Site-Specific Symptom Management
Palliative Radiotherapy for Advanced and Metastatic Lung Cancer
R. Rhome and K. Dharmarajan, Mount Sinai Hospital and the Icahn School of Medicine at Mount Sinai, New York, NY, United States
Abstract
Palliative radiation can be useful in numerous clinical scenarios with respect to primary or metastatic thoracic tumors. Extensive stage small cell lung cancer (SCLC) and locally advanced, unresectable nonsmall cell lung cancer (NSCLC) are the most common causes of symptoms that can be managed even in the setting of incurable underlying disease. Dyspnea, pain, hemoptysis, and superior vena cava syndrome are some manifestations of thoracic tumor invasion that compromise quality of life and survival. Multiple trials have been conducted to assess symptom relief and survival, with some conflicting evidence. Palliation of symptoms is reasonably achieved in most of these studies, and some show benefits in survival or tumor regression. Various fractionation schemas have been studied to determine the optimal symptom control. Metaanalyses have attempted to identify subsets of patients that benefit more, with performance status emerging as consistent predictor of benefit. Patient selection remains crucial to determining the risk–benefit ratio of any regimen.
Keywords
Palliation; lung; thorax; superior vena cava syndrome; endobronchial brachytherapy; oligometastatic disease
Introduction
Management of symptoms from locally advanced or metastatic lung cancer can be challenging due to their multifactorial nature. The use of palliative radiation therapy is generally accepted as a safe and effective means of symptom management in select patients. The doses and fractionation schemes in the literature are varied, though some may be more suitable based on other factors such as performance status, immediacy of symptom relief, and need for durability of control. Treatment-related toxicities vary with the schema and targets, and therapeutic ratio should be considered when offering treatment.
Evaluation
General History and Physical Exam
• When patients present with either locally destructive or metastatic disease in the thorax, evaluation will often be directed by the nature of the complaint.
• Given the diversity of critical structures in the thorax, location of the tumor in question will determine additional workup.
• After a general history and physical exam, a more focused history should be performed.
![]() Evaluate presence of dyspnea at rest, associations with cough or chest pain, and exacerbating factors.
Evaluate presence of dyspnea at rest, associations with cough or chest pain, and exacerbating factors.
![]() Qualifying and documenting dyspnea on exertion in relation to common exertions (i.e., number of blocks before resting, flights of stairs) can be useful in subjectively determining if a patient’s functional respiratory status is improving or declining.
Qualifying and documenting dyspnea on exertion in relation to common exertions (i.e., number of blocks before resting, flights of stairs) can be useful in subjectively determining if a patient’s functional respiratory status is improving or declining.
![]() Assess for causes of baseline dyspnea, given that concomitant pulmonary disorders such as emphysema and chronic obstructive pulmonary disease (COPD) are prevalent in populations with small cell lung cancer (SCLC) and nonsmall cell lung cancer (NSCLC).
Assess for causes of baseline dyspnea, given that concomitant pulmonary disorders such as emphysema and chronic obstructive pulmonary disease (COPD) are prevalent in populations with small cell lung cancer (SCLC) and nonsmall cell lung cancer (NSCLC).
• Further workup depends on presenting symptoms
![]() Initial imaging of the chest often includes chest X-ray followed by computed tomography (CT) of the chest. When medically possible, patients should have contrast-enhanced CT chest.
Initial imaging of the chest often includes chest X-ray followed by computed tomography (CT) of the chest. When medically possible, patients should have contrast-enhanced CT chest.
![]() Dyspnea in the setting of malignancy can be multifactorial, given the possibility for any combination of embolism, infection, tumor, or effusion.
Dyspnea in the setting of malignancy can be multifactorial, given the possibility for any combination of embolism, infection, tumor, or effusion.
– Presence of a pleural effusion should be sampled by thoracentesis if possible. This not only is therapeutic in the setting of removing compressive fluid, but also allows staging and prognostic information in the setting of malignant cells in the effusion.
– Bronchoscopy can also be important for direct evaluation, tissue diagnosis, and the potential opportunity for therapeutic interventions such as endobronchial stents.
– If there is suspicion for overlying infection, standard infectious workup should also be initiated, which would include complete blood count with differential and blood cultures.
– As always, a thorough pain history is required. Duration of pain, character, severity, and exacerbating/alleviating factors are all equally important in thoracic malignancies.
– In patients presenting with chest pain and/or shortness of breath, CT angiogram of the chest is often warranted to rule out pulmonary embolism (given malignancy as predisposition to hypercoagulable state). Differentiating symptoms such as pain or dyspnea related to an embolism versus local effects of tumor can be crucial in initiating effective palliation of symptoms.
• Patients suspected to have superior vena cava syndrome (SVCS) should have a thorough history taken relating to the duration and severity of classical symptoms.
![]() Symptom assessment should include facial edema and cyanosis (refer to the later section on management of SVCS for additional symptoms and implications). Consider a venogram.
Symptom assessment should include facial edema and cyanosis (refer to the later section on management of SVCS for additional symptoms and implications). Consider a venogram.
• Complaints of dysphagia should be evaluated by endoscopy. Esophagogastroduodenoscopy (EGD) can be useful in determining the nature of dysphagia, including differentiating between extrinsic compression from an intrathoracic tumor and a tumor arising from or invading the esophagus directly. This can potentially be therapeutic providing the opportunity to dilate or stent obstructions if appropriate.
• Hoarseness should be further evaluated by fiberoptic nasal endoscopy to assess of the presence of primary laryngeal tumors as opposed to symptoms secondary to recurrent laryngeal nerve paralysis.
