Appendix H
Selected Sampling Methods and Documentation
Primary proof of environmental damage requires evidence, and soil, soil vapor, air, and water samples are the main forms of evidence presented to regulatory agencies and discussed in courtrooms. In ideal conditions, the samples are collected by a licensed professional, such as an experienced professional geologist or professional engineer or by staff under their direction, and the samples are analyzed by an independent third‐party certified laboratory.
The process of assessing spills and leaks in soil, surface water, groundwater, air, and soil vapor relies on standard operating procedures (SOPs), specialized equipment, and detailed record keeping. When the spillage source is unknown, subsurface sampling tends to be an iterative process to define the vertical and lateral extent of a release. The information in this appendix was designed to be a general overview of sampling methods and documentation. Site‐specific challenges, local regulatory policies, or health exposure issues may require other procedures or different techniques than the general methods described below.
General Sampling Procedures
All information pertinent to field investigations is typically kept on forms that comprise the field documents in which all pertinent information about borehole samples and groundwater samples are recorded.
Field Documents
- Daily Field Form
- Workplan with Technical Objectives
- Site‐Specific Safety Plan
- Load List
- Transportation Log and Safety Checklist
- Boring Log Forms
- Water Sampling Data Sheets
- Sample chain‐of‐Custody
The information documented includes the following minimum data:
- Mapped investigation location.
- Sample number.
- Mapped sample location, including boring or well number, and/or depth.
- Name of collector.
- Date and time of collection.
- Analyses to be performed.
- Field observations.
- Field measurements (photoionization detector (PID) readings, pH, conductivity, water levels).
- Mapped GPS readings for sample locations.
- Photographs documenting sampling event.
- Other data forms.
Decontamination
Prior to arriving at a sampling site, all hand augering and direct push technology sampling equipment should be cleaned with a steam cleaner (or more commonly a hot‐water pressure washer) or through a three‐bucket decontamination process using phosphate‐free detergent and rinsed twice with deionized (DI) water, which can be tap water treated by using a standard deionizing resin column (EPA 2015). This procedure should also be carried out on‐site prior to, between, and after collecting each sample. Drilling equipment and samplers should be decontaminated and thoroughly cleaned or bought new from a trusted vendor prior to arriving on‐site and between uses. Decontamination is best conducted on an impermeable surface on a plastic sheet with berms allowing for all effluent to be contained and collected. The drilling equipment including hollow stem augers, flight augers, soil core barrels, etc. are commonly placed on a rack for air drying. All other sampling equipment is typically washed with nonphosphate detergent and rinsed twice with DI water. Water used for decontamination should be stored in labeled containers certified for hazardous materials storage. The decontamination water is disposed of in an approved manner. Labels should contain the following minimum information:
- Type of material contained (e.g. decontamination water).
- Date of first accumulation.
- Client’s name, address of site.
Instrument Calibration and Maintenance
The following field equipment is frequently used during the sample collection of the environmental site investigation and remediation project phase. Calibration procedures and frequency are listed in the equipment manufacturer’s guidebooks:
- Organic vapor meter (OVM) to measure soil vapors.
- PID to measure soil vapors.
- Optional flame ionization detector (FID) to measure soil vapors.
- Water level meter to measure water depth and total depth of well.
- Oil–water interface probe to measure water depth, free product thickness, and depth of well.
- pH/temperature/conductivity meter for water samples.
- Magnetic line locating tools to measure magnetic fields related to subsurface obstructions.
Prior to Drilling Activities
The Underground Service Alert (USA) or state equivalent should be contacted between two days and two weeks prior to drilling or digging into the subsurface. USA contacts local utility companies who will identify the locations where buried utilities enter the property. Because the utility companies do not identify buried utilities on private property, boring locations can be cleared using a magnetic line and cable locator. The exact location and number of borings at each site is usually marked in the field by the project scientist or project engineer based on a prepared work plan.
Soil Sampling
Soil samples should be collected in accordance with local, state, and national regulatory guidelines. Variations in local regulations, guidelines, and requirements do exist and can be quite extensive. Standard US Environmental Protection Agency (EPA) methodologies for sampling and analysis are routinely utilized for environmental projects. When required, a work plan and permit application is submitted to and approved by the lead regulatory agency prior to commencing drilling or excavation activities.
Surface Samples
Undisturbed samples are obtained using a slide hammer and core sampler with a single sampling cup at the end. The sampler typically contains one clean, six‐in‐long‐by‐two‐in‐diameter brass or stainless steel sample tube. The soil core sample is obtained by hammering the cup and sampler into undisturbed soil. The sampler is retrieved and opened, and the sample tube containing the sample is extracted. Soil core samples may be collected from tank pits and soil piles by driving a brass or stainless steel tube into the soil by hand. The top inch or two of soil at the sampling location are first removed to ensure a relatively fresh sample. Some analyses or guidelines allow for a disturbed sample to be dug using a trowel and then placed in a glass jar. Soil samples can also be obtained using handheld continuous coring tools, typically 4 ft (1.2 m) long and 1 in (2.5 cm) in diameter. Made by a variety of manufacturers, these portable core samplers are driven by hand into the soil core using electric rotary hammers or pneumatic hammers. After driving the sampler about 4 ft (1.2 m) deep into the ground, the sampler is extracted, and the inner sampling tube containing the sample is removed. The inner sample tubes are typically composed of polyethylene, brass, or stainless steel.
