Cellular and Physiological Effects of Arginine in Seniors
Vance L. Albaugh, Melissa K. Stewart and Adrian Barbul, Vanderbilt University School of Medicine, Nashville, TN, United States
Abstract
Arginine is a conditionally essential amino acid that plays a number of critical roles in human health and disease. Since discovering arginine to be the nitrogen source for nitric oxide, biomedical research has intensely focused on it and its vasodilatory effects. However, other recent work has highlighted not only these potential cardiovascular benefits of arginine and its metabolites but also the advances in the knowledge of arginine biochemistry, organ–organ trafficking, and insights from clinical studies examining its role of arginine as a therapeutic modality in a variety of disease states. These advances, along with a comprehensive review of arginine biochemistry and physiology, are reviewed within this chapter and aim to highlight this robust area of medical research.
Keywords
Arginine; citrulline; nutritional physiology; metabolism; arginase; nitric oxide; nitric oxide synthase
Introduction
Arginine (2-amino-5-guanidinovaleric acid) is an alpha amino acid that has garnered significant research interest for its involvement in a several physiological and pathophysiological processes in human health and disease. Arginine was first shown to be a constituent of animal proteins in 1895 by Hedin (Rogers and Visek, 1985), which spurred interest in the compound’s biologic actions. The discovery of arginine as the nitrogen source of nitric oxide (Palmer et al., 1988) has reinvigorated basic and clinical research on arginine and its potential uses as a nutraceutical or dietary supplement. Given its broad range of physiological actions, many of which are affected by normal physiological aging as well as pathological processes, the aim of this chapter is to review arginine’s broad reach in human biology and identify the current state of research in many of these areas.
Sources of Arginine
Dietary Arginine and Nutritional Importance
Arginine is considered a “conditionally essential” amino acid, which means that its nutritional status (i.e., essential versus nonessential) depends on the health or developmental status of the organism. The human body has the ability to synthesize arginine de novo, but if arginine demand increases, the endogenous supply can be insufficient. Thus, during developmental periods or time of rapid growth and cellular turnover, arginine requirements are significantly increased and necessitate exogenous arginine supply. This need for additional arginine has been confirmed by numerous studies demonstrating that endogenous production is insufficient to meet the metabolic needs of growing infants and other neonates (e.g., piglets, young rats), of adults under highly catabolic conditions (e.g., sepsis, burn injury, trauma), or following extensive bowel resection (Wakabayashi et al., 1994).
Arginine has three potential sources in humans: (1) dietary arginine as a constituent of consumed protein, (2) recycled arginine from endogenous protein turnover, and (3) arginine synthesized de novo. Dietary arginine is available in a variety of foods but is most notably found in significant quantifies in seeds, nuts, seafood, algae, meats, rice protein concentrate, and soy protein isolate (Ros, 2015). In general, these foods have the highest per weight amounts of arginine (Hu et al., 1998; King et al., 2008). In addition to naturally occurring dietary sources, supplements are commercially available to consumers for several putative indications.
Like other dietary amino acids that are digested and liberated by intestinal peptidases, arginine is taken up in the diet through apical enterocyte membranes. Under normal conditions, approximately 40% (30–44%) of dietary arginine is extracted by the splanchnic tissues (Castillo et al., 1993), which is likely secondary to the relatively high arginase activity in the intestinal mucosa, a tissue with a high rate of cellular turnover. Dietary arginine uptake by enterocytes uses several amino acid transporters. These transport systems continue to be identified and studied but include three major categories: system ![]() (cationic amino acid-specific transporters), systems
(cationic amino acid-specific transporters), systems ![]() and
and ![]() (the sodium-dependent and sodium-independent broad-scope transporters, respectively), and the system
(the sodium-dependent and sodium-independent broad-scope transporters, respectively), and the system ![]() (the cation-modulated broad-scope transporter). These transporters have been extensively reviewed elsewhere and continue to be characterized (for reviews, see Devés and Boyd, 1998). Although a discussion of these transporters is beyond the scope of this chapter, it is important to recognize that they are expressed on a variety of cell types in addition to enterocytes and determine the flux of arginine and other amino acids into and across cells and tissues, depending on the density of expression and cell polarity. Virtually all cells have the capacity for arginine transport across the plasma membrane, which facilitates its wide range of biologic uses (Devés and Boyd, 1998).
(the cation-modulated broad-scope transporter). These transporters have been extensively reviewed elsewhere and continue to be characterized (for reviews, see Devés and Boyd, 1998). Although a discussion of these transporters is beyond the scope of this chapter, it is important to recognize that they are expressed on a variety of cell types in addition to enterocytes and determine the flux of arginine and other amino acids into and across cells and tissues, depending on the density of expression and cell polarity. Virtually all cells have the capacity for arginine transport across the plasma membrane, which facilitates its wide range of biologic uses (Devés and Boyd, 1998).
Endogenous Arginine and Whole-Body Arginine Flux
Arginine homeostasis, like that of most amino acids in the plasma, is a dynamic process. Typical arginine plasma concentrations in adult humans range from 80 to 120 µM, relative to the 2.5-mM concentration of total plasma amino acids (Brosnan, 2003). The half-life of arginine in humans and many other mammalian species is approximately one hour (Wu et al., 2007). Human whole-body arginine flux in the postabsorptive state is approximately 70 µM/h to 90 µM/kg/h (Castillo, 1996; Castillo et al., 1995). Thus, extrapolating that flux to a 24 h rate corresponds to approximately 15–20 g of arginine per day. Given that average daily dietary arginine consumption is only 4–6 g per day (Visek, 1986), a significant amount of recycling has to occur to meet basal arginine requirements.
Aside from exogenous dietary sources, baseline metabolic requirements for arginine are fulfilled by endogenous production. This occurs via three separate pathways with recycling from protein turnover being responsible for approximately 85% of available circulating arginine in humans (Castillo, 1996; Dejong et al., 1998). In addition to arginine recovery from protein turnover, arginine can be synthesized from other amino acid metabolites. In the adult human, de novo arginine synthesis can account for approximately 10–15% of arginine production (Luiking et al., 2012). This endogenous synthesis occurs from the conversion of proline, glutamate or glutamine, and citrulline, a nonproteinogenic amino acid that can be converted to arginine through organ-to-organ flux and metabolism by the intestine and kidney. The de novo synthesis of arginine from citrulline accounts for the largest amount of endogenously synthesized arginine (approximately 60–80%) (Curis et al., 2007; 2005). In fact, oral citrulline supplementation can be used to increase plasma arginine quite effectively, as citrulline has an exceedingly high bioavailability and virtually no citrulline is lost in the urine (Rougé et al., 2007; Urschel et al., 2006).
Biochemistry of Arginine
Our understanding of the regulation of arginine metabolism has significantly increased over the last several decades. Arginine is well known for its role in maintaining long-term nitrogen balance as part of the urea cycle, and as the source of nitrogen in the synthesis of nitric oxide (NO). Our appreciation for arginine biochemistry and homeostasis continues to grow and includes a previously unrecognized organ-to-organ trafficking and subcellular localization of arginine-metabolizing enzymes. Each of these processes adds a number of layers of complexity to the role arginine plays in cellular metabolism. However, we have little knowledge of how the expression of catabolic or anabolic enzymes in these pathways changes with aging. We also do not know if arginine flux changes over an organism’s or human’s lifetime.
