Preparation of cellulose nanocomposites
Abstract:
The preparation of elongated rod-like nanoparticles from lignocellulosic fibers and their use as a reinforcing phase in a polymeric matrix is discussed. The hierarchical structure of natural fibers is described and the expected effects of changing the scale of cellulosic particles from micro to nano are outlined. The preparation methods and morphological features of the ensuing nanofibers are also discussed. The different ways of processing nanocomposite films using a polymer matrix are presented. The main properties of these films, in terms of microstructure, thermal properties, mechanical performances, swelling behavior and barrier properties are reviewed.
3.1 Introduction
Over the last two decades a good deal of work has been dedicated to the use of lignocellulosic fibers as reinforcing elements in polymeric matrices and for the possibility of replacing conventional fibers such as glass by natural fibers in reinforced composites. However, one of the main drawbacks of lignocellulosic fibers, among others, is the important variation of properties inherent to any natural product. Indeed, their properties are related to climatic conditions, maturity, and type of soil. Disturbances during plant growth also affect the plant structure and are responsible for the wide scatter of mechanical plant fiber properties.
One of the basic approaches to achieving improved fibers and composites is to eliminate the macroscopic flaws by disintegrating the natural grown fibers, and separating the almost defect-free highly crystalline fibrils. This can be achieved by exploiting the hierarchical structure of natural fibers. Aqueous suspensions of cellulose nanoparticles can be prepared by a mechanical treatment or acid hydrolysis of the biomass. The object of this latter treatment is to dissolve away regions of low lateral order so that the water-insoluble, highly crystalline residue may be converted into a stable suspensoid by subsequent vigorous mechanical shearing action. The resulting nanocrystals occur as rod-like particles or whiskers, the dimensions of which depend on the nature of the substrate, but range in the nanometer scale. Because these whiskers contain only a small number of defects, their axial Young’s modulus is close to the one derived from theoretical chemistry and potentially stronger than steel and similar to kevlar. These highly stiff nanoparticles are therefore suitable for the processing of green nanocomposite materials.
Cellulosic nanoparticles, the generally accepted and overused trade name being nanocellulose, have generated interest from the scientific community because of their biodegradability, strength and other characteristics. Sustainability and green issues continue as top priorities for many businesses and individuals, stimulating the search for non-petroleum-based structural materials such as bionanocomposites that are biodegradable, high performance and lightweight.
3.2 Hierarchical structure of natural fibers
Cellulose is one of the most important structural elements in plants and some other living species serving to maintain their structure. It is a ubiquitous structural polymer that confers its mechanical properties to higher plant cells. In nature, cellulose occurs as slender rod-like or threadlike entity, which arises from the linear association of crystallites. This entity is called the microfibril (a collection of cellulose chains) and it forms the basic structural unit of the plant cell wall. Each microfibril can be considered as a string of cellulose crystallites, linked along the chain axis by amorphous domains (Fig. 3.1). Their structure consists of a predominantly crystalline cellulosic core. It is covered with a sheath of paracrystalline polyglucosan material surrounded by hemicelluloses (Whistler and Richards, 1970).
These microfibrils are cemented by other polymers such as lignin and hemicelluloses and aggregate further to form the lignocellulosic fibers. Depending on their origin, the microfibril diameters range from about 2 to 20 nm for lengths that can reach several tens of micrometers. As they are almost defect-free, the modulus of these sub-entities is close to the theoretical limit for cellulose. The potential of cellulose-based composites lies in the Young’s modulus of the cellulose crystallite, which was first experimentally studied in 1962 from the crystal deformation of cellulose I using highly oriented fibers of bleached ramie (Sakurada et al., 1962). A value of 137 GPa was reported. This value differs from the theoretical estimate of 167.5 GPa reported by Tashiro and Kobayashi (1991). More recently, Raman spectroscopy technique was used to measure the elastic modulus of native cellulose crystals (Šturcova et al., 2005). A value around 143 GPa was reported. The elastic modulus of single microfibrils from tunicate was measured by atomic force microscopy (AFM) using a three-point bending test (Iwamoto et al., 2009). Values of 145 and 150 GPa were reported for single microfibrils prepared by 2,2,6,6-tetramethylpiperidine–oxyl radical (TEMPO) oxidation and sulfuric acid hydrolysis, respectively.
These impressive mechanical properties make cellulose nanoparticles ideal candidates for the processing of reinforced polymer composites. Incorporating these nanoparticles in a synthetic or natural polymeric matrix consists therefore in biomimicing nature.
3.3 From micro- to nanoscale
As indicated in section 3.1, a major drawback of natural fibers for composite applications is the big variation of properties inherent to the natural products. The properties are related to climatic conditions, maturity, type of soil. Disturbances during plant growth will also affect the plant structure and are responsible for the enormous scatter of mechanical plant fiber properties. For instance, the number of knobby swellings and growth-induced lateral displacement present in plant fibers influence the tensile strength and elongation at break of the fibers obtained. One of the means to get round this problem involves decreasing the size of the natural particles from the micro to the nanoscale. Conceptually, nanoparticles refer to particles with at least one dimension less 100 nm.
When carrying out this scale shift, exploiting the hierarchical structure of natural fibers, important consequences occur. The first one is obviously an increase of the specific area of the particles, from values around a few m2 g−1 to a few 100 m2 g−1. This, in turn, results in an increase of the interfacial area with the polymeric matrix and a decrease of the average interparticle distance as the particle size decreases. Some particle–particle interactions can thus be expected. The homogeneous dispersion of nanoparticles in a continuous medium is generally difficult because the surface energy increases when decreasing their dimensions. Another important typical feature of nanoparticles is the possibility of improving properties of the material for low filler content without a detrimental effect on the impact resistance and plastic deformation. A reduction of gas diffusion (the barrier effect) is also likely to occur. Moreover, cellulosic nanoparticles are distinguished by their liquid crystal behavior when suspended in water, presenting birefringence phenomena under polarized light.
Native cellulose fibers are built up by smaller and mechanically stronger entities, the cellulose fibrils. Fibrils contain both crystalline and noncrystalline domains, the latter being located at the surface and along the main axis. Noncrystalline domains form weak spots along the fibrils. The processes for isolating cellulose nanoparticles include simple mechanical methods or a combination of chemical and mechanical methods. The first method involves submitting the natural fibers to multiple mechanical shearing actions. This disintegration process allows the release of the constitutive cellulosic microfibrils. The resulting aqueous suspensions display outstanding properties with a huge increase of the viscosity for very low nanoparticle contents. These micro or nanofibrils occur as very long thin entangled filaments. These nanoelements can also be obtained by a combination of enzymatic hydrolysis that facilitates the fibrillation and mechanical shearing, thus allowing a reduction in the energy consumption involved in the process. The second method involves submitting the natural fibers to a strong acid hydrolysis treatment followed by a sonication treatment to obtain stiff rod-like nanoparticles as explained in section 3.4. These nanocellulosic nanoparticles are easier to disperse in a polymeric matrix than entangled microfibrils and their better defined form is ideal for modeling rheological and reinforcement behaviors. This chapter mainly deals with acid-hydrolyzed nanoparticles but references are also given to mechanically sheared nanocellulose.
3.4 Preparation of cellulose nanocrystals
The extraction or isolation of crystalline cellulosic regions, in the form of nanocrystals, is a simple process based on acid hydrolysis. Various descriptors have been used in the literature to designate these crystalline rod-like nanoparticles. It is mainly referred to as whiskers, nanowhiskers, cellulose nanocrystals, NCC (nanocrystalline cellulose), monocrystals, microcrystals or microcrystallites, despite their nanoscale dimensions. The terms microfibrils, microfibrillated cellulose (MFC), or nanofibrillated cellulose are used to designate cellulosic nanoparticles obtained by a simple mechanical shearing disintegration process (Fig. 3.2).

3.2 Transmission electron micrograph from a dilute suspension of MFC obtained from Opuntia ficus-indica (reprinted with permission from Malainine et al., 2005, copyright Elsevier).
To prepare cellulose whiskers, the biomass is generally first submitted to a bleaching treatment with NaOH to purify cellulose. After removal of other constituents, such as lignin and hemicelluloses, the bleached material is disintegrated in water, and the resulting suspension is submitted to a hydrolysis treatment with acid. The amorphous regions of cellulose act as structural defects and are responsible for the transverse cleavage of the microfibrils into short nanocrystals under acid hydrolysis. under controlled conditions, this transformation consists of the disruption of amorphous regions surrounding and embedded within cellulose microfibrils. The crystalline segments remain intact, because of the faster hydrolysis kinetics of amorphous domains compared with crystalline ones. The hydronium ions penetrate the cellulosic material in the amorphous domains promoting the hydrolytic cleavage of the glycosidic bonds to release individual crystallites. The resulting suspension is subsequently diluted with water and washed by successive centrifugations. Dialysis against distilled water is then performed to remove free acid in the dispersion. Disintegration of aggregates and complete dispersion of the whiskers is obtained by a sonication step. These suspensions are generally much diluted because of the formation of a gel for low nanoparticle contents. Exact determination of the whisker content can be achieved by weighing aliquots of the solution before and after drying. The dispersions are stored in the refrigerator after filtration to remove residual aggregates. This general procedure has to be adapted depending on the nature of the substrate.
Dong et al. (1998) were among the first researchers studying the effect of hydrolysis conditions on the properties of resulting cellulose nanocrystals. They proved that a longer hydrolysis time leads to shorter monocrystals and also to an increase in their surface charge. The acid concentration was also found to affect the morphology of whiskers prepared from sugar-beet pulp as reported by Azizi Samir et al. (2004b). Beck-Candanedo et al. (2005) reported the properties of cellulose nanocrystals obtained by hydrolysis of softwood and hardwood pulp and investigated the influence of hydrolysis time and acid-to-pulp ratio. It was found that the reaction time is one of the most important parameters to consider in the acid hydrolysis of wood pulp. Moreover, they considered that too long reaction time would digest completely the cellulose to yield its component sugar molecules. On the contrary, a lower reaction time only yields large undispersable fibers and aggregates. It was reported that an increase of the hydrolysis time of pea hull fibers results in a decrease of both length and diameter, whereas the aspect ratio first increases and then decreases (Chen et al., 2009). The effect of the reaction conditions on cellulose nanocrystal surface charge and sulfur content was not significant and it was supposed to be controlled by factors other than hydrolysis conditions. However, chiral nematic pitch decreases when increasing the cellulose concentration and decreasing nanocrystals length. An attempt to find optimized conditions to prepare cellulose nanocrystals from microcrystalline cellulose (MCC) derived from Norway spruce (Picea abies) was also reported (Bondenson et al., 2006). The processing parameters have been optimized by using a response surface methodology.
Cellulose fibers can be oxidized by TEMPO-mediated oxidation creating carboxyl groups at the surface. The TEMPO-oxidized cellulose fibers can be converted to transparent and highly viscous dispersions in water, consisting of highly crystalline individual nanofibers (Fukuzumi et al., 2009; Saito et al., 2006; 2007). At pH 10, optimal conditions were reached, giving cellulose nanofibers 3–4 nm wide and a few micrometers long. It was also shown that the hydrolysis of amorphous cellulosic chains can be performed simultaneously with the esterification of accessible hydroxyl groups to produce surface functionalized whiskers in a single step (Braun and Dorgan, 2009). The reaction was carried out in an acid mixture composed of hydrochloric and an organic acid (acetic and butyric). Resulting nanocrystals are of similar dimensions to those obtained by hydrochloric acid hydrolysis alone. Narrower diameter polydispersity indices indicate that surface groups aid the individualization of the nanowhiskers. The resulting surface-modified cellulose whiskers are dispersible in ethyl acetate and toluene indicating increased hydrophobicity and presumably higher compatibility with hydrophobic polymers.
