Novel technologies for the decontamination of fresh and minimally processed fruits and vegetables
B.A. Niemira, United States Department of Agriculture, Agricultural Research Service (USDA-ARS), USA
Abstract:
The complex challenges of ensuring food safety and quality that producers and processors of fresh produce face require them to seek creative applications of conventional treatments and innovative approaches to develop entirely novel treatments. The variable nature of fresh and fresh-cut produce necessitates developing treatments that are adapted and optimized for each kind of commodity, ranging from leafy greens to whole fruits to processed products. This chapter will examine the state of development and commercialization of a range of novel technologies. These will include advanced aqueous-phase and gas-phase chemical treatments, precision thermal treatments, cold plasma systems, and biological control treatments. The chapter will conclude with a summary of current trends and future prospects for how the industry is working to meet goals for produce safety, quality and integrity for consumers.
11.1 Introduction
Fresh produce commodities vary widely, but all have high market quality standards that must be met. This, coupled with their generally fragile nature and the lack of a broadly applicable antimicrobial process (a ‘kill step’) limits the available options for the fresh produce industry (UFPA 2007; JIFSAN 2007). For consumers of fresh and fresh-cut produce, the incidence of foodborne illness (FBI) resulting from contaminated produce is an increasing cause for concern (Sivapalasingam et al., 2004). Risks to consumers can be reduced by consistent use of good agricultural practices (GAP), good manufacturing practices (GMP) and good handling practices (GHP) in the pre-harvest, post-harvest and supply-chain phases. However, recalls of tomatoes, leafy greens, melons, sprouts, and other fresh produce continue, with concomitant loss of consumer confidence and negative economic impacts for producers, processors and retailers.
Advances have been made with conventional treatments, and the successes in commercialization are detailed elsewhere in this book. This chapter will focus on technologies which are still being developed. In some cases, these will have already met key criteria for commercial utilization and adoption. In others, the technologies are still at pilot-scale or bench-scale research phase. In the coming years, one may reasonably expect that these technologies will be integrated into HACCP and supply chain programs to enhance the safety of fresh produce. The specific technologies this chapter considers include advanced aqueous-phase and gas-phase chemical treatments, precision thermal treatments, cold plasma systems and biological control treatments. Finally, the chapter will examine the means by which communication and collaboration tools can improve the ways industry, consumers and regulators can work together to meet produce safety goals.
11.2 Optimization of existing chemical treatments
This section deals with the optimized application of existing chemical treatments. Building on the established technology and knowledge base associated with these compounds will hopefully lead to further gains in safety and security. It is common practice in the produce industry to incorporate sanitizing solutions at various stages of harvest, pre-pack and processing. These can be in the form of separate washes, but are often used as part of flume water. With respect to amended flume water, it is generally accepted that the primary intent is to prevent pathogen survival and buildup in the flume itself. Aqueous treatments can consist of sodium hypochlorite in the 50–200 ppm range, or ozonated water in the 5–20 mg/L range. These chemical agents will serve to effect 1–2 log cfu reductions, but will not typically achieve significantly more. Avoiding cross-contamination is a material benefit to this usage of chlorine or ozone, even where product contamination levels are not significantly improved.
11.2.1 Electrolyzed water (EW)
EW provides an effective antimicrobial treatment using only inexpensive, nonvolatile inputs: salt, water and electricity. When electricity is passed through a dilute aqueous saline solution, typically ~ 1% sodium chloride, the electrolyzed water yields an acidified stream rich in chloride ions, and a basic stream high in sodium ions (Koseki et al., 2004; Wang et al., 2006).
The antimicrobial efficacy of EW is related to concentration and exposure time, but also by conditions of exposure. Green onions and tomatoes inoculated with a 109 cfu/ml culture cocktail of Escherichia coli 0157:H7, Salmonella Typhimurium, or Listeria monocytogenes were rendered free of contamination (i.e. below detection limit) within 3 minutes of treatment with acidified EW (pH 2.06, free available chlorine concentration 37.5 ± 2.5 mg/l). However, under conditions of high organic material, the efficacy of the process was reduced (Park et al., 2008a). In associated studies, lettuce and spinach leaves were inoculated with a 108-109 cfu/ml cocktail of three strains of each E. coli 0157:H7, Salmonella Typhimurium and L. monocytogenes. As with green onions and tomatoes, treatment with acidic EW (pH 2.06, free available chlorine concentration 37.5 ± 2.5 mg/l) was similarly effective, reducing the pathogens to undetectable levels within 5 minutes, but only when the presence of organic material was low. As the presence of organic material increased, the bactericidal activity decreased (Park et al., 2008b). These results suggest that the most appropriate usage for EW is as a second wash/treatment step, after a primary wash had been effected to remove most of the associated organic material.
11.2.2 Aqueous chlorine dioxide
Wash treatments using an aqueous chlorine dioxide solution have been successfully implemented in a variety of commercial produce packaging and processing facilities worldwide (Gomez-Lopez et al., 2009). Chlorine dioxide has 2.5 times the oxidative capacity of conventional chlorine, and chlorine dioxide is somewhat less susceptible to interference by organic compounds in the food matrix (Beuchat et al., 2004). Another primary reason for its widespread adoption is that aqueous wash treatments are a familiar technology for fruit and vegetable processors.
