Energy-Saving Biomass Processing with Polar Ionic Liquids
Yukinobu Fukaya and Hiroyuki Ohno
Abstract
Ionic liquids (ILs), which are organic salts with melting points of lower than 100°C, have been prepared as potential solvents for extracting cellulose. The hydrogen-bonding basicity (β value) of ILs is an important parameter for evaluating their capability to dissolve cellulose. Some polar ILs with large β values have been synthesized based on the relationship between ion structure and the β value of the corresponding ILs. Some other ILs containing carboxylate anions or phosphonate derivatives have been confirmed to dissolve cellulose under mild conditions. Bran, a typical biomass, was mixed with polar ILs to extract cellulose. The solid crosslinked lignin was filtered out, and the filtrate was mixed with excess methanol to precipitate cellulose and hemicellulose. Because both ILs and methanol can be used repeatedly, this process can be operated in a closed system to separate lignin and polysaccharides (mainly cellulose).
Keywords
Ionic liquid; polarity; hydrogen bond; extraction; cellulose
Chapter Outline
8.1. Cellulose Dissolution and Ionic Liquids
8.1.2. Initial Stage of Ionic Liquid Design as Solvents for Cellulose
8.2. Required Factors of Ionic Liquids for Cellulose Dissolution
8.2.1. Interaction of Cellulose and Ionic Liquids
8.2.2. Hydrogen-Bonding Characteristics of ILs
8.2.3. Polar ILs Containing Carboxylate Anions
8.3. Design of a New Class of Polar Ionic Liquids
8.3.1. Requirement of New Polar ILs
8.3.2. Facile Preparation of Polar ILs
8.3.3. Further Design of Polar ILs
8.4. Polar Ionic Liquids for Biomass Processing without Heating
8.4.1. Physicochemical Properties of New Polar ILs
8.4.2. Extraction of Cellulose from Bran with Phosphonate-Type ILs
8.4.3. Extraction of Cellulose from Bran with Phosphinate-Type ILs
8.1 Cellulose Dissolution and Ionic Liquids
8.1.1 Cellulose Dissolution
Cellulosic biomass is the most abundant bioresource produced on earth. In view of a perpetual supply of food, converting edible food into fuel is out of the question in spite of the easiness of producing bioethanol from starch. The process of producing biofuel from inedible biomass has been developed for the purpose of preserving edible food. Use of ubiquitously available cellulosic biomass should be a key strategy for supplying sustainable energy. The polysaccharides contained in biomass are insoluble in water owing to their intra- and intermolecular hydrogen bonding. Moreover, polysaccharides exist as supramolecular complexes in nature that are almost impossible to dissolve in water at the molecular level; thus, they are not easily extracted from biomass under mild conditions. In order to realize the bioenergy conversion with minimum consumption of energy, implementing the following steps is especially important: (1) dissolution and extraction of cellulose from biomass; (2) depolymerization of cellulose to glucose or oligosaccharides; and (3) energy conversion of these saccharides to generate electrical energy. In any process to generate energy from the biomass, the energy consumed should be kept as low as possible. At present, the energy needed to generate energy from polysaccharides is greater than the total energy yielded.
8.1.2 Initial Stage of Ionic Liquid Design as Solvents for Cellulose
Since it is not easy to produce energy from biomass with molecular liquids, there is increasing attention on using ionic liquids (ILs) as a new class of solvents for enhancing the production of energy from biomass (Rogers and Seddon, 2003). ILs are organic salts that exist as liquid at normal room temperature or ambient temperature below 100°C (Welton, 1999). They have unique characteristics that include extremely low volatility, low flammability, and high thermal stability. Because of these promising features, ILs have been studied extensively to examine their physicochemical properties as well as their potential applications as solvents and electrolytes. There have been two major waves of studying ILs as a new class of solvents (Wassersheid and Welton, 2008) and electrolyte materials used in energy-related devices (Ohno, 2011). The application of ILs in bioscience is set to become the third wave; processing of biomass with ILs to enhance energy conversion from biomass is a big issue (Ohno and Fukaya, 2009).
ILs are composed wholly of ions; they are often mistakenly regarded as highly polar liquids. However, not all ILs show high polarity (Anderson et al., 2002); the physicochemical property essential for using the solvent is its polarity in addition to its hydrogen-bonding ability, affinity with compounds, viscosity, and usable temperature range as liquid. All these properties can be tuned by designing the component ions. The diversity of organic ions leads to the development of ILs with desired characteristics and functions. If designed appropriately, the component ions contained in ILs give rise to polar ILs that exhibit much higher polarity than conventional molecular liquids. Hence, ILs with unusual high polarity should be the next generation of liquids used to dissolve molecular insoluble materials such as components in biomass in an efficient and environmental-friendly closed system for producing bioenergy.
