Palliative Radiotherapy for Advanced and Metastatic Head and Neck Cancers and Skin Metastases
T.J. Wilhite1 and J.D. Schoenfeld2, 1Mayo Clinic, Rochester, MN, United States, 2Dana-Farber Cancer Institute, Boston, MA, United States
Abstract
This chapter outlines a practical approach to palliative radiotherapy in the patient with advanced cancer involving the head and neck region and overlying skin, including primary tumors of the head and neck. Evaluation begins with a thorough history and physical examination aimed at identifying and characterizing tumor burden, acute concerns like airway patency, pain, and symptomatic impairments, such as dysphagia. Goals of care are determined with the patient based on realistic near- and long-term hopes and expectations. If palliative radiotherapy is indicated, selection of dose and fractionation should be grounded in evidence relevant to the clinical scenario. Validated regimens for the head and neck differ in terms of treatment time and toxicity, as well as expected degree of local control. Acute effects of radiation in the head and neck may be especially detrimental to quality of life, prompting the need for early recognition and proactive, multidisciplinary treatment management.
Keywords
Head and neck cancer; intensity modulated radiation therapy (IMRT); re-irradiation; stereotactic body radiotherapy (SBRT); oral cavity; oropharynx; larynx; hypopharynx; nasopharynx
Evaluation
General History & Physical Examination (H&P)
• Cancer in the head and neck region can affect respiratory function, airway patency, and hemodynamic stability; therefore, it is important to obtain vital signs and assess patients for medical stability before proceeding with a full evaluation.
• In a stable patient, evaluation should begin with a general history and physical exam.
![]() Important points to cover in the general history:
Important points to cover in the general history:
– History of the present illness.
![]() Diagnosis, original site of disease.
Diagnosis, original site of disease.
![]() For head and neck tumors originating from the oropharynx, HPV status should be obtained if available.
For head and neck tumors originating from the oropharynx, HPV status should be obtained if available.
![]() Prior treatments received (surgery, radiation, chemotherapy, and experimental agents).
Prior treatments received (surgery, radiation, chemotherapy, and experimental agents).
![]() For surgeries in the head and neck region, review of prior operative notes can be of value.
For surgeries in the head and neck region, review of prior operative notes can be of value.
![]() Time elapsed from the last dose of chemotherapy/restaging exams.
Time elapsed from the last dose of chemotherapy/restaging exams.
![]() Prior radiation therapy: Area treated, duration of treatment and dose, short- and long-term effects of therapy.
Prior radiation therapy: Area treated, duration of treatment and dose, short- and long-term effects of therapy.
![]() Detailed records including radiation plans, dose-volume histograms, on treatment assessments, and completion summaries should be obtained for prior radiation treatment delivered to the head and neck region.
Detailed records including radiation plans, dose-volume histograms, on treatment assessments, and completion summaries should be obtained for prior radiation treatment delivered to the head and neck region.
![]() Current systemic disease status (oligometastatic vs diffuse).
Current systemic disease status (oligometastatic vs diffuse).
![]() Serious health problems, prior operations.
Serious health problems, prior operations.
![]() Contraindications to radiation such as active connective tissue diseases.
Contraindications to radiation such as active connective tissue diseases.
![]() Recurrent pneumonias may be indicative of aspiration. A dedicated assessment for this may be indicated.
Recurrent pneumonias may be indicative of aspiration. A dedicated assessment for this may be indicated.
![]() Social history should note alcohol intake and all forms of tobacco usage.
Social history should note alcohol intake and all forms of tobacco usage.
• A focused history should then assess:
![]() Pain: Accurate assessment of pain in patients with cancers in the head and neck area helps guide effective analgesic treatment.
Pain: Accurate assessment of pain in patients with cancers in the head and neck area helps guide effective analgesic treatment.
– OPQRST: Onset, provocation/palliation, quality, radiation, severity, and time course.
– Because pain may correspond with disease progression, note temporal aspects of pain.
– Distinguish between visceral, somatic, and neuropathic pain.
– Somatic and neuropathic pain are common in the head and neck.
– Although less common, visceral pain can be referred from the heart, lungs, and diaphragm to the jaw and neck.
– Other types of referred pain include head and neck pain from invasion of the clivus. Referred otalgia may result from nerve stimulation/damage secondary to the following causes:
![]() CN V: Trigeminal neuralgia, oral cavity carcinoma.
CN V: Trigeminal neuralgia, oral cavity carcinoma.
![]() CN VII: Dental pain, TMJ dysfunction, parotitis, parotid tumors.
CN VII: Dental pain, TMJ dysfunction, parotitis, parotid tumors.
![]() CN IX: Pharyngitis, tonsillitis, oropharyngeal carcinoma.
CN IX: Pharyngitis, tonsillitis, oropharyngeal carcinoma.
![]() CN X: GERD, laryngopharyngeal carcinoma.
CN X: GERD, laryngopharyngeal carcinoma.
![]() C2 and C3: Neuropathy, metastatic invasion.
C2 and C3: Neuropathy, metastatic invasion.
![]() Bleeding: Patients with bleeding should be evaluated urgently to prevent hemodynamic compromise. Appropriate treatment depends on the underlying etiology.
Bleeding: Patients with bleeding should be evaluated urgently to prevent hemodynamic compromise. Appropriate treatment depends on the underlying etiology.
– Local causes of bleeding include ulceration, exposure of intratumoral vessels, ischemic/inflammatory insult to surrounding vessels, and direct tumor extension into major vessels (e.g., carotids), which may be life-threatening.
– Medications that increase risk of bleeding (e.g., NSAIDs and anticoagulants) should be identified and discontinued, if indicated.
![]() Airway/Breathing: Airway obstruction is the most imminent threat to ventilation and oxygenation. Obstruction of the nasal cavity, oral cavity, and/or oropharynx may produce dyspnea.
Airway/Breathing: Airway obstruction is the most imminent threat to ventilation and oxygenation. Obstruction of the nasal cavity, oral cavity, and/or oropharynx may produce dyspnea.
– Initial presentation of airway obstruction may be inability of patient to lie supine.