Current research efforts are aimed at expanding the commodities suitable for treatment, and at optimizing the process. In one recent study using pathogen-inoculated blueberries (Wu and Kim, 2007), a chlorine dioxide wash reduced L. monocytogenes (4.88 log cfu/g), Pseudomonas aeruginosa (2.16 log cfu/g), Salmonella Typhimurium (3.32 log cfu/g), Staphylococcus aureus (4.56 log cfu/g), and Yersinia enterocolitica (3.49 log cfu/g). Antimicrobial activity was greatest at the highest concentration tested, 15 ppm chlorine dioxide. This level also reduced natural yeasts and molds by 2.82 log cfu/g. The time of treatment to achieve maximum effect varied from 20 minutes to 2 hours, depending on the pathogen.
11.3 Antimicrobial treatments
Many novel treatments are derived from current practices, but applied in new ways. The preceding section discussed standard aqueous treatments practised in industry. An exciting area of research and developments deals with an evolutionary extension of these aqueous antimicrobial compounds as gas-phase treatments. These are new antimicrobial technologies, but which function in the realm of existing produce handling and processing protocols. One of the promising aspects of these treatments is their ability to permeate small spaces of produce tissue which are inaccessible to aqueous treatments. Cracks, stem scars, blossom ends, involutions and other small spaces provide areas that can accommodate bacteria. By forcing antimicrobials into these spaces, enhanced bacterial reductions can be achieved. Another important area of development is the use of precision thermal treatments that conventionally have been used for phytosanitary disinfestation.
11.3.1 Gas phase chlorine dioxide
While aqueous chlorine dioxide washes have gained wide acceptance for ensuring fresh produce safety, the use of gaseous chlorine dioxide is also an effective antimicrobial process. The advantages of gas-phase treatments are their greater penetration of the antimicrobial into microfissures, stomata, blossom or stem scars, etc. (Sapers et al., 2003). Gomez-Lopez et al. (2009) recently reviewed gas-phase chlorine dioxide, and noted the adaptability of the process for a wide range of commodities (Table 11.1). Also, chlorine dioxide, like many other chemical sanitizers, tends to be less effective against wound-associated microorganisms, most likely due to the high levels of organic material present or the low permeation of the gaseous molecules into the tissue and reaching the infecting organisms (Gomez-Lopez et al., 2009).
Table 11.1
Studies on the effect of gaseous ClO2 on pathogenic microorganisms inoculated onto fruits and vegetables
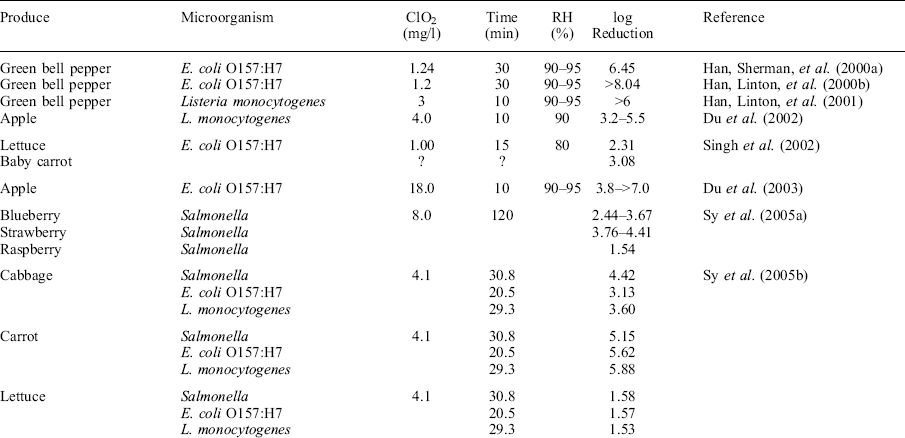
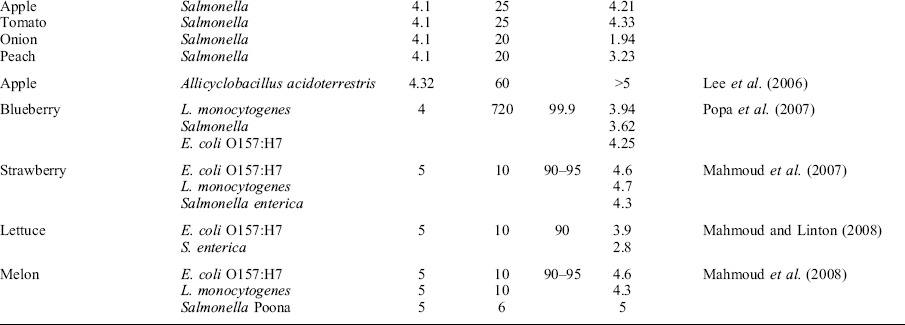
Experiments were performed at 20–23 °C. Used with permission.
Table adapted from Gomez-Lopez et al. (2009)
The efficacy of the gas-phase treatments are influenced by factors such as relative humidity, temperature, concentration and contact time. These constraints will be of primary significance in commercialization. In practice, the composition of the food being treated also exerts an important influence. Vandekinderen et al. (2009) evaluated the antimicrobial potential of a one-minute treatment of 0.08 mg/L gas-phase chlorine dioxide. Lipids and proteins essentially inhibited the antimicrobial activity, while soluble starch and NaCl had little inhibitory effect. The authors postulated that gas-phase chlorine dioxide would be more appropriate for use with carbohydrate-rich foods, rather than high-protein and fatty foods. A recent book chapter considering review of gas-phase chlorine dioxide introduced a novel technology proposed for in-field usage to satisfy the requirements of the military (Setlow et al., 2009). The authors presented a comprehensive summary of this area of technology, and concluded that recent advances in the ability to controllably generate gas-phase sanitizers on-site and at-will signify promising opportunities for commercialization. Results suggested that fresh fruit and vegetable commodities are likely candidates for customized treatments with gas-phase chlorine dioxide designed to avoid oxidation, while enhancing safety and prolonging shelf-life.