Arginine Catabolism
Several pathways degrade arginine for use in vital biologic processes. These enzymes have a wide tissue distribution, and the absence of any one of them can have detrimental effects. One of the most studied arginine-metabolizing enzymes is arginase, the final enzyme of the urea cycle that regenerates ornithine. Unlike the other urea cycle enzymes that exist as single isoforms, two human arginase isoforms are encoded by separate genes: arginase I (AI), which is cytosolic, and arginase II (AII), which localizes to the mitochondrion. (Note: ![]() values for AI and AII isoforms are ~0.1 and ~5 mM, respectively (Moinard et al., 2015). Previously the AI and AII isoforms were referred to as hepatic arginase and extrahepatic arginase, respectively. Both arginase isoforms catalyze the hydrolysis of arginine to form ornithine and urea:
values for AI and AII isoforms are ~0.1 and ~5 mM, respectively (Moinard et al., 2015). Previously the AI and AII isoforms were referred to as hepatic arginase and extrahepatic arginase, respectively. Both arginase isoforms catalyze the hydrolysis of arginine to form ornithine and urea:
![]()
The AI gene was cloned in 1986 by Mori and colleagues (Haraguchi et al., 1987) and has a wide tissue distribution, including a high expression in the liver and, to a lesser extent, erythrocytes, gastrointestinal tract, thymus, skin, uterus, and sympathetic ganglia (Yu et al., 2001, 2003). The AII enzyme was discovered in a patient with known AI deficiency who was noted to have residual arginase activity. The AII gene includes a mitochondrial localization signal that targets it for the mitochondrial matrix. Unlike AI, the AII protein is a ubiquitously expressed enzyme (Morris, 2002) with its highest expression in the kidney, gastrointestinal tract, brain, and lactating mammary gland (Yu et al., 2003).
First discovered in the 1980s, nitric oxide synthase is another well studied enzyme that catalyzes the generation of NO and citrulline from arginine:
There are three isotypes of NOS: endothelial NOS (eNOS), inducible NOS (iNOS), and neuronal NOS (nNOS). NOS1 (neuronal NOS) and NOS3 (eNOS) are constitutive enzymes that are controlled by intracellular calcium and calmodulin (Luiking et al., 2012). NOS2 is inducible at the level of gene transcription, calcium independent, and expressed by macrophages and other tissues in response to (pro)inflammatory mediators (Luiking et al., 2012). From a functional perspective, the ![]() values for arginine of the nNOS, eNOS, and iNOS enzymes are approximately 1.8, 3.6, and 12.5 µM, respectively (Gad, 2010).
values for arginine of the nNOS, eNOS, and iNOS enzymes are approximately 1.8, 3.6, and 12.5 µM, respectively (Gad, 2010).
The relationship between arginase and NOS, and their competition for available arginine, has led to a significant amount of investigation. The biological basis for the so-called arginine paradox remains unknown—that is, the ![]() of NOS is in the micromolar range while the
of NOS is in the micromolar range while the ![]() of arginase is in the millimolar range. These enzymes appear to compete for arginine substrate, although biochemically this cannot be explained by
of arginase is in the millimolar range. These enzymes appear to compete for arginine substrate, although biochemically this cannot be explained by ![]() alone. Thus, the subcellular localization of these enzymes or local concentrations of arginine substrate (or both) must underlie the regulation of these two enzymes. Regardless, several studies have examined the possibilities of arginase contributing to the effects of aging such as endothelial dysfunction and hypertension. Exogenous arginine administration induces the expression of both the AI and AII enzymes and leads to early cellular senescence (Xiong et al., 2014). Increased arginase activity negatively influences eNOS efficiency in producing NO. This negative effect has been observed in hypertensive humans in whom exogenous arginine is ineffective in inducing vasodilation (Holowatz and Kenney, 2007). It is thought that increased arginase expression and activity leads to decreased availability of arginine for NO production. Consistent with this hypothesis, the ornithine produced by excessive arginase activity may be shunted to polyamine biosynthesis for vascular smooth muscle hypertrophy, further exacerbating vascular dysfunction with aging (Durante, 2013). Arginase expression is increased with aging in vascular smooth muscle cells, and this increased activity may decrease local arginine availability for vascular endothelial cells, thus decreasing NO biosynthesis. Not all NOS isoforms behave in the same manner, however, with iNOS actually promoting arginase activation as part of a negative feedback mechanism (Santhanam et al., 2007; Shin et al., 2012). Regardless, these cell-to-cell interactions between arginase and NOS affect each other’s activity by decreasing arginine availability (Johnson et al., 2005; Kim et al., 2009).
alone. Thus, the subcellular localization of these enzymes or local concentrations of arginine substrate (or both) must underlie the regulation of these two enzymes. Regardless, several studies have examined the possibilities of arginase contributing to the effects of aging such as endothelial dysfunction and hypertension. Exogenous arginine administration induces the expression of both the AI and AII enzymes and leads to early cellular senescence (Xiong et al., 2014). Increased arginase activity negatively influences eNOS efficiency in producing NO. This negative effect has been observed in hypertensive humans in whom exogenous arginine is ineffective in inducing vasodilation (Holowatz and Kenney, 2007). It is thought that increased arginase expression and activity leads to decreased availability of arginine for NO production. Consistent with this hypothesis, the ornithine produced by excessive arginase activity may be shunted to polyamine biosynthesis for vascular smooth muscle hypertrophy, further exacerbating vascular dysfunction with aging (Durante, 2013). Arginase expression is increased with aging in vascular smooth muscle cells, and this increased activity may decrease local arginine availability for vascular endothelial cells, thus decreasing NO biosynthesis. Not all NOS isoforms behave in the same manner, however, with iNOS actually promoting arginase activation as part of a negative feedback mechanism (Santhanam et al., 2007; Shin et al., 2012). Regardless, these cell-to-cell interactions between arginase and NOS affect each other’s activity by decreasing arginine availability (Johnson et al., 2005; Kim et al., 2009).
Creatine, a molecule important in transient energy storage from high-energy phosphates of adenosine triphosphate (ATP), is a third molecule synthesized from arginine. The creatine synthetic pathway is important in tissues with fluctuating energy needs such as muscle but is important as a rapid source of ATP-derived energy in many other tissues, including the brain, retina, and spermatozoa (Wyss and Kaddurah-Daouk, 2000). Arginine is metabolized by the enzyme arginine:glycine amidinotransferase (![]() ) (Sipilä, 1980), which catalyzes the condensation of arginine and glycine in the following reaction to form a guanidino intermediate:
) (Sipilä, 1980), which catalyzes the condensation of arginine and glycine in the following reaction to form a guanidino intermediate:
Production of this guanidinoacetate intermediate is the rate-limiting step in creatine production, an irreversible process. Creatine is also obtained from diet, so overall flux of arginine into this metabolic pathway is largely determined by the availability of dietary creatine. It is estimated that 20% of dietary intake of arginine is used for creatine phosphate synthesis (Brosnan and Brosnan, 2010). Significant amounts of dietary creatine can act as a feedback repressor of this enzyme to limit de novo creatine production from arginine (Stead et al., 2001; Wyss and Kaddurah-Daouk, 2000).
The fourth and last pathway that utilizes arginine as a direct precursor is the synthesis of agmatine, a compound involved in the synthesis of polyamines. The polyamines (i.e., putrescine, spermine, and spermidine) are vital to cellular proliferation; dysregulated polyamine synthesis is associated with cancer. Agmatine is an intermediate whose biologic functions have not been completely determined, but recent evidence suggests that it may play an inhibitory role on polyamine biosynthesis (Satriano, 2003). The enzyme that generates agmatine from arginine is arginine decarboxylase (![]() 0.75 mM) (Regunathan and Reis, 2000). As its name implies, it decarboxylates arginine to form agmatine and carbon dioxide:
0.75 mM) (Regunathan and Reis, 2000). As its name implies, it decarboxylates arginine to form agmatine and carbon dioxide:
![]()
The agmatine generated in this reaction can then be acted on by another enzyme, agmatinase (Iyer et al., 2002), which generates putrescine (a polyamine) and urea:
![]()
The biologic actions of agmatine continue to be debated, as both the liver and kidney have high levels of arginine decarboxylase to produce agmatine, two tissues with high capacities for arginine biosynthesis (Lortie et al., 1996; Morrissey et al., 1995). There are conflicting reports on whether or not mammalian cells express significant arginine decarboxylase activity (Morris, 2004). The role of agmatine, agmatinase, and arginine decarboxylase continue to be an area of research and may potentially reveal further roles in mammals for this arginine-derived compound in the future.
