Electrokinetic characterisation of interfacial properties of natural fibres
Abstract:
The benefits of electrokinetic measurements in the study of electrostatic interface forces and electrokinetic surface properties for many industrial, biological and medical applications are reviewed. Measuring ζ-potentials is a useful tool for the characterisation of natural fibres, including acid/base surface characterisation, and evaluating the relative acid/base strength as well as the swelling behaviour of natural fibres.
7.1 Introduction
The qualitative and quantitative characterisation of the surface properties and the reactivity of solid surfaces is of crucial importance for many liquid as well as solid formulations, such as for the stability of dispersions, the adhesion between components in, for instance, composites (Jacobasch et al. 1992; Jacobasch 1993; Mäder et al. 1994), but also for the determination of materials properties, such as biocompatibility of materials (Werner et al. 1995). Measurements of ζ-potential have been used to characterise the influence of various processing parameters and surface treatments on the surface properties of textile (Flath and Saleh 1980; Grosse and Jacobasch 1994), synthetic (Jacobasch and Grosse 1991; Rybicki and Jacobasch 1992) and (sized) glass fibres (Bledzki et al. 1997). However, ζ-potential measurements are particularly useful for the characterisation of rather hydrophilic materials, such as cellulosic materials, because ζ-potential (as well as contact angle) measurements enable the characterisation of surfaces in the wet state (Jacobasch et al. 1995; Ribitsch and Stana-Kleinscheck 1997), which for a number of applications of cellulosic materials, for instance during papermaking (Beck et al. 1980) as well as for membrane separations, is of crucial importance. Moreover, it was found that the adhesion in fibre reinforced polymers correlates with the results obtained from ζ-potential measurements. For instance, it was found that the minimum value of the ζ-potential measured as a function of the KCl concentration (ζ = .f([KCl]) for glass fibres correlates with the adhesion behaviour of these fibres in unsaturated polyester resins (Jacobasch 1984). This correlation depends on the KCl concentration, which is governed by the dispersion forces present on a particular surface.
Dispersion forces determine the adhesion if no specific (direct chemical) interactions occur between the two phases. It was shown that the difference in the adsorption-free energies of the electrolyte ions at the solid surface corresponds to the dispersive forces occurring at the solid/electrolyte interface (Jacobasch et al. 1995). Moreover, ζ-potential measurement is a straightforward and reliable tool for predicting adhesive properties (Häßler and Jacobasch 1994); in this study for an increasing difference in the ζ-potential plateau values determined by measuring the ζ-potential as function of pH between adherent (substrate) and adhesive (matrix), the measured adhesive strength increases. For polymer composites, it was reported that the trend of changing adhesion could be predicted by ζ-potential measurements (Häßler and Jacobasch 1994; Chiu and Wang 1998; Bismarck et al. 1999b; Campagne et al. 2002).
Natural cellulose-based fibres have many advantages but also several disadvantages (see preceding chapters on moisture uptake of natural fibres). Natural fibres contain a high portion of hydroxyl groups, which makes them highly polar; these groups readily form hydrogen bonds at the interfaces in composites (Bellmann et al. 2005). However, natural fibres are usually covered with pectin and waxy substances (Stana-Kleinschek and Ribitsch 1998), which hinder the hydroxyl groups from interacting with polar matrices and, therefore, affect adhesion. Owing to the intrinsic hydrophilic behaviour of natural fibres (highly dependent on the purity of the composition of the fibres and the degree of crystallinity of the cellulose), these fibres swell severely and so take up a significant amount of water (see also chapter 10). Nevertheless, natural fibres have become a favourable constituent for use as reinforcement in green composites. Many natural fibres with very different compositions and properties are available (ranging from bast to leaf and seed fibres). The physical properties of natural fibres, such as their density, mechanical properties and surface area can be easily measured, however, the surface properties, such as surface chemistry and wetting behaviour are more difficult to determine because of the intrinsic hydrophilicity of the fibres and the fact that the fibres are essentially all made up of carbohydrates and lignin (carbon, hydrogen and oxygen-based organic compounds). In order to characterise the surface properties of various natural fibres and to understand the underlying mechanisms that may lead to better natural fibre composites, it is beneficial to explore the electrokinetic properties of these fibres.
