Ethanol Production from Biomass
Haruki Ishizaki and Keiji Hasumi
Abstract
In the New Stone Age, alcoholic drinks were produced by natural fermentation of fruits. As principles of ethanol fermentation have been elucidated, industrial production of ethanol has been developed for applications in beverages, cosmetics, industrial solvents, and pharmaceuticals. Presently, bioethanol is produced primarily from corn starch or sugar cane. As these raw materials are in competition with food and animal feed, ethanol production from lignocellulosic biomass has been eagerly investigated worldwide. Although there are various microorganisms producing ethanol, the best microorganisms for efficient conversion of lignocellulosic biomass into ethanol have not yet been discovered. Genetic engineering of microorganisms and fermentation technology for achieving high ethanol productivities and yields have been studied enthusiastically worldwide. This chapter provides an overview of the principles of ethanol fermentation, ethanol-producing microorganisms, and fermentation technology.
Keywords
Ethanol; metabolic pathway; microorganisms; saccharide; lignocellulosic biomass; fermentation technology
Chapter Outline
10.1.1. Outline of Ethanol Fermentation
10.1.2. Principles of Ethanol Fermentation
10.2. Ethanol-Producing Microorganisms
10.3. Methods of Ethanol Fermentation
10.3.1. Raw Materials for Ethanol Fermentation
10.1 Ethanol Fermentation
10.1.1 Outline of Ethanol Fermentation
In the New Stone Age, there existed alcoholic drinks produced by natural fermentation of fruits. Several thousand years ago, in the region of Mesopotamia, there were descriptions of the production of wine or beer that was offered to God to pray for a good harvest and hunting for ancient people. Nomadic tribes produced kumis from horse milk, and agricultural people produced alcohol such as wine, beer, and rice wine from grapes, wheat, and rice. In the Middle Ages, ethanol was initially developed as medicine, and during the middle of the sixteenth century, whisky and brandy made from beer and wine became alcoholic beverages. After this, ethanol production was developed worldwide to produce beverages, raw materials for medicines, fragrances, and solvents. In 2009, 468 million barrels of bioethanol were produced worldwide for use as an alternative to gasoline.
Antoine Lavoisier (France) made the first scientific studies of alcoholic fermentation. He described how sugar is converted into alcohol and carbon dioxide in 1789, and he determined the composition of both fermentable substances and the products of fermentation. In 1857, Louis Pasteur (France) showed that lactate fermentation is caused by living organisms without air. Emil C. Hansen (Denmark) first isolated Saccharomyces cerevisiae from wort for beer fermentation in 1883. In 1897, Eduard Buchner (German) found that cell-free extract from yeast can also transform glucose to ethanol. This was the first demonstration that enzymes bring about fermentation reactions.
10.1.2 Principles of Ethanol Fermentation
a Embden–Meyerhof Pathway (EM Pathway)
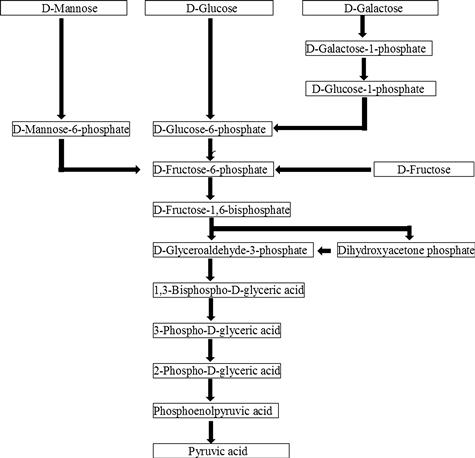
The EM pathway is the main pathway for the anaerobic degradation of carbohydrates. D-Glucose first enters into the EM pathway via phosphorylation to be converted to D-glucose-6-phosphate by hexokinase, with one mole of ATP required as phosphate donor. D-Glucose-6-phosphate is next converted to D-fructose-6-phosphate and then catalyzed by phosphofructokinase to produce D-fructose-1,6-bisphosphate in the follow-up phosphorylation with ATP. D-Fructose-1,6-bisphosphate is subsequently split by fructose-1,6-bisphosphate aldolase into two triosephosphates, i.e. D-glyceraldehyde-3-phosphate and dihydroxyacetone phosphate. D-Glyceraldehyde-3-phosphate is converted to pyruvate by five-step enzyme reactions, and four moles of ATP are generated from two moles of the triosephosphates in this step. Pyruvate is converted to ethanol by pyruvate decarboxylase and alcohol dehydrogenase. Thus, one mole of glucose is converted to two moles of ethanol and two moles of carbon dioxide while generating two moles of ATP.
