Enzymes for Cellulosic Biomass Conversion
Takashi Tonozuka, Makoto Yoshida and Michio Takeuchi
Abstract
Cellulose is one of the most abundant biomass resources on earth. The enzymatic degradation of cellulosic biomass is a key technology for producing cost-effective bioethanol. This chapter provides an introduction to the structure and function of cellulases and related enzymes. A filamentous fungus, Trichoderma reesei, is the most used microorganism in industry as a source of cellulolytic enzymes, and two major cellulases, Cel6A and Cel7A, from T. reesei have been extensively studied. Some other enzymes, β-glucosidases and hemicellulase, are also essential for the degradation of cellulosic biomass. This chapter also includes a brief overview of the structure and function of amylases, which are enzymes conventionally used in the production of bioethanol.
Keywords
Cellulose; cellulase; cellobiohydrolase (CBH); endoglucanase (EG); Trichoderma reesei; β-glucosidase (BGL); hemicellulase; amylase
Chapter Outline
9.1. General Information on Cellulases
9.1.2. General Properties of Cellulases
9.2. Structure and Function of Cellulases
9.2.1. The CAZy Database: A Classification System Based on Structures
9.2.2. Cellulases of Two Well-Studied Organisms, Trichoderma reesei and Clostridium thermocellum
9.2.3. The GH6 and GH7 Enzymes from Trichoderma reesei
9.2.4. Subsite and Catalytic Residues
9.3. Other Biomass-Degrading Enzymes
9.1 General Information on Cellulases
9.1.1 Cellulose
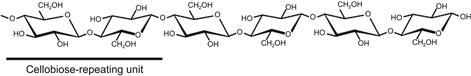
Cellulose is the major carbohydrate component of plant cell walls, and thus is one of the most abundant biomass resources on earth. The cellulose molecule is an insoluble linear homopolymer of β-D-glucopyranose units linked together by the 1 and 4 carbon atoms. Because the adjacent glucose moieties are rotated 180° about the axis of the cellulose backbone chain as shown in Figure 9.1, the basic structural repeating unit of cellulose is cellobiose rather than glucose (Blackwell, 1982; Atalla, 1983). The ends of the cellulose chain contain different types of β-anhydroglucose units, i.e. the reducing end with a free hemiacetal (or aldehyde) group at C-1, and the non-reducing end with a free hydroxyl at C-4. The hydroxyl groups in cellulose are positioned in the ring plane, whereas the hydrogen atoms are in the vertical position. Therefore, a hydrophobic surface is formed in the cellulose chain due to the nature of successive β-anhydroglucose units.
The cellulose molecule does not exist as an isolated individual molecule. It forms a crystalline structure, known as cellulose microfibril (Nishiyama, 2009), through the tight packing of chains by intra- and intermolecular bonds such as hydrogen bonds and van der Waals forces. The crystalline structure gives cellulose high tensile strength and resistance to chemical or enzymatic attack. In nature, therefore, cellulose can be digested only by a limited number of organisms, most of which are bacteria and fungi.
9.1.2 General Properties of Cellulases
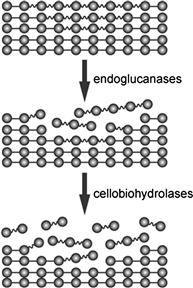
The word “cellulase” is a generic term used to refer to the enzymes that hydrolyze glucan chains in cellulose polymer into cellooligosaccharides or ultimately glucose. Traditionally, cellulases have been divided into two types, i.e. cellobiohydrolases (CBHs; EC 3.2.1.91) and endoglucanases (EGs; EC 3.2.1.4), based on their activity profiles. The former hydrolyzes cellulose molecules from the end of the chain with the release of cellobiose, whereas the latter randomly cleaves the internal β-1,4-glucosidic bond of cellulose. EGs preferentially act on amorphous cellulose and soluble cellulose derivatives such as carboxymethyl cellulose (CMC), resulting in a rapid decrease of the degree of polymerization in cellulose; they lack hydrolytic activity against crystalline cellulose. On the other hand, CBHs possess significant activity against the crystalline region of cellulose, although the majority of CBH-type enzymes show poor activity against CMC. These enzymes act concertedly to degrade cellulose biologically (Streamer et al., 1975; Wood and McCrae, 1978; Henrissat et al., 1985) in three sequential reactions: (1) EGs initially hydrolyze the cellulose chain in the amorphous region; (2) CBHs recognize the newly generated ends of cellulose chains and start the catalytic reaction with the release of cellobiose; and (3) successive hydrolysis of cellobiose by CBHs finally results in degradation of the crystalline region of cellulose. The sequence of these reactions is called the endo–exo mechanism (Figure 9.2).
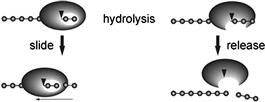
Recently, the endo–exo concept has been considered as insufficient to understand cellulose biodegradation, so that the concept of a processivity mechanism has been suggested to comprehend the behavior of cellulase’s action (Davies and Henrissat, 1995). This concept explains the action of cellulases after the catalytic reaction (Figure 9.3), i.e. a high processive enzyme slides to the next cellobiose unit following hydrolysis of the β-1,4-glucosidic bond of cellulose, whereas a low processive enzyme easily detaches itself from the cellulose chain after the catalytic reaction. A correlation between structural features and processivity has been shown based on three-dimensional structures of cellulases. In particular, a tunnel structure around the active site is critical for expressing processive functions. This is covered in detail in Section 9.2.