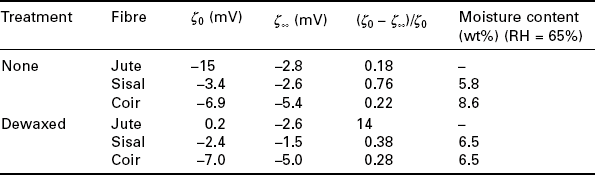
The measurement of ζ-potential is an efficient means of characterising natural fibres. To aid understanding we start by introducing electrokinetic phenomena and ζ-potential. If a solid in chemical and thermal equilibrium is in contact with an aqueous electrolyte solution, an electrochemical double layer (EDL) (Fig. 7.1) forms at the phase boundary, i.e. the interface. This EDL forms because of the dissociation of functional surface groups (should they be present) and/or the preferential adsorption of ions, the adsorption of ionic surface active agents or because of the isomorphic substitution of cations and anions at the interface (Lyklema 1981), which results in an asymmetric distribution of charges in the interface. Any excess charges at the surface are compensated for by counter ions, some of which are partially/strongly adsorbed at the solid surface whereas the remainder (caused by Brownian movement) of the counter ions required for complete charge neutrality is situated at a larger distance away from the surface. The phenomenon is described by the generally accepted Gouy–Chapman–Stern–Grahame (GCSG) model (Stern 1924; Hunter 1981; Börner and Jacobasch 1985) of the electrochemical double layer (Grahame 1947). Although the widely used model was proposed by Grahame in 1947, the EDL concept itself dates back to Helmholtz (1853). The GCSG model (Fig. 7.1) for a solid with negative surface potential ψ, which is experimentally inaccessible, assumes that the EDL consists of two layers of immobile adsorbed charges, the inner (IHP) and outer Helmholtz plane (OHP), and a diffuse layer, called the Gouy or diffuse layer (Fisher 1996), in which the ions are trapped at a large distance away from the solid surface by a balance of attractive electrostatic forces and thermal (Brownian) motion (Werner et al. 1995). The adsorption of anions from the electrolyte phase is driven by attractive interactions on the solid surface by partially losing their hydration shell (specific adsorption), whereas hydrated cations adsorb only by electrostatic forces on the interface. The potential drop across the Helmholtz plane is linear and depends only on the size of the ions as well as the size of the hydration shell of the counter ions (Bergmann and Voinov 1976). However, in the diffuse part of the EDL, the potential drop is exponential and can be described by a Boltzmann distribution. By creating a relative movement between the solid and the liquid, the diffusive part of the EDL is sheared off of the surface creating an electrical potential across the surface, which can be measured. The measured potential is called the ζ-potential, which is the potential at the plane of shear between the immobile and diffusive part of the EDL. The position of the plane of shear is physically unambiguously defined by the equilibrium between the attractive surface forces and the force field causing the relative movement between the solid and liquid phase (Jacobasch et al. 1996a). The relative movement between the solid and the electrolyte phase causes a number of effects on electrokinetic phenomena; which are named, depending on the type of relative movement: electrophoresis, electro-osmosis, streaming potential and sedimentation potential.
7.1 A schematic of the electrochemical double layer: Gouy–Chapman–Stern–Grahame model (Jacobasch et al. 1998).
These electrokinetic phenomena can be quantified by either measuring the speed at which a particle or the electrolyte moves as function of an applied electric field or by measuring the potential difference across a sample when the electrolyte is forced through the sample or particles sediment under gravity. Although electrophoresis is the most frequently used method for measuring the ζ-potential, it is impractical to characterise fibres or any macroscopic surface (Delgado et al. 2007). Electrochemical characterisation (ζ-potential) does not only offer the possibility of estimating the state, type and amount of dissociable surface functional groups on fibre surfaces but also allows dispersion forces proceeding from solid surfaces to be characterised (Uchida and Ikada 2002).
The composition of the EDL can be assessed by measuring the ζ-potentials as function of the composition of the electrolyte phase. Measuring the ζ-potentials as a function of the electrolyte concentration and pH (ζ = f(c, pH)) allows information to be extracted about the mechanisms that lead to the formation of the EDL. Moreover, thermodynamic quantities such as aerial charge densities, molar free adsorption enthalpies and equilibrium constants for the specific adsorption of ions and the dissociation of functional surface groups can be determined (Jacobasch et al. 1996a).
7.2 Streaming potential measurements
The ζ-potentials of macroscopic materials, such as bundles of fibres, powder beds, films or porous media, which can be arranged in a capillary system, are best characterised using the streaming potential measurement method (Jacobasch et al. 1996b). For streaming potential measurements the relative movement between the solid and the electrolyte phase is induced by forcing the electrolyte through the capillary system using a pump, which causes a pressure drop Δp across the sample. The complete or partial shear-off of the ions in the diffusive part of the double layer results in a potential difference, the streaming potential Up across the sample (i.e. the capillary system). The potential at the plane of shear, i.e. the ζ-potential, can be determined from streaming potential measurements using the Smoluchowski equation:
where εr is the dielectric constant of the electrolyte, ε0 = permittivity of vacuum, η = dynamic viscosity of electrolyte and k the conductivity of the electrolyte. Following equation [7.1] it is possible to determine the ζ-potential by measuring the streaming potential as a function of the pressure drop across the sample p = f(U). However, equation [7.1] is only valid for single capillaries. Moreover, it is usually impossible to characterise or describe the geometrically irregular capillary bundles, such as packed fibre beds. Nevertheless, it is possible to determine the ζ-potential of macroscopic solids by correcting for surface conductivity of the solid. This can be achieved by a relative simple means (Fairbrother and Mastin 1924) but is not the topic of this chapter. However, if ζ-potential measurements are used for the qualitative comparison between samples, one does not necessarily have to correct for the surface conductivity, which would, at a given pH and electrolyte concentration only change the amplitude of the ζ-potentials but does not affect the position of the isoelectric point and the shape of ζ = f(pH) (Jacobasch et al. 1996a).