Most environmental projects involve significant soil sampling to evaluate hydrologic and lithologic conditions. Soil samples may be collected in the vadose zone above the water table or within the capillary fringe. Soil samples collected in the saturated or phreatic zone below the water table are used to evaluate the aquifer characteristics. These various soil samples help scientists and engineers to understand groundwater dynamics and the vertical and lateral movement of contaminants in the subsurface. Although groundwater sampling is imperative for groundwater assessments, data from soil samples used in conjunction with groundwater data provides a more complete understanding of subsurface conditions.
More detailed soil sampling information can be obtained from Testa (1994), Jacobs (2000), American Society for Testing and Materials (ASTM) (1984), California Regional Water Quality Control Board (1989), and SWRCB (2012). Although there are numerous minor differences in soil sampling and record‐keeping techniques and methods, regional variations, and specific regulatory requirements that vary from state to state, a generalized summary of selected environmental soil sampling techniques is defined below.
Undisturbed samples are obtained by driving a sampler into the soil, either manually or using a drilling rig. The soil sampler is retrieved and opened, and the sample liner containing the soil sample is extracted. Samples are collected during subsurface investigations in vertical borings with rigs. The inner sample liners are typically composed of polyethylene, brass, or stainless steel. Samples may also be collected from tank pits and soil piles by driving a sampling tube into the soil by hand. Some analyses or guidelines allow for a disturbed sample to be collected using a trowel and then placed in a glass jar. PID are used in the field to measure organic vapors in soil samples. Soil samples should be collected in accordance with regulatory guidelines, which may vary between states and locales.
Lithologic Description
Soils and unconsolidated deposits for environmental projects are commonly described according to ASTM International Method D2488‐17 (ASTM 2017) and the Unified Soil Classification System (USCS) for physical description and identification of soils. Other soil identification systems include the Burmister Soil Identification System (BS) for unconsolidated deposits, which are commonly used, with the USCS, and the Comprehensive Soil Classification System (CSCS) developed by the US Department of Agriculture. The CSCS describes soils as to agricultural productivity potential and best agricultural land use.
The ASTM Soil Classification Flow Chart and the USCS are generally accepted soil description methods used in the engineering and environmental fields. Descriptions for moisture, density, strength, plasticity, etc. are made using ASTM guidelines. Stratigraphic, genetic, and other data and interpretations are usually also recorded on the boring log. For consolidated deposits, including igneous, metamorphic, and sedimentary rocks, the USCS is combined with other geologic characteristics such as weathering, sorting, sphericity, separation, and other features.
Color is correctly described by comparing the soil sample with a Munsell Rock Color Chart (Munsell Color 1988) and applying the correct designations and descriptions. If the Munsell chart is not available, colors should be described using only red, orange, yellow, green, blue, purple, brown, black, and white. Modifiers such as light or dark are acceptable. Dual color descriptions (yellow‐brown) may also be used. Descriptions such as tan, buff, etc. are generally not be used. The soil is described when moist or wet. Each stratum of soil is identified by the following items, in the order given: color, soil type, classification symbol, Munsell color designation (if any), consistency or relative density, moisture, structure (if any), and modifying information such as grain sizes, particle shape, cementation, plasticity, stratification, etc. Some lithologic descriptions follow:
- YELLOWISH‐BROWN SANDY CLAY (CL), 10 YR 5/4; stiff, moist, fissured, with occasional gravel to 1 in (2.5 cm) size; landslide debris. Munsell color system based on hue, value, and chroma: 10 YR 5/4.
- GRAY‐BROWN CLAYEY SAND WITH GRAVEL (SC); medium dense, wet, fine‐grained sand, 15% clay, <10% gravel, (alluvium) (not using Munsell chart).
Soil Texture and Grain Size
A Soil Textural Triangle (Figure H.1) is used to help distinguish different soils. The general size (Table H.1) and water flow characteristics through soil and rock types are shown (Figure H.2).
