A case study in military ration foods: the Quasi-chemical model and a novel accelerated three-year challenge test
Abstract:
Maple-filled French toast (MFFT) enrobed sandwiches are intermediate moisture (IM) bi-layered foods formulated with ‘hurdles’ to prevent the growth of Staphylococcus aureus for product shelf-life (3 years at T = 80 °F ≈ 27 °C). A novel approach to accelerate microbial challenge testing was investigated. The Quasi-chemical model’s secondary model (‘growth-no-growth boundary’) was used to guide the formulation of the MFFT. The novel 3-year test protocol used a five-strain cocktail of S. aureus to challenge MFFT with variations in storage temperature, product formulation, and packaging atmosphere. The inactivation kinetics were evaluated with the modified Quasi-chemical model and determined to be more rapid at T = 35 °C than at T = 25 °C. Removing the oxygen scavenger did not promote S. aureus growth. These results provide a basis for standardizing accelerated microbial challenge tests for new varieties of IM enrobed sandwiches in the pipeline to save time, money, and labor, while ensuring food safety.
21.1 Introduction
Minimally processed intermediate moisture (IM) enrobed sandwiches are convenient on-the-move, eat-out-of-hand (no utensils) nutrient-rich meals that require no end-consumer preparation steps. These ‘pocket’ sandwiches are popular among consumers of military rations because they satisfy consumer demand for safe, more fresh-like foods, while also featuring high quality, and improved organoleptic attributes. One well-known example is the popular barbecue chicken ‘pocket’ sandwich (ABC News–World News Tonight, 2002; BBC News, 2002; CNN.com, 2002; Cook, 2002; Fabricant, 2002; Graham-Rowe, 2002; Yahoo! News, 2002). Military ration systems are steadily increasing the types and varieties of enrobed sandwiches meeting the extended shelf-life requirements (Fig. 21.1). Many types of these enrobed sandwiches are derivatives based on IM pouch bread–a loaf of pouch bread packaged in trilaminate foil with an oxygen scavenger. A relatively recent and popular enrobed sandwich item is Maple-filled French toast (MFFT), a new variety of high quality, minimally processed, IM breakfast sandwich being developed to meet consumer demand (Fig. 21.2).
Fig. 21.1 Many new varieties of ‘pocket’ sandwiches are in development, as shown in (a) and (b), and are popular among consumers for their good taste and convenience as eat-on-the-move foods.
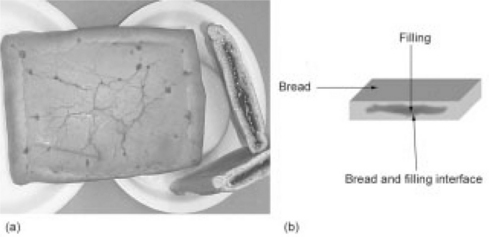
Fig. 21.2 Maple-filled French toast enrobed breakfast sandwich (a) top view and cross-sectional view showing moist maple-flavored filling, and (b) diagram of cut interface for inoculation with 5-strain S. aureus cocktail across the cut surface to include bread, filling, and the bread/filling interface.
IM foods are defined (Karel, 1973) conventionally as having Aw levels ranging from 0.7 to 0.9, and moisture content ranging from 20 to 50%. Minimally processed IM foods, in general, tend to feature more fresh-like character, retain increased sensory attributes such as flavor, texture, and color, have higher nutritive content, and receive higher consumer acceptance than their thermally treated counterparts. It is important that minimally processed IM foods feature not only improved organoleptic attributes and consumer acceptance, but that they are safe microbiologically. Staphylococcus aureus is a notorious pathogen that thrives in the ecological niche (low Aw, low moisture content) characteristic of IM foods (Lotter and Leistner, 1978). The potential microbial hazards associated with S. aureus in IM enrobed sandwiches are the possibilities of post-processing contamination (the sandwiches do not undergo an end-treatment to act as an effective kill step to eliminate pathogenic microorganisms), survival for extended periods in low Aw foods such as powdered dry milk (see Dow Jones/Agence France Presse English, 2000), and for recovery from sub-lethal injury during the protracted 3-year storage period (the ‘Phoenix phenomenon,’ see Jay, 1996) in microdomains of relatively high moisture content and Aw created by moisture migration from the filling to the bread and dispersed inhomogeneously throughout the microstructure of the bread matrix.
A factor confounding the microbiological stability of complex, bi-layered foods such as enrobed sandwiches, cream-filled cakes, and pastries is the potential for moisture transfer to occur between the two separate phases during storage that may render a previously stable phase unstable (Tatini, 1973; Rajkowski et al., 1994). For example, dried salami slices stable at Aw ≤ 0.85 did not support S. aureus growth, however, storage for 48 h of these meat slices in contact with cheese at Aw = 0.91 increased the Aw of these salami slices to Aw = 0.91, rendering them capable of supporting S. aureus growth (Rajkowski et al., 1994). Another factor to consider with regard to moisture migration from the filling to the bread during storage for 3 years at ambient temperatures is potential sogginess. Enrobed sandwiches for military rations must retain physico-chemical stability and high quality without the degradation of texture.
Formulating and designing IM foods to attain stability over shelf-life with respect to preventing microbiological growth and/or degradation reactions is generally referred to as ‘hurdle technology’ (Leistner et al., 1981). Hurdle technology encompasses a number of methods to impart food stability (microbiological and physico-chemical) by controlling intrinsic factors of the food (such as pH, salinity, and Aw by adjusting the levels of various humectants), by adding anti-microbial compounds or the presence of competing microflora, and by controlling extrinsic factors (atmospheric conditions such as storage temperature, relative humidity, and ambient pressure). Achieving this with IM foods generally requires imposing hurdles of low Aw, low pH, and other factors that influence microbial survivability. It is therefore essential to formulate enrobed sandwiches to be nutritious, have high consumer ratings, and be detrimental to the survivability of potential microbial hazards such as S. aureus.
Predicting pathogen growth in foods constrained by ‘hurdles’ (Leistner, 1994; Leistner et al., 1981) has enormous cost-savings potential for food product development by generating guidelines for HACCP plans and reducing the need for extensive and time-consuming microbiological challenge testing. Predictive mathematical models are tools that provide convenient methods for assessing and predicting the criteria that constrain the growth of pathogens or spoilage microorganisms in foods and ensuring food safety or quality (McMeekin et al., 1993). Accordingly, it is essential to know how bacterial populations grow and die in response to the factors that characterize a food product, and to develop appropriate secondary models. Feeherry et al. (2003) examined the ability of military IM pouch bread to support S. aureus growth over the Aw range 0.8360.909 at pH 5.2–5.5, then used these results to define conditions that prevent the growth of S. aureus in pouch bread formulations and other IM foodstuffs. Using the four-parameter logistic equation to model S. aureus growth kinetics as functions of food properties (Aw and pH), Feeherry et al. (2003) noted two things: first, in cases where death-only kinetics occurred, the logistics equation could not model the data without first making some significant modifications; and second, after growth to a stationary phase, the data began to show a decline. This data had to be subjectively removed to be consistent with conventional growth studies. Clearly, a new, more comprehensive model was needed to account for continuous growth-death kinetics, and the model had to be versatile and convenient for use in conditions producing (nonlinear) inactivation kinetics without requiring end-user modifications.
