11. Nonisothermal Reactor Design—The Steady-State Energy Balance and Adiabatic PFR Applications
If you can’t stand the heat, get out of the kitchen.
—Harry S Truman
11.1 Rationale
To identify the additional information necessary to design nonisothermal reactors, we consider the following example, in which a highly exothermic reaction is carried out adiabatically in a plug-flow reactor.
Example 11-1 What Additional Information Is Required?
The first-order liquid-phase reaction
A → B
is carried out in a PFR. The reaction is exothermic and the reactor is operated adia-batically. As a result, the temperature will increase with conversion down the length of the reactor. Because T varies along the length of the reactor, k will also vary, which was not the case for isothermal plug-flow reactors.

Describe how to calculate the PFR reactor volume necessary for 70% conversion and plot the corresponding profiles for X and T.
Solution
The same CRE algorithm can be applied to nonisothermal reactions as to isothermal reactions by adding one more step, the energy balance.
1. Mole Balance (design equation):
2. Rate Law:
Recalling the Arrhenius equation, k = k1 exp
we know that k is a function of temperature, T.
3. Stoichiometry (liquid phase): υ = υ0
4. Combining:

Following the Algorithm
Combining Equations (E11-1.1), (E11-1.2), and (E11-1.4), and canceling the entering concentration, CA0, yields
Combining Equations (E11-1.3) and (E11-1.6) gives us
Oops! We see that we need another relationship relating X and T or T and V to solve this equation. The energy balance will provide us with this relationship.
So we add another step to our algorithm; this step is the energy balance.
5. Energy Balance:
In this step, we will find the appropriate energy balance to relate temperature and conversion or reaction rate. For example, if the reaction is adiabatic, we will show that for equal heat capacities, CPA and CPB , and a constant heat of reaction,
We now have all the equations we need to solve for the conversion and temperature profiles. We simply type Equations (E11-1.7) and (E11-1.8) along with the parameters into Polymath, Wolfram, or MATLAB; click the RUN button; and then carry out the calculation to a volume where 70% conversion is obtained.
Analysis: The purpose of this example was to demonstrate that for nonisothermal chemical reactions we need another step in our CRE algorithm, the energy balance. The energy balance allows us to solve for the reaction temperature, which is necessary in evaluating the specific reaction rate constant k(T).
Why we need the energy balance
T0 = Entering Temperature
ΔHRx = Heat of Reaction
CPA = Heat Capacity of species A
11.2 The Energy Balance
11.2.1 First Law of Thermodynamics
We begin with the application of the first law of thermodynamics, first to a closed system and then to an open system. A system is any bounded portion of the universe, moving or stationary, which is chosen for the application of the various thermodynamic equations. For a closed system, in which no mass crosses the system boundaries, the change in total energy of the system, dE, is equal to the heat flow to the system, SQ, minus the work done by the system on the surroundings, SW. For a closed system, the energy balance is
The δ’s signify that SQ and SW are not exact differentials of a state function.
The continuous-flow reactors we have been discussing are open systems in which mass crosses the system boundary. We shall carry out an energy balance on the open system shown in Figure 11-1. For an open system in which some
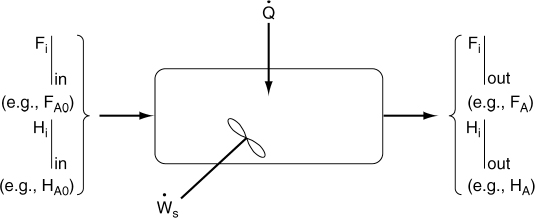
of the energy exchange is brought about by the flow of mass across the system boundaries, the energy balance for the case of only one species entering and leaving becomes
Energy balance on an open system
Typical units for each term in Equation (11-2) are (Joule/s). (Joule bio: http://www.corrosion-doctors.org/Biographies/JouleBio.htm.)
We will assume that the contents of the system volume are well mixed, an assumption that we could relax but that would require a couple of pages of text to develop, and the end result would be the same! The unsteady-state energy balance for an open well-mixed system that now has n species, each entering and leaving the system at its respective molar flow rate Fi (moles of i per time) and with its respective energy Ei (Joules per mole of i), is
We will now discuss each of the terms in Equation (11-3).
The starting point
11.2.2 Evaluating the Work Term
It is customary to separate the work term,
where P is the pressure (Pa) [1 Pa = 1 Newton/m2 = 1 kg·m/s2/m2] and
Flow work and shaft work
Let’s look at the units of the flow-work term, which is
where F¡ is in mol/s, P is in Pa (1 Pa = 1 Newton/m2), and
We see that the units for flow work are consistent with the other terms in Equation (11-3), i.e., J/s (http://www.corrosion-doctors.org/BiographiesfWattBio.htm).
