Palliative Radiotherapy for Brain Metastasis
R.B. Jimenez and H.A. Shih, Massachusetts General Hospital, Boston, MA, United States
Abstract
Brain metastases and primary tumors of the central nervous system (CNS) collectively impact an increasing number of cancer patients. Therefore, it is essential that practitioners understand the most common clinical concerns affecting these patients and are able to appropriately provide for their needs. The purpose of this chapter is to review the frequently utilized radiation therapy approaches, doses, and fractionation schedules for palliative CNS radiation therapy, as well as to outline the proper evaluation and management of these patients’ clinical symptoms.
Keywords
Whole brain radiation therapy; SRS; side effects; CNS; palliation
Introduction
Unlike most site-specific symptoms, the management of a patient with a primary central nervous system (CNS) tumor or brain metastasis is complicated both by the complexity and gravity of neurologic symptoms, as well as the added uncertainty of distinguishing between treatment- and disease-related side effects. Additionally, many patients, due to impairments in speech or cognitive processing, may have difficulty with clearly communicating their concerns.
Therefore given the ramifications of misdiagnosis in this patient population, it is vital to document both a thorough baseline neurologic examination as well as a list of the medications that the patient is taking at the time of evaluation. When there is uncertainty regarding the etiology of a patient’s symptoms, there should be prompt additional investigation with further examination, imaging, or subspecialist input.
Evaluation
• Prompt assessment of severity of acute symptoms with evaluation of performance status and acquisition of vital signs. If patient is in extremis, transfer to the emergency department for urgent brain imaging and neurosurgery/neurology consultation should be pursued.
• If initial evaluation does not necessitate enhanced care procedures, a complete history including neurologic symptoms (headache, nausea, focal neurologic deficits) should be elicited followed by:
![]() Careful examination with a thorough neurologic exam, including evaluation of the cranial nerves, muscle tone and strength, sensation to touch, pain, and temperature, reflexes, and neurocognitive status.
Careful examination with a thorough neurologic exam, including evaluation of the cranial nerves, muscle tone and strength, sensation to touch, pain, and temperature, reflexes, and neurocognitive status.
![]() As appropriate, labwork including a CBC and complete metabolic panel, renal function and liver function tests (LFTs), and/or blood cultures.
As appropriate, labwork including a CBC and complete metabolic panel, renal function and liver function tests (LFTs), and/or blood cultures.
Palliative Radiotherapy Regimens
Whole Brain Radiation Therapy
• Whole brain radiation therapy (WBRT) has been utilized for decades to relieve symptoms related to brain metastases or malignant gliomas, to decrease in-brain progression, and in the case of brain metastases, to reduce the risk of neurologic death [1–4].
• Additionally, WBRT has been demonstrated to increase survival compared to no treatment or to corticosteroids alone both in patients with primary CNS tumors and with brain metastases [5].
• While WBRT for the palliation of multiple brain metastases is becoming increasingly controversial due to concerns for neurocognitive impairment, it remains an appropriate treatment option for patients with diffuse brain metastases, leptomeningeal disease, or gliomatosis cerebri [6–8].
• Wide variation in dose/fractionation schemes exist for WBRT.
![]() Comparisons of different treatment schemas have demonstrated no measurable differences in locoregional control, overall survival, or acute toxicity [9].
Comparisons of different treatment schemas have demonstrated no measurable differences in locoregional control, overall survival, or acute toxicity [9].
![]() Therefore hypofractionated regimens, e.g., 20 Gy in 5 fractions should be considered when estimates of life expectancy (see Chapter 3: Prognostication in Patients Receiving Palliative Radiation Therapy) are less than 12 weeks.
Therefore hypofractionated regimens, e.g., 20 Gy in 5 fractions should be considered when estimates of life expectancy (see Chapter 3: Prognostication in Patients Receiving Palliative Radiation Therapy) are less than 12 weeks.
• For patients with longer life expectancies or when there are concerns for neurocognitive impairment from radiation, more conventional fractionation schemes, e.g., 30 Gy in 10 fractions, 35 Gy in 14 fractions, or 37.5 Gy in 15 fractions, can be employed. Please see section on “Functional Deficits” for additional strategies to reduce neurocognitive decline when considering WBRT.
Partial Brain Radiation Therapy
• Due to a growing concern for the neurotoxic effects of WBRT, alternative treatments that target metastases while providing greater preservation of normal brain tissue have become increasingly popular.
