General Approach to Palliative Care and Palliative Radiation Oncology
C.C. Johnstone1 and S. Lutz2, 1Medical College of Wisconsin, Milwaukee, WI, United States, 2Blanchard Valley Regional Cancer Center, Findlay, OH, United States
Abstract
Cancer is a global problem with an estimated 14.1 million cancer cases worldwide in 2012 and there were 8.2 million cancer deaths. The treatment of patients with advanced cancer is a multidisciplinary effort that requires careful coordination. Prognostication and accurate estimates of life expectancy are essential for goal setting and appropriate treatment selection. Patients with advanced cancer have unmet spiritual needs; simple acronyms and tools can help physicians take a spiritual history and start the conversation as part of a comprehensive patient-centered approach.
Increasingly, quality metrics surrounding the appropriate use of aggressive therapies such as longer course palliative radiation and chemotherapy at the end of life are being developed. Avoidance of radiation therapy (RT), especially long-courses of radiation therapy, at the end of life is important given the delayed palliative effect of RT. Whenever possible, the shortest course of RT likely to have the desired effect should be employed. This maximizes patient comfort and convenience and minimizes cost and toxicity. Similarly, chemotherapy may not be appropriate in the last month of life.
Keywords
Palliative care; radiation therapy; life expectancy; prognostication; spirituality; symptom management
Introduction
The burden of cancer continues to increase in the United States and globally, with an estimated 14.1 million cancer cases worldwide in 2012 that is projected to increase to 19.3 million cases by 2025. In 2012, there were 8.2 million cancer deaths and 32.6 million people living with cancer [1]. Thus, the need for good palliative care (PC) is also increasing globally.
Ideally, PC is a multi- and interdisciplinary effort. Emerging in the PC world is the notion that there are two fundamental categories of PC. The first is generalized PC knowledge that every person who provides health care to patients with cancer should have. The second category is a more specialized skill set that caregivers who focus their time in PC should have [2]. This is partly in recognition of the shortage of PC specialists worldwide [2–4].
The function of PC is to reduce pain and suffering, allow discussions of goals of care, facilitate death with dignity, promote quality-of-life, and support patients, their families, and their caregivers. The assessment includes pain and symptom assessment as well as an assessment of the social and spiritual context. Patients whose spirituality is supported by the medical team have experienced better outcomes and quality of life [5–7]. After a complete assessment, prognostication about the illness trajectory, the expected timelines and maximizing the goals that are important to the patient come into play.
Life Expectancy and Prognostication
Questions about life expectancy and the quality of that remaining life are extremely important to patients with metastatic cancer. Physicians and other health care providers often overestimate life expectancy, by as much as 3 months or more [8]. Accurate estimates of life expectancy are important to patients and physicians for many reasons. It helps set appropriate goals, avoid treatments that will have little or no benefit, and choose supportive care or treatments that will be effective within the remaining time.
From the literature on clinical prediction and prognostication, several themes emerge. Clinical prediction tends to overestimate survival, but those clinical estimates improve over time with repeated encounters. The strongest prognostic indicators are the patient’s performance status, the presence of the symptom cluster known as the terminal syndrome (dyspnea, dysphagia, dry mouth, anorexia, and weight loss) and the presence of cognitive failure or confusion [9].
