Vitamins and Minerals in Older Adults
Causes, Diagnosis, and Treatment of Deficiency
Jennifer Doley, Carondelet St. Mary’s Hospital, Tucson, AZ, United States
Abstract
Many individuals, especially older adults, are at risk of micronutrient deficiencies due to poor-quality diets and medical conditions and their treatments that affect nutrient absorption or utilization. Micronutrient deficiencies can result in significant signs and symptoms that further complicate medical conditions and reduce the intake of nutrient-dense foods. It is critical that the health-care practitioner recognize the risk factors for deficiency, screen for deficiencies in high-risk populations, and properly diagnose and treat micronutrient deficiencies. This chapter will provide an overview of the functions of vitamins and minerals; factors affecting micronutrient needs, intake, absorption, and utilization in older populations; and an overview of the diagnosis and treatment of deficiency.
Keywords
Micronutrient; vitamin; mineral; needs; absorption; utilization; deficiency; diagnosis; treatment
Introduction
Micronutrients have been studied for their role in the prevention of many disease states, although evidence for or against routine supplementation is mixed. The US Preventive Services Task Force released a statement specifically on cancer and cardiovascular disease, stating that there is insufficient evidence to assess the harms and benefits of single or multivitamin and mineral supplements, with the exception of vitamin E. The task force concluded that vitamin E has no effect on either disease (Moyer, 2014). This chapter will thus focus on the cause, diagnosis, and treatment of micronutrient deficiencies in older adults rather than on disease prevention.
Vitamins and minerals play an important role in health during aging, and deficiencies can affect both physical and mental functions. Consuming enough micronutrients can be a challenge for some populations, especially the elderly, as a host of physiological and cognitive age-related changes can affect intake, digestion, absorption, and metabolism of these nutrients. Many micronutrient deficiencies can result in impairment of physical and cognitive functions, which can then cause further nutritional decline.
As with any disease or condition, prevention of micronutrient deficiency is ideal; thus, it is important for the clinician to understand micronutrient needs in older adults and to develop the skills necessary to assess dietary intake and identify factors that may impair intake, absorption and utilization of these nutrients. The ability to identify deficiencies and prescribe appropriate food and supplement regimens to treat deficiency is also crucial.
Vitamins
Vitamins are organic compounds required in limited amounts for normal growth and metabolism, and are typically categorized as fat soluble or water soluble. Thirteen compounds are recognized as vitamins; while choline is not technically classified as a vitamin, it is an essential nutrient and will be included in this review (Mahan et al., 2012; Mueller, 2012; NIH, 2016).
Most vitamins are actually groups of structurally related compounds called vitamers that have similar activities. For example, vitamin D comprises the vitamers calcitriol, ergocalciferol, and cholecalciferol. Vitamins are necessary for a wide variety of biochemical functions; they function as antioxidants and as cofactors for many enzymatic reactions and serve as regulators of mineral metabolism. See Table 14.1 for a list of common functions of vitamins (Mahan et al., 2012; Mueller, 2012; NIH, 2016).
Table 14.1
Common Functions of Vitamins (Mahan et al., 2012; Mueller, 2012; NIH, 2016)
| Vitamin | Common Functions |
| A | Vision, gene expression, reproduction, immune function |
| D | Maintenance of serum calcium and phosphorus levels, bone health |
| E | Antioxidant |
| K | Synthesis of proteins involved in blood clotting and bone metabolism |
| B1 (thiamin) | Carbohydrate and branched-chain amino acid metabolism, nerve function |
| B2 (riboflavin) | Carbohydrate, amino acid, and lipid metabolism; conversion of B6 to its active form |
| B3 (niacin) | Carbohydrate, amino acid, and lipid metabolism |
| B6 (pyridoxine) | Carbohydrate, amino acid, and lipid metabolism; hemoglobin and neurotransmitter synthesis; immune function |
| B12 (cobalamin) | Nucleic and amino acid metabolism, red blood cell maturation, conversion of folate to active form |
| C (ascorbic acid) | Collagen, carnitine, and neurotransmitter synthesis; antioxidant and immune functions; absorption of nonheme iron; cholesterol hydroxylation into bile acids |
| Folic acid | Nucleic and amino acid metabolism, DNA and red blood cell synthesis |
| Pantothenic acid | Carbohydrate, amino acid, and lipid metabolism; heme and sterol synthesis |
| Biotin | Carbohydrate, amino acid, and lipid metabolism |
| Choline | Neurotransmitter and homocysteine synthesis, cell membrane signaling, lipid transport |
Minerals
The term mineral is a misnomer; dietary minerals are actually chemical elements. The four most common elements—hydrogen, carbon, nitrogen, and oxygen—serve organic functions. Other elements known to be necessary for humans include sodium, magnesium, phosphorus, sulfur, chloride, potassium, and calcium. Additional elements considered essential in much smaller amounts are called trace elements and include chromium, manganese, iron, cobalt, nickel, copper, zinc, selenium, bromine, molybdenum, and iodine. Some elements are suspected to play a role in human health, although there is insufficient evidence to clearly describe their biochemical functions or needed amounts. These include lithium, boron, fluorine, silicon, vanadium, and arsenic (Mahan et al., 2012; Mueller, 2012; NIH, 2016).
Some elements such as sodium and potassium function primarily as electrolytes; others such as magnesium and calcium serve a structural function in addition to acting as electrolytes. Many minerals are required for the production and function of a wide variety of enzymes necessary for normal metabolic reactions. Other mineral functions include hormone synthesis, antioxidant activity, cell signaling, and energy production. See Table 14.2 for a list of common mineral functions (Mahan et al., 2012; Mueller, 2012; NIH, 2016).