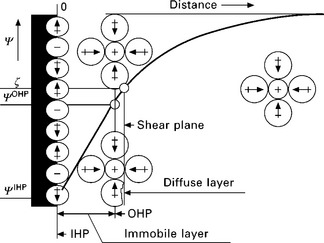
The ζ-potential of macroscopic surfaces, including (natural) fibres, can easily be measured using an electrokinetic analyser (EKA) (Fig. 7.2), made by Anton Paar KG (Graz, Austria). The measuring system (Jacobasch et al. 1986) consists of a chassis for holding the measuring cells and also contains piping for the electrolyte transport, a valve to change the flow direction of the electrolyte, a computer controlled pump, and a differential pressure sensor situated between the inlet and outlet of the measuring cell, as well as a conductivity meter and a pH electrode. The electrolyte is held in a large beaker. A computer is used to control the EKA and to measure the pH, conductivity, pressure drop and streaming potential. The streaming potential is usually measured using two perforated Ag/AgCl electrodes, which sandwich the sample of interest.
7.2 Schematic illustration of the EKA equipped with a cylindrical glass cell used to measure the ζ-potential of (natural) fibres (with kind permission of Dr R. Tahhan). (E = internal conductivity meter, T = thermostat containing the electrolyte, D = fibre measuring cell, K = pump, Δp = pressure sensor, S = valve to control flow direction of electrolyte.
The ζ-potential as property of the EDL is influenced by a number of factors (Jacobasch 1984) the surface potential ψ0, pH, the swelling behaviour of the sample in the electrolyte, temperature, the valence and concentration of the ions in the electrolyte as well as the surface composition of the solid sample. The ζ-potential measurements allow the determination of a number of properties, such as nature (i.e. acid/base (Brønsted) properties) and concentration of dissociable functional groups, the adsorption of water and ions from the electrolyte, as well as the interaction forces that originate from a surface (Rzätzsch et al. 1990).
As mentioned earlier, natural fibres are highly polar owing to the large amount of hydroxyl groups present and, therefore, they take up, i.e. swell readily in, water. According to Kanamaru (1960), the change of the ζ-potential of a solid swelling in an aqueous electrolyte can be described as follows:
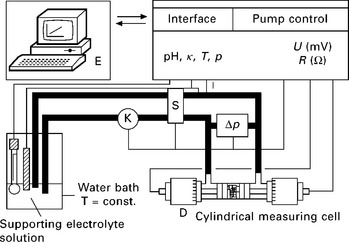
where ζ is the measured ζ-potential value at a certain time, ζ∞ is the potential value which the function ζ = f(t) reaches asymptotically and k is a relative constant, which depends on the structure of the solid, and of the swelling process (Jacobasch 1984; Bismarck et al. 2001). It was found that the quotient Δζ = (ζo – ζ∞)ζo is proportional to the water uptake at 100% relative humidity (the sorption capacity) of the natural fibres (Fig. 7.3) (Baltazar-y-Jimenez and Bismarck 2007b).
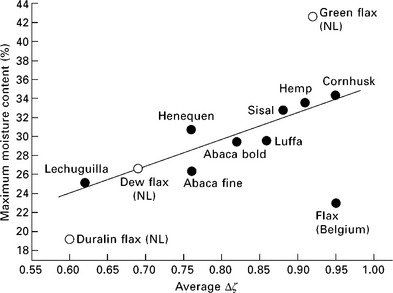
7.3 Maximum moisture content (MC) of natural fibres as a function of the Δζ quotient, which correlates almost linearly with the MC of the fibres. (adapted from Baltazar-y-Jimenez and Bismarck 2007b, Bismarck, et al. 2002)
Time dependent ζ-potential measurements are a relatively fast way to characterise the swelling behaviour of solids, however, it should be noted that investigating the kinetics of water uptake by natural fibres using streaming potential measurements can be problematic. This is because the first step in the measurement of streaming potential involves rinsing the fibre plug to remove all the trapped air. Only after that can the time dependent ζ-potential measurement be started. This, however, does not take into account that water uptake of natural fibres occurs instantly as soon as the fibres are in contact with the water-based electrolyte (1 mM KCl, pH = 5.6). Therefore, the precision of locating the initial starting point ζ0 is difficult. Nevertheless, it has been confirmed that good agreement on determining ζ0 can be obtained by fitting an exponential first order decay function to the measured ζ-potential-time graph and subtracting ζ∞ (Bismarck et al. 2002).
It can be seen from Fig. 7.4 that the value of the ζ-potential decreases rapidly to approach a much smaller value asymptotically, and that the decay of the ζ-potential differs in magnitude for the different fibres. For most natural fibres, with the exception of flax and cornhusk (Fig. 7.4b and 7.4c), the ζ-potential of the fibres increases from a value ζ0 on different time scales asymptotically to a constant but larger value ζ∞. For flax fibres and cornhusk the ζ-potential was initially positive and decreased with time to a much smaller ζ-potential. Moreover, as the measurements advanced and the plateau developed, the flax fibres produced a negative value ζ∞(Fig. 7.4b), whereas the cornhusk produced a positive one (Fig. 7.4c).