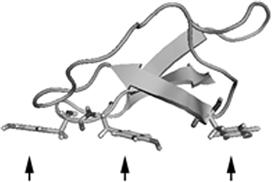
Cellulase generally consists of two domains: (1) a catalytic domain, which catalyzes hydrolysis of the β-1,4-glucosidic bond of cellulose, and (2) a cellulose-binding domain (CBD), the binding domain from fungal enzymes, which is currently called family 1 carbohydrate-binding module (CBM1) and is one of the best characterized CBMs. This domain lacks any catalytic activity; it binds the surface of cellulose via three aromatic amino acids, as shown in Figure 9.4. Removal of this domain causes a significant decrease in both binding and catalytic activities against crystalline cellulose but shows no effects on the hydrolysis of amorphous cellulose (Tomme et al., 1988; Ståhlberg et al., 1993; Linder and Teeri, 1997). These observations suggest that CBM1 is related to the degradation of crystalline cellulose.
9.2 Structure and Function of Cellulases
9.2.1 The CAZy Database: A Classification System Based on Structures
A database designated as CAZy (Carbohydrate-Active enZymes; http://www.cazy.org/; Cantarel et al., 2009) is indispensable for studying the structure and function of cellulases. The CAZy database was developed by Bernard Henrissat and colleagues, and is now used worldwide as the standard classification system of carbohydrate-acting enzymes. In the CAZy database, carbohydrate-acting enzymes are classified based on their structural similarity into four major groups, i.e. glycoside hydrolase (GH), glycoside transferase (GT), polysaccharide lyase (PL), and carbohydrate esterase (CE). Each group is divided into families, which are serially numbered. For example, one of the cellobiohydrolases is classified and designated as glycoside hydrolase (GH) family 6, and the abbreviated name of the family, GH6, is normally used.
The nomenclature of cellulases has recently been developed based on the CAZy database. As described in Section 9.1.2, cellulases are divided into EG (endoglucanase) and CBH (cellobiohydrolase) in terms of their enzymatic properties. The name of cellulases is conventionally given as a combination of EG/CBH and numbers; for example, cellulases from Trichoderma reesei are designated as “EG I” or “CBH II”. According to the recent nomenclature, the name of cellulases is a combination of “Cel” (which means cellulase), the number of the glycoside hydrolase family, and a capital letter. For example, the cellulases “EG I” and “CBH II” from Trichoderma reesei as described above are designated as “Cel7B” and “Cel6A” respectively.
Many carbohydrate-acting enzymes including cellulases consist of a catalytic domain and a carbohydrate-binding domain; the latter plays a crucial role in the enzymatic activity. In the CAZy database, these non-catalytic domains are classified into carbohydrate-binding modules (abbreviated as CBM).
9.2.2 Cellulases of Two Well-Studied Organisms, Trichoderma reesei and Clostridium thermocellum
Cellulases are classified into glycoside hydrolase families, GH5, GH6, GH7, GH8, GH9, GH12, GH44, GH45, GH48, GH51, GH74, and GH124. Enzymes belonging to GH61 also show activities similar to cellulases, but the GH61 enzymes have been reported not to be identified as cellulases; they are identified as copper-dependent monooxygenases (Quinlan et al., 2011). Although cellulases are found in many glycoside hydrolase families, none of the cellulase-producing organisms possesses genes encoding all of these GH members.
A filamentous fungus, Trichoderma reesei, is the most used microorganism in industry as a source of cellulolytic enzymes (Gusakov, 2011), and therefore numerous studies of enzymes derived from this organism have been carried out. The organism has been shown to be the anamorph of the ascomycete Hypocrea jecorina, but is still better known by its former name, T. reesei. The complete genome of T. reesei, which was sequenced in 2008, indicates that the organism possesses genes for GH5 (two genes), GH6 (one gene), GH7 (two genes), GH12 (one gene), GH45 (one gene), and GH61 (three genes) (Martinez et al., 2008). Among these enzymes, the most predominant cellulases secreted by T. reesei are Cel7A (CBH I) and Cel6A (CBH II), which account for 50–60% and ∼20% respectively of the total cellulases. Likewise, a basidiomycete, Phanerochaete chrysosporium, which is also well known as a producer of cellulolytic enzymes, secretes cellobiohydrolase (Cel7D) of up to ∼10% of the total secreted protein in liquid culture (Ubhayasekera et al., 2005).
