monocytogenes in fruit juices. Fems Immun Med Microbiol. 2003; 35:269–273.
Alpas, H., Alma, L., Bozoglu, F. Inactivation of Alicyclobacillus acidoterrestris vegetative cells in model system, apple, orange and tomato juices by high hydrostatic pressure. World J Microbiol Biotech. 2003; 19:619–623.
Balasubramaniam, V.M., Farkas, D. High-pressure food processing. Food Sci Tech Int. 2008; 14:413–418.
Bang, W.-S., Swanson, B.G. Scanning electron microscopy studies of Saccharomyces cerevisiae structural changes by high hydrostatic pressure treatment. Food Sci Biotech. 2008; 17:1102–1105.
Basak, S., Ramaswamy, H.S. Ultra high pressure treatment of orange juice: a kinetic study on inactivation of pectin metyl esterase. Food Res Int. 1996; 29:601–607.
Basak, S., Ramaswamy, H.S., Piette, J.P.G. High pressure destruction kinetics of Leuconostoc mesenteroides and Saccharomyces cerevisiae in single strength and concentrated orange juice. Innov Food Sci Emerg Tech. 2002; 3:223–231.
Baxter, I.A., Easton, K., Schneebeli, K., Whitfield, F.B. High pressure processing of Australian navel orange juices: sensory analysis and volatile flavor profiling. Innov Food Sci Emerg Tech. 2005; 6:372–387.
Bayindirli, A., Alpas, H., Bozoglu, F., Hizal, M. Efficiency of high pressure treatment on inactivation of pathogenic microorganisms and enzymes in apple, orange, apricot and sour cherry juices. Food Control. 2006; 17:52–58.
Bull, M.K., Szabo, E.A., Cole, M.B., Stewart, C.M. Toward validation of process criteria for high-pressure processing of orange juice with predictive models. J Food Prot. 2005; 68:949–954.
Butz, P., Serfert, Y., Fernandez-Garcia, A., Dieterich, S., Lindauer, R., Bognar, A., Tauscher, B. Influence of high-pressure treatment at 25 °C and 80 °C on folates in orange juice and model media. J Food Sci. 2004; 69:117–121.
Buzrul, S., Alpas, H., Largeteau, A., Demazeau, G. Inactivation of Escherichia coli and Listeria monocytogenes in kiwifruit and pineapple juices by high hydrostatic pressure. Int J Food Microbiol. 2008; 124:275–278.
Cano, M.P., Hernández, A., De Ancos, B. High pressure and temperature effects on enzyme inactivation in strawberry and orange products. J Food Sci. 1997; 62:85–88.
Cortés, C., Esteve, M.J., Frígola, A. Effect of refrigerated storage on ascorbic acid content of orange juice treated by pulsed electric fields and thermal pasteurization. Eur Food Res Tech. 2008; 227:629–635.
Da Rocha Ferreira, E.H., Rosenthal, A., Calado, V., Saraiva, J., Mendo, S. Byssochlamys nivea inactivation in pineapple juice and nectar using high pressure cycles. J Food Eng. 2009; 95:664–669.
De Ancos, B., Sgroppo, S., Plaza, L., Cano, P. Possible nutritional and health-related value promotion in orange juice preserved by high-pressure treatment. J Sci Food Agric. 2002; 82:790–796.
Del Pozo-Insfran, D., Del Follo-Martinez, A., Talcott, S.T., Brenes, C.H. Stability of copigmented anthocyanins and ascorbic acid in muscadine grape juice processed by high hydrostatic pressure. J Food Sci. 2007; 72:247–253.
Deliza, R., Rosenthal, A., Silva, A.L.S. Consumer attitude towards information on non conventional technology. Trends Food Sci Technol. 2003; 14:43–49.
Deliza, R., Rosenthal, A., Abadio, F.B.D., Silva, C.H.O., Castillo, C. Application of high pressure technology in the fruit juice processing: benefits perceived by consumers. J Food Eng. 2005; 67:241–246.
Doğan, C., Erkmen, O. High pressure inactivation kinetics of Listeria monocytogenes inactivation in broth, milk, and peach and orange juices. J Food Eng. 2004; 62:47–52.
