Palliative Radiation Oncology for Gastrointestinal Tract Malignancies
P. Venkat, S.E. Hoffe and J.M. Frakes, H. Lee Moffitt Cancer Center and Research Institute, Tampa, FL, United States
Abstract
Gastrointestinal malignancies comprise of a diverse group of diseases with the majority of patients presenting with locally advanced or metastatic disease. This provides a challenge to physicians in adequately providing palliation and improving the quality of life of our patients, highlighting the importance of a multidisciplinary approach. In this chapter we will focus on the most common symptoms associated with each disease site to provide an overview of the literature and summary of treatment recommendations. Patients’ performance status and prognosis are major drivers in determining appropriate type as well as length of treatment. Lastly, we will focus on liver metastases to help guide treatment decisions from a heterogeneous group of diseases.
Keywords
Dysphagia; bleeding; obstruction; jaundice; pelvic pain; liver metastases
Introduction/Background
• Gastrointestinal (GI) tract malignancies include a diverse group of diseases including esophageal, gastric, pancreatic, hepatobiliary, small bowel, colon, rectal, and anal cancers.
• Unfortunately over 50% of these malignancies present at locally advanced or metastatic stages making palliative treatments an integral part of their management.
• Locally advanced disease within the GI tract can cause a variety of symptoms including, but not limited to, dysphagia, bleeding, obstruction, nausea/vomiting, malnutrition, dehydration, and pain [1].
• A multidisciplinary approach is required for optimal palliation.
• This chapter addresses the most common symptoms and their management with a focus on radiation therapy.
• We will conclude by exploring a unique topic within gastrointestinal metastatic disease: liver metastases.
Esophageal Cancer
• Despite advances in the multidisciplinary treatment of esophageal cancer, the 5-year overall survival rate remains low at approximately 15%, with over 50% of patients presenting with locally advanced or metastatic disease [2].
• Local progression of disease within the esophagus can cause severe morbidity including dysphagia, pain, and bleeding.
Dysphagia
• Malignant dysphagia is a complex medical problem with debilitating consequences for the patient.
• It can progress rapidly from difficulty swallowing solids to the inability to swallow liquids and saliva, placing the patient at risk for severe nutritional deficits and aspiration pneumonia.
• Evaluation must include swallow evaluation with consideration of feeding tube placement.
• Many treatment options exist for the management of malignant dysphagia with no clear consensus on optimal treatment paradigms as few studies have directly compared modalities with formal quality of life end points or measurements.
• Many times, in the setting of disseminated disease, systemic therapy can be initiated first with improvement of dysphagia.
• Beyond systemic therapy, options include surgical bypass or resection, external beam radiotherapy (EBRT) or brachytherapy (BT), endoscopic dilation, stenting, or endoluminal ablation [3].
• Given the limited prognosis and generally poor prognosis of these patients, surgical management is often deferred in favor of a less invasive approach.
• Most commonly utilized is stenting and/or radiation therapy [4].
• Endoscopic dilation is often used for immediate relief of symptoms, but on its own requires serial dilations with increased risk of esophageal perforation [5].
• Stenting has also been shown to provide rapid relief of dysphagia. However, the durability of this response is similarly limited due to stent migration and local tumor progression [6].
• Table 15.1 summarizes management recommendations of dysphagia by prognosis.
Table 15.1
Management of Dysphagia Summary
| Prognosis | Treatment Option |
| <3 month | Stenting +/− EBRT (20 Gy in 5 fractions or 30 Gy in 10 fractions) or BT (7–28 Gy in fractions of 5–7 Gy) |
| 3–6 months | EBRT (20 Gy in 5 fractions or 30 Gy in 10 fractions) +/− BT (8 Gy in 2 fractions) |
| >6 months | CRT (30–50 Gy) after initial systemic therapy with stable disease |
EBRT, External beam radiotherapy; BT, brachytherapy; CRT, chemoradiation.
Radiation Therapy
• Radiation therapy has the advantage of treating the cause of dysphagia, gross tumor, rather than just the symptom, resulting in a more durable response.
• Two randomized controlled studies have compared BT to stenting.
![]() Homs et al. compared high dose rate (HDR) BT with stent placement [7].
Homs et al. compared high dose rate (HDR) BT with stent placement [7].
– Between 1999 and 2002, 209 patients from nine hospitals in the Netherlands were randomized to either stent placement or single dose BT. Stents deployed were all self-expanding metal stents, and BT was delivered in a single fraction of 12 Gy.
– Relief of dysphagia occurred more rapidly after stent placement than after BT, but relief persisted longer in the BT group.
– There were also improved quality of life scores and fewer complications in favor of BT (21% vs 33%, p=0.02).
![]() Berquist et al. also compared HDR BT with self-expanding metal stent placement [8].
Berquist et al. also compared HDR BT with self-expanding metal stent placement [8].
– The BT regimen was 7 Gy in 3 fractions delivered over 2–4 weeks.
– A total of 65 patients were randomized.
– At 1 month, the patients who underwent stent placement had significantly higher quality of life scores for dysphagia (p=0.05), but most other quality of life scores declined.
– Dysphagia scores improved at 3 months in the BT group with little deterioration of other quality of life scores.
– Dysphagia recurrence was higher in the stent group.
– When immediate relief of dysphagia is indicated and prognosis is poor, stenting is the preferred approach as onset of relief is rapid.
![]() If prognosis is greater than 3 months, HDR BT is recommended.
If prognosis is greater than 3 months, HDR BT is recommended.
– Dose recommendations from the American Brachytherapy Society are 7–28 Gy in fractions of 5–7 Gy [9].
– Lower doses should be utilized in patients who have previously undergone EBRT to the esophagus for either definitive or palliative management.
• A recent multicenter randomized trial compared conventional metal stent placement with stents combined with low dose rate (LDR) BT [10].
![]() Zhu et al. randomized 160 patients to either a conventional metal stent or a stent loaded with Iodine-125 seeds.
Zhu et al. randomized 160 patients to either a conventional metal stent or a stent loaded with Iodine-125 seeds.
– Overall survival was improved in the BT group with a median overall survival of 177 days versus 147 days (p=0.0046).
– Side effects from treatment were similar between the two groups.
– This approach combines the rapid onset of relief provided by stent placement with the prolonged durability of response provided by BT with no increase in treatment length.
• EBRT alone also provides significant relief of dysphagia.
![]() Multiple retrospective studies have shown a significant palliation of dysphagia in 70–90% of patients undergoing EBRT alone [11–14].
Multiple retrospective studies have shown a significant palliation of dysphagia in 70–90% of patients undergoing EBRT alone [11–14].
![]() Palliative treatment regimens range from 30 Gy over 2 weeks to 50 Gy over 5 weeks.
Palliative treatment regimens range from 30 Gy over 2 weeks to 50 Gy over 5 weeks.
![]() Relief of dysphagia typically takes >2 weeks, so if severe, stenting +/− EBRT or BT is typically preferred.
Relief of dysphagia typically takes >2 weeks, so if severe, stenting +/− EBRT or BT is typically preferred.
![]() Choice of regimen should be based on patient goals, performance status, and preference.
Choice of regimen should be based on patient goals, performance status, and preference.
• EBRT can also be used in combination with stenting, BT, and chemotherapy.