In most instances, the flow-work term is combined with those terms in the energy balance that represent the energy exchange by mass flow across the system boundaries. Substituting Equation (11-4) into (11-3) and grouping terms, we have
The energy F¡ is the sum of the internal energy (Ui), the kinetic energy (
In almost all chemical reactor situations, the kinetic, potential, and “other” energy terms are negligible in comparison with the enthalpy, heat transfer, and work terms, and hence will be omitted, in which case
We recall that the enthalpy, Hi (J/mol), is defined in terms of the internal energy Ui (J/mol), and the product PVi (1 Pa·m3/mol = 1 J/mol):
Convention Heat Added
Heat Removed
Work Done by System
Work Done on System
Enthalpy
Enthalpy carried into (or out of) the system can be expressed as the sum of the internal energy carried into (or out of) the system by mass flow plus the flow work:
Combining Equations (11-5), (11-7), and (11-8), we can now write the energy balance in the form
The energy of the system at any instant in time, Êsys, is the sum of the products of the number of moles of each species in the system multiplied by their respective energies. The derivative of the Esys term wrt time will be discussed in more detail when unsteady-state reactor operation is considered in Chapter 13.
We shall let the subscript “0” represent the inlet conditions. The conditions at the outlet of the chosen system volume will be represented by the unsubscripted variables.
In Section 11.1, we discussed that in order to solve reaction engineering problems with heat effects, we needed to relate temperature, conversion, and rate of reaction. The energy balance as given in Equation (11-9) is the most convenient starting point as we proceed to develop this relationship.
Energy Balance

11.2.3 Overview of Energy Balances
What is the plan? In the following pages we manipulate Equation (11-9) in order to apply it to each of the reactor types we have been discussing: BR, PFR, PBR, and CSTR. The end result of the application of the energy balance to each type of reactor is shown in Table 11-1. These equations can be used in Step 5 of the algorithm discussed in Example E11-1. The equations in Table 11-1 relate temperature to conversion and to molar flow rates, and to the system parameters, such as the product of overall heat-transfer coefficient, U, and heat exchange surface area, a, i.e., Ua, with the corresponding ambient temperature, Ta, and the heat of reaction, ΔHRx.
Examples of How to Use Table 11-1.
We now couple the energy balance equations in Table 11-1 with the appropriate reactor mole balance, rate law, and stoichiometry algorithm to solve reaction engineering problems with heat effects.
TABLE 11-1 ENERGY BALANCES OF COMMON REACTORS
1. Adiabatic (
Conversion in terms of temperature
Temperature in terms of conversion calculated from the energy balance
For an exothermic reaction (−HRx) > 0
2. CSTR with heat exchanger, UA (Ta − T), and large coolant flow rate
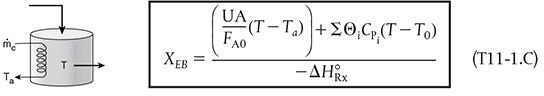
3. PFR/PBR with heat exchange

In general most of the PFR and PBR energy balances can be written as
3A. PFR in terms of conversion
3B. PBR in terms of conversion
3C. PBR in term of molar flow rates
3D. PFR in term of molar flow rates
4. Batch
5. For Semibatch or unsteady CSTR
6. For multiple reactions in a PFR (q reactions and m species)
i = reaction number, j = species
7. Variable heat exchange fluid temperature, Ta
Co-current Exchange
Countercurrent Exchange
The equations in Table 11-1 are the ones we will use to solve reaction engineering problems with heat effects.
Nomenclature:
U = overall heat-transfer coefficient, (J/m2 · s · K);
A = CSTR heat-exchange area, (m2);
a = PFR heat-exchange area per volume of reactor, (m2/m3);
All other symbols are as defined in Chapters 1 through 10.
End results of manipulating the energy balance (Sections 11.2.4, 12.1, and 12.3)
For example, recall the rate law for a first-order reaction, Equation (E11-1.5) in Example 11-1
which will be combined with the mole balance to find the concentration, conversion and temperature profiles (i.e., BR, PBR, PFR), exit concentrations, and conversion and temperature in a CSTR. We will now consider four cases of heat exchange in a PFR and PBR: (1) adiabatic, (2) co-current, (3) countercur-rent, and (4) constant exchanger fluid temperature. We focus on adiabatic operation in this chapter and the other three cases in Chapter 12.
Case 1: Adiabatic. If the reaction is carried out adiabatically, then we use Equation (E11-1.3) for the reaction
Adiabatic
Consequently, we can now obtain –rA as a function of X alone by first choosing X, then calculating T from Equation (T11-1.B), then calculating k from Equation (E11-1.3), and then finally calculating (−rA) from Equation (E11-1.5).
We can use this sequence to prepare a table of (FA0/−rA) as a function of X. We can then proceed to size PFRs and CSTRS. In the absolute worst case scenario, we could use the techniques in Chapter 2 (e.g., Levenspiel plots or the quadrature formulas in Appendix A). However, instead of using a Levenspiel plot, we will most definitely use Polymath to solve our coupled differential energy and mole balance equations.
The algorithm

Levenspiel plot
Cases 2, 3, and 4: Correspond to Co-current Heat Exchange, Countercurrent Heat Exchange, and Constant Coolant Temperature TC, respectively (Ch. 12). If there is cooling along the length of a PFR or PBR, we could then apply Equation (T11-1.D) to this reaction to arrive at two coupled differential equations.
