Pretreatment and Saccharification of Lignocellulosic Biomass
Eika W. Qian
Abstract
Lignocellulosic biomass is the most economical and highly renewable natural resource in the world. The development of renewable energy converted from lignocellulosic biomass as an alternative for fossil fuel is ultimately essential for the survival of the human race. In this chapter, compositions and structure of lignocellulosic biomass are firstly introduced. Then, the saccharification or hydrolysis, which is one of the important processes used to produce liquid fuels such as cellulosic ethanol by transferring the cellulose present in lignocellulosic biomass into sugar monomer units, is introduced. At the same time, the importance of pretreatment as a prior and prerequisite operation for saccharification of lignocellulosic biomass, and a variety of physical and chemical pretreatment methods and their combinations are described. Finally, a novel pretreatment method and saccharification of rice straw using solid acid catalysts are introduced.
Keywords
Lignocellulosic biomass composition; lignocellulosic biomass structure; chemical pretreatment; physical pretreatment; hydrolysis; solid acid catalyst
Chapter Outline
7.1. Composition and Structure of Lignocellulosic Biomass
7.1.1. Renewable, Low-Cost, and Abundant Lignocellulosic Biomass
7.1.2. Composition and Structure of Lignocellulosic Biomass
7.2. Pretreatment of Lignocellulosic Biomass
7.2.1. Importance of Pretreatment
7.3. Pretreatment and Saccharification of Lignocellulosic Biomass using Solid Acid Catalysts
7.3.2. Pretreatment of Rice Straw
7.3.3. Saccharification of Lignocellulosic Biomass using Solid Acid Catalysts
7.1 Composition and Structure of Lignocellulosic Biomass
Biomass is the only known sustainable bioresource that can be used to produce liquid transportation fuels (Qian, 2011). It is produced via photosynthesis to convert light energy and atmospheric carbon, i.e. CO2, into chemical energy to be stored as carbohydrates in plant biomass or living carbon. This is a natural process to capture and store atmospheric CO2. Lignocellulosic biomass is the most economical and highly renewable natural resource in the world. It is estimated that the annual production of lignocellulosic biomass is over 200 billion tons on Earth, and only 3% of this has been used in the pulp industry. In this chapter, the availability, potential, and recalcitrant structure of lignocelluloses are described. Further, saccharification, which is a very important process in the production of bioethanol, is introduced in order to develop a novel process to produce bioethanol for transportation fuels from lignocellulosic biomass.
7.1.1 Renewable, Low-Cost, and Abundant Lignocellulosic Biomass
Plants use solar energy to combine carbon dioxide and water, forming a sugar building block (CH2O)n to produce oxygen, as shown in equation (7.1). The sugar is stored in a polymeric form such as cellulose, starch, and/or hemicellulose. Most biomass is approximately 75% sugar polymer.
![]() (7.1)
(7.1)
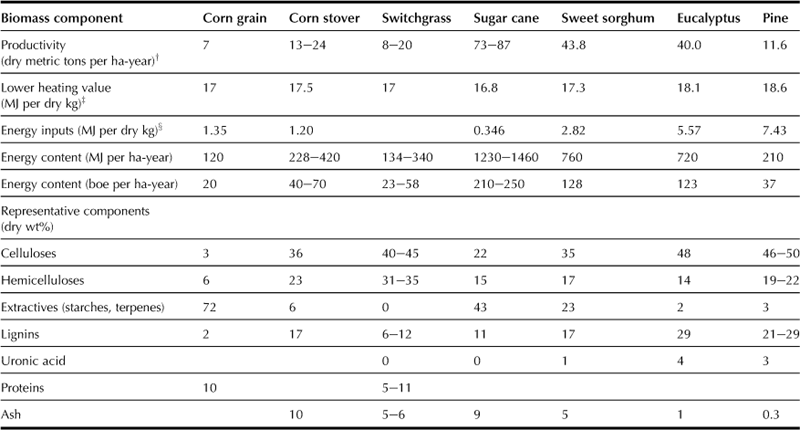
The first step for biofuels production is obtaining an inexpensive and abundant biomass feedstock available from the following sources: waste materials (agriculture wasters, crop residues, wood wastes, urban wastes), forest products (wood, logging residues, trees, shrubs), energy crops (starch crops such as corn, wheat, barley; sugar crops; grasses; woody crops; vegetable oils; hydrocarbon plants), or aquatic biomass (algae, water weed, water hyacinth). Table 7.1 shows the growth rate or productivity, the lower heating value, the total production energy, and the chemical composition of different types of biomass (Towler et al., 2004). The plant growth rate varies with a typical range from 6 to 90 metric tons per ha-year that is equivalent to 19–280 barrels of oil equivalent per ha-year (Klass, 1998). Plants typically capture 0.1–1.0% of solar energy, with the percentage of solar energy capture proportional to the plant growth rate. The energy inputs reported in Table 7.1 include the energy required to make fertilizer as well as the energy needed to transport the harvested crop. The rates and the energy requirements for plant growth are dependent on plant species (Table 7.1). The development of more efficient plant materials with faster growth rates while requiring less energy input will depend on progress in plant breeding, biotechnology, and genetic engineering.
TABLE 7.1
Chemical Composition, Energy Content, and Yield of Various Terrestrial Biomass Species∗
Adapted from: ∗Towler et al. (2004); Lynd et al. (1999); and Klass (1998). †Klass (1998); Towler et al. (2004). ‡O’Sullivan (1997). §Towler et al. (2004).
Although photosynthesis has much lower sunlight-to-chemical energy efficiency than photovoltaic, it is regarded as “the natural solar cell” that collects low-energy-density solar radiation from large areas, fixes CO2, and generates a chemical energy carrier (i.e. biomass) at nearly zero cost. On average, the net primary productivity of terrestrial biomass equals only 0.3% of the average energy density of sunlight. With improvements in plant characteristics by using modern biotechnology and cultivation technologies, a 10% increase in global photosynthesis efficiency for territorial plants would assimilate more than 8.8 billion tons of carbon from the atmosphere (not accounting for carbon re-emission), which is equal to the total global current net carbon emission.
7.1.2 Composition and Structure of Lignocellulosic Biomass
The major components of plant cell walls are cellulose, hemicelluloses, and lignin, which together form a complex and rigid structure (Figure 7.1). Plant cell walls of different plant types vary greatly in appearance and property. The complicated structure of lignocellulosic biomass contributes to its resistance to biological and chemical degradation. For example, in nature, natural biodegradation of lignocellulosic biomass is slow because it requires the collective actions of many hydrolytic enzymes, including cellulase (endoglucanase, cellobiohydrolase, and beta-glucosidase), hemicellulase, and lignin-degrading enzymes. Two main root causes of the recalcitrance of lignocellulosic biomass to cellulose enzymatic hydrolysis are believed to be: (1) low accessibility of (micro)crystalline cellulose fibers, which significantly reduces the efficiency of cellulose, and (2) the presence of lignin (mainly) and hemicellulose on the surface of cellulose, which prevents cellulase from accessing the substrate efficiently.
