Palliative Radiotherapy for Malignant Epidural Spinal Cord Compression
H.-H.M. Yu1, E. Maranzano2 and D. Rades3, 1H. Lee Moffitt Cancer Center and Research Institute, Tampa, FL, United States, 2Santa Maria Hospital, Terni, Italy, 3University of Lubeck; University Hospital Schleswig-Holstein, Luebeck, Germany
Abstract
Metastatic epidural spinal cord compression (MESCC) requires urgent multidisciplinary evaluation and intervention in order to maintain or restore neurological functions. Radiotherapy is the most common treatment modality and can effectively palliate pain and maintain neurological functions. Surgical decompression may be appropriate for selected patients with limited metastasis and good prognosis. Mechanical stability of the spine needs to be evaluated concurrently and may require surgery. Pretreatment ambulatory status is a major prognostic factor for ambulatory function after treatment as well as overall survival.
Keywords
Spinal cord compression; radiation therapy; decompressive surgery; mechanical instability; re-irradiation; prognostic factors
Introduction
Metastatic epidural spinal cord compression (MESCC) is an oncological emergency and needs to be diagnosed early and treated promptly. Patients with MESCC often experience debilitating pain and/or neurological impairment; these symptoms can significantly impact their functional independence and quality of life. Therefore, the primary goal of intervention is to maximize quality of life by relief of pain and preservation/restoration of neurological function as well as to maintain mechanical stabilization of the spine.
Approximately 5–10% of patients with cancer develop malignant spinal cord compression during the course of their disease [1]. The most common location is the thoracic spine (59–78%), followed by the lumbar spine (16–33%), and the cervical spine (4–13%) [2]. The most common histologies include lung, prostate, and breast cancers, each accounting for about 20% of patients with MESCC. Median survival in patients with MESCC is generally short and varies depending on the type of primary tumor. Median survival is about 4–6 months for patients with lung cancer, whereas for patients with breast cancer and multiple myeloma the median survival can be up to 18 months [1,2].
Definition
Definition of epidural spinal cord compression varies in the literature. Some authors suggested that any radiographic evidence of thecal sac indentation should be considered epidural spinal cord compression (ESCC), whereas other authors differentiate between (symptomatic) ESCC and pending ESCC [3,4]. Recently, ESCC grading scale, an MRI-based grading system, was proposed by the Spinal Oncology Study Group [5]. Using the axial T2-weighted images at the site of most severe compression, the degree of ESCC is determined (See Table 12.1).
Table 12.1
Epidural Spinal Cord Compression (ESCC) Grading System
Grade 0: Bone-only disease
Grade 1: Epidural impingement
1a: Epidural impingement without deforming the thecal sac
1b: Thecal sac deformation without spinal cord abutment
1c: Thecal sac deformation, cord abutment, no cord compression
Grade 2: Moderate-grade spinal cord compression with visible CSF around the cord
Grade 3: High-grade spinal cord compression without visible CSF around the cord
This grading system is a guideline from expert consensus but has not been robustly validated. Nevertheless, it may help guide treatment decision-making.
Presentation
Common presenting symptoms include pain and myelopathy or neurological impairment [6–9]:
Pain
• The most common presenting symptom, occurring in approximately 80–95% of patients. Unexplained back pain in cancer patients warrants immediate evaluation.
• Typically precedes neurological deficits.
• Local pain is thought to be due to periosteal stretching from tumor growth and/or local inflammatory process. It often responds to steroids.
• Radicular pain indicates neuroforaminal pathology due to nerve root compression or irritation and tends to be constant with dermatomal distribution of the involved nerve root.
• Mechanical pain is movement-related pain. It is indicative of bone etiology often caused by vertebral body collapse or compression as a result of instability. It is often worse with movement and is often refractory to steroids or narcotics.
Myelopathy/Neurological Impairment
• The presence of neurological impairment is indicative of high-grade ESCC.
• Motor weakness is the most common neurological symptom (indicative of impaired corticospinal tracks) [7–9].
• Approximately 60–85% present with motor weakness at the time of diagnosis [3].
• Sensory impairment (paresthesias, loss of sensation) often accompanies motor impairment (pinprick evaluates spinothalamic tracks; proprioception evaluates posterior columns).
• Sphincter control/continence and autonomic impairment such as urinary retention and hesitancy are often a late finding. Urinary retention is the most common and often correlates with the degree of motor deficit [10].
• Functional assessment can be objectively graded using Frankel classification [11].
| Frankel Classification | |
| Grade A | Paraplegia |
| Grade B | Sensory function only, complete paralysis |
| Grade C | Not ambulatory but has some motor function below compression level |
| Grade D | Ambulatory with some motor function below compression level |
| Grade E | No neurologic symptoms |

• The cause of neurological impairment is thought to be commonly due to edema caused by increased vascular permeability when the epidural venous plexus is compressed by an epidural metastasis.
• Direct compression of the spinal cord by an expanding epidural mass can also lead to spinal cord ischemia and subsequent neurological injury.
• Bone fragments due to posterior displacement of fractured bone metastasis may also result in direct compression on the spinal cord [7].
