Assessing the moisture uptake behavior of natural fibres
Abstract:
Various methods for the determination of moisture uptake and water sorption behaviour of natural fibres are discussed; namely through simple weight gain measurement and dynamic vapour sorption (DVS), respectively. Simple weight gain measurement provides the equilibrium moisture content at a specified relative humidity. DVS, on the other hand, provides water sorption/desorption behaviour of natural fibres. Not only can the water sorption behaviour of natural fibres be determined, water sorption hysteresis can also be studied. A novel method of determining the accessible hydroxyl groups utilising heavy water using DVS apparatus was also discussed. This technique could potentially be applied to study the water sorption kinetics and mechanism of natural fibres.
10.1 Introduction
Understanding the moisture uptake behaviour of natural fibres has become increasingly important owing to their wide applications in numerous industries: the textile industry (Huda et al. 2007), the pulp and paper industry (Eichhorn et al. 2001) and more recently, the composites industry (Bledzki and Gassan 1999). The use of natural fibres is mainly driven by cost considerations and increased environmental concerns and legislations (EU 1999; EU 2000; EU 2003). Natural fibres have proven their application in reinforced polymeric materials for the production of natural fibre reinforced composites (Baltazar-y-Jimenez et al. 2008a; 2008b; Huda et al. 2008; Juntaro et al. 2007; Juntaro et al. 2008; Pommet et al. 2008; Sahoo et al. 2007; Satyanarayana et al. 2009). However, natural fibres’ high moisture sorption properties have proven to be a major disadvantage for composites application (Dhakal et al. 2007; Stamboulis et al. 2000). The high moisture uptake affects not only the fibres’ properties but also the mechanical performance of the resulting composites. High moisture sorption of natural fibres caused a reduction in the fibres’ tensile strengths as a result of water molecules forcing the cellulose molecules in natural fibres apart, thereby reducing the overall rigidity of natural fibres (Stamboulis et al. 2000). The effect of moisture on the mechanical performance of natural fibre reinforced composites was also studied extensively (Akil et al. 2009; Dhakal et al. 2007; Rashdi et al. 2009; Stamboulis et al. 2000). It was found that the mechanical performance of the composites reduces when the composites were exposed to moisture for an extended period of time. This is attributed to the high moisture uptake of natural fibres, which reduces the tensile strength of the fibre (Stamboulis et al. 2000) and weakens the fibre–matrix interface owing to water absorption (Dhakal et al. 2007).
In addition to the application of natural fibres in the textile (Huda et al. 2007) and composites industries, natural fibres also have the potential to be used as an environmental friendly material for building construction, utilising their moisture uptake behaviour (Collet et al. 2008). When used appropriately, new natural fibre based construction materials, such as lime-hemp render, hemp mortar and hemp wool have the potential to be used as an insulating material and a material that can control the humidity in an indoor environment (Hill et al. 2009b). Therefore, an understanding of the physical properties of natural fibres, in particular of the moisture uptake behaviour, is important. With this knowledge in hand, materials engineers will be able to design new environmentally friendly materials for different applications.
10.1.1 Background on the moisture sorption of natural fibres
The major constituents of natural fibres are cellulose, hemicellulose and lignin. Natural fibres are inherently hydrophilic in nature owing to the presence of a large number of hydroxyl (− OH) groups available, particularly in cellulose and hemicellulose. However, not all constituents contribute to the absorption of moisture. Cellulose, which forms the major constituent of natural fibres, is hydrophilic in nature and it absorbs water molecules. Even though cellulose has a high –OH to C ratio, not all –OH groups are exposed or accessible as cellulose is semicrystalline (Pott 2004). The highly crystalline region of cellulose is virtually inaccessible to water molecules but the water molecules are able to penetrate and gain access into the amorphous region of cellulose. Hemicellulose, on the other hand, is predominantly amorphous (Hansen and Plackett 2008). It is highly accessible to water molecules as it has a high –OH to carbon (C) ratio. Lignin, however, has a low –OH to C ratio and it is hydrophobic in nature (Bismarck et al. 2005). As natural fibres absorb water molecules, they swell up owing to water molecules occupying the space in between the microfibrils. This space that water molecules occupy is termed the transient microcapillary network (Hill et al. 2009b). The water molecules within natural fibres can either form a monolayer, which associate closely with the available –OH groups, or form a multilayer at which not all water molecules are in intimate contact with available –OH groups. It has been shown that the water molecules in a monolayer are more mobile than the water molecules located in a multilayer (Walker 2006).
