Developments in in-container retort technology: the Zinetec Shaka® process
Abstract:
This chapter covers the invention, development and commercialization of a novel in-container retorting system. The Shaka® process is one of the latest innovations in a long line stretching back to Appert. Shaka® retorts use vigorous horizontal agitation to mix the contents of the container and transfer heat rapidly from the surface throughout the contents which results in much faster sterilization than existing methods. Sterilization times of just a few minutes result in better tasting more nutritious foods. The process is suitable for a wide variety of food products including those containing particles and many different container types and sizes.
16.1 Introduction
This chapter covers a case study of the introduction of a new in-container retorting system from its inception, development and commercialization up to the point where production size retorts are available. It is, therefore, a little different from the majority of chapters in that it covers the development and commercialization of food producing equipment, rather than the food. Zinetec’s Shaka® process was conceived not through the forces of necessity but due the observation that the technology associated with in-container retorting had not changed in principle for very many years and so perhaps there was an opportunity to innovate and develop an improved system. Current retorting systems fall into four main categories, these are batch and continuous, and with or without agitation.
• Batch retorts without agitation (static) that are capable of sterilizing containers of food product at temperatures greater than 100 °C have been in existence since the middle of the 19th century. These early versions of retorts used steam as the heat transfer medium, as indeed do the majority today. Later developments included the use of water, steam/air, raining and spray water systems, all of which allowed the processing of containers that required counter pressure so enabling them to retain their shape and integrity.
• The Hydrostatic retort is the most common type of continuous cooker without agitation. It is generally used for cans where no counter pressure is required, though versions providing overpressure are available.
• Continuous retorts (cookers) with agitation were first introduced in 1920 by FMC in the form of the Sterilmatic. Agitation of the contents was induced by rolling the cans, thus shortening the process compared with static.
• Batch retorts with agitation were the latest of the four categories to be introduced. These systems generally use ‘end over end’ rotation and were introduced in the 1950s. It was found that this method of rotation was generally better than axial rotation as utilized in the Sterilmatic retort for inducing movement of the product within the container and further shortening process times. This was the last major innovation to provide a significant reduction of process times before the introduction of the Shaka® process.
Both the agitation methods described above suffer from the fundamental limitation that the forces employed to cause the movement of the product within the container are a balance between gravity and centrifugal force. As the speed the container rotates increases, the process time reduces until an optimum is reached when the headspace air bubble moves through the product to cause the maximum amount of mixing. As the speed of rotation is increased further, the process time lengthens until eventually the speed is sufficiently high that no mixing occurs and process times will be virtually the same as a static process.
Advances in retort control systems and other areas have enabled processes to be optimized, too, but this has not generally resulted in reductions in process times sufficient to allow the production of the significantly better products the market now demands.
The Shaka® process is fundamentally different in that it uses reciprocal agitation in addition to gravity. These reciprocal forces are typically two to three times that due to gravity and it is the use of the sum of these forces to cause considerably more movement of the product within the container and shorten the process. It is this much shorter process that enables the production of improved products.
16.2 The Shaka® process
16.2.1 Concept and initial development
If a can of food product in a steam-filled retort is considered, it is evident that there is plenty of thermal energy available on the inner surface of the can wall. The barrier to the flow of heat to the centre of the can is the food product, this barrier being greater with foods that transfer heat by conduction rather than convection. The only way to surmount this barrier is to improve the movement of the food product within the container by devising a better method of agitation.
A transparent model was made up of around 400 g capacity and filled with water (leaving a small headspace), and to this was added a few coloured plastic chips as markers. This model was agitated first by rolling to simulate axial rotation, and second by rotating end over end, to gauge the amount of mixing of the contents induced by the two existing methods. The model was then agitated in a variety of different ways, the simplest and what appeared to be as effective as any, was to place the jar on its side and reciprocate it back and forth with a stroke of around 100 mm. The movement of the water in the jar generated by this motion was far greater than by either of the existing methods of rotation. The agitating experiments were repeated using light coloured cooking oil in the model to simulate a thicker, more viscous, conduction style product again with chips of colored plastic to highlight the movement. The results were similar to those with water. It appeared obvious from the much greater movement of the product within the container that horizontal reciprocation may well increase the rate of heat transfer into the product and hence shorten the process time. This was confirmed when a can containing a thermocouple was reciprocated in a small retort, see Table 16.1.