• Radiation approaches that treat brain metastases while including less than the entire brain in the treatment portal can vary significantly in approach:
![]() Targeting the entire posterior fossa for lesions confined to the cerebellum
Targeting the entire posterior fossa for lesions confined to the cerebellum
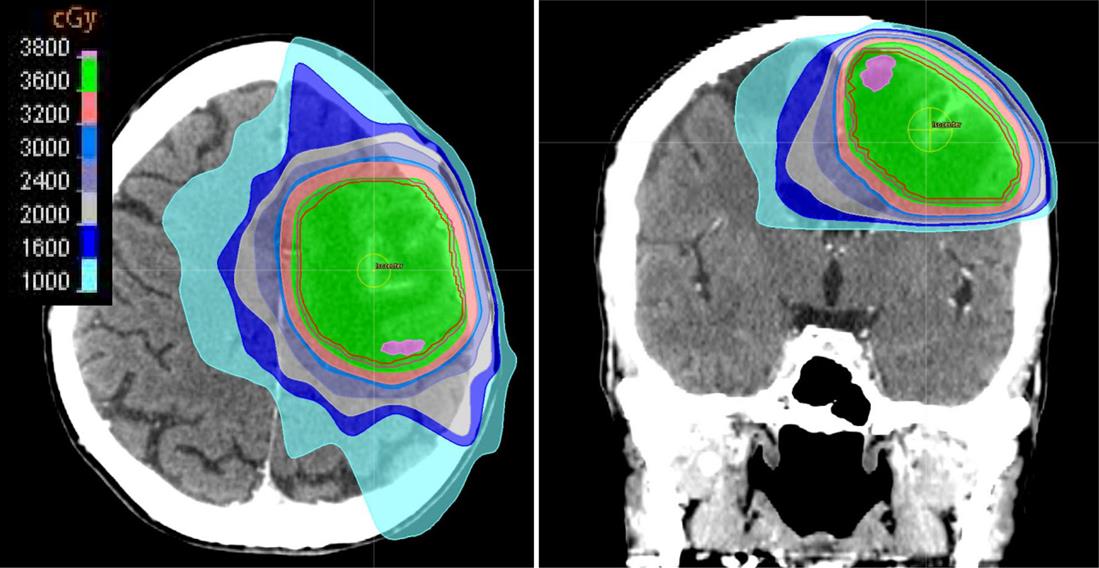
![]() Contouring the gross tumor volume (GTV) of a large single lesion or multiple small lesions and adding a modest expansion of 0.5–1.0 cm to create a partial brain clinical target volume (CTV) (Fig. 11.1).
Contouring the gross tumor volume (GTV) of a large single lesion or multiple small lesions and adding a modest expansion of 0.5–1.0 cm to create a partial brain clinical target volume (CTV) (Fig. 11.1).
![]() In cases of partial brain radiation therapy, where randomized data are lacking, dose and fractionation schemes similar to those used in WBRT are utilized to ensure a similar rate of local control.
In cases of partial brain radiation therapy, where randomized data are lacking, dose and fractionation schemes similar to those used in WBRT are utilized to ensure a similar rate of local control.
Stereotactic Radiosurgery
• Stereotactic radiosurgery (SRS) has become a popular alternative to whole brain or partial brain radiation therapy, most commonly delivering a single, highly focused dose of radiation to a lesion while largely sparing uninvolved brain tissue.
• SRS has proven to be well-tolerated and highly effective for local control, with 1 year local control rates exceeding 90% [3–4,10–11].
• It is a technique that is most appropriate for uniformly shaped lesions smaller than 3 cm that are located at a safe distance from dose-limiting normal structures.
• RTOG 9005 identified the maximum tolerated dose (MTD) for brain lesions by size as: 24 Gy, 18 Gy, and 15 Gy for tumors measuring less than or equal to 2, 2.1–3, and 3.1–4 cm, respectively [11]. In this study, the radionecrosis rate was 11% at 2 years.
• In practice, most physicians rarely employ SRS for lesions exceeding 3 cm and rarely prescribe doses as high as 24 Gy out of concern for inducing radionecrosis and due to efficacy of disease control at lower doses.
• While there is significant variability by institution, many physicians employ single doses of between 18 and 20 Gy for lesions measuring less than 3 cm.
Hypofractionated Stereotactic Radiotherapy
• Hypofractionated stereotactic radiotherapy (hSRT) uses the precision localization techniques of SRS, but rather than deliver radiation in a single dose, it employs smaller fractional doses of radiation for lesions that are not ideal for single dose treatment due to a larger size, irregular shape, or proximity to dose-limiting normal structures, such as the optic apparatus or brainstem.