The challenge of dissolving cellulose with ILs has been considered over the past decade. Rogers et al. (2006) first demonstrated that some ILs are capable of solubilizing cellulose (Swatloski et al., 2002). They studied a series of 1-n-butyl-3-methylimidazolium ([C4mim]) salts as solvents for solubilizing cellulose and found that 1-n-butyl-3-methylimidazolium chloride ([C4mim]Cl) solubilizes cellulose under conventional heating conditions. For example, clear solutions containing 3 wt% and 10 wt% pulp cellulose (DP ≈ 1000) can be obtained by simply mixing the cellulose with [C4mim]Cl at 70 and 100°C respectively. After this pioneering work, numerous papers have been published on cellulose dissolution and cellulose product fabrication, among many other related topics.
In spite of the potential of using chloride-based salts as solvents for dissolving cellulose and even other biomass components, most chloride salts have relatively high melting temperature. In order to improve the physicochemical properties of [C4mim]Cl, several ILs with low melting point and relatively low viscosity have been synthesized to overcome the drawbacks of the chloride salts. For instance, introducing an allyl group instead of a butyl group onto the imidazolium cation was found to give relatively low viscosity and low melting temperature to the resulting chloride salts (Mizumo et al., 2004). While these chloride salts are obtained as liquid at room temperature through the modification of cations, they are still highly viscous and accordingly inadequate to be used as solvents under mild conditions.
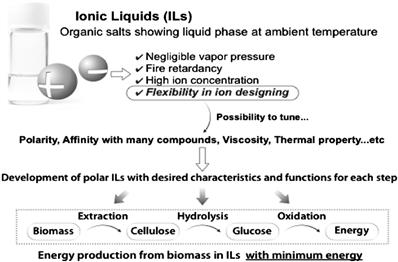
We have focused on the flexibility of ion selection, and developed a new class of polar ILs suitable for carrying out biomass processing under mild conditions to achieve energy-saving biomass processing. Figure 8.1 shows the strategy to obtain energy from biomass using the new class of polar ILs. Three steps are involved in this strategy: (1) extraction of cellulose from biomass; (2) hydrolysis of cellulose into glucose; and (3) electrochemical oxidation of glucose into electric energy. In this chapter, the first step, namely the extraction of cellulose from biomass, is described.
8.2 Required Factors of Ionic Liquids for Cellulose Dissolution
8.2.1 Interaction of Cellulose and Ionic Liquids
The conditions for ILs to dissolve cellulose are first explored in order to develop a new class of polar ILs that is capable of dissolving cellulose under mild conditions. Chloride salts have been used by Rogers and colleagues for studying the solubilizing mechanism of cellulose by ILs using NMR spectroscopy (Moulthrop et al., 2005). The 13C-NMR spectra of cellulose in chloride salts show that cellulose is totally disordered in ILs. Moreover, Monya and colleagues determined the NMR longitudinal (T1) and transverse (T2) relaxation times of [C4mim]Cl, both for the cation (13C) and the chloride anion (35/37Cl) in order to examine the interaction between IL and cellulose at the molecular level (Remsing et al., 2006). These relaxation times were analyzed as functions of concentrations of cellobiose, glucose, and glucose pentaacetate because, in general, variations in relaxation times yield useful information about the molecular dynamics of the compound. Investigation of the relaxation time as a function of increasing cellobiose concentration reveals that the interaction between chloride anion and the sugar unit is very strong; glucose interacts strongly with the anion whereas glucose pentaacetate has almost no effect on the relaxation rate of 35Cl. The dissolving of cellobiose or glucose in [C4mim]Cl involves the formation of hydrogen bonds between the Cl anions of ILs and the hydroxyl groups of sugar with a stoichiometric ratio. Youngs et al. (2006) investigated the interaction between D-glucose (model compound) and [C4mim]Cl by means of molecular dynamic simulation. Their findings reveal that the cations interact only weakly with the sugar; this agrees with the results of previous studies that the most dominant interaction is clearly between chloride anions and the cellulose hydroxyl groups.