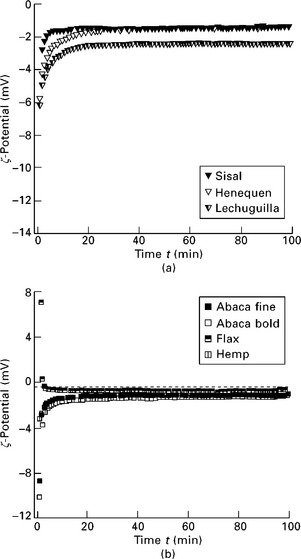
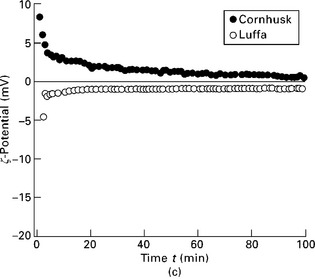
7.4 ζ-Potential as a function of time for (a) leaf fibres (henequen, lechuguilla and sisal; (b) bast fibres (abaca fine, abaca bold, flax and hemp; and (c) fruit fibres (cornhusk and luffa) (Baltazar-y-Jimenez and Bismarck 2007b).
Once the water uptake behaviour of natural fibres has reached saturation, ζ-potential as a function of pH can also be measured. Measurements of ζ-potential as a function of the pH of the electrolyte solutions (ζ = f(pH)) provide information about the presence of Br0nsted acidic and/or basic surface functional groups as well as their relative acid/base strength provided that the formation of the EDL is mainly dominated by the dissociation of functional surface groups according to their pKa (Jacobasch 1989). Figure 7.5 shows schematically the ζ = f(pH) graph. If an investigated material contains only acidic functional groups (i.e. surface-COOH), a plateau region is observed at pH values (dζ/d(pH) = 0) indicating the increasing dissociation of acidic surface groups and, therefore, increased number of negative surface charges on the surface. Once all surface groups are dissociated, the ζ-potential (ζPiateau) stays the same for increasing pH. However, with decreasing pH, the dissociation of acidic surface groups is reduced causing an increase in the ζ-potential until complete protonation of the acid groups, which results in an isoelectric point (iep) at which ζ = 0. A ζ-potential reversal is sometimes observed because of H30 + adsorption on the surface. If, however, a solid only contains basic functional groups (i.e. surface–NH2), at high pH, the dissociation of these groups is suppressed while OH− ions are adsorbed onto the surface. When the pH decreases, the number of positively charged groups increases eventually resulting in a plateau in the ζ = f(pH) curve. The ζ = f (pH) of solids that do not contain dissociable groups has no plateau. In this instance, the ζ-potential is determined by the increasing adsorption of H3O +/OH− ions, respectively (Bismarck et al. 1999a). One should note that even in this instance the iep is located in the acid pH range as OH− ions are smaller and so polarise the surface more effectively (Zimmermann et al. 2009). However, the point at which ζ = 0 depends on the concentration of ions in the electrolyte solution; thus, if iep depends on the concentration of ions in the electrolyte, the specific adsorption of ions from the electrolyte occurs on the materials surface (Jacobasch et al. 1996b). Moreover, if iep is independent of the concentration of the electrolyte, no specific ion adsorption takes place and it is called the point of zero charge (pzc).
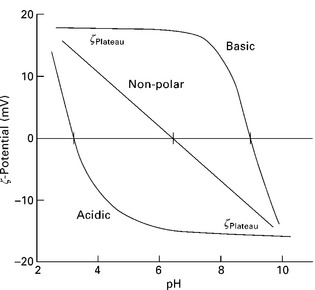
7.5 Schematic illustration of pH dependent ζ-potential curves measured on acidic, basic or non-polar samples.
The sign of the ζ-potential (negative or positive) and the position of the iep provide information about the presence of the acidic or basic surface groups. Amphoteric materials, which contain both acid and basic dissociable functional groups, for instance proteins, wool or polyamides, have a sigmoidal shape of ζ = f(pH).
When the ζ-potential is zero, which is known as the isoelectric point (iep), the surface carries no net electrical charge because the number of negative charges equals the number of positive ones. The position of the iep is determined by the function of the pKa concentration of all functional groups at the surface; if the iep is situated in the low pH range the solid surface has an acidic character whereas an iep at high pH signifies that the surface contains dissociable (Brønsted) basic surface groups (Mäder et al. 1994). According to Ottewill and Shaw (Ottewill and Shaw 1967; Healy and White 1978) the pKa und pKb-values of functional groups present can be determined for small ζ-potentials using the following equation:
where pHζplateau/2 is the pH at 2ζ = ζplateau, F = Faraday constant, R = gas constant, and T = absolute temperature. The pKa of a solid surface is calculated using the measured quantities pHζPlateau/2 and ζPlateau. However, since ζPlateau depends on the ionic strength of the electrolyte, it is necessary to determine ζ = f(pH) for various electrolyte concentrations and to extrapolate the calculated pKa to zero ionic strength. A full derivation of the thermodynamic approach to quantifying the pKa of acidic and basic function groups is available (Hunter 1981; Börner and Jacobasch 1985; Jacobasch 1989).
