Development of Second-Generation Biorefineries
H. Stichnothe1, H. Storz1, D. Meier2, I. de Bari3 and S. Thomas4, 1Thünen Institute of Agricultural Technology, Braunschweig, Germany, 2Thünen Institute of Wood Research, Hamburg, Germany, 3Division of Bioenergy, Biorefinery and Green Chemistry, ENEA Centro Ricerche Trisaia, Policoro, Italy, 4US Department of Energy, Bioenergy Technologies Office, Golden, CO, United States
Abstract
A wide range of nonfood biomass and conversion technologies can be used for the production of bioenergy and biobased products. The fermentation of lignocellulosic-derived sugars and the thermochemical conversion of biomass (eg, fast pyrolysis) are examples of relevant conversion technologies. The main product of fast pyrolysis is bio-oil, which can be used directly in stationary boilers or after upgrading as a drop-in blend component in existing refineries. Bio-oil requires chemical upgrading, before it is suitable as fuel. The commercial use of bio-oil for material/chemical purposes is currently limited to minor food uses (ie, smoke aroma and flavor enhancers). Different pretreatment technologies can be used in the initial conversion of biomass to sugars for fermentation. Technical obstacles in those pretreatment processes differ among the various approaches, but can include insufficient separation of cellulose and lignin, formation of byproducts that inhibit downstream fermentation, high use of chemicals and/or energy, as well as high costs for cellulase enzymes, although the latter has decreased substantially in recent years. There is currently no consensus on a preferred pretreatment method or combination of methods. A wide range of biofuels and biobased chemicals can be produced from sugars via fermentation and/or chemical conversion, including advanced biofuels and chemical intermediates. The integration of different pretreatment and conversion technologies in biorefineries can maximize the use of biomass components and improve the efficiency of the entire value chain. In the mid- to long term, thermochemical and biochemical conversion of lignocellulosic biomass are promising technologies for the production of biofuels and biobased chemicals.
Keywords
Biorefinery; fast pyrolysis; fermentation; pretreatment; bio-oil; biobased chemicals
2.1 Introduction
The use of conventional crops for biofuels and bioenergy is controversial due to direct and indirect land use change perceptions. There are additional concerns, including increases in crop and food prices in developing countries caused by competition for land (Schmidhuber, 2007; Fargione et al., 2008; Searchinger et al., 2008; Bringezu, 2009; Kim et al., 2009, 2012; Dale and Kim, 2011; Kim and Dale, 2011; Kline et al., 2011; O’Hare et al., 2011; Bringezu et al., 2012; Broch et al., 2013). Therefore, the use of nonfood biomass for the production of bioenergy and biobased chemicals is preferred.
A wide range of nonfood biomass and conversion technologies can be used for the production of bioenergy and biobased products. The availability of nonfood biomass is dependent on a number of regional factors. The amount of organic waste and processing residues (eg, from the food industry) depends on the livestock density, and the type and scale of the food industry. In Europe the organic waste and residue availability for bioenergy production is estimated to be in the range of 550 million tonnes1 per year (t/year), of which 375 million t (~68%) are agricultural residues (Searle and Malins, 2014). The amount of surplus agricultural residues in India is approximately 235 million t/year (Hiloidhari et al., 2014) and in China 500 million t/year (Jiang et al., 2012). In the United States (US), primary and secondary agricultural residues are conservatively estimated to be 240 million dry t/year in 2030 at a farm gate price of $66 per metric t or 114 million dry t/year at a price of $40 per metric t (US-DOE et al., 2011). However, the amount of potentially harvestable residues must always take into account the need to avoid any depletion of soil organic carbon stocks (Montanarella and Vargas, 2012). Primary and secondary forest residues from non-Federal land in the US are projected to be at 86 million dry t/year in 2030 at a forest landing price of $66 per dry t or 72 million dry t/year at a price of $40 per t (US-DOE et al., 2011). Dedicated woody and herbaceous energy crops are also useful resources for biorefineries and approximately 342 million dry t/year are projected to be available in the US in 2030 at a farm gate price of $66 per t or 30 million dry t/year at a price of $40 per t (US-DOE et al., 2011). The high cost of establishment of these perennial crops and the lack of a harvestable crop in the first year or two requires a higher farm gate price in order for farmers to be able to make money growing these crops.
The focus of this chapter is mainly on technologies that enable the conversion of nonfood biomass into value-added products. We attempt to identify the most promising technology options and/or combinations for biomass processing.
2.2 Technology and Feedstock Matrix
Fig. 2.12 identifies technologies that facilitate the conversion of a variety of biomass materials to value-added products. However, the main focus here is on the conversion of woody and herbaceous lignocellulosic (nonfood) materials, including agricultural and forestry primary and secondary residues, dedicated energy crops, and the organic fraction of municipal solid waste. Oils and fats are important feedstock of the chemical industry in the past and in the present. Many newly produced biobased chemicals are from sugars and due to the food–fuel debate lignocellulosic-derived sugars (also called second-generation sugars) have recently gained much attention. Thus, this chapter will be focused on the process and technologies for the production of cheap and clean second-generation sugars.
As Fig. 2.1 indicates, there are many process technology options by which a variety of edible and nonedible biomass materials can be converted into useful bioenergy, fuels, and chemicals. The production of heat and power (eg, combustion in district heating systems, waste incineration, or large-scale cocombustion) are mature technologies and thus not discussed here. Anaerobic digestion (AD) and composting (indicated in gray boxes in Fig. 2.1) are briefly described as they are relevant to agricultural residue treatment, the recycling of nutrients, and thus sustainable land management. The main focus of this chapter is the conversion of biomass into value-added products via new or emerging technologies that are indicated by black boxes in Fig. 2.1.
2.2.1 Composting and Anaerobic Digestion
Composting is a biological process in which organic waste such as food waste, manure, garden waste, trimmings, etc., are converted into a humus-like substance by microorganisms under aerobic conditions. Composting is a robust, low-cost, and low-tech option that can handle a variety of organic wastes varying in composition and moisture content. There are three major designs used in composting: static piles, vessels, and windrows. Windrow composting is usually considered the most cost-effective composting option. The organic material is layered into long piles and thoroughly mixed by turning machines. The temperature within windrows can reach 65°C; hence composting can also act as a biological drying process. Therefore, composting is frequently used in combination with biogas production in areas with high livestock density, where surplus nutrients cause considerable problems (Luo et al., 2013). Compost is used as a soil amendment and slow-release organic fertilizer. During the composting process, a large part of the carbon in the organic waste feedstock is metabolized to carbon dioxide, which is usually not captured for recycle into a useful bioproduct, but it provides a low-tech method to reduce the volume of waste streams and provide a useful product.
Compost is one of the best sources of stable organic matter from which new humus can be formed in degraded soils. An estimated 45% of European soils have low organic matter content, principally in southern Europe, but also in areas of France, the UK, and Germany. Standards on the use and quality of compost exist in most countries (eg, Organic Farming Regulation or eco-labels for soil improvers). The standards differ substantially, partly due to differences in national and international soil policies.
In AD processes, organic material is converted in the absence of oxygen into raw biogas (methane plus carbon dioxide) and unmetabolized solids, or digestate, which can be used as fertilizer. After purification, the methane component of biogas (ie, biomethane) is equivalent in energy content to fossil natural gas, and can be used as fuel or as a source of biobased hydrogen or syngas. The carbon dioxide component rejected during the biomethane purification process is generally exhausted to the atmosphere and not captured for reuse. Codigestion facilities are typically agricultural anaerobic digesters that simultaneously accept two or more input materials. Feedstock for AD can include a range of organic materials, including biodegradable waste, such as grass clippings, food waste, sewage, and manure. Anaerobic digesters can also be fed with dedicated energy crops, such as Silphium perfoliatum or silage maize, for biogas production, although this is generally considered to be controversial due to land use issues. Lignocellulosic biomass is not the preferred feedstock for AD, because most anaerobes are unable to degrade lignin. However, the carbohydrate content of plant cell walls is generally useable by AD microbial consortia.