– Acute respiratory distress from airway obstruction should be assessed by physical exam: tachypnea, use of accessory muscles, and inspiratory stridor.
– Oxygen saturation is a poor indicator of acute airway obstruction severity, as saturation may be maintained by compensatory mechanisms in spite of impending clinical demise.
– If airway compromise is imminent, urgent intubation or tracheostomy should be performed in collaboration with anesthesia or ENT surgery until a more permanent solution is achieved.
– Whether to proceed with tracheostomy, debulking, palliative radiation, or observation will depend on degree of airway obstruction and expected potential for shrinkage in response to ongoing or future therapies.
![]() In patients with bulky disease in the midline neck (e.g., anaplastic thyroid cancer), tracheostomy may be more challenging as tumor may impede surgical access.
In patients with bulky disease in the midline neck (e.g., anaplastic thyroid cancer), tracheostomy may be more challenging as tumor may impede surgical access.
![]() Advance planning (i.e., referral to head and neck surgery and multidisciplinary discussion with the patient regarding goals of care) is necessary as emergency tracheostomy may be exceedingly difficult.
Advance planning (i.e., referral to head and neck surgery and multidisciplinary discussion with the patient regarding goals of care) is necessary as emergency tracheostomy may be exceedingly difficult.
![]() Speaking: Speech is produced by a complex series of active and static interactions among structures along the vocal tract.
Speaking: Speech is produced by a complex series of active and static interactions among structures along the vocal tract.
– Optimal articulation requires proper movement and/or alignment of the lips, teeth, tongue, hard palate, soft palate, uvula, epiglottis, and larynx, as well as the nasal cavity, oral cavity, pharynx, and laryngeal cavity.
– Both primary and metastatic cancers may impair speech by disrupting the anatomic relationships between these structures and their functions.
– CNs V, VII, IX, X, XI, and XII all carry efferent fibers involved in speech. Damage to these may produce speech-impairing deficits.
– Speech impairments should prompt timely referral to a speech-language pathologist, who may complete a comprehensive assessment of speech and aid in rehabilitation.
![]() Swallowing: Swallowing depends on complex neuromuscular coordination that enables the passage of solid or liquid from the oral cavity to the esophagus.
Swallowing: Swallowing depends on complex neuromuscular coordination that enables the passage of solid or liquid from the oral cavity to the esophagus.
– The process of swallowing can be divided into three phases, each of which with its own neurologic control.
![]() Oral phase (voluntary): Bolus entry -> oral containment -> salivary moistening -> mastication -> trough formation -> tongue elevation -> bolus propelled into pharynx.
Oral phase (voluntary): Bolus entry -> oral containment -> salivary moistening -> mastication -> trough formation -> tongue elevation -> bolus propelled into pharynx.
![]() Pharyngeal phase (involuntary): Nasopharyngeal closure -> pharyngeal constriction -> oropharyngeal closure -> laryngeal closure -> hyoid elevation -> pharyngeal peristalsis -> bolus propelled into esophagus.
Pharyngeal phase (involuntary): Nasopharyngeal closure -> pharyngeal constriction -> oropharyngeal closure -> laryngeal closure -> hyoid elevation -> pharyngeal peristalsis -> bolus propelled into esophagus.
![]() Esophageal phase (involuntary): Upper esophageal sphincter relaxation -> esophageal peristalsis (striated then smooth muscle) -> lower esophageal sphincter relaxation -> bolus propelled into stomach.
Esophageal phase (involuntary): Upper esophageal sphincter relaxation -> esophageal peristalsis (striated then smooth muscle) -> lower esophageal sphincter relaxation -> bolus propelled into stomach.
– If the mechanisms of swallowing are impaired, pulmonary aspiration may occur, leading to complications ranging from chemical pneumonitis to pneumonia.
– Pulmonary aspiration may be assessed with a bedside swallow test. A patient is asked to swallow a glass of water while oxygen saturation is measured. A desaturation of ≥2% in the minutes after swallowing may indicate silent aspiration.
– Deficiencies of cough, gag reflex, and motor speech functions also suggest an increased risk of aspiration.
– Videofluroscopic swallowing study enables more accurate diagnosis of swallowing dysfunction and may be used to rule out aspiration.
– Multidisciplinary care with involvement of a speech-language pathologist is helpful for diagnosis and treatment of dysphagia and aspiration.
![]() Nausea: While nausea may arise from a multitude of causes, identification of the underlying etiology is essential to guiding therapy, and is usually gleaned from a careful history.
Nausea: While nausea may arise from a multitude of causes, identification of the underlying etiology is essential to guiding therapy, and is usually gleaned from a careful history.
– Common iatrogenic causes include chemotherapy, radiotherapy, and initiation of opioids.
– Other causes include anxiety, severe pain, anticipatory nausea, CN VIII damage/vestibulopathy, constipation, bowel obstruction, brain metastases, uremia, encephalopathy, gastritis, gastric ulcer disease, and metabolic derangements (hyponatremia and hypercalcemia).
– Etiology may be confirmed based on responsiveness to selected treatment.
– Nonpharmacologic interventions, such as frequent small feedings of cold meals and avoidance of triggering stimuli, may be helpful for nausea from variety of causes.
– Pharmacologic/interventional treatments should address the underlying cause: for example, prochlorperazine or ondansetron for chemo-induced and opioid-induced nausea, meclizine for vestibulopathy, dexamethasone for brain metastases, PPI or H2 blockers for gastritis, metoclopramide for gastroparesis, lorazepam for anxiety, and surgery/stenting for bowel obstruction.
![]() Nutrition/Weight loss: Nutritional status may be compromised by dysphagia, nausea, dysgeusia, and loss of appetite, as well as the metabolic effects of cancer itself.
Nutrition/Weight loss: Nutritional status may be compromised by dysphagia, nausea, dysgeusia, and loss of appetite, as well as the metabolic effects of cancer itself.
– History should focus on uncovering specific causes of malnutrition/weight loss. However, in the majority of advanced cancer patients, anorexia occurs secondary to effects of cancer itself.
– Assess ability of patient to increase oral intake, and consult a dietician for careful monitoring of nutritional intake, which should be followed closely throughout treatment and recovery.