Table 14.2
Common Functions of Minerals (Mahan et al., 2012; Mueller, 2012; NIH, 2016)
| Mineral | Common Functions |
| Calcium (Ca) | Structural component of bones and teeth, cell membrane integrity, neuromuscular activity, endocrine secretory function, blood coagulation |
| Chloride (Cl) | Regulation of water distribution, maintenance of acid–base balance, nerve-impulse transmission |
| Copper (Cu) | Red blood cell formation, iron utilization, energy production, connective tissue synthesis, antioxidation |
| Iodine (I) | Thyroid hormone synthesis, nerve muscle function, nutrient metabolism |
| Iron (Fe) | Formation of hemoglobin and myoglobin |
| Magnesium (Mg) | Structural component of bones; enzyme activation for glucose, DNA, and protein metabolism; fatty acid synthesis; cell membrane potential—muscle and nerve function; cardiovascular excitability |
| Manganese (Mn) | Constituent of some metalloenzymes; enzyme activation |
| Molybdenum (Mo) | Enzyme component |
| Phosphorus (P) | Structural component of bones and cell membranes; pH maintenance; glycolysis; muscle and nerve function |
| Potassium (K) | Electrolyte, protein, and glycogen synthesis, membrane potential—muscle and nerve function |
| Selenium (Se) | Cofactor for glutathione and iodine; thyroid function |
| Sodium (Na) | Regulation of water distribution; membrane potential—muscle and nerve function; active transport of molecules across cell membranes |
| Zinc (Zn) | Protein synthesis; immunity; growth of genital organs |
Needs
The US Department of Agriculture has established a recommended dietary allowance (RDA) and estimated average requirement (EAR) for most nutrients. The RDA is an estimate of the amount of a nutrient sufficient to meet the needs of 97–98% of a population, while the EAR is the estimated requirement for 50% of a population. There is insufficient evidence to recommend an RDA for some nutrients, thus adequate intakes (AIs) are established, which are quantities believed to be sufficient to meet the needs of most individuals, although inadequate data prevent specificity in determining what percentage of the population would most benefit. RDAs, EARs, and AIs are divided into age and sex categories; see Tables 14.3 and 14.4 for the RDAs and AIs of vitamins and minerals for older adults (USDA, 2016).
Table 14.3
RDAs or AIs for Vitamins for Older Adults (USDA, 2016)
| Fat Soluble | RDA Men | RDA Women |
| Vitamin A | 900 µg | 700 µg |
| Vitamin D | 600 IU (50–70 year) | 600 IU (50–70 year) |
| 800 IU (>70 year) | 800 IU (>70 year) | |
| Vitamin E | 15 mg | 15 mg |
| Vitamin Ka | 120 µg | 90 µg |
| Vitamin B1 | 1.2 mg | 1.1 mg |
| Vitamin B2 | 1.3 mg | 1.1 mg |
| Vitamin B3 | 16 mg | 14 mg |
| Vitamin B6 | 1.7 mg | 1.5 mg |
| Vitamin B12 | 2.4 µg | 2.4 µg |
| Vitamin C | 90 mg | 75 mg |
| Folic acid | 400 µg | 400 µg |
| Pantothenic acida | 5 mg | 5 mg |
| Biotina | 30 µg | 30 µg |
| Cholinea | 550 mg | 425 mg |
aSignifies AI.
Table 14.4
RDAs or AIs for Minerals for Older Adults (USDA, 2016)
| Mineral | RDA Men | RDA Women |
| Calcium | 1000 mg (51–70 year) 1200 mg (>70 year) | 1200 mg (≥51 year) |
| Chloridea | 2 g | 1.8 g |
| Copper | 900 µg | 900 µg |
| Iodine | 150 µg | 150 µg |
| Iron | 8 mg | 8 mg |
| Magnesium | 420 mg | 320 mg |
| Manganesea | 2.3 mg | 1.8 mg |
| Molybdenum | 45 µg | 45 µg |
| Phosphorus | 700 mg | 700 mg |
| Potassiuma | 4.7 g | 4.7 g |
| Selenium | 55 µg | 55 µg |
| Sodiuma | 1.3 g | 1.2 g |
| Zinc | 11 mg | 11 mg |
aSignifies AI.
Requirements for some micronutrients increase with age, most notably vitamin D and calcium, due in part to the increased incidence and risk for osteopenia and osteoporosis in older adults. Iron requirements decrease for older women because they are no longer of childbearing age. Although the RDAs for other nutrients remain unchanged for older populations, some elders may have needs higher than the RDAs for select micronutrients due to impairments in their absorption or metabolism (USDA, 2016).
Causes of Deficiency
Micronutrient deficiencies can generally be categorized as primary (caused by inadequate intake) or secondary (caused by a medical condition or medication that interferes with the absorption or metabolism of the vitamin or mineral). However, the origin of deficiency is often multifactorial; a specific disease or host of medical problems can reduce food intake, increase needs, decrease absorption and impair metabolism, all of which can contribute to the development of micronutrient deficiencies. See Tables 14.5 and 14.6 for micronutrient deficiency risk factors (Montgomery et al., 2014).
Table 14.5
Risk Factors for Vitamin Deficiencies (Mueller, 2012; Allen et al., 2006; Mahan et al., 2012; NIH, 2016; Grober and Kisters, 2012)
| Vitamin | Deficiency Risk Factorsa |
| A | Severe zinc deficiency, chronic alcoholism, severe malnutrition, poor intake of dairy products |
| D | Limited exposure to sunlight, kidney and liver disease (limits activation of vitamin D), darkly pigmented skin, obesity (vitamin D sequestered in fat cells), medications (antiepileptics, antiretrovirals, glucocorticoids), liver disease, poor intake of dairy products, older age (less ultraviolet exposure, inadequate intake, reduced cutaneous synthesis) |
| E | Fat malabsorptiona |
| K | Fat malabsorptiona |
| B1 | Chronic alcoholism, liver disease, AIDS; poor intake of animal and dairy products and legumes; older age (inadequate intake, combination of chronic diseases with concomitant intake of multiple medications, decreased absorption); chronic or high-dose use of diuretic medications |
| B2 | Chronic alcoholism, liver disease, poor intake of animal and dairy products; usually associated with other B vitamin deficiencies |
| B3 | Poor intake of animal and dairy products |
| B6 | Chronic alcoholism, liver disease, poor intake of animal and dairy products, end-stage renal disease or chronic renal insufficiency, autoimmune disorders, long-term use of antiepileptic medications; usually associated with other B vitamin deficiencies |
| B12 | Chronic alcoholism, poor intake of animal and dairy products, decreased or absent gastric acid production (use of proton pump inhibitors, gastrectomy, gastric bypass, Helicobacter pylori overgrowth), ileal resection |
| C | Poor intake of fruits and vegetables rich in vitamin C, smoking (due to increased needs) |
| Folic acid | Chronic alcoholism, liver disease; poor intake of fruits, vegetables, legumes, and dairy productions; medications (phenytoin, cholestyramine, amphotericin B, metformin) |
| Pantothenic acid | Severe malnutrition; usually associated with other B vitamin deficiencies |
| Biotin | Severe malnutrition; usually associated with other B vitamin deficiencies |
| Choline | Severe malnutrition; usually associated with other B vitamin deficiencies |
aAll micronutrients are affected by gastrointestinal (GI) malabsorptive conditions, including celiac disease, short bowel syndrome, inflammatory bowel disease, pancreatic enzyme insufficiency, and gastric bypass surgery.