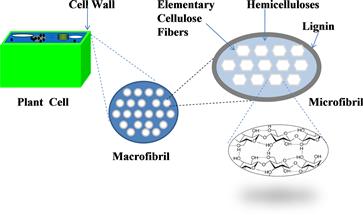
FIGURE 7.1 Schematic structure of a lignocellulosic biomass containing cellulose, hemicellulose, and lignin.
The structured portion of lignocellulosic biomass is composed of cellulose, hemicellulose, and lignin. Cellulose (a crystalline glucose polymer) and hemicellulose (a complex amorphous polymer, whose major component is a xylose monomer unit) make up 60–90 wt% of terrestrial biomass, as shown in Table 7.1. Lignin, which is a large polyaromatic compound, is another major component of biomass. The term “extractives” in Table 7.1 refers to those compounds that are not an integral part of the biomass structure (Huber et al., 2006), and are soluble in solvents such as hot and cold water, ethers, or methanol. Extractives include different types of carbohydrates such as sucrose from sugar cane and amylose from corn grains. Ash listed in Table 7.1 is biomass material that cannot be blazed.
Extractives are mainly starch in crops and its wastes. Starch is a glucose polysaccharide that has α-1,4-glycoside linkages (Huber et al., 2006); they also have a large quantity of α-1,6-glycoside linkages that make the polymer amorphous. Human and animal enzyme systems can easily digest starches due to the presence of α-linkages. Starches are commonly found in the vegetable kingdom, e.g. corn, rice, wheat, beans, and potatoes. When treated in hot water, the starch forms two principal components: water-soluble amylose (10–20 wt%) and water-insoluble amylopectin (80–90%). Amylose contains only α-1,4-glycoside linkages, whereas amylopectin contains both α-1,4- and α-1,6-glycoside linkages with an approximate α-1,4 to α-1,6 linkage ratio of 20:1.
Cellulose is a linear biopolymer of anhydroglucopyranose connected by β-1,4-glycosidic bonds. Coupling of adjacent cellulose chains by orderly hydrogen bonds and van der Waal’s forces lead to parallel alignment and a crystalline structure, resulting in low accessibility to enzymes (Figure 7.1). Recently, quantitative data of cellulose accessibility to cellulase clearly suggest that only a small fraction of β-glucosidic bonds of cellulose, ranging from 0.0023 to 0.041, are accessible by cellulase. The evidence from cell wall biophysics, biosynthesis, genomics, and atomic force microscopy (AFM) images suggests that elementary cellulose fibrils are synthesized by a cellulose synthase complex locus that contains 36-glucan chains with both crystalline and subcrystalline structures (Ding and Himmel, 2006). A number of elementary fibrils coalesce into much larger microfibrils and then into macrofibrils.
Unlike starch, cellulose is a crystalline material with an extended flat twofold helical conformation (Wyman et al., 2005). Hydrogen bonds maintain and reinforce the flat linear conformation of the chain. The top and bottom of the cellulose chains are essentially completely hydrophobic. The sides of the cellulose chains are hydrophilic and capable of bonding hydrogen because all the aliphatic hydrogen atoms are in axial positions, whereas the polar hydroxyl groups are in equatorial positions. The degree of polymerization of cellulose is approximately 10,000 glucopyranose monomer units in wood and up to 15,000 glucopyranose monomer units in cotton. Partial acid hydrolysis breaks down cellulose into cellobiose (glucose dimer), cellotriose (glucose trimmer), cellotetrose (glucose tetramer), and ultimately into glucose when the acid hydrolysis is complete.
Hemicellulose is situated between lignin and a collection of cellulose fibers underneath; it is a sugar polymer that typically consists of 20–40 wt% of biomass (Huber et al., 2006). In contrast to cellulose, which is a polymer of only glucose, hemicellulose is a copolymer of five different sugars. This complex polysaccharide occurs in association with cellulose in cell walls. Five-carbon sugars (usually xylose and arabinose) and six-carbon sugars (galactose, glucose, and mannose), which are highly substituted with acetic acid, are contained in hemicellulose. The most abundant building block of hemicellulose is xylan (a xylose polymer linked at the 1 and 4 positions). Hemicellulose is amorphous because of its branched nature that is relatively easy to hydrolyze into its monomer sugars as compared with cellulose.
Furthermore, consistent with their structural chemistry and side-group substitutions, xylans are interspersed, interweaved, and ester-linked at various points with the overlying “sheath” of lignin, as well as to produce a coat around underlying strands of cellulose via hydrogen bonds. The xylan layer with its covalent linkage to lignin and its non-covalent interaction with cellulose may be important in maintaining the integrity of the plant cell wall in situ, and in helping to protect the fibers against degradation by enzymes. At least two types of covalent crosslinks have been identified between hemicellulose and lignin: (1) diferulic acid bridges and (2) ester linkages between lignin and glucuronic acid attached to xylans.
Ten to twenty-five weight percent of lignocellulosic biomass is typically composed of lignin, which is a highly branched, substituted, mononuclear aromatic polymer found in the cell walls of certain biomass, particularly woody biomass. Lignin is often associated with the cellulose and hemicellulose materials making up lignocellulosic compounds. It is an irregular polymer, which is formed by an enzyme-initiated free-radical polymerization of the alcohol precursors. The bonding in the polymer can occur at many different sites in the phenylpropane monomer due to electron delocalization in the aromatic ring, the double bond-containing side chain, and the oxygen functionalities. The manner in which lignin is produced from lignocellulosic biomass affects its structure and reactivity in hydrolysis. Softwood lignins are formed from coniferyl alcohol as monomer units (Qian, 2011), whereas grass lignin contains coniferyl, sinapyl, and coumaryl alcohol.
7.2 Pretreatment of Lignocellulosic Biomass
7.2.1 Importance of Pretreatment
Lignocellulosic biomass can be converted into liquid fuels via three primary routes, including syngas production by gasification, bio-oil production by pyrolysis or liquefaction, and hydrolysis of lignocellulosic biomass to produce sugar monomer units (Huber et al., 2006). The hydrolysis route to produce liquid fuels includes two subprocesses: (1) hydrolysis of cellulose in the lignocellulosic materials to fermentable reducing sugars, and (2) fermentation of reducing sugars to target products (especially cellulosic ethanol or bioethanol). The hydrolysis is usually catalyzed by using enzymes such as cellulase, and the fermentation is carried out with suitable microorganisms. Bioconversion of lignocellulosic biomass to liquid fuels and commodity products has many potential benefits, with enzymatic hydrolysis of cellulose to glucose, followed by fermentation to ethanol, a particularly attractive route to produce low-cost sustainable liquid transportation fuels (Wyman et al., 2005). However, enzymatic hydrolysis may be the most complex step in the bioconversion process because of the combination of substrate-related and enzyme-related effects. Although the hydrolysis mechanism and the relationship between the structure and function of various cellulases have been studied extensively (Converse, 1993), the complex biomass structure confounds understanding of the relative importance of these features and their roles; reducing one barrier to digestion can enhance or mask the importance of others.