• hSRT is usually administered across 2–9 treatments in doses higher than conventional WBRT fractions, but in doses smaller than those used for single-fraction SRS.
• Published reports using this approach also suggest that it is well-tolerated and results in local control rates of 40–90% [12–15].
• To date, the ideal dose and fractionation scheme for hSRT is not well-defined and may vary by size, location, histology, patient performance status, prior cranial irradiation, and burden of disease both intracranially and extracranially.
![]() Published regimens vary widely, but commonly used schemes include 5 Gy×5 fractions or 8 Gy×3 fractions [16–17].
Published regimens vary widely, but commonly used schemes include 5 Gy×5 fractions or 8 Gy×3 fractions [16–17].
![]() Of note, according to the American Society for Radiation Oncology (ASTRO) billing definitions, hSRT with 2–5 fractions are defined as SRS and is often interchangeably referred to as such [18].
Of note, according to the American Society for Radiation Oncology (ASTRO) billing definitions, hSRT with 2–5 fractions are defined as SRS and is often interchangeably referred to as such [18].
Evaluation and Management of Common Clinical Concerns
• Both alopecia and radiation dermatitis are common side effects experienced by patients receiving radiation to the brain, but the management of these symptoms are well-addressed in Chapter 10, Skin Toxicity in Palliative Radiation Therapy, and will not be readdressed in this chapter.
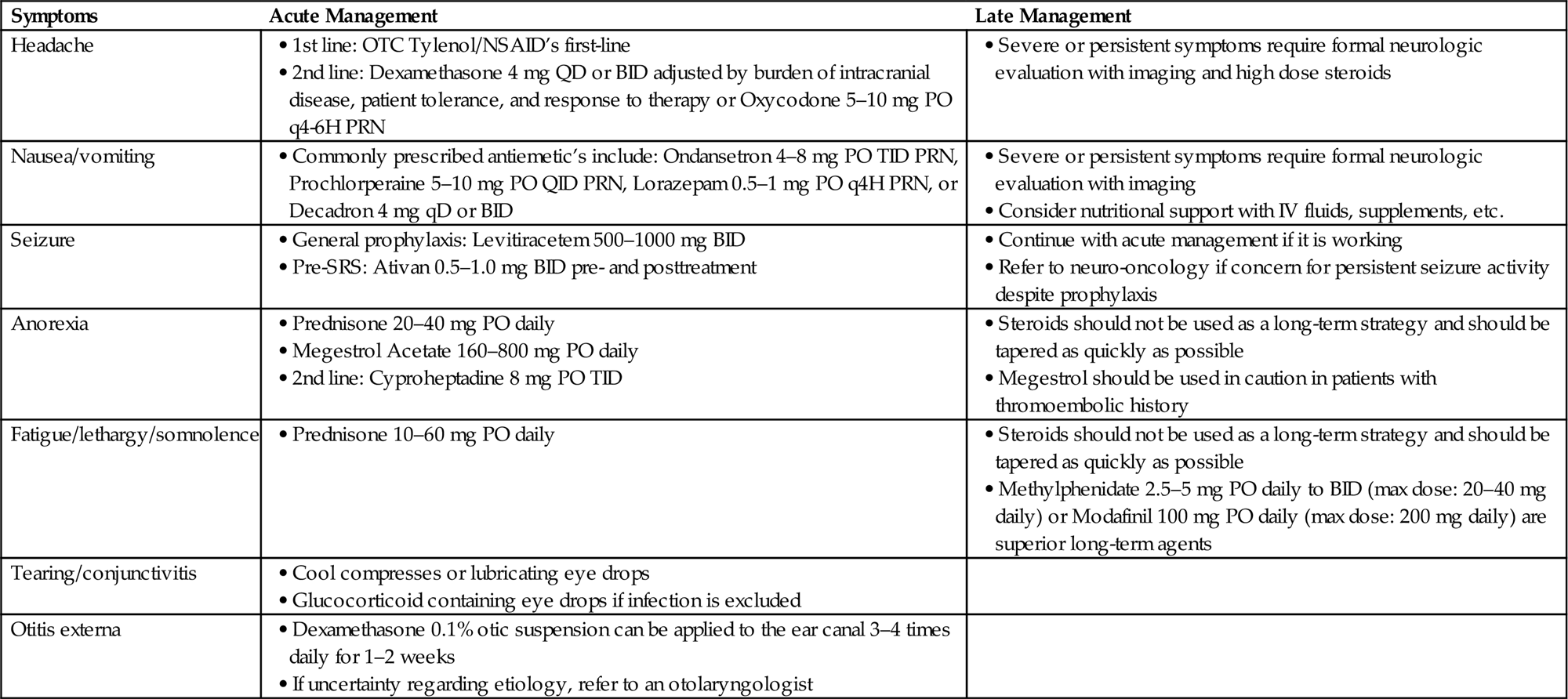
Headache
• Approximately 50% of patients with brain metastases will experience headaches and this symptom remains common among patients receiving WBRT or any other RT to the brain.