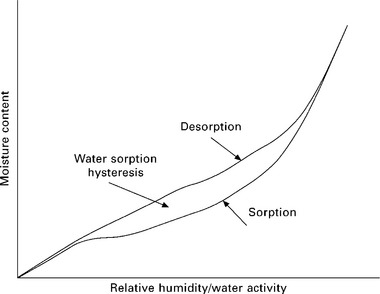
It was found that the moisture sorption isotherm of natural fibres follows an International Union of Pure and Applied Chemistry (IUPAC) type II isotherm. Water sorption hysteresis (the difference between the sorption and desorption loop) has also been observed (Pott 2004; Hill et al. 2009a; 2009b). The sigmoidal nature of the type II isotherm can be further categorised into three regions. Region 1 is typically assigned to relative humidity (RH) of between 0 and 15%, where the dominant moisture sorption mechanism is monolayer adsorption of water molecules onto the cell wall surface of natural fibres. In region 2, the water molecules form a multilayer in the transient microcapillary network between 15 and 70% RH. Beyond 70% RH (region 3), capillary condensation is the dominant mechanism for water molecules absorption. It must be noted that at low RH, adsorption is the dominant mechanism but at higher RH, absorption is the dominant mechanism. In this context, absorption refers to the uptake of water by natural fibres owing to surface tension forces. The heat required to evaporate absorbed liquid is only slightly higher than the energy required to evaporate a drop of liquid from a flat surface (Walker 2006). Adsorption, on the other hand, is based on the attractive forces between the water molecules and natural fibres as a result of hydrogen bonding or van der Waals forces (Walker 2006).
When discussing the moisture uptake of natural fibres, the concept of the fibre saturation point must be introduced. The fibre saturation point is the moisture content at which absorbed water molecules are removed but the cell walls of the fibres are still saturated (Tiemann 1906) by adsorbed water molecules. This point usually occurs around a moisture content of between 25 and 35% RH (Walker 2006). It can also be calculated by fitting the water sorption isotherm to 100% RH (Hill et al. 2009b). However, the fibre saturation point has been shown to be difficult to determine and does not exist experimentally in practice (Hernandez and Pontin 2006; Almeida and Hernandez 2006a; 2006b). The main reason for this lies in the concept of the fibre saturation point. It is assumed that there exist a distinct cut-off point between a fully saturated cell wall and the total removal of absorbed water molecules (Hill et al. 2009b). However, at high RH, capillary condensation begins to fill the lumen of natural fibres, causing a sharp increase in the water sorption curve (Walker 2006).
10.2 Methods of quantifying moisture uptake of natural fibres
In general, the moisture uptake behaviour of natural fibres can be measured by two different methods, involving exposing natural fibres to the required RH, either at constant RH (Baltazar-y-Jimenez and Bismarck 2007) or varying RH as a function of time (Gouanve et al. 2006; Hill et al. 2009b). In addition to these methods, streaming potential (ζ-potential) measurements, which provide information about the formation of the electrochemical double layer between a solid substrate and an electrolyte solution, could also be used to quantify the moisture uptake of natural fibres. Natural fibres swell in water, causing a decay of the ζ-potential as a function of time (Kanamaru 1960). The difference between the starting ζ-potential (at t = 0) and the ζ-potential approaching its asymptotic value (at t = ∞) is proportional to the swelling of natural fibres (Kanamaru 1960; Bismarck et al. 2000; Bismarck et al. 2002; Baltazar-y-Jimenez and Bismarck 2007). The utilisation of streaming ζ-potential to determine the moisture uptake of natural fibres is covered in chapter 7.
10.2.1 Simple weight gain determination of the moisture content of natural fibres
Weight gain measurements utilise humidity chambers or desiccators set up at specified RH using distilled water at room temperature (Bismarck et al. 2002; Baltazar-y-Jimenez and Bismarck 2007). The RH of the humidity chamber can be adjusted using salt solutions. In order to obtain more accurate results, the fibre bundles are dried overnight in an oven before placing them in the chambers. The weight difference before and after exposure to the specified RH were then measured at different time intervals and the moisture content calculated using the equation shown:
where MC = moisture content, m = mass of the sample after exposing to specified RH and m0 = dry mass of natural fibres.