Cellulose nanoparticles are obtained as aqueous suspensions, the stability of which depends on the dimensions of the dispersed species, size polydispersity and surface charge. The use of sulfuric acid to prepare cellulose nanocrystals leads to more stable aqueous suspension than that prepared using hydrochloric acid (Araki et al., 1998). It was shown that the H2SO4-prepared nanoparticles present a negatively charged surface whereas the HCl-prepared nanoparticles are not charged. During acid hydrolysis via sulfuric acid, acidic sulfate ester groups are probably formed on the nanoparticle surface. This creates an electric double layer repulsion between the nanoparticles in suspension, which plays an important role in their interaction with a polymer matrix and with each other. The density of charges on the cellulose nanocrystals surface depends on the hydrolysis conditions and can be determined by elementary analysis or conductimetric titration to determine the sulfur content. The sulfate group content increases with acid concentration, acid-to-polysaccharide ratio and hydrolysis time. Based on the density and size of the cellulose whiskers, Araki et al. (1998, 1999) estimated for a nanocrystal with dimensions of 7 × 7 × 115 nm3, that the charge density is 0.155 e nm−2, where e is the elementary charge. With the following conditions: cellulose concentration of 10 wt% in 60% sulfuric acid at 46 °C for 75 min, the charge coverage was estimated at 0.2 negative ester groups per nm (Revol et al., 1992). Other typical values of the sulfur content of cellulose whiskers prepared by sulfuric acid hydrolysis were reported (Marchessault et al., 1961; Revol et al., 1994). It was shown that even at low levels, the sulfate groups caused a significant decrease in degradation temperature and increase in char fraction confirming that the sulfate groups act as flame retardants (Roman and Winter, 2004).
Cellulose nanocrystals can be prepared from any botanical source containing cellulose. In the many published studies, various cellulosic sources have been used as shown in Fig. 3.3. Regardless of the source, cellulose nanocrystals occur as elongated nanoparticles. The persistence of the spot diffractogram when the electron probe is scanned along the rod during transmission electron microscopy (TEM) observation evidences the monocrystalline nature of the cellulosic fragment (Favier et al., 1995a). Therefore, each fragment can be considered as a cellulosic crystal with no apparent defect. Their dimensions depend on several factors, including the source of the cellulose, the exact hydrolysis conditions and ionic strength.

3.3 Transmission electron micrographs from a dilute suspension of (a) bacterial cellulose (reprinted with permission from Grunert and Winter, 2002, copyright Springer), (b) cotton cellulose (reprinted with permission from Fleming et al., 2000, copyright American Chemical Society, the scale bar corresponds to 400 nm), (c) MCC (reprinted with permission from Kvien et al., 2005, copyright American Chemical Society), (d) sugar beet pulp (reprinted with permission from Azizi Samir et al., 2004a, copyright American Chemical Society), (e) wheat straw nanocrystals (reprinted with permission from Helbert et al., 1996, copyright Wiley Interscience), (f) sisal (reprinted with permission from Siqueira et al., 2009, copyright American Chemical Society), (g) tunicin (reprinted with permission from Anglès and Dufresne, 2000, copyright American Chemical Society), (h) acacia pulp (reprinted with permission from Pu et al., 2007, copyright Elsevier), (i) banana rachis (reprinted with permission from Zuluaga et al., 2007, copyright Springer), (j) eucalyptus wood pulp (reprinted with permission from de Mesquita et al., 2010, copyright American Chemical Society), (k) Luffa cylindrica, (l) ramie (reprinted with permission from Habibi et al., 2008) copyright The Royal Society of Chemistry), (m) Capim Dourado (reprinted with permission from Siqueira et al., 2010a, copyright Springer).
The typical geometrical characteristics for nanocrystals derived from different species are presented in Table 3.1. Even if often composed of a few laterally bound elementary crystallites that are not separated by conventional acid hydrolysis and sonication process (Elazzouzi-Hafraoui et al., 2008), the length and width of hydrolyzed cellulose nanocrystals is generally of the order of a few hundred nanometers and a few nanometers, respectively. It was observed that the length polydispersity has a constant value, whereas the diameter polydispersity depends on the acid used for isolation (Braun et al., 2008). A smaller diameter polydispersity was obtained when using sulfuric acid instead of hydrochloric acid, because of electrostatic charges resulting from the introduction of sulfate ester groups when using the former.
Table 3.1
Geometrical characteristics of cellulose nanocrystals from various sources: length (L), cross-section (D) and aspect ratio (L/D) of rod-like particles obtained from acid hydrolysis
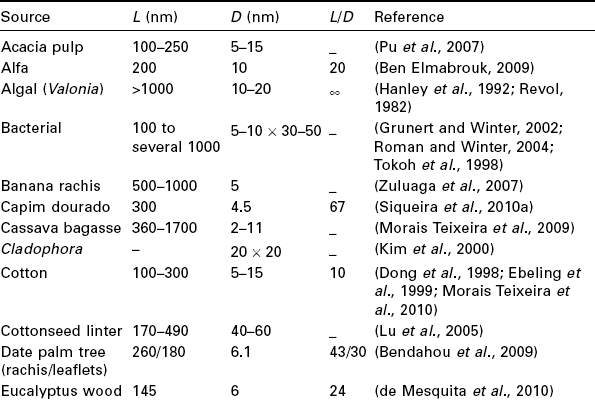
An important parameter for cellulosic whiskers is the aspect ratio, defined as the ratio of the length to the width (Table 3.1), which determined the anisotropic phase formation and reinforcing properties. The average length ranges between 1 μm for nanocrystals prepared for instance from tunicate and around 200 nm for cotton. The cellulose extracted from tunicate, a sea animal, is referred to as tunicin. The diameter ranges between 15 nm for tunicin and 4–5 nm for sisal or wood. The high value reported for cottonseed linter corresponds to aggregates. The aspect ratio varies between 10 for cotton and 67 for tunicin. Relatively large and highly regular tunicin whiskers are ideal for modeling rheological and reinforcement behaviors and were extensively used in the literature. The shape and dimensions of cellulose whiskers can be measured by microscopic observations or scattering techniques. The cross- sections of microfibrils observed by TEM are square, whereas their AFM topography shows a rounded profile owing to convolution with the shape of the AFM tip (Hanley et al., 1992). AFM images of the surface of highly crystalline cellulose microfibrils showed periodicities along the microfibril axis of 1.07 and 0.53 nm that were supposed to correspond to the fiber and glucose unit repeat distances, respectively. Scattering techniques include small-angle light (De souza Lima et al., 2003) and neutron (Orts et al., 1998) scattering.
Layer-by-layer assembled (LBL) tunicin whiskers films show strong antireflection properties having an origin in a novel highly porous architecture reminiscent of a ‘flattened matchsticks pile’, with film-thickness-dependent porosity and optical properties created by randomly oriented and overlapping whiskers (Podsiadlo et al., 2007). There are potential applications of cellulose nanocrystals in the medical field. Dong and Roman (2007) prepared fluorescently labeled cellulosic nanocrystals to be used as indicators in nanomedicine.
3.5 Processing of cellulose nanocomposites
The main challenge with nanoparticles is related to their homogeneous dispersion within a polymeric matrix. The presence of sulfate groups resulting from the acid hydrolysis treatment when using sulfuric acid to prepare cellulose whiskers or nanocrystals induces the stability of the ensuing aqueous suspension. Water is therefore the initially preferred processing medium. A high level of dispersion of the filler within the host matrix in the resulting composite film is expected when processing nanocomposites in aqueous medium.
3.5.1 Polymer latexes
The first publication reporting the preparation of cellulose nanocrystals reinforced polymer nanocomposites was carried out using a latex obtained by the copolymerization of styrene and butyl acrylate, poly(S-co-BuA), and tunicin whiskers (Favier et al., 1995a). The same copolymer was used in association with wheat straw (Helbert et al., 1996) or sugar beet (Azizi samir et al., 2004b) cellulose nanocrystals. Other latexes such as poly(β- hydroxyoctanoate) (PHO) (Dubief et al., 1999; Dufresne, 2000; Dufresne et al., 1999), polyvinylchloride (PVC) (Chazeau et al., 1999a; 1999b; 1999c; 2000), waterborne epoxy (Matos Ruiz et al., 2001), natural rubber (NR) (Bendahou et al., 2009; 2010; Siqueira et al., 2010a), and polyvinyl acetate (PVAc) (Garcia de Rodriguez et al., 2006) were also used as matrix. Recently, stable aqueous nanocomposite dispersions containing cellulose whiskers and a poly(styrene-co-hexyl-acrylate) matrix were prepared via miniemulsion polymerization (Ben Elmabrouk et al., 2009). Addition of a reactive silane was used to stabilize the dispersion. Solid nanocomposite films can be obtained by mixing and casting the two aqueous suspensions followed by water evaporation. Alternative methods include freeze-drying and hot-pressing or freeze-drying, extruding and hot-pressing the mixture.
3.5.2 Hydrosoluble or hydrodispersible polymers
The preparation of cellulosic particles reinforced starch (Anglès and Dufresne, 2000; Kvien et al., 2007; Mathew and Dufresne, 2002; Mathew et al., 2008; Orts et al., 2005; Svagan et al., 2009), silk fibroin (Noishiki et al., 2002), poly(oxyethylene) (POE) (Azizi Samir, 2004a; 2004c; 2004f; 2005b; 2006), polyvinyl alcohol (PVA) (Lu et al., 2008; Paralikar et al., 2008; Roohani et al., 2008; Zimmermann et al., 2004; 2005), hydroxypropyl cellulose (HPC) (Zimmermann et al., 2004; 2005), carboxymethyl cellulose (CMC) (Choi and Simonsen, 2006), or soy protein isolate (SPI) (Wang et al., 2006) has been reported in the literature. The hydrosoluble or hydrodispersible polymer is first dissolved in water and this solution is mixed with the aqueous suspension of cellulose nanocrystals. The ensuing mixture is generally evaporated to obtain a solid nanocomposite film. It can also be freeze-dried and hot-pressed.
3.5.3 Nonaqueous systems
In addition to the use of an aqueous polymer dispersion, or latex, an alternative way to process nonpolar polymer nanocomposites reinforced with cellulose nanocrystals involves their dispersion in an adequate (with regard to matrix) organic medium. Coating with a surfactant or surface chemical modification of the nanoparticles can also be considered. The global objective is to reduce their surface energy in order to improve their dispersibility/compatibility with nonpolar media.