Prognostic Factors
• The neurological status at the time of diagnosis (pretreatment neurological status), especially motor function and ambulatory status, correlates with the prognosis of MESCC.
• Multiple studies have shown that pretreatment ambulatory function is the most important prognostic factor for ambulatory ability after treatment [12,13].
• Rate of motor deficit development due to compression is prognostic for functional outcomes.
![]() More rapid progression of motor weakness prior to intervention predicts a worse outcome [13]. Slower progression predicts a better outcome.
More rapid progression of motor weakness prior to intervention predicts a worse outcome [13]. Slower progression predicts a better outcome.
• Visceral metastases and short-course radiotherapy are associated with inferior local control after radiotherapy [14].
• Longer survival after radiotherapy was associated with absence of visceral metastases, no other bone metastasis, being ambulatory before radiotherapy, longer time interval between diagnosis and cord compression (≤15 vs >15 months), longer duration of developing motor deficits before radiotherapy (1–14 vs >14 days) and breast/prostate/myeloma/lymphoma [14].
• Using these prognostic factors, a prognostic score to predict overall survival has been developed and validated (Table 12.2) [15,16].
• A score that predicts postradiotherapy ambulatory status has also been developed and validated [17,18].
![]() Six prognostic factors were included: tumor type, interval between cancer diagnosis and spinal cord compression, presence of other bone or visceral metastases at the time of radiotherapy, pretreatment ambulatory status, and duration of motor deficits (Table 12.3).
Six prognostic factors were included: tumor type, interval between cancer diagnosis and spinal cord compression, presence of other bone or visceral metastases at the time of radiotherapy, pretreatment ambulatory status, and duration of motor deficits (Table 12.3).
Table 12.2
Prognostic Score to Predict Overall Survival After Radiotherapy for MESCC
| Prognostic Factor | Description | Score |
| Cancer type | Breast cancer | 8 |
| Prostate cancer | 7 | |
| Myeloma/lymphoma | 9 | |
| Lung cancer | 3 | |
| Other | 4 | |
| Interval between cancer diagnosis and cord compression | <15 months | 4 |
| >15 months | 7 | |
| Other bone metastasis | Yes | 5 |
| No | 7 | |
| Visceral metastasis | Yes | 2 |
| No | 8 | |
| Motor function prior to radiotherapy | Ambulatory | 7 |
| Not ambulatory | 3 | |
| Time developing motor deficits prior to radiotherapy | 1–7 days | 3 |
| 8–14 days | 6 | |
| >14 days | 8 |
| Group | Sum Score | Median Survival | 6-Month Overall Survival Rate |
| I | 20–30 | 2.5 months | 16% |
| II | 31–35 | 7.5 months | 48% |
| III | 36–45 | 25 months | 81% |
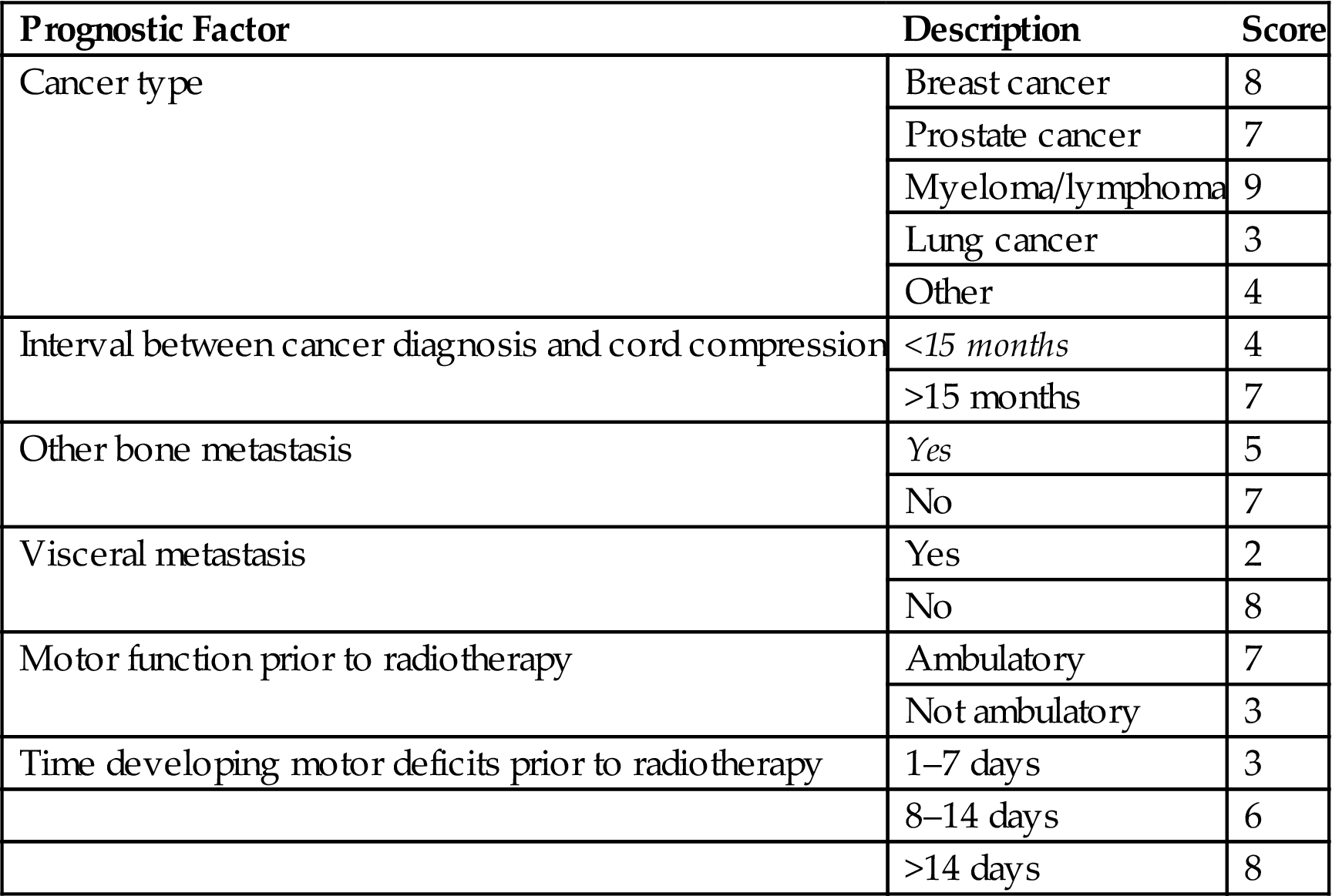

Table 12.3
Prognostic Score to Predict Postradiotherapy Ambulatory Status for MESCC
| Prognostic Factors | Description | Score |
| Cancer type | Breast cancer | 8 |
| Prostate cancer | 7 | |
| Myeloma/lymphoma | 9 | |
| Nonsmall cell lung cancer | 5 | |
| Small cell lung cancer | 6 | |
| Cancer of unknown primary | 5 | |
| Renal cell cancer | 6 | |
| Colorectal cancer | 6 | |
| Other | 6 | |