– Gastrostomy tube should be considered for patients with poor performance status, dysphagia, odynophagia, inadequate hydration, high risk of pulmonary aspiration, 5% weight loss over 1 month, or 10% weight loss over 6 months, if aligned with goals of care.
– Nasogastric tubes are extremely unpleasant for patients and should only be considered for short-term use under extenuating circumstances. Also, they may be difficult to place in a patient with anatomic abnormalities in the head and neck (either due to tumor or due to prior surgeries/radiation).
– If nasogastric tube is indicated in a patient with prior head and neck surgery, consult with surgeon prior to placement.
![]() Neurologic impairment: Numbness, tingling, weakness, and neuropathic pain may result from direct invasion or vascular compromise of nerves in the head and neck secondary to progression of cancer.
Neurologic impairment: Numbness, tingling, weakness, and neuropathic pain may result from direct invasion or vascular compromise of nerves in the head and neck secondary to progression of cancer.
– Focal neurologic deficits in the head and neck may manifest as facial paralysis, dysphagia, speech impairments, and diplopia.
– These deficits negatively impact quality of life and may serve as a great source of frustration to patients.
– Neuropathic pain can be especially challenging to treat.
![]() In many cases neuropathic pain is refractory to gabapentin and pregabalin.
In many cases neuropathic pain is refractory to gabapentin and pregabalin.
![]() Methadone may provide superior relief of neuropathic pain but ought to be prescribed cautiously or in conjunction with a palliative care specialist, as it carries a high risk of unintended overdose.
Methadone may provide superior relief of neuropathic pain but ought to be prescribed cautiously or in conjunction with a palliative care specialist, as it carries a high risk of unintended overdose.
• Physical exam: A focused head and neck exam may include:
![]() Head: Hair, skull, facial contours, salivary glands, skin.
Head: Hair, skull, facial contours, salivary glands, skin.
![]() Eyes: Vision, alignment, movements, pupils, light reflexes, fundi. Exophthalmos, diplopia, and ptosis may indicate the presence of metastases in the orbit or cavernous sinus.
Eyes: Vision, alignment, movements, pupils, light reflexes, fundi. Exophthalmos, diplopia, and ptosis may indicate the presence of metastases in the orbit or cavernous sinus.
![]() Ears: Auricles, canals, tympanic membranes, hearing. Dedicated hearing evaluation may be indicated.
Ears: Auricles, canals, tympanic membranes, hearing. Dedicated hearing evaluation may be indicated.
![]() Nose and Sinuses: Mucosa, septum, sinus tenderness.
Nose and Sinuses: Mucosa, septum, sinus tenderness.
![]() Oral Cavity and Pharynx: Lips, buccal mucosa, gingiva, teeth, retromolar trigone, hard and soft palate, tongue, tonsils, speech, swallow evaluation, assessment for trismus. Consideration should be given for a dental evaluation prior to any treatment in the head and neck region based on symptoms and life expectancy.
Oral Cavity and Pharynx: Lips, buccal mucosa, gingiva, teeth, retromolar trigone, hard and soft palate, tongue, tonsils, speech, swallow evaluation, assessment for trismus. Consideration should be given for a dental evaluation prior to any treatment in the head and neck region based on symptoms and life expectancy.
![]() Neck: Lymph nodes, trachea, thyroid, range-of-motion.
Neck: Lymph nodes, trachea, thyroid, range-of-motion.
• Fiberoptic nasolaryngoscopy, if indicated, enables better visualization of mucosal structures in the nasopharynx, oropharynx, larynx, and hypopharynx and extent of disease in these areas. It also allows for assessment of airway patency, and vocal cord function.
Symptoms
See Table 17.1.
Table 17.1
| Site | Symptoms | Acute Concerns |
| Oral cavity | Dysphagia, odynophagia, dysarthria, bleeding, trismus, tooth loss, infection | Airway compromise, aspiration risk |
| Larynx | Dyspnea, dysarthria, hoarseness | Airway compromise, aspiration risk, vocal cord dysfunction |
| Skin | Cosmetic changes, bleeding, ulceration | Infection, necrosis |
| Neck | Bleeding, dysphagia, dyspnea | Airway compromise, aspiration risk, vessel patency, hemorrhage |
| Neurologic | Cranial nerve palsy, weakness, numbness | Focal neurologic deficits, aspiration risk |
Goals of Care, Performance Status
• Goals of care should be determined by the patient and physician based on a realistic discussion of near- and long-term hopes and expectations.
• Although there is a lack of data regarding the prognostic implications of disease metastatic to the head and neck area, patients with recurrent and/or metastatic head and neck cancers generally have median survival less than 1 year, even with aggressive treatment [1].
• Poor performance status may complicate the logistics of simulation and treatment. Specifically, patients with tumors compressing the tracheal region may be unable to lie flat. Frequent monitoring or individualized radiation techniques and setup may be needed.
• If prognosis is limited and/or performance status is poor, weigh the quality of life benefits of forgoing radiotherapy against the therapeutic benefits of receiving it.
• Encourage patients for whom time is short to make time for personal and professional goals.
• Allow for delays in radiotherapy, if necessary, for patients to fulfill these goals.
Treatment Recommendation
Supportive Care
• Diligent supportive care of advanced cancer patients with disease in the head and neck region requires early recognition and treatment of nausea, nutritional deficiency, anemia, and pain.
• Feeding tubes or parenteral nutrition, if necessary, should be anticipated based on swallowing function, nutritional status, and goals of care.
• If there is concern for airway compromise, ENT specialists or otolaryngologists should be involved and potentially available for airway management, which may include tracheostomy tube placement.
• It is technically challenging and morbid to perform a tracheostomy in proximity to areas of gross disease.
• Referral to experienced head and neck providers and advanced planning, including a thorough discussion of the patient’s goals of care, are indicated.
• Effective treatment of pain should be of high priority. A significant proportion of cancer patients requiring analgesics in the outpatient setting are undertreated [2];
• Cancer patients likely have opioid preferences based on past experience: use this as a starting point.
• Set functional goals to guide pain management (e.g., “I want to be able to eat soft foods”).