11.3.2 Precision thermal treatments
Heat is probably the most reliable and effective antimicrobial process used in food preservation and other applications. Thermal treatments are the most commonly used food processing technologies for shelf-stable food products. However, heat can be extremely damaging to the quality and stability of fresh and minimally processed fruits and vegetables. For this reason, thermal treatments have been restricted to a narrow range of applications for fruits and vegetables. Unripened or incompletely ripened fruits can be given a thermal treatment to kill and/or sterilize insect pests. Even at this more advanced stage of maturity, thermal treatments can compromise produce quality, when applied improperly.
Recently, the use of a precise thermal treatment as an antimicrobial step for cantaloupe has gained attention from industry. A one minute submersion in water heated to 70 °C of intact melons with surface contamination by Salmonella reduced the pathogen population from 4.6 to 0.8 log cfu/cm2, a reduction of 3.8 log cfu/cm2 (Ukuku et al., 2004). A treatment of two minutes at 76 °C reduced Salmonella and total microflora by 3 log cfu/cm2. Cut fruit pieces prepared from the treated melons had higher quality which lasted throughout 28 days of storage at 4 °C (Fan et al., 2006, 2008). Solomon et al. (2006) obtained reductions of 4.6 log cfu/cm2 for Salmonella on melons treated for one minute at 85 °C. In that work, thermal penetration profiles indicated that the internal temperature of treated melons was such that edible flesh 10 mm below the surface was unaffected by thermal conduction from the heated rind. The optimal treatment temperature for cantaloupe appears to be approximately 74–76 °C. Salmonella cannot survive in wash water maintained at this temperature. In actual practice, thermal wash treatments would be combined with conventional chemical rinses and mechanical brushing. When combined with a mechanical brushing step, a 20-second treatment at 75 °C resulted in a 3 log cfu reduction of E. coli (Fallik et al., 2007). Also, the speed of cooling of the thermally treated product can be via relatively slow air-cooling, or, as in the case of the preparation of cut fruit, by the more rapid and expedient method of removing the heated rind.
11.4 Adaptation of existing technologies: plasma, phage treatment and bacteria-based biological controls
The preceding sections introduced some key areas of research in which conventional treatments are being modified either to achieve improved levels of effectiveness or to be used in entirely new ways. This section will consider produce processing technologies which were originally developed for entirely different applications. In many ways, the adaptation of an existing set of tools can be as challenging as de novo development. To take technologies which are mature in their respective context of ink adhesion, electronics manufacture, animal husbandry or field pathology, and adapt them for use to improve the safety of fresh produce, requires setting aside many existing principles and practices.
The promise in leveraging the existing body of knowledge is the potential to speed the development of an effective food processing tool. The challenge is in bringing together the information and perspectives from disparate fields of inquiry, and doing so with the openmindedness necessary to making the technology work under a wholly new set of operational constraints. The examples discussed in this section of the chapter are generally less mature and not ready for commercial implementation compared to many of those discussed earlier. Nevertheless, they hold significant potential for widespread usage as effective tools.
11.4.1 In-package plasma
Cold plasma is a novel sanitizing technology which has shown promise for use on fresh produce. Plasma technologies, and associated terminology, are not particularly common in the context of food processing. Although the technologies used to create plasmas are varied, the underlying mechanisms involve similarities of energy transfer. As energy is added to materials, they change state, going from solid to liquid to gas, with large-scale inter-molecular structure breaking down. As additional energy is added, the intra-atomic structures of the components of the gas break down, yielding plasmas–concentrated collections of ions, radical species and free electrons (Gadri et al., 2000; Niemira and Gutsol, 2009). Although technically it is a distinct state of matter, for all practical purposes, cold plasma may be regarded as an energetic form of gas. For food processing, it is useful to specify that the term ‘cold plasma’ refers to operation at nonthermal temperature ranges, rather than requiring refrigeration as part of the system. This clarification further serves to distinguish cold plasma in the context of produce sanitization from unrelated applications in other areas such as textiles, plastics and electronics manufacturing and processing.
Most cold plasma technologies used for food processing rely on the application of the plasma directly to the food product, or indirectly via a forced air stream. An example of this approach is the gliding arc plasma system (Niemira and Sites, 2008). Treatments of three minutes effectively reduced human pathogens applied to the surfaces of Golden Delicious apples. At the optimal gas flow rate, reductions of Salmonella were 3.4 log cfu/ml, while E. coli O157:H7 was reduced by 3.5 log cfu/ml (see Fig. 11.1). These treatments resulted in minimal color or textural changes to the treated produce.