2.2.2 Preprocessing Technologies
Important issues in the handling and delivery of biomass include low bulk and energy densities and instability in storage, that necessitate conditioning or preprocessing operations prior to introduction into a conversion process. In addition, biomass delivered to the conversion process must possess physical and chemical properties that fall within the design specifications of the biomass conversion process, which vary from one conversion process to another. Preprocessing technologies of potential interest include—but are not necessarily limited to—operations such as drying, milling, fractionation and separation, blending, densification, and torrefaction.
2.2.2.1 Basic Biomass Preprocessing Methods
Chemical-physical properties of interest include moisture, ash, carbohydrates, lignin, and energy content, among others. Moisture in biomass presents problems from several points of view. Firstly, moisture content above 30% (by weight) permits microbial growth in aerobic and anaerobic conditions, which can result in significant mass losses (“shrinkage”) and quality degradation over time in storage. Secondly, moisture in biomass contributes to the weight of material that must be transported from the field to the storage facility or biorefinery, which incurs undesirable transport costs. In addition, most thermochemical and combustion conversion processes prefer very dry (~10% moisture) feedstock materials. The moisture content of herbaceous materials is managed with established agronomic practices, which include chopping and drying crop residues, such as corn stover and wheat straw, in the field using solar radiation, or through natural senescence processes in perennial species, such as switchgrass and miscanthus.
Tropical species, such as energycane, and annual species, such as sweet and biomass sorghum types, that do not naturally senesce and dry in the field may require a just-in-time harvest and delivery strategy, such as that which the sugarcane industry employs today around the world. Woody species, such as hybrid poplar, shrub willow, eucalyptus, and loblolly pine are typically harvested green and usually have a moisture content around 50% at harvest. Partial drying of whole trees in the field after felling is a possibility for some woody species, depending on the harvest strategy. Mechanical drying may be necessary in some cases to preserve biomass quantity and quality in storage, and also to prevent catastrophic spontaneous combustion events due to heating due to microbial activity, but it is extremely energy-intensive, and therefore expensive.
Ash content can also present problems for the biomass conversion industry. In general, ash content should be as low as possible for any conversion process, and therefore harvest, collection, and storage methods should take care not to introduce additional ash from soil contamination. Debarked wood can be very low in ash content (eg, <1% by dry weight). Bark contains significant ash and is also high in lignin, so significantly increases the ash content of whole stem wood. Harvested trees that are skidded to a landing prior to chipping or loading onto trucks can acquire additional ash through contamination by soil. By contrast, very clean herbaceous materials typically contain 4–7% ash, which is significantly higher than clean wood chips, and makes them more suitable for biochemical conversion processes.
As a result of their low ash content and higher energy content, clean, dry wood chips are generally preferred for thermochemical conversion processes over any herbaceous feedstock. Mineral ash contributes nothing to any biomass conversion process yield other than a potential fertilizer coproduct, and generally tends to interfere with conversion processes by reducing yield per tonne of input biomass. The ash content of herbaceous materials can also significantly increase abrasive wear in the moving parts of processing machinery. In thermochemical processes ash can cause slagging in thermochemical reactors and fouling of downstream chemical catalysts. Some mineral species are worse than others in thermochemical processes. In extreme situations, some sort of mechanical or chemical preprocessing step (ie, sieving; washing) may be warranted to reduce the ash content of feedstock materials.
High carbohydrate content is especially important in biochemical conversion processes, as all biofuels and bioproducts are derived from the sugars in cell wall carbohydrates. Due to the oxygen-rich composition of carbohydrates, they have a lower energy content per unit mass than lignin, and are less desirable feedstock components for thermochemical conversion processes.
High lignin content is especially important for thermochemical conversion processes. Lignin has a higher energy content per unit mass than carbohydrate because it is largely comprised of energy-rich aromatic rings and has a lower oxygen content than carbohydrate.
Examples of relevant physical properties include biomass particle size and shape, particle size distribution, fibrous nature, and density. A nominal particle dimension is a typical specification for feedstocks entering a biomass conversion process. A variety of milling processes can be used to achieve this specification, including hammer milling, knife milling, ball milling, each of which has its advantages and disadvantages, depending on downstream needs. A narrow size distribution range is generally more desirable than a broad size distribution profile around the specified nominal dimension. However, a narrow size distribution can be difficult to achieve, given the mechanical properties (brittleness versus elasticity) of biomass materials, and the relative crudeness of most industrial milling processes. In particular, very fine and very coarse particle size fractions are to be avoided, but for different reasons. Larger particles can occlude openings in solid and slurry storage and conveyance systems, while very fine particles tend to settle as sludge once exposed to aqueous conditions. Both of these outcomes can cause process interruptions and considerable downtime. Sieves can be used to fractionate milled materials to remove fines, as well as oversized particles, if necessary.
Recent investigations demonstrate that particle sizing can impact the economics of the production of second-generation sugars via pretreatment and enzymatic hydrolysis. Among the available pretreatment technologies, thermochemical pretreatments have exhibited a wide range of particle sizes below which no increase in pretreatment effectiveness was observed from <0.15 to 50 (Vidal et al., 2011). However, the optimal particle size varies for different combinations of pretreatment technologies and feedstock.
2.2.2.2 Densification and Thermal Pretreatment
Highly fibrous materials tend to be difficult to transport and convey, due to their tendency to tangle, resulting in bridging and blockage of orifices and passageways. Densification of biomass is an option to overcome those obstacles. Densification processes include pelletization and briquetting, which transform loose, fluffy biomass into reasonably uniform (in size, shape, and density), smooth materials that flow evenly and well in standard storage and conveyance equipment.
The combined use of torrefaction and pelletization presents an opportunity to improve stability of biomass in storage, reduce transportation costs, and improve the efficiency of handling operations. During torrefaction, biomass is heated to approximately 200°C in order to reduce and/or remove its water content and drive off volatile compounds. Torrefaction typically reduces the energy content of the torrefied material to approximately 90% of the original material (ie, about 10% of the original biomass is lost during torrefaction). The resulting torrefied material is porous, which provides an opportunity for undesirable moisture uptake, so densification via pelletization is an attractive option.
2.2.3 Pretreatment: Physical, Chemical, and Biochemical
In connection with biocatalytic conversion processes, the term pretreatment is used for a number of single or combined physical, chemical, and biological processes that are used to efficiently convert lignocellulosic biomass, which is usually recalcitrant to enzymatic hydrolysis, into an activated form that can be cost=effectively hydrolyzed enzymatically to produce C5 and C6 sugars in aqueous solution. Thus, the ultimate pretreatment technology would be a low-cost, sustainable process that renders lignocellulosic biomass completely accessible to enzymes and/or microorganisms that efficiently and completely hydrolyze all carbohydrate polymers present in plant cell walls and avoids the formation of toxic degradation products that negatively impact downstream biological or chemical processing, and thereby process yield and profitability.