Table 14.6
Risk Factors for Mineral Deficiencies (Mueller, 2012; Allen et al., 2006; Mahan et al., 2012; NIH, 2016)
| Minerals | Deficiency Risk Factorsa |
| Calcium | Poor intake of dairy products; older age, especially postmenopausal women; medications (glucocorticoids) |
| Copper | Increased GI losses (chronic diarrhea), excess iron or zinc supplementation |
| Iodine | Residence in areas with iodine-poor soil, individuals who do not use iodized salt, long-term administration of iodine-free parenteral nutrition; excess intake of foods high in goitrogens, compounds that inhibit iodine uptake by the thyroid (soy, cassava, and cruciferous vegetables); iron or vitamin A deficiency (also goitrogenic) |
| Iron | Blood loss, vegetarianism and veganism, decreased or absent gastric acid production, poor vitamin C intake, excess copper or zinc supplementation |
| Magnesium | Poor intake of magnesium-rich foods, older age, long-term use diuretic medication; acute hypomagnesemia may occur as a result of electrolyte shifts such as those seen in refeeding syndrome |
| Phosphorus | Poor intake of phosphorus-rich foods, long-term use of phosphorus binders as seen in those with chronic renal disease; acute hypophosphatemia may occur as a result of electrolyte shifts such as those seen in refeeding syndrome |
| Selenium | HIV disease, residence in areas with selenium-poor soil |
| Zinc | Trauma, burns, surgery, increased GI losses (chronic diarrhea), chronic alcoholism, poor intake of animal and dairy products, renal disease, excess iron or copper supplementation |
aAll micronutrients are affected by GI malabsorptive conditions, which include celiac disease, short bowel syndrome, inflammatory bowel disease, pancreatic enzyme insufficiency, and gastric bypass.
Insufficient Intake
Micronutrient intake among older adults, as with other populations, can vary significantly. However, in a recent systematic review of 37 studies involving more than 28,000 community-dwelling older adults in 20 Western countries, researchers concluded that a significant percentage of older men and women had inadequate intake of many micronutrients—especially thiamin, riboflavin, vitamin D, calcium, magnesium, and selenium—when compared to the EAR (ter Borg et al., 2015). Nutrient intake assessment methods varied, making study comparisons challenging, but these data are compelling and suggest adequate micronutrient intake is lacking in many older adults.
The most significant factor affecting micronutrient intake, in the United States and worldwide, is socioeconomic status. The elder population suffers more socioeconomic hardships than younger adults (DiMaria-Ghalili, 2014). Both limited income and access to transportation can reduce the ability to purchase and consume a variety of micronutrient-rich foods (Allen et al., 2006).
Older adults face physical barriers to consuming sufficient amounts of micronutrients. Normal or common physiological changes associated with aging often result in reduced intake of nutrient-rich foods, especially fruits, vegetables, and meats. Changes include poor dentition, altered or decreased taste and smell sensations, decreased appetite, and difficulty chewing and swallowing (DiMaria-Ghalili, 2014). Functional limitations leading to decreased ability to obtain and prepare food are also a concern in the aged population. Functional capacity often declines with age, and the cause is usually multifactorial. Medical conditions such as arthritis, pain, neuromuscular dysfunction, and age-related sarcopenia can all contribute to decreased mobility, physical activity, and manual dexterity.
Psychological problems, such as depression and isolation, and cognitive issues such as dementia are more common in elderly populations and contribute to significant reductions in micronutrient consumption. Isolation and depression are associated with reduced appetite, while individuals with dementia often refuse or forget to eat meals (DiFrancesco et al., 2007; DiMaria-Ghalili, 2014).
The incidence of many chronic health conditions such as cancer, diabetes, heart disease, and hypertension increases as people age. Specific medications necessary for the treatment of these conditions, as well as the total volume of medications taken, can contribute to diminished food intake via decreased appetite, early satiety, taste changes, and GI disturbances such as diarrhea, constipation, and nausea. These challenges can result in the reduction of both the quality and total volume of food consumed (DiMaria-Ghalili, 2014). See Table 14.7 for conditions that reduce intake and their potential causes.
Table 14.7
Common Causes of Decreased Food Intake in Older Adults
| Symptom | Potential Causes |
| Difficulty chewing | Poorly fitting dentures, poor dentition, poor oral hygiene, xerostemia, functional decline |
| Difficulty swallowing | Weakness, functional decline, dysphagia as a result of neurological dysfunction or injury |
| Taste and smell changes | Aging, oral infections, poor oral hygiene, zinc deficiency, xerostemia, medications, some chronic diseases, smoking |
| Poor appetite | Aging, medications, constipation, chronic disease, depression |
| Self-restriction | Strict adherence to therapeutic diets for disease management; constipation, diarrhea, or other GI symptoms; incontinence |
Food Quality
Besides individual conditions that reduce the ability to procure or consume nutrient-rich foods, the other key factor influencing intake is the micronutrient content of the foods consumed. Nutrient content is influenced by food-industry practices, cooking methods, and the environment in which the food is grown.