Bacteria and fungi are important players in degrading the cellulolytic materials in nature. Although bacterial enzymes are not utilized by the industry, numerous studies on bacterial enzymes have been conducted. In particular, several anaerobic bacteria produce a multienzyme complex termed cellulosome, which is an attractive target for the structural biology. The cellulosome was first observed to exist in Clostridium thermocellum, and now several Clostridium species and Ruminococcus species as well as some other organisms have been reported to produce this multienzyme complex (Demain et al., 2005; Doi, 2008). The cellulosome is composed of a non-catalytic subunit called scafoldin that consists of multiple repeats of cohesin domains and cellulosomal enzymes, which typically comprise a catalytic domain and a dockerin domain. Each cohesin domain interacts with the dockerin domain of the cellulosomal enzymes, resulting in the formation of a multienzyme complex, or cellulosome (Figure 9.5C). Cellulases consisting of the cellulosome belong to GH5, GH8, GH9, and GH48, and the majority of the enzymes are classified as either GH9 or GH48. The catalytic domains are composed of an (α/α)6 barrel (Figure 9.5A, B) for GH9 and GH48, and a (β/α)7 barrel and β-sandwich (Figure 9.6A, B) for GH6 and GH7. Thus, the structural folds of cellulosomal enzymes are completely different from those of cellulases from T. reesei.
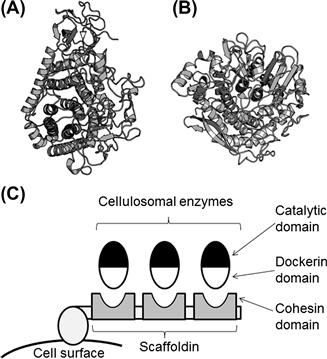
FIGURE 9.5 Cellulases from Clostridium thermocellum.
(A, B) Ribbon representations of: (A) GH9 cellobiohydrolase (PDB ID, 1RQ5) and (B) GH48 cellobiohydrolase (PDB ID, 1L2A). (C) Schematic illustration of a cellulosome. Note that the actual structure is much more complicated.
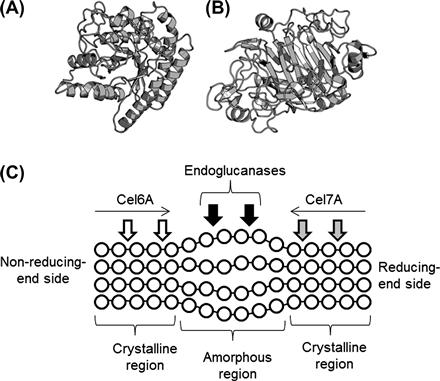
FIGURE 9.6 Cellulases from Trichoderma reesei.
(A–C) Ribbon representations of: (A) Cel6A catalytic domain (PDB ID, 1QJW); (B) Cel7A catalytic domain (PDB ID, 5CEL); and (C) cellulose-binding domain of Cel7A (PDB ID, 1CBH). In (C), three tyrosine residues that are critical for the cellulose-binding are indicated. (D) Schematic illustration of the enzymatic hydrolysis of cellulose. Glucose residues are indicated by circles.
9.2.3 The GH6 and GH7 Enzymes from Trichoderma reesei
Trichoderma reesei mainly produces the GH6 and GH7 CBHs, Cel6A and Cel7A, as described above. The GH6 CBH releases cellobiose units from the non-reducing end of cellulose (EC 3.2.1.91), while the GH7 CBH removes cellobiose units from the reducing end of cellulose (EC 3.2.1.176) (Igarashi et al., 2011). GH7 CBH and GH48 CBH have a unique enzymatic character because there are only a few glycosidases that hydrolyze polysaccharides from the reducing end in an exo manner. In contrast, all the exo-acting amylases degrade starch from the non-reducing end.
A cellulose fiber contains crystalline and amorphous regions. For the T. reesei cellulase system, attacking the crystalline region by GH6 and GH7 CBHs, Cel6A, from the non-reducing end and by Cel7A from the reducing end has been proposed, whereas the amorphous region is accessible to endoglucanase action (Figure 9.6C) (Teeri, 1997). Although no structural homology is found between the catalytic domains of GH6 and GH7, an enclosed tunnel is present at the active site of both GH6 and GH7 CBHs (Figure 9.7A, B). It is noteworthy that the length of the tunnel of T. reesei Cel7A is ∼50 Å (Divne et al., 1998), which is an unusual structure among glycosidases. Some EGs are also classified as GH6 or GH7, but no enclosed tunnel is found in the structures of GH6 and GH7 EGs. Trichoderma reesei Cel6A and Cel7A possess a cellulose-binding domain that is grouped in the CAZy family, CBM1. The cellulose-binding domain of Cel6A is located on the N-terminal side, whereas the cellulose-binding domain of Cel7A is found on the C-terminal side (Linder et al., 1995; Rabinovich et al., 2002). The structural features of CBM1 are described in Section 9.1.2; the CBM1 cellulose-binding domain, which is smaller than the catalytic domain, consists of ∼40 amino acid residues.
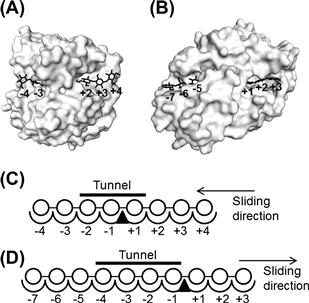
FIGURE 9.7 Subsite structures of Cel6A and Cel7A from Trichoderma reesei.