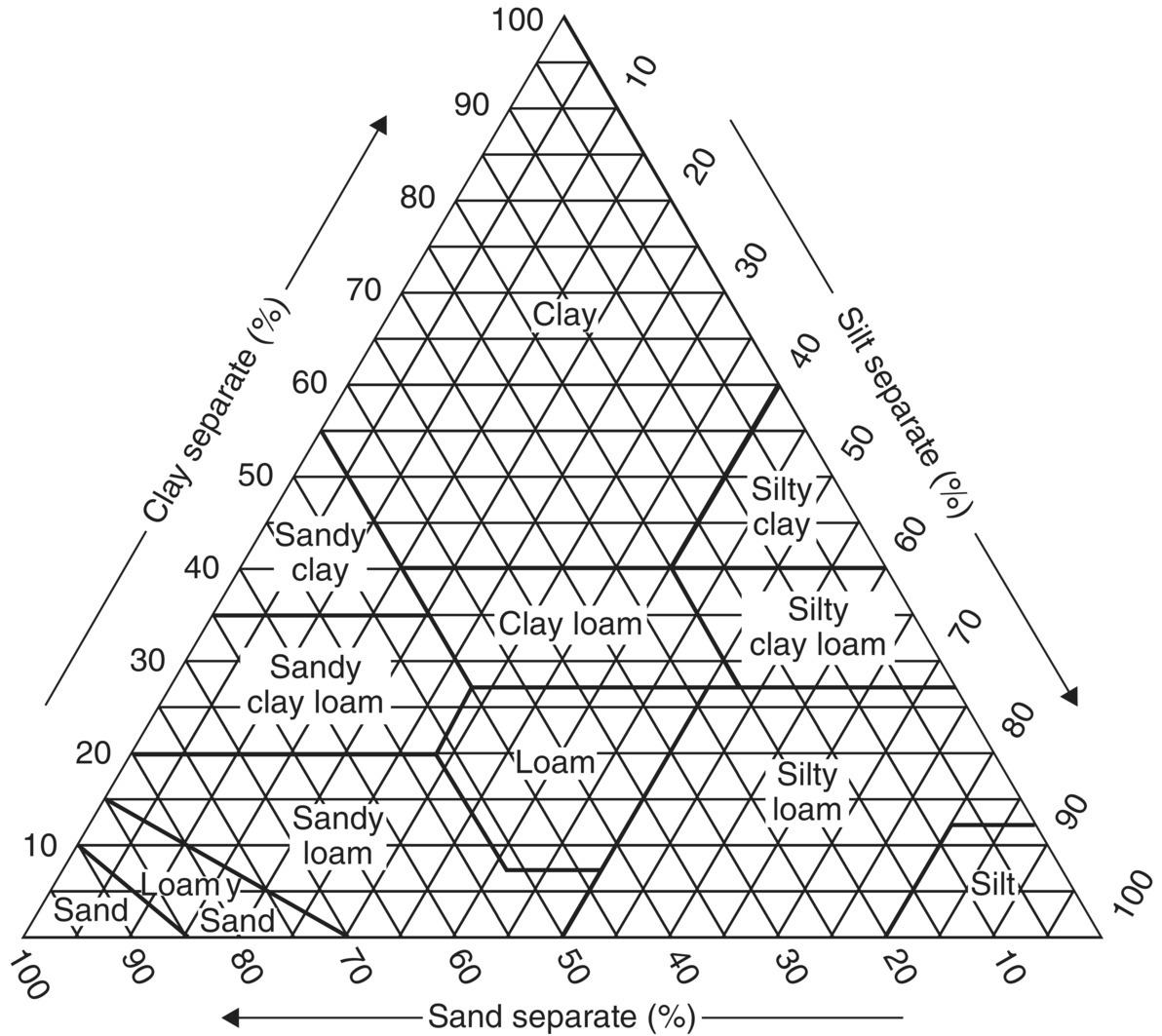
Figure H.1 Soil textural triangle (USDA 2017).
Table H.1 Size of various sediments.
Source: From Heath (1983)
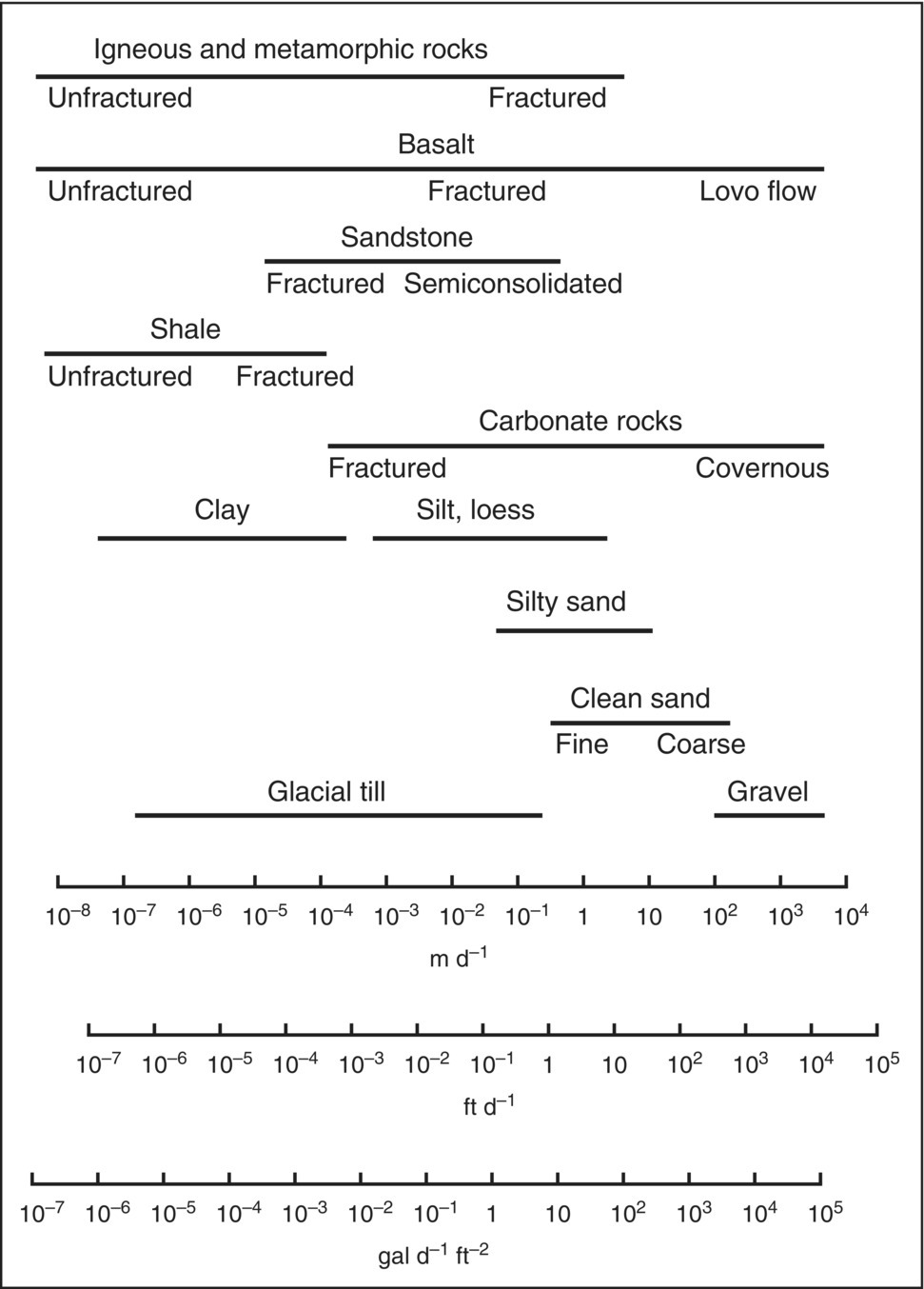

Figure H.2 Soil type and particle diameter limits.