Lignocellulosic biomass has a complicated structure because it contains many components such as cellulose, hemicelluloses, lignin, extractives and ash, as mentioned above (Table 7.1). Thus, these components bring about a barrier to the enzymatic hydrolysis of lignocellulosic biomass. Therefore, removing hemicelluloses and/or lignin before the bioconversion process is a prerequisite to increasing the accessible surface area of cellulose by enzymes to enhance the subsequent enzymatic hydrolysis reactions.
The biological conversion of lignocellulose to fuel generally has three main steps: (1) lignocellulosic biomass pretreatment to convert the recalcitrant lignocellulosic structure into reactive cellulosic intermediates, (2) enzymatic cellulose hydrolysis by cellulase to hydrolyze and convert reactive intermediates to fermentable sugars, e.g. glucose, and (3) fermentation to produce cellulosic ethanol (bioethanol) or other bio-based chemicals, e.g. lactic acid and succinic acid. Pretreating the recalcitrant structure of lignocellulosic biomass efficiently to release the locked polysaccharides is one of the most important factors concerning the success of R&D for the emerging biofuel and other biomass-based industries because lignocellulosic biomass pretreatment is one of the most expensive unit operations and/or processes, and has a major influence on the costs of both prior operations, e.g. reduction of lignocellulosic biomass particle size, and subsequent operations, e.g. enzymatic hydrolysis or fermentation (Wyman et al., 2005).
The purposes of pretreatment are to decrease the crystallinity of cellulose, increase biomass surface area, remove hemicellulose, and break the lignin seal (Mosier et al., 2005). The removal of lignin and/or hemicellulose can substantially reduce the recalcitrance of lignocellulosic biomass to enzymatic hydrolysis (Wyman et al., 2005). This pretreatment changes the biomass structure and improves downstream processing. On the other hand, pretreatment is also one of the least understood processing options. Generally, the following is expected for a desirable attribution of pretreatment (Mosier et al., 2005):
1. Low cost of chemicals for pretreatment, neutralization, and subsequent conditioning.
3. Limited size reduction to avoid energy-intensive and expensive milling operations.
4. Fast reactions and non-corrosive chemicals to minimize pretreatment capital investment.
5. Sugar concentrations higher than 10% derived from hydrolysis of hemicellulose to keep fermentation reactor sizes at a reasonable level and facilitate downstream recovery.
6. Promoting high product yields in subsequent enzymatic hydrolysis or fermentation operations with minimal conditioning costs.
7. By-products not posing additional processing or disposal challenges.
8. Low enzyme loading to realize greater than 90% digestibility of pretreated cellulose in less than 5 days and preferably 3 days.
9. Easy recovery of lignin and other constituents for conversion to valuable co-products and simplification of downstream processing.
7.2.2 Various Pretreatments
A variety of physical and chemical pretreatment methods and their combinations have been developed to improve the accessibility of enzymes to cellulosic fibers (Mosier et al., 2005). Physical pretreatment methods include ball milling, comminution (mechanical reduction of biomass particulate size), and compression milling; chemical pretreatment methods include hydrothermolysis with acid, alkali, and solvents (e.g. H2O2, glycerol, dioxane, phenol and ethylene glycol, ozone). Physicochemical pretreatment methods including uncatalyzed steam explosion, hot compressed water treatment, ammonia fiber explosion, and biological pretreatment techniques have also been used.
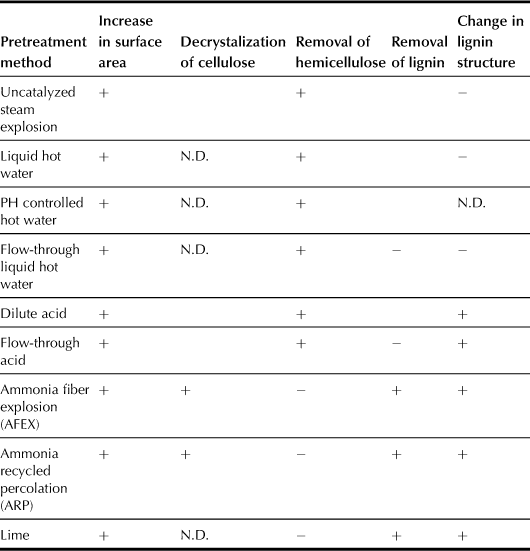
Table 7.2 shows the effect of various pretreatment methods on the chemical and physical structure of lignocellulosic biomass. Uncatalyzed steam explosion is used commercially to remove hemicellulose for manufacturing fiberboard and other related products in the Masonite process (Mosier et al., 2005). High-pressure steam is applied to wood chips for a few minutes without adding chemicals. This process increases the wood surface area and improves the cellulose digestibility significantly without decrystallizing the cellulose.
TABLE 7.2
Effect of Promising Pretreatment Methods on the Structure and Composition of Lignocelluloses Biomass (Mosier et al., 2005)
Hot water treatment using subcritical water (200–250°C) and supercritical water (higher than 374°C and above 22 MPa) treatment at elevated temperatures and pressures can increase the biomass surface area and remove hemicellulose (Mosier et al., 2005; Wyman et al., 2005). Three types of reactors used for hot water pretreatment include co-current (lignocellulose and water flow in the same direction), counter-current (water and lignocellulose move in opposite directions), and flow-through (hot water passes over a stationary bed of lignocellulose). The advantage of hot water treatment is the elimination of chemical usage to achieve size reduction in the pretreatment of biomass, but a major disadvantage is the formation of sugar degradation products (e.g. furfural from xylose and 5-hydroxymethylfurfural (5-HMF) from glucose) in addition to requiring expensive devices because of the high pressure (10–15 MPa).
Pretreatment with dilute sulfuric acid-based chemical pretreatment (Mosier et al., 2005) is the most popular method; it hydrolyzes hemicellulose to sugars with high yields, changes the structure of the lignin, and increases the cellulosic surface area (Mosier et al., 2005; Wyman et al., 2005). This pretreatment process pioneered by Grethlein and others can break the lignin–hemicellulose shield in agricultural residues and woody biomass usually accompanied by degradation of hemicellulose. The acid-catalyzed treatment has been widely assessed from the viewpoint of efficiency improvement, kinetic analysis, and cost-effectiveness, among many others. This mainstream process, however, might have some undesirable effects. For example, the formation of aldehydes such as furfural due to excessive degradation of the produced monosaccharide is essentially inevitable; this results in lowering the conversion yield of polysaccharides and inhibiting the subsequent ethanol fermentation process. An additional problem caused by using this process is that it uses a corrosive acid such as sulfuric acid that needs downstream neutralization in addition to requiring special materials for reactor construction. The recovery of the spent acid also complicates the downstream processing steps.