The Quasi-chemical model is a unique and versatile model suitable for fitting continuous growth-death kinetics and also for fitting nonlinear death-only kinetics without modifying the fundamental structure of the model (Doona et al., 2005; Ross et al., 2005; Taub et al., 2003; Feeherry et al., 2001). Other kinetics models, such as the logistic equation, are sigmoidal functions that, despite their success, do not generally model growth from a lag phase through an asymptotic maximum and followed by death. In fact, experimental conditions are generally configured to isolate growth from inactivation kinetics, and distinct models are applied to evaluate each type of kinetics separately (Gianuzzi et al., 1999). By fitting the growth-death kinetics data, the Quasi-chemical model is a primary model that accurately determines certain useful kinetics parameters (growth rate, lag time). The Quasi-chemical modeling approach was used to interrelate these kinetics parameters with the environmental conditions (Aw, pH, T) and create a unique secondary model called the growth/no-growth boundary line that defines food properties that prevent S. aureus growth based on statistical analysis of the data (Taub et al., 2003). This secondary model was used to predict food formulations for MFFT and other IM foods safe from the growth of S. aureus (Taoukis and Richardson, 2007).
To ensure the safety of foods, microbial challenge studies are designed and carried out in accordance with written guides (Swanson, 2005; US Department of Health and Human Services, Food and Drug Administration, 2001; NSF International, 2000; Powers et al., 1999) for commercial products with substantially shorter shelf-life requirements than military ration components. Microbial challenge studies should be conducted under conditions (temperature packaging, environment, etc.) as similar as possible to those the actual product experiences for durations of (1–1.3) × the product shelf-life to ensure consumer safety. Since pouch bread-based enrobed sandwiches are formulated or designed not to support the growth nor survival of target pathogens or other microorganisms, microbial challenge studies of military ration foods typically involve sampling at appropriate intervals over 6 month storage spans at the temperature of 25 °C to ensure safety. The ‘Phoenix phenomenon’ involves the possible re-growth of sub-lethally injured or non-recoverable organisms after long-term storage (Jay, 1996). Given the long-term survival of S. aureus in dry milk powder (Dow Jones/Agence France Presse English, 2000), the potential for injured cells to recover and grow in the nutrient-rich environment of the MFFT, despite its low Aw and pH that could increase with moisture transfer between layers during 3 years of ambient storage-growth, is conceivable. However, a microbial challenge test of 3–4 years duration, while technically feasible, is uneconomical and impractical from a typical product development standpoint.
As new enrobed, IM food products are developed to meet military requirements for high consumer acceptance, stability, and food safety during prolonged storage at non-refrigerated temperatures (3 years at T = 80 °F ≈ 27 °C), an accelerated microbial challenge test protocol is needed to ensure the safety of these foods while reducing the duration of these studies (microbial challenge tests usually last for product shelf-life, see below). Analogous to how trained sensory panels evaluate foods using accelerated temperatures (6 months at T = 100 °F ≈ 38 °C) to expedite the development process, we have recently studied the effects of temperature on microbiological challenge tests of enrobed bi-layered sandwiches undergoing long-term storage and developed an accelerated temperature microbiological challenge test to shorten the time while ensuring the safety of IM pocket sandwiches with respect to S. aureus, a pathogen notorious for growing (or surviving) in low Aw foods. We compare these results to samples stored for 3 years and evaluate kinetics data using the Quasi-chemical model adapted for kinetics data showing tailing (Doona et al., 2008; Feeherry et al., 2005). Predictive microbiological models help assess whether additional validation testing is needed to prevent the potential growth of the target organism. In this case, the modified Quasi-chemical model provides another tool to demonstrate that the present formulations of MFFT enrobed sandwiches do not support S. aureus growth over 3-year storage.
We apply the Quasi-chemical model to a case study of MFFT and evaluate a novel approach to accelerating the duration of microbial challenge study, using a 5-strain cocktail of S. aureus to challenge MFFT with variations in storage temperature, product formulation, and packaging atmosphere, while ensuring that S. aureus does not survive and re-grow in response to moisture migration during prolonged 3-year storage (the ‘Phoenix phenomenon’). Results of this case study show that MFFT IM bi-layered breakfast sandwiches controlled by appropriate hurdles (Aw = 0.851, pH = 5.36) receive high Hedonic ratings by consumer panels, retain their physico-chemical stability during storage, and maintain microbiological safety with respect to S. aureus for the protracted 3-year storage. Modified atmosphere packaging (MAP) helps inhibit S. aureus growth, but removing the O2 scavenger did not promote S. aureus growth in these circumstances. In fact, only inactivation kinetics of S. aureus were observed, and no re-growth was observed in a 3-year shelf-life study. More surprisingly, the inactivation kinetics of S. aureus were more rapid at T = 35 °C (near the growth optimum of S. aureus) than at T = 25 °C, which may reflect the greater difficulty for injured S. aureus cells to meet their metabolic energy demand near the optimum of their microbial ecological niche than are required at sub-optimal storage conditions. These results may lead to a standardized, accelerated protocol for microbial challenge testing of enrobed, IM foods that can ensure reliable results faster, cheaper, and with less labor than current practices.
21.2 Modeling S. aureus growth in intermediate moisture (IM) bread
Feeherry et al. (2003) conducted a conventional growth study of S. aureus to a stationary phase maximum using standard pouch bread with adjustments to the Aw to specific levels by desorptive (varying the amounts of glycerol added to the dough) or adsorptive (equilibrating in closed chambers of certain relative humidities using saturated salt solutions) techniques, and with adjustments to the pH by adding acidulant to the dough. Growth data of S. aureus in bread with variations in Aw and pH were plotted as colony counts versus time and fit with the 4-parameter logistic function (Eq. 21.1), which in all cases gave excellent fits (R2 = 0.939–0.996).
in which Nt is the survivors at time t; Ni is the enumerated inoculum level; Nf is the survivors at the final measured time; tm is the time of the maximal growth rate; and b is the maximum growth rate, determined as the slope of the line tangent to the curve at tm (Fig. 21.3).