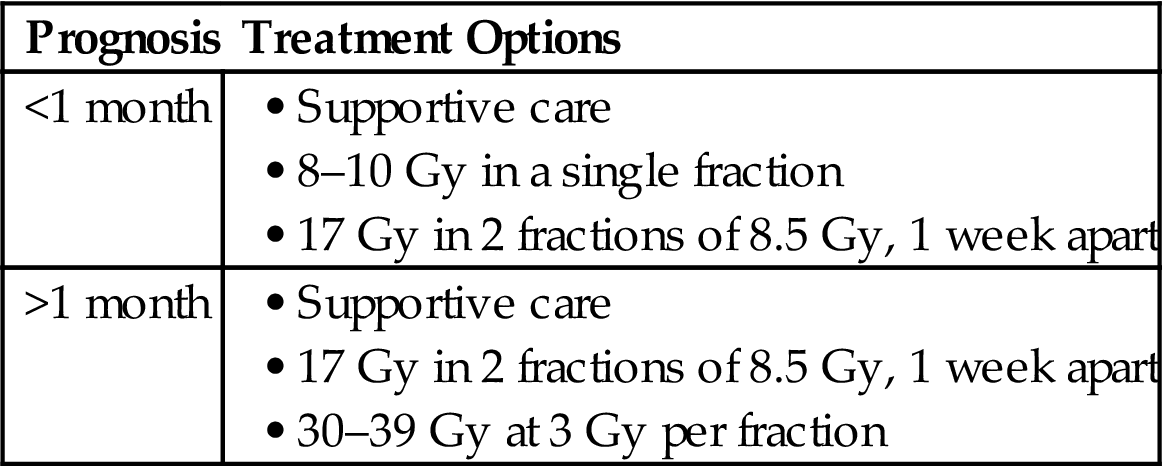
Many of the existing prognostic indicators are best near the EOL. A simple, easy to use and validated tool to predict life expectancy is very much needed. Several tools exist (Table 1.1) and have been studied in patients with advanced or terminal cancer. Each of these tools has limitations. Some are easier to perform and are more generalizable than others. The best use of these tools may be in deciding which patients may not live long enough to see the benefit of a particular treatment. This is particularly true for radiation therapy (RT) as symptom relief typically takes several days to a few weeks for effect. The exception to this is hemostasis, which can often be seen 24–48 hours after the first dose of radiation. Some have advocated chemotherapy delivery within the last month of life as a metric of overutilization of health care [24,25]. Similar metrics may follow for RT [26].
Table 1.1
Tools to Help Assess Life Expectancy in Patients With Cancer
| Tool | Factors | Comment |
| National Hospice Study (NHPCO) [10] | Karnofsky Performance Status (KPS) | Based on hospice patients |
| Anorexia | If KPS ≥50 and none of 5 factors, median survival 6 months, with all 5, 6 weeks | |
| Weight Loss | ||
| Dyspnea | ||
| Dry mouth | ||
| Dysphagia | ||
| Palliative Performance Scale (PPS) [11] | Ambulatory status | Correlated with survival |
| Activity level | Applicable to cancer populations | |
| Disease status | ||
| Self-care | ||
| Intake | ||
| Consciousness | ||
| Palliative Prognostic Index (PPI) [12,13] | KPS | Short-term survival of terminally ill cancer patients |
| Dyspnea at rest | ||
| Oral intake | ||
| Edema | ||
| Delirium | ||
| Palliative Prognostic Score (PaP) [14] | KPS | Valid for terminally ill or advanced cancer patients |
| Anorexia, dyspnea | ||
| High total WBC | ||
| Low lymphocyte percent | ||
| Clinicians prediction of survival (weeks) | ||
| Survival Prediction Score (SPS) [15] | Tumor details | Developed in a palliative radiation oncology setting |
| KPS | ||
| Fatigue | ||
| Anorexia | ||
| Shortness of breath | ||
| Number of Risk Factors (NRF) [16] | KPS | Developed in a palliative radiation oncology setting |
| Primary site | ||
| Metastasis | ||
| Prognosis in Palliative Care Study [17,18] (PiPs) | KPS | Predicts 2 week and 2 month survival |
| Mental test score | Prognostic with or without lab values | |
| Selected laboratory values | ||
| Selected symptoms | ||
| Primary site | ||
| Site of metastasis | ||
| TEACHH [19] | Type of cancer | Developed in a palliative radiation oncology setting |
| ECOGPS | ||
| Age | ||
| Chemotherapy (prior palliative) | ||
| Hospitalizations | ||
| Hepatic metastasis | ||
| Recursive Partitioning Analysis [20] | KPS | Applies to brain metastasis patients only |
| Extent of metastatic disease | ||
| Age | ||
| Graded Prognostic Assessment [21] | KPS | Applies to brain metastasis patients only |
| Extent of metastatic disease | Assessment criteria varies by primary site | |
| Age | ||
| Number of brain metastasis | ||
| Tumor subtype | ||
| Metastatic Spinal Cord Compression Index [22] | Age | Applies to patients with spinal cord compression only |
| Gender | ||
| Primary site | ||
| Number of involved vertebrae | ||
| Other bone metastasis | ||
| Visceral metastasis | ||
| Interval to cord compression | ||
| Ambulatory status | ||
| Time to motor deficits | ||
| Dutch Bone Metastasis Study Group [23] | KPS | Applies to bone metastasis patients only |
| Primary tumor type | ||
| Visceral metastasis |

Religion and Spirituality
Multiple studies have surveyed patients in various settings about their desire to have their health care team inquire about their spiritual or religious beliefs or pray with them. As the severity of illness increases, the proportion of patients who want their spiritual beliefs considered increases. Ninety-four percent of outpatients favor discussion of spirituality in the setting of grave illness [27]. Yet, in another series, 68% of inpatients said that no physician had ever assessed their spiritual or religious needs [28]. Many patients with advanced and life-threatening malignancies do not feel that their spiritual needs are met [29].