Donsí, G., Ferrari, G., Maresca, P. Pulsed high pressure treatment for the inactivation of Saccharomyces cerevisiae: the effect of process parameters. J Food Eng. 2007; 984–990.
Élez, P., Aguiló, I., Martín-Belloso, O. Inactivation of orange juice peroxidase by high-intensity pulsed electric fields as influenced by process parameters. J Sci Food Agric. 2006; 86:71–81.
Erkmen, O., Doğan, C. Kinetic analysis of Escherichia coli inactivation by high hydrostatic pressure in broth and foods. Food Microbiol. 2004; 21:181–185.
Fang, L., Jiang, B., Zhang, T. Effect of combined high pressure and thermal treatment on kiwifruit peroxidase. Food Chem. 2008; 109:802–807.
Fourie, P.C. Fruit and human nutrition. In: Arthey D., Ashurst P.R., eds. Fruit Processing. Glasgow: Blackie Academic & Professional; 1996:20–39.
Garcia-Graells, C., Hauben, K.J.A., Michiels, C.W. High-pressure inactivation and sublethal injury of pressure-resistant Escherichia coli mutants in fruit juices. Appl Environ Microbiol. 1998; 64:1566–1568.
Garcia-Palazon, A., Suthanthangjai, W., Kadja, P., Zabetakis, I. The effects of high hydrostatic pressure on, β-glucosidase, peroxidase and polyphenoloxidase in red raspberry (Rubus idaeus) and strawberry (Fragaria x ananassa). Food Chem. 2004; 88:7–10.
Giese, J. Color measurement in foods as a quality parameter. Food Technol. 2000; 54:62–65.
Giner, J., Ortega, M., Messeguè, M., Gimeno, V., Barbosa-Cánovas, G.V., Martín, O. Inactivation of peach polyphenoloxidase by exposure to pulsed electric fields. J Food Sci. 2002; 67:1467–1472.
Goodner, J.K., Braddock, R.J., Parish, ME., Sims, C.A. Cloud stabilization of orange juice by high pressure processing. J Food Sci. 1999; 64:699–700.
Gregory, J.F., III. Vitamins. In: Fennema O.R., ed. Food chemistry. New York: Marcel Dekker; 1996:559–568.
Hendrickx, M., Ludikhuyze, L., Van Den Broeck, I., Weemaes, C. Effects of high pressure on enzymes related to food quality. Trends Food Sci Technol. 1998; 9:197–203.
Jensen. Alicyclobacillus – a new challenge for the food industry. Food Aus. 1999; 51:33–36.
Jordan, S.L., Pascual, C., Bracey, E., Mackey, B.M. Inactivation and injury of pressure-resistant strains of Escherichia coli O157:H7 and Listeria monocytogenes in fruit juices. J Appl Microbiol. 2001; 91:463–469.
Katz, F. Research priorities move toward healthy and safe. Food Technol. 2000; 54:42–44.
Kouniaki, S., Kajda, P., Zabetakis, I. The effect of high hydrostatic pressure on anthocyanins and ascorbic acid in blackcurrants (Ribes nigrum). Flavour Fragance J. 2004; 19:281–286.
Laboissiere, L.H.E.S., Deliza, R., Barros-Marcellini, A.M., Rosenthal, A., Camargo, L.M.A.Q., Junqueirar, R.G. Effects of high hydrostatic pressure (Hhp) on sensory characteristics of yellow passion fruit juice. Innov Food Sci Emerg Technol. 2007; 8:469–477.
Lavinas, F.C., Miguel, M.A.L., Lopes, M.L.M., Valente mesquita, V.L. Effect ofhigh hydrostatic pressure on cashew apple (Anacardium occidentale L.) juice preservation. J Food Sci. 2008; 73:273–277.
Lee, S.Y., Dougherty, R.H., Kang, D.H. Inhibitory effects of high pressure and heat on Alicyclobacillus acidoterrestris spores in apple juice. Appl Environ Microbiol. 2002; 68:4158–4161.
Lee, S.Y., Chung, H.J., Kang, D.H. Combined treatment of high pressure and heat on killing spores of Alicyclobacillus acidoterrestris in apple juice concentrate. J Food Prot. 2006; 69:1056–1060.