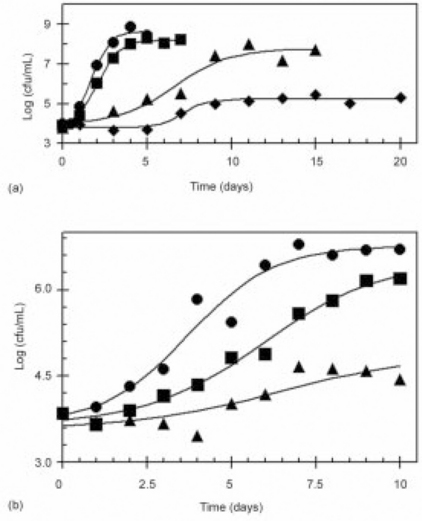
Fig. 21.3 S. aureus growth in bread as functions of (a) Aw varied by adjusting the glycerol level in dough (![]() = 0.0% added glycerol and Aw = 0.909;
= 0.0% added glycerol and Aw = 0.909; ![]() = 3.0% and Aw = 0.891;
= 3.0% and Aw = 0.891; ![]() = 6.3% and Aw = 0.866; and
= 6.3% and Aw = 0.866; and ![]() = 9.0% and Aw = 0.839 andpH ≈ 5.5); (b) pH adjusted by adding glucono-delta-lactone (GDL) (
= 9.0% and Aw = 0.839 andpH ≈ 5.5); (b) pH adjusted by adding glucono-delta-lactone (GDL) (![]() = 0.0% added GDL and pH 5.38;
= 0.0% added GDL and pH 5.38; ![]() = 0.05% and pH 5.36; and
= 0.05% and pH 5.36; and ![]() = 0.1% and pH 5.31 and Aw ≈ 0.86).
= 0.1% and pH 5.31 and Aw ≈ 0.86).
In the nutrient-rich environment of food, bacteria and other organisms tend to exhibit a characteristic pattern called the microbial lifecycle (McMeekin et al., 1993). In this lifecycle, the bacteria or other organisms can survive or be stimulated to grow after an initial relatively quiescent initial period with a relatively slow growth rate that generally produces only a small or modest increase in population (called the lag phase). As the bacteria metabolize and reproduce, the growth rate increases dramatically, leading to a relatively sudden increase in population size (called the exponential growth phase). The rate of growth then declines asymptotically to zero, and the population increases in number until the population density reaches an approximately constant maximum stationary value (called the asymptotic or stationary phase). This so-called stationary phase is not a true steady-state, rather it is indicative of a microbial population in which the rates of growth and death approximately cancel. As the bacterial population ages further, nutrients deplete and excreted metabolic waste products accumulate in the surroundings, and eventually the population density declines due to natural effects (called the death phase and represents dead and injured, non-culturable cells).
Conventional methods involve actually adjusting the experimental conditions to isolate growth (lag through stationary phases) kinetics or inactivation kinetics of the target microorganism, depending on the purpose of the study, and distinctly different predictive models are applied to evaluate the kinetics of each type of response. In the growth study of S. aureus in IM bread using the logistic function, Feeherry et al. (2003) revealed some limitations in the conventional approach to growth studies, notwithstanding the success of the research. After the growth in S. aureus counts reached a maximum, the data started to decline, in a manner consistent with the microbial lifecycle. The logistic function cannot fit such continuous growth-death kinetics data, and data had to be removed subjectively (in accord with convention) to ensure the most accurate fit of the data to the model. However, excluding the declining or so-called death data influences the estimated values of growth rates that are determined from the growth curves and that are used to estimate product shelf-life. Since variations in physico-chemical properties were explored to define the limits of conditions that would support S. aureus growth in IM pouch bread, another limitation was noted for some variations in experimental conditions when only death kinetics were observed. The logistics equation expressed above cannot model death-only kinetics, although it is adaptable to a form capable of describing death-only kinetics to overcome that issue. Recognizing the limitations of the logistic equation, a new type of predictive model was needed that could reduce the subjectivity in handling continuous growth-decline data and that was versatile enough to evaluate death-only data without requiring modification by the end-user of the program. We therefore developed and applied a 5-parameter variant of the Quasi-chemical kinetics model that accounts for tailing (Doona et al., 2008; Feeherry et al., 2005). This 5-parameter version is not a fully mechanistic model, but only one of the simplest mathematical forms capable of representing tailing and the potential for recovery of cells from sub-lethal injury that could render cells dead or non-culturable.
21.2.1 The Quasi-chemical model
The Quasi-chemical kinetics model for growth-death kinetics accounts for all four phases of the microbial lifecycle and has been used to evaluate a variety of nonlinear kinetics characterizing the growth and/or death of pathogenic microorganisms in foods stabilized by hurdles (Doona et al., 2005; Ross et al., 2005; Taub et al., 2003; Feeherry et al., 2001) or treated with the emerging nonthermal processing technologies of high pressure processing (Doona et al., 2005, 2008; Feeherry et al., 2005). As demonstrated below, the model fits continuous growth-death kinetics of S. aureus in IM bread in various conditions of Aw, pH, and temperature, and these results were used to define a unique secondary model called a ‘growth/no-growth boundary line’ based on interrelating variations in environmental conditions of Aw, pH, and temperature with kinetics parameters (maximum growth rate). Continuous modeling of microbial growth-death kinetics in actual foods advances predictive modeling that conventionally separates growth and death models.
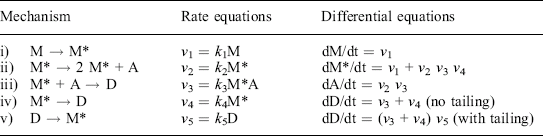
The Quasi-chemical mathematical model (Taub et al., 2003) is derived from a proposed 4-step hypothetical mechanism involving four entities, and the steps proceed according to their respective rates and align essentially as a reaction network involving autocatalytic growth coupled with negative feedback through the activity of a postulated antagonistic metabolite (Fig. 21.4). Analogous to describing the rates of the elementary steps in a chemical reaction system, each step is treated as an individual kinetics process that is described by a corresponding rate expression, and the rate expressions derive a set of ordinary differential equations (ODEs – see Table 21.1) that impart the Quasi-chemical model with several unique and advantageous features. The mathematics of the Quasi-chemical model have been characterized extensively (Ross et al., 2005), including determining relationships among individual rate parameters that impart the model with the ability to model continuous growth-death kinetics or nonlinear death kinetics in foods controlled by hurdles such as Aw, pH, temperature, and in foods subjected to treatments by HPP.