![]() Javed at al. compared metal stenting alone versus stenting followed by EBRT [15].
Javed at al. compared metal stenting alone versus stenting followed by EBRT [15].
– A total of 84 patients were randomized to either metal stent placement alone or metal stent placement followed by 30 Gy in 10 daily fractions of EBRT.
– Dysphagia scores improved in both groups after stent placement, but the response was more durable in the radiation group (7 vs 3 months, p=0.002).
– Overall median survival was also significantly higher in the radiation group (180 vs 120 days, p=0.009).
– Of note, QOL scores improved after stenting and declined immediately following radiation.
– This reinforces the idea that if prognosis is <3 months, stenting alone is a reasonable option.
– However, if prognosis is >3 months, adjuvant EBRT should be strongly considered after stent placement.
– At our institution, we recommend multimodality management with stent placement followed by radiation therapy in patients with moderate to severe dysphagia and prognosis >3 months.
![]() Rosenblatt et al. performed a randomized trial comparing BT alone versus BT followed by EBRT [16].
Rosenblatt et al. performed a randomized trial comparing BT alone versus BT followed by EBRT [16].
– A total of 219 patients were randomized.
– All patients received BT consisting of 8 Gy in 2 fractions within 1 week.
– The EBRT group went on to receive 30 Gy in 10 daily fractions.
– Dysphagia relief was significantly improved with combined therapy, for an absolute benefit of 18% at 200 days from randomization (p=0.019).
– Furthermore, scores for dysphagia (p=0.00005), odynophagia (p=0.006), regurgitation (p=0.00005), chest pain (p=0.0038), and performance status (p=0.0015) were all significantly improved.
– However, weight, toxicities, and overall survival were not different between the study arms.
– Combination of BT and EBRT appears effective and well tolerated and should be considered in patients with reasonable performance status and prognosis approaching 6 months.
![]() A Phase I/II prospective trial from Canada evaluated the efficacy and toxicity of short course EBRT (30 Gy in 10 fractions) with concurrent single course of chemotherapy with 5-flurouracil (5-FU; 1000 mg/m2, days 1–4) and mitomycin-C (10 mg/m2, day 1) [17].
A Phase I/II prospective trial from Canada evaluated the efficacy and toxicity of short course EBRT (30 Gy in 10 fractions) with concurrent single course of chemotherapy with 5-flurouracil (5-FU; 1000 mg/m2, days 1–4) and mitomycin-C (10 mg/m2, day 1) [17].
– Twenty-two patients were enrolled and all completed therapy.
– A complete resolution of dysphagia was achieved in 68% of patients with a median time of complete response of 5 weeks.
– The median dysphagia free interval was 11 weeks, and 73% of these patients remained dysphagia free until death.
– However, 32% of patients had transient worsening of dysphagia secondary to treatment induced esophagitis immediately following therapy.
– Given the increased acute side effects with concurrent chemoradiation, this approach should be reserved for patients with a prognosis >6 months and a good performance status.
![]() At our institution, highly selected patients with a good performance status/prognosis with response to chemotherapy are treated with consolidative chemoradiation to 45–50 Gy over 5 weeks.
At our institution, highly selected patients with a good performance status/prognosis with response to chemotherapy are treated with consolidative chemoradiation to 45–50 Gy over 5 weeks.
Side Effects of Radiotherapy to the Esophagus
• Potential acute side effects of radiotherapy to the esophagus include fatigue, esophagitis/dysphagia, nausea, vomiting, anorexia, weight loss, and pneumonitis.
– Begin with over-the-counter analgesic medications.
– Consider topical anesthetics, such as compounded formulations with viscous lidocaine.
– Systemic opioids, via transdermal patches or liquid formulation, may be required if pain becomes severe.
![]() Nausea and vomiting should be managed aggressively with medications.
Nausea and vomiting should be managed aggressively with medications.
![]() If anorexia, weight loss approaching 5% of the patient’s body weight, dysphagia, or esophagitis are severe, a PEG tube should be considered for nutritional support.
If anorexia, weight loss approaching 5% of the patient’s body weight, dysphagia, or esophagitis are severe, a PEG tube should be considered for nutritional support.
– Treat with prednisone 50–60 mg for 1 week with an extended taper.
– Supplemental oxygen may be required.
– This is a rare, life-threatening event requiring emergent medical intervention.
– Signs and symptoms can include severe chest pain, tachycardia, hemorrhage, and hemodynamic instability.
• The most common late effect of esophageal radiation is stenosis and stricture often requiring serial endoscopic dilatation.
Gastric Cancer
• Cancer of the stomach is the fourth most common cancer and the third leading cause of cancer mortality worldwide [18].
• Local progression of gastric cancer may manifest with gastric outlet obstruction, pain, or bleeding.
• Palliative options include surgical bypass, endoscopic stenting, endoluminal ablation, or radiation therapy.
• Although, EBRT is used widely for palliation of gastric malignancies, little data exists in the literature regarding its efficacy and tolerance.
• Studies show a wide range of symptom relief with EBRT, ranging from 20% to 75% [19,20].
• Common dose fractionation regimens include: 8 Gy in 1 fraction, 20 Gy in 5 fractions, and 30 Gy in 10 fractions.
• Addition of concurrent chemotherapy can be considered in patients with a good performance status in order to obtain a greater and more durable palliation of symptoms.
• Kim et al. retrospectively evaluated symptom relief with radiation therapy in 37 patients with advanced gastric cancers [21].
![]() Nearly 66% of the patients received concurrent chemotherapy; the majority received single agent 5-fluorouracil.
Nearly 66% of the patients received concurrent chemotherapy; the majority received single agent 5-fluorouracil.
![]() A variety of radiation dose fractionation regimens were utilized with a median dose of 35 Gy in 14 fractions.
A variety of radiation dose fractionation regimens were utilized with a median dose of 35 Gy in 14 fractions.
![]() Palliation of bleeding, obstruction, and pain were seen in 70%, 81%, and 86% of patients, respectively.
Palliation of bleeding, obstruction, and pain were seen in 70%, 81%, and 86% of patients, respectively.
![]() Relief from bleeding and obstruction was sustained for a median duration of 11.4 and 6.2 months, respectively.
Relief from bleeding and obstruction was sustained for a median duration of 11.4 and 6.2 months, respectively.
![]() The median duration of pain control had not been reached at last follow-up.
The median duration of pain control had not been reached at last follow-up.
Side Effects of Radiotherapy to the Stomach
• Acute side effects from gastric radiation include fatigue, nausea, vomiting, gastritis/reflux symptoms, enteritis, anorexia, dehydration, or dysphagia.
• Management includes antiemetics, acid reflux medications, antidiarrheal medications, aggressive hydration, and medical pain management.
• If a feeding tube is required, a jejunostomy tube should be placed as opposed to a gastric tube.
Pancreatic Cancer
• Despite advances in multimodality therapy for adenocarcinoma of the pancreas, survival rates remain poor.
• Only 10–25% of patients present with resectable disease and among these patients, 5-year survival rates are as low as 10–20%, with median survival of 13–18 months [22].
• Median survival of patients with locally advanced, unresectable disease is approximately 8–14 months [23].