Fructokinase converts D-fructose to D-fructose-6-phosphate, and hexokinase converts D-mannose to D-mannose-6-phoshate; the latter is subsequently converted to D-fructose-6-phosphate by mannose phosphate isomerase. D-Galactose is converted to D-galactose-1-phosphate by galactokinase, and the resulting product isomerizes to D-glucose-6-phosphate via D-glucose-1-phosphate. D-Fructose-6-phosphate and D-glucose-6-phosphate are metabolized to ethanol through the EM pathway (Figure 10.1).
b Entner–Doudoroff Pathway (ED Pathway)
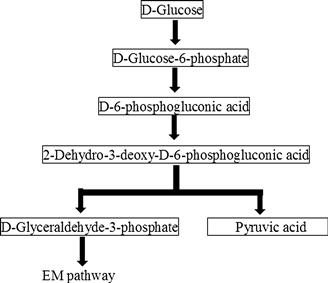
Although the ED pathway is generally considered to be restricted to a limited number of Gram-negative bacteria, the pathway is now known to be present in a diverse group of organisms ranging from archaea to bacteria, to eukarya. Most bacteria utilize the ED pathway to convert glucose to pyruvate anaerobically. D-Glucose enters into the ED pathway after phosphorylation by hexokinase. The resulting D-glucose-6-phosphate is converted to D-6-phosphogluconate using one mole of NADP. D-6-Phosphogluconate is oxidized to 2-keto-3-deoxy-6-phosphogluconate, which is then cleaved by aldolase to pyruvate and D-glyceraldehyde-3-phosphate. D-Glyceraldehyde-3-phosphate is oxidized to pyruvate through the EM pathway, yielding two moles of ATP (Figure 10.2). Pyruvate is converted to ethanol as in the EM pathway. Overall, one mole of glucose is converted to two moles of ethanol and two moles of carbon dioxide by consuming one mole of ATP.
c Pentose Phosphate Pathway
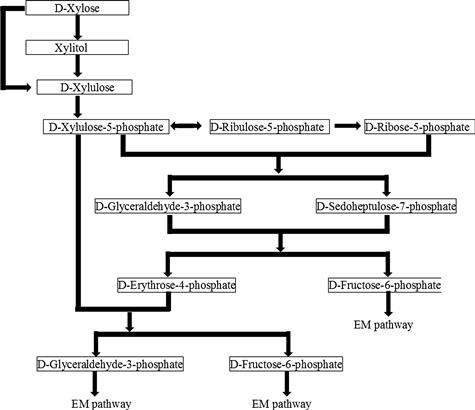
In most bacteria, the D-xylose conversion proceeds via direct isomerization to D-xylulose catalyzed by xylose isomerase without any cofactor. In yeast and most filamentous fungi, D-xylose is first reduced to xylitol by xylose reductase, and the resulting xyitol is subsequently oxidized to D-xylulose by xylitol dehydrogenase. The difference in cofactor preference of xylose reductase (NADPH specific) and xylitol dehydrogenase (NAD specific) leads to redox imbalance and formation of xylitol.