| Interval from cancer diagnosis to cord compression | ≤15 months | 6 |
| >15 months | 8 | |
| Visceral metastasis | Yes | 5 |
| No | 8 | |
| Motor function prior to radiotherapy | Ambulatory without aid | 10 |
| Ambulatory with aid | 9 | |
| Not ambulatory | 3 | |
| Paraplegic | 1 | |
| Time developing motor deficits prior to radiotherapy | 1–7 days | 4 |
| 8–14 days | 7 | |
| >14 days | 9 |
| Group | Sum Score | Postradiotherapy Ambulatory Rate |
| I | 21–28 | 10.6% |
| II | 29–37 | 70.9% |
| III | 38–44 | 98.5% |

Evaluation
History
• A comprehensive history should include characteristics of pain, presence, extent and duration of neurological symptoms, performance status, extent/status of extraspinal metastatic cancer, and GI/GU functions (including urinary retention and continence).
![]() Include any motor, sensory, or autonomic (bladder/bowel) dysfunction.
Include any motor, sensory, or autonomic (bladder/bowel) dysfunction.
![]() Details of onset (acute vs gradual), time since onset, severity, and extent of the neurological symptoms.
Details of onset (acute vs gradual), time since onset, severity, and extent of the neurological symptoms.
• Detailed history of the pain characteristics may elicit whether pain is due to local/axial, radiculopathic, or mechanical causes.
Physical Examination
Imaging
• MRI of the spine with and without gadolinium is the gold standard imaging modality for evaluation and should be performed immediately when metastatic spinal cord compression is suspected.
• MRI determines the location, extent of compression, single versus multiple lesions, and other epidural/cord compression sites. It also differentiates between metastasis and spondylodiscitis [19].
• The entire spine should be imaged because epidural compression may occur at multiple levels.
• To visualize the site of epidural compression:
![]() Sagittal T1-weighted images with and without contrast and T2-weighted images.
Sagittal T1-weighted images with and without contrast and T2-weighted images.
• To confirm the degree of compression:
![]() Axial T1-weighted images with and without contrast and T2-weighted images at the regions of interest based on sagittal imaging finding.
Axial T1-weighted images with and without contrast and T2-weighted images at the regions of interest based on sagittal imaging finding.
• Compression of the thecal sac and structures within the dura including the spinal cord and cauda equina by an extradural mass is diagnostic.
![]() Direct tumor extension from bone metastasis into the epidural space is the most common.
Direct tumor extension from bone metastasis into the epidural space is the most common.
![]() Bone fragment due to vertebral metastasis into the spinal column is another cause.
Bone fragment due to vertebral metastasis into the spinal column is another cause.
• Spinal CT defines bony anatomy and the extent of bone involvement and is especially useful for evaluation of compression fracture, mechanical instability, and posterior bone fragment protrusion.
• Occasionally, biopsy or surgical decompression is considered when pathological diagnosis is needed in patients without known history of cancer diagnosis or if there is no evidence of other metastasis.