• Recently, in the metastatic setting, FOLFIRINOX (leucovorin, 5-fluorouracil, irinotecan, and oxaliplatin) has improved the median survival from 6.8 months with gemcitabine alone to 11.1 months [24].
Local Disease
• Despite the poor prognosis of this disease, patients can benefit from aggressive local therapy for palliation, preventing local progression of disease, subsequently limiting or delaying significant morbidity.
• Local progression of pancreatic cancer can result in severe back and abdominal pain, biliary obstruction, intestinal obstruction, anorexia, cachexia, and fatigue.
• A number of treatment paradigms exist for local control of pancreatic cancer, including supportive care, chemotherapy, EBRT, chemoradiation therapy, and stereotactic body radiation therapy (SBRT).
• Multiple prospective trials have compared various regimens with conflicting results and little consensus.
• Prospective randomized trials are summarized in Table 15.2.
• Given the lack of consensus, any of these approaches are reasonable and the decision should be dictated by patient preference and performance status.
• Further trials with quality of life end points are warranted given the poor prognosis of this disease.
• For patients with good performance we recommend more definitive local therapy with either fractionated concurrent chemoradiation or SBRT.
• A common fractionated regimen is 50.40 Gy in 1.8 Gy daily fractions with concurrent continuous infusion 5-FU.
• More recently SBRT is being utilized with the theory that ablative doses of radiotherapy may improve local control while minimizing acute side effects and duration of treatment, particularly important given the poor prognosis of these patients.
![]() Chang et al. report a retrospective analysis of 77 patients with unresectable adenocarcinoma of the pancreas treated with single fraction SBRT to a dose of 25 Gy [25].
Chang et al. report a retrospective analysis of 77 patients with unresectable adenocarcinoma of the pancreas treated with single fraction SBRT to a dose of 25 Gy [25].
– Local control was good with freedom from local progression at 6 months and 12 months of 91% and 84%, respectively.
– The progression free survival was poor due to metastatic progression; 26% at 6 months and 9% at 12 months.
– The overall survival rates at 6 months and 12 months were 56% and 21%, respectively.
– Acute toxicity was low with only 5% of patient experiencing a grade ≥2 toxicity.
– Late toxicity was significant, however, with 9% of patients experiencing a grade ≥3 toxicity.
– Furthermore, the rates at 6 months and 12 months of grade >2 toxicity were 11% and 25%, respectively.
• Due to the high late toxicity, other SBRT fractionation schemes have been explored.
![]() Moningi et al. report on their single institution experience with 25–33 Gy in 5 fractions for locally advanced pancreatic cancer (LAPC) and borderline resectable pancreatic cancer (BRPC) [26].
Moningi et al. report on their single institution experience with 25–33 Gy in 5 fractions for locally advanced pancreatic cancer (LAPC) and borderline resectable pancreatic cancer (BRPC) [26].
– A total of 88 patients were included, 74 of whom had LAPC.
– The majority of patients received chemotherapy prior to SBRT.
– Radiation targets and doses were modified to achieve strict dose constraints to adjacent structures.
– The PTV was edited to avoid overlap with duodenum, small bowel, and/or stomach, and PTV dose was reduced from 33 Gy if required to meet dose constraints.
– The median overall survival was 18.4 months, and median progression free survival was 9.8 months.
– Surgery was performed in 21.6 % of patients, with margin negative resections in 84%.
– Only three patients experienced acute grade ≥3 toxicity, and only five patients experienced late grade ≥2 toxicity.
– This study highlights the efficacy and safety of fractionated SBRT with strict dosimetric parameters.
• At Moffitt Cancer Center, we have implemented a similar institutional protocol for the treatment of borderline and LAPC.
![]() Mellon et al. reported on 159 patients treated on this protocol, 49 of whom had LAPC [27].
Mellon et al. reported on 159 patients treated on this protocol, 49 of whom had LAPC [27].
– Patients began with chemotherapy for 2–3 months with regimen at the discretion of the treating medical oncologist but the majority being gemcitabine-based.
– Patients then underwent SBRT in five consecutive daily fractions with mean total radiation doses of 30 Gy to tumor and up to 40 Gy to tumor-vessel interfaces with a dose painting technique.
– Restaging scans were performed 4–6 weeks after completion of radiotherapy for evaluation of resectability.
– Median overall survival for LAPC patients was 15.0 months.
– Five LAPC patients underwent R0 resections.
– For those who did not undergo resection, 1 year locoregional control was 78%.
– Only 7% of patients experienced a grade ≥3 toxicity.
• Recently, a multiinstitutional, Phase II, prospective trial was completed that confirmed the safety and feasibility of this approach of a five fraction SBRT regimen following gemcitabine-based chemotherapy [28].
![]() Herman et al. evaluated 49 patients with LAPC treated with up to three doses of neoadjuvant gemcitabine (1000 mg/m2) followed by SBRT to 33 Gy in 5 fractions and adjuvant gemcitabine until disease progression or toxicity.
Herman et al. evaluated 49 patients with LAPC treated with up to three doses of neoadjuvant gemcitabine (1000 mg/m2) followed by SBRT to 33 Gy in 5 fractions and adjuvant gemcitabine until disease progression or toxicity.
– Median overall survival was 13.9 months.
– Rate of acute and late grade ≥2 toxicity was 2% and 11%, respectively.
– Patients reported a significant improvement in pain 4 weeks following SBRT (p=0.001).
– Of note, this trial only included patients with a life expectancy >6 months and an Eastern Cooperative Oncology Group (ECOG) performance status of 0–1.
• These three studies highlight the feasibility, the safety, and the efficacy of fractionated SBRT in LAPC. However, this strategy should be reserved for patients with a good performance status and life expectancy >6 months.
• For patients with poor performance status, we recommend either supportive care alone or chemotherapy alone with radiation therapy reserved for palliation of symptoms.