Food-production practices, including harvesting, transportation, and storage, can subject foods to light and oxygen exposure and time delays between harvest and consumption. Time, light, and oxygen exposure accelerate the ripening process as well as nutrient degradation. Freezing results in minimal micronutrient loss; in addition, produce is typically frozen shortly after harvest, thus maximizing micronutrient content (USDA, 2007). Frozen products may be ideal for some older adults, especially if the frequency with which they can obtain food is limited. Canning may preserve micronutrients if canned shortly after harvest, but minerals can leach from food into surrounding liquid, which is often discarded. Furthermore, adding salt during the canning process may alter the solubility of organometallic compounds, which can affect their bioavailability (Wapnir, 1998).
Other food-industry practices affect micronutrient concentration. Food enrichment is the practice of adding micronutrients back to a food product that were lost during processing, while fortification adds additional micronutrients not present (or present in small amounts) prior to processing. Food fortification has been practiced in industrialized nations for many years; common fortification practices include B vitamins and folic acid in grains, vitamins A and D in milk, iodine in salt, calcium in orange juice and soy products, and a variety of micronutrients in cereals, bottled water, and other beverages. Although less commonly utilized in developing countries, fortification practices including the use of sugar fortified with vitamin A in Central America and iron-fortified fish and soy sauces in Asia have successfully reduced the prevalence of some micronutrient deficiencies (Allen et al., 2006).
Food-preparation methods that utilize higher temperatures, longer cooking times, and large amounts of water or oil (which is then discarded) will increase the loss of some vitamins and minerals, most notably vitamin C. Other factors affecting nutrient retention include pH and exposure to air and light (Bergstrom, 1994). Because of the short cooking time and limited use of water associated with steaming and microwaving, these methods are recommended to minimize micronutrient losses.
The mineral content of plants depends on the mineral concentration of the soil in which it is grown, thus impacting the micronutrient intake of those who consume the plant. Although data are limited for many minerals, research has shown that soil concentrations of zinc and selenium are low in many areas of the world, including China, Africa, India, and parts of North and South America. Low soil concentrations directly correlate with rates of human zinc and selenium deficiency in developing countries but not in industrialized nations. Scientists believe this is due to the wider dietary variety and use of supplements seen in industrialized countries (Udo de Haes et al., 2012). Thus, individuals in these geographic areas may be more likely to have insufficient intake of these minerals if they do not use supplements and lack variety in their diets.
Impaired Absorption
Absorptive issues render older individuals more vulnerable to micronutrient deficiency, even in those whose intakes are considered sufficient for most adults. Micronutrient absorption is affected by several factors, including age, diseases and conditions that affect the GI tract, medications, and the form and amount of the micronutrient consumed. Most micronutrients are largely absorbed in the duodenum and jejunum, with the exception of vitamin B12, which is absorbed in the ileum; thus conditions affecting the proximal GI tract are more likely to result in micronutrient malabsorption.
Human genetic differences may also result in significant variations in micronutrient absorption, as well as nutrient needs and metabolism. Although growing rapidly, the field of nutrigenetics is still in its infancy and insufficient data preclude any changes to current clinical practice related to micronutrients (Baumler, 2012).
Fat-soluble vitamins are absorbed with dietary fat; malabsorption of these vitamins is rare except in conditions resulting in fat malabsorption. Even in cases of decreased absorption, fat-soluble vitamins, with the exception of vitamin D, are rarely deficient in residents of developed countries because of their long-term storage in fatty tissues (Mahan et al., 2012; Mueller, 2012; NIH, 2016).
Absorption of water-soluble vitamins can be influenced by more factors than that of fat-soluble vitamins, and deficiencies are generally more common as body stores of these vitamins are smaller than that of fat-soluble vitamins. Factors that increase the risk of water-soluble vitamin deficiency include alcohol abuse, malnutrition, poor quality or restrictive diets, and malabsorption (Mahan et al., 2012; Mueller, 2012; NIH, 2016).
Mineral absorption is similarly affected by a number of factors, including concentration, form, and concomitant intake of other specific nutrients and dietary components. Dietary minerals are often found in more than one form, and some forms are more bioavailable than others. For example, iron is more efficiently absorbed in the heme form found in animal-based foods than the nonheme iron found in plant-based foods (Mahan et al., 2012; Mueller, 2012; NIH, 2016).
Absorption can also be affected by the amount of mineral consumed, particularly in supplement form; e.g., the percentage of calcium absorbed decreases in doses of more than 500 mg, so it is recommended that daily supplementation be given in divided doses to maximize absorption (NIH, 2016). Zinc excretion in feces also increases when the amount of zinc absorbed increases, so divided doses of zinc supplements may be beneficial (Hambridge et al., 2010). Iron supplementation has been shown to reduce absorption of nonheme iron consumed in foods but not heme iron. Copper absorption also decreases with an increase in intake (Wapnir, 1998).
Excessive or prolonged supplementation of one mineral may result in deficiency of another due to reduced absorption; in particular, zinc, copper, and iron act competitively in the absorption process. Some dietary elements such as phytic and oxalic acid have been shown to reduce micronutrient absorption, while other dietary components such as vitamin C with iron and vitamin D with calcium actually improve mineral absorption (NIH, 2016).
Common physiological changes in aging can also alter micronutrient absorption. While gastric acid production does not necessarily decrease with normal aging, the negative consequences of chronic gastric conditions will manifest with advancing age. In particular, calcium and B12 absorption may be impaired in part due to hypochlorhydria, a common result of atrophic gastritis and the prolonged use of proton pump inhibitors or H2 antagonists. Gastric acid is needed to cleave vitamin B12 from food, and intrinsic factor is needed for B12 absorption, both of which are produced by gastric cells. Hypochlorhydria may also reduce folic acid absorption (Holt, 2007; Salles, 2007).
No marked changes have been noted in the structure or function of small intestinal villi and enterocytes in older adults. Likewise, digestive enzyme production generally does not decrease with age—with the exception of lactase. Lactase production is highest in infancy and childhood and decreases steadily in adulthood. Lactose intolerance itself does not inhibit calcium or other micronutrient absorption, but impaired tolerance of lactose may reduce consumption of dairy products, thus limiting the intake of foods rich in Ca, Mg, P, K, and vitamins D and A (Salles, 2007; Corleto et al., 2014).