There are spiritual coping and methods that health care providers can use to deliver more holistic care to patients with cancer [30]. PC providers can also be taught how to incorporate a discussion of religion and spirituality into the care that they deliver and support those needs of the patients they care for. One commonly cited barrier noted by medical practitioners is the lack of training about how to provide such care [31].
Though many use the terms religion and spirituality interchangeably, there is a distinction between them. Spirituality takes into account one’s view of transcendent and existential questions. Religion is a subset of spirituality surrounding a set of texts, practices, and beliefs shared by a particular community [32].
Though many physicians think religious figures and spiritual care experts should be the ones to discuss spirituality and religion, a national consensus conference determined that all members of the health care team are responsible for addressing patient’s spiritual issues in the context of the biopsychosocial framework. This consensus panel recommended that all patients be screened with a spiritual history and that any spiritual distress should be diagnosed and attended to using validated assessment tools [33].
One such validated tool is the FICA spiritual history tool [34]. This relatively simple tool uses the acronym FICA as follows: F represents faith, belief, or meaning; I stands for importance and influence; C for community; and A represents address or action in care. The key principles of this tool are to assess if a particular person has a set of beliefs or a particular faith that gives meaning to their lives. The next step is to assess how this faith or spirituality helps them cope with stress or how it affects their health care decisions. If they belong to a community of like-minded individuals, how does this community affect their lives? The last step is for the health care team to address these issues as part of the patient’s care (Table 1.2).
Table 1.2
FICA Spiritual History Tool [34]
| F | Faith, belief, or meaning | Do you have faith? What gives your life meaning? |
| I | Importance | Do these beliefs help you cope or make decisions? |
| C | Community | Do you belong to a community? |
| A | Address or action | Health care team incorporates this knowledge |
Relief of Pain and Suffering
Alleviating pain and suffering is a comprehensive multidisciplinary effort that uses a combination of counseling and educating, medications, and therapeutic interventions. This text aims to provide a comprehensive approach to symptom control in patients with advanced cancer [35,36].
Palliative Radiation Therapy
External beam RT is a key component of palliative cancer care. It is useful to treat pain due to osseous metastasis or local tumor invasion, bleeding, obstruction, dyspnea, or cough, and functional impairment due to brain metastasis or impingement of nerve roots or the spinal cord.
Key in the utilization of RT is the selection of the shortest fractionation regimen that is effective to maximize patient and caregiver convenience and minimize toxicity and cost [37–40].
Though many believe that longer courses of RT have a more durable effect, there is no data to support this belief. In the Radiation Therapy Oncology Group (RTOG), patient selection was designed to enroll only those with a long expected survival. There was no difference in efficacy between 8 Gy in a single fraction and 30 Gy in 10 fractions [37]. Similarly, in an analysis of those patients who survived more than 52 weeks in the Dutch Bone Metastasis Study, there was no difference in response rate, time to response, duration of response, and time to progression of pain (Table 1.3) [41]. Randomized trials have confirmed the equivalence of short courses of RT in lung cancer [42–44] and bladder cancer [45] and hypofractionated radiation regimens have been successfully used to treat gynecologic, gastrointestinal, and head and neck malignancies [39].