Non-adiabatic PFR
which are easily solved using an ODE solver such as Polymath. If the temperature of the heat exchanger fluid (Ta) (e.g., coolant) is not constant, we add Equations (T11-1.K) or (T11-1.L) in Table 11-1 to the above equations and solve with Polymath, Wolfram, or MATLAB.
Heat Exchange in a CSTR. Similarly, for the case of the reaction A — B in Example 11-1 carried out in a CSTR, we could use Polymath or MATLAB to solve two nonlinear algebraic equations in X and T. These two equations are the combined mole balance
Non-adiabatic CSTR
and the application of Equation (T11-1.C), which is rearranged in the form
From these three cases, (1) adiabatic PFR and CSTR, (2) PFR and PBR with heat effects, and (3) CSTR with heat effects, one can see how to couple the energy balances and mole balances. In principle, one could simply use Table 11-1 to apply to different reactors and reaction systems without further discussion. However, understanding the derivation of these equations will greatly facilitate the proper application to and evaluation of various reactors and reaction systems. Consequently, we now derive the equations given in Table 11-1.
Why bother to derive the equations in Table 11-1? Because I have found that students can apply these equations much more accurately to solve reaction engineering problems with heat effects if they have gone through the derivation to understand the assumptions and manipulations used in arriving at the equations in Table 11.1, That is, understanding these derivations, students are more likely to put the correct number in the correct equation symbol.
Why bother? Here is why!!
11.3 The User-Friendly Energy Balance Equations
We will now dissect the molar flow rates and enthalpy terms in Equation (11-9) to arrive at a set of equations we can readily apply to a number of reactor situations.
11.3.1 Dissecting the Steady-State Molar Flow Rates to Obtain the Heat of Reaction
To begin our journey, we start with the energy balance Equation (11-9), and then proceed to finally arrive at the equations given in Table 11-1 by first dissecting two terms:
1. The molar flow rates, Fi and Fi0
2. The molar enthalpies, Hi, Hi0[Hi = Hi(T), and Hi0 = Hi(T0)]
Interactive
Computer Modules
An animated and somewhat humorous version of what follows for the derivation of the energy balance can be found in the reaction engineering games “Heat Effects 1” and “Heat Effects 2” on the CRE Web site, http://www.umich.edu/~elements/5e/icm/index.html. Here, equations move around the screen, making substitutions and approximations to arrive at the equations shown in Table 11-1. Visual learners find these two ICGs a very useful resource (http://www.umich.edu/~elements/5e/icm/heatfx1.html).
We will now consider flow systems that are operated at steady state. The steady-state energy balance is obtained by setting (dÊsys/dt) equal to zero in Equation (11-9) in order to yield
Steady-state energy balance
To carry out the manipulations to write Equation (11-10) in terms of the heat of reaction, we shall use the generic reaction
The inlet and outlet summation terms in Equation (11-10) are expanded, respectively, to
and
where the subscript I represents inert species.
We next express the molar flow rates in terms of conversion. In general, the molar flow rate of species i for the case of no accumulation and a stoichiometric coefficient vi is
Specifically, for Reaction (2-2),
Steady-state operation
We can substitute these symbols for the molar flow rates into Equations (11-11) and (11-12), then subtract Equation (11-12) from (11-11) to give
The term in parentheses that is multiplied by FA0X is called the heat of reaction at temperature T and is designated ΔHRx(T).
Heat of reaction at temperature T
All enthalpies (e.g., HA, HB) are evaluated at a temperature T in the reactor and, consequently, [ΔHRx(T)] is the heat of reaction at that specific temperature T. The heat of reaction is always given per mole of the species that is the basis of calculation, i.e., species A (Joules per mole of A reacted).
Substituting Equation (11-14) into (11-13) and reverting to summation notation for the species, Equation (11-13) becomes
Combining Equations (11-10) and (11-15), we can now write the steady-state, i.e., (dÊsys/dt = 0), energy balance in a more usable form:
One can use this form of the steady-state energy balance if the enthalpies are available.
If a phase change takes place during the course of a reaction, this is the form of the energy balance, i.e., Equation (11-16), that must be used.
11.3.2 Dissecting the Enthalpies
We are neglecting any enthalpy changes resulting from mixing so that the partial molal enthalpies are equal to the molal enthalpies of the pure components. The molal enthalpy of species i at a particular temperature and pressure, Hi, is usually expressed in terms of an enthalpy of formation of species i at some reference temperature TR, Hi°(TR), plus the change in enthalpy, ΔHQi, that results when the temperature is raised from the reference temperature, TR, to some temperature T
The reference temperature at which Hi°(TR) is given is usually 25°C. For any substance i that is being heated from T1 to T2 in the absence of phase change
No phase change
Typical units of the heat capacity, CPi, are
A large number of chemical reactions carried out in industry do not involve phase change. Consequently, we shall further refine our energy balance to apply to single-phase chemical reactions. Under these conditions, the enthalpy of species i at temperature T is related to the enthalpy of formation at the reference temperature TR by
If phase changes do take place in going from the temperature for which the enthalpy of formation is given and the reaction temperature T, Equation (11-17) must be used instead of Equation (11-19).