(A) A surface model of the catalytic domain of Cel6A. The cellulose chain was constructed by combining the ligands of the structures, PDB IDs 1OCB and 1QJW, and overlaid on the surface model of 1QJW. (B) A surface model of the catalytic domain of Cel7A. The cellulose chain was constructed by combining the ligands of the structures, PDB IDs 5CEL and 6CEL, and overlaid on the surface model of 5CEL. (C, D) Schematic models of the subsite structures of Cel6A (C) and Cel7A (D). Symbols: circle, glucose residue; triangle, enzymatic cleavage point. The non-reducing end and the reducing end of the substrates are drawn on the left and the right respectively.
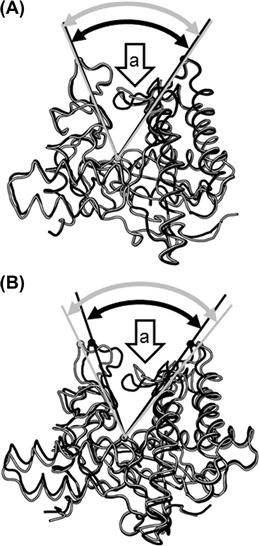
9.2.4 Subsite and Catalytic Residues
The term subsite refers to the structural relationship between an enzyme and a polymer substrate. In the case of glycosidase and polysaccharide substrates, the glycosidase has individual regions called subsites, each of which binds to a specific monosaccharide unit of the polysaccharide substrate. The subsites that bind to the non-reducing end are labeled “minus” and those that bind to the reducing end of the substrate are labeled “plus”; the glycosidase cleaves between the −1 and +1 subsites (Davies et al., 1997). An enclosed tunnel is present at the active site of the GH6 and GH7 CBHs (Figure 9.7A, B). Therefore, the substrate cellulose is initially considered to bind the plus subsites of the GH6 CBH, and then slides through the active center tunnel until it occupies subsites −2 and −1. In the case of GH7 CBH, the plus and minus subsites have opposite signs. For GH6 and GH7 CBHs, subsite −3 and subsite +3 are presumed unnecessary to produce the respective cellobioses, but T. reesei Cel7A has been reported to have 10 subsites from −7 to +3 (Figure 9.7C) (Divne et al., 1998). In T. reesei Cel6A, the existence of eight subsites from −4 to +4 has also been proposed (Figure 9.7D) (Varrot et al., 2003).
Knowledge of the catalytic residues and the concept of subsites is of great importance in understanding the structure–function relationship of enzymes. Reaction mechanisms of glycosidases are divided into two groups: inverting mechanism and retaining mechanism. In the inverting mechanism, the enzymatic reaction is accompanied by an inversion of the anomeric configuration, whereas in the retaining mechanism the anomeric configuration of the substrate is retained. For instance, the “inverting” CBH and the “retaining” CBH produce α-cellobiose and β-cellobiose respectively from cellulose. GH6 and GH7 enzymes act through the inverting mechanism and retaining mechanism respectively. In the inverting mechanism, two amino acid residues that act as a general acid and a general base are found to be the catalytic residues, whereas in the retaining mechanism a nucleophile residue and a general acid/base residue are identified as the catalytic residues. In both mechanisms, aspartic acid and/or glutamic acid residues normally function as the catalytic residues of glycosidases (Rye and Withers, 2000). In T. reesei Cel7A, the nucleophile residue is Glu212 and the general acid/base residue is Glu217 (Divne et al., 1994). In GH6 enzymes, the catalytic general acid residue is identified as a conserved aspartic acid, Asp221 in T. reesei Cel6A, but it is still debatable concerning which residue acts as the catalytic base of the GH6 enzymes. Recent studies show that Asp175 in T. reesei Cel6A is located relatively far from the cleavage site of the substrate, but has been proposed to be the catalytic base. It acts indirectly through the hydrogen bond network of water molecules in what has been called a Grotthus mechanism (Koivula et al., 2002).
In conclusion, the structures and functions of the GH6 and GH7 cellulases are unusual compared with other glycosidases such as amylases. GH6 and GH7 CBHs are characterized by an enclosed tunnel at the active site. GH7 CBHs release cellobiose units from the reducing end of cellulose, and the catalytic base of the GH6 enzymes has been proposed to act indirectly in the so-called Grotthus mechanism. It is likely that these unusual features of cellulases make them suitable for the hydrolysis of cellulose. For more detailed information see Box 9.1.
9.3 Other Biomass-Degrading Enzymes
9.3.1 β-Glucosidases
β-Glucosidases (BGLs; EC 3.2.1.21) catalyze the hydrolysis of β-glucosidic bonds in various β-glucosidic compounds, and thus are involved in various metabolic pathways such as degradation of polysaccharides, cellular signaling, oncogenesis, and host–pathogen interactions. Among these pathways, the enzymes important for cellulose degradation, i.e. cellobiases, are focused in this section. These enzymes are indispensable for the production of glucose, which is then fermented into ethanol by yeast in the subsequent process of bioethanol production.