Coating of cotton and tunicin whiskers by a surfactant such as a phosphoric ester of polyoxyethylene (9) nonyl phenyl ether was found to lead to stable suspensions in toluene and cyclohexane (Heux et al., 2000) or chloroform (Kvien et al., 2005). Coated tunicin whiskers reinforced atactic polypropylene (aPP) (Ljungberg et al., 2005), isotactic polypropylene (iPP) (Ljungberg et al., 2006), or poly(ethylene-co-vinyl acetate) (EVA) (Chauve et al., 2005) were obtained by solvent casting using toluene. The same procedure was used to disperse cellulosic nanoparticles in chloroform and process composites with polylactic acid (PLA) (Kvien et al., 2005; Petersson and Oksman, 2006). Nanocomposite materials were also prepared by dispersing cellulose acetate butyrate (CAB) in a dispersion of topochemically trimethylsilylated bacterial cellulose nanocrystals in acetone and subsequent solution casting (Grunert and Winter, 2002).
Surface chemical modification of cellulosic nanoparticles is another way to decrease their surface energy and disperse them in organic liquids of low polarity. It generally involves reactive hydroxyl groups from the surface. Experimental conditions should avoid swelling media and the peeling effect of surface-grafted chains inducing their dissolution in the reaction medium. The chemical grafting has to be mild in order to preserve the integrity of the nanoparticle. Goussé et al. (2002) stabilized tunicin microcrystals in tetrahydrofuran (THF) by a partial silylation of their surface. Grunert and Winter (2002) reported the preparation of bacterial cellulose nanocrystals topochemically trimethylsilylated. Resulting nanoparticles were dispersed in acetone to process nanocomposites with a cellulose acetatebutyrate matrix. Araki et al. (2001) prepared original sterically stabilized aqueous rod-like cellulose microcrystals suspensions by the combination of HCl hydrolysis, oxidative carboxylation and grafting of poly(ethylene glycol) (PEG) having a terminal amino group on one end using water-soluble carbodi-imide. The PEG-grafted microcrystals displayed drastically enhanced dispersion stability evidenced through resistance to addition of 2 M sodium chloride. They also showed ability to redisperse into either water or chloroform from the freeze- dried state. Alkenyl succinic anhydride (ASA) can be used for acylating the surface of cellulose nanocrystals. Surface chemical modification of tunicin whiskers with ASA was reported by Yuan et al. (2006). The acylated whiskers were found to disperse in medium- to low-polarity solvents. It was shown that by controlling the heating time, whiskers with different dispersibility could be obtained. Nogi et al. (2006a) and Ifuku et al. (2007) were among the first to use acetylated cellulosic nanofibers in the preparation of reinforced clear plastic.
Preparation of stable cellulose whiskers suspensions in dimethylformamide (DMF) (Azizi Samir et al., 2004e; Marcovich et al., 2006), and dimethyl sulfoxide (DMSO) or N-methyl pyrrolidine (NMP) (van den Berg et al., 2007) without either addition of a surfactant or any chemical modification was also reported. From DMF, tunicin whiskers reinforced POE plasticized with tetraethylene glycol dimethyl ether (TEGDME) were prepared by casting and evaporation of DMF (Azizi Samir et al., 2004f). Cross-linked nanocomposites were also prepared by dispersing cellulose nanocrystals in a solution of an unsaturated linear polycondensate, addition of a photoinitiator, casting, evaporating the solvent and UV-curing (Azizi Samir et al., 2004d).
3.5.4 Long-chain grafting
Long-chain surface chemical modification of cellulosic nanoparticles involving grafting agents bearing a reactive end group and a long ‘compatibilizing’ tail was also reported. The general objective was to increase the apolar character of the nanoparticle. In addition, the surface modifications can act as binding sites for active agents in drug delivery systems or for toxins in purifying and treatment systems. These surface modifications may also be able to interdiffuse, upon heating, to form the polymer matrix phase. The covalent linkage between reinforcement and matrix results in near-perfect stress transfer at the interface with exceptional mechanical properties of the composite as a result.
Nanocomposite materials were processed from polycaprolactone (PCL)- grafted cellulose whiskers using the grafting ‘onto’ (Habibi and Dufresne, 2008) and grafting ‘from’ (Habibi et al., 2008) approaches. The ensuing nanoparticles were used to process nanocomposites using PCL as matrix and a casting/evaporation technique from dichloromethane. A co-continuous crystalline phase around the nanoparticles was observed. Cellulose whiskers were also surface-grafted with PCL via microwave-assisted ring-opening polymerization yielding filaceous cellulose whisker-graft-PCL nanocrystals which were incorporated into PLA as matrix (Lin et al., 2009). Epoxy functionality was introduced onto the surface of cellulosic nanoparticles by oxidation by cerium (IV) followed by grafting of glycidyl methacrylate (stenstad et al., 2008). The length of the polymeric chain was varied by regulating the amount of glycidyl methacrylate. The surface of cellulose whiskers was also chemically modified by grafting organic acid chlorides presenting different lengths of the aliphatic chain by an esterification reaction (de Menezes et al., 2009). These functionalized nanoparticles were extruded with low density polyethylene (LDPE) to prepare nanocomposite materials. Cellulose whiskers reinforced waterborne polyurethane nanocomposites were synthesized via in situ polymerization using casting/evaporation technique (Cao et al., 2009). The grafted chains were able to form a crystalline structure on the surface of the nanoparticles and induce the crystallization of the matrix. Cellulose nanoparticles were modified with n-octadecyl isocyanate (C18H37NCO) using two different methods with one consisting of an in situ solvent exchange procedure (siqueira et al., 2010c). Phenol was also enzymatically polymerized in the presence of TEMPO-oxidized cellulosic nanoparticles to prepare nanocomposites under ambient conditions (Li et al., 2010).
3.5.5 Extrusion and impregnation
Very few studies have been reported concerning the processing of cellulose nanocrystals reinforced nanocomposites by extrusion methods. The hydrophilic nature of cellulose causes irreversible agglomeration during drying and aggregation in nonpolar matrices because of the formation of additional hydrogen bonds between amorphous parts of the cellulose nanoparticles. Therefore, the preparation of cellulose whiskers reinforced PLA nanocomposites by melt extrusion was carried out by pumping the suspension of nanocrystals into the polymer melt during the extrusion process (Oksman et al., 2006). An attempt to use PVA as a compatibilizer to promote the dispersion of cellulose whiskers within the PLA matrix was reported (Bondenson and Oksman, 2007). Organic acid chlorides-grafted cellulose whiskers were extruded with LDPE (de Menezes et al., 2009). The homogeneity of the ensuing nanocomposite was found to increase with the length of the grafted chains (Fig. 3.4).

3.4 Photographs of the neat LDPE film and extruded nanocomposite films reinforced with 10 wt% of unmodified and C18 acid chloridegrafted cellulose whiskers (reprinted with permission from de Menezes et al., 2009, copyright Elsevier).
Another possible processing technique of nanocomposites using cellulosic nanoparticles in the dry state involves the filtration of the aqueous suspension to obtain a film or dried mat of particles followed by immersion in a polymer solution. The impregnation of the dried mat is performed under vacuum. Composites were processed by filling the cavities with transparent thermosetting resins such as phenol formaldehyde (Nakagaito and Yano, 2004; 2008; Nakagaito et al., 2005), epoxy (Shimazaki et al., 2007), acrylic (Iwamoto et al., 2008; Nogi et al., 2005; Yano et al., 2005) and melamine formaldehyde (Henriksson and Berglund, 2007). Nonwoven mats of cellulose microfibrils were also used to prepare polyurethane composite materials using a film stacking method (Seydibeyoğlu and Oksman, 2008).
Water-redispersible nanofibrillated cellulose in powder form was recently prepared from refined bleached beech pulp by carboxymethylation and mechanical disintegration (Eyholzer et al., 2010). However, the carboxymethylated sample displayed a loss of crystallinity and strong decrease in thermal stability limiting its use for nanocomposite processing.
3.5.6 Electrospinning
Electrostatic fiber spinning or ‘electrospinning’ is a versatile method for preparing fibers with diameters ranging from several micrometers down to 100 nm through the action of electrostatic forces. Bacterial cellulose whiskers were incorporated into POE nanofibers with a diameter of less than 1 mm by the electrospining process to enhance the mechanical properties of the electrospun fibers (Park et al., 2007). The whiskers were found to be globally well embedded and aligned inside the fibers, even though they were partially aggregated. Electrospun polystyrene (PS) (Rojas et al., 2009), PCL (Zoppe et al., 2009) and PVA (Peresin et al., 2010) microfibers reinforced with cellulose nanocrystals were obtained by electrospinning. Nonionic surfactant sorbitan monostearate was used to improve the dispersion of the particles in the hydrophobic PS matrix.
3.5.7 Multilayer films
The use of the layer-by-layer (LBL) technique is expected to maximize the interaction between cellulose whiskers and a polar polymeric matrix, such as chitosan (de Mesquita et al., 2010). It also allows the incorporation of high amounts of cellulose whiskers, providing a dense and homogeneous distribution in each layer.
Podsiadlo et al. (2005) reported the preparation of cellulose whiskers multilayer composites with a polycation, poly(dimethyldiallylamonium chloride) (PDDA), using the LBL technique. They concluded that the multilayer films presented high uniformity and dense packing of nanocrystals. Orientated self-assembled films were also prepared using a strong magnetic film (Cranston and Gray, 2006a) or spin coating technique (Cranston and Gray, 2006b). The preparation of thin films composed of alternating layers of orientated rigid cellulose whiskers and flexible polycation chains was reported (Jean et al., 2008). Alignment of the rod-like nanocrystals was achieved using anisotropic suspensions of cellulose whiskers. Green composites based on cellulose nanocrystals/xyloglucan multilayers have been prepared using the nonelectrostatic cellulose-hemicellulose interaction (Jean et al., 2009). The thin films were characterized using neutron reflectivity experiments and AFM observations. More recently, biodegradable nanocomposites were obtained by the LBL technique using highly deacetylated chitosan and cellulose whiskers (de Mesquita et al., 2010). Hydrogen bonds and electrostatic interactions between the negatively charged sulfate groups on the nanoparticles surface and the ammonium groups of chitosan were the driving forces for the growth of the multilayered films. A high density and homogeneous distribution of cellulose nanocrystals adsorbed on each chitosan layer, each bilayer being around 7 nm thick, were reported. Self-organized films were also obtained using only charge-stabilized dispersions of cellulose nanoparticles with opposite charges (Aulin et al., 2010) from the LBL technique.
3.6 Properties of cellulose nanocomposites
3.6.1 Microstructure
A visual examination is the simplest way to assess the dispersion of cellulosic nanoparticles within the host polymeric matrix. Because of the nanoscale dimensions of the reinforcing phase the transparency of the nanocomposite film should remain if initially observed for the unfilled matrix. Opacity suggests the presence of aggregates of micrometric sizes (Ljungberg et al., 2005). Optical properties of UV-cured acrylic resin impregnated bacterial cellulose nanofibers were studied by Nogi et al. (2006b). Polarized optical microscopy was used by Azizi Samir et al. (2004c) to observe and follow the growth of POE spherulites in tunicin whiskers reinforced films. For the unfilled POE matrix, birefringent spherulites were clearly identified through the characteristic Maltese cross pattern indicating a spherical symmetry. For a 10 wt% tunicin whiskers reinforced material, the supermolecular structure was found to be quite different. It was observed that the spherulites exhibited a less birefringent character, most probably owing to a weakly organized structure. It was supposed that the cellulosic filler most probably interfered with the spherulite growth and that during growth the whiskers are ejected and then occluded in interspherulitic regions. The high viscosity of the filled medium most probably restricts this phenomenon and limits the size of the spherulites.