Table 15.2
Prospective Randomized Trials (Phase III) for Locally Advanced Pancreatic Cancer
| Trial (Year Published) (References) | Randomization | Number of Patients | Median Survival (Months) | 1 Year Survival (%) |
| CRT vs Chemotherapy | ||||
| ECOG (1985) [29] | EBRT (40 Gy in 20 fractions) + 5-FU | 47 | 8.3 | 26 |
| 5-FU alone | 44 | 8.2 | 32 | |
| GITSG 9283 (1988) [30] | EBRT (54 Gy in 30 fractions) +5-FU and SMF | 22 | 9.7 | 41 |
| SMF alone | 21 | 7.4 | 19 | |
| FFCD/SFRO (2000-2005) [31] | EBRT (60 Gy in 30 fractions) + 5-FU/CDDP | 59 | 8.6 | 32 |
| Gemcitabine alone | 60 | 13 | 53 | |
| ECOG E4201 (2003–2005) [32] | EBRT (50.40 Gy in 28 fractions) + Gemcitabine | 34 | 11.1 | 50 |
| Gemcitabine alone | 37 | 9.2 | 32 | |
| CRT vs EBRT | ||||
| GITSG 9273 (1981) [33] | EBRT (60 Gy in 30 fractions, split course) alone | 25 | 5.3 | 10 |
| EBRT (40 Gy in 20 fractions, split course) + 5-FU | 83 | 9.7 | 35 | |
| EBRT (60 Gy in 30 fractions, split course) + 5-FU | 86 | 9.3 | 46 | |
| ECOG E8282 (2005) [34] | EBRT (59.4 Gy in 33 fractions) alone | 49 | 7.1 | NA |
| EBRT (59.4 Gy in 33 fractions) + 5-FU/MMC | 55 | 8.4 | NA | |
| CRT Regimens | ||||
| GITSG 9277 (1985) [35] | EBRT (60 Gy in 30 fractions, split course) + 5-FU | 73 | 8.5 | 33 |
| EBRT (40 Gy in 20 fractions) + doxorubicin | 70 | 7.6 | 27 | |
| Taipei (2003) [36] | EBRT (50.40–61.2 Gy in 28–34 fractions) + 5-FU | 16 | 6.7 | 31 |
| EBRT (50.4–61.2 Gy in 28–34 fractions) + Gemcitabine | 18 | 14.5 | 56 | |
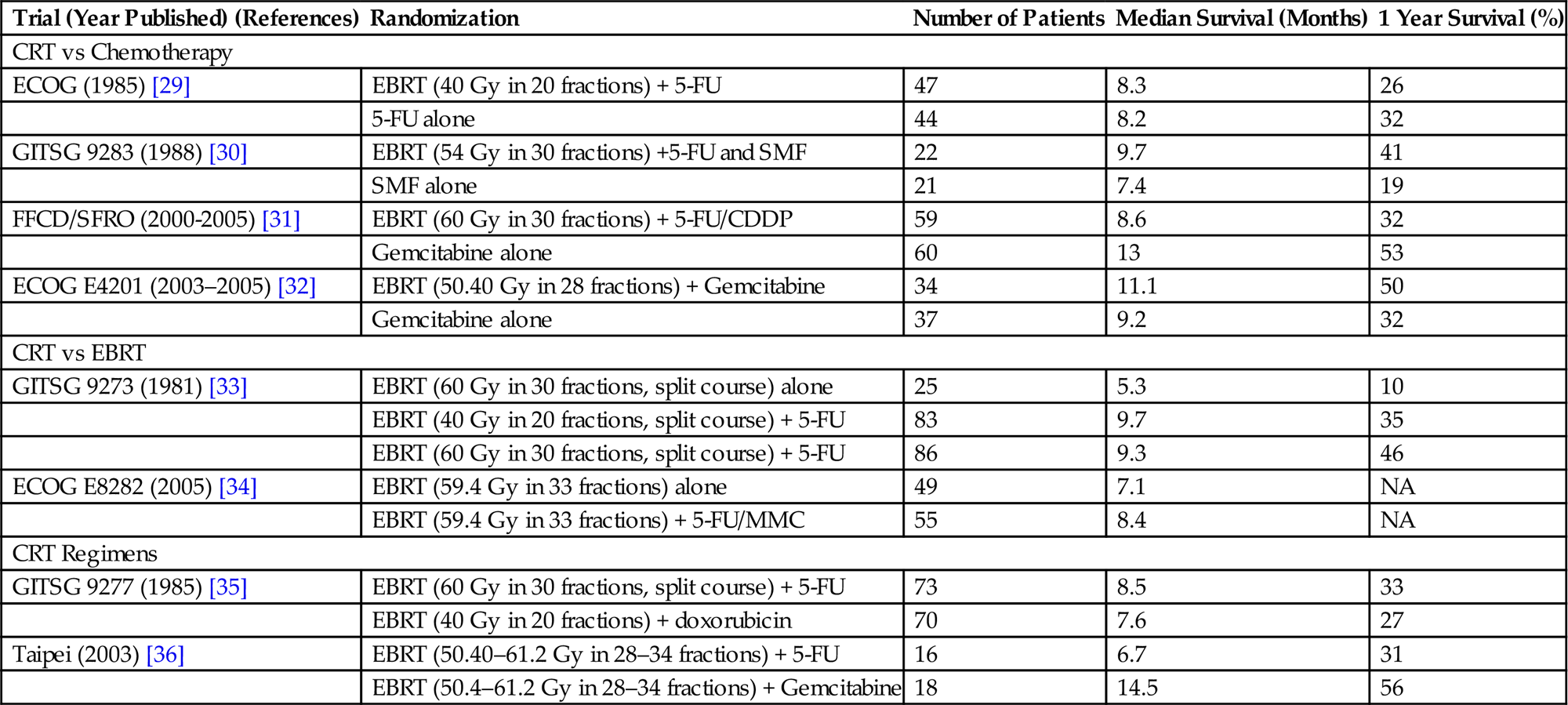
CRT, chemoradiation; ECOG, European Cooperative Oncology Group; EBRT, External beam radiotherapy; 5-FU, 5-flurouracil; GITSG, Gastrointestinal Tumor Study Group; SMF, streptozocin, mitomycin-C, and 5-flurouracil; FFCD/SFRO, Fèdèration Francophone de Cancèrologie Digestive/Sociètè Francophone de Radiothèrapie Oncologique; CDDP, cisplatin; MMC, mitomycin-C.
Symptom Palliation
• Approximately 70% of patients with pancreatic cancer will develop significant abdominal or back pain [37].
![]() The majority of these patients will be managed with systemic opioid analgesics, but approximately one-third of patients will not achieve adequate pain control with oral medications alone [38].
The majority of these patients will be managed with systemic opioid analgesics, but approximately one-third of patients will not achieve adequate pain control with oral medications alone [38].
![]() In these instances, celiac plexus neurolysis should be considered.
In these instances, celiac plexus neurolysis should be considered.
– Eisenberg et al. reported a metaanalysis of 1145 patients treated with celiac plexus neurolysis [39].
– They reported a 70–90% response with acceptable toxicity.
• Gastric outlet obstruction and biliary obstruction are common complications of locally destructive pancreatic cancer.
![]() Management of these clinical syndromes is primarily surgical and endoscopic.
Management of these clinical syndromes is primarily surgical and endoscopic.
![]() Radiation can play a role and will be discussed in the gastric and hepatobiliary sections, respectively.
Radiation can play a role and will be discussed in the gastric and hepatobiliary sections, respectively.
• Gastrointestinal bleeding is a rare, but life-threatening complication from local infiltration of pancreatic cancer, which can present as hematemesis, melena, or hematochezia.
![]() Local infiltration can involve the stomach, duodenum, or colon.
Local infiltration can involve the stomach, duodenum, or colon.
![]() Hemostatic methods for hemorrhage include endoscopic hemostasis, transcatheter arterial embolization, or surgery.
Hemostatic methods for hemorrhage include endoscopic hemostasis, transcatheter arterial embolization, or surgery.
![]() If endoscopic, interventional, and surgical methods fail, EBRT can be considered. Suggested regimens include 30 Gy in 10 fractions, 20 Gy in 5 fractions, or 12 Gy in 3 fractions. Choice of regimen should depend on patient’s clinical status, performance status, and disease burden with more extended courses reserved for patients with a better functional status and lower disease burden and shorter courses preferred for patients with a poor performance status and greater disease burden.