D-Xylulose is converted to D-xylulose-5-phosphate by xylulokinase with the consumption of one mole of ATP. D-Xylulose-5-phosphate is dimerized to D-ribulose-5-phosphate, which then isomerizes to D-ribose-5-phosphate. D-Xylulose-5-phosphate and D-ribose-5-phosphate are converted to a 7-carbon sugar, sedoheptulose-7-phosphate, and a 3-carbon sugar, D-glyceraldehyde-3-phosphate, by transketolase. The resulting 7-carbon sugar and 3-carbon sugar are converted to a 4-carbon sugar, erythrose-4-phosphate, and D-fructose-6-phosphate through the catalysis by transaldolase. Erythrose-4-phosphate and D-xylulose-5-phosphate form D-glyceraldehyde-3-phosphate and D-fructose-6-phosphate via a transketolase-catalyzed reaction. Thus, three moles of xylose is converted to two moles of D-fructose-6-phosphate and one mole of D-glyceraldehyde-3-phosphate; the latter are glycolytic intermediates and enter into the EM pathway (Figure 10.3). As a result, one mole of xylose is converted to 5/3 mole of ethanol.
10.2 Ethanol-Producing Microorganisms
10.2.1 Yeast
a Saccharomyces cerevisiae
Saccharomyces cerevisiae was first isolated in 1883 by Emil C. Hansen; it belongs to ascomycota and multiplies by budding. Saccharomyces cerevisiae converts glucose, fructose, galactose, maltose, and sucrose into ethanol but is not capable of converting pentoses, lactose, and cellobiose. It produces two moles of ethanol and two moles of carbon dioxide from one mole of glucose through the EM pathway. Saccharomyces cerevisiae is among the best known safe microorganisms, and is therefore ideal for producing alcoholic beverages such as wine, beer and Japanese sake, and for leavening.
Saccharomyces cerevisiae has the following beneficial characteristics for ethanol fermentation:
1. It is highly resistant to ethanol and toxic substances compared with bacteria, and is capable of fermenting sugars at low pH values so that the contamination risk is greatly minimized.
2. It can efficiently produce ethanol at high concentrations of ethanol from glucose and sucrose. In Japanese sake manufacturing from boiled rice, over 20% (v/v) ethanol is obtained by fermentation.
3. It has good performance for the production of beverage and bioethanol as well as for use as leavening, because each of these manufacturing processes depends on various characteristic strains.
4. It is GRAS (Generally Recognized As Safe) as a food additive.
5. It is suitable for conducting genetic engineering research and implementation because its whole genome has been elucidated.
Native strains of S. cerevisiae are incapable of utilizing pentoses such as xylose and arabinose, which are abundant in lignocellulosic biomass. For bioethanol production from lignocellulosic biomass, various genetic engineering approaches have been evaluated to efficiently convert pentoses to ethanol (Laluce et al., 2012). These include the expression of heterologous genes encoding xylose reductase, xylitol dehydrogenase and xylose isomerase, compensation of the imbalance of redox cofactors, and improvement of specific pentose transporter systems.
b Schizosaccharomyces pombe
Schizosaccharomyces pombe was isolated from beer in 1893. It is a fission yeast that differentiated from budding yeasts one billion years ago in evolutionary history. In 2002, the entire nucleotide sequence of S. pombe was determined. Schizosaccharomyces pombe tolerates a high concentration of salts, but has lower ethanol resistance than S. cerevisiae.
c Kluveromyces lactis and Kluveromyces marxianus
Kluveromyces lactis and K. marxianus are lactose fermenting yeasts and exhibit the activities of lactose permease and β-galactosidase. Lactose is hydrolyzed to glucose and galactose and the resulting sugars are converted to ethanol via the EM pathway. In Ireland and New Zealand, ethanol for beverages, medical and industrial uses, as well as fuels, are produced from whey by fermentation using K. lactis (Guimarães et al., 2010).