Scanning electron microscopy (SEM) is generally employed for the more extensive morphological inspection of cellulose nanocrystals reinforced polymers by observation of the cryofractured surfaces. By comparing the micrographs showing the surface of fracture of the unfilled matrix and of the composites, the nanoparticles can be easily identified (Fig. 3.5). In fact, they appear as white dots, the concentration of which is a direct function of the particles content in the composite. These shiny dots correspond to the transversal sections of the cellulose whiskers, but their diameter determined by SEM microscopy is much higher than the whiskers diameter. This results from a charge concentration effect owing to the emergence of cellulose whiskers from the observed surface (Anglès and Dufresne, 2000).
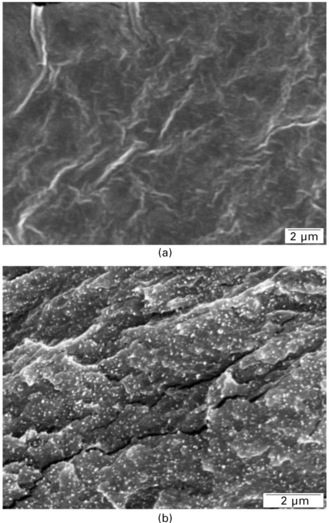
3.5 Scanning electron micrographs from the fractured surfaces of (a) unfilled plasticized starch matrix and related composites filled with (b) 25 wt% tunicin whiskers (reprinted with permission from Anglès and Dufresne, 2000, copyright American Chemical Society).
The dispersion of nanoparticles in the nanocomposite film strongly depends on the processing technique and conditions. SEM comparison between either cast and evaporated or freeze-dried and subsequently hot-pressed composites based on poly(S-co-BuA) reinforced with wheat straw whiskers, demonstrated that the former were less homogeneous and displayed a gradient of whiskers concentration between the upper and lower faces of the composite film (Dufresne et al., 1997; Helbert et al., 1996). It was suggested that the casting/evaporation technique results in films that are more homogeneous and in which the whiskers have a tendency to orient randomly into horizontal planes. A two-dimensional in-plane random network of tunicin whiskers reinforced epoxy was also reported from Raman spectroscopy experiments (Šturcová et al., 2005).
TEM observation can also be performed to investigate the microstructure and dispersion of the nanoparticles in the nanocomposite film. Casting/evaporation was reported to be an efficient processing technique to obtain a high dispersion level (Favier et al., 1995a; 1995b; Kvien et al., 2005; Matos Ruiz et al., 2001; Noishiki et al., 2002; Zimmermann et al., 2005). small angle x-ray scattering (sAXs) and small angle neutron scattering (SANS) are other ways to monitor the dispersion of cellulosic whiskers in the matrix. This latter technique was used to identify an isotropic dispersion of tunicin whiskers in plasticized PVC (Chazeau et al., 1999a). Atomic force microscopy (AFM) imaging has been used to investigate the microstructure of cellulose nanocrystals reinforced polymer nanocomposites (Kvien et al., 2005; Zimmermann et al., 2005). A comparison between field emission (FE) SEM, AFM and bright-field (BF) TEM for structure determination of cellulose whiskers and their nanocomposites with PLA was carried out (Kvien et al., 2005). It was found that AFM overestimated the width of the whiskers owing to the tip-broadening effect. FESEM allowed for a quick examination giving an overview of the sample but with limited resolution for detailed information. Detailed information was obtained from TEM, but this technique requires staining and suffers in general from limited contrast and beam sensitivity of the material.
3.6.2 Thermal properties
The glass–rubber transition temperature, Tg, of cellulose whiskers filled polymer composites is an important parameter, which controls different properties of the resulting composite such as its mechanical behavior, matrix chains dynamics and swelling behavior. Its value depends on the interactions between the polymeric matrix and cellulosic nanoparticles. These interactions are expected to play an important role because of the huge specific area inherent to nanosize particles. For semicrystalline polymers, possible alteration of the crystalline domains by the cellulosic filler may indirectly affect the value of Tg.
No modification of Tg was reported for cellulose whiskers reinforced poly(s-co-BuA) (Dufresne et al., 1997; Favier et al., 1995a; 1995b; Hajji et al., 1996), PHO (Dubief et al., 1999; Dufresne, 2000; Dufresne et al., 1999), PVC (Chazeau et al., 1999a), POE (Azizi Samir et al., 2004a; 2004c), PP (Ljungberg et al., 2005) and NR (Bendahou et al., 2009; Siqueira et al., 2010a). In glycerol plasticized starch based composites, peculiar effects of tunicin whiskers on the Tg of the starch-rich fraction were reported depending on moisture conditions (Angles and Dufresne, 2000). For low loading level (up to 3.2 wt%), a classical plasticization effect of water was reported (Fig. 3.6). However, an antiplasticization phenomenon was observed for higher whiskers content (6.2 wt% and up). These observations were discussed according to the possible interactions between hydroxyl groups on the cellulosic surface and starch, the selective partitioning of glycerol and water in the bulk starch matrix or at whiskers surface, and the restriction of amorphous starch chains mobility in the vicinity of the starch crystallite coated filler surface. For glycerol plasticized starch reinforced with cellulose crystallites prepared from cottonseed linter (Lu et al., 2005), an increase of Tg with filler content was reported and attributed to cellulose/starch interactions. For tunicin whiskers/sorbitol plasticized starch (Mathew and Dufresne, 2002), Tg increased slightly up to about 15 wt% whiskers and decreased for higher whiskers loading. Crystallization of amylopectin chains upon whiskers addition and migration of sorbitol molecules to the amorphous domains were proposed to explain the observed modifications. When using a PVA matrix, an increase in Tg was reported when the cellulose whiskers content increased (Garcia de Rodriguez et al., 2006; Roohani et al., 2008). A similar observation was reported for CMC reinforced with cotton cellulose whiskers (Choi and Simonsen, 2006).

3.6 Glass–rubber transition temperatures associated with the midpoints of the transitions versus water content for glycerol plasticized waxy maize starch filled with 0 (![]() ), 3.2 (
), 3.2 (![]() ), 6.2 (
), 6.2 (![]() ), 16.7 (
), 16.7 (![]() ), and 25 wt% (
), and 25 wt% (![]() ) tunicin whiskers. Solid lines serve to guide the eye (reprinted with permission from Anglès and Dufresne, 2000, copyright American Chemical Society).
) tunicin whiskers. Solid lines serve to guide the eye (reprinted with permission from Anglès and Dufresne, 2000, copyright American Chemical Society).
The melting temperature, Tm, was reported to be nearly independent of the filler content in plasticized starch (Anglès and Dufresne, 2000; Mathew and Dufresne, 2002) and in POE-based materials (Azizi Samir et al., 2004a; 2004c; 2004e) filled with tunicin whiskers. The same observation was reported for CAB reinforced with native bacterial cellulose whiskers (Grunert and Winter, 2002). However, for the latter system, Tm increased when the amount of trimethylsilylated whiskers increased. This difference was attributed to the stronger filler–matrix interaction for chemically modified whiskers. A decrease in both Tm and degree of crystallinity of PVA was reported when adding cellulose nanocrystals (Roohani et al., 2008). However, for electrospun cellulose whiskers reinforced PVA nanofibers, the crystallinity was found to be reduced upon filler addition (Peresin et al., 2010).
A significant increase in crystallinity of sorbitol plasticized starch (Mathew and Dufresne, 2002) was reported when increasing the cellulose whiskers content. This phenomenon was attributed to an anchoring effect of the cellulosic filler, probably acting as a nucleating agent. For POE-based composites the degree of crystallinity of the matrix was found to be roughly constant up to 10 wt% tunicin whiskers (Azizi Samir et al., 2004a; 2004c; 2004e) and to decrease for higher loading level (Azizi Samir et al., 2004c). The nucleating effect of cellulosic nanocrystals appears to be mainly governed by surface chemical considerations (Ljungberg et al., 2006). It was shown from both x-ray diffraction and DSC analysis that the crystallization behavior of films containing unmodified and surfactant-modified whiskers displayed two crystalline forms (α and β), whereas the neat matrix and the nanocomposite reinforced with nanocrystals grafted with maleated polypropylene only crystallized in the α form. It was suspected that the more hydrophilic the whisker surface, the more it appeared to favor the appearance of the β phase. Grunert and Winter (2002) observed from DSC measurements that native bacterial fillers impede the crystallization of the CAB matrix whereas silylated ones help to nucleate the crystallization. The crystallinity of N-octadecyl isocyanate-grafted sisal whiskers reinforced PCL was found to increase upon filler addition, whereas no influence of N-octadecyl isocyanate-grafted sisal MFC was reported (Siqueira et al., 2009). This difference was attributed to the possibility of entanglement of MFC that tends to confine the polymeric matrix and restrict its crystallization.
For tunicin whiskers filled semi-crystalline matrices such as PHO (Dufresne et al., 1999) and glycerol plasticized starch (Anglès and Dufresne, 2000) a transcrystallization phenomenon was reported. For glycerol-plasticized starch-based systems, the formation of the transcrystalline zone around the whiskers was assumed to be caused by the accumulation of plasticizer in the cellulose/amylopectin interfacial zones improving the ability of amylopectin chains to crystallize. This transcrystalline zone could originate from a glycerol–starch V structure. In addition, the inherent restricted mobility of amylopectin chains was proposed to explain the lower water uptake of cellulose/starch composites for increasing filler content. Transcrystallization of PP at cellulose nanocrystal surfaces was identified and found to result from enhanced nucleation owing to some form of epitaxy (Gray, 2008).
The presence of sulfate groups introduced at the surface of the whiskers during hydrolysis with H2SO4 promoted their thermal decomposition (Roman and Winter, 2004; Li et al., 2009). Thermogravimetric analysis (TGA) experiments were performed to investigate the thermal stability of tunicin whiskers/POE nanocomposites (Azizi Samir et al., 2004a; 2004c). No significant influence of the cellulosic filler on the degradation temperature of the POE matrix was reported. Cellulose nanocrystals content appeared to have an effect on the thermal behavior of CMC plasticized with glycerin (Choi and Simonsen, 2006) suggesting a close association between the filler and the matrix. The thermal degradation of unfilled CMC was observed from its melting point (270 °C) and it had a very narrow temperature range of degradation. Cellulose nanocrystals degraded at a lower temperature (230 °C) than CMC, but showed a very broad degradation temperature range. The degradation of cellulose whiskers reinforced CMC was observed between these two limits, but of interest was the lack of steps: the composites were reported to degrade as a unit.
3.6.3 Mechanical properties
Nanoscale dimensions resulting in a very high surface area-to-volume ratio and impressive mechanical properties of rod-like cellulose whiskers have attracted significant interest in the last fifteen years. These characteristics along with the remarkable suitability for surface functionalization make them ideal candidates for improving the mechanical properties of the host material.