7.3 Electrokinetic properties of natural fibres
Numerous investigations have been conducted to investigate various natural fibres using electrokinetic measurements. However, it should be noted that all properties of natural fibres, such as composition, fibre geometry, mechanical properties, even if extracted from the same crop species, are very dependent on the location the plants were grown at, time of harvest, location within the plant that the fibre came from and the extraction method used. Furthermore, natural fibres are often treated or modified to improve the fibre’s properties, which will also influence its surface properties. In conclusion, all natural fibre properties are intrinsically rather variable.
It can be seen from Table 7.1 that electrokinetic properties between different natural fibres, but also the same natural fibres from different regions in the world, do vary widely. This variation is expected as different fibres have different chemical compositions, which determines their surface properties. For example coir and hemp fibres exhibit very different (ζ0 – ζ∞)/ζ0; this is because coir fibres have a high lignin content (up to 45 wt%) and a low cellulose content (as low as 32 wt%), Whereas hemp fibres have a very low lignin content (as low as 3.7 wt%) and a high cellulose content (up to 74 wt%) (Mohanty et al. 2005). Apart from lignin and cellulose content, waxes also make up to 1.7 wt% of the composition of natural fibres and can be mainly found at the surface of the natural fibres. This, in turn, affects the water uptake of natural fibres. The effect that dewaxing of jute, sisal and coir fibres had on the surface properties was studied by Bismarck et al. (Table 7.2) using ζ-potential measurements (Bismarck et al. 2000). It was found that dewaxed jute fibres swell much faster and adsorb more water as shown by ζ = ζ(t) measurements. The natural wax layer of the original jute fibres, consisting of non-water soluble alcohols as well as palmic, oleaginous and stearic acids (Bledzki and Gassan 1997), slows down the water absorption considerably.
Table 7.1
f-Potential measurements determined water uptake at 100% relative humidity (ζ0 – ζ∞)/ζ0) and dissociations of functional groups (ζPlateau) properties of some commonly found natural fibres
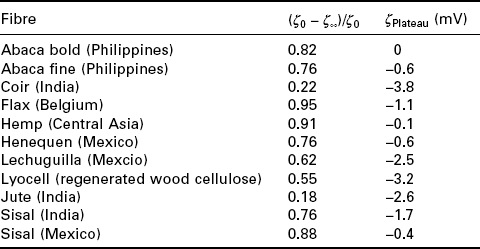
(adapted from Bismarck et al. 2000; 2001; Baltazar-y-Jimenez and Bismarck, 2007b)
The variation of the magnitude of ζPlateau, obtained from ζ = f(pH) reflects the hydrophilic nature of the fibres (Ribitsch and Stana-Kleinscheck 1998). The swelling of natural fibres caused by water uptake resulted in a reduction of the measured ζ-potential because the electrical double layer is withdrawn from mechanical interactions with the electrolyte. This, in turn, leads to a small negative plateau value. The negative ζPlateau of natural fibres is caused by the presence of dissociable carboxyl and hydroxyl groups. For instance, strong acids such as uronic acids are present in hemicellulose whereas weaker acids can be found in residual lignin (Laine 1994).
The increasing utilisation of natural fibres as reinforcement for polymers caused by the huge cost saving potential, but also the increasing environmental awareness, growing global waste problems etc., requires a good understanding of the fibre surface properties that can lead to green composites with improved mechanical properties. Measurement of ζ-potential is a reliable method for the characterisation of natural fibres. The interface between untreated natural fibres and polymer matrices is usually quite poor (Heng et al. 2007). The poor bonding between natural fibres and hydrophobic polymers is usually attributed to the extreme hydrophilic nature of natural fibres and, therefore, extensive research has been focused on the modification of natural fibres to promote better adhesion to intrinsically hydrophobic matrices (Mohanty et al. 2005). It should be noted that x-ray photoelectron spectroscopy (XPS) measurements on natural fibres are difficult to perform because of the complex fibre composition and the fact that almost all components present in natural fibres contain carbon, oxygen and hydrogen. Measurements of ζ-potential have been used to characterise alkali, atmospheric air pressure plasma, and fungal treated natural fibres as well as polymethylmethacrylate (PMMA) grafted natural fibres and fibres modified during novel extraction processes, such as Duralin fibres (Bismarck et al. 2001; Stamboulis et al. 2001; Baltazar-y-Jimenez and Bismarck 2007a; Pickering et al. 2007).