Linton, M., Mcclements, J.M.J., Patterson, M.F. Inactivation of Escherichia coli O157:H7 in orange juice using a combination ofhigh pressure and mild heat. J Food Prot. 1999; 62:277–279.
Linton, M., Mcclements, J.M.J., Patterson, M.F. Survival of Escherichia coli O157:H7 during storage in pressure-treated orange juice. J Food Prot. 1999; 62:1038–1040.
Ludikhuyze, L., Van Loey, A., Oeyi, Hendrickx M. High pressure processing of fruit and vegetables. In: Hendrickx M., Knorr D., eds. Ultra High Pressure Treatments of Foods. New York: Kluwer Academic; 2002:168–171.
Miller, L.G., Kasparc, W. Escherichia coli 0157:H7 acid tolerance and survival in apple cider. J Food Prot. 1994; 57:460–464.
Min, S., Zhang, Q.H. Effects of commercial-scale pulsed electric field processing on flavor and color of tomato juice. Food Chem Tox. 2003; 1600–1606.
Muñoz, M., De Ancos, B., Sánchez-Moreno, C., Cano, M.P. Effects of high pressure and mild heat on endogenous microflora and on the inactivation and sublethal injury of Escherichia coli inoculated into fruit juices and vegetable soup. J Food Prot. 2007; 70:1587–1593.
Nakimbugwe, D., Masschalck, B., Anim, G., Michiels, C.W. Inactivation of Gram-negative bacteria in milk and banana juice by hen egg white and lambda lysozyme under high hydrostatic pressure. Int J Food Microbiol. 2006; 112:19–25.
Nielsen, H.B., Sonne, A.-M., Grunert, K.G., Banati, D., Pollak-Toth, A., Lakner, Z., Olsen, N.V., Zontar, T.P., Peterman, M. Consumer perception of the use of high-pressure processing and pulsed electric field technologies in food production. Appetite. 2009; 52:115–126.
Nienaber, U., Shellhammer, T.H. High-pressure processing of orange juice: kinetics of pectinmethylesterase inactivation. J Food Sci. 2001; 66:328–331.
Noma, S., Tomita, C., Shimoda, M., Hayakawa, I. Response of Escherichia coli O157:H7 in apple and orange juices by hydrostatic pressure treatment with rapid decompression. Food Microbiol. 2004; 21:469–473.
Norton, T., Sun, D.-W. Recent advances in the use of high pressure as an effective processing technique in the food industry. Food Bioprocess Technol. 2008; 1:2–34.
Oey, I., Van Der Plancken, I., Van Loey, A., Hendrickx, M. Does high pressure processing influence nutritional aspects of plant based food systems? Trends Food Sci Tech. 2008; 19:300–308.
Oey, I., Lille, M., Van Loey, A., Hendrickx, M. Effect of high-pressure processing on colour, texture and flavour of fruit-and vegetable-based food products: a review. Trends Food Sci Tech. 2008; 19:320–328.
Palou, E., López-Malo, A., Barbosa-Cánovas, G.V., Welti-Chanes, J., Davidson, P.M., Swansonb, G. Effect of oscillatory high hydrostatic pressure treatments on Byssochlamys nivea ascospores suspended in fruit juice concentrates. Letters Appl Microbiol. 1998; 27:375–378.
Palou, E., López-Malo, A., Barbosa-Cánovas, G.V., Welti-Chanes, J., Swanson, B.G. Polyphenoloxidase activity and color of blanched and high hydrostatic pressure treated banana puree. J Food Sci. 1999; 64:42–45.
Parish, M.E. Orange juice quality after treatment by thermal pasteurization or isostatic high pressure. Lebens Wiss Technol. 1998; 31:439–442.
Pettipher, G.L., Osmundson, M.E., Murphy, J.M. Methods for the detection and enumeration of Alicyclobacillus acidoterrestris and investigation of growth and production of taint in fruit juice and fruit juice-containing drinks. Letters Appl Microbiol. 1997; 24:185–189.