If endoscopic, interventional, and surgical methods fail, EBRT can be considered. Suggested regimens include 30 Gy in 10 fractions, 20 Gy in 5 fractions, or 12 Gy in 3 fractions. Choice of regimen should depend on patient’s clinical status, performance status, and disease burden with more extended courses reserved for patients with a better functional status and lower disease burden and shorter courses preferred for patients with a poor performance status and greater disease burden.
![]() Prognosis is extremely poor in these patients and supportive measures should be initiated.
Prognosis is extremely poor in these patients and supportive measures should be initiated.
Metastatic Disease
• Pancreatic cancer can metastasize to the liver, peritoneum, lung, brain, and bone.
• Radiation therapy can be effective for palliation of metastases to all of these sites.
• Radiation technique, dose, and fractionation should depend on the site of disease, prognosis, performance status, and patient preference.
• See the section “Liver Metastases” for further recommendations.
• For peritoneal metastases, we recommend 30 Gy in 10 fractions to the symptomatic site with bowel being the dose-limiting organ.
• For lung and brain metastases, consider stereotactic techniques if patient has a good performance status and relatively good prognosis. Refer to the corresponding chapters for specific recommendations (see Chapter 14, Site-Specific Symptom Management: Palliative Radiotherapy for Advanced and Metastatic Lung Cancer and Chapter 11, Palliative Radiotherapy for Brain Metastasis).
• For all uncomplicated bone metastases, we recommend 8 Gy in 1 fraction. See the chapter on bone metastases for further recommendations (see Chapter 13, Palliative Radiotherapy for Bone Metastasis).
Hepatobiliary Cancer
• Hepatocellular carcinoma (HCC) is the second leading cause of cancer mortality worldwide [40].
• Primary treatment is surgical resection when feasible.
• Unfortunately, the majority of patients present with unresectable disease secondary to the extent of disease or underlying liver dysfunction.
• Other potentially curative options include liver transplant and percutaneous ablation.
• Palliative treatment options include transarterial chemoembolization (TACE), Yittrium-90 (Y-90) radioembolization, EBRT, or systemic chemotherapy.
Y-90 Radioembolization
• Several retrospective and prospective studies have shown the efficacy and feasibility of Y-90 radioembolization in HCC.
![]() Retrospective studies comparing Y-90 to TACE show similar survival rates and safety profiles [41,42].
Retrospective studies comparing Y-90 to TACE show similar survival rates and safety profiles [41,42].
![]() Salem et al., in fact, reported a better response rate, a longer time to progression, and less toxicity in favor of radioembolization [41].
Salem et al., in fact, reported a better response rate, a longer time to progression, and less toxicity in favor of radioembolization [41].
![]() Y-90 dose ranges from 80 to 135 Gy and is generally well tolerated but can lead to acute fatigue, nausea, vomiting, anorexia, abdominal pain, fever, hepatobiliary toxicity, ulcers of the GI tract, radiation pneumonitis, or radiation-induced liver disease (RILD).
Y-90 dose ranges from 80 to 135 Gy and is generally well tolerated but can lead to acute fatigue, nausea, vomiting, anorexia, abdominal pain, fever, hepatobiliary toxicity, ulcers of the GI tract, radiation pneumonitis, or radiation-induced liver disease (RILD).
Stereotactic Body Radiation Therapy
• SBRT can also be utilized for the management of HCC.
![]() A Phase I/II study used a single fraction of radiotherapy with doses ranging from 14 to 26 Gy [43].
A Phase I/II study used a single fraction of radiotherapy with doses ranging from 14 to 26 Gy [43].
– The treatment was well tolerated with a 98% tumor control rate at 6 weeks.
![]() In a study by Weiner et al., 28 patients were enrolled on a single institution, prospective SBRT protocol [44].
In a study by Weiner et al., 28 patients were enrolled on a single institution, prospective SBRT protocol [44].
– The planned dose was 55 Gy, which was reduced to meet a mean liver dose constraint of <20 Gy or to spare 700 cc to <20 Gy.
– Fiducial markers, respiratory gating, and daily imaging were all used to decrease dose to uninvolved liver.
– Despite these efforts, two patients developed RILD and died.
• Although the risk of RILD is less after SBRT when compared to conventional fractionation regimens, RILD remains a real and devastating risk of SBRT to the liver, and patients must be counseled accordingly.
• Doses should be adjusted based on Child-Pugh score and typically range from 25 to 60 Gy in 3–6 fractions.
• Indeed, other investigators have confirmed the importance of personalizing the dose to the patient based on their underlying liver function and the individual patient’s tumor volume characteristics [45,46].
Biliary Obstruction
• Biliary obstruction is a common manifestation of locally advanced cholangiocarcinoma, adenocarcinoma of the gall bladder, and adenocarcinoma of the pancreatic head.
• Biliary obstruction can present with painless jaundice, pruritis, and clay colored stool.
• It is primarily managed by endoscopic procedures and stenting.
• Percutaneous biliary drainage and surgical management are also commonly utilized.
• When standard treatments fail, palliative radiotherapy can be considered.
Palliative Radiation Therapy for Biliary Obstruction or Pain
• For EBRT, typically doses of 30–50 Gy in 1.8–3 Gy fractions are utilized depending on performance status and prognosis.
• Dose-limiting structures include the duodenum, liver, stomach, and spinal cord.
• To enhance radiation response, intraluminal BT is often employed to increase dose to the gross tumor volume while sparing these dose-limiting organs.
• Either LDR or HDR BT can be utilized, and both are delivered through a percutaneous drainage catheter placed by an interventional radiologist.
• Common BT doses range from 20 Gy to 30 Gy delivered in 3–5 fractions.
• Concurrent chemotherapy, typically 5-fluorouracil, can also be added to enhance tumor response.
Anorectal Cancer
• Approximately 10–15% of patients present with locally advanced or unresectable rectal cancer [47].
• When deep invasion into local structures is present, adequate surgical resection may be extremely morbid or even impossible.
• In these instances, neoadjuvant therapy is recommended in order to allow for potentially curative resection.
![]() A Phase III randomized trial by Braendengen et al. established chemoradiotherapy (CRT) as standard of care over radiotherapy alone for locally advanced rectal cancer [48].
A Phase III randomized trial by Braendengen et al. established chemoradiotherapy (CRT) as standard of care over radiotherapy alone for locally advanced rectal cancer [48].
![]() Patients were randomized to either 50 Gy in 2 Gy per fraction alone or the same radiation regimen with concurrent 5-FU and adjuvant 5-FU for 16 weeks after surgery.
Patients were randomized to either 50 Gy in 2 Gy per fraction alone or the same radiation regimen with concurrent 5-FU and adjuvant 5-FU for 16 weeks after surgery.
![]() An R0 resection was achieved in 84% of the CRT group and 68% in the RT group (p=0.09).
An R0 resection was achieved in 84% of the CRT group and 68% in the RT group (p=0.09).
![]() Local control, time to treatment failure, cancer-specific survival all favored the CRT group.
Local control, time to treatment failure, cancer-specific survival all favored the CRT group.
![]() Acute toxicity was worse with the combined therapy with no difference in late toxicity.
Acute toxicity was worse with the combined therapy with no difference in late toxicity.
• Short course radiotherapy (25 Gy in 5 fractions) can be considered in patients with very limited metastatic disease prior to resection of both the primary and metastases.