Table 16.1
Static and rotary processing compared with reciprocation by hand

Retort temperature – 121 °C. Can – 73 × 110 mm. Product – 5% Bentonite. Headspace – 8 mm.
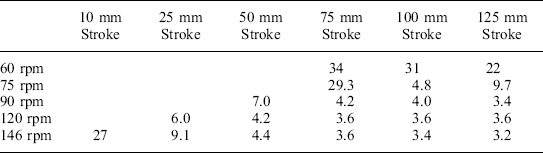
Bentonite is a commonly used product simulant, which, because of its inert nature, is able to be thermally processed repeatedly without its characteristics being changed. This allows the same test containers to be processed many times. As seen in Table 16.1 the heat up and cooling times were much faster by reciprocating than by either the static or rotary motions, and so it appeared sensible to motorize the drive system so that reciprocation could be investigated systematically. The motorized drive system consisted of a crank disc designed to give strokes from 10 to 125 mm and frequencies up to 146 rpm (the maximum speed of the motor). Experiments were carried out across the range of strokes and frequencies using Bentonite, as seen in Table 16.2. The results shown in Table 16.2 are the time in minutes from when steam is turned on to the retort until a thermocouple in the centre of the can containing Bentonite reaches 121 °C for a variety of agitation rates. If no agitation is used, the time taken to reach 121 °C is 62 minutes.
Table 16.2
Heat up time to 121 °C from steam on
Retort temperature – 127 °C. Can – 73 × 110 mm. Product – 5% Bentonite. Headspace – 8 mm. Retort come up time – 2.9 minutes.
The heat up times using the faster agitation rates confirmed the results found with hand agitation and demonstrated that this method of agitation appeared to have significant potential. Further experiments were carried out covering strokes up to 200 mm, different headspaces, thermocouple positions, can sizes and orientation and concentrations of Bentonite to simulate food products of varying thickness. The results of the experiments indicated the following:
• Longer strokes appeared advantageous with higher Bentonite concentrations and smaller headspaces.
• A headspace was necessary as with other agitating retorts to allow the product to move within the container. Sensitivity to variations in headspace did not appear to be too significant.
• Heat up time did not appear effected by position of thermocouple, though a variety of positions were used.
• Cans of 65 × 101, 73 × 110, and 99 × 119 mm showed surprisingly little difference in heat up time. With all three cans, the best orientation seemed to be for the cans to be lying on their sides and for the reciprocation to be along the longitudinal axis.
• Increasing the concentration of Bentonite caused the heat up rate to slow, but these initial experiments showed that this could be largely offset by increasing agitation rates or headspace.
A starch solution plus some real products were processed to ensure the effectiveness of this type of agitation was not limited to Bentonite.
In all cases, the process time by the Shaka® method to achieve a target lethality (Fo) was significantly lower than the corresponding static process time for the same sample type. From these results, there was good evidence to indicate that reciprocal agitation with the Shaka® system could dramatically reduce the process times across a range of food products (Table 16.3).
Table 16.3
Comparison of process times for variety of products
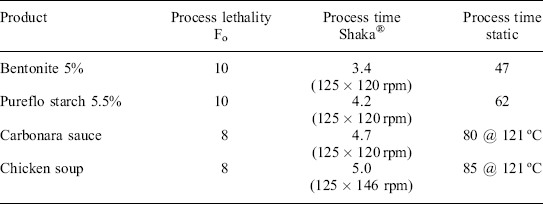
Process time – time in minutes from when retort reaches process temperature (127 °C unless stated) until process lethality reached. Retort come up time – 2.9 minutes. Can – 73 × 110 mm. Headspace – 12 mm.
Some preliminary microbiological challenge experiments were undertaken to see if the lethality of the processes determined by thermocouples and expressed as Fo were correct. Cans were filled with a nutrient broth, inoculated with spores of Bacillus Stearothermophilus to three different levels, and processed through the Shaka® retort to the appropriate Fo. The cans were then incubated and survivors recovered. The results showed good correlation between Fo and bacterial kill, although the number of test cans was small as the pilot retort would only process two cans at a time. At the end of this initial stage the following conclusions were drawn:
• The reciprocal agitation methods employed have resulted in substantial and consistent improvements in the heat transfer rates, as shown by the reduction in heat up times, compared with anything previously possible for in-can sterilization. In fact, the sterilization times attained with the reciprocal agitation methods were near those achieved with some types of ultra-high temperature (UHT) plants. It, therefore, appeared that there was some plausible likelihood of a simple in-can sterilization system that could virtually match UHT for product quality for conduction products.