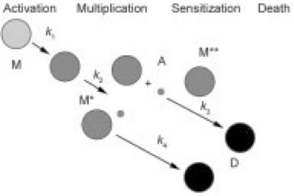
Fig. 21.4 Schematic mechanism of the Quasi-chemical model denoting cells in the stages of: metabolizing (M), multiplying (M*), sensitization to death (M**), and dead and injured (D), and the hypothetical antagonist (A), and proceeding to the next stages with rate constants denoted as k.
The Quasi-chemical model successfully fits the growth-death kinetics or nonlinear death-only kinetics for a generalized set (Doona et al., 2005; Taub et al., 2003) of pathogenic microorganisms (S. aureus, Escherichia coli, and Listeria monocytogenes) in a variety of actual IM food substrates (bread crumb, turkey meat, ham, and cheeses), and as functions of different hurdles, such as Aw (determined adsorptively and desorptively using the humectant glycerol), pH (manipulated through the addition of the acidulant glucono-delta lactone), and storage temperature, and in the presence of anti-microbial compounds commonly found in foods (lactate and plum puree). Figure 21.5 shows fits of the Quasi-chemical C model for S. aureus growth in bread with variations in Aw, pH, and temperature, respectively.

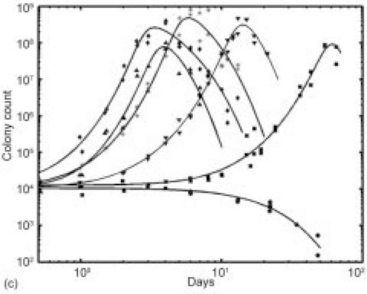
Fig. 21.5 Data and fitted curves of S. aureus kinetics in bread as functions of (a) Aw (![]() = 0.91;
= 0.91; ![]() = 0.87; and
= 0.87; and ![]() = 0.84) at pH = 5.4, T = 35 °C; (b) pH (curves in descending order with pH = 5.38, 5.36, 5.31, 5.19, and 4.97) at Aw ≈ 0.86 and T = 35 °C; and (c) temperature T = 15–40 °C (
= 0.84) at pH = 5.4, T = 35 °C; (b) pH (curves in descending order with pH = 5.38, 5.36, 5.31, 5.19, and 4.97) at Aw ≈ 0.86 and T = 35 °C; and (c) temperature T = 15–40 °C (![]() = 15 °C,
= 15 °C, ![]() = 20 °C, ▼ = 25 °C, * = 30 °C,
= 20 °C, ▼ = 25 °C, * = 30 °C, ![]() = 40 °C, and
= 40 °C, and ![]() = 35 °C) at Aw = 0.90 and pH = 5.23.
= 35 °C) at Aw = 0.90 and pH = 5.23.
Figure 21.6 shows the plot of experimentally determined CFU/mL versus time data (filled circles) and three methods to estimate the maximum growth rate. The solid line depicts the Quasi-chemical model fit using all of the growth-death data, the dotted line represents the Gompertz model fit using the growth phase only data; and the dashed line characterizes the Quasi-chemical model fit using the growth-only data. All of the fits agreed with the growth phase data, with some differences in the estimation of the growth rate (Table 21.2). The Gompertz function estimated a higher value of μ (182) than the corresponding value determined with the Quasi-chemical model (μ = 1.48), or than the Quasi-chemical model fit estimated for the continuous growth-death data (μ = 1.33).
Table 21.2
Comparison of estimated growth rates for the Quasi-chemical and Gompertz models
| Model | μ |
| Quasi-chemical (all data) | 1.33 |
| Gompertz (growth only data, t = 0–7) | 1.82 |
| Quasi-chemical (growth only data, t = 0–7) | 1.48 |
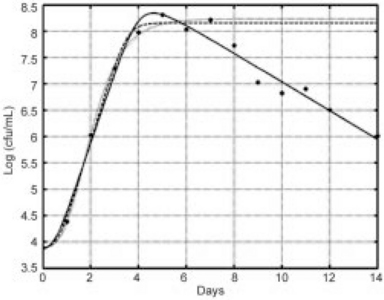
Fig. 21.6 Fitted curves comparing Quasi-chemical model and Gompertz model for estimating maximum growth rate (solid line = Quasi-chemical model of all data); hashed line = Gompertz fit of growth-only data; and heavy dashed line = Quasi-chemical model fit of growth-only data).
The growth/no-growth boundary line is estimated by applying a statistical approach that interrelates the parameters Aw, pH, and μ. Figure 21.7 shows a plot of the (Aw, pH) plane and the observed response of the S. aureus (growth = filled circles, no-growth = filled triangles) at each combination of pH and Aw (at T = 35 °C) used in the characterization of the growth kinetics. The calculated boundary line divides the plane into ‘growth’ (right of the diagonal line) and ‘no-growth’ (left of the diagonal) domains that is concordant with the experimental data with the exception of one point (a fail-safe). The boundary line can be used as a guide for facilitating the formulation and design of pouch bread-based products (e.g., IM enrobed sandwiches) to be in the no-growth domain and safe from supporting the growth of S. aureus (Taoukis and Richardson, 2007).
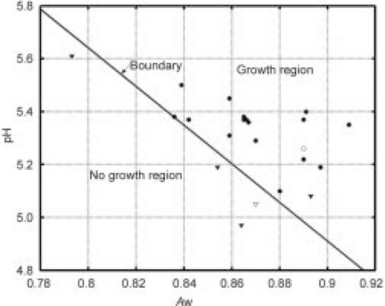
Fig. 21.7 ‘Growth/no-growth’ boundary line is a diagonal line though the (Aw, pH) plane and defining the conditions of pH and Aw in domains of growth (right side of diagonal) and no-growth (left side of diagonal) for S. aureus in bread. Filled circles (![]() ) represent growth, ▼ denote death, and
) represent growth, ▼ denote death, and ![]() and
and ![]() depict validation points.
depict validation points.
21.3 Microbial challenge study of Maple-filled French toast
Microbial challenge studies ensure the safety of foods such as enrobed sandwiches relying on hurdles (Aw, pH, MAP) to control relevant microorganisms (e.g., S. aureus). There is no single universal method for microbial challenge testing of S. aureus or other organisms in all food products, storage conditions, and processing or handling situations. Rather, the variations in microbial challenge testing conducted by food industry and academia, government research laboratories, and other facilities reflect differences in various factors relating to the specific food product and the purpose of the test. To design an appropriate microbial challenge study of the MFFT enrobed IM breakfast sandwich that yields reliable and accurate results of safety, we used L. monocytogenes Challenge Study ‘How To’ Guidelines (Swanson, 2005), Evaluation and Definition of Potentially Hazardous Foods–Ch. 6 Microbiological Challenge Testing (US Department of Health and Human Services, Food and Drug Administration, 2001), Non-potentially Hazardous Foods (NSF International, 2000), and Effect of Water Activity on the Microbiological Stability of Mobility-Enhancing Ration Components (Powers et al., 1999).