Lithologic Interpretation
Once the lithology has been determined, geologists and engineers compare the data to develop geologic cross sections and a geologic model for depositional environment and vertical and lateral extent of subsurface impacts. Modeling allows for prediction of future plume migration.
Soil Sample Shipment
The environmental samples are delivered to a state‐certified laboratory under chain‐of‐custody procedures, usually within 48 hours of sampling. Soil samples are maintained at ~39.2 °F (4 °C) for shipping. Shipping containers are sealed with security tape to assure the sample integrity during shipping. The chain of custody should always accompany the delivered samples.
Sampling Preparation Methods
Disposable Volumetric Core Sampling Devices: The US EPA developed SW‐846 Method 5035 to minimize volatilization from large‐volume soil core samples. While the method is designed for use on samples containing low levels of volatile organic compounds (VOCs), procedures are also provided for collecting and preparing solid samples containing high concentrations of VOCs and for oily wastes (such as an oil spill in soil). For these high‐concentration and oily materials, sample collection and preparation are performed using the procedures described here, and sample introduction is performed using the aqueous purge‐and‐trap procedure in Method 5030. These procedures may be used in conjunction with any appropriate determinative gas chromatographic procedure, including gas chromatography–mass spectrometry (GC–MS) for VOC analysis using EPA Method 8260B/C. The low‐volume soil method uses a hermetically sealed sample vial, the seal of which is never broken from the time of sampling to the time of analysis. Since the soil sample is never exposed to the atmosphere after sampling, the losses of VOCs during sample transport, handling, and analysis are negligible. The applicable concentration range of the low‐volume soil method for oil spills is dependent on the analytical method used, lithologic characteristics, and the target compounds within the crude oil. However, most soil analyses will generally fall in the 0.5–200 μg kg−1 range (US EPA 1996).
Drilling‐derived Waste Disposal
Soil cuttings and excess sampling materials should be properly stored and labeled on‐site in DOT 17H containers pending off‐site disposal. Where excavated soil is left on‐site pending treatment or off‐site disposal, it should be placed in a bermed area upon high density polyethylene (HDPE) liners and covered with UV‐tolerant HDPE sheeting covered with sand bags, concrete blocks etc. to prevent contaminated runoff or leakage.
Sample Preparation, Packaging, and Handling
Soil samples are collected in polyethylene, brass, and stainless steel sample tubes. The tube ends are covered with Teflon tape and plastic end caps, labeled, and sealed in locking plastic bags. Certain soil samples may be collected in laboratory‐approved 8‐oz (227 g) wide‐mouth glass jars with Teflon®‐lined screw‐on lids. Groundwater samples are placed in laboratory‐approved containers that are compatible with the requested analysis. Samples are placed into a cooler containing chemical ice. Padding (i.e. bubble wrap, foam) is used to prevent glass breakage.
Soil Vapor Sampling
After an oil spill or gas leak, the more volatile components of petroleum hydrocarbons, such as methane and benzene, can exist in the vapor phase in the soil pore spaces in the unsaturated zone. Soil vapor sampling provides a quick and relatively low‐cost method of assessment when the spilled hydrocarbon contains volatile components that can be trapped in the soil pore spaces.
The protocols described below are the procedures to be followed during active sampling of soil vapor for an oil spill or gas leak for laboratory chemical analysis. The laboratory must be certified by the appropriate regulating agency for the analyses to be performed.
The procedures presented herein are intended to be of general use and should be supplemented by a work plan and/or health and safety plan. As the work progresses, and if warranted, appropriate revisions may be made by the project manager or project engineer. Detailed procedures in this protocol may be superseded by applicable regulatory requirements.