Diseases or conditions that affect nutrient absorption include short bowel syndrome, which is caused by resection of a significant portion of the small bowel; pancreatic exocrine insufficiency, which is seen in pancreatic resections and cystic fibrosis; total or partial gastrectomy, which is most commonly performed to treat gastric cancer and weight reduction for those who are morbidly obese; untreated celiac disease; an acute phase response typically caused by systemic infections; and genetic disorders resulting in reduced or absent production of specific digestive enzymes. Micronutrient malabsorption is largely influenced by the portion of the GI tract that is dysfunctional or absent. See Tables 14.8 and 14.9 for more details regarding absorption, including physiological and dietary (Mahan et al., 2012; Mueller, 2012; NIH, 2016).
Table 14.8
Absorption of Vitamins (Mueller, 2012; Allen et al., 2006; Mahan et al., 2012; NIH, 2016)
| Vitamin | Approximate % Absorbed | Mechanism | Factors Affecting Absorption |
| A | 90 | Proteases hydrolyze proteins needed to release vitamin A; lipases hydrolyze retinyl esters. Fat and bile acids necessary for incorporation into micelles for passive absorption | Decreased absorption with fat malabsorption |
| D | 50 | Fat and bile acids necessary for incorporation into micelles for passive absorption | Decreased absorption with fat malabsorption |
| E | 20–50 | Fat and bile acids necessary for incorporation into micelles for passive absorption | Absorption decreases as intake increases; at pharmacological doses, absorption can be <10% |
| K | 20–80 | Phylloquinones (K1) absorbed by energy-dependent processes; menaquinones (K2) and menadione (K3) absorbed passively | Decreased absorption with fat malabsorption. Vitamin K in free form (from oils or supplements) absorbed at ~80%; much lower absorption from foods |
| B1 | Phosphatases cleave phosphorylated forms prior to absorption. Energy-dependent active transport mechanism used for absorption with low intake; passive transport used with high intake | Decreased absorption with alcohol consumption (interferes with transport) and folate deficiency (interferes with replication of enterocytes) | |
| B2 | 95 | HCl and phosphatases cleave B2 from protein complexes prior to absorption via an energy-dependent carrier-mediated process | Absorption is proportional to intake. Absorption increases in presence of B2 deficiency. Alcohol consumption and divalent metals (Cu, Zn, Fe, Mn) that form chelates with B2 may reduce absorption |
| B3 | Absorption via carrier-mediated facilitated diffusion | Can be synthesized from tryptophan, although process is insufficient to meet needs alone; B2 and B6 needed for this process | |
| B6 | 75 | Phosphatases are needed to cleave phosphorylated forms from protein prior to absorption via passive diffusion | Absorption is pH dependent |
| B12 | 50 | Pepsin and HCl release B12 from food; B12 forms a complex in the duodenum with intrinsic factor and is then absorbed via active transport in the ileum. B12 is excreted in bile, so it may be reabsorbed via enterohepatic circulation | Absorption is decreased in conditions affecting the function or structure of the stomach or ileum. Absorption decreases for supplemented B12 doses above 1–2 µg, as the capacity of intrinsic factor is exceeded |
| C | 80–90 | Active transport mechanism | Absorption can decrease to <50% at doses above 1 gm/day |
| Folic acid | 50–100 | Zinc-dependent brush border enzymes convert polyglutamate to monoglutamate form before absorption via active transport; passive transport may be used at high doses. Some forms of folate are excreted in bile so they may be reabsorbed via enterohepatic circulation | Absorption of supplemented folate is 80–100%, while approximately 50% of folate naturally occurring in foods is absorbed. Zinc deficiency, alcohol intake, and changes in enteric pH reduce absorption |
| Pantothenic acid | Dietary CoA form hydrolyzed to pantothenic acid prior to absorption via active and passive transport | Inflammatory bowel disease and alcohol intake may decrease absorption | |
| Biotin | Protease and biotinidase cleave biotin from protein and reduce to free biotin before absorption via carrier-mediated diffusion | Inflammatory bowel disease, achlorhydria, and the biotin-binding protein avidin found in raw egg whites may impair absorption | |
| Choline | Hydrolysis of lecithin by lipases releases choline before carrier-mediated and passive diffusion processes | Endogenous de novo synthesis of choline occurs but alone is insufficient to meet needs |

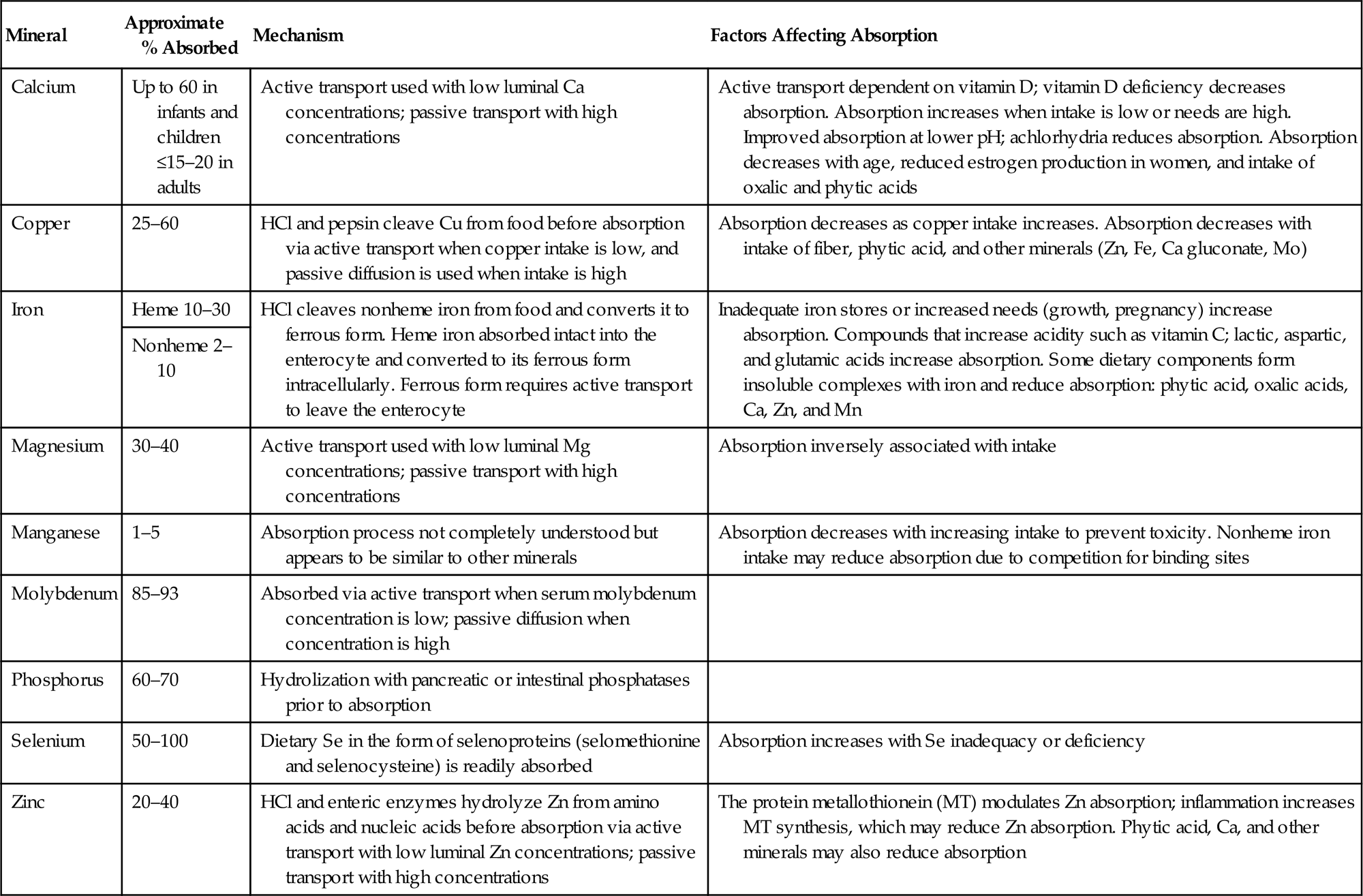
Table 14.9
Absorption of Select Minerals (Mueller, 2012; Allen et al., 2006; Mahan et al., 2012; NIH, 2016)