Kluveromyces marxianus is a thermotolerant yeast that is capable of fermenting various sugars including glucose, mannose, galactose, and xylose at 40°C or 30°C. The capability of xylose fermentation is lowered with more glucose repression at higher temperatures as compared with fermentations at 30°C. Because K. marxianus has thermotolerant characteristics, various genetic engineering research has been conducted for fermenting various sugars efficiently at relatively high temperatures (Rodrussamee et al., 2011).
d Pentose-Fermenting Yeast
In the case of bioethanol production from lignocellulosic biomass, fermenting pentoses efficiently is as important as fermenting hexose. Many pentose-fermenting yeasts have been isolated; these include Pichia stipitis, P. segobiensis, Candida shehatae, Pachysolen tannophilus, and Hansenula polymorpha.
Pichia stipitis, a respiratory yeast, was isolated from decaying wood and larvae of wood-inhabiting insects. It is capable of fermenting glucose, xylose, galactose, and cellobiose under anaerobic conditions. Pichia stipitis has the highest native capacity of xylose fermentation among known microbes, but glucose inhibits xylose transport non-competitively. Additionally, P. stipitis has less ethanol resistance than S. cerevisiae, and controlling the dissolved oxygen at low levels is of great importance for high ethanol yield using P. stipites (Agbogbo and Coward-Kelly, 2008).
Hansenula polymorpha is a thermotolerant xylose fermenting yeast. It grows prolifically at 37°C and survives at temperatures of up to 48°C. While xylose fermentation by H. polymorpha has been studied in various metabolic engineering fields, its ethanol production rate has not yet been improved.
10.2.2 Bacteria
a Zymomonas mobilis
Zymomonas mobilis is a Gram-negative, facultative anaerobic bacterium that was isolated from the alcoholic beverage Mexican pulque in 1928. In Mexico, the distilled spirit tequila is traditionally made from fermentation of juices from the agave plant using Z. mobilis. The microorganism degrades sugar to pyruvic acid via the ED pathway, and pyruvic acid is then converted to ethanol and carbon dioxide. It is GRAS as a food additive. The ED pathway yields only half as much ATP from glucose, as does the EM pathway. This low yield of ATP results in a small yield of cell mass; hence, Z. mobilis has higher yield per unit cell mass than S. cerevisiae (Weber et al., 2010). Zymomonas mobilis is capable of fermenting glucose, fructose, and sucrose but not other forms of sugars. Because pentoses such as xylose and arabinose, which are abundantly contained in lignocellulosic biomass, cannot be effectively fermented by Z. mobilis, various catabolic genes have been transferred into Z. mobilis to broaden the types of sugars that can be effectively fermented by Z. mobilis to produce bioethanol.
b Zymobacter palmae
Zymobacter palmae, a Gram-negative, facultative anaerobic bacterium, was isolated from palm sap in Okinawa prefecture, Japan. It can ferment glucose, fructose, galactose, mannose, sucrose, maltose, melibiose, raffinose, mannitol, and sorbitol. To broaden the range of its fermentable sugar substrates, including the pentose sugar xylose, Escherichia coli genes encoded with the xylose catabolic enzymes such as xylose isomerase, xylulokinase, transaldolase and transketolase, have been introduced into Z. palmae, where their expression is driven by the Z. mobilis glyceraldehyde-3-phosphate dehydrogenase promoter. This new strain produces 91% of the theoretical yield of ethanol from xylose (Yanase et al., 2007).
c Thermophilic Bacteria
Thermophilic bacteria generally possess unique thermostable enzymes for efficient hydrolyses of biomass to ferment a broad range of carbohydrates including pentoses into ethanol (Chang and Yao, 2011). Because thermophilic bacteria are capable of fermenting both hexoses and pentoses, they are suitable for producing bioethanol from lignocellulosic biomass. However, they have a much lower ethanol production rate than S. cerevisiae, and they exhibit low tolerance to ethanol and toxic substances. Therefore, industrial application of thermophilic bacteria is still very limited.