• Treatment of neuropathic pain can be particularly challenging.
• For refractory moderate to severe neuropathic pain, consider methadone; however, prescribe with caution or in collaboration with palliative care specialist given complex pharmacokinetic profile and risk of unintended overdose.
Palliative Chemotherapy
• For patients with advanced, metastatic head and neck cancer, palliative chemotherapy alone is not curative. However, it may provide prolonged survival but with significant risk of associated toxicity.
• EXTREME trial (Vermorken NEJM 2008): 442 patients with untreated recurrent or metastatic SCC of the head and neck were randomized to either [1] cisplatin/carboplatin + 5-FU or [2] cisplatin/carboplatin + 5-FU and cetuximab (EGFR inhibitor).
![]() Patients receiving cetuximab showed improved median survival (10.1 months vs 7.4 months), PFS (5.6 months vs 3.3 months), and increased response rate (36% vs 20%).
Patients receiving cetuximab showed improved median survival (10.1 months vs 7.4 months), PFS (5.6 months vs 3.3 months), and increased response rate (36% vs 20%).
![]() However, toxicity was greater with cetuximab (26 vs 16 patients had adverse events): mostly anemia, neutropenia, and thrombocytopenia, but nine patients in the cetuximab arm had sepsis (vs 1 in the chemo-alone, p=0.02) [1].
However, toxicity was greater with cetuximab (26 vs 16 patients had adverse events): mostly anemia, neutropenia, and thrombocytopenia, but nine patients in the cetuximab arm had sepsis (vs 1 in the chemo-alone, p=0.02) [1].
• Investigations of novel targeted agents and immunotherapy for various metastatic cancers, including head and neck cancers, are ongoing, with promising early results.
• A variety of chemotherapy agents administered to patients with advanced cancers may contribute to symptoms in the head and neck, which may be exacerbated by radiotherapy and hence potentially dose-limiting.
• Mucositis is one of the most common side effects of chemotherapy, in general, and can be treated with frequent saline and analgesic rinses. Other potential treatments include antimicrobials, amifostine, benzydamine hydrochloride, l-glutamine, GM-CSF, and superoxide dismutase inhibitors.
• Other potential head and neck side effects of chemotherapy include dermatitis, xerostomia, tongue swelling/tenderness, dysgeusia, neuropathy, mucosal pain, odynophagia, increased propensity for infection/dental abscess.
• Patients are likely to benefit from regular dental care.
• EGFR-inhibitors are also used in a variety of cancers (head and neck, lung, breast, colorectal) and may cause alopecia, hypertrichosis, dry skin, dermatitis, and mucositis.
Radiotherapy for Aggressive Local Control or Potential Cure
• While most tumors arising in the head and neck region stem from primary tumors within the head and neck, other potential sources of disease include metastases from distant primaries (e.g., prostate, renal cell, bladder, lung, and breast), as well as various cutaneous malignancies.
• If a patient with distant metastases to the head and neck region has a low enough systemic disease burden to justify pursuit of aggressive local treatment (oligometastatic disease), then aggressive local treatment may be indicated.
Head and Neck Primaries
• The most common form of primary treatment failure in head and neck cancer is locoregional recurrence, occurring in 20–30% of patients [3,4].
• Patients with locoregional recurrence who lack distant metastases may still achieve long-term survival if aggressive treatment options are pursued, albeit with significant risks of toxicity.
• In a patient with locoregional recurrence who previously received radiation therapy (RT), the length of disease-free interval (e.g., approximately 6 months) before recurrence is thought to inversely correlate with radioresistance.
• If disease-free interval is <6 months, disease recurrence within the previously irradiated field may not benefit from re-irradiation with curative intent; however, data are limited, and this observation is likely not valid in cases of miss or marginal miss, which may be more common in the intensity modulated radiation therapy (IMRT) era [5].
• Locoregional recurrences in patients with good performance status and limited resectable disease should be treated with curative-intent surgical resection. Postoperative re-irradiation may be appropriate if risk of future recurrence is high [6].
• Unfortunately, most patients with local recurrence are medically or technically inoperable. In this setting, aggressive re-irradiation, potentially given in combination with concurrent systemic therapy, offers palliative benefit and a potential chance of cure, although this is debatable.
• Considerations prior to full-dose re-irradiation with or without chemotherapy include willingness of patient to tolerate potentially morbid treatment with significant mortality risk, degree of radioresistance (based on length of disease-free interval), and anticipated RT dose to critical structures based on pattern of recurrence (e.g., spinal cord, optic nerve, optic chiasm, and carotid arteries).
• Given the high morbidity associated with curative-intent re-irradiation, advanced treatment modalities with increased conformality, such as IMRT, may enable dose-escalation with less risk of severe complications [7–9].
• While concurrent chemotherapy with re-irradiation may address the issue of radioresistance among recurrent tumor cells, re-irradiation alone has conferred survival rates of 13–22% at 3 years [10,11].
• Attention to professional guidelines is indicated, [6] as is consideration for referral to a high volume head and neck center.
![]() Prescription dose and treatment volumes should be tailored to the individual patient and clinical scenario.
Prescription dose and treatment volumes should be tailored to the individual patient and clinical scenario.
– One potential option is to treat to a total dose of 60 Gy with concurrent chemotherapy (ideally different agents than those used during the first course of treatment).
– Treatment volumes should focus on gross disease or areas at highest risk of recurrence in the postoperative bed.
– Given the potential toxicity, elective nodal irradiation is not generally recommended.
![]() Even in the case of re-irradiation, an attempt should be made to spare normal structures as much as possible; however, priority should be given to limiting the dose administered to a previously irradiated spinal cord. Data are limited, but institutional experience has suggested there is likely some recovery of cord tolerance over time.
Even in the case of re-irradiation, an attempt should be made to spare normal structures as much as possible; however, priority should be given to limiting the dose administered to a previously irradiated spinal cord. Data are limited, but institutional experience has suggested there is likely some recovery of cord tolerance over time.
Important Trials
• RTOG 9911 (Langer J Clin Oncol 2007): 105 patients with local H&N SCC recurrence underwent chemo-re-irradiation.