The first demonstration of the reinforcing effect of cellulose whiskers in a poly(S-co-BuA) matrix was reported by Favier et al. (1995a; 1995b), who measured by dynamic mechanical analysis (DMA) a spectacular improvement in the storage modulus after adding tunicin whiskers even at low content into the host polymer. This increase was especially significant above the Tg of the thermoplastic matrix because of its poor mechanical properties in this temperature range. Figure 3.7 shows the isochronal evolution of the logarithm of the relative storage shear modulus (log G′ T/G′200, where G′200 corresponds to the experimental value measured at 200 K) at 1 Hz as a function of temperature for such composites prepared by water evaporation. In the rubbery state of the thermoplastic matrix, the modulus of the composite with a loading level as low as 6 wt% is more than two orders of magnitude higher than the one of the unfilled matrix. Moreover, the introduction of 3 wt% or more cellulosic whiskers provides an outstanding thermal stability of the matrix modulus up to the temperature at which cellulose starts to degrade (500 K). Since this pioneer work, many studies have reported the reinforcing capability of cellulose nanoparticles.

3.7 Logarithm of the normalized storage shear modulus (log G'T/G'200, where G'200 corresponds to the experimental value measured at 200 K) versus temperature at 1 Hz for tunicin whiskers reinforced poly(S-co-BuA) nanocomposite films obtained by water evaporation and filled with 0 (![]() ), 1 (
), 1 (![]() ), 3 (
), 3 (![]() ), 6 (∆) and 14 wt% (
), 6 (∆) and 14 wt% (![]() ) of cellulose whiskers (reprinted with permission from Azizi Samir et al., 2005a, copyright American Chemical Society).
) of cellulose whiskers (reprinted with permission from Azizi Samir et al., 2005a, copyright American Chemical Society).
This outstanding reinforcing effect was ascribed to a mechanical percolation phenomenon (Favier et al., 1995a; 1995b). A good agreement between experimental and predicted data was reported when using the series–parallel model of Takayanagi modified to include a percolation approach. It was suspected that all the stiffness of the material was caused by infinite aggregates of cellulose whiskers. Above the percolation threshold the cellulosic nanoparticles can connect and form a three dimensional continuous pathway through the nanocomposite film. For rod-like particles, such as tunicin whiskers with an aspect ratio of 67, the percolation threshold is close to 1 vol% (Favier et al., 1997a). The formation of this cellulose network was supposed to result from strong interactions between whiskers, such as hydrogen bonds (Favier et al., 1997b). This phenomenon is similar to the high mechanical properties observed for a paper sheet, which result from the hydrogen-bonding forces that hold the percolating network of fibers. This mechanical percolation effect explains both the high reinforcing effect and the thermal stabilization of the composite modulus for evaporated films. Any factor that affects the formation of the percolating whiskers network or interferes with it changes the mechanical performances of the composite (Dufresne, 2006). Three main parameters were reported to affect the mechanical properties of such materials, viz. (i) the morphology and dimensions of the nanoparticles, (ii) the processing method, and (iii) the microstructure of the matrix and matrix/filler interactions.
Morphology and dimensions of the nanoparticles
Cellulose nanocrystals occur as rod-like nanoparticles, the geometrical aspect ratio is an important factor because it determines the percolation threshold value. This factor is linked to the source of cellulose and whiskers preparation conditions. A higher reinforcing effect is obtained for nanocrystals with a high aspect ratio. For instance, the rubbery storage tensile modulus was systematically lower for wheat straw whiskers/poly(S-co-BuA) composites than for tunicin whiskers based materials (Dufresne, 2006). Also, the flexibility and tangling possibility of the nanofibers plays an important role (Azizi samir et al., 2004b; Bendahou et al., 2009; Siqueira et al., 2009). It was reported that entangled MFC induces a higher reinforcing effect than straight whiskers, whereas the elongation at break was lower.
The processing method
The processing method affects the possibility of formation of a continuous whiskers network and thus the final properties of the nanocomposite material. Slow processes such as casting/evaporation were reported to give the highest mechanical performance materials compared with freeze-drying/molding and freeze-drying/extruding/molding techniques. During slow water evaporation, because of Brownian motion in the suspension or solution (the viscosity of which remains low up to the end of the process when the latex particle or polymer concentration becomes very high), the rearrangement of the nanoparticles is possible. They have sufficient time to interact and connect to form a continuous network which is the basis of their reinforcing effect. The resulting structure is completely relaxed and direct contacts between nanoparticles are then created. Conversely, during the freeze-drying/hot- pressing process, the nanoparticle arrangement in the suspension is first frozen, and then, during the hot-pressing stage, because of the polymer melt viscosity, the particle rearrangements are strongly limited.
Microstructure of matrix and matrix/filler interactions
The microstructure of the matrix and the resulting competition between matrix/filler and filler/filler interactions also affect the mechanical behavior of cellulose nanocrystals reinforced nanocomposites. Classical composite science tends to favor matrix/filler interactions as a fundamental condition for optimal performance. For cellulose whiskers based composite materials, the opposite trend is generally observed when the material is processed via casting/evaporation method. This unusual behavior is ascribed to the originality of the reinforcing phenomenon of cellulosic nanoparticles resulting from the formation of a H-bonded percolating network. However, when using a processing route other than casting/evaporation in water medium, the dispersion of the hydrophilic filler in the polymeric matrix is also involved (de Menezes et al., 2009) and improved filler/matrix interactions generally lead to higher mechanical properties. In non-percolating systems, for instance for materials processed from freeze-dried cellulose nanocrystals, strong matrix/filler interactions enhance the reinforcing effect of the filler (Ljungberg et al., 2005). The transcrystallization phenomenon reported for PHO (Dufresne et al., 1999) and plasticized starch (Anglès and Dufresne, 2000) on cellulose whiskers resulted in a decrease of the mechanical properties (Anglès and Dufresne, 2001) because of the coating of the nanoparticles with crystalline domains. When using unhydrolyzed cellulose microfibrils extracted from potato pulp rather than cellulose nanocrystals to reinforce glycerol plasticized thermoplastic starch, a completely different mechanical behavior was reported (Dufresne and Vignon, 1998; Dufresne et al., 2000) and a significant reinforcing effect was observed. It was suspected that a tangling effect contributed to this high reinforcing effect (Anglès and Dufresne, 2001).
3.6.4 Swelling properties
Swelling or kinetics of solvent absorption is a method that can highlight specific interactions between the filler and the matrix. It usually involves first drying and weighing the sample, and then immersing it in the liquid solvent or exposing it to the vapor medium. The sample is then removed at specific intervals and weighed until an equilibrium value is reached. The swelling rate of the sample can be calculated by dividing the gain in weight by the initial weight. Generally, the short-term behavior displays a fast absorption phenomenon whereas in the long term, the kinetics of absorption is low and leads to a plateau, corresponding to the solvent uptake at equilibrium. The diffusion coefficient can be determined from the initial slope of the solvent uptake curve as a function of time. For cellulosic particles reinforced composites, it is generally of interest to investigate the water absorption of the material because of the hydrophilic nature of the reinforcing phase. When a nonpolar polymeric matrix is used, the absorption of a nonaqueous liquid can be investigated.
A higher resistance of thermoplastic starch to water was reported when increasing the cellulose nanoparticles content (Anglès and Dufresne, 2000; Lu et al., 2005; Svagan et al., 2009). Both the water uptake and the diffusion coefficient of water decreased upon nanoparticles addition. These phenomena were ascribed to the presence of strong hydrogen bonding interactions between particles and between the starch matrix and cellulose whiskers. The hydrogen bonding interactions in the composites tend to stabilize the starch matrix when it is submitted to a highly moist atmosphere. Moreover, the high crystallinity of cellulose might also be responsible for the decreased water uptake at equilibrium and diffusion coefficient of the material. A lower water uptake and dependence on cellulose whiskers content were reported when using sorbitol rather than glycerol as plasticizer for the starch matrix (Mathew and Dufresne, 2002). An explanation was proposed based on the chemical structure of both plasticizers, more accessible end hydroxyl groups in glycerol being about twice those of sorbitol. Similar results were reported for cellulose whiskers reinforced SPI (Wang et al., 2006) and CMC (Choi and Simonsen, 2006). Sisal whisker addition was found to stabilize PVA-based nanocomposites with no benefit seen when increasing the whisker content beyond the percolation threshold (Garcia de Rodriguez et al., 2006). A lower water uptake was observed when using MFC instead of cellulose whiskers as a reinforcing phase in NR (Bendahou et al., 2010). This observation was explained by the difference in the structure and composition of both nanoparticles and, in particular, by the presence of residual lignin, extractive substances and fatty acids at the surface of MFC that limits, comparatively, the hydrophilic character of the filler. In addition, assuming that the filler/matrix compatibility was consequently lower for whiskers-based nanocomposites, one can imagine that water infiltration could be easier at the filler/matrix interface. For MFC-based nanocomposites, despite higher amorphous cellulose content, the higher hydrophobic character of the filler favors the compatibility with NR and therefore restricts the interfacial diffusion pathway for water.
Swelling experiments of PVC reinforced with tunicin whiskers were conducted in methyl ethyl ketone (MEK) (Chazeau et al., 1999a). A significant decrease in swelling was observed when increasing the cellulose whiskers content, which was assumed to be caused by the existence of an interphase making a link between nanoparticles, thus allowing the formation of a flexible network. The swelling behavior in toluene of poly(S-co-BuA) reinforced with cellulose fibrils from Opuntia ficus-indica cladodes was reported by Malainine et al. (2005). They observed a strong toluene resistance even at very low filler loading. Although the unfilled matrix completely dissolved in toluene, only 27 wt% of the polymer was able to dissolve when filled with only 1 wt% of cellulose microfibrils. This phenomenon was attributed to the presence of a three-dimensional entangled cellulosic network which strongly restricted the swelling capability and dissolution of the matrix. For higher microfibrils content, no significant evolution was observed because of both the overlapping of the microfibrils restricting the filler–matrix interfacial area and the decrease of the entrapping matrix fraction owing to the densification of the microfibrils network. Similar experiments were conducted with cellulose microfibrils obtained from sugar beet pulp reinforced poly(s-co-BuA) (Dalmas et al., 2006). Toluene resistance of nanocomposites was found to be less significant than for microfibrils from Opuntia ficus-indica cladodes and to evolve with filler content. It was observed that the cohesion of composites prepared by evaporation was higher than the one of freeze-dried/hot-pressed materials. This difference was attributed to the presence of a H-bonded network in the former samples. It was concluded that the solvent did not have any effect on the hydrogen bonds of the cellulose network present in evaporated composites. On the contrary, for freeze-dried/hot-pressed materials, the lower interactions created between fibrils and polymer chains meant that they were able to be more easily disentangled and dissolved by the solvent. The swelling behavior of cellulose whiskers reinforced NR in toluene as reported by Bendahou et al. (2010) was found to strongly decrease even with only 1 wt% of cellulose nanoparticles and to be almost independent of filler content and nature (MFC or whiskers).
3.6.5 Barrier properties
Petrochemical-based polymers predominate in the packaging of foods because of their ease of processing, excellent barrier properties and low cost. However, there is currently an increasing interest in replacing conventional synthetic polymers by more sustainable materials. One promising application area of cellulosic nanoparticles is barrier membranes, where the nano-sized fillers impart enhanced mechanical and barrier properties. Research in this area is burgeoning and evolving rapidly to enhance the barrier properties and to overcome certain limitations. In addition, it is well known that molecules penetrate with difficulty in the crystalline domains of cellulose microfibrils. Moreover, the ability of cellulosic nanoparticles to form a dense percolating network held together by strong inter-particle bonds suggest their use as barrier films.