Clostridium thermocellum has cellulosome, and it converts celluloses and hemicelluloses into ethanol directly at high temperature (60–70°C), which reduces the risk of contamination. However, its ethanol tolerance is as low as 2% (v/v). Thermoanaerobacterium saccharolyticum grows at 45–65°C; it is capable of fermenting hemicellulose and xylan directly. There are many other thermophilic bacteria that have been reported to utilize lignocellulosic biomass. These include Geobacillus thermoglucosidasius, Thermoanaerobacterium saccharolyticum, Thermoanaerobacterium ethanolicum, Thermoanaerobacterium pseudethanolicus, and Thermoanaerobacter brockii.
d Clostridium phytofermentans
Clostridium phytofermentans is a mesophilic, anaerobic Gram-positive bacterium. It is capable of fermenting almost all types of carbohydrates contained in lignocellulosic biomass, including hexoses, pentoses, oligosaccharides, and polysaccharides, to produce ethanol and hydrogen as the major metabolic end products (Warnick et al., 2002).
e Escherichia coli
Escherichia coli has a near complete assimilating pathway for utilization of hexoses and pentoses; they are used as a host microorganism to improve bioethanol production by genetic engineers. Zymomonas mobilis genes for pyruvate decarboxylase and alcohol dehydrogenase are integrated into the E. coli chromosome (Ohta et al., 1991). The modified E. coli, known as strain KO11, is used for industrial ethanol production from waste woods. Disadvantages of the modified E. coli strain include its low resistance to ethanol and incapability of surviving beyond a narrow neutral pH range of 6.0–8.0.
f Corynebacterium glutamicum
Corynebacterium glutamicum is well known as an industrial organism for the production of amino acids. A genetically engineered strain is constructed by introducing pyruvate decarboxylase and alcohol dehydrogenase genes from Z. mobilis. Ethanol production by this modified strain occurs in the absence of cell growth and thus is not affected by the presence of toxic substances such as furfural, 5-hydroxymethyl-2-furaldehyde, and 4-hydroxybenzaldehyde, so that the ethanol production is proportional to cell density (Inui et al., 2004).
10.3 Methods of Ethanol Fermentation
10.3.1 Raw Materials for Ethanol Fermentation
a Saccharides (Sucrose, Glucose, Fructose, and Lactose)
The saccharides sucrose, glucose, and fructose are converted to ethanol by using a simple process that does not involve the saccharification process so that this process is especially useful for starch-based raw materials. These saccharides are contained abundantly in crystallized sugar cane juice, beet juice, and molasses. Because sugar cane juice and beet juice cannot be stored for more than a few days, their use is seasonally limited. Additionally, because these raw materials do not contain insoluble substances, they are suitable for cell-recycle fermentation as currently implemented in Brazil to produce most bioethanol from sugar cane juice and molasses.
Lactose, β-D-galactopyranosyl-(1 → 4)-D-glucose, is a disaccharide found most notably in milk. Lactose fermentation occurs in two steps: (1) lactose is hydrolyzed to glucose and galactose by β-galactosidase, and (2) the resulting sugars are converted to ethanol via the EM pathway. Whey is a by-product during the manufacture of cheese and casein; it contains about 4% lactose that can be used as a raw material for ethanol production. In New Zealand and Ireland, industrial ethanol production from whey started in the 1970s, and fuel ethanol production has been in operation since 2005 (Guimarães et al., 2010).
b Starch
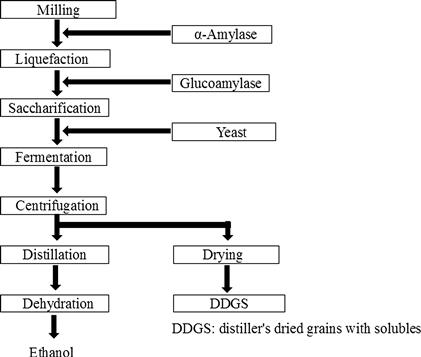
Starchy raw materials such as corn, cassava, sweet potato, rice, and wheat have been used to produce beverages since ancient times. These raw materials are liquefied by α-amylase and then hydrolyzed to glucose by glucoamylase.