![]() RT: 60 Gy/1.5 Gy fx b.i.d. q 2 weeks×4 cycles.
RT: 60 Gy/1.5 Gy fx b.i.d. q 2 weeks×4 cycles.
![]() Chemo: cisplatin 15 mg/m2 IV daily×5 and paclitaxel 20 mg/m2 IV daily×5 q 2 weeks×4 cycles. G-CSF was given days 6–13 of each 2-week cycle.
Chemo: cisplatin 15 mg/m2 IV daily×5 and paclitaxel 20 mg/m2 IV daily×5 q 2 weeks×4 cycles. G-CSF was given days 6–13 of each 2-week cycle.
![]() Median survival=12.1 months, estimated OS 1 year=50.2%, 2 year=25.9%.
Median survival=12.1 months, estimated OS 1 year=50.2%, 2 year=25.9%.
![]() 26% of patients did not complete chemotherapy, 28% had Grade ≥4 acute toxicity, 21% had Grade ≥4 acute hematologic toxicity, and eight treatment-related deaths occurred (8%): five acute, three late (including two carotid hemorrhages) [12].
26% of patients did not complete chemotherapy, 28% had Grade ≥4 acute toxicity, 21% had Grade ≥4 acute hematologic toxicity, and eight treatment-related deaths occurred (8%): five acute, three late (including two carotid hemorrhages) [12].
• RTOG 9610 (Spencer Head Neck 2008): 79 patients with local H&N SCC recurrence underwent chemo-re-irradiation.
![]() 60 Gy/1.5 Gy b.i.d. fx. Chemo: 4 weekly cycles of 5-FU 300 mg/m2 IV bolus and hydroxyurea 1.5 g PO.
60 Gy/1.5 Gy b.i.d. fx. Chemo: 4 weekly cycles of 5-FU 300 mg/m2 IV bolus and hydroxyurea 1.5 g PO.
![]() Median survival was better for patients with > 1 year disease-free interval prior to chemo-re-irradiation (9.8 months vs 5.8 months, p=0.036).
Median survival was better for patients with > 1 year disease-free interval prior to chemo-re-irradiation (9.8 months vs 5.8 months, p=0.036).
![]() Estimated OS rate for all patients were 2 year=15.2% and 5 year=3.8%.
Estimated OS rate for all patients were 2 year=15.2% and 5 year=3.8%.
![]() 25.3% of patients had acute Grade ≥4 toxicity, 19.4% had Grade 3, 3% had Grade 4 late toxicity, and six treatment-related deaths occurred (8%): two hemorrhage, four neutropenia [13].
25.3% of patients had acute Grade ≥4 toxicity, 19.4% had Grade 3, 3% had Grade 4 late toxicity, and six treatment-related deaths occurred (8%): two hemorrhage, four neutropenia [13].
Radiotherapy for Palliation
• For patients with advanced cancer in the head and neck region for whom aggressive treatment is either inappropriate or inconsistent with goals of care, RT with palliative intent may potentially alleviate symptoms and improve quality of life.
• Similar treatment principles apply for patients with advanced, metastatic cancer from a head and neck primary and those with metastatic spread from distant sites, such as GU, lung, and breast.
• For a symptomatic tumor in the head and neck region, RT is typically more likely to produce a local response than chemotherapy.
• Treatment volume for palliative RT
![]() Should target only gross disease with an individualized margin to account for uncertainty and setup error.
Should target only gross disease with an individualized margin to account for uncertainty and setup error.
![]() Focus on areas of tumor responsible for symptoms.
Focus on areas of tumor responsible for symptoms.
• More conformal techniques, such as IMRT, may be considered if durable palliation is desired, if there is concern for intolerable or unwanted treatment-related side effects, or potentially for protection of critical structures in the case of re-irradiation. The benefits of using IMRT or other advanced techniques should be balanced against the increased cost and the need to start treatment within a shorter time frame.
• Skin metastases may bleed or be otherwise symptomatic and thus may also benefit from palliative radiotherapy.
![]() Electrons may be preferable to photons for superficial lesions, depending on depth of invasion and surrounding anatomy.
Electrons may be preferable to photons for superficial lesions, depending on depth of invasion and surrounding anatomy.
![]() The use of a bolus enables the 100% isodose line to be approximated to the surface of the targeted lesion.
The use of a bolus enables the 100% isodose line to be approximated to the surface of the targeted lesion.
![]() If surface anatomy is complex, dose heterogeneity inherent to electron beam therapy may result in hot and cold spots. This can be addressed with a custom bolus.
If surface anatomy is complex, dose heterogeneity inherent to electron beam therapy may result in hot and cold spots. This can be addressed with a custom bolus.
Palliative Radiotherapy Regimens for Head and Neck Cancer
See Table 17.2.
• RTOG 85–02 QUAD SHOT regimen: 44.4 Gy in 3 cycles of 3.7 Gy fx b.i.d. for 2 consecutive days, with 2–3 week intervals in between [14].
![]() Several studies have demonstrated favorable rates of response and acceptably low toxicity profiles with QUAD SHOT [15,16].
Several studies have demonstrated favorable rates of response and acceptably low toxicity profiles with QUAD SHOT [15,16].
![]() MSKCC QUAD SHOT experience [17]: 44 patients completed at least one cycle of QUAD SHOT; 16 patients (36%) completed all 3 cycles. Palliative response (relief of presenting symptom or clinical/radiographic tumor response) was achieved in 75% of all patients, which was significantly associated with increased number of cycles received (p=0.01). Median OS=6.27 months (range 0.23–30.4 months). Grade 3 toxicity=5% (dermatitis and mucositis), Grade 2=30% (fatigue and mucositis) [17].
MSKCC QUAD SHOT experience [17]: 44 patients completed at least one cycle of QUAD SHOT; 16 patients (36%) completed all 3 cycles. Palliative response (relief of presenting symptom or clinical/radiographic tumor response) was achieved in 75% of all patients, which was significantly associated with increased number of cycles received (p=0.01). Median OS=6.27 months (range 0.23–30.4 months). Grade 3 toxicity=5% (dermatitis and mucositis), Grade 2=30% (fatigue and mucositis) [17].