The oxygen permeability of paper was considerably reduced when coated with MFC layer (Syverud and Stenius, 2009). The water vapor transmission rate of cotton nanocrystals reinforced CMC (Choi and Simonsen, 2006) and PVA (Paralikar et al., 2008) films were reported to decrease. Cellulose nanoparticles prepared by drop-wise addition of ethanol/HCl aqueous solution into a NaOH/urea/H2O suspension of MCC were found to decrease the water vapor permeability of glycerol plasticized-starch films (Chang et al., 2010). The water vapor barrier of mango puree based edible (Azeredo et al., 2009) and chitosan films films (Azeredo et al., 2010) were successfully improved by adding cellulose whiskers
3.7 Conclusions and future trends
The potential of nanocomposites in various sectors of research and application is promising and attracting increasing investment. Owing to their abundance, high strength and stiffness, low weight and biodegradability, nano-scale cellulose fiber materials serve as promising candidates for the preparation of bionanocomposites. A broad range of applications of nanocellulose exists even though there are still a high number of unknown possibilities. Tens of scientific publications and experts show its potential even if most of the studies focus on their mechanical properties as reinforcing phase and their liquid crystal self-ordering properties. Packaging is one area in which nanocellulose-reinforced polymeric films can be of interest because of the possibility of producing films with high transparency and improved mechanical and barrier properties. However, although there have been many promising achievements on the laboratory or pilot scale, there are several challenges to overcome in order to be able to produce cellulose-based nanocomposites on an industrial scale.
Two important programs have recently started to produce nanocellulose. One is the creation in 2008 in Finland of the ‘Suomen Nanoselluloosakeskus’ Centre or ‘Finnish Centre for Nanocellulosic Technologies’. One of the challenges is to produce large quantities of microfibrils of uniform quality. The forest industry in Finland is going through a major transition, and the utilisation of new technologies is expected to provide a means for strengthening the competitiveness in the sector. The other program, supported by the Canadian government and represented by FPInnovations, is the creation of the ArboraNano network. The objective of this program for the valorization of nanocellulose (as cellulose nanocrystals) is also to revive the forestry sectors in Canada, strongly affected by the growing competition from emerging countries in Asia and South America.
However, it is worth noting that there are many safety concerns about nanomaterials, as their size allows them to penetrate into cells and eventually remain in the system. There is no consensus about categorizing nanomaterials as new materials.
3.8 References
Anglès, M.N., Dufresne, A. Plasticized starch/tunicin whiskers nanocomposites:1. Structural analysis. Macromolecules. 2000; 33:8344–8353.
Anglès, M.N., Dufresne, A. Plasticized starch/tunicin whiskers nanocomposites:2. Mechanical behavior. Macromolecules. 2001; 34:2921–2931.
Araki, J., Wada, M., Kuga, S. Steric stabilization of a cellulose microcrystal suspension by poly(ethylene glycol) grafting. Langmuir. 2001; 17:21–27.
Araki, J., Wada, M., Kuga, S., Okano, T. Flow properties of microcrystalline cellulose suspension prepared by acid treatment of native cellulose. Colloids Surf. A. 1998; 142:75–82.
Araki, J., Wada, M., Kuga, S., Okano, T. Influence of surface charges on viscosity behavior of cellulose microcrystal suspension. J. Wood Sci.. 1999; 45:258–261.
Aulin, C., Johansson, E., Wågberg, L., Lindström, T. Self-organized films from cellulose I nanofibrils using the layer-by-layer technique. Biomacromolecules. 2010; 11:872–882. [10.1021/bm100075e].
Azeredo, H.M., Mattoso, L.H.C., Avena-Bustillos, R.J., Filho, G.C., Munford, M.L., Wood, D., McHugh, T.H. Nanocellulose reinforced chitosan composite films as affected by nanofiller loading and plasticizer content. J. Food Sci.. 2010; 75:N1–N7. [10.1111/j.1750-3841.2009.01386.x].
Azeredo, H.M., Mattoso, L.H.C., Wood, D., Williams, T.G., Avena–Bustillos, R.J., McHugh, T.H. Nanocomposite edible films from mango puree reinforced with cellulose nanofibers. J. Food Sci. 2009; 74:N31–N35.
Azizi Samir, M.A.S., Alloin, F., Dufresne, A. Review of recent research into cellulosic whiskers, their properties and their application in nanocomposite field. Biomacromolecules. 2005; 6:612–626.
Azizi Samir, M.A.S., Alloin, F., Dufresne, A. High performance nanocomposite polymer electrolytes. Compos. Interfaces. 2006; 13:545–559.
Azizi Samir, M.A.S., Alloin, F., Gorecki, W., Sanchez, J.Y., Dufresne, A. Nanocomposite polymer electrolytes based on poly(oxyethylene) and cellulose nanocrystals. J. Phys. Chem. B. 2004; 108:10845–10852.
Azizi Samir, M.A.S., Alloin, F., Paillet, M., Dufresne, A. Tangling effect in fibrillated cellulose reinforced nanocomposites. Macromolecules. 2004; 37:4313–4316.
Azizi Samir, M.A.S., Alloin, F., Sanchez, J.Y., Dufresne, A. Cellulose nanocrystals reinforced poly(oxyethylene). Polymer. 2004; 45:4033–4041.
Azizi Samir, M.A.S., Alloin, F., Sanchez, J.Y., Dufresne, A. Cross-linked nanocomposite polymer electrolytes reinforced with cellulose whiskers. Macromolecules. 2004; 37:4839–4844.
Azizi Samir, M.A.S., Alloin, F., Sanchez, J.Y., El Kissi, N., Dufresne, A. Preparation of cellulose whiskers reinforced nanocomposites from an organic medium suspension. Macromolecules. 2004; 37:1386–1393.
Azizi Samir, M.A.S., Chazeau, L., Alloin, F., Cavaillé, J.Y., Dufresne, A., Sanchez, J.Y. POE-based nanocomposite polymer electrolytes reinforced with cellulose whiskers. Electrochim. Acta. 2005; 50:3897–3903.
Azizi Samir, M.A.S., Montero Mateos, A., Alloin, F., Sanchez, J.Y., Dufresne, A. Plasticized nanocomposite polymer electrolytes based on poly(oxyethylene) and cellulose whiskers. Electrochim. Acta. 2004; 49:4667–4677.
Beck-Candanedo, S., Roman, M., Gray, D.G. Effect of reaction conditions on the properties and behavior of wood cellulose nanocrystal suspensions. Biomacromolecules. 2005; 6:1048–1054.
Ben Elmabrouk, A., Thielemans, W., Dufresne, A., Boufi, S. Preparation of poly(styrene–co–hexylacrylate)/cellulose whiskers nanocomposites via miniemulsion polymerization. J. Appl. Polym. Sci.. 2009; 114:2946–2955.
Bendahou, A., Habibi, Y., Kaddami, H., Dufresne, A. Physico-chemical characterization of palm from Phoenix Dactylifera L, preparation of cellulose whiskers and natural rubber-based nanocomposites. J. Biobased Mat. Bioenergy. 2009; 3:81–90.
Bendahou, A., Kaddami, H., Dufresne, A. Investigation on the effect of cellulosic nanoparticles’ morphology on the properties of natural rubber based nanocomposites. Eur. Polym. J.. 2010; 46:609–620.
Bondenson, D., Mathew, A., Oksman, K. Optimization of the isolation of nanocrystals from microcrystalline cellulose by acid hydrolysis. Cellulose. 2006; 13:171–180.
Bondenson, D., Oksman, K. Polylactic acid/cellulose whisker nanocomposites modified by polyvinyl alcohol. Composites: Part A. 2007; 38:2486–2492.
Braun, B., Dorgan, J.R. Single-step method for the isolation and surface functionalization of cellulosic nanowhiskers. Biomacromolecules. 2009; 10:334–341.
Braun, B., Dorgan, J.R., Chandler, J.P. Cellulosic nanowhiskers. Theory and application of light scattering from polydisperse spheroids in the Rayleigh–Gans–Debye regime. Biomacromolecules. 2008; 9:1255–1263.
Cao, X., Dong, H., Li, C.M. New nanocomposite materials reinforced with flax cellulose nanocrystals in waterborne polyurethane. Biomacromolecules. 2007; 8:899–904.
Cao, X., Habibi, Y., Lucia, L.A. One-pot polymerization, surface grafting, and processing of waterborne polyurethane-cellulose nanocrystal nanocomposites. J. Mater. Chem.. 2009; 19:7137–7145.
Chang, P.R., Jian, R., Zheng, P., Yu, J., Ma, X. Preparation and properties of glycerol plasticized-starch (GPS)/cellulose nanoparticle (CN) composites. Carbohydr. Polym.. 2010; 79:301–305.
Chauve, G., Heux, L., Arouini, R., Mazeau, K. Cellulose poly(ethylene-co-vinyl acetate) nanocomposites studied by molecular modeling and mechanical spectroscopy. Biomacromolecules. 2005; 6:2025–2031.
Chazeau, L., Cavaillé, J.Y., Canova, G.R., Dendievel, R., Boutherin, B. Viscoelastic properties of plasticized PVC reinforced with cellulose whiskers. J. Appl. Polym. Sci.. 1999; 71:1797–1808.
Chazeau, L., Cavaillé, J.Y., Perez, J. Plasticized PVC reinforced with cellulose whiskers. II. Plastic behavior. J. Polym. Sci. B: Polym. Phys.. 2000; 38:383–392.
Chazeau, L., Cavaillé, J.Y., Terech, P. Mechanical behaviour above Tg of a plasticized PVC reinforced with cellulose whiskers, a SANS structural study. Polymer. 1999; 40:5333–5344.
Chazeau, L., Paillet, M., Cavaillé, J.Y. Plasticized PVC reinforced with cellulose whiskers 1. Linear viscoelastic behavior analyzed through the quasi point defect theory. J. Polym. Sci. B: Polym. Phys. 1999; 37:2151–2164.
Chen, Y., Liu, C., Chang, P.R., Cao, X., Anderson, D.P. Bionanocomposites based on pea starch and cellulose nanowhiskers hydrolyzed from pea hull fibre: effect of hydrolysis time. Carbohydr. Polym.. 2009; 76:607–615.
Choi, Y.J., Simonsen, J. Cellulose nanocrystal-filled carboxymethyl cellulose nanocomposites. J. Nanosci. Nanotechnol.. 2006; 6:633–639.
Cranston, E.D., Gray, D.G. Formation of cellulose-based electrostatic layer-by- layer films in a magnetic field. Sci. Technol. Adv. Mater.. 2006; 7:319–321.
Cranston, E.D., Gray, D.G. Morphological and optical characterization of polyelectrolyte multilayers incorporating nanocrystalline cellulose. Biomacromolecules. 2006; 7:2522–2530.