A variation of this method can be performed by immersing the fibres in distilled water instead of a humidity chamber at specified RH (Sreekala and Thomas 2003). The increase in the weight of the fibres was recorded at specific time intervals. The authors found that the values obtained from this process are highly reproducible. The water uptake by the fibres (in mol percent of water uptake per mass of fibres) can be evaluated using:
where X(t) = moles of water absorbed per unit mass of fibres, mwater(t) = equilibrium mass of water at time t and minitial = initial mass of the fibres. This analysis can be extended further by fitting it into the empirical equation [10.3] (Khinnavar and Aminabhavi 1991; Sreekala and Thomas 2003):
where k and n are parameters fitted through linear regression, k is a parameter that depends on the interaction between the solid substrate and the water molecules, and n gives an indication about the types of mass transport phenomena (Fickian or Knudsen).
10.2.2 Dynamic vapour sorption in measurement of the water sorption isotherm of natural fibres
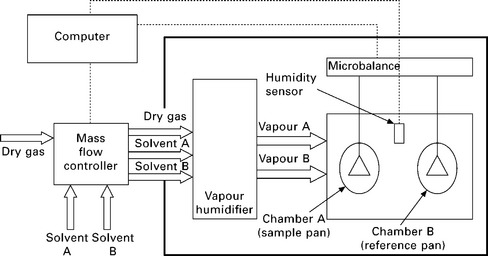
Dynamic vapour sorption (DVS) is very useful in the accurate measurement of sorption isotherms at various temperatures and relative humidity. The vapour phase can also be replaced; instead of water, other organic solvents can be used as long as Antoine’s equation (see for instance: Perry and Green 1997) for the particular solvent is available. Antoine’s equation describes the pure saturated vapour pressure of a solvent as a function of temperature. With this equation, the partial pressure of a solvent in the vapour phase can be predicted accurately. Using different organic solvents proves to be useful when studying the sorption isotherm of (modified) natural fibres. The use of DVS to study the moisture uptake of natural fibres has been studied extensively (Gouanve et al. 2006; Bessadok et al. 2007; Bessadok et al. 2008; Collet et al. 2008; Alix et al. 2009; Hill et al. 2009b). Such extensive use of DVS is not surprising as this technique is well established. In addition to this, natural fibres can be exposed to varying RH (as a function of time and temperature) to study the effect of different RH in situ on the moisture uptake of natural fibres.
The apparatus setup for a DVS instrument is shown in Fig. 10.1. The setup contains two measuring pans (one reference pan and one sample pan) suspended from the arm of an ultra-sensitive microbalance (up to 0.05 μg resolution). The measuring pans are connected to the microbalance by hanging wires and both the pans are situated in their respective chambers. These chambers are located in a temperature-controlled environment. A flow of dry gas along with the correct amount of water vapour is then passed through the chambers to maintain the required RH.
10.3 Moisture uptake behaviour of various natural fibres
10.3.1 Moisture content of natural fibres based on weight gain measurements
Table 10.1 shows the moisture content of various natural fibres evaluated using simple weight gain measurements. The equilibrium moisture content of different natural fibres is around 7 wt% at 100% RH. It is possible to increase the moisture content of sisal fibres from 5.8 wt% to 6.5 wt% by dewaxing the fibres. This can be done by either heating the fibres in a mixture of ethanol and benzene (a ratio of 1:2) for 72 h at 50 °C (Bismarck et al. 2001) or Soxhlet extraction in acetone for 1 h (Mukherjee and Satyanarayana 1984; Juntaro et al. 2008) to remove waxy substances. It seems, however, that dewaxing of coir fibres reduces its moisture content. Unfortunately, no explanation was given by the authors. Alkaline treatment is known to remove fatty acids from the surfaces of natural fibres (Geethamma et al. 1995). As a result, the moisture uptake of alkaline treated sisal and coir fibres increased when compared with neat fibres. Bismarck et al. (2001) also observed an increase in the thermal degradation temperature of sisal and coir fibres. This is thought to be due to the removal of compounds, such as wax or fatty acids, that decompose earlier than the major constituent in natural fibres.