• High-dose palliation may be used for patients with high performance status in whom longer-term local control may provide symptomatic benefit: 50 Gy/2.5 Gy fx or 50 Gy/3.125 Gy fx [18].
• Low-dose palliation is appropriate for patients with poorer performance status or more limited prognosis: 20 Gy in 5 fractions or 30 Gy in 10 fractions [19].
• Low-dose hypofractionated regimen: 30 Gy in 5 fractions at 2 per week, with at least 3 days apart (plus optional 6 Gy boost) [20].
• “0–7–21”: Patients with very limited performance status and prognosis may be treated with 24 Gy in 3 fractions, delivered on day 0, day 7, and day 21 [21].
• Single fraction: 8 Gy × 1 may be used for symptom control when other regimens are not feasible or indicated.
• Hypofractionated palliative RT [22]: 110 patients with unresectable HNSCC received 40 Gy/16 fx, with escalation to 50 Gy when tolerable. Response rate: complete=10%, complete and partial=73%. Percentage of symptoms relieved: <50%=26%, 50–75%=57%, and >75%=17%. Overall PFS at 12 months was 55.1% (95% CI 40.3–69.9%). Statistically significant correlates of PFS were weight >50 kg (p=0.049) and RT dose > 40 Gy (p=0.012). Toxicity was acceptable [22].
• IHF2SQ regimen for palliative chemoradiotherapy [23]: 78 patients with unresectable HNSCC treated with 2 fx of 3 Gy per day (day 1 and 3), during the 1st, 3rd, 5th, and 7th week of concurrent platinum-based chemo. Complete or partial response in 53%. Median OS=12.9 months, median PFS=10.3 months. Toxicity was acceptable [23].
• Comparison of fractionation schemes:
![]() Low toxicity of QUAD SHOT [24]: 66 patients with advanced head and neck cancer treated with palliative radiotherapy.
Low toxicity of QUAD SHOT [24]: 66 patients with advanced head and neck cancer treated with palliative radiotherapy.
![]() Palliative response rates: QUAD SHOT=83%, 70 Gy/35 fx=77%, 30 Gy/10 fx=67%, 37.5 Gy/15 fx=86%, 20 Gy/5 fx=60% (p=0.42).
Palliative response rates: QUAD SHOT=83%, 70 Gy/35 fx=77%, 30 Gy/10 fx=67%, 37.5 Gy/15 fx=86%, 20 Gy/5 fx=60% (p=0.42).
![]() Grade ≥3 toxicity: QUAD SHOT=9%, other regimens=37% (p=0.01) [24].
Grade ≥3 toxicity: QUAD SHOT=9%, other regimens=37% (p=0.01) [24].
![]() Potential benefit of dose escalation [25]: Retrospective review of 148 patients with head and neck cancer who underwent palliative RT. Median dose was 50 Gy (range 2–70); median fraction number was 20 (range 1–40). Median OS=5.2 months. Increasing radiation dose was significantly associated with improved OS (HR 0.97, 95% CI 0.96–0.99, p< 0.01) and treatment response (OR 1.05, 95% CI 1.01–1.08, p < 0.01) [25].
Potential benefit of dose escalation [25]: Retrospective review of 148 patients with head and neck cancer who underwent palliative RT. Median dose was 50 Gy (range 2–70); median fraction number was 20 (range 1–40). Median OS=5.2 months. Increasing radiation dose was significantly associated with improved OS (HR 0.97, 95% CI 0.96–0.99, p< 0.01) and treatment response (OR 1.05, 95% CI 1.01–1.08, p < 0.01) [25].
Table 17.2
Guide to Palliative RT Regimens for Head and Neck Cancer
| Regimen | Dose | Fractionation | Timing | Clinical Role |
| Quad Shot | 44 Gy in 3.7 Gy fx b.i.d. for 2 consecutive days | 3 cycles separated by 2–3 weeks interval | Untreated, incurable, inoperable disease; favorable balance of response/toxicity |
| High-dose palliation | 50 Gy in 2.5 Gy fx or 3.125 Gy fx | daily. Higher doses may also be considered in select cases (e.g., 55–60 Gy) | Good performance status; long-term control symptomatically beneficial |
| Low-dose palliation | 20 Gy in 4 Gy fx or 30 Gy in 3 Gy fx | daily | Poor performance status; limited prognosis |
| Low-dose hypofractionation | 30 Gy in 6 Gy fx, boost to 36 Gy as tolerated | 2 fx per week, at least 3 days apart | Poor performance status; limited prognosis; flexible treatment schedule |
| 0–7–21 | 24 Gy in 8 Gy fx | delivered days 0, 7, 21 | Very limited performance status and/or prognosis |
| Single fraction | 8 Gy × 1 | Other regimens not feasible or indicated |
| Hypofractionated palliation | 40 Gy in 2.5 Gy fx, escalation to 50 Gy as tolerated | daily | Untreated, incurable, inoperable disease |
| IHF2SQ for palliative chemorads | 48 Gy in 3 Gy fx b.i.d. on days 1 and 3 of weeks 1, 3, 5, and 7 of concurrent platinum-based chemo | Untreated, incurable, inoperable locally advanced disease; performance status too low for conventional chemorads |
Stereotactic Body Radiotherapy and Brachytherapy
• Stereotactic body radiotherapy (SBRT) may allow for hypofractionation and dose escalation, potentially improving rates of durable local control in patients with a longer life expectancy. SBRT also offers the practical benefit of reduced treatment time compared with conventional RT.
![]() Several small studies have shown that advanced stage head and neck cancer patients treated with palliative SBRT have favorable symptom control rates with relatively low rates of toxicity [26–28].
Several small studies have shown that advanced stage head and neck cancer patients treated with palliative SBRT have favorable symptom control rates with relatively low rates of toxicity [26–28].
– SBRT for head and neck cancer [29]: 44 patients with primary, recurrent, or metastatic head and neck cancer underwent either single-dose (n=18; 13–18 Gy) or fractionated RT (n=37; 36–48 Gy) to 55 malignant lesions.
![]() Overall complete and partial response rate=77%. Tumor control rates at 1 year: primary=83.3%, recurrent=60.6%. Median OS: primary=28.7 months, recurrent=6.7 months, metastatic=5.6 months.