| Mineral | Approximate % Absorbed | Mechanism | Factors Affecting Absorption |
| Calcium | Up to 60 in infants and children ≤15–20 in adults | Active transport used with low luminal Ca concentrations; passive transport with high concentrations | Active transport dependent on vitamin D; vitamin D deficiency decreases absorption. Absorption increases when intake is low or needs are high. Improved absorption at lower pH; achlorhydria reduces absorption. Absorption decreases with age, reduced estrogen production in women, and intake of oxalic and phytic acids |
| Copper | 25–60 | HCl and pepsin cleave Cu from food before absorption via active transport when copper intake is low, and passive diffusion is used when intake is high | Absorption decreases as copper intake increases. Absorption decreases with intake of fiber, phytic acid, and other minerals (Zn, Fe, Ca gluconate, Mo) |
| Iron | Heme 10–30 | HCl cleaves nonheme iron from food and converts it to ferrous form. Heme iron absorbed intact into the enterocyte and converted to its ferrous form intracellularly. Ferrous form requires active transport to leave the enterocyte | Inadequate iron stores or increased needs (growth, pregnancy) increase absorption. Compounds that increase acidity such as vitamin C; lactic, aspartic, and glutamic acids increase absorption. Some dietary components form insoluble complexes with iron and reduce absorption: phytic acid, oxalic acids, Ca, Zn, and Mn |
| Nonheme 2–10 | |||
| Magnesium | 30–40 | Active transport used with low luminal Mg concentrations; passive transport with high concentrations | Absorption inversely associated with intake |
| Manganese | 1–5 | Absorption process not completely understood but appears to be similar to other minerals | Absorption decreases with increasing intake to prevent toxicity. Nonheme iron intake may reduce absorption due to competition for binding sites |
| Molybdenum | 85–93 | Absorbed via active transport when serum molybdenum concentration is low; passive diffusion when concentration is high | |
| Phosphorus | 60–70 | Hydrolization with pancreatic or intestinal phosphatases prior to absorption | |
| Selenium | 50–100 | Dietary Se in the form of selenoproteins (selomethionine and selenocysteine) is readily absorbed | Absorption increases with Se inadequacy or deficiency |
| Zinc | 20–40 | HCl and enteric enzymes hydrolyze Zn from amino acids and nucleic acids before absorption via active transport with low luminal Zn concentrations; passive transport with high concentrations | The protein metallothionein (MT) modulates Zn absorption; inflammation increases MT synthesis, which may reduce Zn absorption. Phytic acid, Ca, and other minerals may also reduce absorption |
Altered Metabolism
Adequate amounts of micronutrients may be successfully consumed and absorbed, but deficiency can still occur if nutrient utilization is impaired or altered. Some disease states and medications can increase micronutrient losses in the urine or stool, reduce conversion to active forms, and reduce the body’s ability to store or transport micronutrients.
Liver disease in particular has a significant effect on micronutrient utilization, as the liver is a primary storage site for some vitamins, produces transport proteins, and serves as a site for such metabolic reactions as hydroxylation or phosphorylation, which are necessary to convert some vitamins to biologically active forms. For example, adequate liver function is necessary for vitamin D to undergo the first of two hydroxylation reactions needed to convert it to the active form. Many individuals with advanced liver disease already suffer from malnutrition and insufficient nutrient intake; impairment in storage, metabolism, and transport further compromises micronutrient status (Johnson et al., 2013).
The kidneys are also a site for metabolism of some micronutrients, particularly vitamin D. People with chronic kidney disease are unable to produce sufficient amounts of the biologically active form of vitamin D because the damaged kidney cannot sufficiently perform the second hydroxylation needed for this conversion. Vitamin D deficiency is thus more common in this population (Tsiaras and Weinstock, 2011).
Individuals who undergo regular renal replacement therapy such as hemodialysis risk water-soluble vitamin and mineral deficiencies due to increased losses during the dialysis process, increased needs, and inadequate intake. It is recommended that those undergoing dialysis receive water-soluble vitamin supplementation in the presence of inadequate intake or deficiency. Water-soluble vitamin supplements have been specifically formulated for this population (K/DOQI, 2000).