Despite the simplicity of the concept, our initial literature and patent searches indicated the approach to be novel over prior-art, and a larger purpose retort was therefore constructed.
16.2.2 First Shaka® retort
Based on the work done on the prototype retort it was evident that a retort with the following features was required:
• Sufficiently large to hold at least 50 73 × 110 mm cans and have a holding system that held each container separate from its neighbour. The holding system needed to be flexible enough to accommodate a variety of container sizes and types.
• The drive has to be capable of strokes from 25–300 mm and rotational speeds up to about 250 rpm.
• The retort needed to vent and come up to temperature quickly, and have capability of spray or flood cooling.
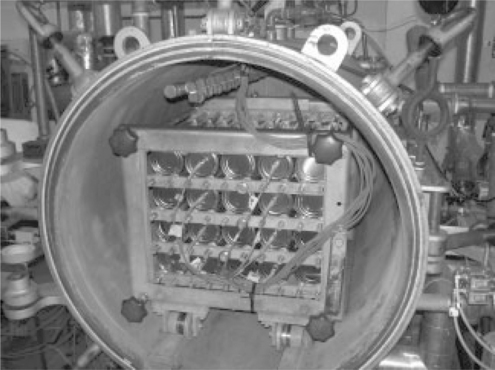
From the images shown in Figs 16.1 and 16.2, it can be seen that the reciprocating drive method adopted was a crank and slider mechanism driving a basket via a drive shaft entering the retort through a boss containing seals and bearings. The basket has flanged, railway style, wheels that run on rails.
16.2.3 Validation and development
After a few minor modifications the retort proved highly satisfactory with good heat distribution, venting in about a minute, and a come up time to reach 130 °C in about 1.5–2.0 min depending on load. Repeating the microbiological challenge experiments on a larger scale was possible with the new retort. The experiments covered two agitation conditions, two process temperatures, and two test organisms (B. stearothermophilus – TH24, and Clostridum sporogenes – PA3679) and consisted of a total of over 1100 inoculated cans. As can be seen from Table 16.4 there was good correlation between the lethality in the cans as measured by the thermocouples and that from the survival level of the test organisms.
Table 16.4
Microbiological challenge experiments comparing Fo and Fs.
| Thermal lethality (Fo) | Bacterial kill (Fs) | |
| B. Stearothermophilus | 12.6–13.2 | 13.0–18.1 |
| Cl. Sporogenes | 4.4–5.7 | 4.0–7.6 |
It was evident from these results that further systematic evaluation of this method of retorting covering the full range of conditions of which the new retort was capable was warranted. A series of experiments were undertaken using the most widely used can size, 73 × 110 mm, covering a range of products and headspaces. The products selected were different concentrations of Bentonite (5, 7, 8, 9, or 10% in water) and headspaces of 4, 8, or 12 mm (gross). All the concentrations of Bentonite selected were sufficient to produce a product that in a static retort would heat by conduction. After some experimentation the thermal treatment decided upon was to measure the time taken from when the steam is turned on to the retort to when a thermocouple in the centre of the can reaches 120 °C with the retort controller set to 130 °C. Once the test cans had all reached 120 °C, cooling commenced using water sprays, and the time measured for the thermocouples to reach 40 °C.
This test was carried out on the different products and headspaces across the range of strokes and frequencies available and tables of results drawn up of the form of Table 16.5 which is shown as an example. The table of results for the different variables showed initially a rapid fall in heat up time as the intensity of agitation increased, that eventually tended to level off, as indicated by a stable area with relatively little change. The intensity of agitation was increased by lengthening the stroke in steps from 25 mm to 300 mm and increasing the frequency of reciprocation within the range 40 rpm to 230 rpm.