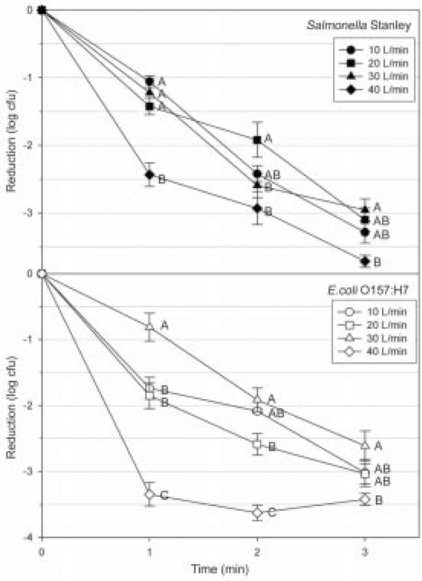
Fig. 11.1 Cold plasma inactivation of Salmonella Stanley (top graph) and E. coli 0157:H7 (lower graph) on golden delicious apples. Feed gas is air, flow rate = 10 liters/min (circle), 20 liters/min (square), 30 liters/min (triangle), or 40 liters/min (diamond). Different letters for each treatment time indicate significant differences (P < 0.05) among flow rates. Bars = standard error. (Adapted from Niemira and Sites, 2008)
A modified design of cold plasma emitters offers the potential for in-package treatment processing (Schwabedissen et al., 2007). Electrically conductive labels are affixed to the inside surface of the container. By inducing a voltage through the packaging, cold plasma may be generated on the label’s edges, generating ozone and other sanitizing plasma species inside the package. A ten-minute treatment using this approach generated ozone concentrations of approximately 2000 ppm within a container. This was sufficient to effect a 4-log cfu/ml reduction of Bacillus subtilis on agar within the package. The process is undergoing optimization by critical analysis and adjustment of the cold plasma generating discharge labels. Factors such as their method of application (screen-printed, vs. applied or bonded) their shape, material, electrical conductivity, etc., all influence the efficacy of plasma generation.
A different type of external plasma generation system used external electrodes to generate ozone within the package (Klockow and Keener, 2009). The voltage applied externally led to the creation of ozone in the 3 mm thick plasma field inside the plastic bag, between the pinched electrodes. The resultant ozone concentrations within the bag were 1.6 and 4.3 mg/L for bags filled with air and oxygen gas, respectively. Spinach leaves inoculated with E. coli O157:H7 demonstrated significant reductions in microbial populations following these treatments, ranging from 3 to 5 log cfu/leaf (Table 11.2). It should be noted that although the treatment was effective in reducing the pathogen, it also had a negative impact on sensory quality. The degree of discoloration was related to the concentration of ozone, with oxygen packaging having a greater negative impact than air packaging.
Table 11.2
E. coli 0157:H7 surviving populations and corresponding ozone concentrations at refrigeration (5 °C)
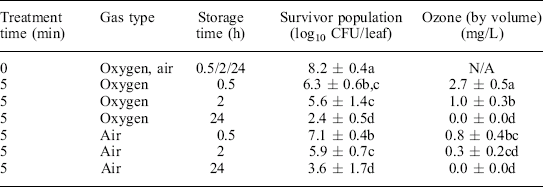
Average population of untreated, unstored, inoculated samples was 7.6 ± 0.6 log10 CFU/leaf. The microbial detection limit for E. coli 0157:H7 (6460) was 2.0 log10 CFU/leaf. Average ozone concentrations after 5 min treatment in air and oxygen were 1.6 ± 0.2 mg/L and 4.3 ± 1.0 mg/L, respectively. Values in survivor population column with different letters are significantly different (P < 0.05). Values in ozone column with different letters are significantly different (P < 0.05). Used with permission.
Reprinted from Klockow and Keener (2009)
Cold plasma is a rapidly developing technology, and holds significant potential for operational application to fruits and vegetables. As with all processing technologies, however, the retention of quality of the treated produce is a fundamental requirement for any antimicrobial treatment. For this reason, a clearer understanding of the sensory impact of efficacious levels of plasma treatment will be an essential part of establishing protocols for commercial use.
11.4.2 Phage treatments
Bacteriophages are viruses which infect and kill bacteria such as L. monocytogenes, E. coli 0157:H7 or Salmonella. Bacteriophages are commonly referred to in the food science community simply as phages. They are regarded as a targeted, self-replicating bio-based antimicrobial tool. The advantage of phage treatments is that they will use the cellular machinery of the pathogenic bacterial host to reproduce, thus amplifying the concentration of viral particles in the presence of the bacterial threat agent. The phage-host interaction is strain specific, with a given isolate of bacteriophage being effective against a single isolate of bacteria, or, at most, against a narrow range of isolates (Sharma et al., 2005). This specificity implies that real-world applications would rely on cocktails of phages to broaden their utility as a food treatment. The USFDA recently approved phage treatments as a means for suppressing and/or eliminating L. monocytogenes from packaged ready-to-eat meat and poultry products (Lang, 2006). A liquid culture of the Listeria-specific phage is applied to the meat or poultry product immediately prior to packaging. The regulations related to foods treated with such phage preparations require the ingredients label on the product to contain the phrase ‘bacteriophage preparation’ (Lang, 2006). Additional descriptive labeling may also be required, depending on the composition of the product.