Ammonia fiber/freeze explosion (AFEX) in which anhydrous ammonia is used to react with lignocellulose can increase the surface area of the biomass, decrease crystallinity of cellulose, dissolve part of the hemicellulose, and remove lignin. Pretreatment of the lignocellulose with a less concentrated ammonia solution is known as ammonia recycled percolation (ARP). The AFEX pretreatment is a novel alkaline pretreatment process that effects a physicochemical alteration in the lignocellulosic ultra and macro structure. Studies have shown that the AFEX pretreatment increases the biomass enzymatic digestibility several folds over the untreated lignocellulosic (Teymouri et al., 2005). The AFEX pretreatment results in the decrystallization of cellulose (Gollapalli et al., 2002), partial depolymerization of hemicellulose, deacetylation of acetyl groups, cleavage of lignin–carbohydrate complex (LCC) linkages and lignin C–O–C bonds, increase in the accessible surface area due to structural disruption (Turner et al., 1990), and increase in wettability of the treated biomass (Sulbaran de Ferrer et al., 1997). The AFEX process has proved to be attractive economically compared with several other leading pretreatment technologies based on a recent study using an economic model (Eggeman and Elander, 2005) for evaluating bioethanol conversion of corn stover.
Wet oxidation (WO) has been shown to be an efficient pretreatment method of wood and wheat straw; it dissolves the hemicellulosic fraction and makes the solid cellulose fraction susceptible for enzymatic hydrolysis and fermentation (Bjerre et al., 1996). The WO process is carried out using hot water under pressure with the addition of oxygen. Once oxygen is not applied, the process is essentially a hydrothermal pretreatment that is comparable to the well-known steam pretreatment. Addition of carbonate during the hydrolysis (alkaline WO) results in low sugar degradation, and reduced formation of 2-furfural (Bjerre et al., 1996) and phenol aldehydes (Klinke et al., 2002). In steaming and dilute acid hydrolysis pretreatments, sugar monomers are produced whereas the alkaline WO treatment of wheat straw only produces soluble polymeric hemicellulose sugars (arabinoxylan).
Silverstein et al. (2007) compared the pretreatment effect for using acid, alkali and wet oxidation (H2O2 and ozone) to pretreat cotton stalks. Cellulose conversion of the pretreated samples after 72 h of enzymatic hydrolysis is shown in Table 7.3. Pretreatment using sodium hydroxide shows the highest cellulose conversion of 60.8%, followed by hydrogen peroxide (49.8%) and sulfuric acid (23.8%). The hydrolysis pretreatment using sodium hydroxide has the highest (62.6%) conversion of xylan to xylose, whereas the hydrogen peroxide pretreatment averages 7.8% conversion. For the acid pretreatment, no xylan is detected in the treated solids during initial carbohydrate, but an average of 14.3 mg xylose per g dry biomass is detected in the supernatant after enzymatic hydrolyses. The difference in cellulose conversion during enzymatic hydrolyses is hence largely dependent on the difference in lignin composition. On the other hand, the ozone pretreatment does not cause any significant changes in lignin, xylan, or glucan contents during the treatment period.
TABLE 7.3
Effect of Various Pretreatment Methods on Glucan and Xylan Conversion after Enzymatic Hydrolysis of Cotton Stalks (Eggeman and Elander, 2005)
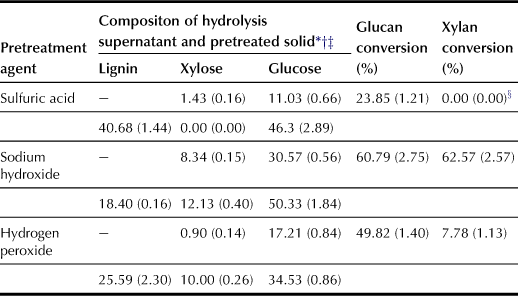
∗Composition percentages calculated from values on a dry weight basis.
†Data are averages of three replicates. Numbers in parentheses standard deviations.
‡Compositions of xylose and glucose in the hydrolysis supernatant are given in the upper rows while compositions of pretreated solids are given in the bottom rows.
Wyman et al. (2005) reported the results of using different pretreatment methods followed by enzymatic hydrolyses to produce sugars from corn stover feedstock; over 90% yields of sugar are obtained with the various pretreatments. Hot water treatment using a flow-through reactor has the highest overall soluble product yield; however, the xylose monomer yield is only 2.4%, indicating that this method does not produce xylose monomers. The dilute acid pretreatment method produces the highest quantity of sugar monomers, with 92% yields. Results are expected to be different with other feedstocks (Yang, and Wyman, 2004).
The order of bioethanol production costs is: dilute acid < AFEX < lime < ARP < hot water. Hot water pretreatment is the most expensive method because it requires more enzymes to break down the xylose oligomers. If the oligomers could be successfully converted into ethanol (or other products), the unit cost of producing ethanol would decrease for the hot water, ARP, and lime methods, because these pretreatment methods yield a significant quantity of oligomers.
Recently, novel pretreatment methods such as microwave treatment (Nakayama and Okamura, 1989), and combined ethanol cooking and ball milling (Teramoto et al., 2008) have also been developed. The latter is similar to organosolv pulping (Aziz and Sarkanen, 1989; Chum et al., 1990; Grethlein and Converse, 1991), which is generally implemented using a strong acid catalyst in order to remove the lignin component completely. This pretreatment alleviates the problems associated with the use of strong acid catalysts so that the production of furfural due to excessive degradation of polysaccharide components is extremely low with insignificant delignification (Teramoto et al., 2008). Therefore, the cooking process is regarded not as a delignification process but a process to activate the original wood. Subsequently, the activated solid products are pulverized by using ball-milling in order to improve their enzymatic digestibility. The enzymatic hydrolysis experiments demonstrate that the conversion of cellulosic components into glucose can attain 100% under optimal conditions.
7.3 Pretreatment and Saccharification of Lignocellulosic Biomass using Solid Acid Catalysts
7.3.1 Solid Acid Catalysts
Various methods to pretreat lignocellulose with advantages and disadvantages have been developed as mentioned above. On the other hand, there is no single best pretreatment method for all types of lignocellulose, including soft biomass such as rice straw, wheat straw, and corn stover, etc. as well as hard biomass such as wood, etc. A new way to efficiently exploit lignocellulose by avoiding the drawbacks associated with the aforementioned technologies is the use of solid acid catalysts. This new method is capable of overcoming the above-mentioned problems such as the use of environment-unfriendly chemicals and the need to purify final products. The solid acid catalyst has an exceptional catalytic performance in pretreating biomass with high selectivity because both the quantity and strength of acid applied can be controlled. Furthermore, the use of solid acid catalyst facilitates the subsequent separation of final products and recovery of the catalyst after pretreatment in addition to eliminating the neutralization step.