Table 10.1
Equilibrium moisture content of various natural fibres at 100% RH (unless indicated). Results are obtained from simple weight gain measurement
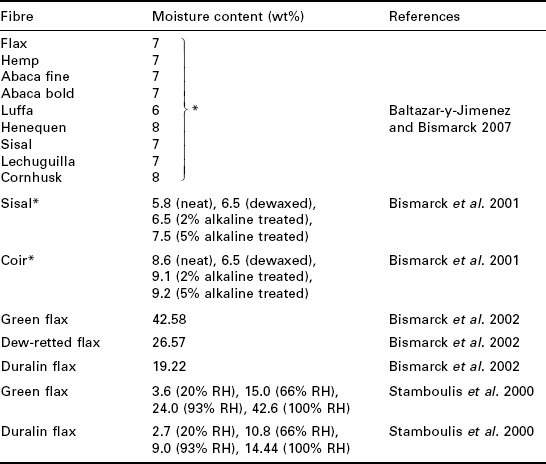
*Moisture content of fibres at 65% RH.
Bismarck et al. (2002) investigated the moisture uptake behaviour of flax fibres extracted from flax stems by various treatments; namely green flax, dew-retted flax and Duralin flax. Dew-retting is a fibre extraction process that relies on the natural colonisation of aerobic fungi in the stalks and stems of fibres just after harvesting. The process to obtain Duralin flax is developed by CERES B.V., which uses deseeded straw of flax fibres as starting material. It turns out that the use of the straw of flax fibres is beneficial for both strength and reproducibility (Stamboulis et al. 2000). More importantly, no retting process is required to extract natural fibres from the straw. A description of the Duralin process can be found in the literature (Stamboulis et al. 2000; Stamboulis et al. 2001; Bismarck et al. 2002). Green flax takes up 40% more moisture than dew-retted flax. Duralin flax, on the other hand, shows the lowest moisture uptake. This is a result of the Duralin process, which depolymerises hemicellulose and lignin into lower molecular weight compounds. These compounds subsequently cure into a water resistant resin (Stamboulis et al. 2000), which leads to the lower moisture uptake of Duralin flax than green flax.
10.3.2 Moisture content of various natural fibres based on DVS
A typical water sorption curve obtained by DVS is shown in Fig. 10.2. The difference between the sorption and desorption loop is termed water sorption hysteresis. Hill et al. (2009b) studied the water sorption behaviour of various natural fibres. From Table 10.2, it can be seen that jute, coir and Sitka spruce (a type of wood fibre) show higher moisture content at 95% RH than hemp, flax and cotton. One of the major differences between these two groups of fibres is their lignin content. Jute, coir and Sitka spruce have a higher lignin content than hemp, flax and cotton (Bismarck et al. 2005). The high moisture uptake of highly lignified natural fibres, such as coir and jute fibres, might be a direct result of the amorphous nature of lignin. Even though amorphous lignin is hydrophobic, it is able to deform to accommodate more water in the cell wall. This argument is also in agreement with the moisture uptake of cotton. Cotton is almost pure cellulose but it has the lowest moisture uptake. The main reason for this observation is that cotton is highly crystalline with low amorphous content. Cotton consists of nearly 90 wt% cellulose molecules with no lignin content (Bismarck et al. 2005) and the crystallinity index of cellulose was found to be 80% (Segal et al. 1959). Therefore, most of the cellulosic –OH groups are not accessible to water molecules, leading to the observed low moisture uptake.
Table 10.2
Moisture uptake of various natural fibres measured using DVS at 95% RH.
| Fibres | Moisture uptake (wt%) | Maximum hysteresis* (wt%) |
| Jute | 25 | 3.1 |
| Coir | 31 | 3.3 |
| Flax | 20 | 1.6 |
| Sitka spruce | 23 | 3.0 |
| Hemp | 25 | 1.8 |
| Cotton | 14 | 2.0 |
*Maximum hysteresis occurs at different RH for different types of fibres.