Table 1.3
Results From the Dutch Bone Metastasis Trial in Patients Surviving >1 Year
| Metric | Single Fraction of 8 Gy | Multiple Fraction 24 Gy in 6 Fractions |
| Response rate | 87% | 85% |
| Complete response rate | 62% | 48% |
| Time to response | 4 weeks | 4 weeks |
| Duration of response (mean/median) | 29 weeks/35 weeks | 30/42 weeks |
| Progression of pain | 55% | 53% |
| Time to progression | 17 weeks | 18 weeks |
One reason commonly cited in favor of multifraction regimens for the treatment of bone metastasis over those with higher dose per fraction regimens is the potential for pathologic fracture. In the analysis of the RTOG 97-14, there was no difference in the long-term risk of pathologic fracture with the single fraction regimen of 8 Gy when compared to multifraction regimen of 30 Gy in 10 fractions [46]. The initial report of the Dutch Bone Metastasis Study did show higher rates of pathologic fracture in the single fraction arm, but a subsequent analysis that corrected for the percent of cortical destruction did not demonstrate a difference in fracture rates between treatment arms [47,48]. This was confirmed in a large meta-analysis [49]. For patients with >30% cortical destruction, prophy-lactic change there may be cases where higher doses of RT are appropriate, including bone metastases with a large soft tissue component, osteolytic lesions with impending pathologic fracture, or patients with a symptomatic pathologic fracture [50]. Longer courses in these settings may help promote remineralization and tumor control, which is important for those patients with a longer life expectancy. After pathologic fracture and surgical intervention, it may be difficult to assess efficacy of single-fraction treatment. Optimal fractionation remains controversial; a single trial of patients with neuropathic pain from bone metastases did not show superiority for either 20 Gy in 5 fractions or a single 8 Gy fraction [51].
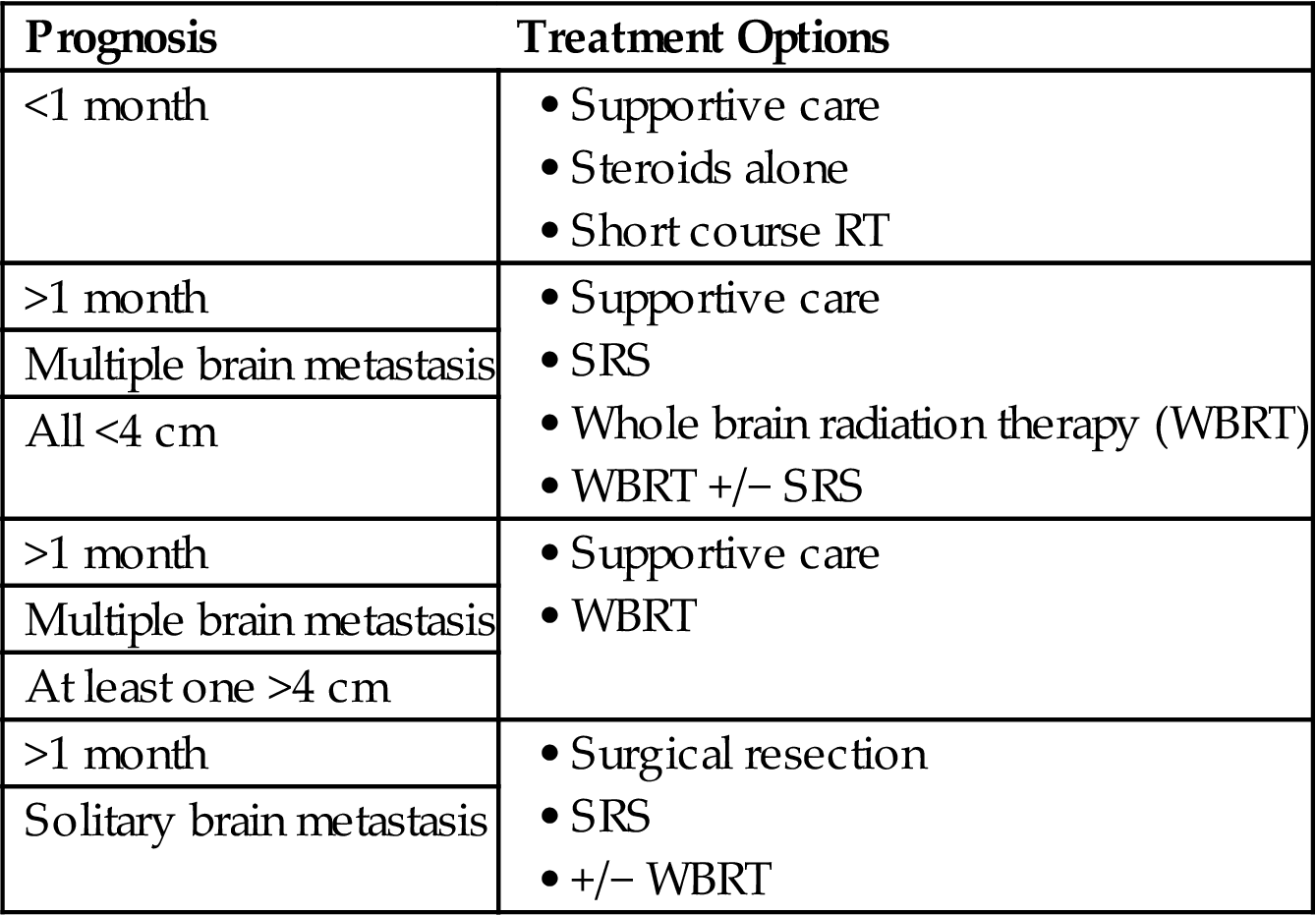
Various clinical scenarios are depicted in Tables 1.4–1.8 with appropriate treatment options based on a patient’s life expectancy.
Table 1.4
Palliative Radiation Fractionation Schemes for Bone Metastasis

Table 1.5
Palliative Radiation Fractionation Schemes for Spinal Cord Compression

Table 1.6
Palliative Radiation Fractionation Schemes for Brain Metastasis