Active Soil Gas Sampling (EPA Method TO‐15)
Active vapor sampling or air sampling by EPA Method TO‐15 uses specially prepared stainless steel air sampling canisters (SUMMA® canisters). The vapor well or vapor probe to be sampled should be purged before vapor sampling in order to remove stagnant or ambient air and therefore to obtain vapor that is representative of general subsurface conditions. The well or probe should be purged and sampled as follows:
Connect the well or probe to be sampled to the extraction device and purge the tube. A site‐specific purge volume versus contaminant concentration test should be conducted as the first soil gas sampling activity. This test should be performed at the test point where the contaminant concentrations are suspected to be the highest. The purge volume should be estimated based on summation of the volume of the sample container, internal volume of tubing used, and annular space around the probe tip. The step purge test of one, three, and seven purge volumes should be conducted as a means to determine the purge volume to be used at all sampling points. If no contaminants are detected during the step purge test, three purge volumes should be extracted prior to sampling at each location.
A leak test should be conducted at every soil gas probe in order to detect and prevent sample dilution with ambient air. A leak check compound, such as soapy water, should be placed where ambient air could enter the sampling system (sample system connection, surface bentonite seal, top of the temporary soil gas probe).
A purge and sample flow rate should be selected to reduce compound partitioning during soil gas sampling. A vacuum device (gastight syringe) should be used between the soil gas sample tubing and the soil gas extraction device (vacuum pump, SUMMA canister) to qualitatively determine if a high vacuum (no‐flow or low‐flow) soil condition is present.
Purging and sampling should be conducted at low flow rates between 100 and 200 milliliters per minute (ml min−1). The purge/sample rate can be modified based on conditions encountered in individual soil gas probes.
Soil Vapor Sample Containers
Soil gas samples should be collected in gastight, opaque/dark containers (e.g. syringes, glass bulbs wrapped in aluminum foil, SUMMA canisters). Tedlar® bags (translucent) should not be used to collect VOC samples. If a SUMMA canister is used, a flow regulator should be placed between the probe and the canister to ensure that the canister is filled at the low flow rate as specified. If a syringe is used for soil vapor sampling, it should be leak‐checked before each use by closing the exit valve and attempting to force ambient air through the needle. If syringe samples are analyzed within five minutes of collection, aluminum foil wrapping should not be applied.
Soil Vapor Quality Control and Decontamination
Prior to its use at a site, each sample container should be assured clean by the analytical laboratory as follows:
- New containers should be determined to be free of contaminants by the supplier.
- Reused/recycled containers: Method blank(s), as specified below, should be used to verify sample container cleanliness. After each use, reusable sample containers should be properly decontaminated as follows:
- Glass syringes or bulbs should be disassembled and baked at 464 °F (240 °C) for a minimum of 15 minutes.
- Soil gas SUMMA canisters should be properly decontaminated as specified by appropriate EPA analytical method.
NOTES: Plastic syringes are not recyclable and should be used only once. Teflon® and Tedlar® are trademarks of DuPont; SUMMA® is a trademark of Molectrics for the proprietary electropolishing process used on the specially prepared sampling canisters.
Passive Soil Gas Sampling (EPA Method TO‐17 and TO‐15)
For a crude oil spill or to sample for VOCs, EPA TO‐17 is another soil gas and air sampling method that is useful as a rapidly deployed and generally low‐cost screening technique.
The TO‐15 is a passive approach that is different from the active soil gas method. For TO‐15 subsurface sampling, synthetic sorbers or carbon sorbers are placed at the bottom of a 1‐in‐diameter borehole to about 30 in depth. The sorber is left in the ground for about 14 days and once retrieved it is stored in a air‐tight tube and capped with a cork or rubber stopper. The passive sorber collects VOCs that are migrating upward from the subsurface. The VOCs adhere to the sorbers and increase in concentration over time. The sorbers are analyzed using a gas chromatograph using mass spectrometry techniques (GC‐MS), or similar analysis. Due to the passive nature of the sorbers, the resulting concentrations of target chemicals is generally known, but the vertical extent of the contamination is not defined, and VOCs could be from nearby soil gas in the unsaturated zone or migrating vapors from shallow or deep groundwater sources. Passive soil gas sampling has been used extensively in oil and gas prospecting as well.
Soil Vapor Sample Log (Chains of Custody)
A sample log form is used to record the following information:
- Sample I.D.
- Duplicate I.D., if applicable.
- Date and time sampled.
- Name of sample collector.
- Probe number.
- Depth at which the soil gas sample is collected.
- Purge volume and purge rate.
- Extraordinary circumstances (if any).
- Number and type of sample container(s).
Groundwater Sampling
Monitoring of depth to water and free product thickness within wells at the site is conducted using a water level meter and an interface probe respectively. For consistency, all measurements is taken from the north side of the wellhead at the survey mark on the lip of casing. To assess potential infiltration of fine‐grained sediments, total well depth should also be measured. To reduce the potential for cross‐contamination between wells, the monitoring is performed in the order from the least to the most contaminated, if known (and according to the last sampling event). Wells containing free product are monitored last. The measuring equipment is decontaminated between wells. Water level data collected from the wells is used to develop a groundwater contour map for the project site. Groundwater flow is perpendicular to equipotential lines drawn on the map. Prior to sample, each well must be developed.
Water Sampling from Monitoring Wells
For newly installed wells, a minimum of 24–72 hours may be required to lapse between well development and sampling. If free product is detected, a product sample is sometimes collected to identify the compound of the free product and later, for source identification. Where several chemical analyses are to be performed for a given well, individual samples are collected to match the methods of analysis in order of decreasing volatility.