Arginine Synthesis
Intestinal–Renal Axis
Arginine is synthesized endogenously through two related pathways. The first pathway is referred to as the intestinal–renal axis. First identified in the 1980s, it involves the coordinated actions of the intestine and kidneys in organ-to-organ metabolite shunting that culminates in de novo renal arginine synthesis and release (Fig. 27.1). Metabolic precursors of arginine are converted to citrulline, a common metabolic intermediate.
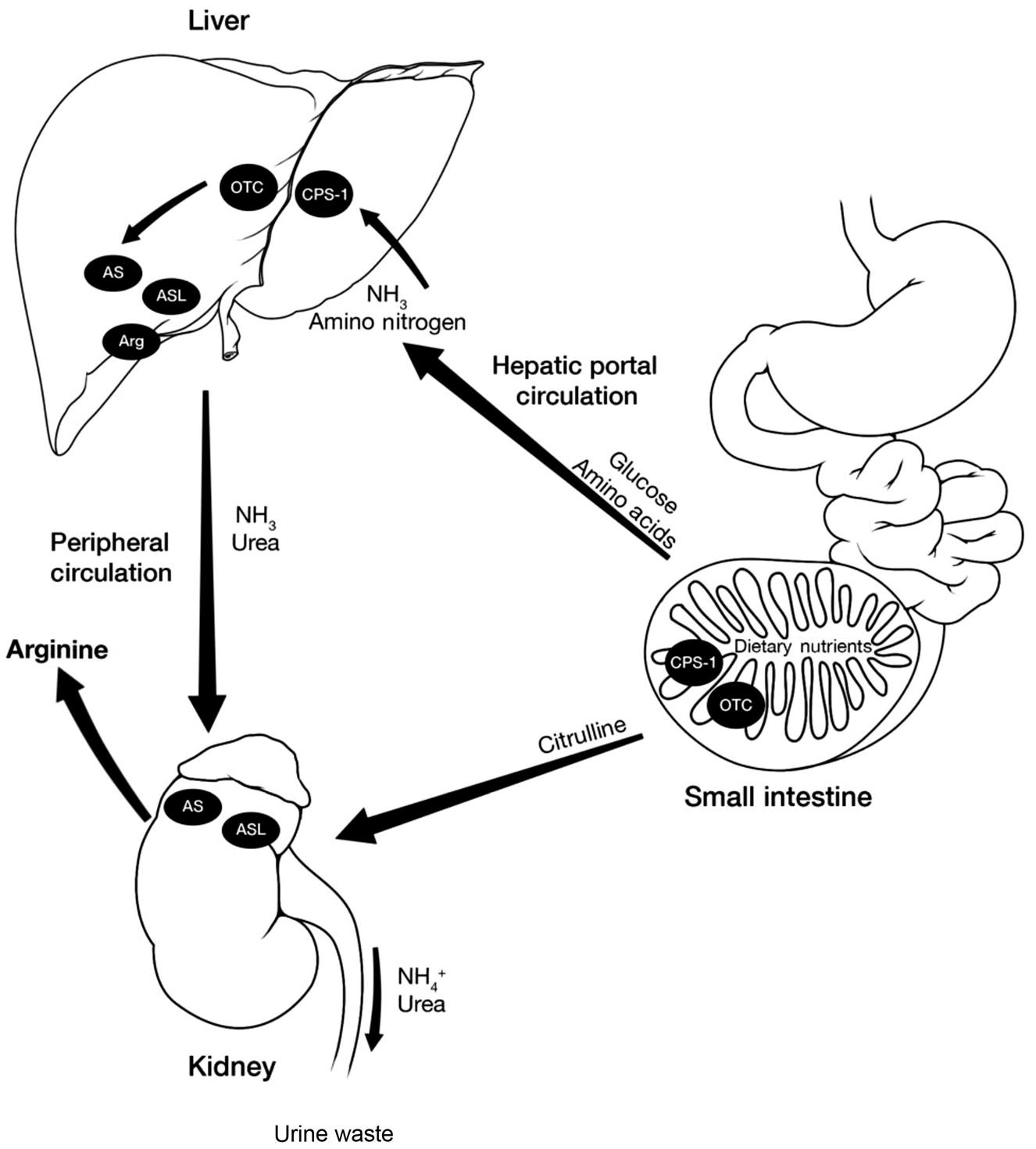
Endogenous arginine synthesis involves a coordinated organ-to-organ trafficking of arginine precursors made possible by the expression of a partial urea cycle in the intestines and kidney, as well as a lack of hepatic citrulline clearance. The complete urea cycle consists of five enzymes: (1) carbamoyl phosphate synthetase 1, (2) ornithine transcarbamoylase (OTC), (3) argininosuccinate synthetase (AS), (4) argininosuccinate lyase (ASL), and (5) arginase (Arg). Dietary nutrients (e.g., amino acids, carbohydrates) are absorbed by CPS-1 and OTC-expressing intestinal enterocytes, which convert arginine precursors to citrulline. This conversion arginine precursors to bypass hepatic portal clearance, which is essentially zero for citrulline. This effectively shunts arginine precursors in the form of citrulline to the kidney. Once in the kidney, citrulline can be converted to arginine by the expression of AS and ASL, releasing arginine into circulation for use by peripheral tissues. Unlike arginine precursors and citrulline, other excess amino acid nitrogen is cleared in the hepatic portal circulation and converted to urea or NH3, which is then excreted into the urine by the kidney.
Cytosolic arginine can be hydrolyzed by arginase in the intestine to produce ornithine and urea:
![]()
Of plasma arginine flux, 15% enters this extrahepatic arginase pathway that degrades arginine to ornithine and urea (Castillo, 1996). Similarly, proline, glutamine, and glutamate can also be metabolized to ornithine, which is subsequently converted to citrulline. Proline is first metabolized to pyrroline-5-carboxylate (P5C) via proline oxidase, and then ornithine aminotransferase coverts P5C to ornithine:
![]()
Glutamine can also be metabolized by glutaminase to form glutamate, which is then converted to P5C and ornithine via ornithine aminotransferase (similar to proline):
![]()
Carbamoyl phosphate and ornithine are then finally condensed in a reaction catalyzed by ornithine transcarbamoylase:
![]()
which generates citrulline in the mitochondria and is then transported to the cytosol ready for export. All of these enzymes that lead to the production of citrulline are expressed within the intestinal enterocytes and represent the expression of a partial urea cycle that exists within the small intestine. This partial urea cycle converts ornithine to citrulline before exporting it from the cell. In addition, dietary ornithine or amino acids that are degraded to ornithine can be recycled to arginine using the classical urea cycle enzymes.
After citrulline is produced by the intestinal enterocytes, it is released into the portal circulation and then recirculates via the arterial circulation to the kidneys. The kidneys efficiently extract the circulating citrulline and convert it to arginine (Fig. 27.1), which becomes available for the rest of the cells and tissues of the body. Experimental data have demonstrated that this de novo arginine synthesis via the intestinal–renal axis is tightly linked to citrulline availability (Luiking et al., 2012). This pathway of converting arginine precursors to a common nonarginine compound (i.e., citrulline) appears advantageous because the liver has virtually zero clearance of citrulline from the portal circulation (Morris, 2002). In stark contrast, the kidney efficiently clears circulating citrulline (Wu and Morris, 2003).
Citrulline comes from several possible sources, including (1) diet; (2) arginine from the NOS pathway; (3) ornithine catabolism of proline, glutamate, or glutamine, and (4) asymmetric dimethyl arginine breakdown. In mice, and potentially humans, the predominant citrulline precursors in this situation are dietary arginine as well as citrulline (Marini, 2012).
Once in the kidney, two cytosolic enzymes that are classically expressed in the urea cycle allow for the conversion of ornithine to arginine. The first enzyme is argininosuccinate synthetase (AS), which condenses citrulline and aspartate to form argininosuccinate. Then the second enzyme, argininosuccinate lyase, generates arginine as well as fumarate as a by-product:
![]()
Note that this is an energetically costly process to the cell, using two molar equivalents of ATP for the cycling process, although the benefit of shunting arginine around the liver appears to be desirable to provide sufficient arginine to the remaining tissues of the body.
Citrulline–Nitric Oxide Cycle
The citrulline–nitric oxide cycle is a theoretical pathway for which the magnitude of flux is inherently difficult to measure and not currently well appreciated. This pathway, however, seems to be active in cells with endogenous NOS activity, potentially as a way to ensure a continuous supply of arginine for NO production. Cells in which arginine would otherwise be limiting can recycle the citrulline produced as a by-product of NOS using Krebs cycle intermediates to continuously generate arginine (Wu and Morris, 2003). Similar to the intestinal–renal axis, the citrulline–NO cycle uses urea cycle enzymes. The citrulline that is produced through the activity of NOS is converted back to arginine via the action of the enzymes argininosuccinate synthase and argininosuccinate lyase. In the first reaction, argininosuccinate synthase condenses citrulline with aspartate:
![]()
Subsequently, argininosuccinate is converted back to arginine by the enzyme argininosuccinate lyase (ASL):
![]()
Hypothetically, this citrulline–NO cycle could continuously generate arginine from citrulline as long as aspartate does not become a limiting factor. Thus, the other vital portion of this cycle is the continuous supply of aspartate. The beauty and simplicity of this cycle, however, is the pathway for the regeneration of aspartate and removal of fumarate that could otherwise build up significant concentrations. The cell appears to handle this via two routes. The first is by the actions of fumarase, which catalyzes the conversion of fumarate to malate, which then can be metabolized by malate dehydrogenase to form oxaloacetate:
![]()
Oxaloacetate can be subsequently acted on by the enzyme aspartate transaminase to regenerate aspartate:
![]()
Alternatively, the fumarate can be converted to malate; then, instead of malate dehydrogenase, malic enzyme can convert the malate to pyruvate. Subsequently, pyruvate can be acted on by pyruvate carboxylase to reform oxaloacetate, which can eventually generate aspartate using the enzymatic conversions above:
![]()
As previously mentioned above, nearly all known cell types express AS and ASL. Thus, further studies are needed to determine the importance and flux rates of these processes in vivo (Morris, 2002). Regardless, experimental data from a number of investigators have suggested that this pathway is induced along with iNOS activity (Mori and Gotoh, 2000), which makes sense teleologically as a means to circumvent a potential arginine deficiency in a time of stress or need (e.g., infection and iNOS activity).
Arginine Physiology and Biological Importance
As mentioned, arginine and its metabolites are important in numerous biologic processes. Arginine is most notable as a urea cycle intermediate and carrier of excretable nitrogen, as well as a precursor of NO that is important in vasodilation and the immune response. More recent studies continue to examine arginine and the arginases, which have critical importance in vascular pathology (Durante et al., 2007). Given our expanded understanding of the biologic actions of arginine, the physiologic effects of arginine supplementation have also been an area of intense investigation. How arginine metabolism changes over time and with aging of the body is less well understood and represent important areas for future research. Each of the aforementioned categories has a specific connection and interest to the aging population and will be broadly discussed in the following section.
Protein Synthesis and Degradation
Other important but less well recognized biological effects involving arginine are its role in targeting proteins for degradation as they come off the ribosome. This so-called N-end rule for protein degradation uses an amino terminal arginine as a marker for protein degradation, which may act as a quality control for cellular protein synthesis (Tasaki and Kwon, 2007; Varshavsky, 1996). Conversely, arginine is a potent stimulator as well as a substrate in protein synthesis. It is estimated that approximately 80% of arginine needed for protein translation comes from recycled arginine (Luiking et al., 2012). Moreover, arginine has been shown in several studies to stimulate the mammalian target of rapamycin (mTOR) pathway, a known regulator of protein translational initiation (Corl et al., 2008; Wang et al., 2012). This effect on protein translation via mTOR is independent of NO (Bauchart-Thevret et al., 2010). Specifically, arginine directly stimulates p70 S6 kinase and phosphorylation of eukaryotic initiation factor 4E binding protein-1 (Ban et al., 2004). In older individuals, evidence suggests that arginine may have protective effects on muscle wasting and could improve lean muscle mass. These studies, however, have examined arginine in combination therapies and not arginine supplementation alone (Clark et al., 2000; Flakoll et al., 2004; May et al., 2002). Overall, these studies have examined functional outcomes in groups with single or multiple amino acid supplements. The effects of these supplements may be either direct effects on skeletal muscle or indirect effects through other hormonal pathways such as growth hormone and insulin and insulin-like growth factor 1.
Acid–Base Homeostasis and the Urea Cycle
In addition to being an important anaplerotic precursor of the Krebs cycle through conversion to glutamate (Owen et al., 2002), arginine and its metabolism are vitally important in humans as an intermediate in the urea cycle. The final step in the pathway is catalysis of arginine by the enzyme arginase, which produces ornithine and urea, allowing the urea to be available for excretion and regenerating ornithine for the cycle to continue to function. It is important to note, as stressed by Morris, that many textbooks erroneously state that a significant amount of arginine comes from its synthesis from the urea cycle. This is far from the case, as virtually no net synthesis of arginine occurs in the liver (Morris, 2009).
Beyond its classic role in nitrogenous waste detoxification, arginine and the urea cycle also play an important role in acid–base homeostasis. This may be less well clinically appreciated, but the urea cycle is bicarbonate consuming (Häussinger, 1986) and critical in the maintenance of whole-body acid–base balance (Häussinger et al., 1984). Aside from the link between the urea cycle and acid–base homeostasis, recent evidence has demonstrated a role for sirtuins, a family of protein deacetylases, as a link between aging and the regulation of the urea cycle (Nakagawa and Guarente, 2009; Nakagawa et al., 2009).
Immunology
As the immune system ages, its capabilities decline and there is increased susceptibility to infection and cancer, with an increased incidence of autoimmune disorders (Agarwal and Busse, 2010). Given such, the use of arginine to prevent or overcome immunosenescence has received much attention.
The role of all amino acids in immunity, including arginine, has been well studied. Arginine is an essential determinant in immune cell metabolism. Specifically, arginine has been shown to be important in T-cell activation and proliferation, B-cell development, and cytokine production and activity (Bronte and Zanovello, 2005; Feldmeyer et al., 2012). Both enzymes of arginine metabolism, NOS and arginase, are felt to be responsible for its immunologic activity.
Absence of L-arginine in culture media results in significant reduction in proliferation of T lymphocytes in response to mitogens (Brittenden et al., 1994). The optimal L-arginine concentration in media for maximal T-lymphocyte proliferation is similar to those found in human plasma (Efron et al., 1991). Arginine is also required for the effective induction of cytotoxic T-cell function in vitro (Moriguchi et al., 1987). Jurkat T cells cultured in media lacking L-arginine manifest rapid reduction in the expression of the T-cell receptor (TCR), CD36ζ, caused by a short half-life of CD36ζ-mRNA(Rodriguez et al., 2002). It is important to note that CD36ζ phosphorylation is the rate-limiting step in the assembly and membrane expression of the TCR (Minami et al., 1987). Such reduction in mRNA decreases TCR internalization, thus leading to a decrease in total TCR cell membrane expression (Bronstein-Sitton et al., 1999; Valitutti et al., 1997).
Arginine has also been identified as being important in a specific stage of murine B-cell development (de Jonge et al., 2002a, b). The investigators engineered a transgenic mouse expressing arginase I under the control of the rat intestinal fatty-acid binding promoter and enhancer element. The mice demonstrated a 30–40% reduction of plasma arginine and showed a disruption in the B-cell development at the progenitor B to precursor B cell interface in the bone marrow. There were also decreased B cells in secondary lymphoid organs such as the spleen and Peyer’s patches in the initial 3 weeks of the neonatal period. The defects were reversed with arginine supplementation (Fafournoux et al., 2000; LeBien, 2002).
Arginine is metabolized in macrophages and lymphocytes by two independent enzymatic pathways, NOS and arginase (Ochoa et al., 2001a). Several clinical conditions—including trauma (Munder et al., 1998), sepsis (Carraway et al., 2015; Witte, 2003), and liver transplantation (Ikemoto et al., 1998; Längle et al., 1995)—have been associated with increased arginase activity, leading to excessive catabolism and subsequent depletion of arginine. This coincides with decreased T-cell proliferation. Supplementation with L-arginine increases CD4+ cells (Kirk et al., 1992), suggesting that arginine may play an important role in reversing the immunosuppression observed during periods of stress (Bernstein, 1996). Specifically, trauma is associated with a greater than 10-fold increase in arginase activity (Ochoa et al., 2001b; 1991). In addition, increased arginase activity in the liver has been implicated in the increased tolerance of the liver to organ rejection (Callery et al., 1991; Schrempf Decker et al., 1983). Moreover, growing evidence suggests that arginine is also an important contributor to intestinal immune function (Li et al., 2007).
Following trauma or surgery, arginine-rich diets were found to reverse the alteration of T-cell function that was initially seen as secondary to the pathologic process. In addition, arginine supplementation in patients undergoing major abdominal operations for gastrointestinal malignancies resulted in increased in vitro immune responses, which correlated to decreased wound infections and decreased length of hospital stay (Daly et al., 1988). T-lymphocyte blastogenesis was also preserved and enhanced in moderately stressed intensive care unit patients given an enteral diet containing large amounts of arginine (Daly et al., 1988). Whether these effects translate into an improvement in clinical outcomes in critically ill patients remains unclear.
Of interest, arginine supplementation following vaccination against Streptococcus pneumoniae in people aged 60 and older resulted in increased neutrophil chemotaxis, natural killer cytotoxicity, and serum concentration of immunoglobulin G. These results suggest that arginine supplementation may enhance the immune response elicited by the pneumococcal vaccine in older people (Moriguti et al., 2005).
Wound Healing
In the United States, chronic wounds affect nearly 6.5 million patients and annually cost in excess of $25 to treat. This burden is expected to grow, in part due to an aging population (Sen et al., 2009). Much research has been focused on the prevention and treatment of wounds. Arginine supplementation has been investigated as a means of enhancing wound healing. Although a lot has been learned, much remains unknown.
Seifter and Levinson first postulated the role of arginine in wound healing in 1978. They demonstrated that arginine-deficient animals subjected to dorsal skin incision and closure had decreased wound breaking strength and wound collagen accumulation when compared to animals fed an normal arginine-containing diet (Seifter et al., 1978). Subsequent experiments revealed that chow-fed rats given a dietary supplement of 1% arginine had enhanced wound healing responses as assessed by wound breaking strength and collagen synthesis when compared to chow-fed controls. Likewise, parenteral fed rats given an amino acid mixture containing high doses of arginine (7.5 g/L) displayed superior wound healing via fresh wound strip breaking strength, fixed breaking strength, and collagen deposition (Barbul et al., 1985). In addition, mature rats fed diets supplemented with arginine and glycine had enhanced wound collagen deposition compared to controls (Chyun and Griminger, 1984).
Using a micromodel described by Goodson and Hunt that allows for studying fibroblastic responses and wound collagen deposition in human (Goodson and Hunt, 1982), 36 healthy human volunteers ages 25–35 years were given daily 17 g or 24.8 g of free arginine supplementation; this significantly increased the amount of hydroxyproline deposition at the wound site (an amino acid-specific for collagen) (Barbul et al., 1990). Subsequently, a study evaluated 30 volunteers >70 years old who were given daily supplements of 17 g of free arginine and demonstrated significantly enhanced wound collagen accumulation. Arginine supplementation had no effect on the rate of epithelialization of a superficial skin defect, indicating that the predominant effect is on wound collagen deposition (Kirk et al., 1993). A more recent study demonstrated that arginine, in combination with other supplements, increases human wound collagen deposition (Williams et al., 2002).
No single theory can account for the observed effects of arginine on wound healing. Several possible mechanisms have been postulated to explain the effects and provide a framework for understanding (Fig. 27.2).
![]() Arginine supplementation provides a substrate for collagen synthesis at the wound site. Although free arginine makes up just a tiny amount of the collagen molecule, less than 5%, there could be utilization of arginine as substrate for proline through the following pathway:
Arginine supplementation provides a substrate for collagen synthesis at the wound site. Although free arginine makes up just a tiny amount of the collagen molecule, less than 5%, there could be utilization of arginine as substrate for proline through the following pathway:![]()
![]() Arginine levels are essentially nondetectable within the wound environment during the later phases of wound healing when fibroplasia predominates. While ornithine levels are higher in the wound than in the plasma, tracer isotope studies revealed that the rate of conversion of ornithine to proline in the wound is actually quite low, making this mechanism of arginine utilization unlikely (Albina et al., 2005).
Arginine levels are essentially nondetectable within the wound environment during the later phases of wound healing when fibroplasia predominates. While ornithine levels are higher in the wound than in the plasma, tracer isotope studies revealed that the rate of conversion of ornithine to proline in the wound is actually quite low, making this mechanism of arginine utilization unlikely (Albina et al., 2005).
![]() Arginine induces collagen synthesis via a pituitary secretagogue mechanism. The beneficial effects of supplemental arginine on wound healing are in many respects similar to those of growth hormone—namely, enhanced wound breaking strength and collagen deposition (Herndon et al., 1990; Jørgensen and Andreassen, 1988; Kowalewski and Yong, 1968). In support of such a mechanism, it has been noted that the effect of arginine on wound healing is abrogated in hypophysectomized animals (Barbul et al., 1983). In addition, arginine supplementation in doses that increase the wound healing response also induces elevations in plasma insulin-like growth factor, the peripheral mediator of growth hormone (Kirk et al., 1993).
Arginine induces collagen synthesis via a pituitary secretagogue mechanism. The beneficial effects of supplemental arginine on wound healing are in many respects similar to those of growth hormone—namely, enhanced wound breaking strength and collagen deposition (Herndon et al., 1990; Jørgensen and Andreassen, 1988; Kowalewski and Yong, 1968). In support of such a mechanism, it has been noted that the effect of arginine on wound healing is abrogated in hypophysectomized animals (Barbul et al., 1983). In addition, arginine supplementation in doses that increase the wound healing response also induces elevations in plasma insulin-like growth factor, the peripheral mediator of growth hormone (Kirk et al., 1993).
![]() Arginine stimulates T-cell responses, thereby reducing the inhibitory effect of injury on T-cell function (Barbul et al., 1980a, b; Fabris and Mocchegiani, 1992). T lymphocytes are essential for normal wound healing as evidenced by decreased wound breaking strength in animals treated with monoclonal antibodies against T lymphocytes (Peterson et al., 1987). T lymphocytes are found immunohistochemically throughout the various phases of wound healing in distinct patterns (Fishel et al., 1987) and facilitating normal repair (Agaiby and Dyson, 1999).
Arginine stimulates T-cell responses, thereby reducing the inhibitory effect of injury on T-cell function (Barbul et al., 1980a, b; Fabris and Mocchegiani, 1992). T lymphocytes are essential for normal wound healing as evidenced by decreased wound breaking strength in animals treated with monoclonal antibodies against T lymphocytes (Peterson et al., 1987). T lymphocytes are found immunohistochemically throughout the various phases of wound healing in distinct patterns (Fishel et al., 1987) and facilitating normal repair (Agaiby and Dyson, 1999).
![]() Arginine is the unique substrate for NO. Several studies suggest that NO plays a critical role in wound healing. Exogenous NO administration has been shown to increase collagen synthesis in cultured dermal fibroblasts (Schäffer et al., 1997). In contrast, inhibitors of NO have been shown to significantly impair healing of cutaneous incisional and colonic anastomotic healing in rodents (Efron, 1999; Schäffer et al., 1999). In models of impaired healing such as diabetes, NO synthesis is impaired together with decreased collagen accumulation, while administration of NO restores wound healing responses toward normal (Shi et al., 2003). Transfection of iNOS DNA into wounds results in supraphysiologic collagen deposition (Thornton et al., 1998). Conversely, mice lacking the iNOS gene have delayed the closure of excisional wounds, an impairment that is remedied by adenoviral transfer of the iNOS gene to the wound bed (Yamasaki et al., 1998). Strongly supporting this mechanism of action are findings that arginine does not stimulate wound healing in iNOS knockout mice, suggesting that the iNOS pathway is at least partially responsible for the enhancement of wound healing observed with the administration of arginine (Shi et al., 2000).
Arginine is the unique substrate for NO. Several studies suggest that NO plays a critical role in wound healing. Exogenous NO administration has been shown to increase collagen synthesis in cultured dermal fibroblasts (Schäffer et al., 1997). In contrast, inhibitors of NO have been shown to significantly impair healing of cutaneous incisional and colonic anastomotic healing in rodents (Efron, 1999; Schäffer et al., 1999). In models of impaired healing such as diabetes, NO synthesis is impaired together with decreased collagen accumulation, while administration of NO restores wound healing responses toward normal (Shi et al., 2003). Transfection of iNOS DNA into wounds results in supraphysiologic collagen deposition (Thornton et al., 1998). Conversely, mice lacking the iNOS gene have delayed the closure of excisional wounds, an impairment that is remedied by adenoviral transfer of the iNOS gene to the wound bed (Yamasaki et al., 1998). Strongly supporting this mechanism of action are findings that arginine does not stimulate wound healing in iNOS knockout mice, suggesting that the iNOS pathway is at least partially responsible for the enhancement of wound healing observed with the administration of arginine (Shi et al., 2000).
Cardiovascular Health
Cardiovascular disease, specifically heart disease, is the leading cause of death in the United States, accounting for more than 600,000 deaths per year (Xu et al., 2016). The role of arginine in cardiovascular health has been extensively studied at the basic science, translational, and clinical levels. Given that cardiovascular disease is a condition of aging, such discussion has implications for the elderly population.
As mentioned, arginine is the precursor for the endogenous synthesis of NO (Palmer et al., 1988; Schmidt et al., 1988). This pathway, although it only accounts for a small portion of arginine metabolism, has attracted much attention given the role of NO in vascular physiology and pathophysiology (Böger et al., 1996; Epstein et al., 1993). Normal plasma arginine concentrations are 80–120 µM, and the ![]() for arginine as substrate for NOS is ~1–10 µM (Brosnan, 2003; Wu et al., 2007). Given such, it appears that there should be a surplus of substrate. Despite this logic, several studies indicate that administration of exogenous arginine increases the generation of NO, a phenomenon now termed the arginine paradox. When examining vascular endothelial function in a lysinuric protein intolerant patient who had a defect in a dibasic amino acid transporter, there was noted impaired uptake of exogenous arginine, leading to a 79% lower plasma concentration. Assessment of the NO-dependent endothelial function revealed 70% lower serum levels of NO. Moreover, the study revealed decreased flow-mediated brachial artery vasodilator response, elevated plasma fibrin degradation products, increased thrombin–antithrombin III complex, and reduced circulating platelet count. Parenteral infusion of arginine reversed all of these effects (Kamada et al., 2001).
for arginine as substrate for NOS is ~1–10 µM (Brosnan, 2003; Wu et al., 2007). Given such, it appears that there should be a surplus of substrate. Despite this logic, several studies indicate that administration of exogenous arginine increases the generation of NO, a phenomenon now termed the arginine paradox. When examining vascular endothelial function in a lysinuric protein intolerant patient who had a defect in a dibasic amino acid transporter, there was noted impaired uptake of exogenous arginine, leading to a 79% lower plasma concentration. Assessment of the NO-dependent endothelial function revealed 70% lower serum levels of NO. Moreover, the study revealed decreased flow-mediated brachial artery vasodilator response, elevated plasma fibrin degradation products, increased thrombin–antithrombin III complex, and reduced circulating platelet count. Parenteral infusion of arginine reversed all of these effects (Kamada et al., 2001).
Generated NO is an important messenger molecule with diverse function. Animal models of human disease—including hypercholesterolemic rabbits (Böger et al., 1997; 1995; Cooke et al., 1991), hyperlipidemic monkeys (Quillen et al., 1991), and hypertensive rats (Lüscher and Vanhoutte, 1986)—have shown that the biologic functions of endothelium-derived NO are impaired, leading to dysregulation of endothelial control of vascular tone and blood flow. In all of these models, arginine supplementation helped restore NO production and improve endothelial dysfunction.
Translated to the human population, a multitude of positive results in the prevention and management of cardiovascular disease and its downstream organ system effects have been reported. Examples include the following:
![]() 4–24 g/day of enteral arginine supplementation significantly lowered both systolic and diastolic blood pressure (Dong et al., 2011);
4–24 g/day of enteral arginine supplementation significantly lowered both systolic and diastolic blood pressure (Dong et al., 2011);
![]() 6.6 g/day of enteral arginine supplementation in patients with peripheral arterial disease resulted in a 66% increase in pain-free walking distance, 23% increase in total walking distance, and improved emotional and social functions per SF-36—effects that were initially noted at two weeks and were sustained at 10 weeks (Maxwell et al., 2000);
6.6 g/day of enteral arginine supplementation in patients with peripheral arterial disease resulted in a 66% increase in pain-free walking distance, 23% increase in total walking distance, and improved emotional and social functions per SF-36—effects that were initially noted at two weeks and were sustained at 10 weeks (Maxwell et al., 2000);
![]() 15 g/day of enteral arginine supplementation in patients with congestive heart failure led to improvement in glomerular filtration rate, natriuresis, and plasma endothelin levels at 5 days (Watanabe et al., 2000);
15 g/day of enteral arginine supplementation in patients with congestive heart failure led to improvement in glomerular filtration rate, natriuresis, and plasma endothelin levels at 5 days (Watanabe et al., 2000);
![]() 6.6 g/day of enteral arginine supplementation in a patient with type 1 diabetes with debilitating exertional angina pectoris resulted in complete amelioration of angina and normalized exercise capacity at 7 days (Schwartz, 2003);
6.6 g/day of enteral arginine supplementation in a patient with type 1 diabetes with debilitating exertional angina pectoris resulted in complete amelioration of angina and normalized exercise capacity at 7 days (Schwartz, 2003);
![]() 8.4 g/day of enteral arginine supplementation in hypercholesterolemic humans showed modest attenuation of platelet reactivity and aggregation (Wolf et al., 1997); and
8.4 g/day of enteral arginine supplementation in hypercholesterolemic humans showed modest attenuation of platelet reactivity and aggregation (Wolf et al., 1997); and
![]() 17 g/day of enteral arginine supplementation in a healthy, nonsmoking, elderly population revealed a decreased serum total cholesterol level with a reduction in low-density lipoprotein cholesterol but not high-density lipoprotein cholesterol. Thus, the ratio of low- to high-density lipoprotein fraction was increased (Hurson et al., 1995).
17 g/day of enteral arginine supplementation in a healthy, nonsmoking, elderly population revealed a decreased serum total cholesterol level with a reduction in low-density lipoprotein cholesterol but not high-density lipoprotein cholesterol. Thus, the ratio of low- to high-density lipoprotein fraction was increased (Hurson et al., 1995).