• In order to determine the etiology of the headache, this symptom deserves careful questioning regarding the nature of the pain (e.g., sharp, dull, worse lying down, or associated with nausea/vomiting) to elicit a possible etiology.
• The differential diagnosis for a headache in a patient with brain metastases or a primary CNS tumor is broad and may range from the benign to the severe (e.g., stress, herniation, hemorrhage, obstructing hydrocephalus).
• If there is concern that the symptom may be related to an acutely life-threatening etiology, such as hemorrhage, these patients should be emergently evaluated and appropriately directed to an emergency room.
• If there is a low level of clinical concern, conservative management is appropriate. This includes ensuring adequate hydration and an adequate blood glucose level.
![]() If no obvious etiology is identified, treatment with over-the-counter analgesics including acetaminophen or NSAIDS is appropriate. Before prescribing, consider comorbidities that could serve as a contraindication to taking these medications.
If no obvious etiology is identified, treatment with over-the-counter analgesics including acetaminophen or NSAIDS is appropriate. Before prescribing, consider comorbidities that could serve as a contraindication to taking these medications.
![]() Stress is also a common source of headaches and reassurance can be helpful to resolving symptoms.
Stress is also a common source of headaches and reassurance can be helpful to resolving symptoms.
• If there is a moderate level of clinical concern, oral steroids, e.g., dexamethasone 4 mg QD or BID, can be initiated.
![]() Dosage should be chosen and adjusted by the burden of intracranial disease, patient tolerance, and response to therapy.
Dosage should be chosen and adjusted by the burden of intracranial disease, patient tolerance, and response to therapy.
![]() Patients with a known significant burden of intracranial disease may benefit from an increase in dosage, up to 16 mg/day.
Patients with a known significant burden of intracranial disease may benefit from an increase in dosage, up to 16 mg/day.
![]() Oral narcotics including short-acting oxycodone or morphine may be considered.
Oral narcotics including short-acting oxycodone or morphine may be considered.
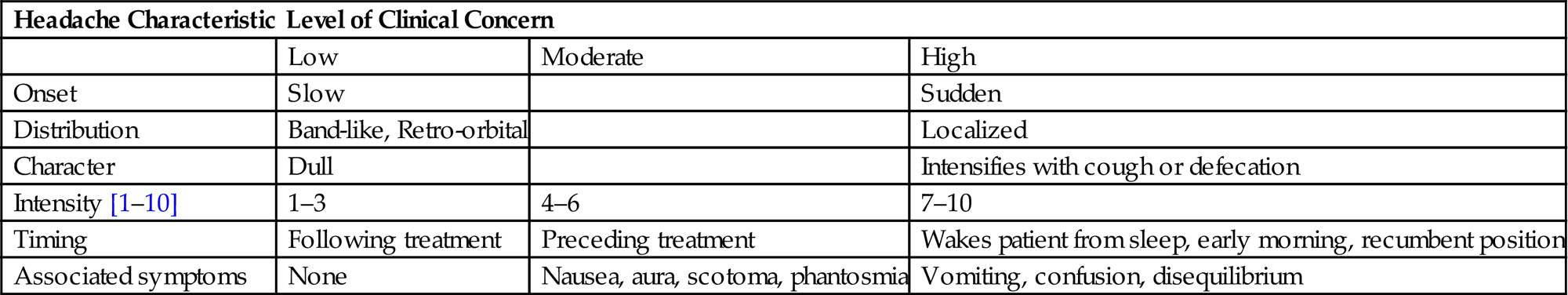
• If there is a high level of concern, prompt imaging and/or formal neurologic evaluation should be executed with management determined by findings. High dose steroids (dexamethasone 8–16 mg/day) may also be initiated in parallel (Table 11.1).