The skin is another organ necessary for maintenance of adequate vitamin D stores because skin cells produce the inactive form of vitamin D when exposed to ultraviolet radiation. With sufficient sunlight, those with limited intake or absorption of vitamin D from foods can still produce enough of the vitamin to maintain adequate serum levels. However, because of a greater awareness of cosmetic changes and skin cancer risks associated with ultraviolet radiation, as well as lifestyle changes such as increasing participation in indoor activities, exposure to sunlight has decreased significantly in industrialized nations. Scientists suspect this has contributed to a greater prevalence of vitamin D deficiency. Older populations are particularly at risk because the skin’s ability to synthesize vitamin D decreases with age. Furthermore, older individuals, especially those who are residents of long term care facilities, often receive little exposure to sunlight (Tsiaras and Weinstock, 2011).
Diagnosis of Deficiency
Accurate diagnosis of micronutrient deficiencies can be challenging. Serum levels of many vitamins are typically not reflective of total body stores; in some cases, serum levels remain within normal ranges until the later stages of deficiency. Serum levels of many minerals are also not indicative of deficiency. In particular, phosphorus and calcium homeostasis is maintained via a complex interplay of hormones, which results in mineral release or deposition from the bones to maintain serum levels, regardless of total body stores or intake (Esper, 2015).
Serum levels of other minerals and some vitamins are also greatly affected by disease states. In particular, liver disease and acute illness or injury resulting in inflammation reduces hepatic production of negative acute-phase serum proteins. Many of these proteins serve as micronutrient transporters, thus serum levels tested in the presence of liver disease or inflammation are less likely to be reflective of total body stores (Bresnahan and Tanumihardjo, 2014). Reductions in serum levels of some vitamins such as A, C, and E, as well as such minerals as Se, Cu, Fe, and Zn, are seen in patients with significant trauma or inflammation (Sriram and Lonchyna, 2009).
Experts disagree on the normal or ideal serum level for some micronutrients, which further complicates the issue of deficiency diagnosis. In particular, nutrition experts have not reached a universal consensus regarding recommended serum vitamin D levels, although the vitamin has been widely studied in recent years and is now recognized as commonly deficient worldwide (Brouwer-Brolsma et al., 2013). There is also significant variability in the methods used to perform biochemical tests by various laboratories, thus reference values are not consistent. See Tables 14.10 and 14.11 for commonly utilized reference values for serum vitamin and mineral levels (Mueller, 2012).
Table 14.10
Commonly Used Assays for Vitamins (Mueller, 2012)
| Vitamin | Assay | Normal Level |
| A | Serum vitamin A | >30 µg/dL |
| D | 25-hydroxyvitamin D | 30–70 ng/mL |
| E | Alpha-tocopherol | 5.5–18 mg/L |
| K | International normalized ratio | 0.8–1.2 |
| Prothrombin time | 10–15 s | |
| B1 | Whole blood thiamin | 70–180 nmol/L |
| Plasma thiamin | 8–30 nmol/L | |
| Erythrocyte transketolase activity | <1.2 µg/mL/h | |
| B2 | Serum riboflavin | 5–50 nmol/L |
| Erythrocyte glutathione reductase | <1.2 IU/g | |
| B3 | Serum niacin | 0.5–15 ng/mL |
| N-methylnicotinamide | >5.8 µmol/day | |
| B6 | Plasma pyridoxal-5 phosphate | 20–125 nmol/L |
| B12 | Serum B12 | 210–911 pg/mL |
| Serum methylmalonic acid | 0–0.4 µmol/dL | |
| C | Plasma vitamin C | 0.2–2 mg/dL |
| Folic acid | Serum folate | 5.4–40 ng/mL |
| RBC folate | 280–903 ng/mL | |
| Pantothenic acid | Whole blood pantothenic acid | >1 µmol/L |
| Urinary excretion | >1 mg/day | |
| Biotin | Serum biotin | 100–400 pmol/L |
| Urinary excretion | >6 µg/day | |
| Choline | Plasma choline | 10 µmol/L |

Table 14.11
Commonly Used Assays for Select Minerals (Mueller, 2012)
| Mineral | Assay | Normal Level |
| Copper | Serum copper | 0.75–1.45 µg/mL |
| Iodine | Serum iodine | 40–92 ng/mL |
| Urinary iodine | 100–199 µg/L | |
| Iron | Serum iron males | 24–336 µg/L |
| Serum iron females | 11–307 µg/L | |
| Transferrin | 170–340 mg/dL | |
| Iron-binding capacity | 240–450 mg/dL | |
| Manganese | Blood manganese | 4.7–18.3 ng/mL |
| Serum manganese | 0–2 µg/L | |
| Molybdenum | Serum molybdenum | 0.58–0.8 µg/L |
| Selenium | Serum selenium | 23–190 µg/L |
| Urinary selenium | 10–35 µg/day | |
| Plasma glutathione peroxidase | >10.5 U/mL | |
| Zinc | Plasma zinc | 70–120 µg/dL |
| RBC zinc | 1000–1600 µg/dL |
B12 status can be particularly challenging to assess because serum levels do not clearly reflect body stores. Both B12 and folate deficiency can cause megaloblastic anemia, although mean corpuscular volume, which indicates the size of the red blood cell, may not necessarily be elevated when B12 or folate deficiency is present or if there is a concurrent iron deficiency. Serum methylmalonic acid and homocysteine can be measured; levels of these compounds may be elevated in B12 deficiency. It is important to note that treatment of folate deficiency may correct megaloblastic anemia, which will then mask the presence of a B12 deficiency; while the anemia is corrected, the neurological effects of B12 deficiency may still be present and can become permanent if the deficiency is not treated (Chan and Mike, 2014).
Because of these confounding factors related to diagnosis, the clinician must examine not only biochemical indices but also estimate dietary intake, assess risk factors, and conduct a physical examination to identify possible signs of deficiency. Micronutrient deficiencies are most likely to manifest in body structures that can be easily examined, including the skin, hair, nails, eyes, mouth, and tongue. See Table 14.12 for physical signs of micronutrient deficiency (Esper, 2015; Pogatshnik and Hamilton, 2011).