Despite the evidence supporting the use of arginine for cardiovascular health, other studies have failed to show any benefit. In one randomized clinic trial, there was no improvement in vascular stiffness measurements or ejection fraction with the addition of arginine in the acute postinfarction period (Schulman et al., 2006). Moreover, in men with stable angina, oral supplementation with 15 g/day of arginine was not associated with improvement in endothelium-dependent vasodilation, oxidative stress, or exercise performance (Walker et al., 2001). Lastly, oral supplementation of 6 g/day of arginine in patients with coronary artery disease did not affect exercise-induced changes in QT-interval duration, QT dispersion, or the magnitude of ST-segment depression (Bednarz et al., 2000).
Though the clinical and therapeutic benefit of arginine supplementation on mitigating cardiovascular disease varies, arginine supplementation may be favorable in at-risk patient populations. The possible role of arginine in maintaining or improving cardiac function prior to onset of severe disease merits further study.
Neurologic Function
Neurodegeneration is characterized by progressive deterioration in cognitive ability and capacity for independent living. The arising clinical syndrome—dementia—is typically a condition that affects older people and leads to disability and dependence (Prince et al., 2013). Given the rising epidemic secondary to population aging, much research has been undertaken on the topic.
Given its role in NO production via neuronal NOS, the function of arginine supplementation on neurologic function has yet to be fully investigated. However, studies exploring the importance of NO on memory and learning via synaptic plasticity is growing (Böhme et al., 1991; Susswein et al., 2004; Zhou and Zhu, 2009). In vitro experiments have shown that NO serves to increase glutamate release and increase synaptic effectiveness, an occurrence termed potentiation that is thought to improve memory (Epstein et al., 1993). In vivo, the same physiology is likely present, for studies reveal that inhibiting NO impairs learning behavior (Chapman et al., 1992). A few investigations have explored the role of arginine supplementation on age-related degenerative neurologic disorders, specifically senile dementia and Alzheimer’s disease. The former study revealed improved cognitive function and reduced lipid peroxidation in 16 elderly patients following arginine supplementation (Ohtsuka and Nakaya, 2000). The latter concluded that arginine might provide a protective effect through the redox stress and inflammatory process and regulation of synaptic plasticity and neurogenesis (Yi et al., 2009).
Sexual Function
Sexual dysfunction is highly prevalent in both men and women and is a topic of particular importance to the elderly population because sexual dysfunction is clearly associated with advancing age (Laumann et al., 1999). Given the prevalence, a multitude of investigations have focused on sexual dysfunction, including a possible role for arginine supplementation. Thus far, there is no clear evidence of benefit. It is known that NO, through the synthesis of cyclic guanosine 5'-monophosphate, functions to initiate and maintain increased intracavernous pressure, penile vasodilatation, and penile erection. Thus, it is postulated that the production of NO via arginine, through the NOS pathway, may be of importance in sexual physiology.
In the 1950s, researchers first showed that adult men fed an arginine-deficient diet for nine days had an approximately 90% decreased sperm count and a 10-fold increase in the percentage of nonmotile sperm (Albanese, 1952). A subsequent study showed that supplementing infertile men with oral arginine for 6–8 weeks increased sperm counts and motility (Tanimura, 1967). The role of arginine in erectile dysfunction has also been studied. Studies in mice (Moody et al., 1997) and humans (Chen et al., 2001; Zorgniotti and Lizza, 1994) revealed improved erectile response and, in humans, subjective improvement in sexual function following arginine supplementation. A more robust study, however, utilizing a crossover design, failed to show benefit in arginine supplementation versus placebo in the management of impotence (Klotz et al., 1999). Despite the unclear benefit, arginine remains an active ingredient in most over-the-counter aphrodisiacs and sexual enhancement formulas.
Endocrinology
Endocrine disturbances are common in the aging population. A study in 2010 revealed that 9.6% of people 65 and older carried the diagnosis of diabetes mellitus (DM). An additional 9.3% of the population met clinical criteria for the disease but had not yet been diagnosed (Harris, 1990). Given such prevalence, the role of arginine on the endocrine system has been studied and will be discussed in the following.
Intravenous infusions of essential amino acids, specifically arginine, induce the release of insulin in humans (Floyd et al., 1966). Further, enteral intraduodenal arginine infusions result in sustained increases in plasma insulin levels (Dupre et al., 1969) with no observed hypoglycemic events. Later studies revealed that arginine supplementation also increases concentrations of glucagon (Palmer et al., 1976) and growth hormone (Merimee et al., 1965). The role of arginine in growth hormone secretion was further studied and revealed mixed results (Isidori et al., 1981). Arginine has also been found to stimulate the release of prolactin (Rakoff et al., 1973), pancreatic polypeptides, and somatostatin (Weir et al., 1978). Despite evidence of the endocrine-related actions of arginine, the underlying mechanism remains unclear. One proposed method is through arginine-derived NO (Schmidt et al., 1992).
Diabetes is associated with reduced levels of plasma arginine (Pieper et al., 1996) and elevated levels of the NOS inhibitor asymmetric dimethylarginine (Abbasi et al., 2001). Arginine supplementation may be an effective way to improve endothelial function and insulin sensitivity in DM (Giugliano et al., 1997; Wascher et al., 1997). Arginine also reduces microangiopathic complications of DM by counteracting lipid peroxidation (Lubec et al., 1997). A robust double-blind study revealed that oral administration of 9 g/day of arginine significantly improved peripheral and hepatic insulin sensitivity (Piatti et al., 2001). Again, the mechanisms remains unclear, but both NO-dependent (Schmidt et al., 1992) and NO-independent (Thams and Capito, 1999) pathways have been postulated to be operational.
Arginine Dosing and Supplementation
Throughout this chapter, we have discussed many putative effects of arginine based on the growing appreciation of arginine biochemistry and biology since the 1980s. The discovery of arginine as the precursor of nitric oxide led to increasing numbers of studies examining the potential effects in patients with coronary artery disease, peripheral artery disease, and other diseases that may benefit from increased NO production. However, many other conditions may also have therapeutic benefits, including wound healing and immunologic responses. Consumers have many over-the-counter supplements available to them, all of which are various formulations of arginine or arginine in combination with one or more other nutrients or compounds. Overall there is no good systematic data identifying which if any of these supplements might be beneficial. A number of studies examining oral arginine supplementation can be found in Table 27.1, which demonstrates the diversity in dosing and indications for arginine supplementation that have been examined in humans. When considering supplementation with arginine, an arginine-containing supplement, or any nutritional supplement, medical advice should always be obtained.