Alkali treatment removes some, if not all, of the wax components from the fibre surface via saponification (Ribitsch et al. 1996; Bledzki and Gassan 1997). Such treatment increases the active fibre surface simultaneously with accessibility of the dissociable surface groups. For sisal fibres, the ζplateau of the fibres after treatment remains at − 1.7 ± 1 mV, which suggests that the surface properties were not affected by alkali treatment.
Atmospheric air pressure plasma treatment of natural fibres (flax, hemp and sisal) led to an increase in positively charged surface functional groups, such as carbonyl and carboxyl groups, at the fibre/electrolyte interface, as shown by the increased ζPlateau (Baltazar-y-Jimenez and Bismarck 2007a). This is attributed to the air plasma functionalisation and/or the plasma-induced change in the surface morphology, which resulted in an increased exposure and accessibility of zwitterionic functional compounds (Homola and James 1977; Rendall and Smith 1978).
Stamboulis et al. (2001) studied the ζ = f(pH) of Duralin treated flax fibres. The process to obtain Duralin flax was developed by CERES B.V., which uses simply deseeded flax straw as starting material (Stamboulis et al. 2000). More importantly, no retting process is required to extract natural fibres from the straw. A description of the Duralin process can be found in the literature (Stamboulis et al. 2000; Stamboulis et al. 2001; Bismarck et al. 2002). During the Duralin fibre extraction process, hemicellulose and lignin are depolymerised into lower molecular weight aldehyde and phenolic compounds, which recombine into a water-resistant resin, which affects the composition of the flax fibres and thus the water uptake behaviour as well as the ζ = f(pH) curve. It was observed that as a result of the Duralin fibre extraction process, the surface contained a large number of acidic surface groups. Furthermore, as compared with the control, it was reported that the ζPlateau became more negative as the fibres are more moisture sensitive. This upgrading treatment of the flax fibres does not affect the mechanical properties of the fibres.
Pickering et al. (2007) applied a fungal treatment to hemp fibres and observed that the ζPlateau decreased compared with the original fibres, suggesting that the removal of non-cellulosic materials from the fibre’s surface leads to the exposure of more reactive hydroxyl sites. In the meantime it was also reported that fungal treatment led to surface roughening of the fibres, owing to the action of the fungal hyphae, which can produce fine holes in the hemp fibre cell wall, supposedly resulting in more negative ζPlateau. The removal of non-cellulosic materials also leads to a drop in the tensile strength of the fibres (Li et al. 2009).
Methylmethacrylate (MMA) grafting on waxed and dewaxed jute fibres was studied by Bismarck et al. (2000). A small decrease in the ζPlateau was found when the original fibres were grafted with a large amount of PMMA. However, if the grafting took place after the fibres were dewaxed, a higher grafting density could be achieved. This would lead to a less negative ζplateau, implying that a higher pH would be required to protonate all the available ether functions on the surface. A mechanism was proposed (Bismarck et al. 2000).
7.4 Conclusion
During the last couple of decades, there has been a renewed interest in using natural fibres as a substitute for synthetic fibres. This motivation is driven by potential advantages of weight savings, low costs, advantages in recycling and ecological aspects. Natural fibres are largely made up of cellulose, hemicelluloses, lignin, pectin, and wax, and much research has been undertaken with the aim of optimising the properties of natural fibres in order to ultilise them more widely as reinforcement for polymers. Surface treatments applicable to natural fibres have been developed and characterised using streaming ζ-potential measurements so that surface properties could be better understood. Natural fibres exhibit a very hydrophilic surface owing to the presence of hydroxyl groups, which is responsible for their unique swelling behaviour. Most natural fibres contain acidic surface functional groups which result in pronounced ζPlateau in the alkaline region of ζ = f(pH). By employing ζ-potential measurements for the characterisation of natural fibres together with other characterisation methods, valuable information about the surface properties of natural fibres can be gathered. The most important outcome is probably the fact that the results of measuring ζ = f(t) correlate well with the maximum moisture uptake of natural fibres, which makes this type of measurement a fast and reliable method for characterising the swelling behaviour of natural fibres.
7.5 References
Baltazar-y-Jimenez, A., Bismarck, A. Surface modification of lignocellulosic fibres in atmospheric air pressure plasma. Green Chemistry. 2007; 9:1057–1066.
Baltazar-y-Jimenez, A., Bismarck, A. Wetting behaviour, moisture up-take and electrokinetic properties of lignocellulosic fibres. Cellulose. 2007; 14(2):115–127.
Beck, U., Müller, F., Rohloff, E. Zetapotentialmessungen in der Papierindustrie: Neuere Meßmethoden und Anwendungen. Acta Polymerica. 1980; 31:504–509.
Bellmann, C., Caspari, A., Albrecht, V., Loan Doan, T.T., Mäder, E., Luxbacher, T., Kohl, R. Electrokinetic properties of natural fibres. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 2005; 267(1–3):19–23.
Bergmann, E., Voinov, M. Extension of Gouy–Chapman double-layer theory to interface between a liquid and a solid electrolyte. Journal of Electroanalytical Chemistry. 1976; 67(2):145–154.