Overall complete and partial response rate=77%. Tumor control rates at 1 year: primary=83.3%, recurrent=60.6%. Median OS: primary=28.7 months, recurrent=6.7 months, metastatic=5.6 months.
![]() Grade 3+ toxicity in 7 patients (16%) [29].
Grade 3+ toxicity in 7 patients (16%) [29].
– Phase II study of SBRT for re-irradiation with cetuximab [30]: 56 patients with recurrent head and neck cancer treated with SBRT to 36 Gy/6 fx with concomitant cetuximab (2 week treatment course).
![]() At 3 months, response rate=58.4%, disease control rate=91.7%; 1 year OS=47.5%.
At 3 months, response rate=58.4%, disease control rate=91.7%; 1 year OS=47.5%.
![]() Patients with Grade ≥3 toxicity=18 (32%). Skin toxicity: all grades=41 (73%), Grade ≥3=5 (9%). Treatment-related death=1 (hemorrhage) [30].
Patients with Grade ≥3 toxicity=18 (32%). Skin toxicity: all grades=41 (73%), Grade ≥3=5 (9%). Treatment-related death=1 (hemorrhage) [30].
![]() SBRT may be a reasonable alternative to curative intent fractionated RT for primary treatment of inoperable head and neck cancer in elderly patients and those with poor performance status. While data is limited, published control rates, survival, and toxicity are encouraging [31,32].
SBRT may be a reasonable alternative to curative intent fractionated RT for primary treatment of inoperable head and neck cancer in elderly patients and those with poor performance status. While data is limited, published control rates, survival, and toxicity are encouraging [31,32].
• Brachytherapy may also provide therapeutic advantages in both curative and palliative situations by enabling dose-escalation, normal tissue avoidance, and the practical benefit of shortened treatment times.
![]() Regimens vary widely across institutions, but high-dose-rate intraoperative techniques are most commonly utilized [33].
Regimens vary widely across institutions, but high-dose-rate intraoperative techniques are most commonly utilized [33].
![]() Modern studies of high-dose-rate brachytherapy for head and neck cancer recurrences report local control rates at 1–2 years approaching 70%, with Grade ≥3 toxicity occurring in <20% of patients [34,35].
Modern studies of high-dose-rate brachytherapy for head and neck cancer recurrences report local control rates at 1–2 years approaching 70%, with Grade ≥3 toxicity occurring in <20% of patients [34,35].
Expected Acute Side Effects From Radiation
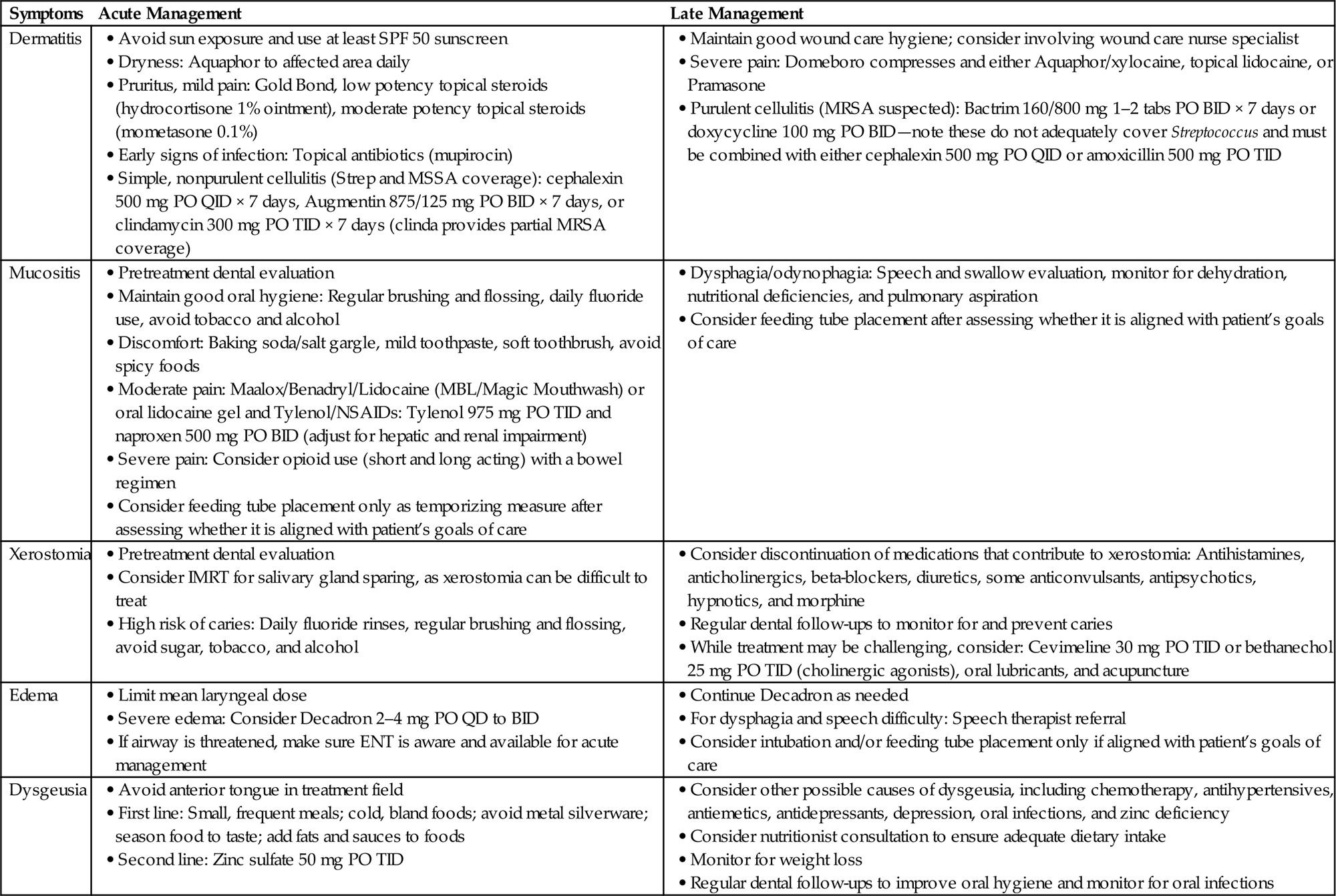
Dermatitis
• Radiation dermatitis occurs in the majority of patients who receive radiotherapy for advanced cancer in the head and neck region. Risk varies based on total RT dose, fraction size, treatment time, beam type and energy, and skin volume exposed [36].