Dalmas, F., Chazeau, L., Gauthier, C., Cavaillé, J.Y., Dendievel, R. Large deformation mechanical behavior of flexible nanofiber filled polymer nanocomposites. Polymer. 2006; 47:2802–2812.
de Menezes, A.J., Siqueira, G., Curvelo, A.A.S., Dufresne, A. Extrusion and characterization of functionalized cellulose whisker reinforced polyethylene nanocomposites. Polymer. 2009; 50:4552–4563.
de Mesquita, J.P., Donnici, C.L., Pereira, F.V. Biobased nanocomposites from layer-by-layer assembly of cellulose nanowhiskers with chitosan. Biomacromolecules. 2010; 11:473–480.
De Souza, Lima M.M., Wong, J.T., Paillet, M., Borsali, R., Pecora, R. Translational and rotational dynamics of rodlike cellulose whiskers. Langmuir. 2003; 19:24–29.
Dong, S., Roman, M. Fluorescently labeled cellulose nanocrystals for bioimaging applications. J. Am. Chem. Soc.. 2007; 129:13810–13811.
Dong, X.M., Revol, J.F., Gray, D.G. Effect of microcrystallite preparation conditions on the formation of colloid crystals of cellulose. Cellulose. 1998; 5:19–32.
Dubief, D., Samain, E., Dufresne, A. Polysaccharide microcrystals reinforced amorphous poly(β-hydroxyoctanoate) nanocomposite materials. Macromolecules. 1999; 32:5765–5771.
Dufresne, A. Dynamic mechanical analysis of the interphase in bacterial polyester/cellulose whiskers natural composites. Compos. Interfaces. 2000; 7:53–67.
Dufresne, A. Comparing the mechanical properties of high performances polymer nanocomposites from biological sources. J. Nanosci. Nanotechnol.. 2006; 6:322–330.
Dufresne, A., Cavaillé, J.Y., Helbert, W. Thermoplastic nanocomposites filled with wheat straw cellulose whiskers. Part II: Effect of processing and modeling. Polym. Compos.. 1997; 18:198–210.
Dufresne, A., Dupeyre, D., Vignon, M.R. Cellulose microfibrils from potato cells: processing and characterization of starch/cellulose microfibrils composites. J. Appl. Polym. Sci.. 2000; 76:2080–2092.
Dufresne, A., Kellerhals, M.B., Witholt, B. Transcrystallization in mcl–PHAs/cellulose whiskers composites. Macromolecules. 1999; 32:7396–7401.
Dufresne, A., Vignon, M.R. Improvement of starch films performances using cellulose microfibrils. Macromolecules. 1998; 31:2693–2696.
Ebeling, T., Paillet, M., Borsali, R., Diat, O., Dufresne, A., Cavaillé, J.Y., Chanzy, H. Shear-induced orientation phenomena in suspensions of cellulose microcrystals, revealed by small angle x-ray scattering. Langmuir. 1999; 15:6123–6126.
Elazzouzi-Hafraoui, S., Nishiyama, Y., Putaux, J.L., Heux, L., Dubreuil, F., Rochas, C. The shape and size distribution of crystalline nanoparticles prepared by acid hydrolysis of native cellulose. Biomacromolecules. 2008; 9:57–65.
Eyholzer, Ch., Bordeanu, N., Lopez-Suevos, F., Rentsch, D., Zimmermann, T., Oksman, K. Preparation and characterization of water-redispersible nanofibrillated cellulose in powder form. Cellulose. 2010; 17:19–30.
Favier, V., Canova, G.R., Cavaillé, J.Y., Chanzy, H., Dufresne, A., Gauthier, C. Nanocomposites materials from latex and cellulose whiskers. Polym. Adv. Technol.. 1995; 6:351–355.
Favier, V., Canova, G.R., Shrivastava, S.C., Cavaillé, J.Y. Mechanical percolation in cellulose whiskers nanocomposites. Polym. Eng. Sci.. 1997; 37:1732–1739.
Favier, F., Chanzy, H., Cavaillé, J.Y. Polymer nanocomposites reinforced by cellulose whiskers. Macromolecules. 1995; 28:6365–6367.
Favier, V., Dendievel, R., Canova, G.R., Cavaillé, J.Y., Gilormini, P. Simulation and modeling of three-dimensional percolating structures: case of a latex matrix reinforced by a network of cellulose fibers. Acta Mater.. 1997; 45:1557–1565.
Fleming, K., Gray, D.G., Prasannan, S., Matthews, S. Cellulose crystallites: a new and robust liquid crystalline medium for the measurement of residual dipolar couplings. J. Appl. Polym. Sci.. 2000; 113:927–935.
Fukuzumi, H., Saito, T., Iwata, T., Kumamoto, Y., Isogai, A. Transparent and high gas barrier films of cellulose nanofibres prepared by TEMPO-mediated oxidation. Biomacromolecules. 2009; 10:162–165.
Garcia de Rodriguez, N.L., Thielemans, W., Dufresne, A. Sisal cellulose whiskers reinforced polyvinyl acetate nanocomposites. Cellulose. 2006; 13:261–270.
Goussé, C., Chanzy, H., Exoffier, G., Soubeyrand, L., Fleury, E. Stable suspensions of partially silylated cellulose whiskers dispersed in organic solvents. Polymer. 2002; 43:2645–2651.
Gray, D.G. Transcrystallization of polypropylene at cellulose nanocrystal surfaces. Cellulose. 2008; 15:297–301.
Grunert, M., Winter, W.T. Nanocomposites of cellulose acetate butyrate reinforced with cellulose nanocrystals. J. Polym. Environ.. 2002; 10:27–30.
Habibi, Y., Dufresne, A. Highly filled bionanocomposites from functionalized polysaccharides nanocrystals. Biomacromolecules. 2008; 9:1974–1980.
Habibi, Y., Goffin, A.L., Schiltz, N., Duquesne, E., Dubois, P., Dufresne, A. Bionanocomposites based on poly(ε-caprolactone)-grafted cellulose nanocrystals by ring opening polymerization. J. Mat. Chem.. 2008; 18:5002–5010.
Hajji, P., Cavaillé, J.Y., Favier, V., Gauthier, C., Vigier, G. Tensile behavior of nanocomposites from latex and cellulose whiskers. Polym. Compos.. 1996; 17:612–619.
Hanley, S.J., Giasson, J., Revol, J.F., Gray, D.G. Atomic force microscopy of cellulose microfibrils: comparison with transmission electron microscopy. Polymer. 1992; 33:4639–4642.
Helbert, W., Cavaillé, J.Y., Dufresne, A. Thermoplastic nanocomposites filled with wheat straw cellulose whiskers. Part I. Processing and mechanical behavior. Polym. Compos.. 1996; 17:604–611.
Henriksson, M., Berglund, L.A. Structure and properties of cellulose nanocomposite films containing melamine formaldehyde. J. Appl. Polym. Sci.. 2007; 106:2817–2824.
Heux, L., Chauve, G., Bonini, C. Nonflocculating and chiral-nematic self-ordering of cellulose microcrystals suspensions in nonpolar solvents. Langmuir. 2000; 16:8210–8212.
Ifuku, S., Nogi, M., Abe, K., Handa, K., Nakatsubo, F., Yano, H. Surface modification of bacterial cellulose nanofibres for property enhancement of optically transparent composites: dependence on acetyl-group DS. Biomacromolecules. 2007; 8:1973–1978.
Iwamoto, S., Abe, K., Yano, H. The effect of hemicelluloses on wood pulp nanofibrillation and nanofiber network characteristics. Biomacromolecules. 2008; 9:1022–1026.
Iwamoto, S., Kai, W., Isogai, A., Iwata, T. Elastic modulus of single cellulose microfibrils from tunicate measured by atomic force microscopy. Biomacromolecules. 2009; 10:2571–2576.
Jean, B., Dubreuil, F., Heux, L., Cousin, F. Structural details of cellulose nanocrystals/polyelectrolytes multilayers probed by neutron reflectivity and AFM. Langmuir. 2008; 24:3452–3458.
Jean, B., Heux, L., Dubreuil, F., Chambat, G., Cousin, F. Non-electrostatic building of biomimetic cellulose-xyloglucan multilayers. Langmuir. 2009; 25:3920–3923.
Kim, U.J., Kuga, S., Wada, M., Okano, T., Kondo, T. Periodate oxidation of crystalline cellulose. Biomacromolecules. 2000; 1:488–492.
Kvien, I., Tanem, B.S., Oksman, K. Characterization of cellulose whiskers and their nanocomposites by atomic force and electron microscopy. Biomacromolecules. 2005; 6:3160–3165.
Kvien, I., Sugiyama, J., Votrubec, M., Oksman, K. Characterization of starch based nanocomposites. J. Mater. Sci.. 2007; 42:8163–8171.
Li, R., Fei, J., Cai, Y., Li, Y., Feng, J., Yao, J. Cellulose whiskers extracted from mulberry: a novel biomass production. Carbohydr. Polym.. 2009; 76:94–99.
Li, Z., Renneckar, S., Barone, J.R. Nanocomposites prepared by in situ enzymatic polymerization of phenol with TEMPO-oxidized nanocellulose. Cellulose. 2010; 17:57–68.
Lin, N., Chen, G., Huang, J., Dufresne, A., Chang, P.R. Effects of polymer-grafted natural nanocrystals on the structure and mechanical properties of poly(lactic acid): a case of cellulose whisker-graft-polycaprolactone. J. Appl. Polym. Sci.. 2009; 113:3417–3425.
Ljungberg, N., Bonini, C., Bortolussi, F., Boisson, C., Heux, L., Cavaillé, J.Y. New nanocomposite materials reinforced with cellulose whiskers in atactic polypropylene: effect of surface and dispersion characteristics. Biomacromolecules. 2005; 6:2732–2739.
Ljungberg, N., Cavaillé, J.Y., Heux, L. Nanocomposites of isotactic polypropylene reinforced with rod-like cellulose whiskers. Polymer. 2006; 47:6285–6292.
Lu, Y., Weng, L., Cao, X. Biocomposites of plasticized starch reinforced with cellulose crystallites from cottonseed linter. Macromol. Biosci.. 2005; 5:1101–1107.
Lu, Y., Weng, L., Cao, X. Morphological, thermal and mechanical properties of ramie crystallites-reinforced plasticized starch biocomposites. Carbohydr. Polym.. 2006; 63:198–204.
Lu, J., Wang, T., Drzal, L.T. Preparation and properties of microfibrillated cellulose polyvinyl alcohol composite materials. Composites: Part A. 2008; 39:738–746.
Malainine, M.E., Mahrouz, M., Dufresne, A. Thermoplastic nanocomposites based on cellulose microfibrils from Opuntia ficus-indica parenchyma cell. Compos. Sci. Technol.. 2005; 65:1520–1526.
Marchessault, R.H., Morehead, F.F., Joan Koch, M. Hydrodynamics properties of neutral suspensions of cellulose crystallites as related to size and shape. J. Colloid Sci.. 1961; 16:327–344.
Marcovich, N.E., Auad, M.L., Belessi, N.E., Nutt, S.R., Aranguren, M.I. Cellulose micro/nanocrystals reinforced polyurethane. J. Mater. Res.. 2006; 21:870–881.
Mathew, A.P., Dufresne, A. Morphological investigation of nanocomposites from sorbitol plasticized starch and tunicin whiskers. Biomacromolecules. 2002; 3:609–617.
Mathew, A.P., Thielemans, W., Dufresne, A. Mechanical properties of nanocomposites from sorbitol plasticized starch and tunicin whiskers. J. Appl. Polym. Sci.. 2008; 109:4065–4074.