The heat capacity at temperature T is frequently expressed as a quadratic function of temperature; that is
However, while the text will consider only constant heat capacities, the PRS R11.1 on the CRE Web site has examples with variable heat capacities (http://www.umich.edu/~elements/5e/11chap/prof.html).
To calculate the change in enthalpy (Hi - Hi0) when the reacting fluid is heated without phase change from its entrance temperature, Ti0, to a temperature T, we integrate Equation (11-19) for constant CPi to write

References Shelf
Substituting for Hi and Hi0 in Equation (11-16) yields
Result of dissecting the enthalpies
11.3.3 Relating ΔHRx(T), ΔH°Rx(TR), and ΔCP
Recall that the heat of reaction at temperature T was given in terms of the enthalpy of each reacting species at temperature T in Equation (11-14); that is
where the enthalpy of each species is given by
If we now substitute for the enthalpy of each species, we have
For the generic reaction
The first term in brackets on the right-hand side of Equation (11-23) is the heat of reaction at the reference temperature TR
The enthalpies of formation of many compounds, Hi° (TR), are usually tabulated at 25°C and can readily be found in the Handbook of Chemistry and Physics and similar handbooks.1 That is, we can look up the heats of formation at TR, then calculate the heat of reaction at this reference temperature. The heat of combustion (also available in these handbooks) can also be used to determine the enthalpy of formation, Hi° (TR), and the method of calculation is also described in these handbooks. From these values of the standard heat of formation, Hi° (TR), we can calculate the heat of reaction at the reference temperature TR using Equation (11-24).
1 CRC Handbook of Chemistry and Physics, 95th ed. (Boca Raton, FL: CRC Press, 2014).
The second term in brackets on the right-hand side of Equation (11-23) is the overall change in the heat capacity per mole of A reacted, ΔCP,
Combining Equations (11-25), (11-24), and (11-23) gives us
Heat of reaction at temperature T
Equation (11-26) gives the heat of reaction at any temperature T in terms of the heat of reaction at a reference temperature (usually 298 K) and the ΔCP term. Techniques for determining the heat of reaction at pressures above atmospheric can be found in Chen.2 For the reaction of hydrogen and nitrogen at 400°C, it was shown that the heat of reaction increased by only 6% as the pressure was raised from 1 atm to 200 atm!
Heat of reaction at temperature T
Calculate the heat of reaction for the synthesis of ammonia from hydrogen and nitrogen at 150°C in terms of (kcal/mol) of N2 reacted and also in terms of (kJ/mol) of H2 reacted.
Solution
N2 + 3H2
Calculate the heat of reaction at the reference temperature TR using the heats of formation of the reacting species obtained from Perry’s Chemical Engineers’ Handbook or the Handbook of Chemistry and Physics.3
The enthalpies of formation at 25°C are
Note: The heats of formation of all elements (e.g., H2, N2) are by definition zero at 25°C.
To calculate
2 N. H. Chen, Process Reactor Design (Needham Heights, MA: Allyn and Bacon, 1983), p. 26.
3 D. W. Green, and R. H. Perry, eds., Perry’s Chemical Engineers’ Handbook, 8th ed. (New York: McGraw-Hill, 2008).
or in terms of kJ/mol (recall: 1 kcal = 4.184 kJ)
Exothermic reaction
The minus sign indicates that the reaction is exothermic. If the heat capacities are constant or if the mean heat capacities over the range 25°C to 150°C are readily available, the determination of AHRx at 150°C is quite simple.
in terms of kJ/mol
The heat of reaction based on the moles of H2 reacted is
Analysis: This example showed (1) how to calculate the heat of reaction with respect to a given species, given the heats of formation of the reactants and the products, and (2) how to find the heat of reaction with respect to one species, given the heat of reaction with respect to another species in the reaction. Finally, (3) we also saw how the heat of reaction changed as we changed the temperature.
Now that we see that we can calculate the heat of reaction at any temperature, let’s substitute Equation (11-22) in terms of ΔHR(TR) and ΔCP, i.e., Equation (11-26). The steady-state energy balance is now
Energy balance in terms of mean or constant heat capacities
From here on, for the sake of brevity we will let
unless otherwise specified.
In most systems, the work term, Ws, can be neglected (note the exception in the California Professional Engineers’ Registration Exam Problem P12-6B at the end of Chapter 12). Neglecting Ws, the energy balance becomes
In almost all of the systems we will study, the reactants will be entering the system at the same temperature; therefore, we will set Ti0 = T0.
We can use Equation (11-28) to relate temperature and conversion and then proceed to evaluate the algorithm described in Example 11-1. However, unless the reaction is carried out adiabatically, Equation (11-28) is still difficult to evaluate because in nonadiabatic reactors, the heat added to or removed from the system can vary along the length of the reactor or Q can vary with time. This problem does not occur in adiabatic reactors, which are frequently found in industry. Therefore, the adiabatic tubular reactor will be analyzed first.