Table 11.1
Headache Characteristics and Associated Level of Concern
| Headache Characteristic | Level of Clinical Concern | ||
| Low | Moderate | High | |
| Onset | Slow | Sudden | |
| Distribution | Band-like, Retro-orbital | Localized | |
| Character | Dull | Intensifies with cough or defecation | |
| Intensity [1–10] | 1–3 | 4–6 | 7–10 |
| Timing | Following treatment | Preceding treatment | Wakes patient from sleep, early morning, recumbent position |
| Associated symptoms | None | Nausea, aura, scotoma, phantosmia | Vomiting, confusion, disequilibrium |
Nausea/Vomiting
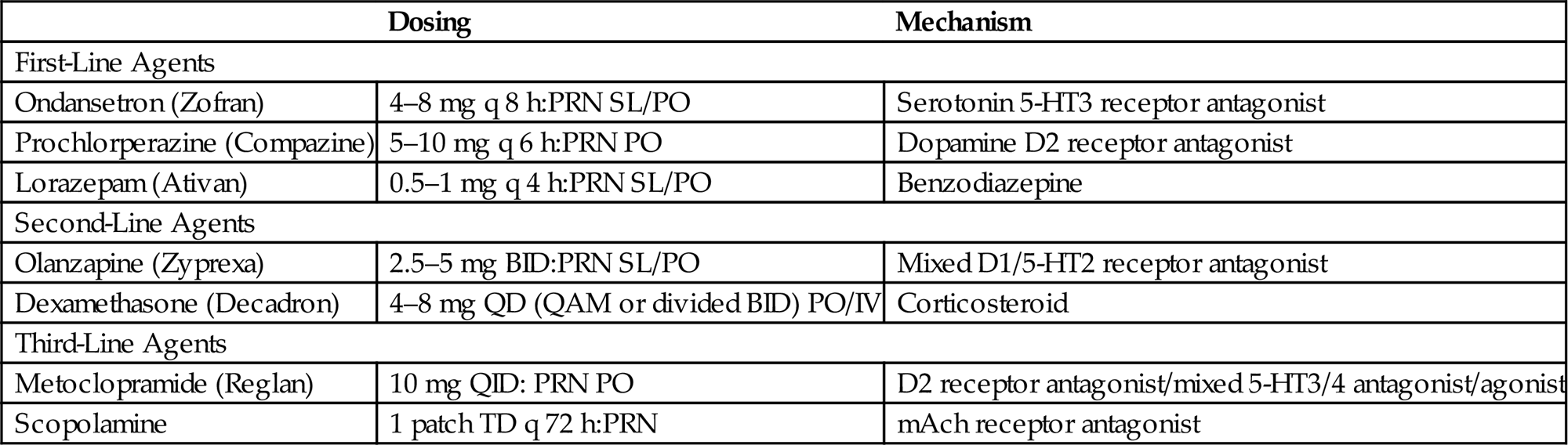
• Nausea and vomiting are common symptoms in the CNS patient. A stepwise approach to the treatment of nausea and vomiting is outlined in Table 11.2.
• While prophylactic treatment is not necessary, once a patient develops nausea and vomiting, premedication prior to irradiation can be helpful in preventing symptoms.
• Patients should always be evaluated prior to medical management to rule out a life-threatening etiology (e.g., increased intracranial pressure from obstructive hydrocephalus) that would require other immediate intervention.
• Multiple agents can be used to manage nausea and vomiting, however, agents of the same or similar class should not be administered concurrently.
• Additionally, the use of third-line agents should be used judiciously as metoclopramide increases gastrointestinal motility and may exacerbate symptoms, while the elderly and infirm may be particularly susceptible to the anticholinergic effects of scopolamine.
• If a patient reports persistent nausea/vomiting despite antiemetics, a detailed drug history may uncover other potential etiologies.
![]() Changes in narcotics to newer generation drugs may be a source of worsening nausea.
Changes in narcotics to newer generation drugs may be a source of worsening nausea.
![]() Patients receiving chemotherapy may be symptomatic from the chemotherapeutic agent, and this may resolve with adjustment of antiemetic dosage or regimen, but is best determined by the prescribing oncologist.
Patients receiving chemotherapy may be symptomatic from the chemotherapeutic agent, and this may resolve with adjustment of antiemetic dosage or regimen, but is best determined by the prescribing oncologist.
![]() Alternatively, a patient on a steroid taper may require a slower taper to resolve his or her symptoms.
Alternatively, a patient on a steroid taper may require a slower taper to resolve his or her symptoms.
• Of note, many patients are prescribed steroids prior to the initiation of radiation therapy to decrease expected reactive inflammation and to prevent or reduce neurologic symptoms. However, steroids are often continued in these patients despite resolution of their symptoms.