Pretreatment technologies for biocatalytic conversion technologies can be broadly divided into two categories: pretreatment and fractionation.3 The goal of fractionation is to separate biomass into its three main components: hemicellulose, cellulose, and lignin. The quality of the manufactured cellulose can be of similar quality as obtained by conventional pulping processes. Although many research groups have tried to find a more economically attractive application for lignin, it is usually used as fuel for the conversion of cellulose- and hemicellulose-derived sugars into value-added products, which can be energy-intensive. Hemicellulose is commonly treated as a secondary sugar stream from lignocellulosics-based biorefineries. This is often due to the lack of efficient and robust fermentation processes for the conversion of C5 sugars to bioethanol or other product. The quality of the hemicellulose stream, as defined by its sugar composition and the level of sugar degradation byproducts, depends on the specific biomass pretreatment employed. In this regard, optimized pretreatment and fractionation schemes are necessary to ensure the maximum exploitation of the biomass carbohydrates.
The development of pretreatment technologies for lignocellulose is driven by the production of second-generation bioethanol. Therefore pretreatment methods are the subject of intensive research activities (Mosier et al., 2005; Yang and Wyman, 2008; Gírio et al., 2010; Goh et al., 2011; Shi et al., 2011; Panagiotopoulos et al., 2012; Chiesa and Gnansounou, 2014).
One example of a pretreatment strategy is dilute acid hydrolysis, which is probably the oldest pretreatment option available. Dilute acid pretreatment processes typically employ a dilute (eg, 0.1%) strong acid, such as sulfuric acid, high temperature (eg, 150–200°C), and a fairly short residence time (eg, 10 minutes) to break the lignin seal and nearly completely hydrolyze hemicellulose into its constituent sugars. The net effect is to increase the surface area of cellulose fibers that is accessible to subsequently added cellulase enzymes. This approach ultimately results in increasing the concentration and amount of fermentable sugars from a given amount of input biomass.
There are a variety of physical and physio-chemical methods that have advantages and disadvantages (Linde et al., 2008; Hendriks and Zeeman, 2009; Alvira et al., 2010; Goh et al., 2011; Lai et al., 2014; Reisinger et al., 2014; Papa et al., 2015). In general, ammonia fiber explosion, wet oxidation, and liquid hot water are more suitable for agricultural residues and dedicated herbaceous energy crops, while steam explosion is applicable for both herbaceous and woody biomass. Hardwoods are typically less recalcitrant to dilute acid pretreatment than softwoods because their hemicelluloses are composed of acetylated xylans that rapidly hydrolyze in water at elevated temperatures. Another reason for the recalcitrance of softwood species is the presence of a more heavily crosslinked lignin in softwood cell walls relative to hardwoods and herbaceous species. Hardwoods also contain lower contents of readily fermentable C6 sugars in their hemicellulose than softwoods, as do agricultural residues and herbaceous crops (Gnansounou, 2008). This is because softwood hemicelluloses contain higher amounts of the C6 sugars, mannose and galactose, while hardwood and herbaceous species hemicellulose contains primarily C5 sugars, mainly xylose and arabinose. Furthermore, in acid pretreatment conditions pentose sugars are more easily degraded to byproducts, such as short-chain organic acids and furan compounds. Thus, the preferred pretreatment should facilitate high hemicellulose sugar recovery yields, while maintaining an acceptable downstream cellulose enzymatic hydrolysis rate and glucose yield.
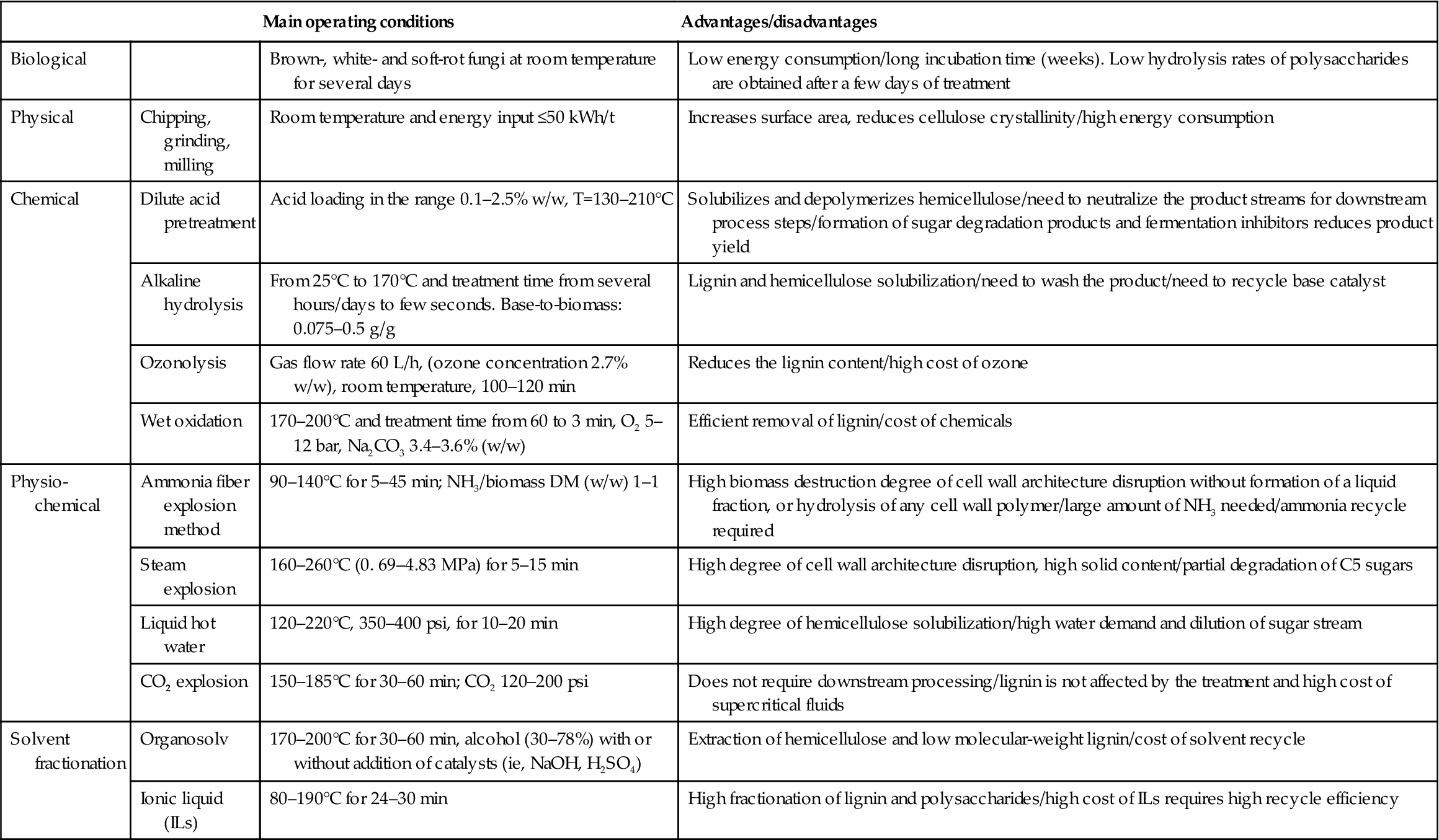
Pretreatment processes are frequently classified as biological, physical, chemical, physicochemical, and solvent-assisted. Most chemical/biochemical pretreatments result in sugars in aqueous solution and a solid fraction. However, sugar degradation products are often also produced during pretreatment, which can negatively affect subsequent purification and/or conversion steps. The types and relative amounts of the sugars produced depend on process conditions and feedstock composition. Table 2.1 shows selected process conditions for various pretreatment methods as well as advantages and disadvantages of the methods.