| Prognosis | Treatment Options |
| <1 month | |
| >1 month | |
| Multiple brain metastasis | |
| All <4 cm | |
| >1 month | |
| Multiple brain metastasis | |
| At least one >4 cm | |
| >1 month | |
| Solitary brain metastasis |
Efficacy of Palliative Radiation Therapy
RT provides time-efficient and effective pain relief with few side effects. It can be extremely useful in patients who are intolerant of opioid analgesics and can decrease the need for opioids thus reducing the systemic side effects of opioids. The vast majority of patients respond to RT, with 60–80% having a partial response and 25–30% experiencing a complete response to treatment. Radioresistant tumors, such as sarcoma or renal cell carcinoma, are palliated by RT and may benefit from fractionation schemes with higher dose per fraction [52].
Pain relief is not immediate after the initiation of RT. Patients can experience some relief within a few days or a week after the completion of treatment but the full palliative may not be seen until 4–6 weeks after the completion of treatment. Patients who are not expected to live this long may be better palliated using pain medications or other interventions. Since pain relief is not immediate, and RT requires patient immobilization for 15–30 minutes for treatment delivery, adequate pain control prior to the initiation of therapy is important. A helpful guide to pain medication dosing has been described by the World Health Organization [53]. These regimens may include nonsteroidal antiinflammatory agents, narcotic analgesics, or adjuvant pain medicines such as corticosteroids, nerve-stabilizing medicines, or antidepressants.
The pain relief provided by RT is variable, but typically lasts several months or longer. In patients who experience progression of pain, retreatment can be considered. In the NCIC study of retreatment, the initial course of RT varied and included single-fraction and multifraction schedules with daily fraction sizes of 3–8 Gy [54]. Patients with persistent pain at 4 weeks were randomized to 8 Gy in a single fraction versus 20 Gy in five fractions. There was no difference at two months in the overall response to treatment, pathologic fracture, or development of spinal cord or cauda equina compression. Acute toxicities such as anorexia and diarrhea were less frequent in the single fraction arm [54].