Table 16.5
Heat up times to 120 °C from steam on for variety of agitation conditions: product – 8% bentonite; headspace – 12 mm; retort controller set point – 130 °C
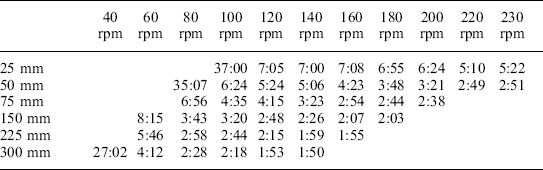
No agitation (static) – time to 120 °C = 50 minutes
The top row shows the revolutions per minute of the crank reciprocating the basket, the left-hand column gives the movement (stroke) of the basket in millimetres and the figures are the times in minutes and seconds to reach 120 °C.
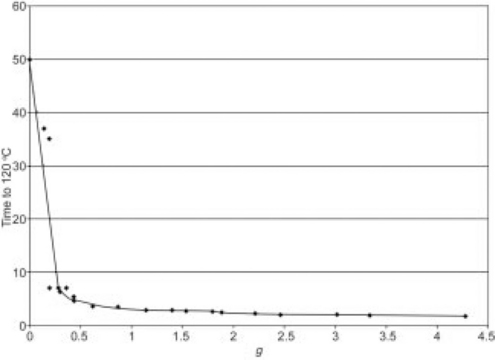
The intensity of agitation can conveniently be expressed as a single value by calculating the maximum acceleration for each combination of stroke length and frequency of reciprocation. A number of results from Table 16.5 were selected and their strokes and frequencies used to calculate the maximum acceleration. These have been plotted in Fig. 16.3, expressed as g (acceleration of gravity), versus heat up time to 120 °C. For further detail see the following equation:
where ω = angular velocity in radians, r = radius of crank (half stroke), l = length of connecting rod.
From Fig. 16.3 it will be seen that with this particular product and headspace combination there is a steep fall in the heat up time to around 0.5 g and that, once past 1.0 g, there is very little subsequent change. This area indicates that the process is stable, meaning that small variations in either the stroke or frequency will tend to have little effect on the rate of heating and hence the process time. With other combinations of product and headspace the form of the graph will be similar, although the position where the steep fall changes to the stable zone can move.
Table 16.6 is similar to Table 16.5, but these results are times for cooling to 40 °C. As can be seen, the results show the same type of pattern as seen for heating.
Table 16.6
Cooling times from 120 °C to 40 °C for a variety of agitation conditions: product – 7% bentonite; headspace – 12 mm
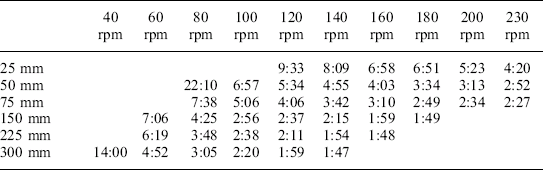
No agitation (static) – cooling time to 40 °C = 43.25 minutes
This work had provided sufficient understanding of the process for a patent to be drafted. As has already been mentioned, initial searches had revealed no prior art. However, subsequent searches uncovered a number of related patents, the most significant of which was a US patent from 1938 (Pat No. 2,134,817) that described an agitating retort which used reciprocation. Compared to the work described herein, it was evident that the intensity of agitation described in Frank Gerber’s 1938 patent would not have been sufficient to produce the reductions in heat up and process times generated by the Shaka® process. The granted patent (European Patent number EP0804095, US Patent number 5,857,312) covers reduction in heat up time and stability of the process. Four further patents on the Shaka® process have been applied for or granted covering further aspects of the process as well as process engineering and mechanical engineering aspects.
16.2.4 Food products
The most significant benefit of the Shaka® process is its ability to produce higher quality food products because of the shortened thermal process, which is especially applicable for conduction products where processing times in conventional retorts are the longest. There are some advantages with convection products, but they are not as significant as with conduction products.
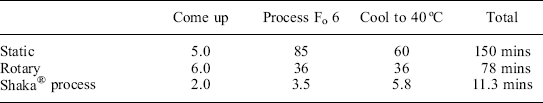
The results in Table 16.7 are for Béchamel sauce, a conduction product, in 73 × 110 mm cans processed in either static, end over end rotary, or Shaka® retorts. The rotary process conditions were optimized with regard to temperature and speed of rotation, 121 °C and 15 rpm. The optimal process being the one showing least colour change after processing. The same temperature was used for the static process. The conditions for the Shaka® process were 130 °C and 150 mm × 120 rpm agitation.