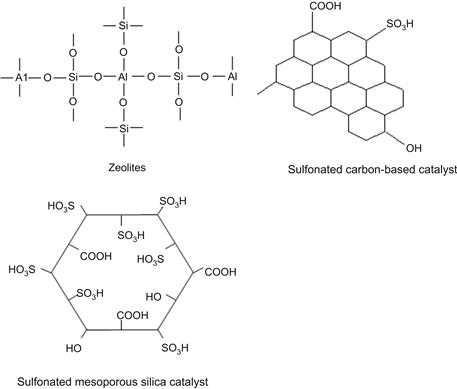
Using heterogeneous solid acid catalysts to replace conventional homogeneous acids has been applied across many fields, including petroleum refining, organic synthesis, and fine chemicals. Solid acid catalysts offer the great advantages of efficient recovery, low or even no corrosion, and mild reaction conditions. As shown in Figure 7.2, the solid catalysts include silica–alumina, zeolites, niobic acid, sulfonated carbon-based catalyst, mesorporous silica-based catalyst and strong ion-exchangeable resins, as well as sulfated transition metal oxides including zirconia, titania, etc., which have equivalent acidity or stronger acidity than liquid acid. In fact, using solid acid catalysts to hydrolyze lignocellulosic biomass has been studied recently. Patents awarded to methods for lignocellulosic biomass saccharification and liquefaction have been published by Qian and Hosomi. (2009a, b). The hydrolysis of cellulose using amorphous carbon-bearing sulfonic acid groups (Suganuma et al., 2008) and acidic ionic liquid-modified silica catalysts (Amarasekara and Owereh, 2010; Goderis et al., 2010) have been carried out.
In this section, the utilization of solid acid catalysts in pretreatment and direct saccharification of lignocellulosic biomass is presented.
7.3.2 Pretreatment of Rice Straw
a Pretreatment of Rice Straw using Solid Acid Catalysts
A novel pretreatment process that is mild and cost-effective for enzymatic hydrolyses of lignocellulosic biomass needs to be developed. Most hemicelluloses and some lignin and ash are removed after catalytic hydrothermal saccharification of rice straw using solid acid catalysts, and the major solid residues are cellulose (Li and Qian, 2011). Thus, solid acid catalysts are feasible in the hydrothermal pretreatment of soft biomass such as rice straw, grass, etc. Novel processes to pretreat lignocellulosic biomass using solid acid catalysts have thus been investigated, and the influence of pretreatment conditions such as pretreated temperature, pretreated time, and types of catalyst on the efficiency of subsequent enzymatic saccharification was evaluated.
The hydrothermal pretreatment of rice straw using solid acid catalysts was carried out in an 80-cm3 autoclave using a commercial solid acid catalyst (Amberlyst 35 Dry), sulfated zirconia (SA-J1), and a novel sulfonated mesoporous silica catalyst (MPS-1). After pretreatment, the solid acid catalyst was recovered, and the mixed slurry of rice straw and water was put in an L-type glass reactor to be hydrolyzed using cellulase (Onozuka R-10). Enzymatic saccharification of the pretreated rice straw was carried out at 45°C for 1–3 days to investigate the efficiency of solid acid catalysts.
The various chemical species before and after enzymatic saccharification of the pretreated rice straw at 180°C for different reaction times using SA-J1 catalyst were investigated. Before enzymatic hydrolyses, the yield of monosaccharide increased as pretreatment time increased from 10 to 60 min (Li et al., 2012a). At the same time, low levels of by-products such as furfural, 5-HMF and organic acids are formed, and their quantities increase with longer pretreatment time. On the other hand, there is no significant change in the yield of oligosaccharide. However, in the subsequent enzymatic saccharification of pretreated rice straw, the yield of monosaccharide increases as pretreatment time is extended from 10 to 30 min, but then remains constant up to 60 min. In comparison with the pretreatment process, the yield of oligosaccharide decreases significantly, indicating that oligosaccharide is converted into monosaccharide during the process of enzymatic saccharification.
Various by-products such as organic acids, furfural etc. are formed after pretreatment. Also, no significant variations in the yields of various by-products after enzymatic saccharification are observed for all samples pretreated for different durations. This means that no additional by-products are formed during the process of enzymatic saccharification.
b Effect of Pretreatment Conditions and Solid Acid Catalysts
In order to investigate the effects of pretreatment temperature on the enzymatic saccharification of pretreated rice straw, studies on pretreating rice straw were carried out at 110–180°C for 3 h. With the pretreatment temperature elevated from 110 to 150°C, the yields of all products except glucose increased. The yields of oligosaccharide and monosaccharide reached 250 and 105 g kg−1 respectively when the pretreatment was maintained at 150°C for 3 h. Further, enzymatic saccharification of the pretreated rice straw was carried out at 45°C for 3 days. As shown in Figure 7.3, in the case of the enzymatic saccharification of pretreated rice straw, the yield of monosaccharide rises with increasing pretreatment duration up to 3 h at 150°C, and the maximum yield of 450 g kg−1 monosaccharide is obtained. Based on a composition analysis of raw rice straw, approximately 76% holocellulose in rice straw is converted into monosaccharide. In addition, the higher pretreatment temperature accelerates the enzymatic hydrolysis rate of the rice straw. As listed in Table 7.4, the decreasing rates of xylan and glucan are accelerated at higher pretreatment temperatures for the same pretreatment duration. Lignin has the same tendency to decrease this rate as xylan and glucan.
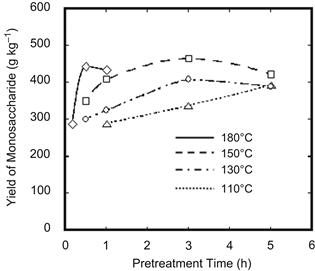
FIGURE 7.3 Effects of pretreatment temperature and time on yields of monosaccharides after enzymatic hydrolysis of pretreated rice straw using SA-J1 catalyst.
TABLE 7.4
Effects of Pretreated Time and Pretreated Temperature on the Change in Composition of Pretreated Rice Straw using SA-J1 Catalyst
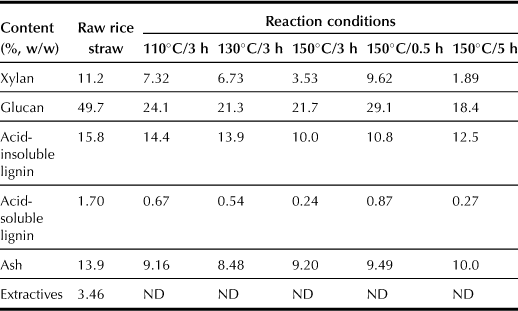
Scanning electron microscope (SEM) images show that the raw rice straw sample exhibits rigid and highly ordered fibrils, whereas pretreated samples have loose and fluty fibers with a more disordered orientation for higher pretreatment temperature. The microfibers are also separated from the initial connected structure and fully exposed, thus increasing the external surface area and internal porosity, which enhances ability of the cellulase to attack cellulose contained in the pretreated rice straw. In addition, with increasing pretreatment temperature, the CrI index of cellulose in rice straw before and after enzymatic hydrolysis increases from 13.6% to 44.9%. Both the surface area and pore volume are higher after pretreatment than those of the untreated raw rice straw. At the same time, the molecular weight of cellulose in the pretreated rice straw decreases from 622,490 to 579,802, which indicates the reduced degree of polymerization of cellulose after pretreatment.