![]() Prolonged steroid usage can be associated with serious side effects including agitation, insomnia, weight gain, gastritis, myopathy and associated weakness, hyperglycemia, and increased risk of infection.
Prolonged steroid usage can be associated with serious side effects including agitation, insomnia, weight gain, gastritis, myopathy and associated weakness, hyperglycemia, and increased risk of infection.
• Therefore steroids should be weaned as quickly as possible. If the patient was asymptomatic when steroids were initiated and received fewer than 5 days worth of medication, they can be stopped immediately and without taper.
• Patients with refractory severe nausea and vomiting and escalating symptoms should be directed to a local emergency department for prompt evaluation and management.
Table 11.2
Approach to Antinausea and Antiemetic Management
| Dosing | Mechanism | |
| First-Line Agents | ||
| Ondansetron (Zofran) | 4–8 mg q 8 h:PRN SL/PO | Serotonin 5-HT3 receptor antagonist |
| Prochlorperazine (Compazine) | 5–10 mg q 6 h:PRN PO | Dopamine D2 receptor antagonist |
| Lorazepam (Ativan) | 0.5–1 mg q 4 h:PRN SL/PO | Benzodiazepine |
| Second-Line Agents | ||
| Olanzapine (Zyprexa) | 2.5–5 mg BID:PRN SL/PO | Mixed D1/5-HT2 receptor antagonist |
| Dexamethasone (Decadron) | 4–8 mg QD (QAM or divided BID) PO/IV | Corticosteroid |
| Third-Line Agents | ||
| Metoclopramide (Reglan) | 10 mg QID: PRN PO | D2 receptor antagonist/mixed 5-HT3/4 antagonist/agonist |
| Scopolamine | 1 patch TD q 72 h:PRN | mAch receptor antagonist |
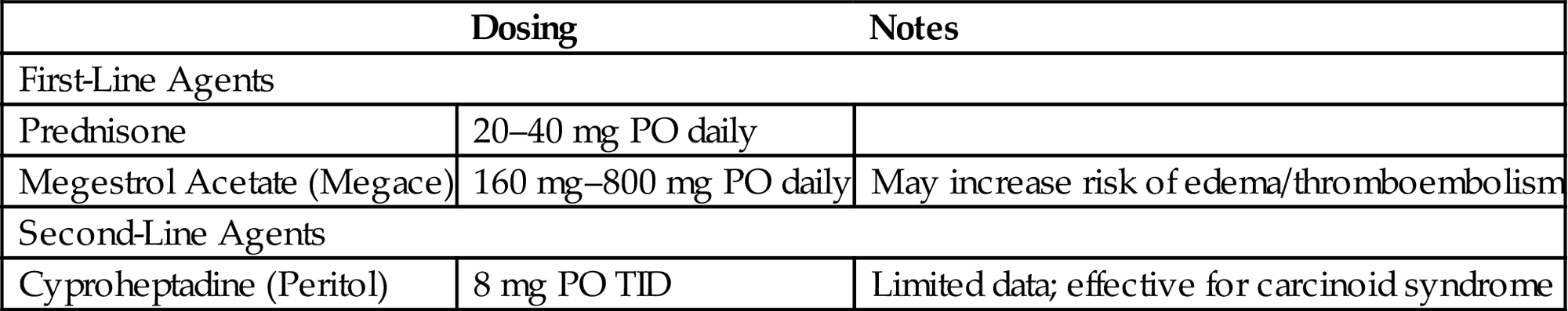
Anorexia
• Many patients with CNS malignancies experience a loss of appetite.
• While a loss of appetite is a subjective concern, assessing the patient’s weight, physical appearance, and serum electrolytes/albumin can provide additional information regarding their nutritional status and aid in determining if medical intervention is necessary.
• Anorexia can be caused as a direct effect of radiation therapy to the brain, but the cause is more often multifactorial and may be owed to medication-induced anorexia or dysgeusia, pain, dysphagia, persistent nausea, or depression/anxiety, as well as tumor-mediated anorexia/cachexia effects in the case of very ill patients.
• A thorough history, including the timing of the onset of symptoms in relation to the patient’s treatment course, may be helpful in determining the etiology of the anorexia and determine if a simple modification of the patient’s medication regimen or optimization of their antiemetics may be sufficient to resolve this concern.
• Alternatively, if the symptoms appear to be radiation or directly malignancy-related, the agents in Table 11.3 can be trialed.
• Cannabinoids, including Dronabinol and Marinol, while frequently cited as an effective appetite stimulant, have not proven efficacious in the advanced cancer population compared to megestrol acetate, either alone or in combination [19].