The differences in process times to achieve Fo = 6 are dramatic. The Shaka® process reduces process time by better than 20-fold compared with static, and better than 10-fold compared with rotary. The sauce resultant from the Skaka® method was still white, virtually indistinguishable from the unprocessed product, compared with the rotary retorted samples which were noticeably brown, and the static, which was an even darker brown, especially against the walls of the can. The reduction in process times seen in Table 16.7 are typical for many foodstuffs and have been seen across a wide range of products including soups, sauces, ready meals, baby foods, desserts, etc. The reduction in process time is partly due to the more efficient mixing produced by the Shaka® method of agitation and partly the higher temperatures that can be used in conjunction with the better mixing. In comparing process times for 121 °C and 130 °C, a little under half the improvement is due to the better agitation with the remainder due to the higher temperature that the better agitation facilitates. In the Béchamel Sauce example, slightly higher temperatures (125 °C) for the rotary process were tried as part of optimization. However, due to the more limited mixing that the rotary agitation provides, more burning occurred against the wall of the container than at 121 °C, and produced an inferior quality product even though the process time was shorter.
Considering now products containing particulates, the reduction in process times depends on the size of the particulate, as heat can only get into the centre of the particulate by conduction; the bigger the particulate, the longer the process. Obviously, with solid packs such as tuna or ham, there is no reduction in process time. From work done on the Shaka® process, the particulates of 3–4 mm diameter or less heat at the same rate as the carrier liquid, and so they make no difference on the process time. Larger particulates up to around 12 mm diameter may add a minute or so to the process time. However, if particulates are significantly bigger than this, it may mean that the process has to be lengthened to the extent that there is a danger of the carrier liquid (sauce) becoming overcooked together with the outer part of the particle.
Another factor to be considered with particulate products because of the vigorous agitation is possible damage to the particles. Process conditions, that is the time, temperature and intensity of agitation must take into account the nature of the particulate matter and the effect of the process on this nature. In other words, it is important to understand the intrinsic properties of the particulate before and after cooking, and how well does it withstand the agitation. For instance, uncooked potato is hard and quite tough, but on cooking becomes fragile and can easily disintegrate. For example, it has been found that a high level of agitation can be used through most of the heating phase while the potato pieces are still quite firm, but, to prevent damage through the latter part of heating and through cooling when the particles are most fragile, the intensity of agitation should be reduced. By this means damage was largely avoided.
The Shaka® process has demonstrated significant advantages in quality across a very wide range of particulate and homogeneous products compared with the existing methods of static and rotary processing and can match UHT in some areas as has already been mentioned.
When the cook values products received from the Shaka® process were compared to those from UHT systems using scraped surface exchangers similar values were found. In the comparison the total cook values received during the heating, holding and cooling stages were calculated. As the cook values were similar product quality from the two systems should also be similar. A comparative study carried out across a range of products confirmed this to be the case.
16.2.5 Container types and sizes
As will have been noted, most of the initial work on the Shaka® system used 73 × 110 mm cans, the most common retail size. However, as part of the commercialization of the process it was necessary to be able to process a variety of other containers and container sizes, including larger cans, glass jars, plastic containers, and pouches, the majority of these needing counterpressure during sterilization. To accommodate these types of containers, the Shaka® retort was therefore converted so that not only saturated steam, but steam/air mixtures could also be used where counterpressure was required, the movement of the basket being used to mix the steam and air. Modifications were also made to the cooling system so that two-stage cooling was possible using hot water for the first stage to prevent the cracking of glass containers due to thermal shock.
Large cans (153 × 178 mm or similar) proved the easiest, held separately and on their sides as with the smaller cans these processed well at 130 °C using steam and standard pressure cooling. With homogeneous conduction products or products containing small particulates, process times were typically about 10 min to achieve Fo = 8–10, compared to 3–4 h in a static retort, with enormous improvements in product quality.
A variety of sizes of glass container have been successfully processed with both push-on and screw-on lids using steam/air and two-stage cooling. The jars were all significantly taller than their diameter, so were all process on their sides and shaken along their long axis. It was found that process times were 50–100% longer than for cans due to the lower thermal conductivity of glass. Jars with larger diameter lids for a given volume also processed faster.
Plastic containers with both heat seal closures and double seamed ends have been processed. The heat seal containers were all shallow trays and were processed with the closure uppermost, the tray being supported by its flange in a plate with suitable apertures to take the body of the container and shaken along the longer axis. Processing was carried out using steam/air and spray cooling with the selection of the agitation rate and control of the overpressure being fairly critical to prevent distortion of the containers. Again, due to the poor conductivity of plastic compared with metal, the process times were somewhat longer.