Regulations are not yet in place to allow for phage treatments of fresh produce. However, it is clear that phage-based treatments for fruits and vegetables would most likely be applied as a dip or spray, and possibly in combination with other antimicrobial treatments. A phage treatment reduced L. monocytogenes on apples and melons by 0.4 and 5.3 log cfu/g, respectively. A nisin treatment reduced L. monocytogenes on apples and melons by 0.9–2.0 and 3.0 log cfu/g, respectively. Combining the nisin and phage treatment reduced L. monocytogenes on apples and melons by 1.5–2.3 and 6.4 log cfu/g, respectively (Leverentz et al., 2003). Phage KH1 reduced E. coli O157:H7 attached to stainless steel coupons by 1–2 log cfu, and cells living in mature, protective biofilms were not significantly reduced (Sharma et al., 2005). Abuladze et al. (2008) demonstrated the efficacy of a bacteriophage cocktail against E. coli O157:H7 on tomato, spinach and broccoli. A 5 minute treatment with phage cocktail preparations of 108, 109, and 1010 PFU/ml resulted in ∼ 1.2-3.0 log cfu/g reductions on the vegetable commodities, levels comparable to those obtained with similarly inoculated inert surfaces or with beef products. Reductions were enhanced by increasing the concentration of phage particles applied.
The optimization of phage treatments for fresh produce is a matter of ongoing research, in advance of regulatory approval. Possible applications include pre-harvest (i.e., in-field) application, or post-harvest (i.e., during processing or packaging). The economics of scale come into play with respect to any intervention that is proposed for a pre-harvest application. Even for leafy vegetables such as lettuce or spinach, only a portion of the plant is harvested. Therefore, in applications to plants growing in the field, a measurable proportion of the phage cultures applied would be as a prophylactic measure, rather than curative. It remains to be seen if such a methodology could be made practical, even with phage cocktails capable of targeting a broad pathogen range.
11.4.3 Bacterial-based biological control
Research on the use of bacterial biological controls has been going on for a number of years. These preparations may involve a single isolate or a mixed culture, defined or natively derived. This approach has shown some success with applications to control fungal phytopathogens such as Alternaria alternata (Wang et al., 2008). Competitive exclusion has found applications in altering the intestinal microflora of poultry and swine to prevent the establishment of Salmonella (Atterbury 2009). This provides chicks and immature pigs with beneficial gut microflora weeks or months before they would have acquired it otherwise. The heightened disease resistance of a mature GI microflora makes it virtually impossible for Salmonella to multiply. This reduces Salmonella in the environment, in general, while protecting the specific animal being treated.
While the use of biocontrol and competitive exclusion is being used in animal systems and against phytopathogens, the development of an effective biocontrol for plant-contaminating enteric human pathogens has been more challenging. The goal is to develop a readily applied isolate or cocktail of isolates that has specific bactericidal or bacteriostatic potential. The microflora on and in fresh produce can range from 102 to 109 cfu/g. Interactions among the bacteria, yeasts and fungi which make up this population can exert positive or negative influences on human pathogen growth and/or survival (Fett, 2006; Liao, 2008). Native microflora derived from alfalfa seeds and from baby carrot effectively inhibited the enteric pathogens Salmonella, E. coli and L. monocytogenes when inoculated on bell pepper disks (Liao, 2007). One of the primary species in this suppressive microflora is a strain of Pseudomonas fluorescens designated Pf 279. This strain was originally isolated as a biocontrol agent of a phytopathogen that attacks the roots of wheat plants. It has since been found that Pf 2–79 effectively suppresses enteric pathogens on sprouting seeds. The environment required for sprout production, with ample nutrition in a humid, almost aqueous environment, is considered to be one of the primary reasons for a series of sprout-related food borne illness outbreaks in the last 15 years. Using this biocontrol agent as a pre-treatment, Salmonella growth was retarded by 2–3 log cfu/g relative to the control (Liao, 2008).
The work to scale up effective biocontrol and competitive exclusion treatments has been a difficult process. Lessons have been learned from the successes of this type of intervention in animal cultivation and in phytopathogen suppression. However, the relatively low population densities and sporadic distributions of enteric pathogens on fresh produce make it a challenge to completely eliminate them with bioactive methods.
11.5 Future trends
Despite the difficulty of accurately projecting trends, an observer of a few decades ago would have been able to foresee some of the developments of recent years. Efforts have long been underway to harmonize international regulations, in support of increasing globalization of the food supply chain. The obverse of these efforts has been the occasional use of regulations by various nations to achieve unilateral trade balance goals. The competition from international and overseas suppliers of fresh produce has become more intense; at the same time, global partnerships in production and supply chains have broadened the number of products available in domestic markets. The increased demand for convenient processed foods has led to new categories of food products. However, the increased complexity of production of these multi-component convenience foods has magnified the potential for difficulties in ensuring food safety and in component tracebacks compared to raw or unprocessed commodities.
From a food safety perspective, it has become increasingly clear that fresh and fresh-cut produce is comparable to meat, poultry and seafood in terms of the research attention it deserves. An extensive summary of specific areas of key research has recently been presented (Niemira et al., 2009). Regulators, producers and processors have been working with researchers to improve sanitation controls, detection, traceback and epidemiology. The goal of this section is to identify the major factors that will influence the future of produce processing. Rather than an ungrounded attempt to predict which food processing technology will be the ‘next big thing’, the discussion will be oriented on extrapolating existing trends towards likely future directions.