When designing a microbiological challenge test, a standard set of several critical factors must be taken into account, and the microbial challenge test for MFFT must take into account the unique attributes of MFFT and the purpose of this test to meet long-term military storage requirements. The particular points that needed to be taken into special consideration in addressing enrobed, IM, bi-layered MFFT breakfast sandwiches involve study duration, moisture transfer between layers, the potential for the Phoenix phenomenon to occur, and how to accelerate the time of the study like sensory studies to save time, money, and labor, while determining the safety of the MFFT. Since microbiological challenge tests should last (1–1.3) × product shelf-life at T = 25 °C, a study length of 36–48 months would be needed to satisfy military shelf-life requirements. The potential for moisture migration occurring during storage may induce changes in the physico-chemical properties of regions of the food to become supportive of S. aureus growth or allow a small number of injured cells to recover and grow in the product during long-term storage (the so-called Phoenix phenomenon–see Jay, 1996) is a particular concern in connection with the outbreak of S. aureus in dry milk powder (Dow Jones/Agence France Presse English, 2000). It is also important to monitor the physico-chemical properties of both layers of the enrobed MFFT sandwiches (Aw and pH) during storage. The factor under consideration for accelerating the microbial challenge test here is temperature. Since the observed growth optimum for S. aureus in bread is T = 35 °C (Fig. 21.5(c)) and MFFT is a pouch bread-based product, the question therefore arises whether T = 35 °C is an optimum growth condition for S. aureus growth in MFFT that can reliably shorten the duration of the microbial challenge study while rigorously ensuring food safety to save time, money, and labor.
MFFT samples for this microbial challenge test were prepared either in-house at Natick or by a commercial food product manufacturer. One difference, however, was in the ingredients. The in-house samples used calcium sulfate, and the commercial manufacturer prepared MFFT using appropriate amounts of calcium carbonate, although both products satisfied military specification requirements. The ingredients of the commercial product are compiled in Table 21.3.
Table 21.3
Ingredients of MFFT enrobed sandwiches
| Modified MRE bread | Moist maple filling |
| Flour | Maple syrup |
| Water | HFCS Staley Isosweet 100 |
| Shortening | Corn Syrup, Staley 1300 |
| Glycerol 4.63% | National 104 |
| Yeast | Ultra Tex 4 |
| Salt | Kelgum |
| Sucrose ester | Dextrose |
| Gum arabic | Glycerol 4.70% |
| Calcium sulfate | Water |
| Xantham gum | Maple flavor |
| Sorbic acid | |
| Maple flakes | |
| Cinnamon flakes | |
| French toast flavor | |
| Yellow lake |
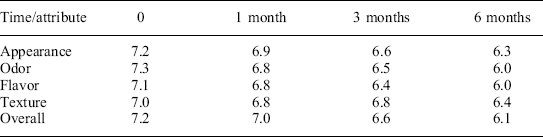
The relatively high consumer acceptance ratings over accelerated storage for 6 months at T = 35 °C are shown in Table 21.4. Ratings were consistently high (> 7) for all of the considered attributes at time zero, and decreased only relatively slightly over accelerated storage. These high ratings indicate widespread consumer acceptance among diverse panelists, and also indicate an ability to resist rapid degradation reactions that could compromise the product quality and consumer acceptance ratings of other foods during the abusive temperatures of accelerated storage.
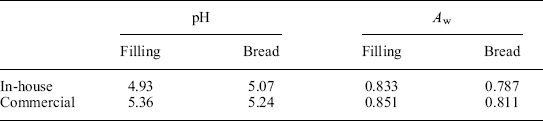
The variations in formulation between the in-house samples and the commercial manufacturer rendered some corresponding different characteristics in Aw and pH of the end-product. Table 21.5 shows the physical properties of the MFFT enrobed sandwich components, the modified bread formulation, and the moist, maple-flavored jelly/viscous liquid filling.
According to the written guides, each component of a non-potentially hazardous (non-sterile and non-refrigerated) food should have pH ≤ 4.6, have Aw ≤ 0.85, and not support the growth of infectious microorganisms like S. aureus. According to the growth/no-growth boundary line for S. aureus in IM pouch bread (Fig. 21.7), the Aw and pH values of the bread component (Table 21.5) place it significantly in the no-growth regime, indicating that S. aureus growth would not be supported. The filling has higher Aw and pH values slightly above the boundary line and in the growth domain. Noting from the formulation of the MFFT (Table 21.3) the comparable humectant content of the layers with respect to glycerol content suggests this similarity helps harmonize the Aw of the respective layers and mitigates moisture migration during storage to some extent. In these circumstances, however, the issue of whether moisture transfer from the filling to the bread is significant enough to create conditions in spatially inhomogeneous microdomains capable of supporting growth of S. aureus during the 3-year shelf life is still a plausible concern. Figure 21.8(a) shows a model bread-filling (barbecue chicken) interface, and Fig. 21.8(b) shows the equilibration of Aw between the filling and bread during storage (within 23 days, and faster at 120 °F ≈ 49°C than at room temperature).
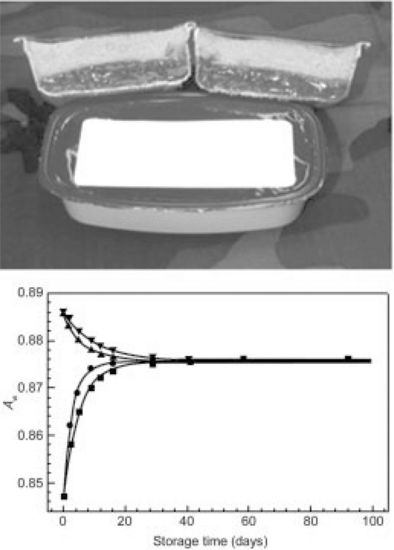
Fig. 21.8 Moisture migration from filling to bread in (a) model barbecue chicken sandwich, and (b) equilibration of Aw between the filling (A and ▼ at 120 °F ≈ 49 °C and room temperature, respectively) and bread (![]() and
and ![]() at 120 °F ≈ 49 °C and room temperature, respectively) during storage. Equilibration occurred within 23 days (faster at 120 °F ≈ 49 °C than at room temperature).
at 120 °F ≈ 49 °C and room temperature, respectively) during storage. Equilibration occurred within 23 days (faster at 120 °F ≈ 49 °C than at room temperature).