The effect of using four different catalysts (Lewis-type solid acids: SA-J1 and SA-J2; Brönsted-type solid acids: MPS-1 and Amberlyst 35 Dry) on the enzymatic hydrolysis of rice straw was elucidated. After pretreatment, there is no significant difference among the liquefaction rates of the pretreated rice straw using these four catalysts. However, SA-J1 and SA-J2 show higher yields of oligosaccharide after pretreatment than MPS-1 and Amberlyst 35 Dry catalysts. In contrast, MPS-1 and Amberlyst 35 Dry have higher yields of monosaccharide (xylose and glucose) than SA-J1 and SA-J2 catalysts. These results indicate the possibility of a direct saccharification of lignocellulosic biomass using a solid acid catalyst with stronger acidity such as a Brönsted-type solid acid without subsequent enzymatic hydrolysis. After enzymatic hydrolysis of the pretreated rice straw, SA-J1 and SA-J2 catalysts have higher yields of oligosaccharide than MPS-1 and Amberlyst 35 Dry catalysts. In addition, the oligosaccharide yield obviously decreases after enzymatic saccharification of the pretreated rice straw using SA-J1 and SA-J2 catalysts; it remains relatively constant if MPS-1 and Amberlyst 35 Dry are used. Most monosaccharides obtained using MPS-1 and Amberlyst 35 Dry catalyst treatment come from pretreated rice straw, whereas most monosaccharaides using SA-J1 and SA-J2 catalyst pretreatment come from the enzymatic hydrolysis of pretreated rice straw. As shown in Table 7.5, the Amberlyst 35 Dry catalyst has a quicker rate of decrease of xylan in the pretreated rice straw than SA-J1 catalyst. However, the rates of decrease of glucan, lignin, and ash in rice straw pretreated using SA-J1 catalyst are faster than those using Amberlyst 35 Dry catalyst.
c Hydrothermal Pretreatment of Rice Straw using Solid Acid Catalysts
The effects of hydrothermal pretreatment of rice straw using solid acid catalysts are shown in Scheme 7.1. In hydrothermal pretreatment of rice straw in the presence of a solid acid catalyst, part of the hemicellulose in rice straw is first attacked by protons bonded to the functional groups of the solid acid catalyst. At the same time, part of the lignin soluble in water decomposes via hydrothermal hydrolyses. This leads to the structure of rice straw being broken up because lignin is soluble in water so that part of the hemicelluloses is removed. Hemicellulose is mainly converted into oligosaccharide via catalytic hydrolyses and/or hydrothermal hydrolyses. As the pretreatment process proceeds, hemicelluloses are removed completely, and the oligosaccharide is converted into monosaccharide. Simultaneously, part of the cellulose is converted into oligosaccharide, and the structure of rice straw becomes more irregular and disordered, resulting in low crystallinity of cellulose or an amorphous structure. Thus, the resulting celluloses become more susceptible to enzymatic hydrolysis in the subsequent enzymatic treatment.
7.3.3 Saccharification of Lignocellulosic Biomass using Solid Acid Catalysts
As described in the above section, when a stronger Brönsted acid catalyst such as MPS-1 is used in the pretreatment of rice straw, the formation of monosaccharide is observed. This suggests that direct saccharification of lignocellulosic biomass using a solid acid catalyst without subsequent enzymatic hydrolysis is possible. In this section, direct saccharification of lignocellulosic biomass using novel acid catalysts is introduced.
Selective and efficient hydrolyses of cellulose to glucose is a challenging task; the application of solid catalysts has been studied in recent years. Hara and co-workers demonstrated that a sulfonated carbon prepared from cellulose is capable of hydrolyzing cellulose to glucose and oligosaccharides at a low temperature of 373 K (Suganuma et al., 2008). Likewise, sulfonated carbon materials (e.g. activated carbon, silica–carbon composites, and a mesoporous carbon, CMK-3) convert cellulose into glucose with 40–75% yields for 24 h reaction time (Onda et al., 2008). Meanwhile, Rinaldi et al. (2008) showed that as a solid acid catalyst, a polystyrene sulfonated resin (Amberlyst 15DRY) in [BMIM]Cl can hydrolyze cellulose, although this observation deviates from the fact that the resin merely works as an acidifier under the aqueous conditions without the ionic liquid. On the other hand, lignocellulosic biomass is a solid and is known to be composed of cellulose, hemicelluloses, lignin, extractives, and other components. Hence, increasing the porous size and surface area of the solid catalyst is expected to improve the contact between the solid biomass and solid acid catalysts. On the other hand, mesoporous silicas such as SBA-15, MCM-41, etc. with a large surface area and mesoporous pore size of 2–50 nm have been developed recently. Further, the capability of sulfonated groups to be incorporated on to the surface of mesoporous silica SBA-15 has been reported (Margolese et al., 2000). Thus, novel solid acid catalysts with mesopores and large surface area to hydrolyze lignocellulosic biomass directly can be developed (Li et al., 2012a, b). In this section, the catalytic activities of several sulfonated group-bearing mesoporous silica SBA-15 catalysts in the saccharification of cellulose and rice straw, which is selected as a resource for bioethanol because of its abundance in Japan and Asia, have been assessed.