Bismarck, A., Wuertz, C., Springer, J. Basic surface oxides on carbon fibers. Carbon. 1999; 37(7):1019–1027.
Bismarck, A., Richter, D., Wuertz, C., Springer, J. Basic and acidic surface oxides on carbon fiber and their influence on the expected adhesion to polyamide. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 1999; 159(2–3):341–350.
Bismarck, A., Aranberri-Askargorta, I., Springer, J., Mohanty, A.K., Misra, M., Hinrichsen, G., Czapla, S. Surface characterization of natural fibers; surface properties and the water up-take behavior of modified sisal and coir fibers. Green Chemistry. 2001; 3(2):100–107.
Bismarck, A., Aranberri-Askargorta, I., Springer, J., Lampke, T., Wielage, B., Stamboulis, A., Shenderovich, I., Limbach, H.-H. Surface characterization of flax, hemp and cellulose fibers: surface properties and the water uptake behavior. Polymer Composites. 2002; 23(5):872–894.
Bismarck, A., Springer, J., Mohanty, A.K., Hinrichsen, G., Khan, M.A. Characterization of several modified jute fibers using zeta-potential measurements. Colloid and Polymer Science. 2000; 278(3):229–235.
Bledzki, A., Gassan, J. Handbook of engineering polymeric materials. New York, Dekker: N. Cheremisinoff; 1997.
Bledzki, A.K., Lieser, J., Wacker, G., Frenzel, H. Characterization of the surfaces of treated glass fibres with different methods of investigation. Composite Interfaces. 1997; 5(1):41–53.
Börner, M., Jacobasch, H.-J. Electrokinetische Erscheinungen, Dresden, 1985.
Campagne, C., Devaux, E., Perwuelz, A., Caze, C. Electrokinetic approach of adhesion between polyester fibres and latex matrices. Polymer. 2002; 43(25):6669–6676.
Chiu, H.T., Wang, J.H. The relationship between zeta-potential and pull-out shear strength on modified UHMWPE fiber reinforced epoxy composites. Polymer Composites. 1998; 19(4):347–351.
Delgado, A.V., Gonzalez-Caballero, F., Hunter, R.J., Koopal, L.K., Lyklema, J. Measurement and interpretation of electrokinetic phenomena. Journal of Colloid and Interface Science. 2007; 309(2):194–224.
Fairbrother, F., Mastin, H. Studies in electro-endosmosis. Journal of Chemical Society. 1924; 125:2319–2330.
Fisher, A.C. Electrode dynamics. Oxford Science Publication; 1996.
Flath, H.J., Saleh, N. Zur Bedeutung des Zetapotentials von Faserstoffen für den Färbeprozeß. Acta Polymerica. 1980; 31:510–517.
Grahame, D. The electrical double layer and the theory of electrocapillarity. Chemical Reviews. 1947; 41:441–501.
Grosse, I., Jacobasch, H.J. Grenzflächenchemische Grundlagen der Präparation von Textilfaserstoffen. Tenside Surfactants Detergents. 1994; 31:377–384.
Häßler, R., Jacobasch, H.-J. Wird Adhäsion endlich meßbar? Kleben & Dichten: Adhäsion. 1994; 38:36–38.
Healy, T.W., White, L.R. Ionizable surface group models of aqueous interfaces. Advances in Colloid and Interface Science. 1978; 9(4):303–345.
Helmholtz, H. L. F. v. Ueber einige Gesetze der Vertheilung elektrischer Ströme in körperlichen Leitern mit Anwendung auf die thierisch-elektrischen Versuche. Annalen der Physik und Chemie. 1853; 89(222–233):354–377.
Heng, J.Y.Y., Pearse, D.F., Thielmann, F., Lampke, T., Bismarck, A. Methods to determine surface energies of natural fibres: a review. Composite Interfaces. 2007; 14(7–9):581–604.
Homola, A., James, R.O. Preparation and characterisation of amphoteric polystyrene lattices. Journal of Colloid and Interface Science. 1977; 59(1):123–134.
Hunter, R. Zeta potential in colloid science. New York: Academic Press; 1981.
Jacobasch, H.-J. Oberflächenchemie faserbildender Polymerer. Berlin: Akademie Verlag; 1984.
Jacobasch, H.-J. Characterization of solid-surfaces by electrokinetic measurements. Progress in Organic Coatings. 1989; 17(2):115–133.
Jacobasch, H.-J. Surface phenomena at polymers. Makromolekulare Chemie: Macromolecular Symposia. 1993; 75:99–113.
Jacobasch, H.-J., Grosse, I. Chemische Modifizierung und Charakterisierung von Chemiefaseroberflächen. Chemiefasern und Textilindustrie. 1991; 93:1294–1300.
Jacobasch, H.-J., Simon, F., Warner, C., Bellmann, C. Determination of the zeta potential from streaming potential and streaming current measurements. Technisches Messen. 1996; 63(12):447–452.