Treatment Recommendations
Patients with new or worsening respiratory symptoms often present with a suite of interrelated characteristics such as dyspnea, cough, and pleuritic chest pain. In this section we will explore treatment recommendations for general pulmonary symptoms as well as some specific scenarios such as SVCS.
General Pulmonary Symptoms
• Radiation has been shown to improve general pulmonary symptoms [1–13].
• Various fractionation schemes for radiation therapy have been studied. Table 14.1 has a summary of more commonly used and/or studied fractionation schemes and the relative benefit for several clinically relevant endpoints such as symptom control, toxicity, and survival.
• In 2001 [1], a Cochrane review of palliative radiation regimens for locally advanced NSCLC examined the contributions of available randomized evidence at the time. Though updated later in 2006 [2] and most recently in 2015 [3], the recommendations in these reviews have been largely unchanged.
![]() As of the 2015 release, fourteen randomized trials were analyzed.
As of the 2015 release, fourteen randomized trials were analyzed.
![]() Inclusion required that the studies compare at least two radiation regimens based on effectiveness in palliation of symptoms from NSCLC.
Inclusion required that the studies compare at least two radiation regimens based on effectiveness in palliation of symptoms from NSCLC.
![]() One goal of these reviews was to identify subsets of patients that would benefit more from a particular regimen on the basis of symptom relief, performance status, convenience, or even survival.
One goal of these reviews was to identify subsets of patients that would benefit more from a particular regimen on the basis of symptom relief, performance status, convenience, or even survival.
– While all trials reported improvement in symptoms with radiation, only three of the trials included showed a difference in symptom control with respect to the radiation regimen.
– Teo et al. [4] compared 45 Gy in 18 daily fractions with 31.2 Gy in 4 fractions given once weekly. While there were no differences in survival or radiologic tumor regression, they reported significant improvement in symptoms (assessed monthly) in the 45 Gy arm.
– Bezjak et al. [5] randomized patients with 20 Gy in 5 fractions or 10 Gy in 1 fraction. Patients in the 20 Gy arm had significant improvement in lung cancer-related symptoms and patient-reported global quality of life (QOL) without any differences in toxicity. There was also a survival benefit (6 months compared to 4.2 months) in favor of the fractionated regimen.
– Erridge et al. [6] compared a more commonly used regimen of 30 Gy in 10 fractions with a single fraction of 10 Gy. They found no differences in survival, toxicity, or QOL between the arms. While a significant difference was found with respect to symptom palliation (dyspnea, chest pain) in favor of the 30 Gy regimen, this still met their prespecified parameters for equivalence.
![]() Survival: Multiple trials showed survival differences between the arms studied.
Survival: Multiple trials showed survival differences between the arms studied.
– As mentioned earlier, the Bezjak et al. study [5] showed improved survival in the fractionated arms as compared to the hypofractionated regimens.
– Three randomized trials from the Medical Research Council (MRC) were included in these reviews and each looked at slightly different regimens.
![]() The earliest of the MRC studies compared 30 Gy in 10 fractions, 27 Gy in 6 daily fractions, or 17 Gy in 2 weekly fractions [7].
The earliest of the MRC studies compared 30 Gy in 10 fractions, 27 Gy in 6 daily fractions, or 17 Gy in 2 weekly fractions [7].
![]() The second compared the use of 17 Gy in 2 weekly fractions with 10 Gy in a single fraction in patients with poor performance status [8].
The second compared the use of 17 Gy in 2 weekly fractions with 10 Gy in a single fraction in patients with poor performance status [8].
![]() The final MRC study included compared 36–39 Gy in 12–13 fractions with 17 Gy in 2 weekly fractions in patients with good performance status [9]. Overall, there was a statistically significant survival difference in favor of the more protracted fractionated regimen (31% vs 36% at 1 year and 9% vs 12% at 2 years) and difference in symptom control was reported between these regimens in all three MRC trials.
The final MRC study included compared 36–39 Gy in 12–13 fractions with 17 Gy in 2 weekly fractions in patients with good performance status [9]. Overall, there was a statistically significant survival difference in favor of the more protracted fractionated regimen (31% vs 36% at 1 year and 9% vs 12% at 2 years) and difference in symptom control was reported between these regimens in all three MRC trials.
– Kramer et al. [10] randomized patients to 30 Gy in 10 fractions or 16 Gy in 2 weekly fractions. There was a 1-year survival advantage in favor of the 30 Gy/10 fx arm (19.6% vs 10.9%). On subgroup analysis, this advantage was only significant in the patients with better performance status.
– Another study enrolled asymptomatic unresectable patients with locally advanced NSCLC and randomized them to either 50 Gy in 25 fractions to the primary tumor/mediastinum or 20 Gy in 5 fractions to the same targets followed by another 20 Gy again 4 weeks later [11]
![]() Survival favored the standard fractionation regimen (18% vs 6%), though this trial was criticized for split course design with a significant interval break, theoretically allowing for accelerated tumor repopulation.
Survival favored the standard fractionation regimen (18% vs 6%), though this trial was criticized for split course design with a significant interval break, theoretically allowing for accelerated tumor repopulation.
– Senkus-Konefka et al. [12] randomized patients to either 20 Gy in 5 fractions or 16 Gy in 2 fractions given 1 week apart. Survival was statistically significantly higher in the more hypofractionated arm (5.3 months vs 8 months in the 16 Gy arm).
– The metaanalysis of survival in these trials showed an overall difference in 1-year survival barely achieving significance but favoring more fractionated regimens.
• Toxicity: Practitioners often balance potential differences in symptom relief durability with an increased chance of toxicities.