Management
Urgent consultation with both Neurosurgery and Radiation Oncology and multidisciplinary approach involving radiologists, neurosurgeons, radiation oncologists, medical oncologists, and neurologists provides the best assessment for intervention. Risk and benefit profiles of specific interventions are based on patient’s neurologic status, performance status, extent of epidural disease, stability of the spine, status of extraspinal metastasis, and life expectancy. Treatment decision is often individualized based on these factors.
Dexamethasone
• All patients with suspected MESCC should receive prompt administration of dexamethasone when MESCC is suspected.
• A randomized trial by Sorensen et al. investigated patients treated with radiotherapy alone for spinal cord compression who were randomized to high-dose dexamethasone 96 mg/day vs. none; 81% of the patients who received dexamethasone were ambulatory, compared to 63% in the control arm, demonstrating the benefit of high-dose steroids [20].
• No consensus data on optimal dosing schedules of dexamethasone.
• High-dose dexamethasone is associated with severe gastrointestinal toxicity including bowel perforation, ulcer, and bleeding.
![]() Sorensen et al. reported 11% of patients had serious GI side effects including perforation [20].
Sorensen et al. reported 11% of patients had serious GI side effects including perforation [20].
• A common inpatient regimen is immediate administration of dexamethasone 10 mg IV bolus followed by 4–8 mg q 6–8 hours, with taper completed in 2 weeks.
• An alternative is administration of a medium dose of dexamethasone (8 mg twice a day) from the day of MESCC diagnosis until the end of radiotherapy with taper completed in 2 weeks [21].
• Recommendation for outpatient regimen: initial dose of 4 mg q 6 hours of dexamethasone, with a taper started after surgery and/or radiotherapy. Taper usually consists of an empiric reduction in dose of 2–4 mg every 1–3 days as tolerated.
• Common side effects of dexamethasone include stomach irritation, insomnia, irritability, and fluid retention/lower extremity swelling. Prophylaxis against dexamethasone-induced ulceration with proton pump inhibitor or ranitidine 150 mg BID should be used.
Radiotherapy
Radiotherapy Planning Technique
• Either fluoroscopic simulation or CT simulation are appropriate.
• PA, AP/PA, or posterior oblique field design is commonly used, depending on the distance between body surface and spinal cord. Opposed lateral fields for mid/upper cervical spine may be used.
![]() Posterior oblique may decrease the skin dose but may increase the dose to the lung for treatment to the thoracic spine.
Posterior oblique may decrease the skin dose but may increase the dose to the lung for treatment to the thoracic spine.
• Radiation portals are centered on the site of ESCC. If PA or AP/PA fields are used, the field generally encompasses two vertebral bodies above and below the level of compression with a width of 8–10 cm (unless there is paraverterbral extension which should be included in the treatment field).
• With fluoroscopic simulation, dose is prescribed at cord depth or at mid-plane.
• 3D conformal treatment planning has become more popular in recent years. CT simulation has multiple advantages, including (1) more accurate definition of the treatment site and incorporation of paravertebral extension and (2) more accurate dose calculation accounting for body habitus.
• 30 Gy in 3 Gy per fraction × 10 fractions is most commonly used.
![]() Other fractionations, e.g., 2 Gy × 20 fractions, 2.5 Gy × 15 fractions, 4 Gy × 5 fractions, and 8 Gy × 1 fraction may be considered(please see below for more information on dose/fractionation).
Other fractionations, e.g., 2 Gy × 20 fractions, 2.5 Gy × 15 fractions, 4 Gy × 5 fractions, and 8 Gy × 1 fraction may be considered(please see below for more information on dose/fractionation).
Side Effects
• Side effects are generally mild and depend on the location in the spine being treated.
• Acute (reversible): fatigue, nausea/vomiting, mucositis, esophagitis, bowel irritation.
• Late (very rare): myelopathy, pathological vertebral fractures.
• Antiemetics (typically a 5-hydroxytriptamine antagonist) can be administered 30–60 minutes prior to radiotherapy when treating fields between T8 and L3.
Dose/Fractionation
The majority of patients with MESCC receive radiotherapy, either alone or preceded by surgery. Various radiotherapy fractionation regimens were investigated. While studies have consistently shown that radiotherapy is an effective way to control pain and improve ambulatory outcomes, there is no evidence that neurological outcomes are improved with higher doses of radiotherapy. Dose/fractionation varies in published data.
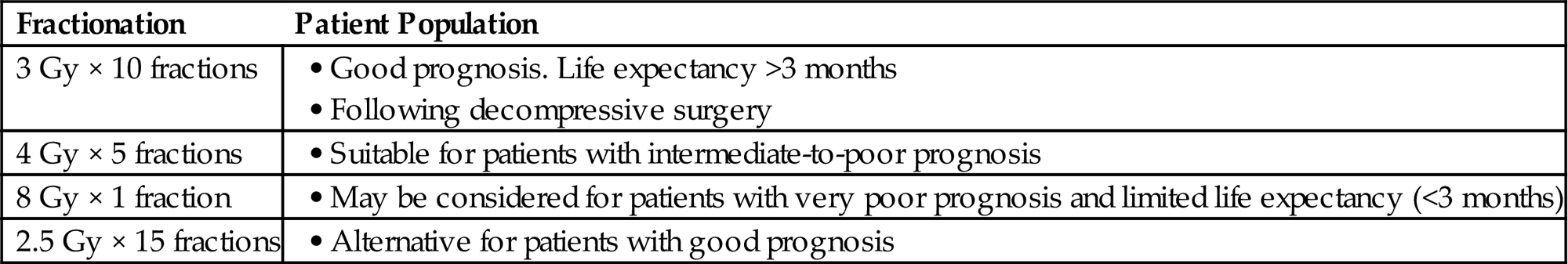
Common fractionation used for MESCC:
| Fractionation | Patient Population |
| 3 Gy × 10 fractions | |
| 4 Gy × 5 fractions | |
| 8 Gy × 1 fraction | |
| 2.5 Gy × 15 fractions |
Data From Randomized Clinical Trials
• Four trials compared short-course versus long-course radiotherapy for patients with short life expectancy without spinal instability.