RT palliates symptoms from tumors in the lung with varying frequencies. Hemoptysis is palliated 80–90% of the time, while more complex symptoms such as cough and dyspnea have lower rates of palliation, 60–90% of the time for cough and 40–60% of the time for dyspnea [55–60]. Symptoms from pelvic malignancies are palliated 60–94% of the time [61,62]. Palliative radiation to patients with rectal cancer can help avoid colostomy [63]. The efficacy of palliative radiation will also be addressed in the chapters that follow.
Emerging Technologies
Several emerging technologies, such as stereotactic body radiation therapy (SBRT) are capable of delivering highly conformal high dose radiation to tumor sites while minimizing dose to adjacent normal tissues [64]. It requires fastidious attention to dose planning, patient setup, and localization and may be ideally suited for retreatment situations where the spinal cord has reached tolerance due to the initial definitive course of RT or a previous palliative course of treatment [65]. Treatment regimens for spine lesions include 30 Gy in 5 fractions, 27 Gy in 3 fractions, 40 Gy in 5 fractions, or 16–24 Gy in a single fraction [66–68]. Early results are promising and prospective, randomized data are likely to help define the best use of this technology [69]. Relatively little data exists on the long-term toxicity of very large single doses and there may be a higher risk of long-term side effects than is typically seen with more established treatment approaches [70]. Spine radiosurgery is an area of active clinical investigation and the subject of a current randomized trial [71]. Routine use should be avoided until sufficient evidence justifies the substantive increase in cost relative to standard RT.
Radiation Therapy Planning and Delivery
Patients with documented metastasis are referred for consultation with a radiation oncologist. All of the relevant clinical data and radiographic studies are reviewed and a history and physical are performed. Communication with the other oncologic and PC providers follows that evaluation as do orders for any additional diagnostic testing or procedures. If the multidisciplinary team determines that RT is the most appropriate palliative treatment for a patient, a simulation or radiation planning session is scheduled. At simulation the patient is positioned in a comfortable and reproducible position and a CT scan of the affected area that includes all of the organs at risk for dose calculations is obtained. Fluoroscopic simulation is an alternative to CT simulation.
Behind the scenes, a dedicated team of professionals works with the radiation oncologist to select the best means by which to deliver dose to the intended target and minimize the dose delivered to adjacent normal tissues. In the palliative setting, where patients often have difficulty with transfers or travel, the consultation, simulation, and initiation of single fraction therapy can be done in a single visit. Once the dosimetric analysis is complete, the physician and the physicist review the plan for accuracy. A verification simulation is performed prior to the delivery of dose to ensure that what was planned actually conforms to the patient setup on the treatment table. Portal images and/or CT verify that the setup is correct for the area being treated. Patients are in the treatment room for 15–20 minutes while the radiation is delivered. Treatment is generally without immediate side effects, other than the potential for discomfort on the treatment table or in the transfer to the treatment table. Generally, no special preparation or fasting is required. An antiemetic may be given prior to treatment if radiation-induced nausea is anticipated.
Side Effects of Radiation Therapy
Palliative RT is generally well tolerated with few acute or long-term side effects. The acute side effects are often predictable based on the region being treated. They are usually mild and manageable with conservative measures. Fatigue is the main systemic side effect; this fatigue is typically less than that associated with the disease or other treatment modalities. Local side effects include skin irritation, nausea, diarrhea, esophagitis, and mucositis. Side effects are related to both the daily dose of radiation and the total dose delivered and can occur acutely, subacutely, or in the long term. Fewer acute side effects have been associated with single-fraction palliative radiation when compared to multifraction regimens [37,72]. A “pain flare” is a transient increase in bone pain that occurs around the first few fractions of radiation for bone metastasis. It is caused by tumor cell kill and has been reported in between 20% and 40% of patients [73]. This pain flare can be mitigated by the use of nonsteroidal or steroidal antiinflammatory medications.