In the USA, bioethanol has been produced using corn by means of wet or dry milling, and nowadays dry milling is the preferred method of processing (Figure 10.4). In this process, ground corn kernels are blended with water and α-amylase to hydrolyze starch into smaller sugar chains at 90–110°C. These fragments are saccharified to glucose by glucoamylase at 50–60°C, and the resulting glucose is converted to ethanol by S. cerevisiae at 30°C.
c Lignocellulosic Biomass
Lignocellulosic biomass refers to agricultural residues such as rice straw, wheat straw, corn stover, bagasse, and plant residues. These biomasses that are the most abundant sustainable raw materials worldwide and are attractive feedstock for bioethanol production because their use as raw materials for bioenergy does not deplete sources of food and animal feed.
Ethanol production from these raw materials is more complicated than that from starchy raw material. Lignocellulosic biomass must be hydrolyzed to fermentable monosaccharide, hexoses and pentoses, but there are problems with this process. Cellulose and hemicellulose are densely packed by layers of lignin that protect cellulose and hemicellulose against enzymatic hydrolyses. Hence, breaking the lignin layers to expose cellulose and hemicellulose by decreasing the crystallinity of cellulose, increasing biomass surface area, removing hemicellulose, and breaking the lignin seal to facilitate subsequent enzyme action is a necessary but expensive pretreatment. Hence, an efficient, rapid, and complete enzymatic hydrolysis to pretreat the biomass is one of the major technical and economical bottlenecks in the overall bioconversion process of lignocellulose to bioethanol. There is another concern in the production of bioethanol from lignocellulosic biomass; a microorganism to produce ethanol efficiently from the second abundant sugar, xylose, has not yet been discovered. Therefore, much genetic engineering research has been devoted to finding microorganisms that are able to ferment xylose efficiently.
10.3.2 Fermentation Technology
a Batch Fermentation
In batch fermentation, substrate and microorganism are loaded into the fermenter batchwise, and this is the most popular and simple method for ethanol production. Batch fermentation has the advantages of low investment costs, simple control and operations, and easy-to-maintain complete sterilization. However, seed culture is needed for each new batch. Bioethanol from corn in the USA is almost entirely produced using batch fermentation.
A higher initial sugar concentration is required in order to achieve more efficient ethanol production; however, a high sugar concentration will inhibit the growth and function of fermenting microorganisms due to excessive osmosis to result in a low fermentation yield with a prolonged fermentation period. The batchwise fermentation method will alleviate this drawback. Fermentation is started with a low initial sugar concentration to allow robust growth of the microorganisms at an early stage, and sugar is added periodically when it is consumed.
b Semi-Continuous Fermentation
(i) Melle–Boinot Method
In semi-continuous processes, a portion of fermented broth is withdrawn at intervals and fresh medium is added to the system. There is no need to inoculate the reactor except during the initial startup.
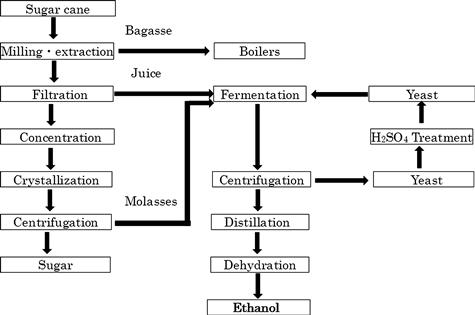
The Melle–Boinot method is the most common fermentation process in Brazil to produce about 85% ethanol from cane juice or cane molasses. The initial processing stage for bioethanol production is basically the same as that for sugar production (Figure 10.5). Fermentation is carried out with sugar cane juice, water-diluted molasses, or mixtures of juice and molasses within 6–12 hours. At the end of fermentation, the ethanol concentration reaches 7–11% (v/v). The fermented broth is centrifuged to separate yeasts from liquid containing ethanol. The liquid is distilled to yield ethanol whereas the recovered yeast cells are treated with sulfuric acid (pH 2.0–2.5) to prevent bacterial contamination. After 2–3 hours of acid treatment, the yeast cells are returned to fermentation tanks for the next round of fermentation. Yeast cells are recycled 400–600 times during the sugar cane harvest season. However, there is a high risk of contamination and mutation of the yeast during the long-term cultivation and periodic handling (Amorim et al., 2011).