Adapted from Hill et al. 2009b
Table 10.2 also shows that the highly lignified fibres have larger water sorption hysteresis than flax, hemp and cotton fibres. Owing to the presence of amorphous lignin, the water molecules follow different pathways during absorption and desorption; that is, water sorption and desorption occur in two different physical states of the natural fibres (Lu and Pignatello 2004). Tvardovski et al. (1997) proposed that the deformation of sorbents is one of the main causes for sorption hysteresis. This concept could be applied to natural fibres. Swelling of natural fibres occurs as a result of the motion of incoming water molecules during water sorption, creating more voids. This swelling effect is confirmed by ζ-potential measurements (Stana-Kleinschek and Ribitsch 1998; Stana-Kleinschek et al. 1999; Bismarck et al. 2000; Bismarck et al. 2001; Bismarck et al. 2002; Baltazar-y-Jimenez and Bismarck 2007). Upon desorption of water molecules, there might be a lag between the water molecules leaving the voids and the relaxation of the sorbent to its original state (Lu and Pignatello 2002), which causes the observed hysteresis because sorption and desorption occur in two different physical environments.
10.3.3 Determination of exposed –OH groups of cellulose by using heavy water
Cellulose is a semicrystalline polymer with ordered regions (lower chemical reactivity) and disordered regions (higher reactivity). Therefore, it is of particular interest to characterise the ‘accessible’ regions of cellulose. This can be done by hydrogen–deuterium exchange. It is well known that deuterium can replace hydrogen in cellulose (Frilette et al. 1948; Sepall and Mason 1961). When cellulose is annealed at 260 °C in 0.1 M NaOD, all the hydrogen atoms (including the crystalline core of cellulose) will be converted to deuterium atoms (Wada et al. 1997). By exposing the deuterium-exchanged cellulose to water under normal condition, the accessible –OD groups will be converted back to –OH, whereas the core of cellulose will stay as –OD groups. By obtaining the IR spectra or measuring the mass increase owing to hydrogen–deuterium exchange of cellulose, the accessibility and crystallite size of cellulose can be obtained (Marrinan and Mann 1954; Horikawa and Sugiyama 2008; Lee et al. 2010). Table 10.3 tabulates the exposed –OH to core –OH ratio of cellulose from various sources.
Table 10.3
Accessible OH groups from various cellulose sources
| Types of cellulose | Exposed OH to core OH ratio | References |
| Cotton | 0.695 | Marrinan and Mann 1954 |
| Viscose rayon | 2.125 | Marrinan and Mann 1954 |
| Cellulose microfibrils (Valonia ventricosa) | 0.934 | Horikawa and Sugiyama 2008 |
| Bacterial cellulose (Nata-de-coco) | 0.705 | Lee et al. 2010 |
The same analysis can be extended to determine the exposed –OH groups of cellulose in a DVS setup (Lee et al. 2010). The authors pre-conditioned highly crystalline bacterial cellulose (BC) for 10 h at 0% RH to remove any adsorbed water molecule. The RH of D2O was then increased to 90% for 2 h and reduced to 0% for 2 h. This cycle was repeated 10 times to enable the adsorption of D2O molecule on the surface of BC but avoiding bulk sorption of BC by D2O. BC was then post-conditioned for 10 h at 0% RH to remove any adsorbed D2O molecules. The amount of exposed –OH groups can be determined from the mass increase of BC (deuterium is 1.67 × 10− 27 kg heavier than hydrogen, see equation [10.4]). This method could potentially be applied to natural fibres to determine the amount of exposed –OH groups or to study the moisture uptake behaviour of natural fibres (Hill et al. 2009b).
where Δm = mass change after hydrogen–deuterium exchange (mg), DSS = degree of surface substitution, mi = initial mass of sample (mg), A = Avogadro’s number and mn = mass of a neutron (mg).
10.4 Summary
Traditionally, natural fibres such as cotton, hemp or flax are widely used in the textile industry for the production of fabrics and yarns. Currently, there is a growing interest in utilising natural fibres as reinforcement for renewable polymers. One of the major problems of utilising natural fibres in composite materials is the high moisture uptake of natural fibres, which leads to poorer mechanical properties of the natural fibres reinforced composites. Therefore, it is important to study the moisture uptake behaviour of natural fibres.
The moisture uptake of natural fibres is complex owing to the composite nature of natural fibres themselves; the fibres contain amorphous and crystalline cellulose, hemicellulose and lignin. Water molecules do not form a single layer associated with the –OH groups. Instead, multiple layers of water molecules are associated with the –OH groups, depending on the humidity. Water sorption hysteresis is also widely encountered in the moisture uptake of natural fibres. So far, no plausible mechanism has been proposed to describe this phenomenon. In terms of the measurement of water sorption behaviour of natural fibres, there are two methods that are used widely; weight gain measurement and DVS. Both measurements give valuable information regarding the moisture uptake and water sorption of natural fibres.