• This regimen can also be utilized in patients with poor performance status, poor prognosis, or symptoms (pain/bleeding). If patients cannot tolerate five treatments, consider 8 Gy in a single fraction.
• Neoadjuvant CRT is preferred when significant downstaging of disease is required for complete surgical resection [49].
• For patients who cannot undergo surgical resection after neoadjuvant treatment, either due to extent of disease or due to medical comorbidities, higher radiation doses in excess of 60 Gy should be considered in order to maximize local control and palliation [50].
Recurrent Disease
• Recurrent disease in patients who have not had prior radiation therapy is approached similarly to unresectable or locally advanced disease with an aggressive multimodality approach.
• No standard regimen exists, but a number of studies have shown efficacy of a neoadjuvant approach with radiation or CRT followed by surgical resection with or without intraoperative radiotherapy (IORT) or adjuvant chemotherapy.
• Recurrent disease in a previously irradiated patient, presents a more challenging clinical scenario.
• Re-irradiation is possible, but puts patients at risk for significant toxicity.
![]() Mohiuddin et al. presented long-term results of re-irradiation in 103 patients [51].
Mohiuddin et al. presented long-term results of re-irradiation in 103 patients [51].
– Re-irradiation consisted of 30 Gy in 1.2 Gy BID fractions or 36 Gy in 1.8 Gy daily fractions followed by a boost to GTV to a dose of 6–20 Gy with concurrent continuous infusion 5-FU.
– Radiation was delivered with opposed laterals or a three-field technique to the presacral area and GTV.
– Late toxicity, consisting of severe, persistent diarrhea, small bowel obstruction, fistula formation, and stricture occurred in 22 (21%) patients.
– The authors report effective palliation of symptoms with 100% resolution of bleeding with control lasting till time of death in 80% of patients.
– Pain completely resolved in 55% of patients, and partially responded in 28% of patients with median duration of pain control of 9 months.
![]() Das et al. reported on 50 patients treated with a hyperfractionated regimen [52].
Das et al. reported on 50 patients treated with a hyperfractionated regimen [52].
– Patients received 30–39 Gy in 1.5 Gy BID fractions.
– The majority of patients received concurrent chemotherapy.
– The 3-year freedom from progression was 33% and 3-year overall survival was 39%.
– The 3-year rate of late grade 3–4 toxicity was 35%.
– Both regimens show significant late toxicity, although many of these toxicities (SBO, fistula, or stricture) may be due to tumor progression as opposed to radiation effect.
Metastatic Disease
• Most common sites of metastasis include liver, lung, brain, and bone.
• Palliative treatment recommendations should take into account site of the lesion, patient prognosis, performance status, and preference.
• See corresponding chapters in section 3 for detailed recommendations (Brain metastasis and primary CNS tumors, Spinal cord compression, and Bone Metastases).
• See Table 15.3 for a summary of anorectal treatment recommendations.
Table 15.3
Anorectal Cancer Treatment Recommendations
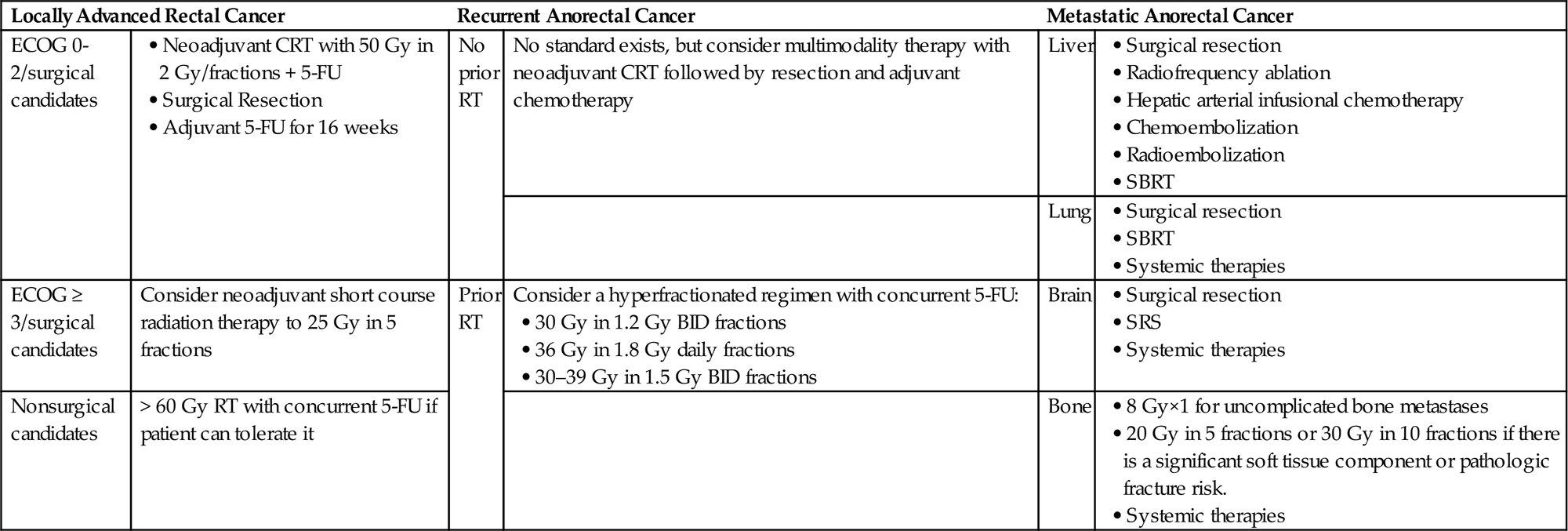
ECOG, Eastern Cooperative Oncology Group; CRT, chemoradiation; 5-FU, 5-fluorouracil; RT, radiation therapy; SBRT, stereotactic body radiation therapy; SRS, stereotactic radiosurgery; BID, twice daily at least 6 h apart.
Liver Metastases
• Liver metastases can originate from almost any primary site.
• Common malignancies to metastasize to the liver are colorectal, breast, and lung cancers.
• Liver metastases are often asymptomatic and are found on staging and follow-up imaging.
• However, they can also cause a variety of local and systemic symptoms including abdominal pain/distention, nausea, vomiting, anorexia, night sweats, or fevers.
• Prognosis is generally poor, but varies widely with primary site of disease, prior therapies, and extent of hepatic and extrahepatic disease.
• Treatment recommendations strongly depend on these same characteristics.
Surgical Resection
• In patients without extrahepatic disease, determination of whether the patient’s liver tumors are surgically resectable, either initially or following downstaging therapy, is key to determine whether there may be any future potential for curative intent treatment.
• Although criteria for surgical removal can vary between institutions and among surgical oncologists, the presence of adverse preoperative prognostic features has historically been cause for caution.
![]() For example, in a series of over 1000 consecutive cases of liver metastases from colorectal cancer, findings of the largest tumor >5 cm, more than one liver metastasis, a disease-free interval <12 months, a preoperative CEA >200 ng/mL, and the presence of a node positive primary tumor were found to be predictive when combined as a clinical risk score [53].
For example, in a series of over 1000 consecutive cases of liver metastases from colorectal cancer, findings of the largest tumor >5 cm, more than one liver metastasis, a disease-free interval <12 months, a preoperative CEA >200 ng/mL, and the presence of a node positive primary tumor were found to be predictive when combined as a clinical risk score [53].