Cross‐contamination between wells is avoided by careful decontamination procedures. Purging will proceed from the least to the most contaminated well, if known or indicated by field evidence. The well is purged until indicator parameters (pH, temperature, and conductivity) are stabilized (within 10%). A minimum of three wetted casing volumes is commonly removed from the well by bailing or pumping. If the aquifer is slow to recharge, purging will continue until the well is pumped dry. Once the well is sufficiently purged, a sample may be collected after the water level has reached 80% of its initial volume. Where water level recovery is slow, the sample may be collected after two hours.
Samples are collected using a disposable polyethylene bailer with a bottom siphon and nylon cord. If a Teflon or stainless steel bailer is used, it is decontaminated between wells. The groundwater collected in the transparent bailer is inspected for the presence of free product. Samples are transferred to clean laboratory‐supplied containers. Some regulators are now allowing micro‐purging, where minimal purging is performed.
Because VOCs may be lost if the sample is aerated, glass vials for VOC analysis (40 ml) are filled using a restricted flow dispenser at the end of the bailer to minimize sample agitation. The vial is filled to above the top of the opening to form a positive meniscus. No headspace should be present in the vial once it is sealed. After the vial is capped, it should be inverted to check for air bubbles. If bubbles are present, the sample should be discarded and redrawn. If it is not possible to collect a sample without air bubbles, the problem should be noted in the daily field log and chain of custody. Where several types of analyses are to be performed for a given well, individual samples are collected in the following order:
- VOCs
- Purgeable organic compounds
- Purgeable organic halogens
- Total organic compounds
- Total organic halogens
- Extractable organic compounds
- Total metals
- Dissolved metals
- Phenols
- Cyanide
Quality Control
A quality control (QC) program that is independent from the laboratory’s program is maintained. This program includes the submittal of duplicates, field blanks, and travel blanks to the laboratory. Spiked samples are not usually supplied from the field. The QC samples are packaged and sealed in the same manner as the other samples. A QC program is designed to include groundwater, soil, and soil vapor media sampling.
Water Sample Preservation
Specific analytes for water samples require specific sample preservation techniques or additives, such as hydrochloric acid (HCl). Sealed ice is placed in the coolers to maintain samples at a temperature of 39.2 °F (4 °C).
Sample Labeling
Sample containers are labeled before or immediately after sampling with self‐adhesive tags with the information written in waterproof ink on weather proof labels:
- Company or organization name.
- Project name.
- Project number.
- Sample I.D. number.
- Date and time sample was collected.
- Initials of sample collector.
- Contact phone number or email address.
- Labels can be prepared in the office with the unchanging data ahead of time.
Field Quality Control Samples
In order to evaluate the precision and accuracy of analytical data, QC samples of various media are prepared as described below. These samples are collected, or prepared, and analyzed by a government‐certified laboratory, as specified below.
Travel Blanks
A travel blank is provided by which is laboratory with each shipping container of samples which is to be analyzed for crude oil and other VOCs. Analysis of the travel blanks shows whether a sample bottle was contaminated during shipment from the container manufacturer, while in bottle storage, in shipment to the laboratory, or during analysis at a laboratory. Travel blanks consist of an aliquot of DI water sealed in a sample bottle, prepared either by the analytical laboratory prior to shipping the sample bottles to site, or the travel blanks are prepared by field sampling technicians before they collect the samples (BNL 2003). For high‐profile cases, if samples are shipped off‐site for analysis that contain VOCs, such as lighter‐end crude oils, a minimum of one travel blank per day is collected and analyzed for the target compound. Travel blanks for soil vapor sample projects, consisting of laboratory‐grade ultrapure air (or sampled media), are prepared in the field to evaluate sample cross‐contamination during shipment. The travel blank is assigned an independent sample number and analyzed by the laboratory using the same analysis as the submitted samples.
Field Blanks
Field blanks are collected to check for cross‐contamination that might occur during sample collection. For example, in a groundwater monitoring program, one field blank might be collected for every 20 samples, or one per sampling round, whichever is more frequent. A DI water rinse of soil sampling equipment can be collected in the field to evaluate decontamination procedures. Soil gas sampling also can use field blanks as a QC measure.
For example, soil gas field blanks are prepared during sampling activities using reused/recycled sampling containers (e.g. glass syringes). At a minimum one decontaminated soil vapor sample container per 20 samples (5%) or per every 12 hours, whichever is more often, is used as a field blank to verify and evaluate the effectiveness of decontamination procedures and to detect any possible interference from ambient air.
On any given day, the field blanks are analyzed for the same parameters as the groundwater samples. Contaminants in travel, field, and equipment blanks might include specific identifiable compounds, such as methylene chloride, acetone, and/or toluene. These compounds are commonly detected in blanks and do not pose significant problems with the reliability of the analytical results. When these contaminants are detected, validation and/or verification procedures are used, where applicable, to qualify the associated data as non‐detects or below laboratory reporting levels (BNL 2003). The field blank is assigned an independent sample number.
Field Duplicates
Water Samples
Field duplicate samples are analyzed to check the reproducibility of sampling and analytical results. For example, in a groundwater monitoring program, duplicates are collected for 5% (1 in 20 samples) of the total number of samples collected for a project per sampling round. The duplicate sample is acquired by filling separate containers from the same well bailer as the actual sample. The contents of the bailer are evenly divided between the actual and duplicate samples to ensure duplication. The duplicate sample is labeled as a duplicate without identifying the well location on either the chain of custody or the sample container. The well location and sample number of the duplicate sample is noted on the Water Sampling Data Form.