Simple weight gain measurements describe the moisture content at a fixed relative humidity. This measurement can mimic the real condition where the natural fibres will be utilised. By using DVS, the moisture uptake of natural fibres at different RH (and temperature) is obtained and studied. The results are fitted into various models to further study the water sorption of natural fibres. A novel method based on hydrogen–deuterium exchange using DVS provides information regarding the accessibility of cellulosic –OH groups, and thus further information regarding the water sorption behaviour of natural fibres.
The challenge now is to understand the moisture uptake mechanism of natural fibres by combining different measuring approaches. This could include separating the effects of different chemical constituents on the water sorption of natural fibres at different RH. Because the water sorption of natural fibres is always in equilibrium with the exposed humidity, a thermodynamic approach should be included, along with sorption/desorption isotherm to describe the kinetics and mechanism of the moisture uptake of natural fibres.
10.5 Acknowledgements
The authors would like to thank the UK Engineering and Physical Science Research Council (EPSRC) for funding (EP/F032005/1) and the Deputy Rector’s award (Imperial College London) for funding KYL.
10.6 References
Akil, H.M., Cheng, L.W., Ishak, Z.A.M., Bakar, A.A., Rahman, M.A.A. Water absorption study on pultruded jute fibre reinforced unsaturated polyester composites. Composites Science and Technology. 2009; 69(11–12):1942–1948.
Alix, S., Philippe, E., Bessadok, A., Lebrun, L., Morvan, C., Marais, S. Effect of chemical treatments on water sorption and mechanical properties of flax fibres. Bioresource Technology. 2009; 100(20):4742–4749.
Almeida, G., Hernandez, R.E. Changes in physical properties of tropical and temperate hardwoods below and above the fiber saturation point. Wood Science and Technology. 2006; 40(7):599–613.
Almeida, G., Hernandez, R.E. Changes in physical properties of yellow birch below and above the fiber saturation point. Wood and Fiber Science. 2006; 38(1):74–83.
Baltazar-y-Jimenez, A., Bismarck, A. Wetting behaviour, moisture up-take and electrokinetic properties of lignocellulosic fibres. Cellulose. 2007; 14(2):115–127.
Baltazar-y-Jimenez, A., Bistritz, M., Schulz, E., Bismarck, A. Atmospheric air pressure plasma treatment of lignocellulosic fibres: impact on mechanical properties and adhesion to cellulose acetate butyrate. Composites Science and Technology. 2008; 68(1):215–227.
Baltazar-y-Jimenez, A., Juntaro, J., Bismarck, A. Effect of atmospheric air pressure plasma treatment on the thermal behaviour of natural fibres and dynamical mechanical properties of randomly-oriented short fibre composites. Journal of Biobased Materials and Bioenergy. 2008; 2(3):264–272.
Bessadok, A., Marais, S., Gouanvé, F., Colasse, L., Zimmerlin, I., Roudesli, S., Métayer, M. Effect of chemical treatments of Alfa (Stipa tenacissima) fibres on water-sorption properties. Composites Science and Technology. 2007; 67(3–4):685–697.
Bessadok, A., Marais, S., Roudesli, S., Lixon, C., Métayer, M. Influence of chemical modifications on water-sorption and mechanical properties of Agave fibres. Composites Part A-Applied Science and Manufacturing. 2008; 39(1):29–45.
Bismarck, A., Aranberri-Askargorta, I., Springer, J., Lampke, T., Wielage, B., Stamboulis, A., Shenderovich, I., Limbach, H.-H. Surface characterization of flax, hemp and cellulose fibers: surface properties and the water uptake behavior. Polymer Composites. 2002; 23(5):872–894.
Bismarck, A., Aranberri-Askargorta, I., Springer, J., Mohanty, A.K., Misra, M., Hinrichsen, G., Czapla, S. Surface characterization of natural fibers: surface properties and the water up-take behavior of modified sisal and coir fibers. Green Chemistry. 2001; 3(2):100–107.
Bismarck, A., Mishra, S., Lampke, T. