Thermochemical Transformation of Biomass
Kenichi Yakushido, Yuichi Kobayashi and Hitoshi Kato
Abstract
The generation of energy from biomass is promoted as a measure to combat global warming. There are five main methods for processing biomass: combustion (including for the generation of electricity), carbonization, gasification (again including for the generation of electricity), gasification for methanol synthesis, and ethanol fermentation. Although agricultural biomass is abundant, it is thinly distributed over a wide area, so its practical use requires much time and effort for harvest, preparation, and storage. In particular, because agricultural biomass initially has high water content, it must be dried before it can be used. In addition, biomass frequently contains chlorinated compounds, which generate dioxins at low temperatures, and alkali metals and silicic acid, which melt at high temperatures during combustion. Therefore, the thermochemical transformation of agricultural biomass requires ingenuity to overcome these concerns. This is why the use of agricultural biomass for energy is not popular at the present time. However, increasing demand for structural innovation through energy policies due to rising fuel costs and global warming has stimulated the development of new technologies to use agricultural biomass as an energy source for achieving sustainable management of global energy sources and alleviating emissions of greenhouse gases.
Keywords
Combustion; power generation; carbonization; gasification; methanol synthesis; co-generation
Chapter Outline
12.1. Need for Biomass Utilization Technology in Japan
12.2. Solidification of Biomass Fuel
12.3.2. Problems in Biomass Combustion
12.4. Gasification with Methanol Synthesis
12.5. Energy Production from Livestock Wastes
12.1 Need for Biomass Utilization Technology in Japan
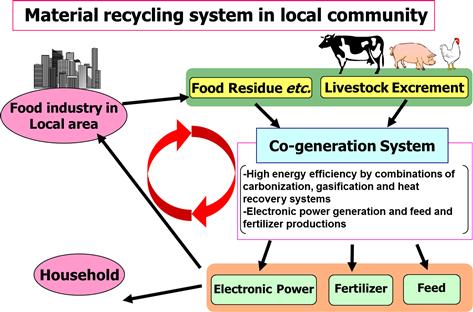
In the EU, the main demand for biomass energy is for heat, and biomass resources are plentiful; hence, traditional technologies can be used to obtain heat energy from biomass. In Japan, in contrast, biomass energy is used mainly for the production of electricity as well as both liquid and gaseous fuels. However, geographic conditions in Japan make it difficult to transport biomass; technologies that can accomplish high-efficiency energy transformation on a small scale are needed. In addition, the expenditures in terms of funds and energy needed for collecting and transporting the widespread and sparsely distributed biomass can be saved if a resource-circulating society is developed in Japan (Figure 12.1).
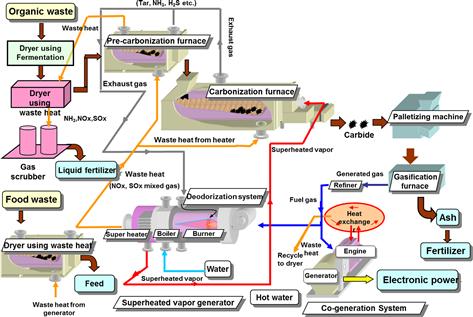
Figure 12.2 shows material flows in a full-scale model of a co-generation system that uses livestock urine and feces as well as food wastes as inputs. A herd of 1300 head of beef cattle generates 34 t of urine and feces per day. These materials can be used to generate power and heat in a co-generation system that simultaneously performs carbonization and gasification to supply 4000 kWh per day of electricity. The heat captured by the co-generation system can dry 7 t of food residues to produce 1.6 t of feed for feeding 500 pigs. Additionally, 1.7 t of carbide can be generated from the gasification operation of the co-generation system to be used as fertilizer.
12.2 Solidification of Biomass Fuel
Through photosynthesis, plants store light energy in plant tissues that can be combusted to release the energy as heat. The combustion also generates ash, which contains various essential and trace elements. The calorific value of plant material depends on both the species and individual plants. In general, slow-growing trees contain less ash and more energy than fast-growing grasses.
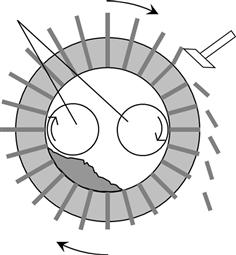
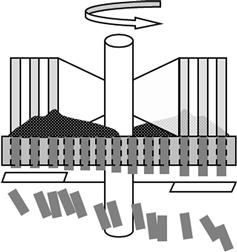
Compression of biomass into pellets or briquettes concentrates the energy density by increasing the bulk mass density. The compression process facilitates storage and transportation; the uniformity of shape and quality of the compressed biomass facilitates the control of combustion, improves heat utilization efficiency, and manages contaminant emissions. Major methods for molding pellet-form fuels include a ring-die system (Figure 12.3) and a flat-die system (Figure 12.4). In the ring-die system, a column rotating along the inner circumference extrudes pellets from the cavity. In the flat-die system, a column rolling on a disk with a cavity extrudes pellets from the top downward.
12.3 Combustion
12.3.1 Combustion System
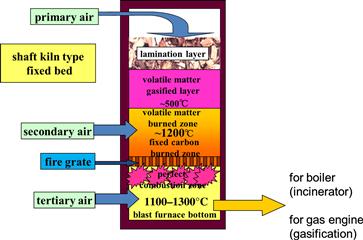
Combustion systems include the fluidized-bed furnace, fire-grate furnace, rotary kiln furnace, and vortex combustion furnace (JSME, 1996). Fire-grate furnaces can be divided into upflow and downflow systems. In an upflow system, the gas generated by thermal decomposition of biomass in a fire grate goes upwards to be burned. The combustion rate increases with biomass input because the heat produced by combustion decomposes the biomass efficiently. In a downflow system, gas generated by biomass combustion in a fire grate is forced downwards to burn beneath the grate (Figure 12.5). The gas can be burned at a constant rate regardless of the biomass input rate because only a small portion of the combustion heat is used for thermal decomposition of the biomass. The downflow fire-grate furnace generates extra heat by providing tertiary air. If the supply of this tertiary air is blocked, the furnace becomes an imperfect gasification furnace.
12.3.2 Problems in Biomass Combustion
Although biomass is typically burned at 600–700°C, agricultural biomass must be burned at temperatures higher than 800°C to reduce the emission of dioxins from fertilizers and chlorinated compounds derived from the soil. Unlike wood, which consists of 99.7% organic matter, agricultural biomass contains appreciable quantities of ionic compounds and silicic acid that will melt to a lava-like slurry to choke the furnace. For example, rice straw from Hokkaido can melt at temperatures exceeding 1000°C, whereas rice straws from Kyushu to Kanto cause no problem at the same temperature. In addition, wild bamboo does not melt but fertilized bamboo grown for edible shoots melts easily. The melting point of agricultural biomass depends on fertilizer application, harvest time, and cultivar. Therefore, the melting temperature of a material should be determined before it is used to assess the material’s suitability for combustion.
12.4 Gasification with Methanol Synthesis
12.4.1 Gasification Method
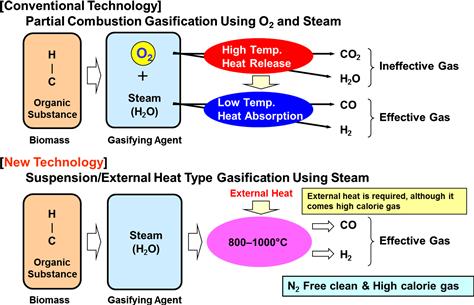
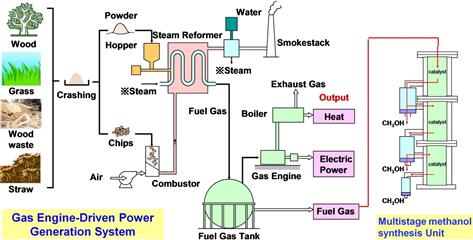
Organic matter decomposes at high temperature to generate combustible gases such as carbon monoxide (CO), hydrogen (H2), and methane (CH4) (Nishizaki, 2008) in addition to generating tar that is then decomposed to form gas at temperatures above 900°C. In traditional internal combustion, incomplete combustion occurs in a conventional furnace. In a new external combustion system, the furnace is externally heated to ∼600°C (Figure 12.6) to gasify the biomass (new technology). The temperature is further raised to ∼1000°C by adding air and oxygen in order to remove the residual tar.
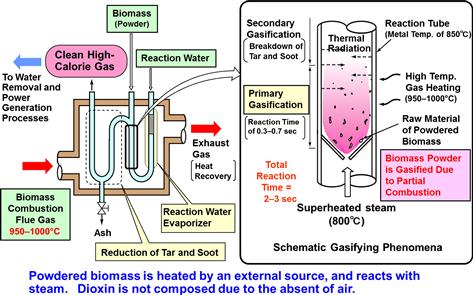
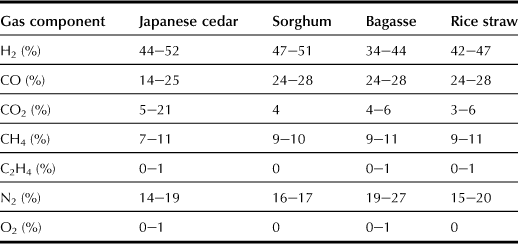
In a new suspension/externally heated system developed for instant gasification, water vapor and biomass particles of ∼3 mm size are converted to gas in a reaction tube with temperature maintained at 800–1000°C (Figure 12.7). The reaction tube that is heated by hot gas drawn from a furnace is used to convert the organic matter into high-calorific gas fuel (∼16 MJ Nm−3); the resulting gas is typically composed of 35–50% H2, 20–30% CO, 7–15% CH4, 1–4% ethylene (C2H4), and 10–20% CO2 (Table 12.1) (Sakai, 1998, 2011). The efficiency of gasification, including external combustion heat, is 85%. In other words, 85% of the biomass energy can be converted into a gas fuel similar to household natural gas. Because there is no internal combustion involved, the final product is a high-calorific gas with a high hydrogen content. The mixed gas can be used for electricity generation with a gas engine to achieve an efficiency of 15–30% for producing electricity from a few kilowatts to several hundred kilowatts. The method can also co-generate heat with the electricity.
12.4.2 Methanol Synthesis
The biomass gas contains H2 and CO as the main ingredients in a ratio of 2:1; it has attracted attention as a source for synthesizing methanol at pressure of 1–2 MPa using copper and zinc as catalysts:
![]()
This exothermic reaction occurs at 200–250°C. When cooled to lower than 60°C, ∼96% of methanol and ∼4% of hydrocarbon-type light fuel and water are produced.
Figure 12.8 outlines a demonstration plant designed to use materials such as tree thinnings, sawdust, bark, rice straw, Napier grass, and sweet sorghum as inputs to produce liquid methanol fuel. For 1 t of dried rice straw, 340 L of methanol can be produced in a multi-stage synthesizing tower operating at a pressure of less than 1 MPa. Unreacted gas is passed to the generator.
12.5 Energy Production from Livestock Wastes
12.5.1 Introduction
Since the commencement of the Livestock Excrement Law of Japan in 1999, the production of livestock manure compost has increased rapidly. Because of oversupply, some compost is exported or is diverted to carbonization or combustion for energy.
Composting is cheaper than carbonization and combustion. When the unit cost of composting is given a value of 1, the cost of processing per unit of input is 1.7–2.0 for carbonization, 3.7 for the production of activated carbon, and 2.0 for combustion. The cost of processing per unit of output is 1.5–2.5 for carbonization, 4.3 for activated carbon, and 8.0 for combustion. Therefore, composting is the most cost-effective use of livestock manure. Other uses of livestock manure are considered only when the cost of its distribution is too high for the composting practice to be cost-effective.