Matos Ruiz, M., Cavaillé, J.Y., Dufresne, A., Graillat, C., Gérard, J.F. New waterborne epoxy coatings based on cellulose nanofillers. Macromol. Symp. 2001; 169:211–222.
Morais Teixeira, E., Corrêa, A.C., Manzoli, A., Leite, F.L., De Oliveira, C.R., Mattoso, L.H.C., Cellulose nanofibres from white and naturally colored cotton fibers. Cellulose 2010; 17:595–606, doi: 10.1007/s10570-010-9403-0.
Morais Teixeira, E., Pasquini, D., Curvelo, A.A.S., Corradini, E., Belgacem, M.N., Dufresne, A. Cassava bagasse whiskers reinforced plasticized cassava starch. Carbohydr. Polym.. 2009; 78:422–431.
Nakagaito, A.N., Iwamoto, S., Yano, H. Bacterial cellulose: the ultimate nano-scalar cellulose morphology for the production of high-strength composites. Appl. Phys. A. 2005; 80:93–97.
Nakagaito, A.N., Yano, H. The effect of morphological changes from pulp fiber towards nano-scale fibrillated cellulose on the mechanical properties of high-strength plant fiber based composites. Appl. Phys. A. 2004; 78:547–552.
Nakagaito, A.N., Yano, H. Toughness enhancement of cellulose nanocomposites by alkali treatment of the reinforcing cellulose nanofibers. Cellulose. 2008; 15:323–331.
Nogi, M., Abe, K., Handa, K., Nakatsubo, F., Ifuku, S., Yano, H. Property enhancement of optically transparent bionanofiber composites by acetylation. Appl. Phys. Lett.. 2006; 89:233123–233125.
Nogi, M., Handa, K., Nakagaito, A.N., Yano, H. Optically transparent bionanofiber composites with low sensitivity to refractive index of the polymer matrix. Appl. Phys. Lett. 2005; 87:243110–243112.
Nogi, M., Ifuku, S., Abe, K., Handa, K., Nakagaito, A.N., Yano, H. Fiber-content dependency of the optical transparency and thermal expansion of bacterial nanofiber reinforced composites. Appl. Phys. Lett.. 2006; 88:133124–133126.
Noishiki, Y., Nishiyama, Y., Wada, M., Kuga, S., Magoshi, J. Mechanical properties of silk fibroin–microcrystalline cellulose composite films. J. Appl. Polym. Sci.. 2002; 86:3425–3429.
Oksman, K., Mathew, A.P., Bondeson, D., Kvien, I. Manufacturing process of cellulose whiskers/polylactic acid nanocomposites. Compos. Sci. Technol.. 2006; 66:2776–2784.
Orts, W.J., Godbout, L., Marchessault, R.H., Revol, J.F. Enhanced ordering of liquid crystalline suspensions of cellulose microfibrils: a small angle neutron scattering study. Macromolecules. 1998; 31:5717–5725.
Orts, W.J., Shey, J., Imam, S.H., Glenn, G.M., Guttman, M.E., Revol, J.F. Application of cellulose microfibrils in polymer nanocomposites. J. Polym. Environ.. 2005; 13:301–306.
Paralikar, S.A., Simonsen, J., Lombardi, J. Poly(vinyl alcohol)/cellulose nanocrystals barrier membranes. J. Membrane Sci.. 2008; 320:248–258.
Park, I., Kang, M., Kim, H.S., Jin, H.J. Electrospinning of poly(ethylene oxide) with bacterial cellulose whiskers. Macromol. Symp.. 2007; 249–250:289–294.
Peresin, M.S., Habibi, Y., Zoppe, J.O., Pawlak, J.J., Rojas, O.J. Nanofiber composites of polyvinyl alcohol and cellulose nanocrystals: manufacture and characterization. Biomacromolecules. 2010; 11:674–681.
Petersson, L., Oksman, K. Biopolymer based nanocomposites: comparing layered silicates and microcrystalline cellulose as nanoreinforcement. Compos. Sci. Technol.. 2006; 66:2187–2196.
Podsiadlo, P., Choi, S.Y., Shim, B., Lee, J., Cuddihy, M., Kotov, N.A. Molecularly engineered nanocomposites: layer-by-layer assembly of cellulose nanocrystals. Biomacromolecules. 2005; 6:2914–2918.
Podsiadlo, P., Sui, L., Elkasabi, Y., Burgardt, P., Lee, J., Miryala, A., Kusumaatmaja, W., Carman, M.R., Shtein, M., Kieffer, J., Lahann, J., Kotov, N.A. Layer-by-layer assembled films of cellulose nanowires with antireflective properties. Langmuir. 2007; 23:7901–7906.
Pu, Y., Zhang, J., Elder, T., Deng, Y., Gatenholm, P., Ragauskas, A.J. Investigation into nanocellulosics versus acacia reinforced acrylic films. Composites Part B: Engineering. 2007; 38:360–366.
Revol, J.F. On the cross-sectional shape of cellulose crystallites in Valonia ventricosa. Carbohydr. Polym.. 1982; 2:123–134.
Revol, J.F., Bradford, H., Giasson, J., Marchessault, R.H., Gray, D.G. Helicoidal self-ordering of cellulose microfibrils in aqueous suspension. Int. J. Biol. Macromol.. 1992; 14:170–172.
Revol, J.F., Godbout, L., Dong, X.M., Gray, D.G., Chanzy, H., Maret, G. Chiral nematic suspensions of cellulose crystallites: phase separation and magnetic field orientation. Liq. Cryst. 1994; 16:127–134.
Rojas, O.J., Montero, G.A., Habibi, Y. Electrospun nanocomposites from polystyrene loaded with cellulose nanowhiskers. J. Appl. Polym. Sci.. 2009; 113:927–935.
Roman, M., Winter, W.T. Effect of sulfate groups from sulfuric acid hydrolysis on the thermal degradation behavior of bacterial cellulose. Biomacromolecules. 2004; 5:1671–1677.
Roohani, M., Habibi, Y., Belgacem, N.M., Ebrahim, G., Karimi, A.N., Dufresne, A. Cellulose whiskers reinforced polyvinyl alcohol copolymers nanocomposites. Eur. Polym. J.. 2008; 44:2489–2498.
Saito, T., Kimura, S., Nishiyama, Y., Isogai, A. Cellulose nanofibers prepared by TEMPO-mediated oxidation of native cellulose. Biomacromolecules. 2007; 8:2485–2491.
Saito, T., Nishiyama, Y., Putaux, J.L., Vignon, M.R., Isogai, A. Homogeneous suspensions of individualized microfibrils from TEMPO-catalyzed oxidation of native cellulose. Biomacromolecules. 2006; 7:1687–1691.
Sakurada, I., Nukushina, Y., Ito, T. Experimental determination of the elastic modulus of crystalline regions oriented polymers. J. Polym. Sci.. 1962; 57:651–660.
Seydibeyoğlu, M.Ö., Oksman, K. Novel nanocomposites based on polyurethane and micro fibrilatted cellulose. Compos. Sci. Technol.. 2008; 68:908–914.
Shimazaki, Y., Miyazaki, Y., Takezawa, Y., Nogi, M., Abe, K., Ifuku, S., Yano, H. Excellent thermal conductivity of transparent cellulose nanofiber/epoxy resin nanocomposites. Biomacromolecules. 2007; 8:2976–2978.
Siqueira, G., Abdillahi, H., Bras, J., Dufresne, A. High reinforcing capability cellulose nanocrystals extracted from Syngonanthus nitens (Capim Dourado). Cellulose. 2010; 17:289–298.
Siqueira, G., Bras, J., Dufresne, A. Cellulose whiskers vs. microfibrils: influence of the nature of the nanoparticle and its surface functionalization on the thermal and mechanical properties of nanocomposites. Biomacromolecules. 2009; 10:425–432.
Siqueira, G., Bras, J., Dufresne, A. Luffa cylindrica: a new lignocellulosic source of fiber, microfibrillated cellulose and cellulose nanocrystals. BioResources. 2010; 5:727–740.
Siqueira, G., Bras, J., Dufresne, A. New process of chemical grafting of cellulose nanoparticles with a long chain isocyanate. Langmuir. 2010; 26:402–411.
Stenstad, P., Andresen, M., Tanem, B.S., Stenius, P. Chemical surface modifications of microfibrillated cellulose. Cellulose. 2008; 15:35–45.
Šturcova, A., Davies, G.R., Eichhorn, S.J. Elastic modulus and stress-transfer properties of tunicate cellulose whiskers. Biomacromolecules. 2005; 6:1055–1061.
Svagan, A.J., Hedenqvist, M.S., Berglund, L. Reduced water vapour sorption in cellulose nanocomposites with starch matrix. Compos. Sci. Technol.. 2009; 69:500–506.
Syverud, K., Stenius, P. Strength and barrier properties of MFC films. Cellulose. 2009; 16:75–85.
Tashiro, K., Kobayashi, M. Theoretical evaluation of three-imensional elastic constants of native and regenerated celluloses: role of hydrogen bonds. Polymer. 1991; 32:1516–1526.
Tokoh, C., Takabe, K., Fujita, M., Saiki, H. Cellulose synthesized by Acetobacter xylinum in the presence of acetyl glucomannan. Cellulose. 1998; 5:249–261.
Van den Berg, O., Capadona, J.R., Weder, C. Preparation of homogeneous dispersions of tunicate cellulose whiskers in organic solvents. Biomacromolecules. 2007; 8:1353–1357.
Wang, B., Sain, M., Oksman, K. Study of structural morphology of hemp fiber from the micro to the nanoscale. Appl. Compos. Mater.. 2007; 14:89–103.
Wang, Y., Cao, X., Zhang, L. Effects of cellulose whiskers on properties of soy protein thermoplastics. Macromol. Biosci.. 2006; 6:524–531.
Whistler, R.L., Richards, E.L. The carbohydrates, 2A. New York: Academic Press, 1970; 447.
Yano, H., Sugiyama, J., Nakagaito, A.N., Nogi, M., Matsuura, T., Hikita, M., Handa, K. Optically transparent composites reinforced with networks of bacterial nanofibers. Adv. Mater. 2005; 17:153–155.
Yuan, H., Nishiyama, Y., Wada, M., Kuga, S. Surface acylation of cellulose whiskers by drying aqueous emulsion. Biomacromolecules. 2006; 7:696–700.
Zimmermann, T., Pöhler, E., Geiger, T. Cellulose fibrils for polymer reinforcement. Adv. Eng. Mater.. 2004; 6:754–761.
Zimmermann, T., Pöhler, E., Schwaller, P. Mechanical and orphological properties of cellulose fibril reinforced nanocomposites. Adv. Eng. Mater.. 2005; 7:1156–1161.
Zoppe, J.O., Peresin, M.S., Habibi, Y., Venditti, R.A., Rojas, O.J. Reinforcing poly(ε-caprolactone) nanofibers with cellulose nanocrystals. ACS Appl. Mater. Interfaces. 2009; 1:1996–2004.
Zuluaga, R., Putaux, J.L., Restrepo, A., Mondragon, I., Gañán, P. Cellulose microfibrils from banana farming residues: isolation and characterization. Cellulose. 2007; 14:585–592.