11.4 Adiabatic Operation
Reactions in industry are frequently carried out adiabatically with heating or cooling provided either upstream or downstream. Consequently, analyzing and sizing adiabatic reactors is an important task.
11.4.1 Adiabatic Energy Balance
In the previous section, we derived Equation (11-28), which relates conversion to temperature and the heat added to the reactor, Q. Let’s stop a minute (actually it will probably be more like a couple of days) and consider a system with the special set of conditions of no work,
For adiabatic operation, Example 11.1 can now be solved!
In many instances, the ΔCP (T - TR) term in the denominator of Equation (11-29) is negligible with respect to the
Relationship between X and T for adiabatic exothermic reactions
Equation (11-29) applies to a CSTR, PFR, or PBR, and also to a BR (as will be shown in Chapter 13). For Q = 0 and Ws = 0, Equation (11-29) gives us the explicit relationship between X and T needed to be used in conjunction with the mole balance to solve a large variety of chemical reaction engineering problems as discussed in Section 11.1.

We can rearrange Equation (11-29) to solve for temperature as a function of conversion; that is
Energy balance for adiabatic operation of PFR
11.4.2 Adiabatic Tubular Reactor
Either Equation (11-29) or Equation (11-30) will be coupled with the differential mole balance
to obtain the temperature, conversion, and concentration profiles along the length of the reactor. The algorithm for solving PBRs and PFRs operated adia-batically using a first-order reversible reaction A ⇄ B as an example is shown in Table 11-2 using conversion, X, as the dependent variable.
The solution to reaction engineering problems today is to use softwar packages with ordinary differential equation (ODE) solvers, such as Polymath, MATLAB, or Wolfram, to solve the coupled mole balance and energy balance differential equations.
We will now apply the algorithm in Table 11-2 and the solution procedure in Table 11-3 to a real reaction.
TABLE 11-2 ADIABATIC PFR/PBR ALGORITHM
| The elementary reversible gas-phase reaction | ||
| is carried out in a PFR in which pressure drop is neglected and species A and inert I enter the reactor. | ||
| 1. Mole Balance: | (T11-2.1) | |
| 2. Rate Law: | (T11-2.2) | |
| (T11-2.3) | ||
| and for ΔCP = 0 | ||
| (T11-2.4) | ||
| 3. Stoichiometry: | Gas, ɛ = 0, P = P0 ∴ p ≡ 1 | |
| (T11-2.5) | ||
| (T11-2.6) | ||
| 4. Combine: | (T11-2.7) | |
| At equilibrium ™rA ≡ 0, and we can solve Equation (T11-2.7) for the equilibrium conversion, Xe | ||
| (T11-2.8) | ||
| 5. Energy Balance: | ||
| To relate temperature and conversion, we apply the energy balance to an adiabatic PFR. If all species enter at the same temperature, Ti0 = T0. | ||
| Solving Equation (11-30) for the case of ΔCP = 0 and when only Species A and an inert I enter, we find the temperature as a function of conversion to be | ||
| (T11-2.9) | ||
| Using either Equation (11-29) or Equation (T11-2.9), we find the conversion predicted as a function of temperature from the energy balance is | ||
| (T11-2.10) | ||
| We have added the subscript to the conversion to denote that it was calculated from the energy balance, rather than from the mole balance. Equations (T11-2.1) through (T11-2.10) can easily be solved using either Simpson’s rule or an ODE solver. | ||

Following the Algorithm
TABLE 11-3 SOLUTION PROCEDURES FOR ELEMENTARY ADIABATIC GAS-PHASE PFR/PBR REACTOR
| Moles as the Dependent Variable | ||
| 1. Mole Balances | ||
| 2. Rates | ||
| Recall that for δ ≡ 0, KC ≡ Ke | ||
| 3. Stoichiometry | ||
| 4. energy | ||
| 5. Parameter Evaluation | ||
| T1 = 298 K | Θ1 = 1 | CT0 = 1.0 mol/dm3 |
| T2 = 298 K | KC2 = 75,000 | ΔH°Rx = ™14,000 cal/mol |
| k1 = 3.5 × 10™5 min™1 | E = 10,000 cal/mol | |
| FA0 = 5 mol/min | ||
| 6. Entering Conditions | ||
| When V = 0, FA0 = 5, FB = 0, and T0 = 480 K | ||
| These values are also used in LEP 11-6 on the Web site. | ||

Living Example Problem
Example 11-3 Adiabatic Liquid-Phase Isomerization of Normal Butane
Normal butane, C4H10 , is to be isomerized to isobutane in a plug-flow reactor. Isobutane is a valuable product that is used in the manufacture of gasoline additives. For example, isobutane can be further reacted to form iso-octane. The 2014 selling price of n-butane was $1.5/gal, while the trading price of isobutane was $1.75/gal.†
† Once again, you can buy a cheaper generic brand of n-C4H10 at the Sunday markets in downtown Riça, Jofostan, where there will be a special appearance and lecture by Jofostan’s own Prof. Dr. Sven Köttlov on February 29th, at the CRE booth.