The MFFT microbial challenge test at two different temperatures (T = 25 °C and T = 35 °C) is a novel approach that used a 5-strain cocktail of S. aureus isolated from foodborne illnesses on two different MFFT formulations (in-house and commercial vendor) with slightly different physical properties of Aw and pH reflecting minor differences in some of the ingredients commonly used in manufacturing within the allowable constraints of military specifications. The temperatures selected are ambient conditions (T = 25 °C) required of the microbial challenge study and the optimal growth temperature for S. aureus (T = 35 °C), to determine if the optimal growth temperature expedites the challenge study results to a more convenient timeframe while still acting as a guarantor of food safety. The following critical factors were considered and handled as discussed below. Steps d, e, and f involved innovations particular to the microbial challenge study for MFFT, as detailed below.
a. Selection of challenge organisms. A 5-strain cocktail of S. aureus was selected to account for variability in strain responses from strains that were isolated from cases of foodborne illnesses, including S. aureus A-100, ATCC 14458, 993, ATCC 13567, and ATCC 27154.
b. Inoculum level. Samples were cut into 25 g squares and inoculated to 104 cfu/g (Fig. 21.2(b)).
c. Inoculum preparation. S. aureus cultures maintained on slants of tryptic soy agar supplemented with 0.5% yeast extract were transferred individually to trypticase soy broth and incubated for 18 h at 35 °C. The process was repeated for another 18 h, after which 0.3 mL of inoculum from the trypticase soy broth were spread-plated on Plate Count Agar (PCA) and incubated for 18 h at 35 °C. Cells were harvested from the PCA and suspended in Butterfield’s phosphate buffer to give a Klett54 reading of about 125–135 units (109 cells/mL). The cultures were mixed in equal proportions and diluted appropriately to produce an inoculum level of approximately 104 CFU/g.
d. Inoculation method. The inoculation method used was innovative for the MFFT. Samples were cut into 25 g squares and inoculated across the cut surface to include bread, filling and the bread/filling interface (see Fig. 21.2(b)). The sample was held in place inside a 400 mL sterile Stomacher bag during inoculation. To try and minimize the introduction of any local areas of higher Aw that could support the growth of the inoculum, the sandwich sample was hand-mixed to some extent to mix the inoculum into the filling, and the sample placed in a Stomacher for 2 min to flatten the sandwich sample.
e. Storage conditions. The storage conditions were varied in a unique manner for the MFFT. After mixing, the inoculated sample was rolled up in the stomacher bag and placed inside a standard trilaminate MRE pouch with a commercial oxygen scavenger, and sealed (not under vacuum). For comparative purposes, one sample set was vacuum-sealed without an oxygen absorber. Samples were held in independent temperature-controlled incubators set at T = 25 and 35 °C, respectively.
f. Duration of study. The microbial challenge study was unique for the MFFT to accommodate military shelf life requirements and requirements for conducting such studies (1–1.3 × product shelf-life, which is 3 years or 1095 days at T = 25 °C for military ration food items).
g. Sample analysis and enumeration. Samples were enumerated in duplicate. Series dilutions were made in Butterfield’s phosphate buffer and plated on Baird-Parker Agar supplemented with egg yolk-tellurite and incubated at 35 °C for 48 h. Uninoculated controls were assessed for Aw, pH, aerobic plate counts (APC), E. coli and Coliforms (EC), Yeasts and molds (YM), and inhabitant S. aureus.
h. Pass/fail evaluation criteria. Product passes if there is ≤ 1 log of growth for each sample by the endpoint. Product fails if growth > 1 log occurs for any sample at 2 time points or at the endpoint.
21.4 Results of the microbial challenge study
Over the period of study, uninoculated control samples were evaluated using standard microbiological quality control techniques for APCs, EC, YMs, and S. aureus. From days 0–270, the counts in all cases were very low and represented no potential hazard to the consumer (Table 21.6).
Table 21.6
Incidental microflora (APCs, EC, YMs, and S. aureus) in uninoculated control MFFT samples
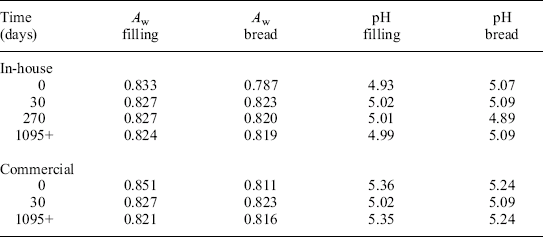
The two types of MFFT enrobed sandwiches (either made in-house or by a commercial manufacturer) were evaluated for changes in Aw and pH during 1095 days (3 years) of ambient storage (T = 25 °C). In both cases, the Aw and pH roughly equilibrated within the first 30 days of storage (Table 21.7). For the in-house sample, the Aw(filling) decreased from 0.833 to 0.827, while the Aw(bread) increased from 0.787 to 0.823. Similarly for the commercial sample, the Aw(filling) decreased from 0.851 to 0.827, while the Aw(bread) increased from 0.811 to 0.823. Similar trends and changes were observed with respect to the corresponding measured pH values of both samples.
MFFT sandwich samples inoculated to 104 cfu/g using a 5-strain cocktail of S. aureus and stored at T = 25 °C or T = 35 °C showed only inactivation kinetics, and counts fell below 250 cfu/g by 21 days until the end of the 3-year challenge study. An oxygen scavenger provided an additional hurdle, and the effects of packaging atmosphere were determined. No re-growth of S. aureus was observed over 3 years of storage. Temperature-dependent inactivation kinetics were observed in all cases, and the inactivation kinetics were evaluated using the Quasi-chemical model adapted for tailing.
21.4.1 MFFT challenge study (T = 25 °C)
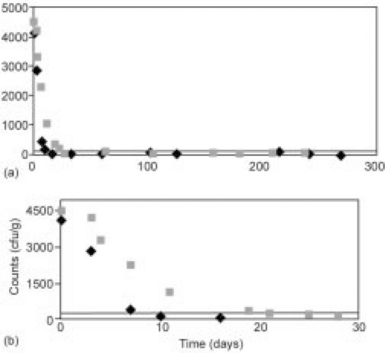
MFFT sandwich samples inoculated to 104 cfu/g using a 5-strain cocktail of S. aureus and stored at T = 25 °C showed inactivation kinetics that dropped below detectable limits within 12 days for all cases of in-house or commercially made samples. Figure 21.9(a) shows the death of the inoculum for both types of samples over 300 days, and Fig. 21.9(b) shows an expanded view of the first 30 days of evaluation.
21.4.2 MFFT challenge study (T = 35 °C)
Similar to inoculated samples stored at T = 25 °C, MFFT sandwich samples inoculated to 104 cfu/g using a 5-strain cocktail of S. aureus and stored at T = 35 °C also showed inactivation kinetics that fell below detectable limits within 5 days for all cases of in-house or commercially made samples, and samples stored without an oxygen scavenger as an additional hurdle (Fig. 21.10). Figure 21.10(a) shows the death of the inoculum for all three types of samples over 300 days, and Fig. 21.10(b) shows an expanded view of the first 10 days of evaluation.
21.4.3 The Quasi-chemical model
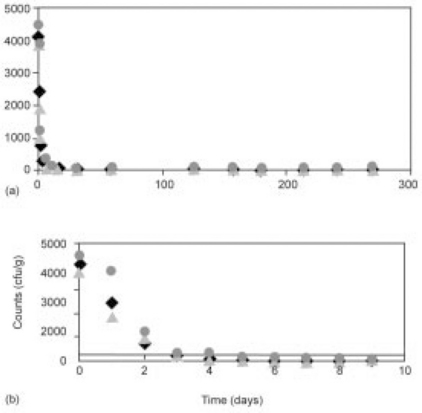
A modified 5-parameter version of the Quasi-chemical model adapted to describe tailing kinetics (see Table 21.1) was used to evaluate the inactivation kinetics of S. aureus in the MFFT samples, including those made in-house and commercially, stored at T = 25 and 35 °C, and those vacuum packaged or packaged with an oxygen scavenger. Figure 21.11 shows fits of the 5-parameter Quasi-chemical tailing model of the inoculated MFFT samples. The modeling results of the in-house MFFT sample indicates a low-level of S. aureus survivors persisting (below reliable detection limits), but otherwise indicates that the inoculum decayed toward a near-zero value. In the case of the commercial samples, after an initial slight shoulder, the data proceeded toward zero in a roughly linear fashion. By comparison, the linear (first-order) fit of the inactivation data of the in-house sample does not agree well with the data.

Fig. 21.11 Quasi-chemical model analysis of S. aureus inactivation kinetics at T = 25 °C for in-house (![]() , —) and commercially made samples (
, —) and commercially made samples (![]() , – –). The first-order model does not fit well the in-house data (
, – –). The first-order model does not fit well the in-house data (![]() , ·– · –).
, ·– · –).
Similarly, Fig. 21.12 shows fits of the inactivation kinetics data for in-house and commercially made MFFT samples with the 5-parameter Quasi-chemical tailing model. The Quasi-chemical model indicates a persistent, low-level population of S. aureus survivors significantly below the 250 cfu/g reliable detection limit for both the in-house and the commercially made samples. For comparative purposes, the linear (first-order) fit of the inactivation data of the in-house sample fit the data reasonably well, and estimated a time to reach 10 cfu/g fairly close to that of the Quasi-chemical model (7.25 and 6.5 days, respectively). In the case of the commercial MFFT samples, the Quasi-chemical model appeared relatively linear in fitting the fast-decaying inactivation data (time to reach 10 cfu/g was 8.5 days) with no initial slight shoulder in the fitted curve.
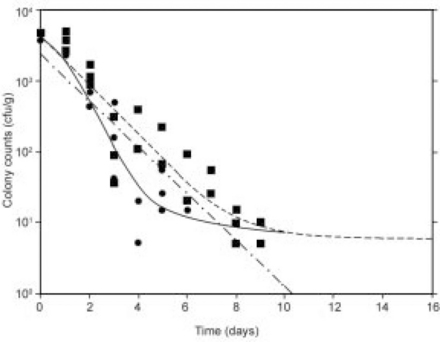
Fig. 21.12 Quasi-chemical model analysis of S. aureus inactivation kinetics at T = 35 °C for in-house (![]() , —) and commercially made samples (
, —) and commercially made samples (![]() , – –). The first-order model does not fit well the in-house data (
, – –). The first-order model does not fit well the in-house data (![]() ,·– · –).
,·– · –).
Figure 21.13 shows fits of the Quasi-chemical and linear model fits of S. aureus inactivation kinetics data in MFFT stored at T = 35 °C with vacuum packaging and no oxygen absorber (generally S. aureus grows more rapidly under aerobic than anaerobic conditions). The 5-parameter Quasi-chemical tailing model indicates a low-level ofpersistent S. aureus survivors significantly below the 250 cfu/g reliable detection limit, which is reached in less than 10 days. Even with this rapid decline in the inoculum, the linear (first-order) model does not fit the inactivation data well, mainly because the fit is distorted by the long tailing data determined during the course of this protracted study. In either case, however, the cumulative data (Figs 21.11, 21.12, and 21.13) clearly indicate that the MFFT breakfast sandwich product has high consumer acceptance and does not support the growth of S. aureus over the time course of prolonged 3-year storage at ambient temperature (T = 25 °C) or at an accelerated temperature of T = 35 °C.
21.5 Conclusions and future trends
MFFT enrobed IM bi-layered breakfast sandwiches receive high Hedonic ratings by consumer panels. The stability of the sandwich layers (physico-chemical and microbiological) are controlled by hurdles of relatively low Aw and pH, and although there were differences initially between the layers, Aw and pH converged to common values within the first 30 days of storage (Aw = 0.851, pH = 5.36). The Quasi-chemical secondary model (‘growth/no-growth boundary’) was helpful in determining suitable formulations to prevent S. aureus growth in IM bread-based products. Removing the O2 scavenger did not promote S. aureus growth. The novel 3-year microbial challenge study of MFFT enrobed IM sandwiches with S. aureus stored at T = 25 °C and at an accelerated temperature of T = 35 °C both demonstrated inactivation of S. aureus, with the rate of inactivation dependent on temperature. The rate of inactivation was more rapid at T = 35 °C (near the optimal growth temperature for S. aureus) than at T = 25 °C, and the inactivation kinetics were fit with the 5-parameter Quasi-chemical model. The 5-parameter variant of the Quasi-chemical model, while not fully mechanistic, provides a simple mathematical representation of potential cell recovery from sub-lethal injury that could render cells dead or non-culturable.
Challenging this product with S. aureus at 35 °C provided an expedient result that predicted the eventual outcome for this product stored for 3 years at 25 °C. This successful 3-year challenge study of S. aureus in MFFT provides a basis for standardizing future microbial challenge studies with new varieties of IM enrobed sandwiches in pipeline (Bacon-Cheddar Pocket, Cheese Bagels) designed as on-the-move, eat-out-of-hand products that feature high consumer acceptance. If this result could be generalized to similar types of enrobed IM foods, this microbial challenge test protocol could help accelerate the development of safe, wholesome, and nutritious minimally processed ration food items with tremendous potential savings in time, money, and labor. So the question of whether standardized microbiological challenge tests at accelerated temperatures (e.g., T = 35 °C) can provide effective bio-markers of Food Safety that save time, money, and labor while ensuring the safety new food items that need to satisfy extended shelf-life requirements of military, appears to have been answered in the affirmative, with the successful conclusion of the MFFT microbiological challenge study.