a Novel Solid Acid Catalysts
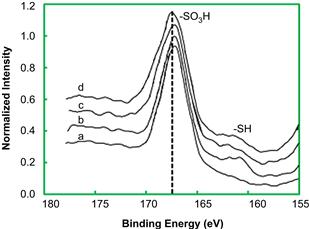
X-ray photoelectron spectra (XPS) of the novel sulfonated mesoporous solid acid catalysts developed recently are shown in Figure 7.4 (Li et al., 2012a). The chemical groups that influence the acidity of the catalyst include OH, COOH, and SO3H groups, and their distributions depend upon the conditions used to prepare the catalysts. The S2p XPS peak at 168 eV is assigned to the sulfo group, which is consistent with the study of Nakajima et al. (2006). IR spectra confirm that the sulfo groups and –OH groups are bonded on the prepared catalyst. The X-ray diffraction (XRD) patterns show that the SBA-15 material structure has formed successfully after the preparation of the silica-based catalyst. When aged for 24 h (MPS-A and MPS-C), the surface area and pore volume increase with lower aging temperature. If aged at 90°C (MPS-B, MPS-C, and MPS-D), the surface area and pore volume increase with longer aging time. Furthermore, decreasing the molar ratio of MPTMS/H2O2 (MPS-A, MPS-E, and MPS-F) from 11.1% to 5.6% under the same aging conditions, the surface area increases initially and then decreases but the pore volume keeps increasing. For cases using 24-h aging time (MPS-A and MPS-C), the total quantity of acid increases with decreasing aging temperature. With 90°C aging temperature (MPS-B, MPS-C, and MPS-D), the total amount of acid increases with increasing aging time. With decreasing molar ratio of MPTMS/H2O2 (MPS-A, MPS-E, and MPS-F) under the same aging conditions, the total quantity of acid increases. The reason why the aging conditions enhance the quantity of acid contained in the prepared catalysts so significantly is not clear. Also, for these catalysts, the total quantity of acid determined by using a titration method is higher than that calculated from sulfo groups determined using XPS. There is no doubt that the sulfo groups originate from MPTMS; however, it has been reported that the total amount of acid from OH or COOH groups exist on the catalyst. Therefore, it was considered that the amount of OH or COOH groups is also affected by aging conditions.
b Saccharification of Rice Straw
The catalysts mentioned above were tested in the saccharification of rice straw to investigate their catalytic characteristics (Li et al., 2012b). The mixture of 3 g rice straw and 30 g distilled water was treated with 0.5 g catalyst (weight ratio of 0.5 g/30 g/3 g for catalyst/distilled water/rice straw) at 180°C for 1 h. Results of post-reaction analyses of the liquid products show low content of monosaccharide and low levels of by-products in a blank sample without catalyst (NSC). In contrast, samples treated with various solid acid catalysts show the presence of monosaccharide as the main product along with various organic acids, furfural, and 5-HMF.
In addition, both the liquefaction rate of rice straw and yields of by-products increase with more surface area, higher pore volume, and a greater amount of solid acid catalyst applied. The yield of total monosaccharide initially increases and then decreases. For the three solid acid catalysts studied, MPS-D catalyst has the highest activity for the saccharification of rice straw.
The effect of reaction temperature on saccharification of rice straw using MPS-D catalyst was investigated. Increasing reaction temperature leads to a higher liquefaction rate of rice straw and yields of furfural, as well as more 5-HMF and total organic acids produced. At 220°C for 1 h, the liquefaction yield of rice straw reaches 40%. The yield of total monosaccharide initially increases with higher reaction temperature up to 180°C, but decreases thereafter at temperatures higher than 180°C. The maximum yield of total monosaccharide is 38% at 180°C for 1 h. When the reaction temperature increases from 180 to 220°C, the yields of glucose and xylose decrease while the yields of furfural, 5-HMF, and total organic acids increase.
The effect of reaction time on saccharification of rice straw using MPS-D catalyst at 180°C was investigated. With the reaction time increased from 0.5 to 2 h, the liquefaction rate of rice straw and the yields of furfural and 5-HMF as well as total organic acids increase. The liquefaction yield of rice straw is 38.9% at 180°C for 2 h. The yield of total monosaccharide initially increased with longer reaction times up to 1 h, but then decreased thereafter. The maximum yield of total monosaccharide is 38% at 180°C for 1 h. The yield of monosaccharides decreases while the yields of furfural, 5-HMF, and total organic acids increase when the reaction time increases from 1 to 2 h.
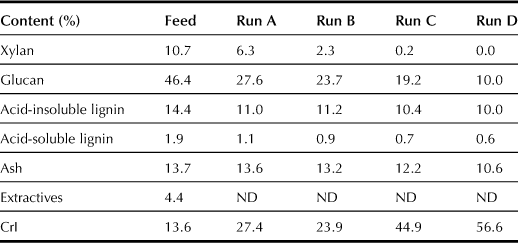
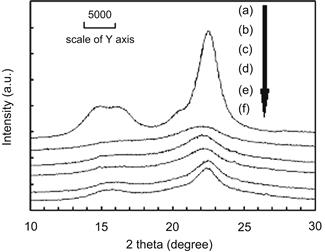
Changes in the property of rice straw before and after saccharification at different reaction temperatures for 1 h were determined by following the procedure described in the NREL Chemical Analysis and Testing procedure and XRD respectively. The XRD patterns and CrI index of α-cellulose in rice straw before and after saccharification are shown in Table 7.6 and Figure 7.5. With the reaction temperature raised from 150 to 220°C, the content of xylan in rice straw decreases from 10.7% to 0%, the content of glucan decreases from 46.4% to 10.0%, and the content of acid-soluble lignin decreases from 1.9% to 0.7%. Quantities of acid-insoluble lignin and ash also decrease slightly. In Figure 7.5, (a) is the reference of the XRD pattern for cellulose, and (b)–(f) are the XRD patterns of rice straw before and after saccharification. The CrI index of α-cellulose in rice straw increases from 13.6% (before saccharification) to 56.6% (after saccharification).
c Hydrolysis Mechanism of Rice Straw on Solid Acid Catalysts
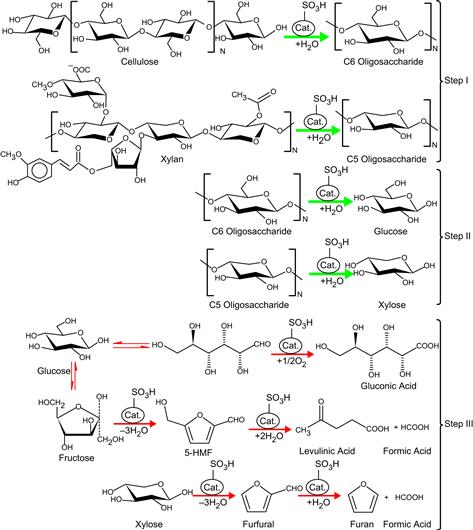
The mechanism for saccharification of rice straw proposed in Scheme 7.2 includes three steps (Li et al., 2012a, b). In Step I, part of the hemicellulose and cellulose contained in rice straw are attacked by protons in the functional groups bonded to a catalyst and converted into various water-soluble oligosaccharides with different molecular weights via catalytic hydrolyses and/or partial hydrothermal hydrolyses. In Step II, water-soluble oligosaccharides with small molecular weight are adsorbed into the pores of the catalyst and then mainly converted into xylose and glucose via catalytic hydrolyses. In Step III, part of the monosaccharide produced inside the pore of the catalyst was further converted into by-products because of overreaction via catalytic hydrolyses. Xylose will be converted further into furfural, which will subsequently be converted into furan (not detected in this study because of its high volatility), formic acid, and/or lactic acid and acetic acid. Similarly, isomerization of glucose into fructose will also occur. Allotropes of glucose and fructose will be converted into 5-HMF, which will further be converted into levulinic acid and formic acid. The prepared catalyst plays a major role in all hydrolysis steps.