11.5.1 Tolerances
Compared to standard practices of the past, fresh produce today must meet exacting metrics for handling and safety. The visual and ‘hand-on-pallet’ load inspections, which may have once sufficed for both suppliers and purchasers, have been replaced by microbiological testing reports, automated temperature data loggers, and computerized process controls. The increasingly widespread use of rigorous HACCP plans signify the specific tolerances for sensory quality and microbiological safety at every step in the chain. Buyers are requiring tighter controls from processors, who in turn have more stringent standards of their grower suppliers. Guidance for the appropriate standards for each phase of an operation–irrigation water quality, worker hygiene practices, flume water amendment protocols, packing line swab testing, etc.–comes from various sources. Industry trade groups regularly issue recommendations. Scientific bodies such as the Institute of Food Technologists and the International Association for Food Protection convene panels of experts from industry, government and academia to review the science and offer guidance. Regulators such as the FDA serve by fostering and supporting these discussions, and in implementing guidelines based on the sound science that arises from them. Finally, independent testing service providers play a key role in applying the relevant science when reviewing the facilities and practices of growers and processors. Integration and coordination of these activities will be the means by which the industry will meet ever more stringent tolerances. Weak links in the chain will be identified and addressed, not as a one-and-done approach, but as a continual process of improvement.
11.5.2 Traceback
Part of the tighter tolerances of the future will be to have structures in place which will facilitate traceback. In the past, a traceback exercise may have led back to an individual grower or supplier. Currently, some, but not all, supply chains can be traced back to a particular field and specific date of harvest. It is common to see barcoded inventory supply labels on pallets and individual boxes, ready to be cross-referenced to supply and delivery manifests. The data management structures in use today serve the needs of normal commerce. Particularly in a commodity environment such as tomatoes, where repack from several suppliers is not unusual, traceback of the source of individual items is not a simple task. The trend in the fresh and fresh-cut produce industry is to adjust product coding and data management so as to enhance efficiency of the normal commerce, but also to better serve the needs of the traceback process. These improvements may derive from developments in technology, such as the use of RFID tags on pallets and boxes, or by better use of existing inventory management and tracking tools. In part, recent changes in country of origin labeling (COOL) will better serve this process. Supply chain control and validation will be at the center of development.
11.5.3 Technology
While new technologies for epidemiological analysis will serve traceback needs, and new inventory tracking tools will serve supply chain management, the primary technological drivers for the fresh produce industry will be in the areas of communications and systems integration. This trend is already visible in the increasing use of common standards for microbiological quality at various points of growing and processing. Information about where and when a particular commodity load was grown and harvested will be most useful and valuable when it is readily available. It may be that the entire history of each pallet or box, from planting date to harvesting date, may accompany it through the supply chain. Much more information will be shared, evaluated and used on a more proactive basis than ever before. It is a widely cited truism that the world is getting smaller and more ‘talkative’ through increased communications. It is as true in the area of fruit and vegetable production and processing as it is in every other sphere of life. The accelerating pace of technology development will ensure that this trend will continue, and will allow for coordination and cooperation among involved industry partners.
11.6 Sources of further information and advice
This present work notwithstanding, many valuable reference materials exist in electronic form as continually updated web sites, RSS outlets, news aggregator feeds, etc. The online sources listed below, current as of March 2010, are some of the means by which producers and consumers can benefit from recent advances in connectivity and communications. The realities of the global market environment means that real-time access to information will be a cornerstone of rapid response to product recalls and compliance issues. The ability to share, discuss, evaluate and act on information will allow the industry to maximize efficiency and control for every stage of production and distribution. While neither exhaustive nor unchangeable, the following list of sources are a valuable entry point into the global network of communication related to the safety and security of fresh produce, and food processing technologies.
• http://twitter.com/FoodSafety (USDA – National Agricultural Library)
• http://twitter.com/USDA nass (USDA – National Agricultural Statistics Service)
• http://twitter.com/USDAFoodSafety (USDA – Food Safety Inspection Service)
• http://www.fsis.usda.gov/News_&_Events/Feeds/index.asp (USDA – FSIS RSS feeds, podcasts and open blogs)
• http://twitter.com/FDArecalls (FDA – Recalls, Market Withdrawals and Safety Alerts)
• http://www.fda.gov/oc/rss/ (FDA – RSS feeds)
• http://twitter.com/CDCemergency (US Centers for Disease Control and Prevention)
• http://bites.ksu.edu/ (BITES Food Safety Network listserv summary and archives)
• http://twitter.com/FoodProcessing (Industry trade journal)
11.7 Acknowledgements
The author would like to thank Ms L. Cheung for technical assistance in preparation of this manuscript, and Drs X. Fan and Y. Liu for their critical reviews. Mention of trade names and commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the US Department of Agriculture.
11.8 References
Abuladze, T., Li, M., Menetrez, M., Dean, T., Senecal, A., Sulakvelidze, A. Bacteriophages reduce experimental contamination of hard surfaces, tomato, spinach, broccoli, and ground beef by Escherichia coli 0157:H7. Appl Environ Microbiol. 2008; 74(20):6230–6238.
Atterbury, R.J., Bacteriophage biocontrol in animals and meat products. Microbial Biotechnol 2009;, doi: 10.1111/j.1751-7915.2009.00089.x.
Beuchat, L.R., Pettigrew, C.A., Tremblay, M.E., Roselle, B.J., Scouten, A.J. Lethality of chlorine, chlorine dioxide, and a commercial fruit and vegetable sanitizer to vegetative cells and spores of Bacillus cereus and spores of Bacillus thuringiensis. J Food Prot. 2004; 67:1702–1708.
Du, J., Han, Y., Linton, R.H. Inactivation by chlorine dioxide gas (ClO2) of Listeria monocytogenes spotted onto different apple surfaces. J Food Prot. 2002; 19:481–490.
Du, J., Han, Y., Linton, R.H. Efficacy of chlorine dioxide gas in reducing Escherichia coli 0157:H7 on apple surfaces. Food Micro. 2003; 20:583–591.