(ii) Ethanol Fermentation by Flocculent Yeast
The use of flocculent yeast reduces the cost of recovering yeast cells because flocculent cells are easily separated from medium without centrifugation during ethanol production in batch or fed-batch fermentation. The fermented broth is left quiescent without agitation for a short time, and yeast cells agglomerate to one another to form large flocs that settle rapidly to the bottom of the fermenter. After 70–90% volume of fermented broth is withdrawn, fresh sugar solution is added to the fermenter to start the next round of fermentation.
Flocculent yeast fermentation has a major advantage in that efficient ethanol production is achieved with a short fermentation period because a high density of yeast culture can be accumulated in the fermenter. The recovery of yeast cells is easier than the recovery of non-flocculent microbial mass from semi-batch fermentation such as the Melle–Boinot method, and the process is not as complicated as the yeast immobilization method. The mechanism of yeast flocculation, which is controlled by cell wall components, is very complicated. Flocculation is affected by many factors such as morphology of cells, ethanol concentration, nutrients, dissolved oxygen, pH, metals, and fermentation temperature, among many others.
c Continuous-Flow Fermentation
(i) Cell Recycling
In a continuous-flow process, sugar solution is continuously fed to a fermenter while the fermenter content is simultaneously withdrawn. The withdrawn fermented broth is fed to a centrifuge to be separated from the solution; the separated yeast cells are returned to the fermenter, and the liquid is fed to a distillation process to recover ethanol. Most of the ethanol-depleted fermented broth is pumped back to the fermenter to reduce water consumption. Because fermentation and distillation are conducted simultaneously to keep sugar concentrations in the fermenter at a low level, inhibition of ethanol production due to high sugar concentrations is alleviated in this method.
(ii) Immobilized Microbial Cells
Microbial cells are immobilized by mixing them with a polysaccharide such as sodium alginate or κ-carrageenan. The mixture is dropped into salt solutions to form gel particles. Trace dissolved oxygen in the solution will enable the microorganisms to grow prolifically near the surface of the gel particles. Therefore, the overall cell density in the reactor during fermentation becomes about 10 times higher than that in batch fermentation. As a result, the fermentation period can be shortened and the productivity of ethanol becomes highly efficient. Advantages of this method are smaller reactor size, greater feasibility of continuous processing, and shorter startup time. The reactor behaves as a fluidized bed that is agitated by the carbon dioxide produced during fermentation in the reactor. However, there are risks of contamination and poor activities for the microbial cells trapped in the gel.
10.3.3 Fermentation Process
a Separate Hydrolysis and Fermentation (SHF)
SHF is a method by which enzymatic hydrolysis and fermentation are performed sequentially. In this process, enzymatic saccharification of starchy biomass or pretreated lignocellulosic biomass is carried out first at the optimal temperature of the saccharifying enzyme. Subsequently, appropriate microorganisms are added to ferment the saccharified solution. In the SHF process, the temperatures of the enzymatic hydrolysis and fermentation can be optimized independently. Because enzymatic hydrolyses are performed at optimal temperature, this process requires a smaller quantity of saccharifying enzymes than the simultaneous saccharification and fermentation process.
Additionally, because saccharified solution containing fermentable sugar can be sterilized, the risk of contamination is reduced. However, the SHF process is carried out in two separate processes that use two independent reactors for the saccharification and fermentation processes; the capital cost is therefore higher than that for the simultaneous process.
b Simultaneous Saccharification and Fermentation (SSF)
SSF is a method by which enzymatic hydrolysis and fermentation are performed simultaneously in the same reactor. The combination of saccharification and fermentation can decrease the number of vessels needed and thereby reduce the initial costs. Using the SSF process eliminates the inhibition of saccharifying enzyme by sugars because the resulting sugars are immediately converted to ethanol by fermentation microorganisms (see Box 10.1). However, the SSF process has disadvantages when compared with the SHF process. The optimum temperature for yeast fermentation is typically lower than that for enzymatic hydrolysis. Therefore, the saccharification process requires more enzyme than the SHF process. Thermotolerant microorganisms have been developed to ferment sugars at an elevated temperature that is optimal for saccharification. In the case of ethanol production using lignocellulosic biomass as raw material, recycling yeast is very difficult because the fermented broth contains many insoluble solids.