![]() The three MRC trials mentioned showed a nonsignificant increase in severe (Grade 3–4) rates of esophagitis in shorter regimens, though the results were very heterogeneous and no definitive relationship was found.
The three MRC trials mentioned showed a nonsignificant increase in severe (Grade 3–4) rates of esophagitis in shorter regimens, though the results were very heterogeneous and no definitive relationship was found.
![]() Radiation-induced myelopathy was described in a small number of patients in the MRC trials [7–9] (five total patients) and another trial that had a single incident in the 50 Gy/25 fraction arm [13]. No clear relationship was found with respect to regimen, though this was a very rare event.
Radiation-induced myelopathy was described in a small number of patients in the MRC trials [7–9] (five total patients) and another trial that had a single incident in the 50 Gy/25 fraction arm [13]. No clear relationship was found with respect to regimen, though this was a very rare event.
– The authors of the metaanalysis calculated the BED1.7 (for spinal cord) of each of the regimens where radiation myelopathy was noted, and each had (biologically equivalent dose) BED >100.
– A prospective nonrandomized study of patients treated with 16 Gy in 2 fractions given 1 week apart (BED1.7=91 Gy) showed no incidents of myelopathy [14].
– While the analysis of this uncommon toxicity has many weaknesses, use of regimens with BED1.7 <100 or the use of a spinal cord block should be considered.
![]() Pneumonitis rates ranged from 1.6% to 6.0% in these trials, but did not show a statistically significant difference in lower or higher fraction regimens.
Pneumonitis rates ranged from 1.6% to 6.0% in these trials, but did not show a statistically significant difference in lower or higher fraction regimens.
• Endobronchial Brachytherapy (EBB): In 2011, the American Society of Radiation Oncology (ASTRO) released guidelines for palliative thoracic radiation therapy [15] that draws on many of the same randomized trials as above and has similar conclusions with respect to optimal external beam radiotherapy (EBRT) regimens. The ASTRO guidelines also evaluated and included EBB trials.
![]() EBB is a different method of delivering radiation therapy by direct insertion of a radioactive source into a target within a major bronchus.
EBB is a different method of delivering radiation therapy by direct insertion of a radioactive source into a target within a major bronchus.
![]() The guidelines identified six randomized trials that studied various combinations of EBB alone or in conjunction with EBRT.
The guidelines identified six randomized trials that studied various combinations of EBB alone or in conjunction with EBRT.
![]() Inclusion criteria required at least a portion of the tumor within a central (main or lobar) bronchus in order to achieve potential benefit from EBB.
Inclusion criteria required at least a portion of the tumor within a central (main or lobar) bronchus in order to achieve potential benefit from EBB.
![]() Langendijk et al. [16] randomized patients with a luminal tumor component to EBRT with or without EBB. Dose of EBRT was at the discretion of the treating physician and the dose of EBB was 15 Gy in 2 fractions (insertions).
Langendijk et al. [16] randomized patients with a luminal tumor component to EBRT with or without EBB. Dose of EBRT was at the discretion of the treating physician and the dose of EBB was 15 Gy in 2 fractions (insertions).
– There was no difference in median survival, but there was a significantly increased rate of lung re-expansion (57% vs 35%) and improved dyspnea in the arm including EBB.
![]() With regards to EBB alone, Stout et al. [17] randomized patients to either 30 Gy in 8 fractions EBRT or a single 15 Gy EBB insertion. This showed improved palliation of symptoms with EBRT (83% vs 59%) with a slightly increased survival (9.4 vs 8.2 months).
With regards to EBB alone, Stout et al. [17] randomized patients to either 30 Gy in 8 fractions EBRT or a single 15 Gy EBB insertion. This showed improved palliation of symptoms with EBRT (83% vs 59%) with a slightly increased survival (9.4 vs 8.2 months).
![]() Dose optimization studies with respect to EBB have not shown a clear benefit with any particular regimen [18,19].
Dose optimization studies with respect to EBB have not shown a clear benefit with any particular regimen [18,19].
![]() A recent updated Cochrane metaanalysis of EBB reported no conclusive evidence that the addition of EBB to EBRT improved palliation but that EBB should be considered in patients previously treated by EBRT [20].
A recent updated Cochrane metaanalysis of EBB reported no conclusive evidence that the addition of EBB to EBRT improved palliation but that EBB should be considered in patients previously treated by EBRT [20].
![]() No obvious external beam radiotherapy regimen is overall superior for palliation of general respiratory symptoms.
No obvious external beam radiotherapy regimen is overall superior for palliation of general respiratory symptoms.
![]() Higher BED regimens may improve symptoms and even survival, but possibly with higher risk of toxicities (See Table 14.1).
Higher BED regimens may improve symptoms and even survival, but possibly with higher risk of toxicities (See Table 14.1).
![]() EBB could be considered in patients previously treated with EBRT or in conjunction with EBRT in properly selected patients with a luminal tumor component to potentially improve dyspnea and lung re-expansion, though metaanalysis of evidence did not produce strong recommendations.
EBB could be considered in patients previously treated with EBRT or in conjunction with EBRT in properly selected patients with a luminal tumor component to potentially improve dyspnea and lung re-expansion, though metaanalysis of evidence did not produce strong recommendations.
– Selection of a regimen should take into account the patient’s performance status and prognosis, patient convenience, durability of response, and anticipated toxicities, as patients with poorer performance status may be considered for more hypofractionated regimens.