8.2.2 Hydrogen-Bonding Characteristics of ILs
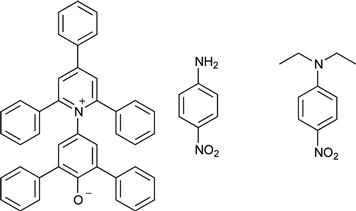
The hydrogen-bonding characteristics of ILs have been empirically determined based on their solvent-dependent absorptions or emissions measured with a variety of solvatochromic probes (Crowhurst et al., 2003; Reichardt, 2005). While ET(30) (in kcal mol−1) values proposed by Crowhurst et al. have been widely used as an empirical polarity scale, the ET(30) values are generally affected by the basic structure of cations as well as by anion and alkyl chain length to some extent. On the other hand, three Kamlet–Taft parameters, i.e. the hydrogen-bonding acidity (α), hydrogen bonding basicity (β), and dipolarity/polarizability (π∗), are quite useful for discussing the polarity of ILs (Kamlet and Taft, 1976). These parameters are determined by using visible spectral measurements of prove dyes as shown in Chart 8.1.
8.2.3 Polar ILs Containing Carboxylate Anions
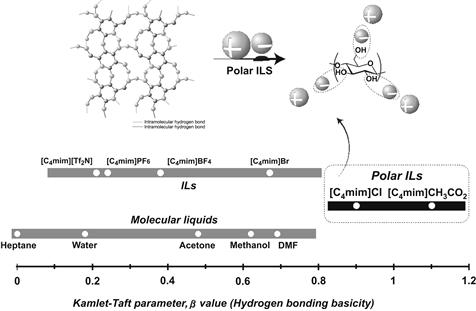
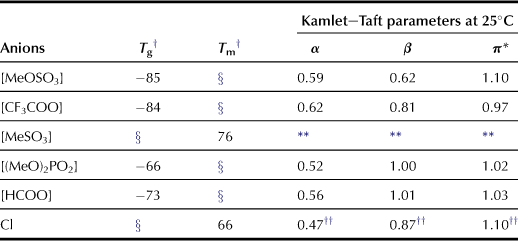
The basic properties of a series of ILs have been analyzed especially for their hydrogen-bonging basicity, i.e. Kamlet–Taft parameter β value. Previous studies on the Kamlet–Taft parameters have shown that the β value strongly depends on the anion structure. Results of Kamlet–Taft parameter measurements reveal that chloride anions show strong hydrogen-bonding basicity. On the other hand, salts containing Br−, PF6−, or BF4− show lower hydrogen-bonding basicity so that they are considered inappropriate for dissolving cellulose.
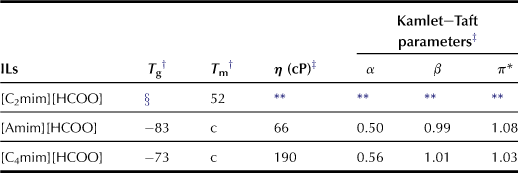
A series of alkylimidazolium salts were prepared by coupling with various anions, and the carboxylate-based ILs show higher β values than conventional ILs (Fukaya et al., 2006). Alkylimidazolium acetate dissolves cellulose at lower temperature than chloride salts (Figure 8.2). The authors examined a series of carboxylate salts with different side chains, i.e. formate, propionate, and butylate. These carboxylate series show higher hydrogen-bonding characteristics (Table 8.1); the results clearly indicate that carboxylate anions are effective for preparing highly polar ILs with strong hydrogen-bonding basicity.
TABLE 8.1
Transition Temperature and Kamlet–Taft parameters of ILs
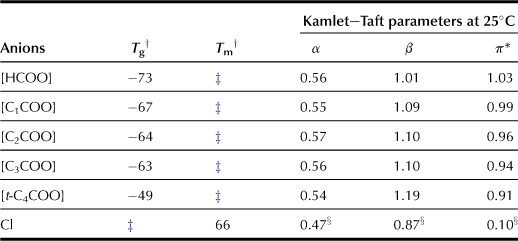
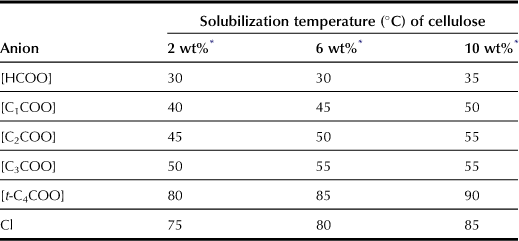
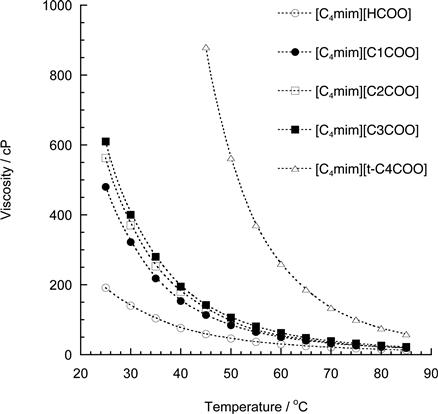
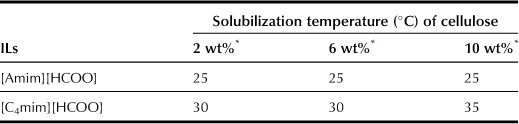
Compared with chloride salts, carboxylate-based ILs are effective for cellulose dissolution (Table 8.2). For example, to dissolve 10 wt% cellulose, [C4mim]Cl needs to be heated to 85°C whereas [C4mim][HCOO] will dissolve 10 wt% cellulose at 35°C. As mentioned in previous paragraphs, the hydrogen bond basicity is necessary to dissolve cellulose by weakening the inter- and intramolecular hydrogen bonds of the cellulose chains. Because [t-C4COO] salt exhibits higher hydrogen bond basicity (β value) than any other carboxylate salts, it is expected to dissolve cellulose more efficiently than the latter. However, the temperature for [t-C4COO] salt to solubilize cellulose is higher than that for other ILs evaluated in this study. The alkyl side chain of carboxylate anions may have an effect on the solubilization of cellulose. The cellulose solubilization temperature increases with larger alkyl side chains for the imidazolium cation but the temperature tends to decrease with decreasing viscosity (Figure 8.3). The viscosity of ILs appears to be a further important factor in dissolving cellulose under mild conditions because cellulose powder is dispersed more efficiently in ILs with lower viscosity.