Plaza, L., Sánchez-Moreno, C., Élez-Martínez, P., De Ancos, B., Martín-Belloso, O., Cano, M.P. Effect of refrigerated storage on vitamin C and antioxidant activity of orange juice processed by high-pressure or pulsed electric fields with regard to low pasteurization. Eur Food Res Technol. 2006; 223:487–493.
Polydera, A.C., Stoforos, N.G., Taoukis, P.S. Comparative shelf-life study and vitamin loss kinetics in pasteurized and high pressure processed reconstituted orange juice. J Food Eng. 2003; 60:21–29.
Polydera, A.C., Galanou, E., Stoforos, N.G., Taoukis, P.S. Inactivation kinetics of pectin methylesterase of greek navel orange juice as a function of high hydrostatic pressure and temperature process conditions. J Food Eng. 2004; 62:291–298.
Ramaswamy, H.S., Riahi, E., Idziak, E. High-pressure destruction kinetics of E. coli (29055) in apple juice. J Food Sci. 2003; 68:1750–1756.
Raso, J., Calderon, M.L., Gongora, M.M., Barbosa-Canovas, G.V., Swanson, B.G. Inactivation of Zygosaccharomyces cerevisiae in fruit juices by heat, high hydrostatic pressure and pulsed electric fields. J Food Sci. 1998; 63:1042–1044.
Rastogi, N.K., Raghavarao, K.S.M.S., Balasubramaniam, V.M., Niranjan, K., Knorr, D. Opportunities and challenges in high pressure processing of foods. Cri Rev Food Sci Nutr. 2007; 47:69–112.
Reyns, K.M.F.A., Soontjens, C.C.F., Cornelis, K., Weemaes, C.A., Hendrickx, M.E., Michiels, C.W. Kinetic analysis and modelling of combined high-pressure-temperature inactivation of the yeast Zygosaccharomyces bailii. Int J Food Microbiol. 2000; 56:199–210.
Rivas, A., Rodrigo, D., Martinez, A., Barbosa-Canovas, G.V., Rodrigo, M. Effect of Pef and heat pasteurization on the physical-chemical characteristics of blended orange and carrot juice. Lebens Wiss Technol. 2006; 39:1163–1170.
Rodrigo, D., Van Loey, A., Hendrickx, M. Combined thermal and high pressure colour degradation of tomato puree and strawberry juice. J Food Eng. 2007; 79:553560.
Ruxton, C.H.S., Gardner, E.J., Walker, D. Can pure fruit and vegetable juices protect against cancer and cardiovascular disease too? A review of the evidence. Int J Food Sci Nutr. 2006; 57:249–272.
Sado, P.N., Jinneman, K.C., Busby, G.J., Sorg, S.M., Omiecinskic, J. Identification of Listeria monocytogenes from unpasteurized apple juice using rapid test kits. J FoodProt. 1998; 58:1199–1202.
Sampedro, F., Rodrigo, D., Hendrickx, M. Inactivation kinetics of pectin methyl esterase under combined thermal-high pressure treatment in an orange juice-milk beverage. J Food Eng. 2008; 86:133–139.
Sampedro, F., Geveke, D.J., Fan, X., Zhang, Q.H. Effect of Pef, Hhp and thermal treatment on Pme inactivation and volatile compounds concentration of an orange juice-milk based beverage. Innov Food Sci Emerg Technol. 2009; 10:463–469.
Sampedro, F., Geveke, D.J., Fan, X., Rodrigo, D., Zhang, Q.H. Shelf-life study of an orange juice-milk based beverage after Pef and thermal processing. J Food Sci. 2009; 74:107–112.
San Martín, M.F., Barbosa-Cánovas, G.V., Swanson, B.G. Food processing by high hydrostatic pressure. Crit Rev Food Sci Nutr. 2002; 42:627–645.
Sánchez-Moreno, C., Plaza, L., De Ancos, B., Cano, P. Effect of high-pressure processing on health-promoting attributes of freshly squeezed orange juice (Citrus sinensis L.) during chilled storage. Eur Food Res Technol. 2003; 216:18–22.
Sánchez-Moreno, C., Plaza, L., De Ancos, B., Cano, P. Vitamin C, provitamin A carotenoids, and other carotenoids in high-pressurized orange juice during refrigerated storage. J Agric Food Chem. 2003; 51:647–653.