• In some states, medical marijuana has been approved for the treatment of pain, nausea, and chronic fatigue, but patients should be referred to a designated prescriber for additional evaluation.
Seizure
• Seizure is an uncommon, but potentially serious secondary effect of radiation therapy to the brain, most commonly triggered by reactive edema.
• Patients with disease involving the motor cortex or meninges are at a relatively higher risk of seizure.
• Among patients receiving SRS or hSRT, approximately 5–15% of patients will experience a seizure following treatment due to the rapid onset of edema associated with high dose radiation [16,20–21]. In some practices, a short course of lorazepam 0.5–1.0 mg BID or other antiepileptic agent is prescribed prophylactically in the days surrounding an SRS procedure, when radiation-induced seizures are most likely.
• In general, patients with primary brain tumors or those with brain metastases are placed on antiepileptic therapy only if they have already experienced a seizure or have undergone brain surgery.
![]() In these cases, levetiracetam is frequently utilized at doses of 500–1000 mg BID for prophylaxis.
In these cases, levetiracetam is frequently utilized at doses of 500–1000 mg BID for prophylaxis.
![]() Levetiracetam can be tapered in the weeks following surgery for patients who remain seizure free.
Levetiracetam can be tapered in the weeks following surgery for patients who remain seizure free.
Fatigue/Lethargy/Somnolence
• While fatigue is a widely recognized side effect of radiation therapy, it can be particularly profound among patients receiving radiation to the brain.
• The reasons for fatigue in this patient population are often multifactorial and can include the disease, depression, anxiety, pain, poor nutrition, dehydration, physical inactivity, and medications in addition to the radiation itself.
• When rapid onset of fatigue or somnolence is observed, care should be taken to rule out serious causes of lethargy including cerebral infarction/hemorrhage or cerebral edema.
• Once more serious causes are excluded, the agents in Table 11.4 can be trialed, preferably with the oversight of neurologic or palliative care services.
Table 11.4
Medical Management of Lethargy and Somnolence
| Agent | Dosing | Notes |
| Prednisone | 10–60 mg PO daily | |
| Methylphenidate (Ritalin) | Initial dosing: 2.5–5 mg PO QD-BID Maximal dose: 20–40 mg daily |
May exacerbate headaches, anorexia, or insomnia in CNS patients |
| Modafinil (Provigil) | Initial dosing: 100 mg PO daily Maximal dose: 200 mg daily |
May exacerbate headaches, nausea, or insomnia in CNS patients |
Tearing/Conjunctivitis
• For patients receiving WBRT or craniospinal irradiation, the posterior orbit is within the radiation treatment field and the lacrimal glands are often partially irradiated. Consequently, excessive eye tearing can be seen.
• With partial brain or periorbital radiation treatments that include partial eye irradiation, radiation-induced conjunctivitis causing a red eye with or without mucoid discharge can be experienced.
![]() Cool compresses or lubricating eye drops can be administered as needed for relief.
Cool compresses or lubricating eye drops can be administered as needed for relief.
![]() Glucocorticoid containing eye drops should be avoided if infection cannot be excluded as steroids may cause corneal damage in the presence of a viral or bacterial nidus.
Glucocorticoid containing eye drops should be avoided if infection cannot be excluded as steroids may cause corneal damage in the presence of a viral or bacterial nidus.
Otitis Externa
• Otitis externa is an inflammatory reaction involving the outer ear or ear canal that can result from whole brain or partial brain irradiation.
• Typically, patients will report having an earache or ear congestion that is exacerbated by pulling or tugging on the external ear.
• A thorough examination including otoscopic examination should be performed and can reveal diffuse inflammation with eczematous changes of the ear canal. Frequently, swelling of the canal can prevent visualization of the tympanic membrane.
• Rarely, the inflammation and pain can be accompanied by discharge from the ear.
• If the practitioner is confident that the symptoms are radiation-induced and noninfectious, a course of dexamethasone 0.1% otic suspension can be applied to the ear canal 3–4 times daily for 1–2 weeks.
• If there is uncertainty regarding the etiology of the inflammation, referral to an otolaryngologist is recommended.
Functional Deficits
• Functional deficits are a common presentation for brain metastases or primary CNS tumors and can be wide ranging from changes to affect, memory, and comprehension to discrete impairments in speech, vision, sensation, or motor function.
![]() During radiation treatment, the new onset of functional deficits should trigger a prompt and thorough evaluation with possible additional imaging if an etiology is not identified.