ILs composed of imidazolium salts with a short alkyl side chain tend to have low viscosity. Moreover, allylimidazolium-based salts have been reported to result in ILs with low viscosity as well. Hence, novel formate salts can be prepared with both 1-ethyl-3-methylimidazolium ([C2mim]) and 1-allyl-3-methylimidazolium ([Amim]) cations to render lower viscosity to the resulting salts (Chart 8.2). Because [Amim][HCOO] has a low viscosity of 66 cP at 25°C, and high hydrogen bond basicity, cellulose can be dissolved in concentrated [Amim][HCOO] under mild conditions (Tables 8.3 and 8.4).
In spite of their strong hydrogen-bonding basicity and potential to solubilize cellulose, carboxylate salts have relatively poor thermal stability. Furthermore, carboxylate-type ILs need to be prepared by a three-step reaction: (1) imidazole quternizes with alkyl halide; (2) it is converted to hydroxide; and (3) it is subsequently coupled with the desired carboxylate anion. Designing a new class of ILs that are easy to prepare with sufficient polarity to dissolve cellulose is of great importance. The drawbacks associated with current ILs have motivated us to develop further new polar ILs with satisfactory thermal stability.
8.3 Design of a New Class of Polar Ionic Liquids
8.3.1 Requirement of New Polar ILs
As previously discussed, ILs such as 1,3-dialkylimidazolium chloride salts are capable of dissolving cellulose without any pretreatment under conventional heating conditions. 1,3-Dialkylimidazolium chloride salts can be readily prepared easily by using the one-pot procedure. However, this procedure has two major disadvantages: (i) it is a time-consuming process and (ii) an excess of alkyl halide is required to achieve good yield. The latter renders an undesired polluting nature to the quaternization process, especially when long chain derivatives are produced. Alkyl halides with long alkyl chains are difficult to remove from the reaction mixture due to their high boiling point. This disadvantage makes the procedure costly and inefficient for synthesizing 1,3-dialkylimidazolium chloride. Moreover, most 1,3-dialkylimidazolium chloride ILs are obtained as a solid or sticky paste at room temperature; they must be handled under heating. This makes the processing of cellulose in 1,3-dialkyl-imidazolium chloride even more costly.
For efficient processing of cellulose, heating should be avoided to reduce the energy cost. In Section 8.2, we proposed a series of novel ILs, namely 1,3-dialkylimidazolium carboxylate, as potential candidates for solubilization of cellulose. These 1,3-dialkylimidazolium carboxylates were obtained as low-viscosity liquids at room temperature. Moreover, they are more effective in solubilizing cellulose at lower temperature than chloride salts. However, 1,3-dialkylimidazolium carboxylates show relatively poor thermal stability. Furthermore, 1,3-dialkylimidazolium carboxylates are prepared by following the aforementioned three-step reactions in which the halide counter anion of the imidazolium cation is first converted to hydroxide, then coupled with the desired carboxylate anion (Scheme 8.1).
8.3.2 Facile Preparation of Polar ILs
To overcome the above drawbacks, a new class of easily preparable ILs having sufficient polarity to dissolve cellulose is necessary. Preparing ILs based on the reaction between tertiary amines and alkyl esters of organic acids to form quaternized salts with desired anions derived from acid esters can be carried out using a straightforward one-pot procedure (Scheme 8.2).