Jacobasch, H.-J., Simon, F., Werner, C., Bellmann, C. Electrokinetic measuring methods: Principles and applications. Technisches Messen. 1996; 63(12):439–446.
Jacobasch, H.J., Simon, F., Weidenhammer, P. Adsorption of ions onto polymer surfaces and its influence on zeta potential and adhesion phenomena. Colloid and Polymer Science. 1998; 276(5):434–442.
Jacobasch, H.-J., Bauböck, G., Schur, J. Comparative measurements of zeta-potential of fibers by electroosmosis and streaming current streaming potential. Monatshefte für Chemie. 1986; 117(10):1133–1144.
Jacobasch, H.-J., Grundke, K., Werner, C. Surface characterization of cellulose materials. Papier. 1995; 49(12):740–745.
Jacobasch, H.-J., Grundke, K., Mäder, E., Freitag, K.-H., Panzer, U. Application of surface free-energy concept in polymer processing. Journal of Adhesion Science and Technology. 1992; 6(12):1381–1396.
Kanamaru, K. Wasseraufnahme in ihrer Beziehung zur zeitlichen Erniedrigung des ζ-Potentials von Fasern in Wasser. Colloid & Polymer Science. 1960; 168(2):115–121.
Laine, J. Surface properties of unbleached kraft pulp fibres, determined by different methods, 1994. [Laboratory of Forest Products Chemistry Report].
Li, Y., Pickering, K.L., Farrell, R.L. Analysis of green hemp fibre reinforced composites using bag retting and white rot fungal treatments. Industrial Crops and Products. 2009; 29(2–3):420–426.
Lyklema, H. Colloidal Dispersions. London: Royal Society of Chemistry; 1981.
Mäder, E., Grundke, K., Jacobasch, H.-J., Wachinger, G. Surface, interphase and composite property relations in fibre-reinforced polymers. Composites. 1994; 25(7):739–744.
Mohanty A.K., Misra M., Drzal L.T., eds. Natural Fibers, Biopolymers, and Biocomposites. CRC Press, 2005.
Ottewill, R.H., Shaw, J.N. Studies on the preparation and characterisation of monodisperse polystyrene lattices. Colloid & Polymer Science. 1967; 218:34–40.
Pickering, K.L., Li, Y., Farrell, R.L., Lay, M. Interfacial modification of hemp fiber reinforced composites using fungal and alkali treatment. Journal of Biobased Materials and Bioenergy. 2007; 1(1):109–117.
Rätzsch, M., Jacobasch, H.-J., Freitag, K.-H. Origin, characterisation and technological effects of interfaction forces proceeding from polymer surfaces. Advances in Colloid and Interface Science. 1990; 31(3–4):225–320.
Rendall, H.M., Smith, A.L. Surface and electrokinetic potentials of interfaces containing 2 types of ionizing group. Journal of the Chemical Society-Faraday Transactions I. 1978; 74:1179–1187.
Ribitsch, V., Stana-Kleinscheck, K., Characterization of textile fiber surfaces by streaming potential measurementsBeitrag zum Zeta Potential Workshop. Austria: Graz, 1997.
Ribitsch, V., Stana-Kleinscheck, K. Characterizing textile fiber surfaces with streaming potential measurements. Textile Research Journal. 1998; 68(10):701–707.
Ribitsch, V., Stana Kleinschek, K., Jeler, S. The influence of classical and enzymatic treatment on the surface charge of cellulose fibres. Colloid and Polymer Science. 1996; 274(4):388–394.
Rybicki, E., Jacobasch, H.-J. Electrokinetic studies on synthetic fibres in surfactant solutions. Tenside Surfactants Detergents. 1992; 29:311–314.
Stamboulis, A., Baillie, C.A., Peijs, T. Effects of environmental conditions on mechanical and physical properties of flax fibers. Composites Part A-Applied Science and Manufacturing. 2001; 32(8):1105–1115.
Stamboulis, A., Baillie, C.A., Garkhail, S.K., van Melick, H.G.H., Peijs, T. Environmental durability of flax fibres and their composites based on polypropylene matrix. Applied Composite Materials. 2000; 7(5–6):273–294.
Stana-Kleinschek, K., Ribitsch, V. Electrokinetic properties of processed cellulose fibers. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 1998; 140(1–3):127–138.
Stern, O. The theory of the electrolytic double-layer. Zeitschrift für Elektrochemie und Angewandte Physikalische Chemie. 1924; 30:508–516.
Uchida, E., Ikada, Y., Zeta-potential of polymer surfacesEncyclopedia of surface and colloid science. New York: A. Hubbard, 2002. [Marcel Dekker: 5657].
Werner, C., Jacobasch, H.-J., Reichelt, G. Surface characterisation of hemodialysis membranes based on streaming potential measurements. Journal of Biomaterials Science: Polymer Edition. 1995; 7(1):61–76.
Zimmermann, R., Rein, N., Werner, C. Water ion adsorption dominates charging at nonpolar polymer surfaces in multivalent electrolytes. Physical Chemistry Chemical Physics. 2009; 11(21):4360–4364.