Late effects are rare and occur several months to years following the delivery of radiation. They are generally irreversible and more serious than the acute side effects of RT. Larger daily doses of radiation correlate with a higher risk of long-term side effects, though the risk of serious side effects is still very low with established regimens. Patients with metastatic cancer may not live long enough to commonly suffer late side effects but with improvements in systemic therapies, some patients may be at risk for the development of late complications that can be associated with short course, high dose per fraction therapy. To date, this has not been clinically significant given the relatively short survival of patients with metastatic cancer and the modest total doses employed.
Guidelines and Quality Measures
Bone Metastasis
Multiple randomized trials have compared single-fraction approaches to palliative radiation to multiple different multifraction approaches for the treatment of bone metastasis. The overwhelming evidence suggests equivalence in efficacy increased cost and inconvenience of multifraction approaches, yet there is still a great deal of variability of approaches in use by radiation oncologists. One survey revealed that 101 different dose fractionation schemes were employed worldwide for this single clinical circumstance [74]. The American Society for Radiation Oncology (ASTRO) and the American College of Radiology (ACR) have developed guidelines; four fractionation schemes are considered equivalent in the successful management of uncomplicated painful bone metastases [75–77]. The use of one of the four approved fractionation schemes is considered a measure of quality as determined by the National Quality Forum (NQF) [78]. In addition, single fraction RT for painful uncomplicated bone metastasis has been incorporated into the American Board of Internal Medicine’s “Choosing Wisely” campaign, a program designed to help physicians become better financial stewards of health care use [79].
End-of-Life Radiation Therapy
The delivery of chemotherapy in the last month of life has been proposed as a metric of overutilization of resources [24]. The delivery of RT in the last two weeks of life may be a similar indicator since palliative radiation takes time to achieve its effect and there are few conditions, namely bleeding and spinal cord compression, where radiation may be indicated in the last month of life in addition to other supportive measures.
Approximately 50% of patients receiving radiation at the EOL do not complete the planned course of radiation [26,80]. In the series described by Toole et al., 6 patients died during the course of radiation and 43 (68%) received radiation within 10 days of death. Thirteen patients (21%) spent more than half of their last month receiving RT [26]. In patients who died after being diagnosed with incurable lung cancer in the National Comprehensive Cancer Network (NCCN) NSCLC Outcomes Database, 10% received RT within 14 days of death and 16% of patients died during treatment [80].
As there is a delay between the delivery of RT and its therapeutic benefit, most patients at the very EOL rarely benefit from RT. Accurate estimates of life expectancy help patients avoid costly care that is unlikely to benefit them or relieve their suffering.
Conclusion
The treatment of patients with advanced cancer is a multidisciplinary effort requiring coordination between the radiation oncologist and other specialists including medical oncologists, surgeons, palliative medicine specialists, and psychiatrists. Prognostication and accurate estimates of life expectancy are essential for goal setting and provision of appropriate care while avoiding treatments unlikely to provide a benefit. Many patients have unmet spiritual needs at the EOL and a simple acronym (FICA) can help physicians take a spiritual history and start the conversation.
RT is an important tool for palliating symptoms such as pain due to osseous metastasis or local tumor invasion, bleeding, obstruction, dyspnea or cough, and functional impairment due to brain metastasis or impingement of nerve roots or the spinal cord. Short course treatments effectively palliate pain and other symptoms. Since pain relief is not immediate, adequate pain control prior to the initiation of therapy is important. The short- and long-term toxicity associated with palliative RT is typically self-limited and can be managed conservatively. Highly conformal RT shows great promise, especially in patients with recurrent pain in the spine after prior conventionally fractionated curative therapy.
Increasingly, quality metrics surrounding the appropriate use of palliative radiation are being utilized. Avoidance of RT, especially long courses of RT, at the EOL is important given the delay between delivery of RT and the palliative effect of RT. Whenever possible, the shortest course of RT likely to have the desired effect should be employed. This maximizes patient comfort and convenience and minimizes cost and toxicity.
List of Abbreviations
ECOGPS Eastern Cooperative Oncology Group Performance Status
Gy gray (unit of radiation therapy)
KPS Karnofsky Performance Status