In nature, various cellulolytic microorganisms produce BGLs during the course of cellulose degradation. Fungal enzymes are especially regarded as the most promising BGL in bioethanol production. Filamentous fungi mainly produce three types of BGLs, i.e. extracellular, intracellular, and cell wall-associated enzymes (Lynd et al., 2002); extracellular and intracellular enzymes have been well characterized so far. These enzymes, which are retaining enzymes, catalyze the hydrolysis of cellobiose and cellooligosaccharides with the release of glucose. In addition to their hydrolytic activities, the enzymes often show transglycosylation activities. Because the activity of BGL decreases at higher concentrations of glucose, improving the glucose tolerance is one of the many important targets for conducting BGL research.
The extracellular BGLs belong to the GH3 family in the CAZy database, and some of the enzymes possess CBM1 beside the catalytic domain. Because the enzymes catalyze the hydrolysis of cellobiose, which is the main product of cellulose hydrolysis by cellulases, the cellobiases are considered to have an important role in the final step of extracellular cellulose degradation (Sternberg, 1977; Eriksson, 1978). Previous studies on extracellular BGL obtained from Phanerochaete chrysosporium reveal interesting results. The enzyme prefers laminaribiose (disaccharide with β-1,3-glucosidic bond) instead of cellobiose, and the reaction efficiency against β-1,3-oligosaccharides increases with higher degree of polymerization of the substrates. This observation suggests that the enzyme is β-1,3-glucanase (Igarashi et al., 2003). Hence, not all extracellular BGLs from cellulolytic fungi are cellobiases. On the other hand, intracellular BGLs belong to the GH1 family, and the intracellular localization of the BGLs requires the uptake of cellobiose into the cell. In this regard, T. reesei is expected to be a putative diglucoside permease involved in the transport of cellobiose into the cell (Kubicek et al., 1993). In addition, intracellular BGL from T. reesei is reported to produce sophorose, which is a very strong inducer of cellulases (Mandels et al., 1962), via transglycosylation activities (Saloheimo et al., 2002), although the induction of cellulases by sophorose is observed only in a few limited fungal species, such as T. reesei.
9.3.2 Hemicellulases
Hemicelluloses, which represent about 20–35% of the plant cell wall, are heterogeneous polymers of various sugars such as xylose, arabinose, glucose, mannose, and galactose. The composition of hemicellulose depends on the source and type of plant biomass. Hardwood and graminaceous plants contain 4-O-methylglucuronoxylan and arabinoxylan as the major hemicellulose respectively, whereas hemicellulose of softwood mainly consists of glucomannan and glucuronoxylan. Xylans, such as 4-O-methylglucuronoxylan, arabinoxylan, and glucuronoxylan, have a backbone of 1,4-linked β-D-xylopyranose units. In addition to xylopyranoside residues, xylans contain various substituents, i.e. arabinoside residues, O-acetyl groups, ferulic acid, p-coumaric acid, and 4-O-methylglucuronic acid (Wilkie and Woo, 1977). Glucomannan is a mainly linear heteropolymer consisting of β-1,4-linked D-mannose and D-glucose (Timell, 1967). Xyloglucan is a highly branched heteropolymer with D-glucose and D-xylose. Xylan and glucomannan are located in the secondary wall, whereas xyloglucan is contained in the primary wall. Therefore, xyloglucan accounts for only a small fraction of the plant cell wall.
The word “hemicellulase” is a generic term used to refer to enzymes that have a role in hemicellulose degradation, and thus includes not only glycosidases but also esterases (de Vries and Visser, 2001).
Endo-1,4-β-D-xylanases (EC 3.2.1.8) are enzymes that randomly hydrolyze β-1,4-xylosidic bonds of xylan backbone (Polizeli et al., 2005). They belong to GH5, GF7, GH8, GH 10, GH11, and GH43 in the CAZy database; GH10 and GH11 have been well characterized. The GH10 enzymes act preferentially on soluble substrates and can readily hydrolyze small xylooligosaccharides such as xylotriose. Unlike GH10 enzymes, GH11 enzymes are most active in the hydrolysis of insoluble xylans, although the activity is more likely to be inhibited by the presence of arabinose decorations. Because xylanase enhances the hydrolysis of cellulose by cellulases, it has been considered as a major accessory enzyme in the saccharification of plant biomass.
α-L-Arabinofuranosidase (EC 3.2.1.55) catalyzes the hydrolysis of α-1,2-, α-1,3- or α-1,5-L-arabinofuranosidic bonds in arabinose-containing polysaccharides (Numan and Bhosle, 2006). It has been classified as GH family 3, 43, 51, 54, and 62 in the CAZy database. Some α-L-arabinofuranosidases demonstrate broad substrate specificity, i.e. acting on arabinofuranosyl residues at O-2, O-5 and/or O-3 positions as a single substituent. On the other hand, α-L-arabinofuranosidases that specifically catalyze the release of αα-1,3-L-arabinofuranosyl substituents from doubly substituted xylose residues have also been found. These enzymes work synergistically on arabinoxylan degradation with xylanases.
Acetylxylan esterases (EC 3.1.1.72) release acetate from xylan and xylooligosaccharides. The enzymes have been classified as carbohydrate esterase (CE) families 1, 2, 3, 4, 5, 6, 7, and 12 in the CAZy database. CE16 is also reported to contain acetyl esterase that releases acetate from 4-nitrophenyl-β-D-xylopyranoside monoacetate (Li et al., 2008). The removal of acetyl groups by acetylxylan esterases improves the access of xylanases to the xylan backbone and facilitates the degradation of xylans.
In addition to the enzymes described above, various other enzymes such as α-glucuronidases, xyloglucanases, galactosidases, glucuronoyl esterases, feruroyl esterases, mannanases, endoglucanases, and xylosidases are required to degrade hemicellulose (de Vries and Visser, 2001). The synergistic action of these enzymes leads to the hydrolysis of the complicated structure of hemicelluloses.
9.3.3 Starch-Hydrolyzing Enzymes: Amylases
Although producing ethanol from starch adversely affects food supplies, the process is still important to convert non-edible biomass such as crop waste into ethanol. The term “amylase” is defined as an enzyme that hydrolyzes starch, and this section includes a synopsis of amylases.
Starch is a polymer of glucose units linked predominantly via α-1,4-glucosidic linkages and some α-1,6-glucosidic linkages to form branch points. Amylases are classified according to their enzymatic reactions into the following four groups (Figure 9.9A):
1. α-Amylase (EC 3.2.1.1) is an endo-acting enzyme that hydrolyzes internal α-1,4-glucosidic linkages of starch via a retaining mechanism. The enzyme belongs to GH13, GH57, and GH119 in the CAZy database, and is produced by a wide range of organisms, including archaea, bacteria, plants, and animals.
2. Starch-debranching enzymes that hydrolyze α-1,6-glucosidic linkages of starch are further classified as isoamylase (EC 3.2.1.68) and pullulanase (EC 3.2.1.41) based on their substrate specificities. Both isoamylase and pullulanase belong to GH13, and are produced by plants and some archaea and bacteria.
3. β-Amylase (EC 3.2.1.2) is an exo-acting enzyme that hydrolyzes the non-reducing ends of starch to produce β-maltose via an inverting mechanism. The enzyme belongs to GH14 in the CAZy database, and is produced by plants and some bacteria.
4. Glucoamylase (EC 3.2.1.3) is an exo-acting enzyme that hydrolyzes the non-reducing ends of starch to produce β-glucose via an inverting mechanism. The enzyme belongs to GH15 in the CAZy database, and is produced by some archaea, bacteria, and fungi.
In the CAZy database, numerous amylases are members of GH13, which was originally known as the α-amylase family. There are four conserved amino acid sequences in the original definition of the α-amylase family (Kuriki and Imanaka, 1999), and three catalytic residues (i.e. aspartic acid, glutamic acid, and aspartic acid residues) are located in the second, third, and fourth conserved regions. Now the α-amylase family is defined as a clan GH-H composed of three glycoside hydrolase families, including GH13, GH70, and GH77 (MacGregor et al., 2001). The essential structure of GH13 is composed of domains A, B, and C; domain A consists of a (β/α)8 barrel with a small domain, i.e. domain B, protruding from it. Domain C is located at the C-terminal side of domains A and B, and it forms a β-sandwich (Figure 9.9B) (Kuriki and Imanaka, 1999). Isoamlase, pullulanase, and many other GH13 enzymes have some extra domains other than A, B, and C.
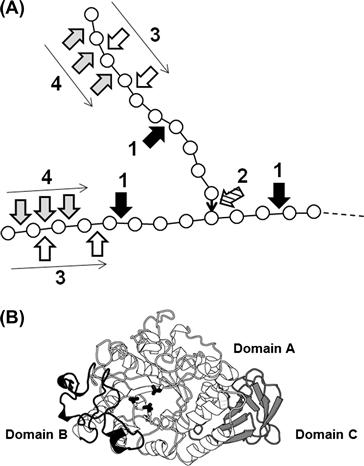
FIGURE 9.9 Action and structure of amylases.
(A) Schematic action pattern of amylases. Symbols: circle, glucose; bold line, α-1,4-glucosidic linkage; ↓, α-1,6-glucosidic linkage; arrow 1, cleavage points of α-amylase; arrow 2, cleavage points of debranching enzymes; arrow 3, cleavage points of β-amylase; arrow 4, cleavage points of glucoamylase. (B) Structure of a typical α-amylase, Aspergillus oryzae α-amylase. Domains are shown in different gray scales. Three catalytic residues conserved in amylase family enzymes are indicated in a stick representation.
References
1. Atalla RH. The structure of cellulose: Recent developments. New York: Academic Press; 1983.
2. Blackwell J. The macromolecular organization of cellulose and chitin. New York: Plenum; 1982.
3. Cantarel BL, Coutinho PM, Rancurel C, Bernard T, Lombard V, Henrissat B. The Carbohydrate-Active EnZymes database (CAZy): An expert resource for Glycogenomics. Nucleic acids research. 2009;37:D233–D238.
4. Davies G, Henrissat B. Structures and mechanisms of glycosyl hydrolases. Structure. 1995;3:853–859.
5. Davies GJ, Wilson KS, Henrissat B. Nomenclature for sugar-binding subsites in glycosyl hydrolases. The Biochemical journal. 1997;321:557–559.
6. Demain AL, Newcomb M, Wu JH. Cellulase, clostridia, and ethanol. Microbiology and molecular biology reviews: MMBR. 2005;69:124–154.