Table 14.1
Select Commonly Used Regimens With Available Randomized Evidence. Gray Boxes Indicate Insufficient Information for That Endpoint for a Particular Regimen. For Symptom Control, Durability, QOL, and OS, + Indicates Strength/Abundance of Evidence for Benefit. For Toxicities, + Indicates Tolerability of Treatment and – Indicates Increased Toxicity. For Tx Length, Higher + Indicates Longer Total Treatment Time
| Regimen | BED10 (Gy) | Symptom Control | Durability | QOL | Tx Length | Toxicities | OS |
| 30 Gy/10 fx [6,7,10] | 39.00 | +++ | ++ | ++ | +++ | + | ++ |
| 20 Gy/5 fx [5,12] | 28.00 | +++ | ++ | ++ | + | + | |
| 17 Gy/2 fx (weekly) [7–9] | 31.45 | ++ | + | ++ | +a | − | |
| 16 Gy/2 fx (weekly) [7,12] | 28.80 | ++ | − | ++ | + | + | |
| 10 Gy/1 fx [5,6,8] | 20.00 | + | +/− | + | + | − | |
| 39 Gy/13 fx [7] | 50.70 | ++ | ++++ | +/−b | + | ||
| 45 Gy/18 fx [4] | 56.25 | ++ | + | ++++ | + | + | |
| 50 Gy/25 fx [11] | 60.00 | ++ | +++++ | +/− | + |
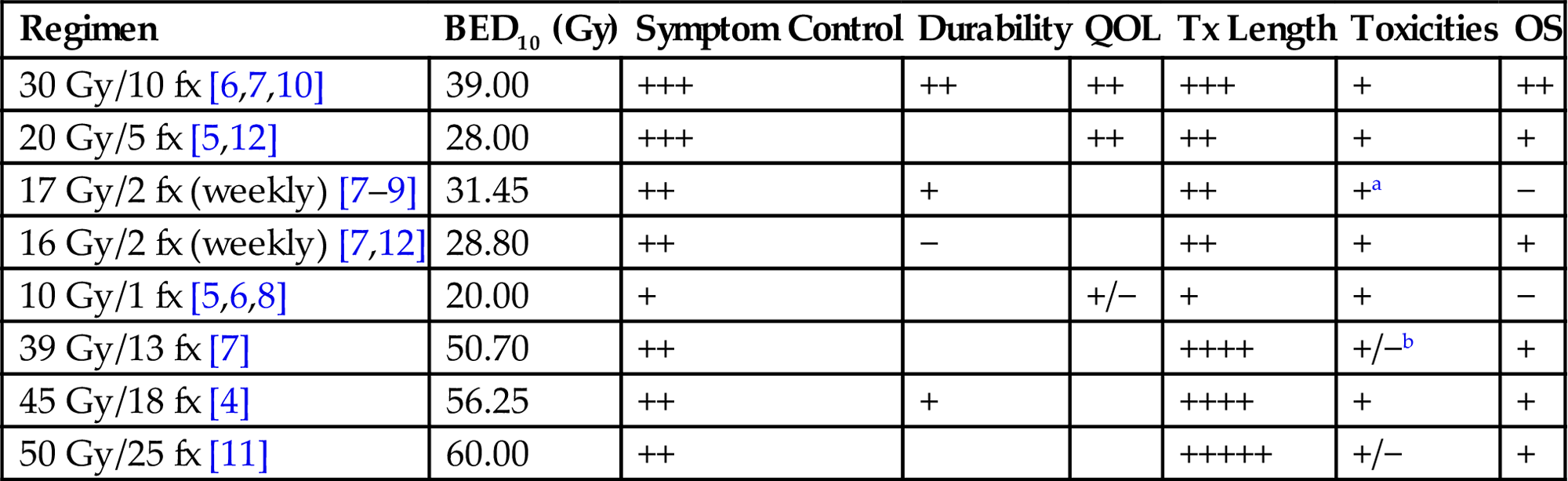
fx, fractions; Gy, Gray; BED10, biologically equivalent dose assuming alpha/beta=10 Gy for tumor control, QOL, quality of life; Tx, treatment.
aSelect instances of radiation myelopathy in this group.
bIncreased acute esophagitis.
Hemoptysis
• Outside of the Cochrane analyses, another notable review source analyzed many of the same trials with some exceptions. Fairchild et al. (2008) [21] attempted to further specify the various pulmonary symptoms to be palliated, and examined BED-related trends in responses. Hemoptysis was specifically addressed in five of the trials included in this review. When divided by higher dose or lower BED arms, the respective rates of hemoptysis resolution were 73.7% and 68.9% (not statistically significant).
• Use of EBB after previous EBRT in the setting of hemoptysis has been studied, with up to 92% relief of hemoptysis rate if coming from endobronchial lesions [22].
• For additional details on the management of hemoptysis, refer to the chapter on hemoptysis in this textbook (See Chapter 9: Malignant Bleeding).
Postobstructive Pneumonia
• In addition to direct pulmonary symptoms such as dyspnea, cough, and pain mentioned earlier, there are also symptomatic issues that are secondary to the malignancy such as recurrent postobstructive pneumonia.
• Both EBB and EBRT may be used for relief of obstruction that leads to pneumonia (EBB for intraluminal causes and EBRT for external compression and intraluminal causes).
• One study of patients that had an endobronchial recurrence after previous EBB or EBRT showed an 82% response rate with respect to postobstructive pneumonia after repeat EBB (8–10 Gy single insertion) [22].
• An earlier study showed a comparable rate (88% response in postobstructive pneumonia) after EBB, though many of these patients also received EBRT with variable dose fractionation schemes [23].
• It is generally recommended that palliative radiation to an obstructive lesion be performed after resolution of acute infectious symptoms treated with an appropriate course of empiric antibiotics.
Mesothelioma
• While mesothelioma can present with any of the same above symptoms to palliate, there have been variable responses in this particular disease.