Table 2.1
Description of pretreatment methods for lignocellulosic biomass based on (Mergner et al., 2013)
| Main operating conditions | Advantages/disadvantages | ||
| Biological | Brown-, white- and soft-rot fungi at room temperature for several days | Low energy consumption/long incubation time (weeks). Low hydrolysis rates of polysaccharides are obtained after a few days of treatment | |
| Physical | Chipping, grinding, milling | Room temperature and energy input ≤50 kWh/t | Increases surface area, reduces cellulose crystallinity/high energy consumption |
| Chemical | Dilute acid pretreatment | Acid loading in the range 0.1–2.5% w/w, T=130–210°C | Solubilizes and depolymerizes hemicellulose/need to neutralize the product streams for downstream process steps/formation of sugar degradation products and fermentation inhibitors reduces product yield |
| Alkaline hydrolysis | From 25°C to 170°C and treatment time from several hours/days to few seconds. Base-to-biomass: 0.075–0.5 g/g | Lignin and hemicellulose solubilization/need to wash the product/need to recycle base catalyst | |
| Ozonolysis | Gas flow rate 60 L/h, (ozone concentration 2.7% w/w), room temperature, 100–120 min | Reduces the lignin content/high cost of ozone | |
| Wet oxidation | 170–200°C and treatment time from 60 to 3 min, O2 5–12 bar, Na2CO3 3.4–3.6% (w/w) | Efficient removal of lignin/cost of chemicals | |
| Physio-chemical | Ammonia fiber explosion method | 90–140°C for 5–45 min; NH3/biomass DM (w/w) 1–1 | High biomass destruction degree of cell wall architecture disruption without formation of a liquid fraction, or hydrolysis of any cell wall polymer/large amount of NH3 needed/ammonia recycle required |
| Steam explosion | 160–260°C (0. 69–4.83 MPa) for 5–15 min | High degree of cell wall architecture disruption, high solid content/partial degradation of C5 sugars | |
| Liquid hot water | 120–220°C, 350–400 psi, for 10–20 min | High degree of hemicellulose solubilization/high water demand and dilution of sugar stream | |
| CO2 explosion | 150–185°C for 30–60 min; CO2 120–200 psi | Does not require downstream processing/lignin is not affected by the treatment and high cost of supercritical fluids | |
| Solvent fractionation | Organosolv | 170–200°C for 30–60 min, alcohol (30–78%) with or without addition of catalysts (ie, NaOH, H2SO4) | Extraction of hemicellulose and low molecular-weight lignin/cost of solvent recycle |
| Ionic liquid (ILs) | 80–190°C for 24–30 min | High fractionation of lignin and polysaccharides/high cost of ILs requires high recycle efficiency |
2.2.4 Saccharification of Cellulose and Hemicellulose
The use of hydrolytic enzymes to degrade cellulose to fermentable sugar is generally considered more effective than the use of concentrated mineral acids, because enzymes are highly specific and can work at mild process conditions, for example, 45–50°C and pH 4.8–5 for cellulases derived from the filamentous fungus, Trichoderma reesei. In addition, the mild chemical conditions employed for enzymatic processes obviate the need for extremely expensive, corrosion-resistant materials of construction that are required when using concentrated mineral acids. Concentrated mineral acids also degrade sugars to unfermentable products, which results in yield losses.
Saccharification of lignocellulosic material requires the use of several enzymes with complementary activities: endoglucanase, which attacks regions in the interior of linear cellulose chains; exoglucanases or cellobiohydrolases, which hydrolyze cellobiose units from the ends of cellulose chains; and β-glucosidase, which converts cello-oligosaccharides and cellobiose into glucose. There are also several complementary enzymes that attack hemicelluloses, including glucuronidase, xylan acetylesterase, xylanase, β-xylosidase, galactomannase, and glucomannase. Filamentous fungi, such as Trichoderma viride, T. reesei, and Trichoderma longibrachiatum, are the principal producers of commercially available cellulases. These commercial products are cocktails containing mixtures of several enzyme types. Prior to their use in the production of second-generation biofuels, cellulases were included in detergent formulations and employed in the manufacture of “stonewashed” jeans.
The economics of biomass saccharification is sensitive to the cost of cellulase enzymes. The low specific activity of cellulases requires large amounts of enzyme to effectively saccharify the cellulose in biomass in a reasonable amount of time. In addition, unproductive binding of the enzymes to biomass increases the amount of enzyme required and therefore operational costs in the process. Addition of surfactants can be useful to decrease nonproductive binding of cellulases to lignin and polysaccharide–lignin complexes (Cui et al., 2011).
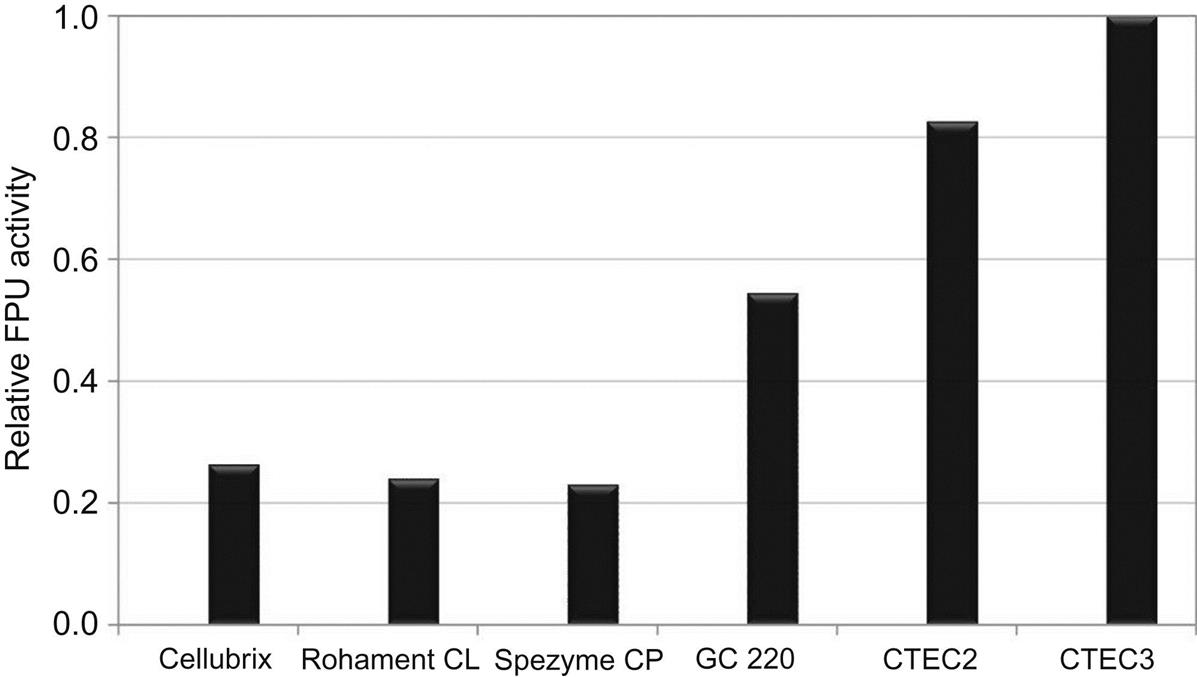
Enzyme companies and others have made significant progress toward reducing enzyme cost by streamlining enzyme production and formulation processes, inclusion of new enzymes in cellulase enzyme cocktails, and increasing the enzyme specific activity using enzyme engineering strategies. For example, with funding from the U.S. Department of Energy (US DOE), Genencor, Novozymes, and DSM each have dramatically reduced the cost and improved the quality of cellulase enzyme cocktails for use in cellulosic biomass conversion processes. Genencor has launched their Accellerase Duet product produced by a genetically modified strain of T. reesei, and which contains both cellulases and xylanases. Similarly, Novozymes has released their CELLIC product family (CTEC2 and CTEC3, and HTEC). These new cellulase products have been designed especially for use in the production of second-generation bioethanol. Dyadic is another US company that is producing cellulase enzyme cocktails for industrial use. Fig. 2.2 describes the improvement of the commercial blends.