• 8 Gy × 2 a week apart versus split course (5 Gy × 3 then 3 Gy × 5 with 4-day interval) [22].
![]() Pain relief was reported in approximately 55% of the patients and was similar in these two groups.
Pain relief was reported in approximately 55% of the patients and was similar in these two groups.
![]() The posttreatment ambulatory rates were approximately 70%, similar between the two groups.
The posttreatment ambulatory rates were approximately 70%, similar between the two groups.
– About 90% of ambulatory patients remained ambulatory posttreatment,
– but only roughly 30% of nonambulatory patients regained ambulation after radiotherapy.
![]() CONCLUSION: 8 Gy × 2 had equivalent palliation versus split course.
CONCLUSION: 8 Gy × 2 had equivalent palliation versus split course.
• 8 Gy × 1 versus 8 Gy × 2 a week apart [23].
![]() No difference between the two arms for pain relief or ambulatory outcomes.
No difference between the two arms for pain relief or ambulatory outcomes.
– Approximately 50% of patients had pain relief.
![]() Up to 90% of ambulatory patients remained ambulatory.
Up to 90% of ambulatory patients remained ambulatory.
![]() But only 4% of paraplegic patients regained walking ability.
But only 4% of paraplegic patients regained walking ability.
![]() The median duration of response=5 months. Median overall survival=4 months in both groups.
The median duration of response=5 months. Median overall survival=4 months in both groups.
![]() CONCLUSION: 8 Gy in 1 versus 8 Gy in 2 had equivalent palliation effect.
CONCLUSION: 8 Gy in 1 versus 8 Gy in 2 had equivalent palliation effect.
• 10 Gy × 1 versus 4 Gy × 5 [24]
![]() Response to RT=improvement or no progression of motor function at 5 weeks.
Response to RT=improvement or no progression of motor function at 5 weeks.
– 79% after 10 Gy × 1 versus 68% after 4 Gy × 5 (not statistically significant).
![]() Only 38% evaluable at 5 weeks.
Only 38% evaluable at 5 weeks.
![]() CONCLUSION: 10 Gy × 1 was not inferior for motor function versus 4 Gy × 5.
CONCLUSION: 10 Gy × 1 was not inferior for motor function versus 4 Gy × 5.
• 4 Gy × 5 versus 3 Gy × 10 for poor-to-intermediate prognosis [25].
![]() Response to RT=improvement or no progression of motor function at 4 weeks.
Response to RT=improvement or no progression of motor function at 4 weeks.
– About 40–44% improved, 45% stable, and 12% deteriorated.
![]() Ambulatory rate at 1 month: 82% versus 75% (NS).
Ambulatory rate at 1 month: 82% versus 75% (NS).
![]() 6-month overall survival ~40%.
6-month overall survival ~40%.
![]() CONCLUSION: 4 Gy × 5 was not inferior for motor function and ambulatory status versus 3 Gy × 10.
CONCLUSION: 4 Gy × 5 was not inferior for motor function and ambulatory status versus 3 Gy × 10.
Data From Prospective Studies
• A prospective observational multi-center study comparing 30 Gy in 10 fractions with 40 Gy in 20 fractions found that 30 Gy in 10 fractions was associated with similar outcomes, less treatment time, and lower costs [26].
• Another prospective study comparing short course (8 Gy × 1 or 4 Gy × 5) versus long course (3 Gy × 10, 2.5 Gy × 15, 2 Gy × 20) radiation schemes found similar motor function improvement (~35%) for various fractionations while improved local control and progression-free survival were associated with protracted fractionation [27].
Retrospective Studies
• The largest retrospective study evaluated five common radiotherapy dose-fractionation schedules received by 1304 patients with MESCC: 8 Gy × 1, 4 Gy × 5, 3 Gy × 10, 2.5 Gy × 15, and 2 Gy × 20 [28,29].
![]() The functional outcomes were similar; approximately 30% of the patients had improvement in motor function, and about 70% of the patients were ambulatory posttreatment.
The functional outcomes were similar; approximately 30% of the patients had improvement in motor function, and about 70% of the patients were ambulatory posttreatment.
![]() However, in-field recurrence differed significantly; patients who received protracted fractionation had lower 2-year in-field recurrence rates (~25% for 8 Gy × 1 and 4 Gy × 5, 14% for 3 Gy × 10, ~8% for 2.5 Gy × 15 and 2 Gy × 20).