• Though curative-intent treatment regimens for mesothelioma include surgery with adjuvant chemotherapy and radiation, this rare often-fatal malignancy can often blur the lines between definitive and palliative treatments.
• More so than parenchymal lesions from NSCLC, mesothelioma often presents with more pleuritic pain symptoms.
• Given the low incidence of mesothelioma in the general population, evidence for treatment comes often from retrospective series and small prospective trials.
![]() MacLeod et al. [24] reviewed the available literature related to mesothelioma treated with radiation therapy specifically for pain symptoms. All studies were level 2 or 3 evidence, and heterogeneity precluded quantitative evaluation. Two trials prospectively evaluated pain, with mixed results.
MacLeod et al. [24] reviewed the available literature related to mesothelioma treated with radiation therapy specifically for pain symptoms. All studies were level 2 or 3 evidence, and heterogeneity precluded quantitative evaluation. Two trials prospectively evaluated pain, with mixed results.
![]() Linden et al. [25] did not find a significant pain control benefit to the addition of radiation (40 Gy in 20 fractions).
Linden et al. [25] did not find a significant pain control benefit to the addition of radiation (40 Gy in 20 fractions).
![]() Bissett et al. [26] used older radiation techniques (Cobalt 60 machines) and found 68% pain improvement measured by prospective pain scores. The review authors were unable to make definitive conclusions on pain relief with radiation based on limited evidence, though options are often limited in this disease.
Bissett et al. [26] used older radiation techniques (Cobalt 60 machines) and found 68% pain improvement measured by prospective pain scores. The review authors were unable to make definitive conclusions on pain relief with radiation based on limited evidence, though options are often limited in this disease.
![]() Jenkins et al. [27] published one of the larger retrospective series from a single institution where patients were treated to 36 Gy in 12 fractions.
Jenkins et al. [27] published one of the larger retrospective series from a single institution where patients were treated to 36 Gy in 12 fractions.
– 54% rate of reduction in chest pain symptoms with radiation.
– Four patients here presented with SVCS, of which three had symptom relief.
– One patient presented with dysphagia and had relief after radiation.
– Only two patients developed grade three toxicities (one pneumonitis, one nausea).
– Response to radiation was predicted by performance status and prognostic category.
![]() The largest retrospective study available looked at 189 patients with mesothelioma that received radiation for any reason [28].
The largest retrospective study available looked at 189 patients with mesothelioma that received radiation for any reason [28].
– 50% local reduction in pain with median dose of 36 Gy.
– The median duration of pain relief was significantly higher in patients treated with fraction sizes higher than 4.0 Gy (98 days vs 69 days).
– Potentially confounded because patients with disease encompassing >66% of the hemithorax were generally given less than 4.0 Gy fractions.
![]() Cochrane review of radiation in mesothelioma was published, but the lack of randomized evidence prevented them from making any definitive conclusions [29].
Cochrane review of radiation in mesothelioma was published, but the lack of randomized evidence prevented them from making any definitive conclusions [29].
![]() Recent European Society for Medical Oncology (ESMO) clinical practice guideline recommended consideration of palliative radiation in the setting of chest wall pain/invasion with mesothelioma [30].
Recent European Society for Medical Oncology (ESMO) clinical practice guideline recommended consideration of palliative radiation in the setting of chest wall pain/invasion with mesothelioma [30].
![]() Currently there are open trials in the United Kingdom studying postsurgical radiation to the surgical tract and will reportedly include pain evaluation.
Currently there are open trials in the United Kingdom studying postsurgical radiation to the surgical tract and will reportedly include pain evaluation.
![]() Overall, while radiation-related pain relief in mesothelioma shows variable response rate in the literature, performance status, prognosis, and patient goals of care can help identify subsets that may have higher chance of benefit.
Overall, while radiation-related pain relief in mesothelioma shows variable response rate in the literature, performance status, prognosis, and patient goals of care can help identify subsets that may have higher chance of benefit.
Superior Vena Cava Syndrome
• SVCS is a clinical diagnosis made based on the presence of characteristic features in the setting of obstruction of the SVC. The constellation of symptoms is related to the reduction of venous flow through the SVC, causing an increase in venous pressure in more proximal vessels.
![]() Can lead to facial plethora/edema, cyanosis, edema in upper extremity, and distention of subcutaneous vessels of the neck and chest.
Can lead to facial plethora/edema, cyanosis, edema in upper extremity, and distention of subcutaneous vessels of the neck and chest.
![]() Symptoms of laryngeal edema (stridor) and cerebral edema (headaches, visual changes, syncope) can occur, and can be potentially life threatening. The neurologic symptoms occur in 2–10% of documented cases, while stridor was noted in 4% of patients [31].
Symptoms of laryngeal edema (stridor) and cerebral edema (headaches, visual changes, syncope) can occur, and can be potentially life threatening. The neurologic symptoms occur in 2–10% of documented cases, while stridor was noted in 4% of patients [31].
• While compression by a malignancy is more common [32], impingement of the SVC by any process can lead to a similar clinical picture. Within malignant causes, primary lung carcinomas comprise a majority of cases [32], though SVCS can also be caused by lymphomas, metastatic parenchymal lesions, malignant lymphadenopathy, or other less common thoracic tumors.
• Historically, the presence of SVCS was regarded as a relative emergency, at least partially due to rarer complications related to cerebral or laryngeal edema.
• An older review of SVCS outcomes looked at 1986 patients, of which a single death was documented as the result of SVCS [33].
• Initial management of patients with SVCS is often guided by the severity of symptoms and the nature of the underlying malignancy.
• Strategies aimed at relief of the obstruction must be weighed against treatment of the overall oncologic process.