Feedstock composition and the specific requirements of downstream processes determine the preferred pretreatment strategy. The types and relative amounts of sugars present in the feedstock are a function of the plant species, but the availability of those sugars for downstream conversion processes are a function of how well the pretreatment and enzymatic saccharification processes perform. Hence, the preferred combination of pretreatment and conversion technology depends on a number of factors and therefore no one-size-fits-all technology option currently exists. The ideal pretreatment process should result in high yields of fermentable sugars from hemicellulose and cellulose, avoid the generation of toxic compounds and yield losses due to sugar degradation, minimize enzyme usage and associated cost, and require little or no energy or chemical inputs. The following section describes biochemical conversion technologies, as well as their connection to pretreatment processes.
2.2.5 (Bio)-Catalytic Production of Bioethanol and Various Chemicals
Bioethanol has become the most commonly used biofuel worldwide. Bioethanol can be blended with gasoline or used in the synthesis of ethyl tertiary-butyl ether, which is used as an antiknocking agent in gasoline. Both compounds serve as oxygenates in gasoline, and were first used in the US to improve air quality in densely populated urban areas.
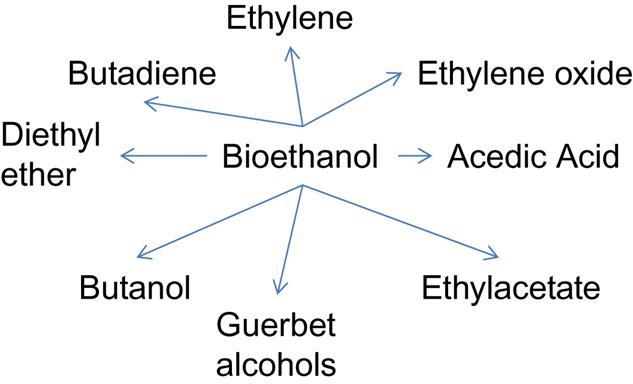
Besides its use as a transportation fuel blending agent, ethanol is also considered a potent platform chemical for the synthesis of other value-added chemicals. Catalytic conversion of bioethanol has been successfully applied to the production of important chemicals such as ethylene, n-butanol, diethyl ether, etc. (Posada et al., 2013). Important bulk chemicals that can be derived from ethanol are shown in Fig. 2.3. The conversion of ethanol to bulk chemicals is still in its infancy, but this family of chemical conversions is probably one of the most promising near-term pathways to a biobased economy. Moreover, the conversion of ethanol to other chemicals would allow for the maintenance of existing downstream processing infrastructure. Ethanol production can be used as a proxy for other products derived from cellulosic sugars via biochemical conversion routes (eg, butanol, furan derivatives, etc.) because the equipment for purification and upgrading of those products is similar to that used for ethanol.
For the production of second-generation biofuels and biobased chemicals, hydrolysis and fermentation steps can be combined in simultaneous saccharification and fermentation (SSF) of hexoses or simultaneous saccharification and cofermentation (SSCF) of both hexoses and pentoses. Consolidated bioprocessing (CBP) is a system in which cellulose enzyme production, substrate hydrolysis, and fermentation take place simultaneously in the same reactor vessel using a fermentation organism that produces the enzymes that saccharify the cellulose. CBP offers the potential for lower biofuel production costs due the process integration, which eliminates the capital and operating costs associated with the separate production of enzymes and ensures higher efficiencies than separate hydrolysis and fermentation (SHF) or SSF (van Zyl et al., 2013). The Saccharomyces cerevisiae strain developed by the Mascoma Corporation is the best CBP organism engineered so far, as this strain can convert several cellulosic substrates to ethanol with addition of minimal exogenous enzymes (Mcbride, 2010). Various recombinant microorganisms have demonstrated impressive saccharification potential (Ha et al., 2011), although their use for large-scale industrial processes still requires fine-tuning of the entire process. The (simultaneous) fermentation of mixed C5 and C6 sugar hydrolysates is still one of the most important challenges in the production of biofuels and biobased chemicals.
Apart from bioethanol, a number of platform chemicals and advanced biofuels can be produced from sugars derived from lignocellulosic materials. The US DOE identified the top 12 chemicals potentially derived from biomass in 2004 (Werpy et al., 2004). The list was extended in 2010 to include alcohols, acids, amino acids, and terpenes (Bozell and Petersen, 2010). Table 2.2 lists chemicals that can be derived from sugars either through fermentation, for example, C4 acids or chemical conversion, for example, furan derivatives produced via catalyzed dehydration of sugars.
Table 2.2
Top chemicals derived from biomass identified by the US Department of Energy (US DOE)
| List in 2004 (Werpy et al., 2004) | List in 2010 (Bozell and Petersen, 2010) |
| 3-Hydroxybutyrolactone | Biohydrocarbons |
| Aspartic acid | Ethanol (C2) |
| Glycerol | Lactic acid (C3) |
| Glucaric acid | Furans |
| Glutamic acid | |
| 3-Hydroxypropanoic acid | |
| Itaconic acid | |
| Levulinic acid | |
| 2,5-Furan dicarboxylic acid | |
| Sorbitol | |
| C4 acids (succinic, fumaric and malic acid) | |
| Xylitol (C5) |
Succinic acid, a C4-dicarboxylic acid platform chemical, has received increasing attention for the production of new polyesters with good mechanical properties combined with full biodegradability. Succinic acid is widely used, especially for the production of renewable polyesters. Succinate concentration as high as 110 g/L has been achieved from glucose by the rumen organism Actinobacillus succinogenes (Liu, 2000). It can also be produced by Anaerobiospirillum succiniciproducens using glucose, lactose, sucrose, maltose, or fructose as carbon sources. The reduction of the carboxylate groups is one of the available routes to 1,4-butandiol (BDO), a building block for the production of various polyesters.
Several other organic acids are also considered to be potential platform molecules. Lactic acid is probably the most prominent. It can dimerize and subsequently form lactide, an intermediate for polymerization to the biodegradable plastic, polylactic acid. The monomer acid is typically produced by glucose fermentation using Lactobacillus delbrueckii (Datta, 2006). Luo et al. (1997) indicated that using the SSF strategy for lactic acid production offers the advantage that enzymatic hydrolysis and lactic acid fermentation work at similar process conditions (eg, temperature and pH). Other organic acids may be a suitable platform for specialty chemicals or niche markets, but the large volumes of waste products produced (ie, gypsum) are problematic and require the development of a more efficient process design.
Other biobased chemicals and advanced fuels obtained through the fermentation of sugars include alcohols higher than ethanol, diols, microbial lipids, and advanced hydrocarbons. Some of these are described below.
Biobutanol has a higher energy density and is less hydrophilic than ethanol, both of which make it a better blending agent for gasoline than ethanol. It can be produced by acetone-butanol-ethanol (ABE)-type anaerobic fermentation organisms (Atsumi et al., 2008). Clostridium beijerinckii ATCC 55025 can utilize hexoses and pentoses simultaneously to produce butanol (Liu et al., 2010). The main problem associated with the industrial production of biobutanol is the high energy required for purifying the product from fermentation liquors at low butanol concentrations. The combination of fermentation with pervaporation has been proposed to separate butanol during fermentation and increase the process efficiency (Qureshi et al., 2001). Gevo and Butamax have successfully converted cellulose-derived isobutanol produced via a fermentation process into isobutylene and paraffinic kerosene (jet fuel) via well-understood chemical processes.