Most of the work on double seamed plastic containers was with 400 g bowls closed with 99 mm diameter an aluminium easy-open end. By experimentation, it was found that processing was best carried out with the double seamed end uppermost and agitation in line with the end. Again, processing was carried out with steam/air mix and spray water cooling, and control of the overpressure was found not to be so critical with these containers. From Table 16.8 the effect of the lower conductivity of plastic compared with metal can be seen.
Table 16.8
Comparative sterilization times: 99 mm diameter plastic bowl and metal can. Product – 7% bentonite; process temperature – 130 °C; agitation – 150 mm × 150 rpm

Both stand up and pillow style pouches have been processed using steam/air and spray water cooling. As would be expected, the direction of agitation is along the longer axis with the pouches lying on their backs. To achieve undamaged pouches at the end of processing it is vital not only to select the correct agitation rate and overpressure profile but the pouches must also be held in custom-made racks in which each pouch is securely located.
16.2.6 Critical factors and determinination of process conditions
For the Shaka® process, the critical factors specifically associated with an agitating process are the same as for rotary retorting – fill weight/headspace, product consistency (viscosity), solid–liquid ratio, particulate size, agitation rate, and container orientation. In determining the thermal process required, the variability of all of these factors must be known in order to assess the worst case conditions that are used when the production process is determined.
If the Shaka® and rotary processes are compared, variations in some of the above critical factors will have a greater effect on one method and some on the other. For instance, because of the stable nature of the Shaka® process (Fig. 16.3) variations in the agitation rate will probably have less effect than with the rotary process; however, variations in the fill weight/headspace and the product consistency may have a greater effect. Rotary processing was carried out safely for many years before the advent of fillers of the accuracy of those available today or inline check weighers, viscometers, etc., and sophisticated data analysis by computer. A modern filling line should, therefore, be able to produce filled and sealed containers of sufficient consistency that not only assures safety, but that the safety margin required is such that the quality of the product is in no way compromised.
Before determination of the thermal process it is firstly necessary to decide upon the container orientation, fill weight and agitation rate. The most appropriate orientation of the container to achieve the best mixing at the lowest rates of agitation may be obvious, if the container has one axis significantly longer than any other. If this is not the case the best orientation can only be determined by experimentation. A number of factors will influence the decision as to what fill weight to use but the maximum fill weight (minimum headspace) for a particular container size will be determined by the requirements of the process. The Shaka® process, along with all agitating processes, requires a headspace to allow the product to move within the container. The minimum required will depend on the nature of the product, type of container and agitation rate. For homogeneous products, the agitation rate selected will depend largely on the thickness of the product and minimum headspace, but it will generally be on the high side so as to facilitate the shortest process. However, if the product contains particulates, then the nature of the particulate will need to be considered. Delicate particulates such as many vegetables when cooked can be damaged if agitation rates are too high, so the rate chosen will have to be determined by experiment and, as has already been mentioned, may have to be varied through the process so as to get the best balance between the length of the process and particulate damage. Having decided upon the container orientation, fill weight and agitation rate the presence of a ‘cold spot’ must be considered. With the Shaka® process, because of the good mixing induced by the type and intensity of agitation employed, all the contents of the container heat evenly and so, generally, there is no ‘cold spot’. This cannot be assumed to be the case without confirmatory data.
Finally it is necessary to decide on the process temperature to be used. The Shaka® process generally uses higher temperatures than more conventional retorts, with temperatures of around 130 °C being common. However, the main arbiter will be product quality and this should be product quality determined on containers filled to worst case conditions and using real-time heat penetration to determine the process. The method of carrying out a heat penetration determination is fairly straightforward as conventional equipment can be used as long as the thermocouples are thin. (1.5–2.0 mm diameter) The only difference is that the leads to the thermocouples must be arranged to allow for the movement of the containers and the data from the thermocouples needs to be recorded at least every 2 seconds because of the speed of the process. Temperature loggers could also be used such as Ellab’s TrackSense or Mesa Labs DataTrace though they are not ideal as data are not real time.