References
1. Amarasekara AS, Owereh OS. Synthesis of a sulfonic acid functionalized acidic ionic liquid modified silica catalyst and applications in the hydrolysis of cellulose. Catalysis Communications. 2010;11:1072–1075.
2. Aziz S, Sarkanen KV. Organosolv pulping—A review. Tappi J. 1989;72:169–175.
3. Bjerre AB, Olesen AB, Fernqvist T, Plöger A, Schmidt AS. Pretreatment of wheat straw using alkaline wet oxidation and alkaline hydrolysis resulting in convertible cellulose and hemicellulose. Biotechnology and Bioengineering. 1996;49:568–577.
4. Chum HL, Johnson DK, Black SK. Organosolv pretreatment for enzymatic-hydrolysis of poplars 2 Catalyst effects and the combined severity parameter. Industrial and Engineering Chemistry Research. 1990;29:156–162.
5. Converse AO. Substrate factors limiting enzymatic hydrolysis. In: Saddler JN, ed. Bioconversion of forest and agricultural plant residues. Wallingford: CAB International; 1993;93–106.
6. Ding SY, Himmel ME. The maize primary cell wall micro-fibril: A new model derived from direct visualization. Journal of Agricultural and Food Chemistry. 2006;54:597–606.
7. Eggeman T, Elander RT. Process and economic analysis of pretreatment technologies. Bioresource Technology. 2005;96(18):2019–2025.
8. Goderis B, Jacobs PA, Sels BF. Sulfonated silica/carbon nanocomposites as novel catalysts for hydrolysis of cellulose to glucose. Green Chemistry. 2010;12:1560–1563.
9. Gollapalli LE, Dale BE, Rivers DM. Predicting digestibility of ammonia fiber explosion (AFEX) treated rice straw. Applied Biochemistry and Biotechnology. 2002;98–100:23–35.
10. Grethlein HE, Converse AO. Common aspects of acid prehydrolysis and steam explosion for pretreating wood. Bioresource Technology. 1991;36:77–82.
11. Huber GW, Iborra S, Corma A. Synthesis of transportation fuels from biomass: Chemistry, catalysts, and engineering. Chemical Reviews. 2006;106:4044–4098.
12. Klass DL. Biomass for renewable energy, fuels and chemicals. San Diego: Academic Press; 1998.
13. Klinke HB, Ahring BK, Schmidt AS, Thomsen AB. Characterization of degradation products from alkaline wet oxidation of wheat straw. Bioresource Technology. 2002;82:15–26.
14. Li S, Qian EW. Direct saccharification of rice straw using a solid acid catalyst. Journal of the Japan Institute of Energy. 2011;90:1065–1071.
15. Li S, Qian EW, Hosomi M, Fukunaga T. Preparation of sulfo group bearing silica-based solid acid catalysts and its application to direct saccharification. Journal of Chemical Engineering of Japan. 2012a;45:1–9.
16. Li S, Qian EW, Shibata T, Hosomi M. Catalytic hydrothermal saccharification of rice straw using sulfonated mesoporous silica-based solid acid catalysts. Journal of the Japan Petroleum Institute. 2012b;55:250–260.
17. Lynd LR, Wyman CE, Gerngross TU. Biocommodity engineering. Biotechnology Progress. 1999;15:777–793.
18. Margolese D, Melero JA, Christiansen SC, Chmelka BF, Stucky GD. Direct syntheses of ordered SBA-15 mesoporous silica containing sulfonic acid groups. Chemistry of Materials. 2000;12:2448–2459.
19. Mosier N, Wyman C, Dale B, et al. Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresource Technology. 2005;96:673–686.
20. Nakajima K, Tomita I, Hara M, Hayashi S, Domen K, Kondo JN. Development of highly active SO3H-modified hybrid mesoporous catalyst. Catalysis Today. 2006;116:151–156.
21. Nakayama E, Okamura K. Influence of a steam explosion and microwave irradiation on the enzymatic hydrolysis of a coniferous wood. Mokuzai Gakkaishi. 1989;35:251–260.
22. Onda A, Ochi T, Yanagisawa K. Selective hydrolysis of cellulose over solid acid catalysts. Green Chemistry. 2008;10:1033–1037.
23. O’Sullivan AC. Cellulose: the structure slowly unravels. Cellulose. 1997;4:173–207.
24. Qian EW. Development of production method and catalysts for biomaterials. Journal of the Japan Institute of Energy. 2011;90:518–525.
25. Qian EW, Hosomi M. Saccharification method of cellulosic biomass. J P Patent 2009a; 254283A.
26. Qian EW, Hosomi M. Method of cellulosic biomass liquefaction. J P Patent 2009b; 296919A.
27. Rinaldi R, Palkovits R, Schüth F. Depolymerization of cellulose using solid catalysts in ionic liquids. Angewandte Chemie International Edition. 2008;47:8047–8050.
28. Silverstein RA, Chen Y, Sharma-Shivappa RR, Boyette MD, Osborne J. A comparison of chemical pretreatment methods for improving saccharification of cotton stalks. Bioresource Technology. 2007;98:3000–3011.
29. Suganuma S, Nakajima K, Kitano M, et al. Hydrolysis of cellulose by amorphous carbon bearing SO3H, COOH, and OH groups. Journal of the American Chemical Society. 2008;130:12787–12793.
30. Sulbaran de Ferrer B, Ferrer A, Byers FM, Dale BE, Aristiguieta M. Sugar production fron rice straw. Archivos Latinoamericanos de Production Animal. 1997;5(Suppl. 1):112–114.
31. Teramoto Y, Tanaka N, Lee S-H, Endo T. Pretreatment of eucalyptus wood chips for enzymatic saccharification using combined sulfuric acid-free ethanol cooking and ball milling. Biotechnology and Bioengineering. 2008;99(1):75–85.
32. Teymouri F, Laureano-Perez L, Alizadeh H, Dale BE. Optimization of the ammonia fiber explosion (AFEX) treatment parameters for enzymatic hydrolysis of corn stover. Bioresource Technology. 2005;96(18):2014–2018.
33. Towler GP, Oroskar AR, Smith SE. Development of a sustainable liquid fuels infrastructure based on biomass. Environmental Progress. 2004;23:334–341.
34. Turner ND, McDonough CM, Byers FM, et al. Disruption of forage structure with an ammonia fiber explosion process. Proceedings, Western Section, American Society of Animal Science. 1990;41.
35. Wyman CE, Decker SR, Himmel ME, Brady JW, Skopec CE, Vikari L. In: Dumitriu S, ed. Polysaccharides. 2nd ed. New York: Marcel Dekker; 2005.
36. Yang B, Wyman CE. Effect of xylan and lignin removal by batch and flow through pretreatment on the enzymatic digestibility of corn stover cellulose. Biotechnology and Bioengineering. 2004;86:88–95.