During radiation treatment, the new onset of functional deficits should trigger a prompt and thorough evaluation with possible additional imaging if an etiology is not identified.
![]() Neurosurgical consultation may be warranted for concerns of progressive tumor growth, cerebral edema, infarction, or hemorrhage.
Neurosurgical consultation may be warranted for concerns of progressive tumor growth, cerebral edema, infarction, or hemorrhage.
• Following completion of radiation therapy to the brain, patients and families may specifically report the delayed, and often, insidious onset of neurocognitive slowing or memory impairment.
![]() Some studies estimate that up to 50% of long-term survivors develop late cognitive deficits caused by radiation-induced injury of normal brain tissue [6–8].
Some studies estimate that up to 50% of long-term survivors develop late cognitive deficits caused by radiation-induced injury of normal brain tissue [6–8].
![]() Older patients and those patients receiving WBRT are particularly vulnerable to this late effect of treatment for reasons that are not fully understood, though radiologic changes correlated with neurocognitive decline include ventriculomegaly and diffuse cortical leukoencephalopathy.
Older patients and those patients receiving WBRT are particularly vulnerable to this late effect of treatment for reasons that are not fully understood, though radiologic changes correlated with neurocognitive decline include ventriculomegaly and diffuse cortical leukoencephalopathy.
![]() Animals models have also demonstrated damage to the microvascular environment as well as direct neuronal loss following radiation to the brain [22–24].
Animals models have also demonstrated damage to the microvascular environment as well as direct neuronal loss following radiation to the brain [22–24].
• Due to concerns for neurocognitive decline following radiation therapy, two recent RTOG trials have shown preliminary success in reducing the extent of decline in patients receiving WBRT with the addition of either protective drug therapy or improved radiation techniques.
![]() RTOG 0614 used memantine drug therapy as a neuroprotectant and demonstrated that patients displayed a longer time to cognitive decline and a decreased probability of cognitive function failure at 6 months with the addition of memantine.
RTOG 0614 used memantine drug therapy as a neuroprotectant and demonstrated that patients displayed a longer time to cognitive decline and a decreased probability of cognitive function failure at 6 months with the addition of memantine.
– In this study, memantine was administered coincident with the first day of radiation treatment and continued for a total of 6 months [25].
– The dose schedule for memantine is as follows: 5 mg by mouth daily for week 1, 5 mg twice daily for week 2, 10 mg in the morning and 5 mg in the evening for week 3, and 10 mg twice daily for the remaining weeks 4–24.
– Careful consideration of the benefits of memantine in this setting is warranted, however, these findings have yet to be replicated and other criticisms of the findings, including a high patient dropout rate, have been raised.
![]() RTOG 0933 sought to minimize dose to the hippocampal region of the brain, an area that is thought to be associated with the consolidation of long-term memory.
RTOG 0933 sought to minimize dose to the hippocampal region of the brain, an area that is thought to be associated with the consolidation of long-term memory.
– In this study, patients received hippocampal sparing WBRT using an IMRT-based plan.
– Cognitive and quality-of-life assessments were performed on these patients at baseline, and at 2, 4, and 6 months following radiation therapy.
– The relative decline in measurements of delayed recall from baseline to 4 months was significantly lower than those observed in historical controls [26].
– Concerns remain with this technique as the effect size was modest and practitioners may not feel comfortable with intentional underdosing of some brain tissue, particularly in patients with a high tumor burden.
![]() While both memantine and hippocampal sparing radiation therapy are not considered the standard of care for all CNS patients, they represent promising interventions for patients receiving WBRT and may be useful in select cases, at the discretion of the physician. At our institution, patients with a life expectancy greater than 1 year are considered the most ideal candidates for receipt of both memantine and hippocampal sparing strategies.
While both memantine and hippocampal sparing radiation therapy are not considered the standard of care for all CNS patients, they represent promising interventions for patients receiving WBRT and may be useful in select cases, at the discretion of the physician. At our institution, patients with a life expectancy greater than 1 year are considered the most ideal candidates for receipt of both memantine and hippocampal sparing strategies.
Conclusion
The management of patients with CNS malignancies can be a complicated but ultimately a rewarding pursuit. Advancements in medical therapies and an increase in variety of radiation techniques available have led to increased survival in both patients with metastatic disease to the brain and in patients with primary brain tumors. This has increased the frequency of need for symptom management. Patients with tumors of the CNS require careful monitoring during and following treatment to recognize and relieve symptoms related to therapy (Table 11.5).