2,3-Butandiol (BDO) is a promising compound with three stereo-isomer forms. It can be used both as liquid fuel and as a platform chemical. Both the levo- and dextro-isomers of BDO are chiral components for asymmetric synthesis reactions. Various microrganisms, namely Klebsiella pneumoniae (Ma et al., 2009), Klebsiella oxytoca (Cheng et al., 2010), Paenibacillus polymyxa (Gao et al., 2010), and Enterobacter aerogenes (Zeng, 1991), have been tested for the production of BDO by using various glucose sources. BDO can be converted to 1,3-butadiene, an intermediate for the synthesis of rubbers, polyesters, and polyurethanes, or to methyl-ethyl-ketone, a solvent and commonly used fuel additive.
Among polyalcohols, xylitol, a natural noncaloric sweetener with anticariogenic properties, has been considered as the main alternative to ethanol produced from C5 sugars. It is currently produced from the nickel-assisted hydrogenation of xylose. Sorbitol can also be biotechnologically produced by the bacterium Zymomonas mobilis, which uses fructose and glucose (Silveira, 2002).
Microbial lipids (predominantly triglycerides) obtained through the fermentation of lignocellulosic hydrolyzates with oleaginous microorganisms can be used for the production of free fatty acids, fatty alcohols (Metzger, 2006), and fatty acid methyl (or ethyl) esters, which can be further converted to several intermediates for the production of biodiesel, hydroprocessed esters and fatty acids, lubricants (Chowdhury, 2013), and surfactants. However, the commercialization of microbial lipids is still cost-prohibitive. More efficient and integrated processes are necessary for better techno-economics of this process (Gong et al., 2013).
Advanced biohydrocarbons are similar to conventional hydrocarbon fuels such as gasoline, diesel or jet fuels, but are produced from biomass feedstocks. Recently, Amyris engineered the isoprene metabolic pathways in some microorganisms for the production of terpenoids such as farnesene, a 15-carbon branched hydrocarbon that can be chemically hydrogenated to a drop-in diesel or jet fuel component (farnesane).
The major part of the current chemicals-based biorefineries use first-generation sugars but very little second-generation sugars. In Table 2.3, chemicals derived from lignocellulosic material are summarized together with selected process conditions.
Table 2.3
Process conditions for chemicals derived from lignocellulosic material
| Value-added product | Feedstock | Fractionation technology | Polysaccharide degradation | Micro-organism | Yield % or concentration | References | Applications |
| Ethanol | Sugarcane bagasse | Steam explosion | Enzymatic hydrolysis | S. cerevisiae | 56.3 g/L | Amores et al. (2013) | Fuel, chemicals |
| Butanol | Wheat straw | Dilute sulfuric acid hydrolysis | Enzymatic hydrolysis | Clostridium beijerinckii P | 0.44 g/g sugars; 0.36 g/L per h | Qureshi et al. (2008) | Fuel, chemicals |
| Xylitol | Wheat straw | Acid hydrolysis | Candida tropicalis (AS2. 1776) | 42% (xylitol/xylose) | Zhuang et al. (2009) | Sweetener | |
| Lactic acid | Water hyacinth | Na2SO3 Pretreatment in NaOH | Enzymatic hydrolysis | Lactobacillus acidophilus | 0.86 g Lactic acid/g sugars consumed | Idreesa et al. (2013) | Personal care, bioplastics |
| Biosuccinic acid | Cotton stalks | Steam explosion | Enzymatic hydrolysis | Actinobacillus succinogenes | 64% total sugars | Li et al. (2013) | Surfactant, foaming agent, antimicrobial agent, etc. |
| Triacyl- glycerides | Corn stover | Mechanical pretreatment | Enzymatic hydrolysis | Cryptococcus curvatus | 86 mg lipid/g feedstock; 7.4 g/L | Gong et al. (2013) | Fuel |
| Glutamic acid | Corncob fibers hydrolysates | Enzymatic hydrolysis | Bacillus subtilis HB-1 | 24.92 g/L | Zhu et al. (2013) | Food, medical, cosmetics industries, etc. |

2.2.5.1 Chemicals From Glycerol
The production of first-generation biodiesel will continue, particularly in Malaysia and Indonesia. Combining palm oil and biodiesel production would produce refined palm oil, biodiesel, and surplus electricity as primary products, as well as bleaching earth, palm fatty acid distillate (PFAD), and glycerol as byproducts. PFAD is a light brown solid at room temperature, which currently is used for soap and animal feed, but also could serve as feedstock for the oleochemical industry. PFAD also contains a significant amount of extractable vitamin E (Santosa, 2008).
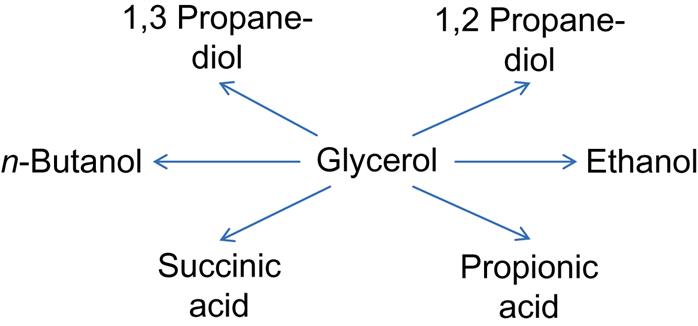
Purified glycerol from biodiesel production is already a marketable product. However, vastly increased biodiesel production could lead to significant glycerol quantities that the market might not be able to absorb without causing a drop in glycerol price. If the most recent biodiesel targets are fulfilled in Indonesia, then the amount of glycerol will increase from 10,000 t in 2009 to 750,000 t in 2016. Vlysidis et al. (2011) conducted a techno-economic analysis of biodiesel refineries. Their results indicate the importance of glycerol as a key building block for the production of commodity chemicals and as feedstock for various fermentation processes (Fig. 2.4).
Another option for the utilization of glycerol is the catalytic dehydration of glycerol to acrolein, a versatile intermediate. Acrolein derivatives, such as acrylic acid, an ingredient for paints and coatings, offer high industrial value. Currently, acrolein is mostly used for the synthesis of methionine. Methionine is a sulfur-containing amino acid that is required as a supplement in animal feed. Methionine has a global annual demand of approximately 850,000 t (Willke, 2014) and an expected annual growth of 5% (Liu et al., 2012).
Food-grade L-methionine, mainly used in human nutrition and medicine, amounts to only 5% of the whole methionine market, but offers a substantially higher margin. Biobased methionine serves the animal feed market in organic farming, in which legislation prohibits or limits the use of fossil-based feed additives (Willke, 2014).
2.2.6 Thermochemical Conversion: Fast Pyrolysis
In addition to the biochemical conversion, thermal conversion for relatively dry, woody materials is a suitable option. Currently, fast pyrolysis is a promising thermal treatment option for converting lignocellulosic biomass into liquid energy carriers or as a drop-in compound in existing refineries.
In fast pyrolysis, biomass decomposes quickly at approximately 475°C in the absence of oxygen, generating primarily condensable vapors and aerosols, as well as smaller amounts of char and noncondensable gases. After cooling and condensation, a dark brown, homogeneous, mobile liquid with a heating value about half that of conventional fuel oil is formed. The essential features of the utilization of fast pyrolysis for producing liquids are:
1. Very high heating rates and very high heat transfer rates at the biomass particle reaction interface, usually requiring a finely ground biomass feed of typically less than 3 mm, as biomass generally has a low thermal conductivity;
2. Carefully controlled pyrolysis reaction temperature of around 500°C to maximize the liquid yield for most biomass;
3. Short hot vapor residence times of approximately 2 seconds to minimize secondary reactions;
4. Rapid and complete removal of product char to minimize cracking of vapors; and
5. Rapid cooling of the pyrolysis vapors to give the bio-oil product.
The main product, bio-oil, is obtained at yields of up to 75 wt% on a dry feed basis, together with byproduct char and noncondensable gas, which can be used within the system to provide the process heat requirements. Flue gas and ash are the only waste products. However, liquid yield depends on biomass, temperature, hot vapor residence time, char separation, and biomass ash content and composition. The latter two have a catalytic effect on vapor cracking at high temperatures.