Because many types of acid esters are commercially available, several alkylimidazolium salts having alkylsulfate, alkylsulfonate, and alkylphosphate anion derivatives can now be prepared by using the one-step quaternization procedure. However, there has been no evaluation of the ILs thus prepared as solvents for cellulose. Therefore, the preparation of a series of ILs with fixed cations, i.e. 1-n-butyl-3-methylimidazolium ([C4mim]) cation, and a wide variety of anions using the one-pot procedure is described in this chapter.
Here the ILs that are readily prepared are emphasized. Among numerous methods for preparing ILs, the straightforward one-pot reaction between tertiary amines and alkyl esters to form quaternized salts with anions derived from acid esters (Fukaya et al., 2008) is presented. The 1-butyl-3-methylimidazolium ([C4mim]) salts, with anions such as methanesulfonate ([MeSO3]), methylsulfate ([MeOSO3]), and dimethylphosphate ([(MeO)2PO2]), were synthesized and evaluated as solvents for dissolving cellulose. The polarity and hydrogen-bonding characteristics of these ILs were evaluated as the Kamlet–Taft parameters at room temperature. Among these prepared ILs, [C4mim][(MeO)2PO2] exhibits higher hydrogen bond basicity (β = 1.00) than [MeOSO3] (β = 0.61) or [C4mim][MeSO3] (β = 0.70). As expected based on the β value, only [C4mim][(MeO)2PO2] is confirmed to dissolve cellulose (Table 8.5).
8.3.3 Further Design of Polar ILs
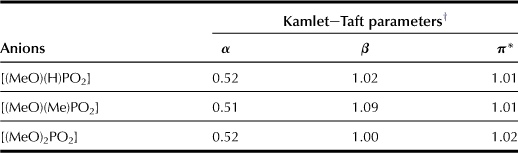
To improve the physicochemical properties and cellulose solubility of the prepared ILs, further investigation was undertaken in order to reveal better anions. As discussed in previous sections, alkyl chains attached to the anion strongly affect solvent properties of the ILs. The reaction between 1-ethylimidazole and the corresponding dimethyl phosphite or dimethyl phosphonate among the many anions examined including alkyl phosphonate and alkyl phosphite can be used for easy preparation of both [C4mim][(MeO)(H)PO2] and [C4mim][(MeO)(Me)PO2].
These ILs have almost similar π∗ and α values. On the other hand, the ILs prepared here have high β value that exceeds 1.0, e.g. 1.07 for the β value of [C4mim][(MeO)(Me)PO2]. The phosphate derivative ILs as prepared here generally has better thermal stability than previously reported polar ILs; the prepared [C4mim][(MeO)(R)PO2] salts are thermally stable at temperatures up to 260–290°C. Their viscosity at 25°C is in the range of 100–500 cP, which is much lower than that for chloride salts (Tables 8.6 and 8.7).
TABLE 8.6
Thermal Properties of [C4mim]-Type Salts
| Anion | Tg∗ | Tm∗ |
| [(MeO)(H)PO2] | −77 | –† |
| [(MeO)(Me)PO2] | −65 | –† |
| [(MeO)2PO2] | −66 | –† |
∗Temperature (°C) at signal peak.
†Not detected.
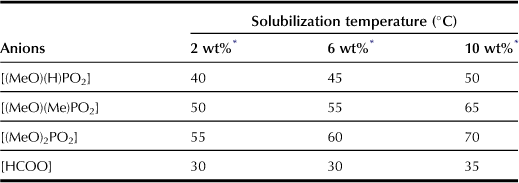
These ILs are capable of dissolving cellulose owing to their strong hydrogen-bonding basicity (Table 8.8). The temperature needed to solubilize the same amount of cellulose powder depends strongly on the anionic structure of the ILs. Cellulose powder of 2.0 wt% concentration is dissolved completely in these ILs at room temperature (25°C) within 3 hours, and the 4.0 wt% cellulose powder is completely dissolved within 5 hours. These ILs also act as solvents for a series of naturally obtained cellulose samples of high molecular weights, including cotton. These results strongly suggest that using phosphate and phosphonate derivative anions is effective in designing ILs with high polarity, low viscosity, and high thermal stability.