This elementary reversible reaction is to be carried out adiabatically in the liquid phase under high pressure using essentially trace amounts of a liquid catalyst that gives a specific reaction rate of 31.1 h-1 at 360 K. You are to process 100,000 gal/day (163 kmol/h) and achieve 70% conversion of n-butane from a mixture 90 mol % n-butane and 10 mol % i-pentane, which is considered an inert. The feed enters at 330 K.
(a) Set up the CRE algorithm to calculate the PFR volume necessary to achieve 70% conversion.
(b) Use an ODE software package (e.g., Polymath) to solve the CRE algorithm to plot and analyze X, Xe, T, and -TA down the length (volume) of a PFR to achieve 70% conversion.
(c) Show the hand calculation for the step-by-step procedure to calculate T, k, KC, Xe, and –rA in order to generate a table of
(d) Use the table you generated in part (c) to calculate the PFR volume to achieve 70% conversion.
(d) Calculate the CSTR volume for 40% conversion.
The economic incentive $ = 1.75/gal versus 1.50/gal
Additional information:
Solution
n-C4H10 ⇄ i-C4H10
A ⇄ B
Problem statement (a) said to set up the CRE algorithm to find the PFR volume necessary to achieve 70% conversion. This problem statement is risky. Why? Because the adiabatic equilibrium conversion may be less than 70%! Fortunately, it’s not so for the case discussed here, 0.7 < Xe. In general, it would be much safer and better to ask for the reactor volume to obtain 95% of the equilibrium conversion, Xf = 0.95 Xe.
It’s risky business to ask for 70% conversion in a reversible reaction
Part (a) PFR algorithm
1. Mole Balance:
2. Rate Law:
with, for the case of δ = 0, KC = Ke,
The algorithm
3. Stoichiometry (liquid phase, v = v0):
4. Combine:
5. Energy Balance: Recalling Equation (11-27), we have

Following the Algorithm
From the problem statement
Applying the preceding conditions to Equation (11-27) and rearranging gives
Nomenclature Note
6. Parameter Evaluation:
Because n-butane is 90% of the total molar feed, we calculate FA0 to be
where Τ is in degrees Kelvin.
Substituting for the activation energy, T1, and k1 in Equation (E11-3.3), we obtain
Substituting for
Recalling the rate law gives us
7. Equilibrium Conversion:
At equilibrium
and therefore we can solve Equation (E11-3.7) for the equilibrium conversion
Because we know KC (T), we can find Xe as a function of temperature.
After carrying out the Polymath solution we will carry out the hand calculation to help give an intuitive understanding of how the parameters Xe and -rA vary with conversion and temperature.
Part B is the Polymath solution method we will use to solve most all CRE problems with “heat” effects.
Part (b) PFR computer solution to plot conversion and temperature profiles We now will use Polymath to solve the preceding set of equations to find the PFR reactor volume and to plot and analyze X, Xe, -rA, and T down the length (volume) of the reactor. The computer solution allows us to easily see how these reaction variables vary down the length of the reactor and also to study the reaction and reactor by varying the system parameters such as CA0 and T0 (c.f. LEP P11-1A (b)).
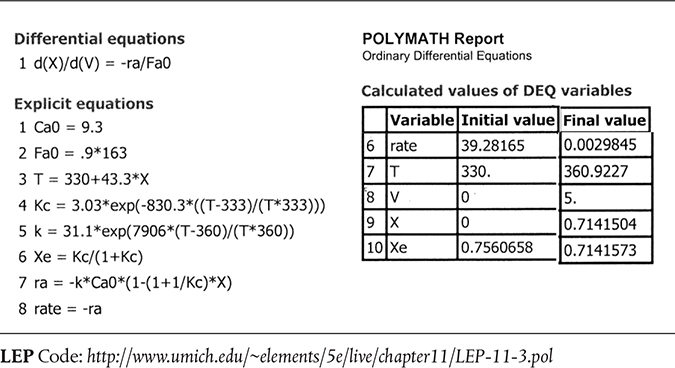
The Polymath program using Equations (E11-3.1), (E11-3.7), (E11-3.9), (E11-3.10), (E11-3.11), and (E11-3.12) is shown in Table E11-3.1.
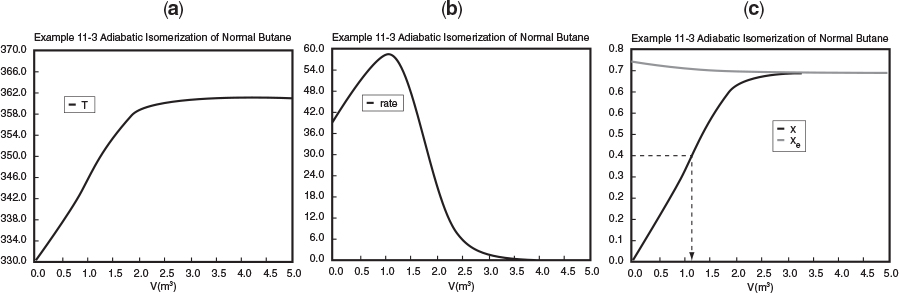
Figure E11-3.1 (a) Adiabatic PFR temperature (K), (b) Reaction rate (kmol/m3/h), and (c) conversion profiles X and Xe.