The basic fast pyrolysis process is depicted in Fig. 2.5. Fig. 2.5 also demonstrates the versatility of fast pyrolysis processes and the most relevant process option at each process stage. The product quality and composition can be influenced through:
![]() pretreatment steps, such as ash removal, catalyst addition, biopolymer separation by various processes;
pretreatment steps, such as ash removal, catalyst addition, biopolymer separation by various processes;
![]() changing pyrolysis conditions by varying parameters such as temperature, pressure, time, and atmosphere and addition of alcohols and catalysts;
changing pyrolysis conditions by varying parameters such as temperature, pressure, time, and atmosphere and addition of alcohols and catalysts;
![]() posttreatment, ie, both physical methods such as staged condensation and extraction and chemical procedures such as solvent addition, derivatization, hydrodeoxygenation, and gasification.
posttreatment, ie, both physical methods such as staged condensation and extraction and chemical procedures such as solvent addition, derivatization, hydrodeoxygenation, and gasification.
2.2.6.1 Fast Pyrolysis Reactors
Reactors serve as the heart of a fast pyrolysis process. Although it most likely represents only about 10–15% of the total capital cost of an integrated system, most research and development has focused on developing and testing varying reactor configurations on a variety of feedstocks. More recently, increased attention has been paid to control and improve liquid quality and liquid collection systems. Several comprehensive reviews of fast pyrolysis processes for bio-oil production are available (Kersten et al., 2005; Mohan et al., 2006; Bridgwater, 2009).
The main conversion reactor technologies include bubbling fluid beds, circulating fluid beds, and transported beds, the rotating cone, a type of transported bed reactor, and ablative pyrolysis, which are all described in Bridgwater (2012). The key requirements in the design and operation of a fast pyrolysis process are heat transfer and char removal, as char and ash are catalytically active (Bridgwater, 2012) and must be removed from the pyrolysis reactor as quickly as possible in order to preserve the quality of the bio-oil product.
2.2.6.2 Pyrolysis Liquid: Bio-Oil
Crude pyrolysis liquid, or bio-oil, is dark brown, viscous, and similar to biomass in elemental composition. Bio-oil is composed of a very complex mixture of oxygenated hydrocarbons with a relatively high proportion of water. It is typically unstable during storage.
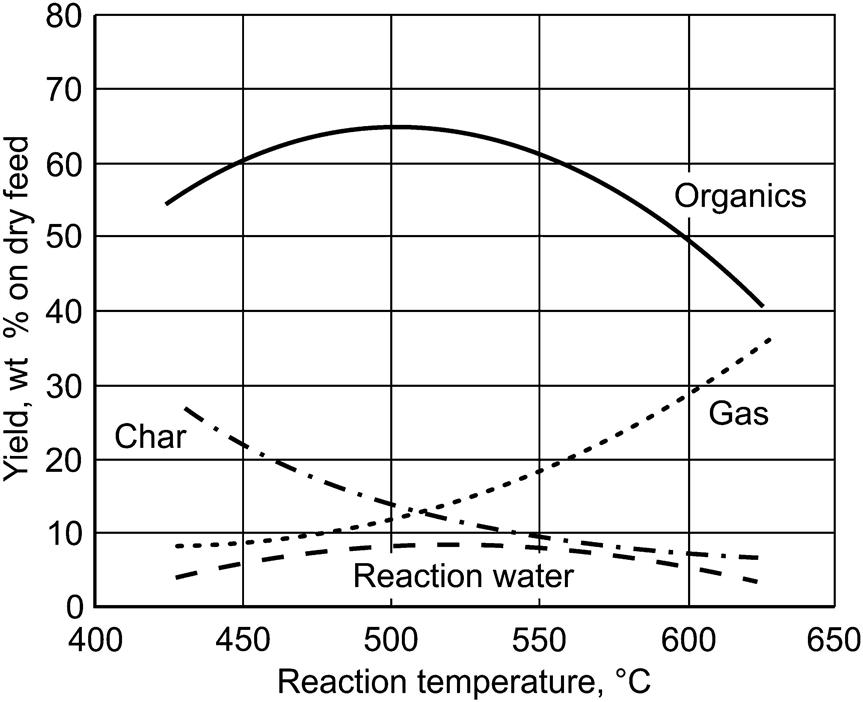
Typical organic yields and their variation with temperature for a woody feedstock are shown in Fig. 2.6. Similar results are obtained for most biomass feedstocks, although the maximum yield can occur between 480°C and 520°C, depending on the feedstock. Grasses, for example, tend to give maximum bio-oil yields of around 55–60 wt% on a dry feed basis at the lower end of this temperature range.
Bio-oil is formed by rapidly quenching, and thus “freezing,” the intermediate products of flash degradation of hemicellulose, cellulose, and lignin. The bio-oil thus contains many reactive species, which contribute to its unique attributes. Bio-oil can be considered a microemulsion in which the continuous phase is an aqueous solution of holocellulose decomposition products, that stabilizes the discontinuous phase of pyrolytic lignin macromolecules through mechanisms such as hydrogen bonding. Aging or instability is believed to result from a breakdown in this microemulsion.
2.2.6.3 Bio-Oil Characteristics4
Fast pyrolysis liquid contains approximately 25 wt% water and has a heating value of about 17 MJ/kg. Although the fast pyrolysis liquid is widely referred to as “bio-oil,” it will not mix with any hydrocarbon liquids. It is composed of a complex mixture of oxygenated compounds that provide both the potential and challenge for utilization. For any application, there are many particular characteristics of bio-oil that require consideration. These have been extensively reviewed (Bridgwater, 2011, 2012). Key properties of bio-oil are summarized in Table 2.4.
Table 2.4
Typical properties of wood-derived crude bio-oil
| Physical property | Typical value |
| Water content | 25% |
| pH | 2.5 |
| Specific gravity | 1.2 |
| Elemental analysis (on dry basis) | C 56% |
| H 6% | |
| O 38% | |
| N 0–0.1% | |
| HHV (as produced) | 17 MJ/kg |
| Viscosity (20°C and 25% water) | 40–100 mPas |
| Solids (char) | 0.1% |
| Vacuum distillation residue | Up to 50% |
Depending on the initial feedstock and the mode of fast pyrolysis, the color can be almost black, through dark red-brown to dark green, being influenced by the presence of microcarbon in the liquid and chemical composition. Hot vapor filtration gives a more translucent red-brown appearance owing to the absence of char. High nitrogen content can impart a dark green tinge to the liquid. The bio-oil contains varying quantities of water, and forms a stable single-phase mixture, ranging from about 15 wt% to an upper limit of about 30 wt% water, depending on the feed material, how it was produced, and subsequently collected.
Bio-oil can tolerate the addition of some water, but too much water will lead to phase separation. The addition of water reduces viscosity, which is useful; reduces heating value, which means that more bio-oil is required to meet a given duty; and can improve stability. The effect of water in bio-oil is therefore complex and important.
Bio-oil cannot be dissolved in water, and is miscible with polar solvents such as methanol, acetone, and is totally immiscible with petroleum-derived fuels. This is due to the high oxygen content of around 35–40 wt%, which is similar to that of biomass, and explains many of its chemical characteristics. Complex catalytic processes are necessary to remove this oxygen. Detail on this upgrading process is provided below.