Sánchez-Moreno, C., Plaza, L., Élez-Martinez, P., De Ancos, B., Martin-Belloso, O., Cano, P. Impact of high pressure and pulsed electric fields on bioactive compounds and antioxidant activity of orange juice in comparison with traditional thermal processing. J Agric Food Chem. 2005; 53:4403–4409.
Sánchez-Moreno, C., Plaza, L., De Ancos, B., Cano, P. Impact of high-pressure and traditional thermal processing of tomato puree on carotenoids, vitamin C and antioxidant activity. J Sci Food Agric. 2006; 86:171–179.
Sancho, F., Lambert, Y., Demazeau, G., Largeteau, A., Bouvier, J.-M., Narbonne, J.-F. Effect of ultra-high hydrostatic pressure on hydrosoluble vitamins. J Food Eng. 1999; 39:247–253.
Sauberlich, H.E., Kretsch, M.J., Skala, J.H., Johnson, H.L., Taylor, P.C. Folate requirements and metabolism in nonpregnant women. Amer J Clin Nutr. 1987; 46:1016–1028.
Schlesser, J., Parisi, B. Inactivation of Yersinia pseudotuberculosis 197 and Francisella tularensis Lvs in beverages by high pressure processing. J Food Prot. 2009; 72:165–168.
Sharma, R. Market trends and opportunities for functional dairy beverages. Aus J Dairy Technol. 2005; 60:196–199.
Talcott, S.T., Brenes, C.H., Pires, D.M., Del Pozo-Insfran, D. Phytochemical stability and color retention of copigmented and processed muscadine grape juice. J Agric Food Chem. 2003; 51:957–963.
Teo, A.Y.-L., Ravishankar, S., Sizer, C.E. Effect of low-temperature, high-pressure treatment on the survival of Escherichia coli O157:H7 and Salmonella in unpasteurized fruit juices. J Food Prot. 2001; 64:1122–1127.
Tijskens, L.M.M., Schijvens, E.P.H.M., Biekman, E.S.A. Modeling the change in colour broccoli and green beans during blanching. Innov Food Sci Emerg Technol. 2001; 2:303–313.
Torregrosa, F., Cortés, C., Esteve, M.J., Frígola, A. Effect of high-intensity pulsed electric fields processing and conventional heat treatment on orange-carrot juice carotenoids. J Agric Food Chem. 2005; 53:9519–9525.
Tsou, C.L. Location of active sites of some enzymes in limited and flexible molecular regions. Trends Biochem Sci. 1986; 67:3058–3062.
Voldrich, M., Dobiáš, J., Tichá, L., Čeřovský, Krátká, J. Resistance of vegetative cells and ascospores of heat resistant mould Talaromyces avellanus to the high pressure treatment in apple juice. J Food Eng. 2004; 61:541–543.
Whitney, B.M., Williams, R.C., Eifert, J., Marcy, J. High-pressure resistance variation of Escherichia coli O157:H7 strains and Salmonella serovars in triptic soy broth, distilled water, and fruit juice. J Food Prot. 2007; 70:2078–2083.
Who/Fao Expert Consultation. Diet, nutrition and the prevention of chronic diseases, 2003. [Who Tech Report Ser, 916].
Wright, A.O., Cardello, A.V., Bell, R. ‘Consumer evaluations of high pressure processed Foods’, in Doona C J and Feeherry F E, High pressure processing of foods. Weinheim, Wiley-Vch: Institute of Food Technologists Series; 2007.
Yeom, H.W., Streaker, C.B., Zhang, Q.H., Min, B. Effects of pulsed electric fields on the quality of orange juice and comparison with heat pasteurization. J Agric Food Chem. 2000; 48:4597–4605.
Zabetakis, I., Leclerc, D., Kajda, P. The effect of high hydrostatic pressure on strawberry anthocyanins. J Agric Food Chem. 2000; 48:2749–2754.
Zook, C.D., Parish, M.E., Braddock, R.J., Balaban, M.O. High pressure inactivation kinetics of Saccharomyces cerevisiae ascospores in orange and apple juices. J Food Sci. 1999; 64:533–535.