7. de Vries RP, Visser J. Aspergillus enzymes involved in degradation of plant cell wall polysaccharides. Microbiology and molecular biology reviews: MMBR. 2001;65:497–522.
8. Divne C, Ståhlberg J, Reinikainen T, et al. The three-dimensional crystal structure of the catalytic core of cellobiohydrolase I from. Trichoderma reesei Science. 1994;265:524–528.
9. Divne C, Ståhlberg J, Teeri TT, Jones TA. High-resolution crystal structures reveal how a cellulose chain is bound in the 50 Å long tunnel of cellobiohydrolase I from Trichoderma reesei. Journal of molecular biology. 1998;275:309–325.
10. Doi RH. Cellulases of mesophilic microorganisms: Cellulosome and noncellulosome producers. Annals of the New York Academy of Sciences. 2008;1125:267–279.
11. Eriksson KE. Enzyme mechanisms involved in cellulose hydrolysis by the white-rot fungus Sporotrichum pulverulentum. Biotechnology and bioengineering. 1978;70:317–332.
12. Gusakov AV. Alternatives to Trichoderma reesei in biofuel production. Trends in biotechnology. 2011;29:419–425.
13. Henrissat B, Driguez H, Viet C, Schulein M. Synergism of cellulases from Trichoderma reesei in the degradation of cellulose. Biotechnology. 1985;3:722–726.
14. Igarashi K, Tani T, Kawai R, Samejima M. Family 3 β-glucosidase from cellulose-degrading culture of the white-rot fungus Phanerochaete chrysosporium is a glucan 1,3-β-glucosidase. Journal of bioscience and bioengineering. 2003;95:572–576.
15. Igarashi K, Uchihashi T, Koivula A, et al. Traffic jams reduce hydrolytic efficiency of cellulase on cellulose surface. Science. 2011;333:1279–1282.
16. Koivula A, Ruohonen L, Wohlfahrt G, et al. The active site of cellobiohydrolase Cel6A from Trichoderma reesei: The roles of aspartic acids D221 and D175. Journal of the American Chemical Society. 2002;124:10015–10024.
17. Kubicek CP, Ressner R, Gruber F, Mandels M, Kubicek-Pranz EM. Triggering of cellulase biosynthesis by cellulase in Trichoderma reesei: Involvement of a constitutive, sophorose-inducible, glucose-inhibited β-diglucoside permease. The Journal of biological chemistry. 1993;268:19364–19368.
18. Kuriki T, Imanaka T. The concept of the alpha-amylase family: Structural similarity and common catalytic mechanism. Journal of bioscience and bioengineering. 1999;87:557–565.
19. Li XL, Skory CD, Cotta MA, Puchart V, Biely P. Novel family of carbohydrate esterases, based on identification of the Hypocrea jecorina acetyl esterase gene. Applied and environmental microbiology. 2008;74:7482–7489.
20. Linder M, Teeri TT. The role and function of cellulose-binding domains. Biotechnology journal. 1997;57:15–28.
21. Linder M, Mattinen ML, Kontteli M, et al. Identification of functionally important amino acids in the cellulose-binding domain of Trichoderma reesei cellobiohydrolase I. Protein science: a publication of the Protein Society. 1995;4:1056–1064.
22. Liu Y, Igarashi K, Kaneko S, et al. Characterization of glycoside hydrolase family 6 enzymes from Coprinopsis cinerea. Bioscience, biotechnology, and biochemistry. 2009;73:1432–1434.
23. Liu Y, Yoshida M, Kurakata Y, et al. Crystal structure of a glycoside hydrolase family 6 enzyme, CcCel6C, a cellulase constitutively produced by Coprinopsis cinerea. The FEBS journal. 2010;277:1532–1542.
24. Lynd LR, Weimer PJ, van Zyl WH, Pretorius IS. Microbial cellulose utilization: Fundamentals and biotechnology. Microbiology and molecular biology reviews: MMBR. 2002;66:506–577.
25. MacGregor EA, Janecek S, Svensson B. Relationship of sequence and structure to specificity in the α-amylase family of enzymes. Biochimica et Biophysica Acta. 2001;1546:1–20.
26. Mandels M, Parrish FW, Reese ET. Sophorose as an inducer of cellulase in Trichoderma viride. Journal of bacteriology. 1962;83:400–408.
27. Martinez D, Berka RM, Henrissat B, Saloheimo M, Arvas M, et al. Genome sequencing and analysis of the biomass-degrading fungus Trichoderma reesei (syn Hypocrea jecorina). Nature biotechnology. 2008;26:553–560.
28. Mertz B, Kuczenski RS, Larsen RT, Hill AD, Reilly PJ. Phylogenetic analysis of family 6 glycoside hydrolases. Biopolymers. 2005;79:197–206.
29. Nishiyama Y. Structure and properties of the cellulose microfibril. J Wood Sci. 2009;55:241–249.
30. Numan MT, Bhosle NB. α-L-Arabinofuranosidases: The potential applications in biotechnology. Journal of industrial microbiology & biotechnology. 2006;33:247–260.