Simultaneous saccharification and co-fermentation (SSCF) of hexoses and pentoses is a process similar to the SSF process except that hexose and pentose fermentation occurs simultaneously. SSCF offers the potential of streamlined processing while reducing capital costs.
c Consolidated Bioprocessing (CBP)
In the CBP process, enzymetic hydrolysis and fermentation take place in one vessel using a single species of microorganism or a co-culture of microorganisms. The CBP process is simple, and therefore both operation and capital costs associated with enzyme production are reduced.
Naturally occurring microorganisms are incapable of producing saccharolytic enzymes to convert the released sugars into ethanol simultaneously. Hence, engineered microorganisms need to be developed in order to make this process suitable for industrial applications.
References
1. Agbogbo FK, Coward-Kelly G. Cellulosic ethanol production using the naturally occurring xylose-fermenting yeast Pichia stipitis. Biotechnology letters. 2008;30:1515–1524.
2. Amorim HV, Lopes ML, Castro Oliveira JV, Buckeridge MS, Goldman GH. Scientific challenges of bioethanol production in Brazil. Applied microbiology and biotechnology. 2011;91:1267–1275.
3. Chang T, Yao S. Thermophilic, lignocellulolytic bacteria for ethanol production: Current state and perspectives. Applied microbiology and biotechnology. 2011;92:13–17.
4. Guimarães PMR, Teixeira JA, Domingues L. Fermentaion of lactose to bio-ethanol by yeasts as part of integrated solution for the valorization of cheese whey. Biotechnology advances. 2010;28:375–384.
5. Inui M, Kawaguchi H, Murakami S, Vertès AA, Yukawa H. Metabolic engineering of Corynebacterium glutamicum for fuel ethanol production under oxygen-deprivation conditions. Journal of molecular microbiology and biotechnology. 2004;8:243–254.
6. Laluce C, Schenberg ACG, Gallard JCM, Coradello LFC, Pombeiro-Sponchiado SR. Advances and developments in strategies to improve strains of Saccharomyces cerevisiae and processes to obtain the lignocellulosic ethanol – A review. Applied biochemistry and biotechnology. 2012;166:1908–1926.
7. Ohta K, Beall DS, Mejia JP, Shanmugam KT, Ingram LO. Genetic improvement of Escherichia coli for ethanol production: Chromosomal integration of Zymomonas mobilis genes encoding pyruvate decarboxylase and alcohol dehydrogenase II.. Applied and environmental microbiology. 1991;57:893–900.
8. Rodrussamee N, Lertwattanasakul N, Hirata K, et al. Growth and ethanol fermentation ability on hexose and pentose sugars and glucose effect under various conditions in thermotolerant yeast Kluveromyces marxianus. Applied microbiology and biotechnology. 2011;90:1573–1586.
9. Warnick TA, Methe BA, Leschine SB. 11.2.2.4 Clostridium phytofermentans sp.nov., a cellulolytic mesophile from forest soil. International journal of systematic and evolutionary microbiology. 2002;52:1155–1160.
10. Weber C, Farwick A, Benisch F, et al. Trends and challenges in the microbial production of lignocellulosic bioethanol fuels. Applied microbiology and biotechnology. 2010;87:1303–1315.
11. Yanase H, Sato D, Yamamoto K, Matsuda S, Yamamoto S, Okamoto K. Genetic engineering of Zymobacter palmae for production of ethanol from xylose. Applied and environmental microbiology. 2007;73:2592–2599.