8.4 Polar Ionic Liquids for Biomass Processing without Heating
8.4.1 Physicochemical Properties of New Polar ILs
Recent efforts have been concentrated on dissolving naturally obtained cellulosic biomass, and several previous reports have indicated that certain ILs can dissolve cellulosic biomass. Previously, the extraction of polysaccharides from plant biomass using ILs required continuous stirring at temperatures above 100°C for at least 24 hours. Much energy can be saved if the extraction is carried out rapidly at 50°C or below. As mentioned above, 1-ethyl-3-methylimidazolium methylphosphonate is effective for processing cellulosic biomass that consists of three main fractions: cellulose, hemicellulose, and lignin. The authors focused on the factors relevant to using ILs to dissolve and extract component cellulose from biomass under mild conditions (Abe et al., 2010). Because there is a wide variety of commercially available phosphonate-derived acid esters and alkyl halides, it is a good idea to combine diverse dialkylimidazolium cations with phosphonate-derived materials as anions in the search for polar ILs. The extraction of polysaccharides from biomass using a series of alkylimidazolium salts coupled with phosphonate-derived anions was investigated with respect to the requirements for efficient biomass processing under mild conditions.
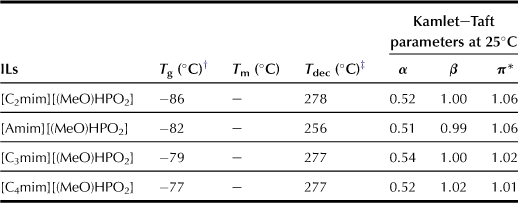
To determine the principal factor governing the degree of extraction and find the ILs that are better suited for extracting cellulose, the authors have prepared a series of new ILs through modification of both cations and anions. The effect of alkyl side chain attached to the imidazolium cation ring on the extraction of polysaccharides from bran was analyzed by studying the effect of four cations with different alkyl side chain lengths on the amount of polysaccharide extracted (Chart 8.3). All the ILs prepared in this study were in liquid form at room temperature. They have one glass transition temperature at around −80°C, and no crystallization was detected when these ILs were kept at −20°C for more than 2 weeks. Results of thermogravimetric analysis (TGA) show that all these ILs are stable under temperature of up to 250°C (Table 8.9).
The viscosity of these ILs were subsequently determined, and the results reveal that they are liquids of relatively low viscosity. These physicochemical properties clearly indicate that the ILs prepared in this study are potential solvents for extracting cellulose and other polysaccharides.
8.4.2 Extraction of Cellulose from Bran with Phosphonate-Type ILs
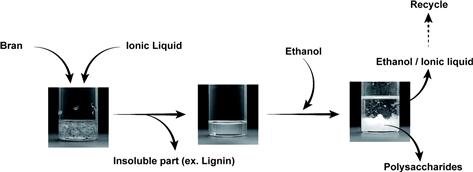
The extraction of polysaccharides from bran is described herein as an example of cellulose extraction from biomass. Bran is the hard shell of wheat and comprises complexes of polysaccharides and lignin, thus making it a sparingly soluble material. The extraction and dissolution of polysaccharides from bran (Figure 8.4) was carried out by mixing bran (0.15 g) with ILs (2.85 g), and gently stirring the mixtures at 50°C to disperse bran in the ILs. Some polysaccharides were extracted within 10 min under gentle stirring at 50°C. The ability of these ILs to solubilize major component polysaccharides of bran such as cellulose, hemicellulose, and residual starch is thus confirmed. The remaining insoluble portion that consists of lignin and its complexes was filtered out. Excess ethanol was then added to the filtrate under continuous stirring. The resulting precipitate was collected by a second filtration and washed repeatedly with ethanol to remove residual ILs. The extracted materials were analyzed using FT-IR, NMR, and TGA measurements, and the results confirm that the extracted materials are a mixture of cellulose, hemicellulose, and residual starch.
The efficiency of extracting polysaccharides from bran using the four ILs depends on the cation structure. Moreover, these ILs are capable of dissolving polysaccharides at 50°C within 10 min. As mentioned in the previous section, dissolution of cellulose within a short mixing period depends to a great extent on the viscosity and hydrogen bond basicity of ILs. These ILs show almost the same hydrogen-bonding basicity. The degree of extraction using these four ILs is, however, independent of their β value. Furthermore, there is no relation between the hydrophobicity of the cations and the degree of extraction. Figures 8.1 and 8.3 show that ILs with lower viscosity are capable of extracting more polysaccharides because of better bran dispersion in ILs with less viscosity.
As described above, 1-ethyl-3-methylimidazolium salts are efficient in extracting polysaccharides. Four 1-ethyl-3-methylimidazolium salts coupled with phosphonate derivative anions having different alkyl side chains, i.e. methylphosphonate, ethylphosphonate, isopropylphosphonate, and n-butylphosphonate anions, were prepared. As shown in Table 8.10, these ILs are stable supercool liquids showing only glass transition at around −80°C. Results of TGA analyses reveal that these ILs remain stable at temperatures of up to 250°C.