Soil Samples
Small variations in soil lithology within several inches can lead to large differences in the results of the chemical analysis. Duplicate soil samples should be collected as near as possible to the actual soil sample, in the same lithologic zone. Duplicate soil samples, if required, are recommended to be collected at a minimum one sample in 20 or 5% of the samples. To maintain sample integrity, soil samples are delivered to certified laboratories using chain‐of‐custody procedures. Duplicates are analyzed only for the parameters relevant to the program they monitored. A successful QC program using field duplicates would show consistent laboratory results between the laboratory and field sampling technicians. The duplicate samples are frequently sent to an independent third‐party certified laboratory to verify the results of the primary laboratory.
Matrix Spike
In the laboratory matrix spike and matrix spike duplicates are performed to determine whether a sample matrix (water, soil, air, etc.) adversely affected the sample analysis. A spike of a known amount of analyte is added to a sample. Matrix spikes are performed at a rate specified by each environmental program. In the case of a groundwater monitoring program, that rate is ~1 per 20 samples (5%) collected per project. For groundwater samples, if no significant matrix effects are observed, then the particular matrix is appropriate. Reactions of the spike chemical, such as acetone, and the sample matrix may cause a significant positive or negative effect on the recovery and may render the chosen analytical method, monitoring, or process ineffectual for that sample matrix (ASTM 2015).
The use of QC on samples is described below.
Sample Handling, Storage, and Transportation
Exposure to light and changes in temperature and pressure will accelerate sample degradation. To protect sample integrity, the following steps will be undertaken:
Soil gas samples
- Soil gas samples (canisters and sorbents) will not be chilled.
- If condensation is observed in the sample container, the sample will be discarded and a new sample will be collected.
- Soil gas samples will be analyzed within 30 minutes by an on‐site mobile laboratory.
- Soil gas samples collected in Summa canisters will be analyzed within 72 hours after collection.
Soil samples
- Soil samples are placed in a cooler or other refrigerated environment at 39.2 °F (4 °C).
- The tubes are capped first with Teflon tape and then capped with plastic end caps. Duct tape or other adhesive tape should not be used as a seal due to residual toluene present.
- Capped soil samples should be double bagged to prevent any air exchange between the sample and the cooler.
Water samples
- Water samples are placed in a cooler or other refrigerated environment at 39.2 °F (4 °C).
- Water samples should not be frozen.
- Care should be taken that no air bubbles are present in the sample.
- Should effervescence be observed in a preserved sample, the water should be re‐sampled using sample bottles with the acid preservative removed.
- Voas being striped must be individually bubble wrapped to prevent any glass to glass contact.
Documentation
Field Data Sheets
A Field Investigation Daily Log is typically completed for each day of field work. Information recorded on the Field Investigation Daily Log includes a description of any deviations from the SOPs that were necessitated by field conditions, such as equipment failure, wells that could not be sampled, etc. Sample numbers may also be recorded on the Field Investigation Daily Log as a means of identifying and tracking the samples. Following review by the project manager, the original records will be kept in the project file. Dated and labeled photographs are frequently included in the project file, as appropriate.
Sample Control
Proper identification, preparation, packaging, handling, shipping, and storage of samples obtained in the field are the responsibility of field personnel. Samples must be readily identifiable and should be as representative as possible of in situ conditions.
Sample Labels
Each sample container is labeled at the time of collection with time, date and sample number. The label is attached to the individual sample containers. In order to submit them to the lab, the labels should contain the following minimum information:
- Project name and number and address.
- Date and time of collection.
- Name of collector.
- Sample number.
- Location of sample collection (i.e. boring name and depth or well number).
- Preservation or special handling employed.
If a sample is known to have a high chemical concentration, field personnel should make a note on the chain of custody so that dilutions may be made in the laboratory.
Chain‐of‐custody Procedures
After samples have been collected and labeled, they will be maintained under chain‐of‐custody procedures. These procedures document the transfer of custody of samples from the field to the laboratory. Each sample sent to the laboratory for analysis will be recorded on a chain‐of‐custody form, which will include instructions to the laboratory for analytical services and special turnaround times. Information contained on the chain‐of‐custody record will include:
- Sample address.
- Project name.
- Project number.
- Signature of sampler(s).
- Date and time sampled.
- Sample identification number.
- Number of sample containers in sampling set.
- Sample matrix (i.e. soil vapor, soil, or water).
- Analyses description and method number.
- Description of sample and container(s).
- Remarks, including preservatives, special conditions, special directions or specific QC measures.
- Turnaround time and person to receive laboratory report.
- Release signature of sampler(s) and signatures of all people assuming custody.
- Condition of samples, including temperature, when received by laboratory.
- Inclusive dates and times of possession.
Blank spaces on the chain of custody are crossed out and initialed by the sampler between the last sample listed and the signatures at the bottom of the sheet. The field sampler signs the chain of custody and records the time and date at the time of transfer to the laboratory or to an intermediate person. A set of signatures is required for each relinquished/reserved transfer, including internal transfer. Commonly, the chain of custody is provided by the laboratory as a triplicate form. The original imprint of the chain‐of‐custody record accompanies the sample containers to the laboratory. A duplicate of the chain of custody is given to the consultant and placed in the project file, with the owner getting the triplicate.