Fallik, E., Rodov, V., Horev, B., Sela, S., Alkalai-Tuvia, S., Vinokur, Y. Hot water rinsing and brushing technology for the fresh-cut industry. Acta Hort (Ishs). 2007; 746:229–236.
Fan, X., Annous, B.A., Sokoraik, J., Burke, A.M., Mattheis, J.P. Combination of hot water surface pasteurization of whole fruit and low dose irradiation of fresh-cut cantaloupe. J Food Prot. 2006; 69:912–919.
Fan, X., Annous, B.A., Beaulieu, J., Sites, J. Effect of hot water surface pasteurization of whole fruit on shelf life and quality of fresh-cut cantaloupes. J Food Sci. 2008; 73:M91–M98.
Fett, W.F. Inhibition of Salmonella enterica by plant-associated pseudomonads in vitro and on sprouting alfalfa seed. J Food Prot. 2006; 69:719–728.
Gadri, R.B., Roth, J.R., Montie, T.C., Kelly-Wintenberg, K., Tsai, P., Helfritch, D.J., Feldman, P., Sherman, D.M., Karakaya, F., Chen, Z. Sterilization and plasma processing of room temperature surfaces with a one atmosphere uniform glow discharge plasma (OAUGDP). Surface Coatings Technol. 2000; 131:528–542.
Gómez-Lopez, V.M., Rajkovic, A., Ragaert, P., Smigic, N., Devlieghere, F. Chlorine dioxide for minimally processed produce preservation: a review. Trends Food Sci Tech. 2009; 20(1):17–26.
Han, Y., Sherman, D.M., Linton, R.H., Nielsen, S.S., Nelson, P.E. The effects of washing and chlorine dioxide gas on survival and attachment of Escherichia coli 0157:H7 to green pepper surfaces. Food Micro. 2000; 17:521–533.
Han, Y., Linton, R.H., Nielsen, S.S., Nelson, P.E. Inactivation of Escherichia coli 0157:H7 on surface-uninjured and -injured green bell pepper (Capsicum annuum L.) by chlorine dioxide gas as demonstrated by confocal laser scanning microscopy. Food Micro. 2000; 17:643–655.
Han, Y., Linton, R.H., Nielsen, S.S., Nelson, P.E. Reduction of Listeria monocytogenes on green peppers (Capsicum annuum L.) by gaseous and aqueous chlorine dioxide and water washing and its growth at 7 C. J Food Prot. 2001; 64:1730–1738.
Jifsan (Joint Institute For Food Safety And Applied Nutrition). Tomato Safety Research Needs Workshop. Available from: http://www.jifsan.umd.edu/events/event_record.php?id=18, 2007. [(accessed 5 March 2010)].
Klockow, P.A., Keener, K.M. Safety and quality assessment of packaged spinach treated with a novel ozone-generation system. Lwt–Food Science and Technology. 2009; 42(6):1047–1053.
Koseki, S., Isobe, S., Itoh, K. Efficacy of acidic electrolyzed water ice for pathogen control on lettuce. J Food Prot. 2004; 67:2544–2549.
Lang, L. Fda approves use of bacteriophages to be added to meat and poultry products. Gastroenterology. 2006; 131(5):1370.
Lee, S.Y., Dancer, G.I., Chang, S.S., Rhee, M.S., Kang, D.H. Efficacy of chlorine dioxide gas against Alicyclobacillus acidoterrestris spores on apple surfaces. Int J Food Microbiol. 2006; 108:364–368.
Leverentz, B., Conway, W.S., Camp, M.J., Janisiewicz, W.J., Abuladze, T., Yang, M., Saftner, R., Sulakvelidze, A. Biocontrol of Listeria monocytogenes on fresh-cut produce by treatment with lytic bacteriophages and a bacteriocin. Appl Env Microbiol. 2003; 69:4519–4526.
Liao, C.H. Inhibition of foodborne pathogens by native microflora recovered from fresh peeled baby carrot and propagated in cultures. J Food Sci. 2007; 72:M134–M139.
Liao, C.H. Growth of Salmonella on sprouting seeds as affected by the inoculums size, native microbial load and Pseudomonas fluorescens 2-79. Lett Appl Microbiol. 2008; 46:232–236.
Mahmoud, B.S.M., Linton, R.H. Inactivation kinetics of inoculated Escherichia coli O157:H7 and Salmonella enterica on lettuce by chlorine dioxide gas. Food Micro. 2008; 25:244–252.
Mahmoud, B.S.M., Bhagat, A.R., Linton, Rh. Inactivation kinetics of inoculated Escherichia coli O157:H7, Listeria monocytogenes and Salmonella enterica on strawberries by chlorine dioxide gas. Food Micro. 2007; 24:736–744.
Mahmoud, B.S.M., Vaidya, N.A., Corvalan, C.M., Linton, R.H. Inactivation kinetics of inoculated Escherichia coli O157:H7, Listeria monocytogenes and Salmonella Poona on whole cantaloupe by chlorine dioxide gas. Food Micro. 2008; 25:857–865.
Niemira, B.A., Gutsol, A. Non-thermal plasma as a novel food processing technology. In: Zhang H.Q., Barbosa-Canovas G., Balasubramaniam V.M., Dunne P., Farkas D., Yuan J., eds. Non-thermal Processing Technologies for Food. Ames, IA: Blackwell Publishing, 2009.