![]() More recent data, however, have reported no survival difference between patients undergoing an R0 resection of 1–3, 4, 5–7, or 8–11 metastases [54].
More recent data, however, have reported no survival difference between patients undergoing an R0 resection of 1–3, 4, 5–7, or 8–11 metastases [54].
• Thus, patients with limited hepatic disease may benefit from a multidisciplinary initial consultation to determine whether, even if the disease is initially unresectable, an anatomic liver resection might be feasible with downstaging and enough future liver remnant function.
• Moreover, the presence of upfront poor prognostic factors does not necessarily preclude ultimate resection and long-term cure.
![]() In a recent report of 612 consecutive patients with metastatic colorectal cancer, 102 were 10 year survivors and of these, 7% had synchronous disease, 36% had a disease-free interval <12 months, 25% had bilobar liver metastases, 50% had a node positive primary cancer, 39% had >1 metastasis, and 35% had a tumor size >5 cm [55].
In a recent report of 612 consecutive patients with metastatic colorectal cancer, 102 were 10 year survivors and of these, 7% had synchronous disease, 36% had a disease-free interval <12 months, 25% had bilobar liver metastases, 50% had a node positive primary cancer, 39% had >1 metastasis, and 35% had a tumor size >5 cm [55].
• For initially unresectable liver metastases, a number of other treatment options in addition to the standard of histology specific systemic therapy are available, such as hepatic arterial infusional chemotherapy for colorectal cancers [56], local ablation therapies that can be delivered percutaneously in interventional radiology [57], chemoembolization strategies [58], radioembolization, and SBRT [59].
Y-90 Radioembolization
• Radioembolization with Y-90 can also be utilized in the metastatic setting for local control [60].
• Although Y-90 therapy is well tolerated without significant toxicity and is given in the outpatient setting, it is still unclear when patients might benefit the most from such liver directed therapy when systemic therapy is an option.
![]() The SIRFLOX randomized, multicenter trial compared Y-90 radioembolization combined with mFOLFOX6 to mFOLFOX6 alone in the first line setting [61].
The SIRFLOX randomized, multicenter trial compared Y-90 radioembolization combined with mFOLFOX6 to mFOLFOX6 alone in the first line setting [61].
– PFS was 20.5 months with the addition of Y-90 compared to 12.6 months.
– Despite this improvement, there was no significant difference in overall PFS or response rate (RR).
– There was, however, an improvement in hepatic RR, 78.7% versus 68.8%, p=0.042 without a significant increase in toxicity.
– This study suggests there may be benefit to combining therapies earlier in the disease course of mCRC liver metastases.
• Thus, for palliation of patients with significant hepatic disease burden, there may be an emerging role for radioembolization as an adjunct to systemic therapy.
Stereotactic Body Radiation Therapy
• With SBRT, investigators have reported on the high rates of local control and the low toxicity rates in studies that enrolled patients with liver metastases from multiple sites, typically 1–5 in number and <6 cm in size [62], with some investigators treating larger lesions [63].
• Recent data suggests excellent local control with regimens such as 60 Gy in 5 fractions [64] and a single dose of 35–40 Gy [65].
• Although the dosing parameters vary, most series confirm that with precision ablative radiotherapy, patients can benefit from this noninvasive modality that offers them convenience, absence of significant toxicity, and excellent hepatic control.
• Local control of liver metastases with SBRT ranges from 70% to 100% at 1 year and 60–90% at 2 years (Table 15.4).
• A recent survey showed that the majority of US practices surveyed had adopted SBRT, with over half incorporating liver SBRT into the practice [66].
Table 15.4
Prospective Trials (Phase I/II) of Stereotactic Body Radiation Therapy for Liver Metastases
| Series | Dose/Fraction # | # of Patient/# of Tumors | Outcomes | Toxicity |
| Herfarth et al. [67] | 14–26 Gy/1 (dose escalation trial) | 35/55 | No significant toxicity | |
| Schefter et al. [68] | 36–60 Gy/3 | 18/NR | 60 Gy in 3 fractions was found to be safe and is currently being tested in a Phase II study | No dose-limiting toxicity |
| Mendez-Romero et al. [69] | 30–37.5 Gy/3 | 25/17 | 2 patients with grade 3 liver toxicity | |
| Hoyer et al. [70] | 45 Gy/3 | 64/141 | One patient died due to hepatic failure. One patient required surgery for a colonic perforation. Two patients developed duodenal ulcers | |
| Rusthoven et al. [62] | 30–60 Gy/3 (dose escalation trial) | 47/49 Lesions assessable for LC; 63 for survival | One patient (2%) developed Grade ≥3 toxicity | |
| Lee et al. [63] | 27.7–60 Gy/3 | 68/NR | 10% Grade 3/4 acute toxicity | |
| Goodman et al. [71] | 18–30 Gy/1 (dose escalation trial) | 26/40 | No grade ≥3 toxicities | |
| Scorsetti et al. [72] | 75 Gy/3 | 42/52 | No grade ≥3 toxicities |
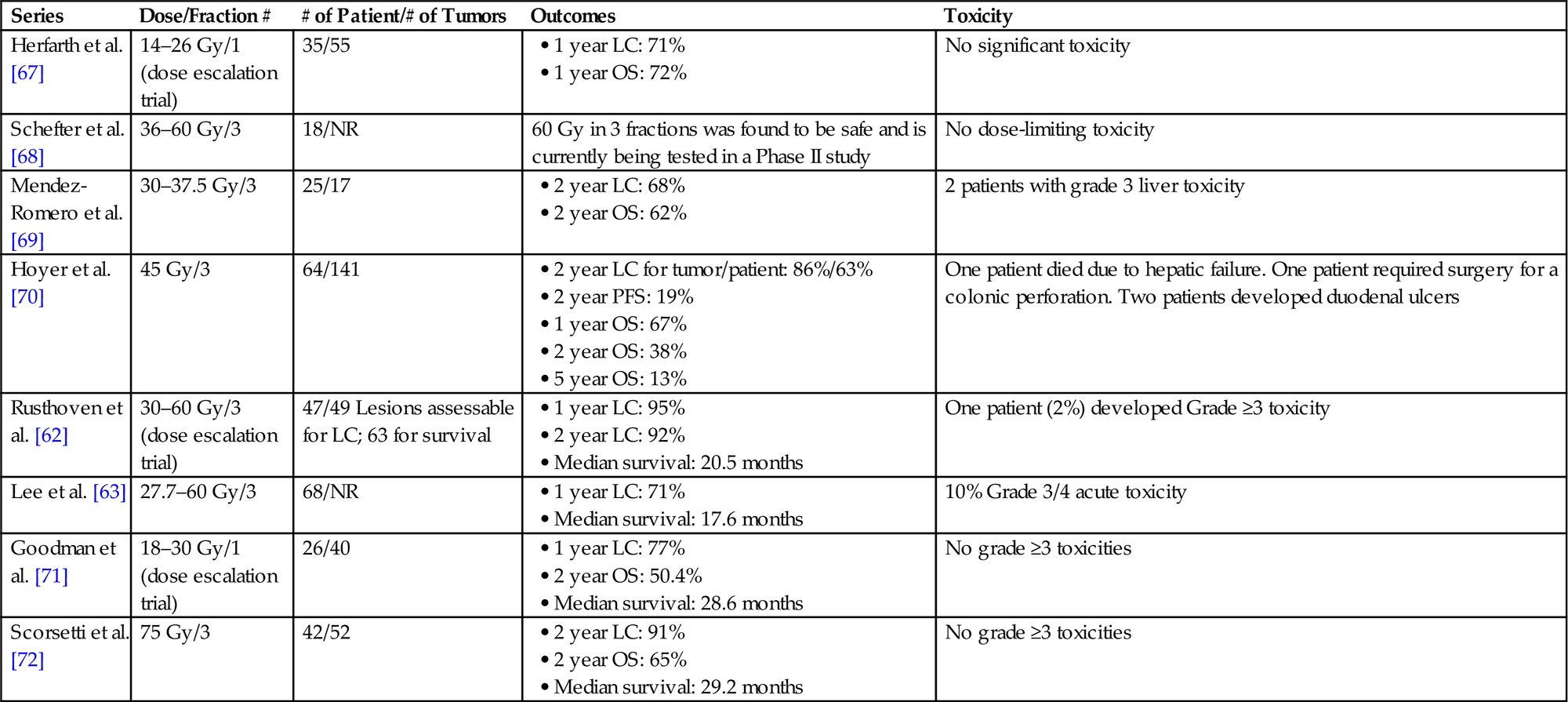
LC, local control; OS, overall survival; NR, not reported; PFS, progression free survival.
