About Electrons and Electronics
Electricity is a lot like water. It flows from one place to another, it can be shut off with appropriate valves, it can be stored for a while, and it can be used to power big mills. A steady flow of electricity is even called a current.
Like water, electricity likes to flow downhill. In electrical terms, that means it flows from wherever there is an excess of electrons to anyplace there is a shortage. Benjamin Franklin labeled these positive (+), for the “uphill” side where electricity is plentiful and negative (–) for the “downhill” side. We've used his nomenclature ever since, and you can see it marked on batteries.
Electricity even has water pressure. The amount of electricity flowing from one place to another (from the positive source to the negative drain) is called the voltage. A big, wide garden hose can move a lot of water, and big, thick wires can carry a lot of voltage. Water pressure determines how far the water squirts out of the garden hose, and it can fluctuate at different times of the day. Likewise, electricity can have high or low pressure, called amps. Lots of volts means lots of electrical flow; lots of amps means lots of electrical pressure.
That describes electrical current, but what is electricity, really? Once again, we can compare it to water. The current in a river is made up of an uncountable number of water molecules, and by the same token, electric currents are made of individual electrons. What are electrons?
We've all seen pictures of an atom like the one in Figure 9.6, which shows electrons circling around a nucleus, like planets around the sun. (It turns out that physicists don't consider this 1950s-era model to be accurate anymore, but it's close enough for our purposes.) There happen to be three electrons in our example, but if you remember your high-school chemistry, there can be dozens of electrons per atom, depending on what material it is. Silicon has 14 electrons in every atom, for example.
Figure 9.6. This very simplified diagram of an atom shows three electrons circling around the nucleus. The electrons have a negative electric charge and the nucleus has a positive electrical charge, so they cancel each other out.

It so happens that electrons can be stripped off an atom and given to another atom. This creates an imbalance that the electrons themselves try to fix, as shown in Figure 9.7. They are attracted back to their “home” atom like magnets to a refrigerator door. If we strip a few electrons off of Atom A and give them to Atom B, then Atom B will have a surplus and Atom A will have a shortage. If the atoms are close together, the excess electrons migrate back to Atom A all by themselves. If we space the atoms too far apart, however, our emigrant electrons won't be able to make the trek and the two atoms will be permanently imbalanced. They will keep trying, however.
Figure 9.7. Electron shortages tend to balance themselves when atoms borrow missing electrons from their neighbors. The flow of electrons from atom to atom makes an electrical current. The number of electrons migrating is called the voltage.
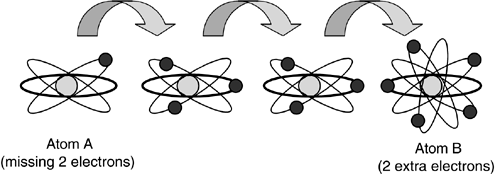
If we place some other atoms between Atom A and Atom B, the lonely electrons will eventually find their way back home. They do this in a kind of “bucket brigade” fashion. Atom A (which is missing two electrons) will steal two electrons from its nearest neighbor atom. That atom will, in turn, make up its own shortage by stealing two electrons from its neighbor, on so on. Eventually, this chain of compulsive theft ends at Atom B, which had two extra electrons anyway, and all our atoms are once again in balance.
Sometimes this works better than others. It depends on what kinds of atoms are between Atom A and Atom B. Many types of atoms won't give up their own electrons easily, so they put a stop to this migration. Other types of atoms are more than happy to cooperate. We call the first class of materials insulators. Plastic, rubber, porcelain, wood, and many other materials are insulators, which is why the electrical outlets in your home have plastic safety covers to prevent the electricity from migrating into your hands. Materials in the second category are called conductors. Metal makes a good conductor, which is why all your house wiring and electrical plugs are made of metal. A few materials fall somewhere in between, and we call these semiconductors.
Tech Talk
In a sense, the electrons don't really migrate from Atom B back to Atom A. It's really the shortage of electrons that moves from A over to B. Benjamin Franklin was wrong; it's the electron holes that move from the positive side to the negative side, not the other way around.
Electronics is all about controlling the flow of electrons and electron holes from where they are to where they want to be. We use metal wires when we want the electricity to flow fast and easily, and rubber or plastic insulators to keep it from places we don't want it to go. We construct elaborate chips out of semiconductors when we want to control when, how, and where the electrons should migrate. That, dear reader, is how semiconductors work.