Niemira, B.A., Sites, J. Cold plasma inactivates Salmonella Stanley and Escherichia coli O157:H7 inoculated on golden delicious apples. J Food Prot. 2008; 71:1357–1365.
Niemira, B.A., Fan, X., Gravanir, B., Doona, C.J., Feeherry, F.E. Research needs and future directions. In: Fan X., Niemira B.A., Doona C.J., Feeherry F.E., Gravani R.B., eds. Microbial Safety of Fresh Produce: Challenges Perspectives and Strategies. Ames, Ia: Blackwell Publishing; 2009:421–426.
Park, E.J., Alexander, E., Taylor, G.A., Costa, R., Kang, D.H. The decontaminative effects of acidic electrolyzed water for Escherichia coli O157:H7, Salmonella typhimurium, and Listeria monocytogenes on green onions and tomatoes with differing organic demands. Food Micro. 2008; 26(4):386–390.
Park, E.J., Alexander, E., Taylor, G.A., Costa, R., Kang, D.H. Effects of organic matter on acidic electrolysed water for reduction of foodborne pathogens on lettuce and spinach. J Applied Micro. 2008; 105(6):1802–1809.
Popa, I., Hanson, E.J., Todd, E.C.D., Schilder, A.C., Ryser, E.T. Efficacy of chlorine dioxide gas sachets for enhancing the microbiological quality and safety of blueberries. J Food Prot. 2007; 70:2084–2088.
Sapers, G.M., Walker, P.N., Sites, J.E., Annous, B.A., Eblen, D.R. Vapor-phase decontamination of apples inoculated with Escherichia coli. J Food Sci. 2003; 68:1003–1007.
Schwabedissen, A., Lacinski, P., Chen, X., Engemann, J. PlasmaLabel–a new method to disinfect goods inside a closed package using dielectric barrier discharges. Contrib Plasma Phys. 2007; 47:551–558.
Setlow, P., Doona, C.J., Feeherry, F.E., Kustin, K., Sisson, D., Chandra, S. Enhanced safety and extended shelf-life of fresh produce for the military. In: Fan X., Niemira B.A., Doona C.J., Feeherry F.E., Gravani R.B., eds. Microbial Safety of Fresh Produce: Challenges Perspectives and Strategies. Ames, Ia: Blackwell Publishing; 2009:263–288.
Sharma, M., Ryu, J.H., Beuchat, L.R. Inactivation of Escherichia coli O157:H7 in biofilm on stainless steel by treatment with an alkaline cleaner and a bacteriophage. J Appl Microbiol. 2005; 99:449–459.
Singh, N., Singh, R.K., Bhunia, A.K., Stroshine, R.L. Efficacy of chlorine dioxide, ozone, and thyme essential oil or a sequential washing in killing Escherichia coli O157:H7 on lettuce and baby carrots. Lebensmittel-Wissenschaft und -Technologie. 2002; 35:720–729.
Sivapalasingam, S., Friedman, C.R., Cohen, L., Tauxe, R.V. Fresh produce: a growing cause of outbreaks of foodborne illness in the United States, 1973 through 1997. J FoodProt. 2004; 67:2342–2353.
Solomon, E.B., Huang, L., Sites, J.E., Annous, B.A. Thermal inactivation of Salmonella on cantaloupes using hot water. J Food Sci. 2006; 71(2):M25–M30.
Sy, K.V., Mcwatters, K.H., Beuchat, L.R. Efficacy of gaseous chlorine dioxide as a sanitizer for killing Salmonella, yeasts and molds on blueberries, strawberries, and raspberries. J Food Prot. 2005; 68:1165–1175.
Sy, K.V., Murray, M.B., Harrison, M.D., Beuchat, L.R. Evaluation of gaseous chlorine dioxide as a sanitizer for killing Salmonella, Escherichia coli O157:H7, Listeria monocytogenes, and yeasts and molds on fresh and fresh-cut produce. J Food Prot. 2005; 68:1176–1187.
UFPA (United Fresh Produce Association). Leafy Greens Food Safety Research Conference. Available from: http://www.unitedfresh.org/newsviews/leafy_greens_food_safety_research, 2007. [(accessed 5 March 2010)].
Ukuku, D.O., Pilizota, V., Sapers, G.M. Effect of hot water and hydrogen peroxide treatment on survival of Salmonella and microbial quality of whole cantaloupe and fresh-cut cantaloupe. J Food Prot. 2004; 67:432–437.
Vandekinderen, I., Devlieghere, F., Van Camp, J., Kerkaert, B., Cucu, T., Ragaert, P., De Bruyne, J., De Meulenaer, B. Effects of food composition on the inactivation of foodborne microorganisms by chlorine dioxide. Int J Food Micro. 2009; 131(2–3):138–144.
Wang, H., Feng, H., Luo, Y. Dual-phasic inactivation of Escherichia coli O157:H7 with peroxyacetic acid, acidic electrolyzed water and chlorine on cantaloupes and fresh-cut apples. J Food Safety. 2006; 26:335–347.
Wang, Y., Bao, Y., Shena, D., Feng, W., Yu, T., Zhang, J., Zheng, X.D. Biocontrol of Alternaria alternata on cherry tomato fruit by use of marine yeast Rhodosporidium paludigenum Fell & Tallman. Int J Food Micro. 2008; 123(3):234–239.
Wu, V.C.H., Kim, B. Effect of a simple chlorine dioxide method for controlling five foodborne pathogens, yeasts and molds on blueberries. Food Micro. 2007; 24(7–8):794–800.