From Table 11-3.1 and Figure E11-3.1 (c) one observes that at the end of the 5 dm3 PFR both the conversion, X, and equilibrium conversion, Xe, reach 71.4%. One also notes in Figures E11-3.1 (a) and (b) that the temperature reaches a plateau of 360.9 K where the net reaction rate approaches zero at this point in the reactor. Be sure to explore each of the three parts of Figure E11-3.1 using Wolfram.
Wolfram Sliders
Analysis: Parts (a) and (b) for the PFR. The graphical output for the PFR is shown in Figure E11-3.1. We see from Figure E11-3.1(c) that a PFR volume 1.15 m3 is required for 40% conversion. The temperature and reaction-rate profiles are also shown. Notice anything strange? one observes that the rate of reaction
Look at the shape of the curves in Figure E11-3.1. Why do they look the way they do?
goes through a maximum. Near the entrance to the reactor, T increases as does k, causing term A to increase more rapidly than term B decreases, and thus the rate increases. Near the end of the reactor, term B is decreasing more rapidly than term A is increasing as we approach equilibrium. Consequently, because of these two competing effects, we have a maximum in the rate of reaction. Toward the end of the reactor, the temperature reaches a plateau as the reaction approaches equilibrium (i.e., X ≡ Xe at (V ≡ 3.5 m3)). As you know, and as do all chemical engineering students at Jofostan University in Riça, at equilibrium (–rA ≅ 0) no further changes in X, Xe, or T take place.
Part (c) Solution by hand calculation to perhaps give greater insight and to build on techniques in Chapter 2
We will now integrate Equation (E11-3.8) using Simpson’s rule after forming a table (E11-3.1) to calculate (FA0/ —rA) as a function of X. This procedure is similar to that described in Chapter 2. We now carry out a sample calculation to show how Table E11-3.1 was constructed.
For example, for X = 0.2 , follow the downward arrows for the sequence of the calculations.
We are only going to do this once!!

Sample calculation for Table E11-3.1
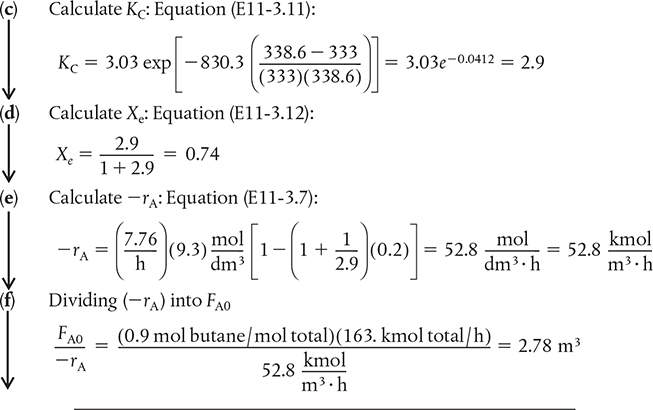
Continuing in this manner for other conversions, we can complete Table E11-3.2.
I know these are tedious calculations, but someone’s gotta

TABLE E11-3.2 HAND CALCULATION
| X | T(K) | k(h-1) | KC) | Xe) | –rA)(kmol/m3 · h) | |
| 0 | 330 | 4.22 | 3.1 | 0.76 | 39.2 | 3.74 |
| 0.2 | 338.7 | 7.76 | 2.9 | 0.74 | 52.8 | 2.78 |
| 0.4 | 347.3 | 14.02 | 2.73 | 0.73 | 58.6 | 2.50 |
| 0.6 | 356.0 | 24.27 | 2.57 | 0.72 | 37.7 | 3.88 |
| 0.65 | 358.1 | 27.74 | 2.54 | 0.718 | 24.5 | 5.99 |
| 0.7 | 360.3 | 31.67 | 2.5 | 0.715 | 6.2 | 23.29 |
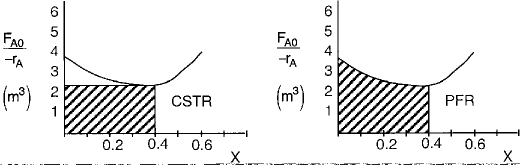
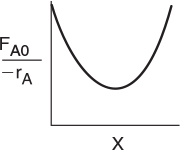
Use the data in Table E11-3.2 to make a Levenspiel plot, as in Chapter 2.
(e) The reactor volume for 70% conversion will be evaluated using the quadrature formulas. Because (FA0/–rA) increases rapidly as we approach the adiabatic equilibrium conversion, 0.71, we will break the integral into two parts
Using Equations (A-24) and (A-22) in Appendix A, we obtain
Why are we doing this hand calculation? If it isn’t helpful, send me an email and you won’t see this again.