However, in-field recurrence differed significantly; patients who received protracted fractionation had lower 2-year in-field recurrence rates (~25% for 8 Gy × 1 and 4 Gy × 5, 14% for 3 Gy × 10, ~8% for 2.5 Gy × 15 and 2 Gy × 20).
![]() Based on this result, the authors recommended 8 Gy × 1 for patients with poor expected survival and 3 Gy × 10 for other patients.
Based on this result, the authors recommended 8 Gy × 1 for patients with poor expected survival and 3 Gy × 10 for other patients.
• Multiple retrospective studies also demonstrated improved pain in 50–70% of patients and preservation of ambulatory ability in ambulatory patients with radiotherapy alone.
• However, radiotherapy has limited ability to improve motor deficit; <30% of the patients regained the ability to ambulate [3].
• Patients with a very good survival prognosis (prognostic score >36 in Table 12.2) appear to benefit from radiotherapy with higher total doses (>30 Gy) and lower doses per fraction (<3 Gy) in terms of better local control and survival of MESCC [30].
Highly Conformal Radiotherapy: Stereotactic Radiosurgery/Stereotactic Body Radiation Therapy (SRS/SBRT)
• SRS/SBRT permits high dose conformal photon radiation therapy to deliver higher biologically ablative doses to improve local control while sparing spinal cord with a steep dose gradient, using a single-fraction or hypofractionated regimen.
• SRS/SBRT was initially investigated in vertebral metastases without epidural extension and has demonstrated excellent pain relief of approximately 90% and local control of 80% regardless of histology [31,32].
• However, SRS/SBRT has limited application in most patients with MESCC since many patients who present with MESCC have poor performance status, extensive disease, and/or poor prognosis.
• A recent prospective study from a single institution by Ryu et al. demonstrated radiosurgical decompression of epidural metastasis using a single fraction of 16 Gy is feasible.
![]() An overall 81% improvement in neurological function, with mean epidural tumor volume reduction of 65% at 2 months after radiosurgery [33].
An overall 81% improvement in neurological function, with mean epidural tumor volume reduction of 65% at 2 months after radiosurgery [33].
![]() Dose constraint of the spinal cord is a major limitation: the portion of epidural mass directly compressing the spinal cord has to receive less therapeutic dose to protect the spinal cord.
Dose constraint of the spinal cord is a major limitation: the portion of epidural mass directly compressing the spinal cord has to receive less therapeutic dose to protect the spinal cord.
• The ASTRO evidence-based guideline for palliative radiotherapy for bone metastases recommended SRS/SBRT be limited within a prospective trial [34].
![]() Only selected patients without high-grade epidural compression, with isolated bone metastasis confined to 1–2 vertebral bodies, with no neurological deficits, and good performance status may be considered for SRS/SBRT.
Only selected patients without high-grade epidural compression, with isolated bone metastasis confined to 1–2 vertebral bodies, with no neurological deficits, and good performance status may be considered for SRS/SBRT.
• Toxicities for SRS/SBRT are generally mild and include esophagitis, mucositis, dysphagia, diarrhea, radiculitis, paresthesia, and pain flare.
![]() Radiation-induced myelopathy is approximately 0.6% [35].
Radiation-induced myelopathy is approximately 0.6% [35].
![]() Vertebral fracture has been reported as a delayed complication [36].
Vertebral fracture has been reported as a delayed complication [36].
Surgery
• Prompt neurosurgical evaluation is critical for patients with suspected spinal cord compression. Goals of surgery include circumferential decompression of the spinal cord to preserve neurological function and preservation or restoration of mechanical instability.
• Postoperative radiotherapy usually follows surgery.
• Patient selection is an important consideration; combined surgery and postop radiotherapy should be limited to surgical candidates with an expected survival of minimum of 3 months, good performance status (Karnofsky performance score (KPS) of ≥70), and with limited MESCC.
• Radiosensitive tumors, such as lymphoma, myeloma, and germ cell tumors, may be treated with radiotherapy without upfront surgical decompression.
Surgical Decompression
• The current surgical standard is direct decompressive surgery with maximal debulking, resection of tumor and bone in the canal, decompression of the neural elements, and appropriate reconstruction of the spine to provide stabilization.
• Decompressive laminectomy with or without radiotherapy did not show benefit and is no longer used in clinical practice [38].
![]() Laminectomy removes posterior elements of the spinal column only but without tumor resection.
Laminectomy removes posterior elements of the spinal column only but without tumor resection.
![]() Spinal cord is not immediately decompressed with laminectomy.
Spinal cord is not immediately decompressed with laminectomy.
![]() Furthermore instability is possible due to resection of the posterior elements.
Furthermore instability is possible due to resection of the posterior elements.
• The treatment paradigm of decompressive surgery followed by postoperative radiotherapy was established by a randomized clinical trial by Patchell et al. [39].
![]() One hundred one patients with a single site of metastatic spinal cord compression from solid malignancies were randomized to decompressive surgery followed by radiotherapy versus radiotherapy alone.