Central Biliary Obstruction
• Central biliary obstruction from metastatic tumor can be problematic, precluding the administration of systemic therapy.
• Another role for palliative radiotherapy is to offer external beam radiation in the hope of decreasing tumor burden enough to improve local biliary outflow so that patients can resume systemic therapy.
• This can be delivered with a short course of EBRT or with SBRT depending on extent of disease burden.
• For those patients with disease suitable for SBRT, recent data supports the efficacy and safety of this approach [73].
Whole Liver Irradiation
• Finally, Whole Liver Irradiation (WLI) with EBRT can be considered if the patient has pain from local extent of disease distending the liver capsule and is not a candidate for other modalities [74].
• Improvement in pain has been reported in up to 90% of patients.
On Treatment Management
• Given the anatomical extent of the gastrointestinal tract, radiation therapy for GI malignancies can lead to a variety of acute symptoms.
• Aggressive symptom management for radiation-induced side effects may be required (Table 15.5).
Table 15.5
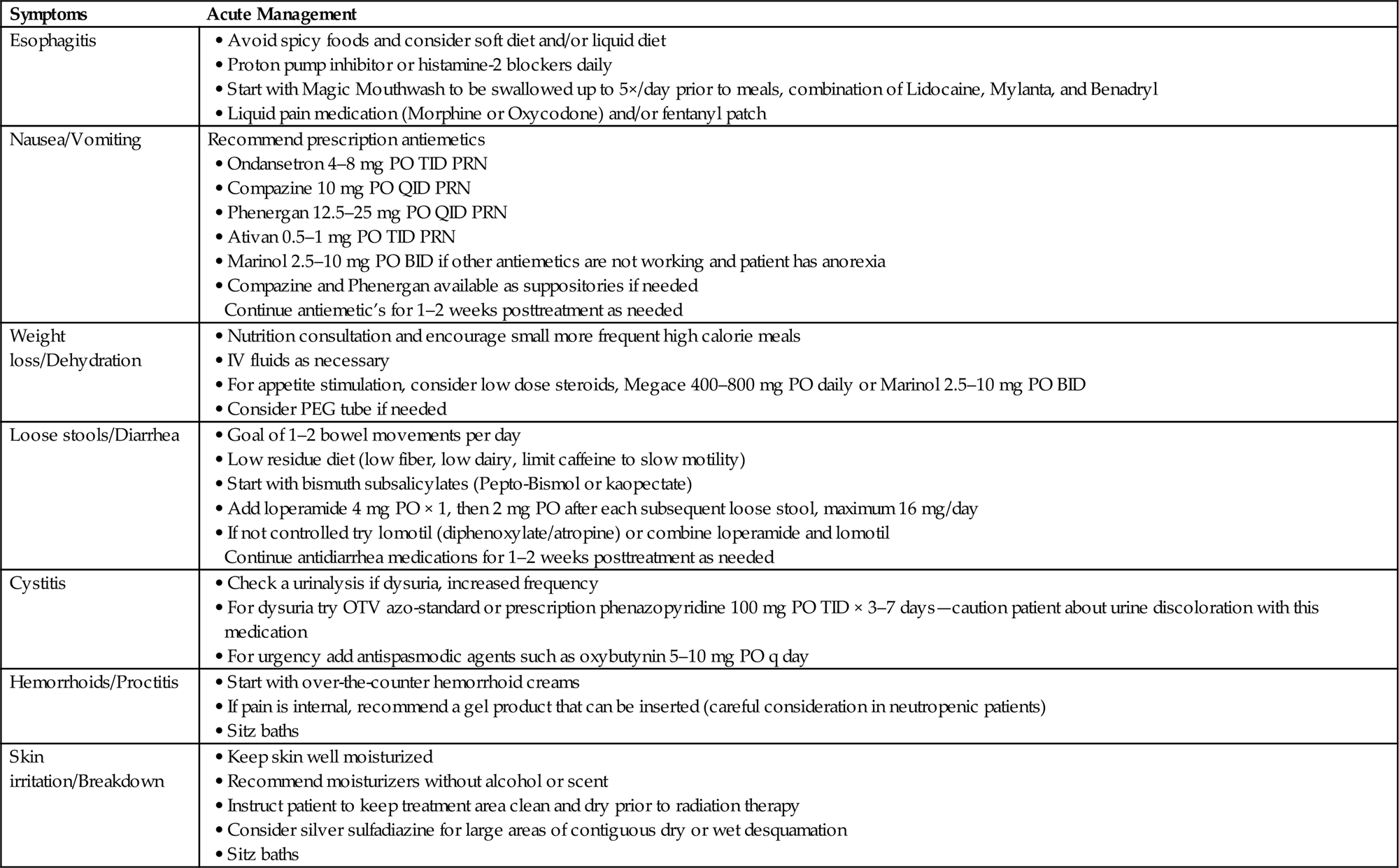
Conclusion
• Locally advanced, metastatic and recurrent gastrointestinal malignancies can cause significant morbidity.
• Palliative management of these diseases demands multidisciplinary management.
• Treatment recommendations should be tailored to the individual patient and guided by prognosis, performance status, and patient preference.
• Radiation therapy can provide significant relief of symptoms while decreasing tumor burden and providing local tumor control.
• Advances in radiation therapy will hopefully allow us to increase palliative effects while minimizing acute and late toxicities.
• Further studies are needed to compare different treatment regimens with regards to palliative outcomes and quality of life measurements.
List of Abbreviations
BRPC borderline resectable pancreatic cancer
EBRT external beam radiotherapy
IORT intraoperative radiotherapy
LAPC locally advanced pancreatic cancer
RILD radiation-induced liver disease
SBRT stereotactic body radiation therapy