If the samples are to be shipped to the laboratory, the original chain‐of‐custody form is sealed inside a plastic bag within the ice chest, and the chest is sealed with custody tape that has been signed and dated by the last person listed on the chain‐of‐custody form. US Department of Transportation shipping requirements are followed. The sample shipping receipt is retained in the project files as part of the permanent chain‐of‐custody document. The shipping company (e.g. Federal Express, UPS) does not sign the chain‐of‐custody forms as a receiver; instead the laboratory signs the chain‐of‐custody forms as a receiver when the samples are received.
References
- ASTM International (ASTM) (1984). ASTM D 1586‐84, Penetration Test and Split Barrel Sampling of Soils. West Conshohocken, PA: ASTM International 9 p.
- ASTM International (ASTM) (2015). ASTM D5810‐96(2015), Standard Guide for Spiking into Aqueous Samples, 6 p.
- ASTM International (ASTM) (2017). Method D 2488‐17, Standard Practice for Description and Identification of Soils (Visual‐Manual Procedures), 13 p.
- BNL (2003). Brookhaven National Laboratory 2003 Site Environmental Report; Retrieved 17 June 2012; http://www.bnl.gov/bnlweb/PDF/03SER/Chapter_9.pdf.
- California Regional Water Quality Control Board (RWQCB) (1989). Leaking Underground Fuel Tank (LUFT) Field Manual: Guidelines for Site Assessment, Cleanup, and Underground Storage Tank Closure, October 1989.
- Heath, R.C. (1983). Basic Ground‐Water Hydrology, U.S. Geological Survey Water‐Supply Paper, vol. 2220. Boulder, CO: U.S. Geological Survey revised 2004, 86 p. https://pubs.er.usgs.gov/djvu/WSP/wsp_2220.pdf accessed 21 October 2018.
- Jacobs, J. (2000). Monitoring well construction and sampling techniques. In: Standard Handbook of Environmental Science, Health and Technology (ed. J. Lehr), 11.46–11.68. New York: McGraw‐Hill.
- Munsell Color (1988). Munsell Soil Color Charts. Baltimore, MD: Munsell Color.
- State Water Resources Control Board (SWRCB), California (2012). Leaking Underground Fuel Tank Guidance Manual, September, 366 p.
- Testa, S.M. (1994). Geologic Aspects of Hazardous Waste Management, 145–187. Boca Raton, FL: CRC Press.
- U.S. Department of Agriculture (USDA) (2017). Natural Resources Conservation Service, Soil Textural Diagram and Soil Textural Calculator; Retrieved 22 October 2017; https://www.nrcs.usda.gov/wps/portal/nrcs/detail/soils/survey/?cid=nrcs142p2_054167.
- U.S. Environmental Protection Agency (EPA) (1996). Method 5035 Closed‐System Purge‐and‐Trap and Extraction for Volatile Organics in Soil and Waste Samples, December, 24 p.; Retrieved 17 June 2012; http://www.epa.gov/osw/hazard/testmethods/sw846/pdfs/5035.pdf.
- U.S. Environmental Protection Agency (EPA) (2015). Operating Procedure, Field Equipment Cleaning and Decontamination; 18 December, SESD Operating Procedure, SESDPROC‐205‐R3, 18 p.
Suggested Reading
- California Department of Water Resources (DWR) (1981). California Well Standards, Bulletin 74–81.
- California Department of Water Resources (DWR) (1990). California Well Standards, Bulletin 74–90, January 1990.
- California Regional Water Quality Control Board (RWQCB) (1990). Tri‐Regional Board Staff Recommendations for Preliminary Investigation and Evaluation of Underground Tank Sites, 10 August 1990.
- Hartman, B. and Jacobs, J. (2000a). Soil vapor principles. In: Standard Encyclopedia of Environmental Science and Technology (ed. J. Lehr), 11.87–11.95. New York, NY: McGraw‐Hill.
- Hartman, B. and Jacobs, J. (2000b). Applications and interpretation of soil vapor data to volatile organic compound contamination. In: Standard Encyclopedia of Environmental Science and Technology (ed. J. Lehr), 11.96–11.112. New York, NY: McGraw‐Hill.
- Jacobs, J. (1994). Overview of site evaluation and in‐situ remediation technologies by direct penetration method. 1994 Annual Meeting, Groundwater Resources Association, Napa, CA (29 September), Abstracts.
- Jacobs, J. (1995). Vertical and horizontal direct push technology and in‐situ remediation delivery systems. 1995 Annual Meeting, Groundwater Resources Association, Sacramento, CA (6 October 1995); Abstracts, p. 59.
- Jacobs, J., Kram, M., and Lieberman, S. (2000). Direct push technology sampling. In: Standard Encyclopedia of Environmental Science and Technology (ed. J. Lehr), 11.151–11.163. New York, NY: McGraw‐Hill.
- Nielsen, D.M. and Nielsen, G. (2006). The Essential Handbook of Ground‐Water Sampling. Boca Raton, FL: CRC Press, 328 p.
- Sisk, S.J. (1981). NEIC Manual for Groundwater/Subsurface Investigations at Hazardous Waste Sites, EPA‐330/9‐81‐002. Denver, CO: U.S. Environmental Protection Agency, July, 1981.