21.6 References
ABC NEWS–World News Tonight. Abc News, Inc, 22 May 2002.
BBC NEWS. Us military unveils ‘super sandwich’. Available from: http://news.bbc.co.uk/hi/english/world/americas/newsid_1923000/1923054.stm, 2002. [(accessed 11 April 2002)].
CNN.COM. Soldiers snack on 3-year sandwich. Available from: http://www.cnn.com/2002/Tech/science/04/11/us.food/index.html, 2002. [(accessed 11 April 2002)].
Cook, G., Army Develops Sandwich to Stand Test of Time. The Boston Globe, 2002 13 April 2002. Available from: http://www.boston.com/globe/
Doona, C.J., Feeherry, F.E., Ross, E.W. A Quasi-chemical model for the growth and death of microorganism in foods by non-thermal and high-pressure processing. International Journal of Food Microbiology. 2005; 100:21–32.
Doona, C.J., Ross, E.W., Feeherry, F.E. Comparing the Quasi-chemical and other models for the High Pressure Processing inactivation of Listeria monocytogenes. Acta Horticulturae. 2008; 802:351–357.
Dow Jones/Agence France Presse English. Over 1100 people ill after drinking Snow brand milk. Available from: http://www.plant.uoguelph.ca/safefood/archives/fsnet-archives, 2000. [(accessed 30 June 2000)].
Fabricant, F., Food Stuff; Hardtack gets a battlefield promotion. New York Times, 2002 1 May 2002. Available from: http://www.nytimes.com
Feeherry, F.E., Ross, E.W., Taub, I.A. Modeling the growth and death of bacteria in intermediate moisture foods. Acta Horticulturae. 2001; 566:123–128.
Feeherry, F.E., Doona, C.J., Taub, I.A. Effect of water activity on the growth kinetics of Staphylococcus aureus in ground bread crumb. Journal of Food Science. 2003; 68(3):982–987.
Feeherry, F.E., Doona, C.J., Ross, E.W. The Quasi-chemical kinetics models for the inactivation of microbial pathogens using High Pressure Processing. Acta Horticulturae. 2005; 674:245–251.
Gianuzzi, L., Contreras, E., Zaritzky, N. Modeling the aerobic growth and decline of Staphylococcus aureus as affected by pH and potassium sorbate concentration. Journal of Food Protection. 1999; 62(4):356–362.
Graham-Rowe, D. Us military creates indestructible sandwich. Available from: http://www.newscientist.com/news/print.jsp?id=ns99992151, 2002. [(accessed 10 April 2002)].
Jay, J.M. Modern Food Microbiology, 5th ed. New York: Chapman & Hall; 1996.
Karel, M. Recent research and development in the field of low-moisture and intermediate-moisture foods. Crc Critical Reviews in Food Technology. 1973; 3:329–373.
Leistner, L. Food Design by Hurdle Technology and Haccp. Kulmbach, Germany: Adalbert-Raps-Foundation; 1994.
Leistner, L., Rodel, W., Krispien, K. Microbiology of meat and meat products in high- and intermediate-moisture ranges. In: Lb Rockland, Stewart G.F., eds. Water Activity: Influences on Food Quality. New York: Academic Press; 1981:855–916.
Lotter, L.P., Leistner, L. Minimal water activity for enterotoxin A production and growth of Staphylococcus aureus. Applied and Environmental Microbiology. 1978; 36(2):377–380.
Mcmeekin, T.A., Olley, J.N., Ross, T., Ratkowsky, D.A. Predictive Microbiology: Theory and Application. Somerset: Research Studies Press Ltd; 1993.
NSF Internationa. Nsf/Ansi 75–2000, Non-potentially hazardous foods, 2000.
Powers, E.M., Briggs, J., Defao, A., Lee, C., Racicot, K., Richardson, M., Senecal, A., Wong, C. Effect of water activity on the microbiological stability of mobility- enhancing ration components, 1999. [Technical Report Natick/Tr-00/003. Us Army Soldier and Biological Chemical Command, Soldier Systems Center, Natick, Ma 01760–5018].
Rajkowski, K.T., Schultz, F., Negron, F., Dicello, A. Effect of water activity on the growth of Staphylococcus aureus at meat-cheese interfaces. Journal of Food Safety. 1994; 14:219–227.
Ross, E.W., Taub, I.A., Doona, C.J., Feeherry, F.E., Kustin, K. The mathematical properties of the Quasi-chemical model for microorganism growth-death kinetics in foods. International Journal of Food Microbiology. 2005; 99:157–171.
Swanson, K.M.J., L. monocytogenes Challenge Study ‘How To’ Guidelines June/July. Available from: Food Safety Magazine. 2005 http://www.foodsafetymagazine.com/article.asp?id=922&sub=sub1 [(accessed 17 April 2010)].
Taoukis, P.S., Richardson, M. Principles of intermediate-moisture foods and related technology. In: Barbosa-Canovas G.V., Fontana A.J., Schmidt S.J., Labuza T.P., eds. Water Activity in Foods: Fundamentals and Applications. Ames, IA: IFT Press–Blackwell Publishing; 2007:273–312.
Tatini, S.R. Influence of food-environments on growth of Staphylococcus aureus and production of various enterotoxins. Journal of Milk Food Technology. 1973; 36(11):559–563.
Taub, I.A., Feeherry, F.E., Ross, E.W., Kustin, K., Doona, C.J. A Quasi-chemical kinetics model for the growth and death of Staphylococcus aureus in intermediate moisture bread. Journal of Food Science. 2003; 68(8):2530–2537.
Us Department Of Health And Human Services, Food And Drug Administration, Microbiological Challenge Testing (updated June 18, 2009) Available from:. Evaluation and Definition of Potentially Hazardous Foods, A Report of the Institute of Food Technologists, 2001 http://www.fda.gov/Food/ScienceResearch/ResearchAreas/SafePracticesforFoodProcesses/ucm094154.htm [(accessed 17 April 2010)].
Yahoo! News. New Secret Weapon–the Indestructible Sandwich. Available from: http://story.news.yahoo.com/news?tmpl+story&u=/nm/20020411/od_nm/sandwiches_dc_1, 2002. [(accessed 11 April 2002)].