![]() If life-threatening symptoms occur, such as airway compromise or symptoms of cerebral edema, relief of compression may take precedent over other diagnostic tests or treatments.
If life-threatening symptoms occur, such as airway compromise or symptoms of cerebral edema, relief of compression may take precedent over other diagnostic tests or treatments.
![]() In the presence of any neurologic symptoms with SVCS, evaluation for concomitant brain metastases is crucial given the high prevalence of brain metastasis.
In the presence of any neurologic symptoms with SVCS, evaluation for concomitant brain metastases is crucial given the high prevalence of brain metastasis.
![]() If symptoms are nonurgent or stable for a period of time (as in a majority of presentations), the patient may be better served by completing the staging workup and obtaining tissue diagnosis for those without a diagnosis of malignancy. Some evidence exists that empiric radiation therapy prior to tissue diagnosis in emergent thoracic scenarios can obscure the subsequent biopsy results [34].
If symptoms are nonurgent or stable for a period of time (as in a majority of presentations), the patient may be better served by completing the staging workup and obtaining tissue diagnosis for those without a diagnosis of malignancy. Some evidence exists that empiric radiation therapy prior to tissue diagnosis in emergent thoracic scenarios can obscure the subsequent biopsy results [34].
![]() When treating the obstruction itself, several approaches exist, including early conservative measures, steroids, radiation, chemotherapy, and endovascular stenting. A Cochran review of treatment strategies is summarized here:
When treating the obstruction itself, several approaches exist, including early conservative measures, steroids, radiation, chemotherapy, and endovascular stenting. A Cochran review of treatment strategies is summarized here:
– Early conservative measures can include elevation of the patient’s head to reduce pressure secondary to edema, though there is no empirical evidence that this is effective [31].
– Steroids: No studies directly evaluate the role of steroids in palliation on SVCS. However, these can be used during radiation management in order to mitigate temporary worsening obstruction due to tumor edema.
– Chemotherapy versus Radiotherapy: similar response rates with either.
![]() In patients with SCLC, the authors of the Cochran review noted relief in 76.9% with chemotherapy and 77.6% with radiation therapy.
In patients with SCLC, the authors of the Cochran review noted relief in 76.9% with chemotherapy and 77.6% with radiation therapy.
![]() There was a slightly lower response rate in NSCLC for both treatment types (59% with chemotherapy and 63% with radiation).
There was a slightly lower response rate in NSCLC for both treatment types (59% with chemotherapy and 63% with radiation).
– Data from randomized trials were included:
![]() Spiro et al. reports an unplanned subset of a randomized trial with SCLC receiving chemoradiation versus chemotherapy: 37 patients had SVCS, and no difference in SVCS recurrence [35].
Spiro et al. reports an unplanned subset of a randomized trial with SCLC receiving chemoradiation versus chemotherapy: 37 patients had SVCS, and no difference in SVCS recurrence [35].
![]() A randomized phase II trial included 34 patients with NSCLC presenting with SVCS, randomized to multiagent chemotherapy or radiation therapy with cisplatin [36]. The rates of SVCS relief and relapse were not different between the two arms.
A randomized phase II trial included 34 patients with NSCLC presenting with SVCS, randomized to multiagent chemotherapy or radiation therapy with cisplatin [36]. The rates of SVCS relief and relapse were not different between the two arms.
![]() Stenting is the most rapid and effective treatment with respect to relief of symptoms in patients that are able to tolerate the procedure.
Stenting is the most rapid and effective treatment with respect to relief of symptoms in patients that are able to tolerate the procedure.
![]() Another advantage to stenting is that tissue diagnosis is not required, nor does upfront stenting obscure future pathologic diagnosis attempts.
Another advantage to stenting is that tissue diagnosis is not required, nor does upfront stenting obscure future pathologic diagnosis attempts.
![]() This is a technique that requires technical expertise and may be difficult to generalize to all centers.
This is a technique that requires technical expertise and may be difficult to generalize to all centers.
• Several fractionation schemes have been studied.
![]() Overall consensus is to use fractions of 3.0–4.0 Gy.
Overall consensus is to use fractions of 3.0–4.0 Gy.
![]() Commonly used fractionation schemes include 30 Gy in 10 fractions, 37.5 Gy in 15 fractions, and 20 Gy in 5 fractions.
Commonly used fractionation schemes include 30 Gy in 10 fractions, 37.5 Gy in 15 fractions, and 20 Gy in 5 fractions.
![]() Multiple studies show that the time to beginning of improvement after radiation therapy is about 72 hours. This should be considered when weighing the timing of other treatments.
Multiple studies show that the time to beginning of improvement after radiation therapy is about 72 hours. This should be considered when weighing the timing of other treatments.
• Treatment recommendations by SVCS classification
![]() Life threatening symptoms (~5% of patients [37]).
Life threatening symptoms (~5% of patients [37]).
– Assess for signs of significant cerebral or laryngeal edema.
– Immediate endovascular stenting is recommended for these patients.
– Immediate RT or chemotherapy should be considered (histology dependent) if stenting not available/unsafe.
![]() SCLC and other chemoradiosensitive histologies: Initiation of chemotherapy if medically possible.
SCLC and other chemoradiosensitive histologies: Initiation of chemotherapy if medically possible.
![]() NSCLC and other nonchemoradiosensitive histologies: Consideration of early radiation or stenting.
NSCLC and other nonchemoradiosensitive histologies: Consideration of early radiation or stenting.
Asymptomatic Metastases
• The treatment of asymptomatic metastases to the lung/thorax is sometimes controversial.
• Traditional canon is that palliative treatments should be used for active/impending symptoms.