The method used to determine process times in particulate products are similar to those used for rotary processing. To determine the rate of heat penetration the largest particle from the product is impaled with the thermocouple. The particulate will almost certainly need to be held onto the thermocouple in some way to prevent it from being displaced by the agitation. It has been found with products containing dry particulate matter, such as pasta, that because of the shortness of the process it is possible they are not fully re-hydrated during the heating phase of the process. It is therefore vital to check that this is not the case or sterility will be compromised.
16.3 Product quality and the Shaka® process
The major advantage of the Shaka® process is in its ability to produce better quality food products. This has been demonstrated with soups, sauces, ready meals, spreads, dips, desserts, beverages, chopped vegetables, baby food and pet food. The step increase in quality that the Shaka® process can produce is likely to have major effects on the food market. For example, it gives packers in the ambient shelf stable sector the opportunity to produce a wider range of higher value products with better flavour, colour and texture. The much lower thermal burden imposed on products by the Shaka® process means that added flavour enhancers such as salt and artificial colours can likely be reduced or eliminated. There are also indications that the same applies to stabilizers, emulsifiers and modified starches leading overall to products with much ‘cleaner labels’.
The improvement in quality seen is often such that products produced by the Shaka® process can compete with similar products produced by freezing, aseptic processing, or for distribution under chill. This gives producers new opportunities and the consumer increased choice, and it will be especially welcome in countries where chill and frozen distribution are not as well developed as they are, for instance, as in western Europe. This can also help address important environmental issues and the necessity to control global warming. Walmart, Tesco, Sainsburys, and Marks and Spencer have all announced their commitment to reducing carbon footprints, some aiming to be carbon neutral by 2012. Shaka® process ambient preserved foods are considerably ‘greener’ than both chill and frozen, chill mainly due to the very high levels of wastage caused by the short shelf life and frozen due to the energy needed to freeze and keep frozen. This should provide extra incentive to grow Shaka® processed foods at the expense of chilled and frozen.
Rising levels of affluence created, to a degree, by working longer hours and both partners in the family earning and so having less free time, have generated demand for pre-prepared premium foods, a major growth area for the supermarkets. This sector does not seem as price sensitive as the commodity area, for instance, in the UK a pack of premium soup often costs five or more times the supermarkets’ cheapest offering. Therefore, if products of appropriate quality can be produced, there will be benefits for the environment, the packer, and the consumer.
16.4 Commercialization of the Shaka® process
It is evident from the data above that the Shaka® process had significant benefits over the existing methods of in-container sterilizing, and these benefits were available via what appeared to be a relatively easy low-tech route; namely, essentially a standard retort with a relatively simple agitation device added. Therefore the decision to try and commercialize the Shaka® process was easy. A business plan was drawn up and discussed below are a few of the more important issues in the plan.
The first decision for Zinetec was how to proceed. Possible routes were to manufacture the retorts ourselves or in partnership. Or, as it was a patented process, license the technology. The decision was relatively straightforward, as we had neither the expertise or financial resources to manufacture retorts either alone or in partnership. Having decided on the licensing route, it was necessary to determine the funds and expertise required.
With regard to funding, Zinetec, being a small company, did some research in the small business community in the UK. This clearly showed that if it was possible to self-fund from existing income streams, this mode was preferable. Other small businesses’ experiences with banks, business angles, etc., at this early stage in the development of a new product or process, where timescales and cash flows are extremely difficult to predict, were mixed to say the least.
The expertise needed was in licensing/technology transfer and in patenting. There are a number of mainly small companies operating in the licensing/technology transfer area, but because of Zinetec’s decision to self-fund the Shaka® project, at least initially, from existing funds, it was necessary to find one that would come into partnership for a percentage of future earnings from the project. The factors in selecting a partner were what percentage they would require for their commitment to the project, and, at a personal level, how well both companies got along, we were likely to be working together closely for a significant period. Fortunately, we managed to find a company that more than satisfied these requirements.
The main Shaka® process patent was in place, but Zinetec had a number of complementary ideas it wished to patent. It was vital to see if the costs of drawing up patents could be reduced. Large companies in the bigger cities charged significantly more than small out of town companies. A small local company was selected which proved entirely satisfactory at a fraction of the cost. An additional benefit is that in always dealing with the same attorney, he builds up a good knowledge of the process enabling the writing of subsequent patents to be done more quickly and at less cost.