Table 27.1
Representative Studies Examining Oral Arginine Administration in Human Subjects on Clinical Endpoints
| Study | Year | Subjects | Time Course | Total Daily Arginine Dose | Endpoints |
| Rector et al. (1996) | 1996 | Heart failure (n=15); crossover | 6 weeks | 5.6–12.6 g | Increased forearm blood flow |
| Wolf et al. (1997) | 1997 | Hypercholesterolemia (n=15 arginine, 8 placebo) | 2 weeks | 8.4 g | Decreased platelet aggregation |
| Ceremużyński et al. (1997) | 1997 | Prior MI; stable angina (n=12 arginine, 10 placebo) | 3 days | 6 g | Increased time to maximal ST depression during exercise; decreased maximal ST depression |
| Lerman et al., (1998) | 1998 | CAD (n=13 arginine, 13 placebo) | 6 months | 9 g | Increased acetylcholine-mediated coronary blood flow; improved symptom scores |
| Blum et al. (2000) | 2000 | CAD patients (n=30); crossover | 1 months | 9 g | Increased plasma arginine; no change in nitrogen oxides, flow-mediated brachial artery dilation, or vascular adhesion molecules |
| Mullen et al. (2000) | 2000 | Type 1 diabetes (ages 18–47; n=22 arginine, 22 placebo) | 6 weeks | 14 g | No change in endothelial dysfunction |
| Maxwell et al. (2000) | 2000 | PAD (intermittent claudication) (n=12/15 arginine, 14 placebo) | 8 weeks | 0, 3.3, 6.6 g | Increased pain-free walking and walking distance |
| Maxwell et al. (2002) | 2002 | CAD (chronic stale angina) (n=36); crossover | 2 weeks | 6 g (food sup) | Improved flow-mediated arterial dilation, increased treadmill exercise time |
| Lekakis et al. (2002) | 2002 | Essential hypertension (n=18 arg, 17 placebo) | 1.5 h | 6 g | Flow-mediated dilation of the brachial artery increased |
| Staff et al. (2004) | 2004 | Pre-eclampsia (n=15 arginine, 15 placebo) | 5 days | 12 g | No change in diastolic blood pressure |
| Oka et al. (2005) | 2005 | PAD (n=18 placebo, 18/17/19 arginine); | 12 weeks | 0, 9, 18, 27 g | Nonsignificant trends for increased speed and distance |
| VINTAGE MI Trial* (Schulman et al., 2006) | 2006 | 60 years or older (n=78 arginine, 75 placebo); confirmed STEMI | 6 months | 9 g | No effect on vascular stiffness or ejection fraction; *trial halted for increased mortality in arginine group |
| NO-PAIN Study (Wilson et al., 2007) | 2007 | PAD; intermittent claudication (n =67 placebo, 66 arginine) | 6 months | 3 g | No changes in nitrogen oxides, vascular reactivity, or walking distance |
| Ruel et al. (2008) | 2008 | Surgical three-vessel CAD (n=19 total, divided into 4 groups) | 3 months | 6 g vs. placebo (all patients receiving VEGF intraop) | Increased myocardial perfusion and contractility |
| Lucotti et al. (2009) | 2009 | Nondiabetic CAD (n=32 arginine, 32 placebo) | 6 months | 6.4 g | Increased insulin sensitivity; decreased IL-6, MCP-1 |

CAD, coronary arterial disease; PAD, peripheral arterial disease; STEMI, ST-elevation myocardial infarction; MI, myocardial infarction; MCP-1, monocyte chemotactic protein-1; IL-6, interleukin-6; VEGF, vasculoendothelial growth factor; VINTAGE MI, Vascular Interaction with Age in Myocardial Infarction.
Pharmacokinetics of Arginine and Citrulline
The biologic effects of arginine appear to be influenced by its plasma concentration, and several studies have made kinetic measurements following single or multiple oral doses (Bode-Böger et al., 1998). More recently, the pharmacokinetics of citrulline have been investigated given the close relationship of arginine and citrulline (i.e., the citrulline–NO pathway), as well as the observation that citrulline increases circulating arginine via endogenous synthesis. For example, Moinard and colleagues found that citrulline supplementation increased plasma arginine concentrations without altering urea or urine urea nitrogen excretion, thus increasing nitrogen balance (Rougé et al., 2007).
The average plasma concentration of arginine in fasted humans is approximately 80–100 µM (Bode-Böger et al., 1998; Cynober, 2002; Tangphao et al., 1999), while citrulline concentration is significantly less at approximately 40 µM (Cynober, 2002). Two particular studies have reported both 6 g (Bode-Böger et al., 1998) or 10 g (Tangphao et al., 1999) oral supplementation of arginine leading to maximal concentrations of approximately 300 µM that are reached 60–90 min following ingestion. Maximal concentration is probably in this range for any single tolerated dose, given that increasing arginine concentration appears to plateau at greater administered amounts. When comparing arginine ingestion and citrulline ingestion at increasing doses, citrulline is actually more effective at increasing arginine concentration compared to supplemental arginine itself (Schwedhelm et al., 2008). As one might expect, this is consistent with the endogenous pathway of arginine synthesis mentioned previously in this chapter, which involves intestinal release of citrulline that completely bypasses the liver and is transported to the kidney, where citrulline is used for endogenous synthesis of arginine (Fig. 27.1) (Crenn et al., 2008).
The oral bioavailability of a single oral dose of arginine appears to plateau prior to a 6 g administration, as 6 g orally is associated with approximately 70% bioavailability (Bode-Böger et al., 1998) while 10 g appears to saturate transport and be only 20% bioavailable (Tangphao et al., 1999). In contrast to the bioavailability of arginine, however, citrulline bioavailability appears markedly increased, though there appears to be a lack of studies that fully evaluate the kinetics (Cynober, 2007). Regardless, no experiments appear to have used sufficient doses to saturate citrulline absorption by the gastrointestinal tract. The interesting finding is that citrulline lacks these common gastrointestinal (GI) side effects from similarly structural amino acids, indicating that the transport of citrulline does not appear to be limited (Breuillard et al., 2015; Schwedhelm et al., 2008).
Longevity of supplementation and risk of exposure to excess arginine or citrulline and its effect on long-term maintenance of metabolite concentrations remains unknown. It is important to note that most studies examine either acute (single dose) or subacute (days to weeks of treatment) dosing regimens and not chronic dosing (Schwedhelm et al., 2008). It is unknown how these different lengths of exposure affect the expression of metabolizing enzymes or other compensatory mechanisms given the increased arginine or citrulline delivered via supplementation. Also, it has yet to be elucidated whether the biologic effect could wane or subside with time or prolonged exposure.
Practical Considerations
There are several practical considerations regarding arginine supplementation. The obvious hurdle to overcome with increasingly larger doses of oral arginine supplementation is that arginine has an unpleasant taste, at least at doses of >7 g orally at a single administration (Evans et al., 2004). Larger doses (approximately 9 g per single oral dose and greater) tend to be poorly tolerated in terms of gastrointestinal cramping, bloating, and diarrhea. These GI effects are likely related to the osmotic effects of slow enterocyte uptake (Grimble, 2007), but there are data to suggest that these may be mediated by local increases in NO concentration in the enterocyte milieu as well. Note that supplementation is typically on a background dietary intake of arginine that typically ranges from 2.5 to 5 g/day (Gad, 2010). Despite previous studies reporting supplemental doses of up to 30 g/day (in split doses) being generally well tolerated, more recent studies suggest that nausea and diarrhea become nearly universal when single dosing is escalated—especially amounts of arginine from 15 to 30 g per single oral dose (Gad, 2010; Moinard et al., 2008).
As mentioned, the role of citrulline in arginine homeostasis has been studied, especially whether or not citrulline might be a more effective supplement compared to arginine. Citrulline is better tolerated based on the absence of reported gastrointestinal side effects (Moinard et al., 2008). Oral citrulline increases nitrogen balance and plasma arginine concentrations (Rougé et al., 2007). In fact, when the appearance of both citrulline and arginine in the plasma are measured following oral citrulline dosing, plasma arginine concentration increases in response to citrulline more than arginine supplementation. These results are consistent with the conversion of citrulline to arginine in the kidney being a saturable process (Moinard et al., 2008).
As previously mentioned, the GI side effects of arginine supplementation further make the potential for citrulline more attractive as a long-term, high-dose supplement. The intestinal dibasic amino acid transporter responsible for arginine absorption (as well as other amino acids) is a high-affinity and low-capacity system (Grimble, 2007). Arginine, ornithine, citrulline, and cystine share the same enterocyte transporter (Grimble, 2007), but citrulline remains free from many of these side effects associated with significant arginine intake. What is particularly interesting and currently unknown is why citrulline, but not the other metabolites sharing this transporter, lacks GI side effects.
Arginine Dosing and Aging
Whether or not aging affects how arginine dosing should or can be administered is not well studied. Moreover, the exact mechanisms of how aging affects the absorption of nutrients in general is not completely understood in human or animal models, and many questions remain (Holt, 2007; Keller and Layer, 2014; Schiller, 2009). Aging has a range of effects on macro- and micronutrient absorption, and it is likely that these effects could significantly vary between individuals. Clearly, many questions need to be answered about arginine metabolism in general as well as potentially innovative ways identified to take advantage of biology to augment endogenous responses to amino acids for therapeutic purposes. Could citrulline supplementation be a reasonable way to augment endogenous arginine availability? Could citrulline be advantageous in aged individuals or others with intrinsic intestinal pathology leading to poor GI absorption? These as well as many other questions are important but currently remain unanswered.