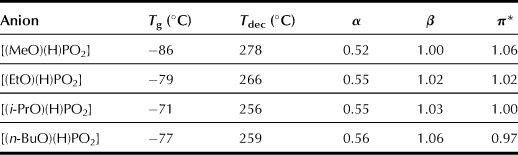
These ILs have relatively strong hydrogen-bonding characteristics, especially the β value, which is generally affected mainly by the nature of the anionic species. Additionally, they have similar but slightly different β values in the following order of magnitude: [(MeO)(H)PO2] (β = 1.00) < [(EtO)(H)PO2] (β = 1.02) < [(i-PrO)(H)PO2] (β = 1.03) < [(n-BuO)(H)PO2] (β = 1.06). Because the series of anions studied here have the same basic unit structure ([(R)(H)PO2]), the disparity between β values of these ILs can be explained by the difference in the electron-releasing capability of the alkyl side chain. As mentioned above, the viscosity of ILs that is an important factor influencing the extraction of polysaccharides from bran depends on the anionic structure of ILs due to the bulkiness of the alkoxyl groups on the anion. Hence, alkylphosphonate with a shorter alkyl chain should be used for preparing low-viscosity ILs.
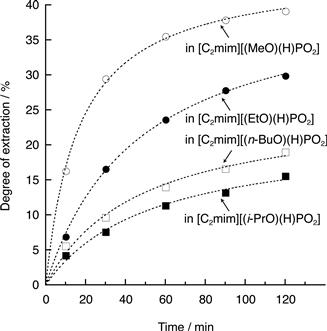
Figure 8.5 shows the degree of extraction of polysaccharides from bran by using this series of phosphonate-derived salts at 50°C. The degree of cellulose extraction after 2 hours of mixing shows the following order of magnitude: [C2mim][(i-PrO)(H)PO2] (16%) < [C2mim][(n-BuO)(H)PO2] (19%) < [C2mim][(EtO)(H)PO2] (30%) < [C2mim][(MeO)(H)PO2] (39%).
The solubility of polysaccharides depends mainly on the β value of the ILs. However, the degree of extraction of polysaccharides from bran in this experiment is not observed to depend on the β value. Among these four ILs, the β value is highest for [C2mim][(n-BuO)(H)PO2] and lowest for [C2mim][(MeO)(H)PO2]. However, [C2mim][(MeO)(H)PO2] is capable of extracting more polysaccharide than [C2mim][(n-BuO)(H)PO2]. We infer that for a series of ILs having the β value within a certain range, the amount of polysaccharide extracted from bran within a limited period is influenced more by the IL viscosity than any other factors. Among these ILs, [C2mim][(n-BuO)(H)PO2] has a larger alkyl side chain so that it has better capacity to extract polysaccharides than [C2mim][(i-PrO)(H)PO2]. The properties and interaction force estimated based on the size of the ion are not therefore primary factors that affect the degree of extraction; the IL viscosity is closely related to the degree of extraction. The ILs with lower viscosity have the best capacity to extract polysaccharides within a short period under mild conditions. Low-viscosity ILs are capable of extracting a large amount of polysaccharides at 50°C within the given time. For polar ILs with similar β values, the degree of extraction in a limited time depends strongly on the viscosity. Although [C2mim][(MeO)HPO2] and [C2mim][(i-PrO)HPO2] have similar β values (1.00 and 1.03), [C2mim][(i-PrO)HPO2] (η = 65 cP at 50°C) needs 120 min stirring at 50°C to extract ca. 15% of polysaccharides from bran, whereas [C2mim][(MeO)HPO2] (η = 31 cP at 50°C) needs only 10 min under similar conditions to extract the same quantity of polysaccharides. The solubility of cellulose in these ILs is independent of their viscosity over a very long time, but faster solubilization can be carried out using less viscous ILs. The bran is dispersed more easily in ILs with lower viscosity so that the ILs may penetrate into the complex of cellulose and lignin more easily, which leads to the observed greater solubility under mild conditions and over short times.