The commercial strategy developed with our licensing/technology transfer partner was to license separately the food packers to use the process and suitable retort manufacturers to produce the equipment. Within this overall strategy, ways were planned in which activities could bring income to the project before full commercialization occurred. These included charging for packing trials, royalties from sale and rent of pilot retorts, development licences, etc. The income was not large, but it was vital in making the project affordable.
It seemed evident that in order to interest the retort manufacturers, it was first necessary to interest some of the food packers. The major food manufacturers therefore became the prime target with the initial aim of persuading them to undertake trials on the Shaka® retort being installed at CCFRA (now Campden BRI). The retort installed at CCFRA was the one used for the majority of the trials described above. Once the interest of a number of food companies had been established, a number of retort manufacturers were approached and three offered licences to manufacture Shaka® process retorts. Three were selected, as it gives food manufacturing customers a reasonable choice of retorts, provides a certain amount of competition, and, at the same time, gives each retort producer a potentially valuable market share to justify its initial design, building, testing, and marketing investment. The three selected were Steriflow (France), Satori (Germany), and Allpax (USA). The retort manufacturers introduced their first pilot machines in 2006 with full-scale production machines planned for 2008 and 2009. Unfortunately Satori became victims of the recession and ceased trading in 2009.
The interest of the major food manufacturers was aroused not only by the ability of the process to produce quality products, but also by its simplicity and low cost, especially compared with UHT/Aseptic systems. The cost of Shaka® retorts for a given rate of production should be less due to a Shaka® retort being able to process many times faster. Therefore a Shaka® retort of similar size to a rotary retort may cost more but has a much higher throughput. The ability of the Shaka® process to produce many batches per hour means that the handling of the baskets of containers in and out of the retorts will need to be much faster than was necessary with conventional retorts to maximize throughput. The simplicity of the Shaka® process means that installation, commissioning and training costs will be little higher than for other batch retorts and as it is only a retort it should be compatible with the rest of existing filling lines. Recent trials have shown, somewhat surprisingly, that Shaka® retorts can be more energy efficient than many of the existing types of batch retorts. It had always been thought that small savings were likely but this recent work has shown significant savings are possible.
The process opens up a number of marketing opportunities including, as already mentioned, the possibility to compete with chill and frozen products in some product areas, the ability to make products previously not possible by in-container sterilization, the chance to significantly upgrade existing products where appropriate, and, perhaps for the first time, produce premium products in the Food Service sector. A number of licences have already been signed with food companies in both Europe and the USA to allow them to explore the technology with commercial licences to produce products, hopefully, to follow. If the Shaka process is the success it looks like, though the current world recession has delayed its introduction, a large number of factors will have contributed. Notably, a good and close working relationship with our licensing/technology transfer partner and with CCFRA together with a fair measure of luck.
16.5 Future trends
With the growth of the premium sector expected to continue, the market potential for better quality ambient preserved foods is very large. Fresher tasting, better looking, value-added ambient foods, produced at cost levels comparable with current ambient foods, is the strongest ‘driver’ for the technology. However, until recently, a major constraint was the lack of Shaka® production retorts to make this development work worthwhile. During 2007 and 2008 we have seen the rent and sale of pilot retorts to many of the most sophisticated food companies in the world. The sale and rent of pilot retorts has continued with production machines now also available. Zinetec are anticipating that 2010/11 should see rapid commercialization with the first food products appearing on the market. However, in the present economic climate predicting the future is even more problematic than normal.
In the slightly longer term, if predictions for global warming are accurate, ‘green’ issues will become more important. Shaka® retorts can achieve significant energy savings compared with many existing retorts. Additionally, Shaka® thermally sterilized long shelf life ambient stored products score well compared with chill and frozen foods. Together, these concomitant improvements in energy efficiency and food quality of the Shaka® process could force the market in this direction.
In the two largest countries in terms of population, China and India, together with other parts of what is currently the developing world, it is doubtful whether widespread chill and frozen distribution and storage will exist in the foreseeable future. The reason will not only be environmental pressure, but also the high cost of setting up the infrastructure and geography – the size of the countries concerned. It is noticeable that chill products make up a much smaller proportion of supermarket space in the USA than in Western Europe where the countries are small with high population densities making the distribution of short shelf life chill products more viable. Therefore, in these emerging countries the food market could be very largely split between fresh and long shelf life ambient storage including thermally processed products with the Shaka® process having a significant role.