One hundred one patients with a single site of metastatic spinal cord compression from solid malignancies were randomized to decompressive surgery followed by radiotherapy versus radiotherapy alone.
![]() Conclusion: Patients with single metastatic spinal cord compression treated with decompressive surgery and radiotherapy were able to maintain ambulatory ability longer and were more likely to regain ambulatory ability, when compared to radiotherapy alone.
Conclusion: Patients with single metastatic spinal cord compression treated with decompressive surgery and radiotherapy were able to maintain ambulatory ability longer and were more likely to regain ambulatory ability, when compared to radiotherapy alone.
– More patients remained ambulatory after surgery plus radiotherapy compared to radiotherapy alone (84% vs 57%).
– Those who were not ambulatory prior to treatment: 62% in the surgery and radiotherapy group regained the ability to walk after therapy, compared to only 19% of the patients who received radiotherapy only.
– Patients treated with surgery maintained the ability to walk much longer (122 days vs 13 days).
– The need for corticosteroids and analgesics was reduced in the surgical plus radiotherapy group.
– In 20% of the patients in the radiotherapy only group, significant progressive motor dysfunction during radiotherapy was noted and these patients underwent surgery.
– Survival time improved with surgery followed by radiotherapy (median 126 days vs 100 days).
– Criticisms of the study include [40]:
![]() Poorer outcomes for radiotherapy-only patients compared to historical data (patients maintained ambulatory ability for median of 13 days).
Poorer outcomes for radiotherapy-only patients compared to historical data (patients maintained ambulatory ability for median of 13 days).
![]() Statistical power is questioned because the study ended prematurely after meeting an early stopping rule.
Statistical power is questioned because the study ended prematurely after meeting an early stopping rule.
![]() Slow accrual: 101 patients over 10 years.
Slow accrual: 101 patients over 10 years.
![]() The results of the highly selected cohort of the Patchell study could not be confirmed in a match pair analysis more likely reflecting clinical routine [41], which showed similar rates of motor function improvement and posttreatment ambulatory rate between the surgery/radiation and radiation alone arms.
The results of the highly selected cohort of the Patchell study could not be confirmed in a match pair analysis more likely reflecting clinical routine [41], which showed similar rates of motor function improvement and posttreatment ambulatory rate between the surgery/radiation and radiation alone arms.
| Patchell et al. (2005) [40] (Surgery/Radiation vs Radiation Alone) | Rades et al. (2010) [41] (Surgery/Radiation vs Radiation Alone) | |
| Improved motor function% | 27 vs 26 | |
| Posttreatment ambulatory rate% | 84 vs 57 | 69 vs 68 |
| % of nonambulatory patients regained ambulation | 62 vs 19 | 30 vs 26 |
| 1-year local control% | 90 vs 91 |
• In recent years separation surgery, or limited decompressive surgery, has recently been proposed to combine with radiotherapy.
![]() Separation surgery decompresses epidural mass with spinal stabilization and provides a separation between the tumor and the spinal cord.
Separation surgery decompresses epidural mass with spinal stabilization and provides a separation between the tumor and the spinal cord.
![]() This allows optimal and safe delivery of highly conformal radiation therapy (SRS/SBRT) to treat the remaining cancer.
This allows optimal and safe delivery of highly conformal radiation therapy (SRS/SBRT) to treat the remaining cancer.
![]() This approach may decrease the perioperative risk related to radical decompression and at the same time allow decompression of high-grade ESCC.
This approach may decrease the perioperative risk related to radical decompression and at the same time allow decompression of high-grade ESCC.
![]() Single institution experience reported promising durable local control including 1-year control of 95% in 186 patients in one series [42].
Single institution experience reported promising durable local control including 1-year control of 95% in 186 patients in one series [42].
Surgical Stabilization
• Mechanical instability includes fracture-dislocation, translational deformity, and significant collapse of vertebral body with associated mechanical pain.
• This can lead to mechanical injury to the spinal cord resulting in detrimental neurological function.
• The spinal instability neoplastic score (SINS) was developed by expert consensus from the Spine Oncology Study Group to evaluate mechanical stability [43,44].
![]() See Appendix E (Palliative Care Toolkit) for SINS classification.
See Appendix E (Palliative Care Toolkit) for SINS classification.
![]() Factors that predict instability include >50% vertebral body collapse, bilateral facet destruction, pain associated with movement, progressive deformity, presence of subluxation, and location in the junctional segments of the spine.
Factors that predict instability include >50% vertebral body collapse, bilateral facet destruction, pain associated with movement, progressive deformity, presence of subluxation, and location in the junctional segments of the spine.
• Surgical stabilization should be strongly considered in patients with mechanical instability (e.g., high SINS score).
• Percutaneous cement augmentation such as vertebroplasty and kyphoplasty is not indicated for patients with spinal cord compression.
![]() Although this approach may be effective in reducing pain due to compression fracture in cancer patients, patients with epidural metastasis were excluded in the CAFÉ study [45].
Although this approach may be effective in reducing pain due to compression fracture in cancer patients, patients with epidural metastasis were excluded in the CAFÉ study [45].