Table 14.12
Physical Signs of Deficiency for Select Micronutrients (Mueller, 2012; Mahan et al., 2012; Pogatshnik and Hamilton, 2011; Esper, 2015)
| Micronutrient | Physical Sign or Symptom |
| Vitamin A | Poor wound healing, abnormally dry skin, follicular hyperkeratosis; pale, mottled, or poor blanching in nails; night blindness, conjunctival xerosis, Bitot’s spots, keratomalacia |
| Vitamin D | Swollen painful joints, rickets, bowleg |
| Vitamin K | Petechiae, ecchymosis |
| Vitamin B1 | Stomatitis, pitting edema, motor weakness, peripheral neuropathy |
| Vitamin B2 | Nasolabial seborrhea, angular palpebritis, stomatitis, cheilosis, angular stomatitis, glossitis, atrophic filiform papillae |
| Vitamin B3 | Pellagrous dermatitis, nasolabial seborrhea, angular palpebritis, cheilosis, angular stomatitis, glossitis, edematous tongue, dementia |
| Vitamin B6 | Stomatitis, nasolabial seborrhea, angular palpebritis, cheilosis, angular stomatitis, glossitis, peripheral neuropathy |
| Vitamin B12 | Pale conjunctivae, glossitis, atrophic filiform papillae, peripheral neuropathy, dementia |
| Vitamin C | Poor wound healing, perifolliculosis, petechiae, ecchymosis; pale, mottled, or poorly blanching nails; splinter hemorrhages on nails, stomatitis, bleeding spongy gums, pitting edema |
| Folic Acid | Pallor, pale conjunctivae, stomatitis, glossitis, atrophic filiform papillae |
| Biotin | Pallor, stomatitis, hair loss, dermatitis, glossitis |
| Copper | Pallor, corkscrew hair, hair loss, depigmentation of hair, peripheral neuropathy |
| Iodine | Goiter or enlarged thyroid |
| Iron | General pallor, pale conjunctivae, poor capillary refill, koilonychia, angular stomatitis, atrophic filiform papillae |
| Zinc | Poor wound healing, generalized dermatitis, impaired night vision, alopecia, dys- and hypogeusia |
Treatment
Treatment of micronutrient deficiencies should include three key components: (1) identification and treatment of the underlying cause of the deficiency if possible, (2) education and counseling for patients on ways to increase micronutrient consumption and absorption from foods if appropriate, and (3) implementation of an appropriate treatment regimen, including the appropriate chemical form of the supplement, dose, route and timing of administration, and duration of treatment. When determining appropriate treatment regimens, many factors should be considered, including the severity of deficiency, presence of medical conditions that affect nutrient absorption or utilization (especially renal and hepatic function), and reliability and reputation of the supplement manufacturer.
In the presence of untreated fat malabsorption, aqueous forms of fat-soluble vitamins may be used to treat or prevent deficiency. In patients with short bowel syndrome or another malabsorptive condition difficult to treat medically, various forms and doses of water-soluble vitamins are available; increasing the dose, even above the tolerable upper level established by the US Department of Agriculture, may be necessary to effectively treat deficiency. Oral supplementation may be available in tablet, liquid, or chewable forms, especially multivitamin with mineral supplements.
If oral supplementation is ineffective, other forms may be utilized, most notably intravenous solutions, although these are generally used in patients who cannot consume food by mouth and require parenteral nutrition. Vitamin B12 is commonly supplemented via a monthly intramuscular injection, especially for those with gastric or ileum resections that prevent B12 absorption. B12 is also available in the form of nasal sprays or gels.
The chemical form of the nutrient should also be considered when choosing a supplement, especially for minerals. Many forms of Ca and Fe are readily available, and some forms are more easily absorbed than others. It is important to carefully read supplement labels to determine the elemental amount that the product provides, which is the amount expected to be absorbed.
Absorption considerations also need to be addressed when determining a dosing schedule. For some micronutrients, absorption decreases with increasing intake, thus several smaller doses should be taken daily to maximize absorption capacity. Some dietary components can either increase or decrease absorption of specific micronutrients, so prescription instructions should include whether the supplement should be taken with or without food. See Tables 14.8 and 14.9 for more details.
The duration of treatment will depend on the underlying cause of the deficiency; if the primary cause is difficult to treat medically, then long-term supplementation may be necessary. Periodic reassessment of micronutrient status via biochemical assay and physical exam is necessary to determine if signs and symptoms have resolved, and to prevent toxicity. Toxicity is more likely with fat-soluble vitamins because excess amounts of water-soluble vitamins are generally excreted in the urine.
The effect of micronutrient supplementation on the prevention and treatment of numerous disease states has been studied extensively; while an exhaustive review is outside the scope of this chapter, two common conditions seen in older populations bear mention. First, in patients who are critically ill and require nutrition support, it is recommended that antioxidant vitamins be provided—namely, vitamins E and C and some trace minerals such as Se, Zn, and Cu—because evidence indicates that supplementation may reduce overall mortality, although infectious complications and the length of intensive care unit and hospital stays do not appear to be affected (McClave et al., 2016). Iron, however, should not be supplemented in this population as it may contribute to microorganism proliferation (Bresnahan and Tanumihardjo, 2014).
Second, individuals who undergo cancer treatments such as chemotherapy or radiation are advised to avoid supplements that contain antioxidants because some research suggests this antioxidant effect may protect cancer cells, thus reducing the efficacy of cancer therapies. This effect has not been seen with the intake of antioxidant rich foods; individuals should be encouraged to continue consuming nutrient-rich foods (Lawenda et al., 2008). General multivitamin and mineral supplementation may benefit some individuals with cancer, although patients should be advised to consult their oncologist before beginning a micronutrient supplement.
Conclusion
The use of multivitamin and mineral supplements in the United States is increasing (Gahche et al., 2011). Despite this, many individuals, especially older adults, are at risk for deficiencies due to poor-quality diets and medical conditions and their treatments that affect the absorption or utilization of micronutrients. Micronutrient deficiencies can result in significant signs and symptoms that further complicate medical conditions and reduce the intake of nutrient-dense foods. It is critical that the health-care practitioner recognize the risk factors for deficiency, screen for deficiencies in high-risk populations, and properly diagnose and treat micronutrient deficiencies.