Since the enforcement of the Act on Special Measures Against Dioxins in 1999, an alternative to the traditional chicken manure boiler has emerged in the form of a large-scale power generation plant fired by chicken manure. The plant, which can burn more than 100 t day−1, is designed to reduce dioxin emissions to alleviate adverse environmental effects. The plant produces high-temperature, high-pressure vapor to generate electricity using turning steam turbines. Currently, such plants with a total capacity of 300 t day−1 are operated in the Miyazaki and Kagoshima prefectures; the plant in Miyazaki prefecture is a co-combustion system that burns cattle, swine, and chicken manure. Both prefectures have a surplus of manure, and thus a lower demand for chicken manure compost than cattle and swine manure composts. The difficulties in the distribution of broiler manure compost have favored the introduction of the plants. In addition, small- to medium-scale livestock waste boilers that can be used to heat livestock barns and to produce hot water for washing eggs have been commercialized.
12.5.2 Use of Livestock Manure by Combustion
a Combustion Method
Various combustion methods are available. In the stoker furnace, manure is burned in a fixed fire grate. In the fluidized-bed furnace, superheated air is blown through a sand layer to fluidize the sand to be mixed with the combustible input. Although the fluidized-bed furnace consumes a great deal of power to fluidize the sand, it has been used in three plants because the temperature is easy to control. Large-scale power plants use these two systems. In the rotary kiln furnace, combustibles are burned in a cylindrical rotating furnace; the rotary kiln furnace is appropriate for small-scale plants to process animal manure for heating.
The stoker furnace that is often used to burn wood chips has been built in Miyazaki prefecture to process broiler chicken manure.
b Considerations in Combustion of Livestock Manure
Because of its high content of potassium and sodium, manure melts easily during combustion. For example, beef cattle manure starts to melt at 900–1000°C, broiler manure melts at 1200–1250°C, and layer manure melts at more than 1350°C. Therefore, to prevent melting, the temperature of the fluidized bed must be maintained at 700–750°C, and inflammable gases are used in order to keep the furnace temperature higher than 900°C. In addition, because floating ash can melt on to the wall of the furnace and the inside of the heat-exchange pipes as clinker, the heat-exchange unit must be designed for ease of cleaning.
c Treatment of the Exhaust
Because livestock manure is an industrial waste, it is regulated under the Act on Special Measures Against Dioxins. To reduce dioxin emissions, the furnace exhaust is burned at 800°C for more than 2 s in a secondary combustion unit and then rapidly cooled. Particles of ash contained in the exhaust are removed by using a cyclonic filter or bag filter.
The exhaust can also contain NOx, SOx, and HCl. Although their concentrations usually meet environmental standards, equipment for water washing and calcium hydroxide processing should be added when the capacity of the system is larger or the limits become stricter.
d Use of Ash After Combustion
The ash of combusted manure contains phosphate, potassium, and calcium, but little or no nitrogen. It can be incorporated into fertilizer or added to compost.
12.5.3 Future Issues
A manure-fired power plant processing 300 t of manure per day can generate ∼10 MW of electricity at an efficiency of 20–25%. Even when vapor is used, the total thermal efficiency is only about 50%, and the rest of the energy is lost as waste heat. The effective use of this waste heat remains to be addressed, perhaps through a heat utilization annex to power plants.
References
1. Japanese Society of Mechanical Engineers (JSME). Combustion handbook. Tokyo: Maruzen; 1996; (p. 5).
2. Nishizaki J. Creative energy engineering science. Tokyo: Kodansha; 2008; (pp. 84–102).
3. Sakai M. The 21st century energy that biomass breaks up. Tokyo: Morikita; 1998; (pp. 48–57).
4. Sakai M. Present conditions and development of suspension/external heat type high-calorie gasification system. Tokyo: CMC; 2011; (pp. 70–77).