Recurrence
• In-field recurrence after initial radiotherapy for MESCC has been shown to occur in approximately 10–25% of patients [14,28,29].
![]() A match-pair study comparing 8Gy × 1 versus 4 Gy × 5 for life expectancy <6 months reported need for in-field re-irradiation did not differ: 18% versus 9% at 6-months (NS) [46].
A match-pair study comparing 8Gy × 1 versus 4 Gy × 5 for life expectancy <6 months reported need for in-field re-irradiation did not differ: 18% versus 9% at 6-months (NS) [46].
• If patients are surgical candidates with life expectancy >3 months, decompressive surgery for recurrence in the previously irradiated area is preferred.
• Re-irradiation may be the only suitable intervention for many patients due to poor performance status or short expected survival. It is well tolerated and effective when the cumulative biologically equivalent dose (BED) is limited to BED ≤120 Gy2 (using alpha/beta of two for radiotherapy-induced myelopathy) [47,48].
• An analysis of 579 evaluable patients entered on two randomized trials that assessed radiotherapy for MESCC [47] concluded that re-irradiation was safe and effective.
![]() One-half of patients with in-field recurrences after different doses and fractionations received re-irradiation.
One-half of patients with in-field recurrences after different doses and fractionations received re-irradiation.
![]() Ambulatory capacity before re-irradiation was the strongest prognostic factor for functional outcome [47].
Ambulatory capacity before re-irradiation was the strongest prognostic factor for functional outcome [47].
![]() Mean interval between radiotherapy courses was about 5 months.
Mean interval between radiotherapy courses was about 5 months.
![]() Ambulation was maintained in 86% of the ambulatory patients.
Ambulation was maintained in 86% of the ambulatory patients.
![]() However, all five nonambulatory patients did not regain ambulatory function after radiotherapy [47].
However, all five nonambulatory patients did not regain ambulatory function after radiotherapy [47].
• Motor function after re-irradiation was associated with performance status, time to developing motor deficits, visceral metastases, and whether first course improved motor function [48].
• Spinal re-irradiation using short-course radiotherapy (8 Gy × 1, 3 Gy × 5, or 4 Gy × 5) was showed to have equivalent effectiveness.
![]() Eighty-five percent of the patients had stable or improved motor function [49].
Eighty-five percent of the patients had stable or improved motor function [49].
![]() Six of sixteen nonambulatory patients regained ambulation after radiotherapy.
Six of sixteen nonambulatory patients regained ambulation after radiotherapy.
• If the cumulative BED is >120 Gy2, surgery should be considered, if possible. SBRT is an alternative for those who cannot undergo surgery.
• Re-irradiation with SBRT may be considered in selected patients with isolated local recurrence, and good performance status and life expectancy.
![]() Improvement of neurological deficits has been described in a retrospective single institutional study [50].
Improvement of neurological deficits has been described in a retrospective single institutional study [50].
![]() Neurological outcomes correlate with pretreatment neurological status. Thus diagnosis before development of neurological deficit is crucial.
Neurological outcomes correlate with pretreatment neurological status. Thus diagnosis before development of neurological deficit is crucial.
• Clinical decision for re-irradiation should be made on a case-by-case basis, with at least a 5 month interval between the radiotherapy courses.
Summary
• Patients with history of cancer who present with persistent back pain should be promptly evaluated for MESCC because early diagnosis and intervention of spinal cord compression results in the best prognosis of ambulation.
• Surgical decompression is the best initial therapy for selected patients with moderate-grade (compression of the thecal sac) or high-grade (compression of the spinal cord) ESCC, followed by radiotherapy. Exceptions include patients with a highly radiosensitive tumor, patients with multiple levels of MESCC, patients with a poor performance status, and patients with a very limited survival prognosis.
• Those patients who are not surgical candidates should be considered for radiotherapy alone.
• Radiotherapy planning uses AP/PA, PA only, or a posterior oblique field arrangement. The treatment field should encompass the site of compression and two uninvolved vertebral bodies above and below the level of compression with a width of 8–10 cm.
• The radiation fractionation regimen depends on the survival prognosis. Patients with a poor survival prognosis should receive 8 Gy × 1 or 4 Gy × 5, those with an intermediate prognosis 3 Gy × 10, and those patients with a very good prognosis 2.5 Gy × 15 or 2 Gy × 20.
• Retreatment for recurrent MESCC with external beam radiotherapy is safe and feasible. Cumulative radiotherapy dose (BED) should be kept to 120 Gy2 or below. Otherwise, neurosurgery (if possible) or SBRT should be considered in patients with good survival prognosis.
• SRS/SBRT should be preferentially limited within a prospective trial for patients with a very good survival prognosis and limited metastatic epidural extension, as recommended by the ASTRO Evidence-Based Guideline. This requires multidisciplinary evaluation by neurosurgery, radiation oncology, and medical oncology.
• Spinal stability needs to be evaluated by neurosurgery; stabilization surgery may be needed. Percutaneous cement augmentation such as vertebroplasty and kyphoplasty is not indicated for patients with spinal cord compression.
• Additional randomized trials are required to further optimize the treatment of MESCC.