The density of the bio-oil is very high at around 1.2 kg/L, compared with light fuel oil at around 0.85 kg/L. This means that the bio-oil has about 42% of the energy content of fuel oil on a weight basis, but 61% on a volumetric basis. This has implications for the design and specification of equipment such as pumps and atomizers in boilers and engines.
Viscosity is important in many fuel applications (Diebold et al., 1997). The viscosity of produced bio-oil can vary from as low as 25 m2/second to as high as 1000 m2/second (measured at 40°C). However, even high viscosities can result, depending on the feedstock, the water content of the bio-oil, the amount of light ends collected, and the extent to which the oil has aged.
Bio-oil cannot be completely vaporized. If the oil is heated to more than 100°C, it rapidly reacts and eventually produces a solid residue of around 50 wt% of the original liquid, and some distillate containing volatile organic compounds and water. While bio-oil has been successfully stored for several years in normal storage conditions in polyolefin plastic drums without any deterioration that would prevent its use in any of the applications tested to date, it does change slowly with time. Most noticeably, there is a gradual increase in viscosity. More recent samples that have been distributed for testing have shown substantial improvements in consistency and stability, demonstrating the improvement in process design and control as the technology develops.
Aging is a well-known phenomenon caused by continued slow secondary reactions in the bio-oil which manifests as an increase in viscosity with time. It can be reduced or controlled by the addition of alcohols such as ethanol or methanol. It is exacerbated or accelerated by the presence of fine char (Diebold, 2002).
Approximately 300 chemical components are detectable by gas chromatography in bio-oil. They can be divided into chemical groups such as organic acids, nonaromatic carbonyls and alcohols, heterocyclic furans and pyrans, aromatics, phenols, and sugars (mainly anhydrosugars).
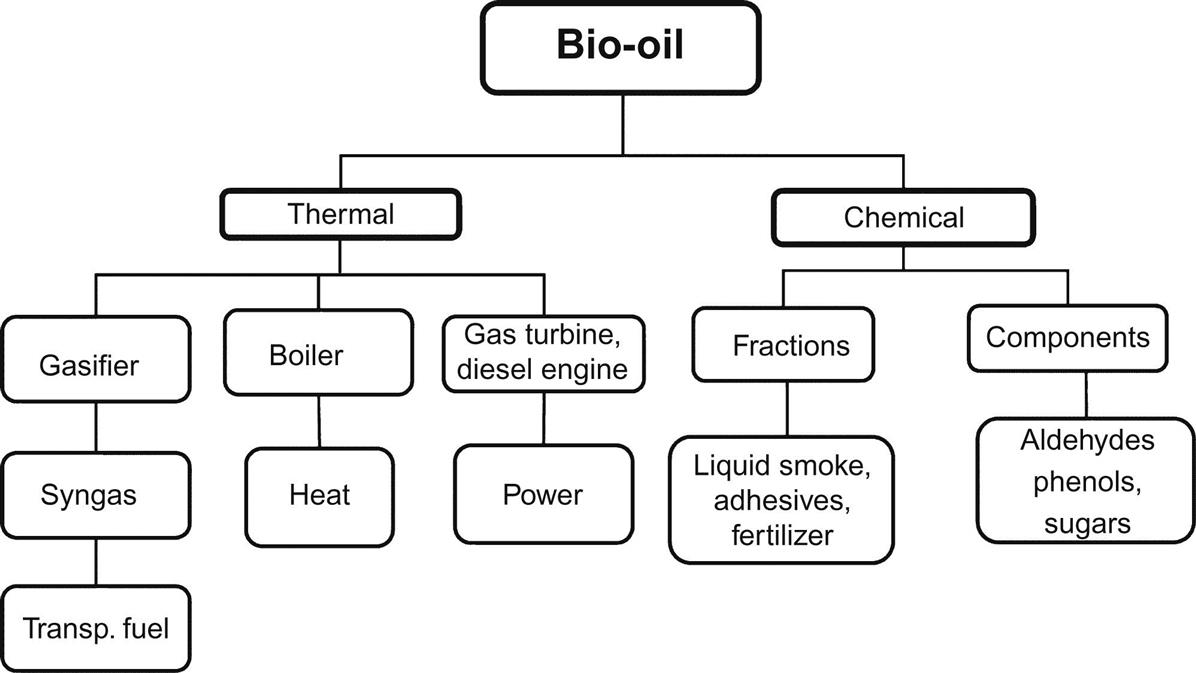
2.2.6.4 Applications of Bio-Oil
Bio-oil is primarily used in thermal and/or chemical applications. It can substitute for fuel oil in static applications such as boilers and turbines (Czernik and Bridgwater, 2004). Experiments have also been carried out with stationary diesel engines (Solantausta et al., 1994). Gasification of bio-oil is an ongoing activity at Karlsruhe Institute of Technology, Germany. In their bioliq process, bio-oil from straw pyrolysis is mixed with char to form a slurry which is subsequently gasified in a pressurized entrained flow gasifier. The resulting syngas is cleaned and used for the synthesis of dimethyl-ether, primarily used as transportation fuel (Dahmen et al., 2012).
Fig. 2.7 shows various use options of bio-oil. The only nonenergy application is the production of liquid smoke aroma and flavor enhancer. In the European Community, several companies have applied for product permission in the European market (Theobald et al., 2012). All other chemical applications are being studied at the laboratory or technical scale.
2.3 Summary
Today, the use of nonfood biomass for the production of bioenergy and biobased chemicals is preferred over the use of food crops. A wide range of nonfood biomass and conversion technologies can be used for the production of bioenergy and biobased products. The fermentation of lignocellulosic-derived sugar and the thermochemical conversion of biomass (eg, fast pyrolysis) are relevant conversion technologies. For both, the (lignocellulosic) biomass must be pretreated or at least conditioned prior to conversion.
Fast pyrolysis produces bio-oil, which can be used directly in stationary boilers. Bio-oil needs upgrading before it is suitable as fuel in transport systems such as aviation or shipping. For example, desired characteristics of jet fuels are being miscible with fossil jet fuels, remaining stable during storage, and having a suitable freeze and boiling point. Hydrocracking and hydrodeoxygenation are the two main approaches for upgrading of bio-oil to hydrocarbons that would be suitable blending agents with petroleum-derived fuels. Currently economic constraints inhibit the large-scale use of bio-oil-derived transport fuels; the same applies for bio-oil-derived materials. The use of bio-oil for material/chemical purposes is currently extremely limited.
Different technologies are involved in the initial conversion of biomass to sugars, since pretreatment is common to all lignocellulosic-sugar value chains. Technical obstacles in existing pretreatment processes for fermentation include insufficient separation of cellulose and lignin, formation of byproducts that inhibit downstream fermentation, high use of chemicals and/or energy, as well as high costs for enzymes, although the latter has decreased substantially recently. There is no single preferred pretreatment method or combination of methods, but mild acid hydrolysis and steam explosion, dilute and concentrated acid, and mild alkaline processes will be applied in the near future. Feedstock pretreatment, conversion, and product purification are linked processes and must be optimized as a whole system.
The most relevant products derived from sugars are chemicals having bifunctional groups suitable as C2–C6 building blocks. Ethanol, acetone, and butanol are also fermentation products. The catalytic conversion of intermediates, such as ethanol and glycerol, to a range of large-volume chemicals will most likely be part of the technology portfolio, particularly in regions where downstream infrastructure exists.
The integration of different pretreatment and conversion technologies in biorefineries can maximize the use of all biomass components and improve eco-efficiency of the whole value chain. In the long term, thermochemical and biochemical conversion of lignocellulosic biomass are promising technologies for the production of biofuels and biobased chemicals.