8.4.3 Extraction of Cellulose from Bran with Phosphinate-Type ILs
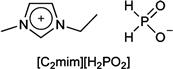
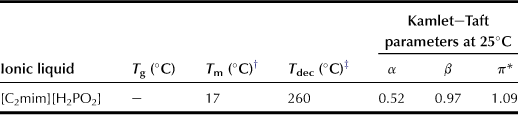
The aforementioned results strongly suggest that ILs with lower viscosity are efficient to extract polysaccharides within a short period of time when the ILs have adequate polarity. Hence, a novel IL with high hydrogen-bonding basicity and low viscosity can be designed. Imidazolium cations with short alkyl side chains generally leading to ILs with low viscosity have been confirmed by the results obtained with a short alkyl side chain of phosphonate-derived anions. The IL, i.e. 1-ethyl-3-methylimidazolium phosphinate ([C2mim][H2PO2]; Chart 8.4), is a newly designed extraction reagent. [C2mim][H2PO2] is a liquid at a room temperature (Tm) of 17°C. Results of TGA measurements indicate that this IL is stable at temperatures of up to 250°C. The Kamlet–Taft parameter measurement reveals that [C2mim][H2PO2] exhibits relatively strong hydrogen-bonding basicity. Compared with the alkylphosphonate series salts (1.00 < β < 1.06; see Table 8.11), the β value of [C2mim][H2PO2] is relatively low at 0.97. Additionally, it has the lowest viscosity in these phosphate derivative ILs. As a result of the strong hydrogen-bonding characteristics of [C2mim][H2PO2] and its lower viscosity, this IL is expected to be a superior solvent for extracting polysaccharides from bran.
As expected, [C2mim][H2PO2] is demonstrated to extract polysaccharides better than any other phosphonate salts evaluated in this study. It is capable of extracting 42% of polysaccharides from bran, whereas [C2mim][(MeO)HPO2] can extract only 39% of polysaccharides from bran at 50°C with gentle stirring for 2 hours. As described in the previous section, the authors successfully dissolved cellulose using a series of alkylphosphonate derivative salts without heating, and expect that [C2mim][H2PO2] can also be used to extract polysaccharide components from bran without heating. The test results show that, using [C2mim][H2PO2] with gentle stirring at room temperature for 5 hours, 14% of polysaccharides can be extracted. Although the efficiency of extraction is much lower than at 50°C (>35% for 2 hours), the results clearly demonstrate the possibility of extracting polysaccharides without heating. To confirm the IL stability, NMR spectra of the ILs after extraction experiments have been examined to confirm the stability of the newly designed IL, and the results confirm that the methylphosphonate salt has not been hydrolyzed during the polysaccharide extraction process. This observation strongly indicates that these newly designed polar ILs can be recycled and reused efficiently in closed systems for biomass processing.
8.5 Conclusion and Future Aspects
Some polar ILs have been designed and examined for their capability to dissolve cellulose. They are also tested for extracting cellulose from biomass under mild conditions. Only polar ILs are capable of extracting cellulose from biomass without heating. Additionally, because these ILs are thermally stable, they can be used repeatedly, suggesting that the system for processing biomass can be operated cost-effectively. The only concern is that ILs are costly on the current market; it is expected that a dramatic increase of future applications and demand for this reagent will significantly lower its cost, or novel types of ILs with higher efficiency and lower cost will be developed and made available for cost-effective extraction of polysaccharides from biomass.
References
1. Abe M, Fukaya Y, Ohno H. Green Chemistry. 2010;12:1274–1280.
2. Anderson JL, Ding J, Welton T, Armstrong DW. Journal of American Chemical Society. 2002;124:14247.
3. Crowhurst L, Mawdsley PR, Perez-Arlandis JM, Salter PA, Welton T. Physical Chemistry Chemical Physics. 2003;5:2790–2794.
4. Fukaya Y, Sugimoto A, Ohno H. Biomacromolecules. 2006;7:3295–3297.
5. Fukaya Y, Hayashi K, Wada M, Ohno H. Green Chemistry. 2008;10:44–46.
6. Kamlet MJ, Taft RW. Journal of American Chemical Society. 1976;98:377.
7. Mizumo T, Marwanta E, Matsumi N, Ohno H. Chemistry Letters. 2004;33:1360.
8. Moulthrop JS, Swatloski RP, Moyna G, Rogers RD. Chemical Communications. 2005; 1557.
9. Ohno H. Electrochemical aspects of ionic liquids. Wiley Interscience 2011.
10. Ohno H, Fukaya Y. Chemistry Letters. 2009;38:2–7.
11. Reichardt C. Green Chemistry. 2005;7:339–351.
12. Remsing RC, Swatloski RP, Rogers RD, Moyna G. Chemical Communications. 2006; 1271–1273.
13. Rogers RD, Seddon KR. Science. 2003;302:792–793.
14. Swatloski RP, Spear SK, Holbrey JD, Rogers RD. Journal of American Chemical Society. 2002;124:4974–4975.
15. Welton T. Chemical Reviews. 1999;99:2071–2083.
16. Wassersheid P, Welton T. Ionic liquids in synthesis. Wiley-VCH 2008.
17. Youngs TGA, Holbrey JD, Deetlefs M, Nieuwenhuyzen M, Gomes MFC, Hardacre C. ChemPhysChem. 2006;7:2279–2281.