• In recent years, treatment of oligometastatic disease has been an increasingly popular topic [38]. In certain malignancies, metastatic disease can be controlled effectively for years with a variety of systemic/targeted therapies and local treatments such as metastasectomy [39].
• While still not definitive therapy per se, targeting thoracic metastases with local therapy such as surgery or radiation in the setting of disseminated disease is an increasingly studied paradigm.
• Stereotactic body radiation therapy (SBRT) for selected patients with oligometastasis to the lung has been established in recent years with safe delivery of higher doses per fraction to small defined metastases.
![]() Currently there are no randomized data comparing SBRT to surgery or no local treatment, but several prospective trials have shown promising results.
Currently there are no randomized data comparing SBRT to surgery or no local treatment, but several prospective trials have shown promising results.
– One multiinstitutional Phase I/II trial was conducted enrolling patients with 1–3 lung metastases from a variety of different primary sites [40].
![]() The Phase I aspect of the trial escalated the dose from 48 Gy to 60 Gy in 3 fractions with no dose-limiting toxicities and all patients in the Phase II aspect were prescribed 60 Gy in 3 fractions.
The Phase I aspect of the trial escalated the dose from 48 Gy to 60 Gy in 3 fractions with no dose-limiting toxicities and all patients in the Phase II aspect were prescribed 60 Gy in 3 fractions.
![]() Local control (actuarial) with SBRT was 100% and 96% at 1 and 2 years, respectively.
Local control (actuarial) with SBRT was 100% and 96% at 1 and 2 years, respectively.
![]() Median survival was 19 months and 2-year survival was 38%.
Median survival was 19 months and 2-year survival was 38%.
![]() The treatment was well-tolerated, with 7.9% incidence of any grade 3 toxicity (including a single incident of grade 3 pneumonitis) and no grade 4–5 toxicities.
The treatment was well-tolerated, with 7.9% incidence of any grade 3 toxicity (including a single incident of grade 3 pneumonitis) and no grade 4–5 toxicities.
– Another recent study examined patients with oligometastatic NSCLC specifically with ≤5 active metastases that were treated to 50 Gy in 10 fractions [41].
![]() Grade 3 pulmonary toxicity (cough, pneumonitis) was 8% with no toxicities of grade 4–5.
Grade 3 pulmonary toxicity (cough, pneumonitis) was 8% with no toxicities of grade 4–5.
![]() Partial metabolic response rate (by PET) at 30% and a complete response rate of 30% for a total 60% response.
Partial metabolic response rate (by PET) at 30% and a complete response rate of 30% for a total 60% response.
![]() In-field failures occurred in 15% of patients.
In-field failures occurred in 15% of patients.
![]() The median survival was 23 months and 1-year overall survival of 67%.
The median survival was 23 months and 1-year overall survival of 67%.
![]() This study had a slightly lower lesion control rate than others, likely partially attributed to lack of respiratory motion control compensation in the SBRT technique and the use of a lower BED treatment.
This study had a slightly lower lesion control rate than others, likely partially attributed to lack of respiratory motion control compensation in the SBRT technique and the use of a lower BED treatment.
– A third recent prospective trial combined SBRT with erlotinib in patients with metastatic NSCLC of limited scope, defined as less than six sites of extracranial disease and no more than three pulmonary lesions [42]. Multiple fractionation schemes were permitted, including 19–24 Gy for single fraction, 27–33 Gy for 3 fractions, or 35–40 Gy in 5 fractions.
![]() Median OS was 20.4 months and median progression-free survival was 14.7 months.
Median OS was 20.4 months and median progression-free survival was 14.7 months.
![]() Of the 47 evaluable sites treated, three showed in-field progression (6.4%).
Of the 47 evaluable sites treated, three showed in-field progression (6.4%).
• Patient selection is key in predicting benefit from local therapies in disseminated disease.
![]() De Vin et al. [43] proposed a prognostic model for patients with oligometastatic disease treated with SBRT.
De Vin et al. [43] proposed a prognostic model for patients with oligometastatic disease treated with SBRT.
– Patients with adenocarcinoma fared significantly better with respect to survival compared to nonadenocarcinoma, though this may reflect differences in natural disease history rather than response to SBRT.
– BED10 of ≥75 Gy compared to <75 Gy was a significant predictor of survival.
– Several patient-related factors identified that predicted survival after SBRT including dose, histology, primary location, and gender (female patients had improved survival).
![]() A metaanalysis examined individual data on patients with oligometastatic NSCLC treated with either surgery or SBRT [44].
A metaanalysis examined individual data on patients with oligometastatic NSCLC treated with either surgery or SBRT [44].
– Patients were divided into risk groupings based on recursive partitioning analysis that identified lower nodal stage, histologic type, and metachronous presentation as positive predictors of survival after local therapy.
– Though there was no control group in this analysis, these patient-related factors may help guide the decision to offer local therapy to oligometastatic disease.
• In summary, while SBRT can be used safely and effectively for localized or oligometastatic thoracic lesions, randomized trials will be needed to demonstrate that it provides disease-free or overall survival benefits for patients. Patient selection is an important part of determining the potential benefit of these local therapies, and BED of the SBRT regimen chosen may predict response.
Expected Acute Side Effects
• Acute toxicities of radiation therapy are dose and fractionation-dependent.
• In general, patients may experience standard toxicities, such as skin changes (erythema, pruritis, desquamation) and fatigue.
• Treatment of larger fields that are potentially occupied by remaining healthy lung tissue should be done with care.
• Standard lung constraints should be respected, though these were generally developed for either standard fraction sizes (such as 1.8 Gy to 2.0 Gy per fraction) or SBRT.