31. Polizeli ML, Rizzatti AC, Monti R, Terenzi HF, Jorge JA, Amorim DS. Xylanases from fungi: Properties and industrial applications. Applied microbiology and biotechnology. 2005;67:577–591.
32. Quinlan RJ, Sweeney MD, Lo Leggio L, et al. Insights into the oxidative degradation of cellulose by a copper metalloenzyme that exploits biomass components. Proceeding of the National Academy of Sciences of the United States of America. 2011;108:15079–15084.
33. Rabinovich ML, Melnick MS, Bolobova AV. The structure and mechanism of action of cellulolytic enzymes. Biochemistry (Moscow). 2002;67:850–871.
34. Rouvinen J, Bergfors T, Teeri T, Knowles JK, Jones TA. Three-dimensional structure of cellobiohydrolase II from. Trichoderma reesei Science. 1990;249:380–386.
35. Rye CS, Withers SG. Glycosidase mechanisms. Current opinion in chemical biology. 2000;4:573–580.
36. Saloheimo M, Kuja-Panula J, Ylosmaki E, Ward M, Penttila M. Enzymatic properties and intracellular localization of the novel Trichoderma reesei β-glucosidase BGLII (cel1A). Applied and environmental microbiology. 2002;68:4546–4553.
37. Ståhlberg J, Johansson G, Pettersson G. Trichoderma reesei has no true exo-cellulase: All intact and truncated cellulases produce new reducing end groups on cellulose. Biochimica et biophysica acta. 1993;1157:107–113.
38. Stajich JE, Wilke SK, Ahrén D, et al. Insights into evolution of multicellular fungi from the assembled chromosomes of the mushroom Coprinopsis cinerea (Coprinus cinereus). Proceeding of the National Academy of Sciences of the United States of America. 2010;107:11889–11894.
39. Sternberg D, Vijayakumar P, Reese ET. β-Glucosidase: Microbial production and effect on enzymatic hydrolysis of cellulose. Canadian journal of microbiology. 1977;23:139–147.
40. Streamer M, Eriksson KE, Pettersson B. Extracellular enzyme system utilized by the fungus Sporotrichum pulverulentum (Chrysosporium lignorum) for the breakdown of cullulose Functional characterization of five endo-1,4-β-glucanases and one exo-1,4-β-glucanase. European Journal of Biochemistry. 1975;59:607–613.
41. Tamura M, Miyazaki T, Tanaka Y, Yoshida M, Nishikawa A, Tonozuka T. Comparison of the structural changes in two cellobiohydrolases, CcCel6A and CcCel6C, from Coprinopsis cinerea: A tweezer-like motion in the structure of CcCel6C. The FEBS journal. 2012;279:1871–1882.
42. Teeri TT. Crystalline cellulose degradation: New insight into the function of cellobiohydrolases. Trends in biotechnology. 1997;15:160–167.
43. Timell TE. Recent progress in the chemistry of wood hemicelluloses. Wood Science and Technology. 1967;1:45–70.
44. Tomme P, Van Tilbeurgh H, Pettersson G, et al. Studies of the cellulolytic system of Trichoderma reesei QM 9414 Analysis of domain function in two cellobiohydrolases by limited proteolysis. European Journal of Biochemistry. 1988;170:575–581.
45. Ubhayasekera W, Muñoz IG, Vasella A, Ståhlberg J, Mowbray SL. Structures of Phanerochaete chrysosporium Cel7D in complex with product and inhibitors. The FEBS journal. 2005;272:1952–1964.
46. Varrot A, Hastrup S, Schülein M, Davies GJ. Crystal structure of the catalytic core domain of the family 6 cellobiohydrolase II, Cel6A, from Humicola insolens, at 1.92 Å resolution. Journal of biochemistry. 1999a;337:297–304.
47. Varrot A, Schülein M, Davies GJ. Structural changes of the active site tunnel of Humicola insolens cellobiohydrolase, Cel6A, upon oligosaccharide binding. Biochemistry. 1999b;38:8884–8891.
48. Varrot A, Frandsen TP, von Ossowski I, et al. Structural basis for ligand binding and processivity in cellobiohydrolase Cel6A from Humicola insolens. Structure. 2003;11:855–864.
49. Wilkie KCB, Woo SL. A heteroxylan and hemicellulosic materials from bamboo leaves, and a reconsideration of the general nature of commonly occurring xylans and other hemicelluloses. Carbohydrate research. 1977;57:145–162.
50. Wood TM, McCrae SI. The cellulase of Trichoderma koningii Purification and properties of some endoglucanase components with special reference to their action on cellulose when acting alone and in synergism with the cellobiohydrolase. The Biochemical journal. 1978;171:61–72.
51. Yoshida M, Sato K, Kaneko S, Fukuda K. Cloning and transcript analysis of multiple genes encoding the glycoside hydrolase family 6 enzyme from Coprinopsis cinerea. Bioscience, biotechnology, and biochemistry. 2009;73:67–73.
52. Zou J, Kleywegt GJ, Ståhlberg J, et al. Crystallographic evidence for substrate ring distortion and protein conformational changes during catalysis in cellobiohydrolase Ce16A from Trichoderma reesei. Structure. 1999;7:1035–1045.