![]() Grade 1: Faint erythema or dry desquamation.
Grade 1: Faint erythema or dry desquamation.
![]() Grade 2: Moderate to brisk erythema; patchy, most desquamation mostly confined to skinfolds; moderate edema.
Grade 2: Moderate to brisk erythema; patchy, most desquamation mostly confined to skinfolds; moderate edema.
![]() Grade 3: Moist desquamation other than skinfolds; bleeding with minor trauma/abrasion.
Grade 3: Moist desquamation other than skinfolds; bleeding with minor trauma/abrasion.
![]() Grade 4: Skin necrosis or ulceration of full thickness dermis; spontaneous bleeding.
Grade 4: Skin necrosis or ulceration of full thickness dermis; spontaneous bleeding.
• Skin changes are sharply demarcated, as they reflect the associated treatment field.
• Other risk factors include poor nutrition, preexisting dermatologic conditions, overlapping skinfolds, concurrent cetuximab, connective tissue diseases, HIV, diabetes, receipt of radiosensitive agents, and inherited disorders of DNA repair [36,37].
• Basic management strategies: Counseling about skin care, daily use of Aquaphor, and consideration of low to moderate dose topical steroids (e.g., mometasone 0.1%). Consider topical antibiotic (e.g., mupirocin) if evidence of infection.
Mucositis
• Radiation-induced mucositis is a common side effect of radiation to the head and neck that may potentially result in pain, dysphagia, dehydration, nutritional deficiencies, weight loss, feeding tube dependency, and pulmonary aspiration [38].
• Stomatitis (mucositis of the oral cavity) is of particular concern with chemoradiation, as various chemotherapy agents are independently associated with stomatitis, frequently in the anterior structures of the oral cavity.
• There is no reliable prophylaxis for radiation-induced mucositis; nonetheless, patients should be encouraged to maintain excellent oral hygiene and undergo dental evaluation prior to treatment.
• Aggressive use of analgesics may be required if pain is function limiting.
• In the event of dysphagia/odynophagia, speech and swallow evaluation and therapy may be indicated.
• Feeding tube placement should be considered only as a temporizing measure and must be aligned with patient’s goals of care.
• Basic management strategies: consider regular oral rinses with baking soda/salt solution. Mild to moderate pain: consider Maalox/Benadryl/Lidocaine (MBL/Magic Mouthwash), or application of oral lidocaine gel to specific areas of pain. Acetaminophen and NSAIDs can also be considered, although care should be taken in patients with liver or renal dysfunction or those receiving systemic chemotherapy. Moderate to severe pain: consider opioid use (both short- and long-acting), potentially in conjunction with a bowel regimen.
Xerostomia
• Xerostomia is caused by hyposalivation that results from irradiation of the salivary glands.
• Xerostomia may impair speech, chewing, and swallowing, cause pain and ulceration, and predispose to infection as well as rapidly progressing caries [39].
• IMRT and proton beam therapy may enable improved sparing of salivary glands, which may be worth pursuing in selected patients considering that management of xerostomia is challenging.
• Potential therapies include the cholinergic agonists cevimeline and bethanechol, acupuncture, and oral lubricants [39].
• Avoidance of sugar and good oral hygiene practices, with prophylactic daily fluoride rinses, may help prevent rapidly progressing caries.
• Medications that may contribute to xerostomia include antihistamines, anticholinergics, beta-blockers, diuretics, some anticonvulsants, antipsychotics, hypnotics, and morphine.
Edema
• Radiotherapy may cause lymphedema of the larynx and pharynx, leading to difficulty speaking and dysphagia.
• Laryngeal edema is often more pronounced in the case of re-irradiation.
• RTOG Scale for laryngeal edema (to be determined by flexible fiberoptic exam) [40]:
• To prevent laryngeal edema, mean laryngeal dose should be ≤43.5 Gy [41].
• If airway is threatened, make sure ENT surgery is aware and available for acute management.
Dysgeusia
• Temporary radiation-induced dysgeusia occurs as a result of damage to the taste buds and/or chorda tympani.
• Avoidance of the anterior tongue in the treatment field is an effective strategy for preserving taste, even if the base of the tongue is irradiated [42].
• Dysgeusia may have a large negative impact on quality of life, contributing to weight loss and nutritional deficiencies.
• Other causes of dysgeusia include chemotherapeutics, especially taxanes and vincristine.
• Consultation with a nutritionist may be required to ensure that patient is able to maintain adequate dietary intake.
On Treatment Management
Multidisciplinary
Steroids
Skin Care
• Moderate potency topical steroids, such has 0.1% mometasone, have been shown to be effective as prophylaxis against discomfort, burning, and itching associated with radiation dermatitis [43].
• Skin dryness can be alleviated by Aquaphor or other moisturizes.
• Pruritis can be treated with Gold Bond or 1% hydrocortisone ointment.
• Sun exposure to treatment site should be limited either by covering affected area or with SPF 50 or greater sunscreen.
• For moderate to severe radiation dermatitis, Domeboro compresses and Aquaphor/xylocaine, topical lidocaine, or Pramasone may be beneficial.
Oral Care
• Prior to receiving head and neck radiation, patients should undergo a dental evaluation to assess baseline oral health and determine the need for restorative work or extractions.
• Typical recommendations for maintaining good oral hygiene include: regular brushing, soft toothbrush, mild toothpaste, baking soda gargle, floss gently as tolerated, use a humidifier, reduce intake of spicy foods, and avoid intake of alcohol and use of tobacco products.
• Daily prophylactic fluoride use is also recommended, as patients are at increased risk for caries.
• Close dental follow-up is recommended, as patients are at high risk for dental complications (Table 17.3).
Table 17.3