• Doses associated with palliative radiation can range from 2.0 Gy to 8.0 Gy in fraction-size, and therefore exist between conventional EBRT and SBRT dose constraints. If constraint violation is in question, calculation of BED is recommended to aid clinical decision-making.
• Hypofractionated regimens have been associated with some acute onset symptoms within 24–48 hours of treatment including fevers, rigors, chest pain, or sweating [45]. These symptoms are not serious and rarely require intervention, but should be part of expectant counseling for patients.
• While nausea/vomiting can occur in patients on treatment in general, patients receiving treatment for mesothelioma seem to experience this at a higher rate.
On-Treatment Management
• General management on treatment should include at least weekly vital signs including orthostatic blood pressure readings to monitor for dehydration and accurate weight to monitor for accelerated weight loss.
• Skin changes like erythema and dry desquamation should be managed with topical emollients.
• Though uncommon in doses such as these, moist desquamation should be treated with antimicrobial topical agents such as silver sulfadiazine.
• Radiation-induced esophagitis
![]() Can be managed with topical analgesics such as viscous lidocaine alone or in mixtures with antacids and antihistamines (occasionally referred to as “magic mouthwash” or “oncology mouthwash”). These often offer only temporary relief of esophagitis symptoms but can be effective if optimally timed for meals or oral intake.
Can be managed with topical analgesics such as viscous lidocaine alone or in mixtures with antacids and antihistamines (occasionally referred to as “magic mouthwash” or “oncology mouthwash”). These often offer only temporary relief of esophagitis symptoms but can be effective if optimally timed for meals or oral intake.
![]() Escalated pain symptoms in this setting sometime require narcotics for management. Transdermal narcotics can be effective at providing long-acting analgesia without requiring oral administration.
Escalated pain symptoms in this setting sometime require narcotics for management. Transdermal narcotics can be effective at providing long-acting analgesia without requiring oral administration.
![]() Overuse of narcotics should be regarded with caution given the potential for respiratory depression in a population that may have significant dyspnea at baseline.
Overuse of narcotics should be regarded with caution given the potential for respiratory depression in a population that may have significant dyspnea at baseline.
• Occasionally costochondritis can develop and cause mechanical chest pain.
![]() This may be more common with SBRT and can be acute or subacute (6 months to 1 year) after treatment.
This may be more common with SBRT and can be acute or subacute (6 months to 1 year) after treatment.
![]() Usually conservative measures including over-the-counter nonsteroidal antiinflammatory medications are adequate to treat this until they resolve spontaneously.
Usually conservative measures including over-the-counter nonsteroidal antiinflammatory medications are adequate to treat this until they resolve spontaneously.
• Acute cardiac toxicities are uncommon, and may manifest as pericarditis or pericardial effusion.
![]() If pericarditis is suspected on the basis of chest pain or hemodynamic changes, evaluation by cardiology or emergency medicine is recommended.
If pericarditis is suspected on the basis of chest pain or hemodynamic changes, evaluation by cardiology or emergency medicine is recommended.
![]() Pericardial friction rub on exam and diffuse ST segment elevations on electrocardiogram are characteristics. This can usually be managed conservatively with antiinflammatories.
Pericardial friction rub on exam and diffuse ST segment elevations on electrocardiogram are characteristics. This can usually be managed conservatively with antiinflammatories.
![]() Constraints proposed by an MD Anderson review of pericardial effusion in treatment of esophageal tumors with definitive radiation include mean dose <26 Gy and V30 <46% [46], though these may not be directly applicable to the various dose regimens and fraction sizes used in palliative thoracic radiation.
Constraints proposed by an MD Anderson review of pericardial effusion in treatment of esophageal tumors with definitive radiation include mean dose <26 Gy and V30 <46% [46], though these may not be directly applicable to the various dose regimens and fraction sizes used in palliative thoracic radiation.
![]() Another study pooling protocol patients at a single institution reported an actuarial rate of 3.9% for pericarditis at 6 months, which suggested that fraction size may predict development of pericarditis or effusion [47].
Another study pooling protocol patients at a single institution reported an actuarial rate of 3.9% for pericarditis at 6 months, which suggested that fraction size may predict development of pericarditis or effusion [47].
• Possibly the most challenging on-treatment toxicities to manage are those related to the patients’ respiratory symptoms.
![]() Patients may present with a history of respiratory issues and are often receiving palliative radiation due to an acute exacerbation of these symptoms.
Patients may present with a history of respiratory issues and are often receiving palliative radiation due to an acute exacerbation of these symptoms.
![]() Worsening dyspnea or cough on treatment could represent radiation toxicity, tumor progression, embolism, infection, or development of a pleural effusion.
Worsening dyspnea or cough on treatment could represent radiation toxicity, tumor progression, embolism, infection, or development of a pleural effusion.
![]() As these are all managed in very different ways, there should be a low threshold to order repeat diagnostic imaging or infectious workups while on treatment.
As these are all managed in very different ways, there should be a low threshold to order repeat diagnostic imaging or infectious workups while on treatment.
![]() Depending on etiology, a trial of inhaled bronchodilators may be useful.
Depending on etiology, a trial of inhaled bronchodilators may be useful.
![]() As earlier, concurrent steroids may be effective in reducing transient tumor swelling when treating compression-related symptoms. Working closely with the patient’s pulmonologist is advised (Table 14.2).
As earlier, concurrent steroids may be effective in reducing transient tumor swelling when treating compression-related symptoms. Working closely with the patient’s pulmonologist is advised (Table 14.2).
Table 14.2
On-Treatment and Chronic Management of Toxicities