Analysis: Part (c) You probably will never ever carry out a hand calculation similar to the one shown above. So why did we do it? Hopefully, we have given you a more intuitive feel for the magnitude of each of the terms and how they change as one moves down the reactor (i.e., what the computer solution is doing), as well as a demonstration of how the Levenspiel Plots of (FA0/-rA) vs. X in Chapter 2 were constructed. At the exit, V = 2.6 m3, X = 0.7, Xe = 0.715, and T = 360 K.
Later, 10/10/19: Actually, since this margin note first appeared in 2011, I have had 2 people say to keep the hand calculation in the text so I kept it in.
Is VPFR > VCSTR or is VPFR < VCSTR?
Part (d) CSTR Solution
First, take a guess. Let’s now calculate the adiabatic CSTR volume necessary to achieve 40% conversion. Do you think the CSTR will be larger or smaller than the PFR?
The CSTR mole balance is
Using Equation (E11-3.7) in the mole balance, we obtain
From the energy balance, we have Equation (E11-3.9):
Using Equations (E11-3.10) and (E11-3.11) or from Table E11-3.1, at T = 347.3 K, we find k and KC to be
Then
We see that the CSTR volume (1 m3) to achieve 40% conversion in this adiabatic reaction is less than the PFR volume (1.15 m3).
By recalling the Levenspiel plots from Chapter 2, we can see that the reactor volume for 40% conversion is smaller for a CSTR than for a PFR. Plotting (FA0/ — rA) as a function of X from the data in Table E11-3.1 is shown here.
In this example, the adiabatic CSTR volume is less than the PFR volume.
The PFR area (volume) is greater than the CSTR area (volume).
Analysis: In this example we applied the CRE algorithm to a reversible-first-order reaction carried out adiabatically in a PFR and in a CSTR. We note that at the CSTR volume necessary to achieve 40% conversion is smaller than the volume to achieve the same conversion in a PFR. In Figure E11-3.1(c) we also see that at a PFR volume of about 3.5 m3, equilibrium is essentially reached about halfway through the reactor, and no further changes in temperature, reaction rate, equilibrium conversion, or conversion take place farther down the reactor.
AspenTech: Example 11-3 has also been formulated in AspenTech and can be loaded on your computer directly from the CRE Web site.

For reversible reactions, the equilibrium conversion, Xe, is usually calculated first.
11.5 Adiabatic Equilibrium Conversion
The highest conversion that can be achieved in reversible reactions is the equilibrium conversion. For endothermic reactions, the equilibrium conversion increases with increasing temperature up to a maximum of 1.0. For exothermic reactions, the equilibrium conversion decreases with increasing temperature.
11.5.1 Equilibrium Conversion
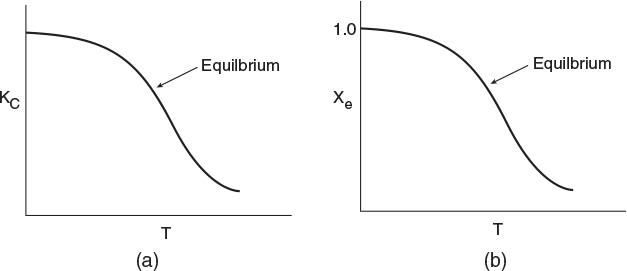
Exothermic Reactions. Figure 11-3(a) shows the variation of the concentration equilibrium constant, KC as a function of temperature for an exothermic reaction (see Appendix C), and Figure 11-3(b) shows the corresponding equilibrium conversion Xe as a function of temperature. In Example 11-3, we saw that for a first-order reaction, the equilibrium conversion could be calculated using Equation (E11-3.12)
First-order reversible reaction
Consequently, Xe can be calculated as a function of temperature directly using either Equations (E11-3.12) and (E11-3.4) or from Figure 11-3(a) and Equation (E11-3.12).
For exothermic reactions, the equilibrium conversion decreases with increasing temperature.
We note that the shape of Xe versus T curve in Figure 11-3(b) will be similar for reactions that are other than first order.
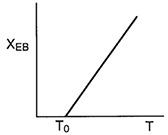
To maximum the maximum conversion that can be achieved in an exothermic reaction carried out adiabathelly, we find the intersection of the equilibrium conversion as a function of temperature (Figure 11-3(b)) with temperature-conversion relationships from the energy balance (Figure 11-2 and Equation (TII-I.A)), as shown in Figure 11-4.
Figure 11-3 Variation of equilibrium constant and conversion with temperature for an exothermic reaction (http://www.umich.edu/~elements/5e/11chap/summary-biovan.html).

Figure 11-4 Graphical solution of equilibrium and energy balance equations to obtain the adiabatic temperature and the adiabatic equilibrium conversion Xe.
Adiabatic equilibrium conversion for exothermic reactions
This intersection of the energy balance line, XEB, with the thermodynamic equilibrium Xe curve gives the adiabatic-equilibrium conversion and temperature for an entering temperature T0.
If the entering temperature is increased from T0 to T01, the energy balance line will be shifted to the right and be parallel to the original line, as shown by the dashed line. Note that as the inlet temperature increases, the adi-abatic equilibrium conversion decreases.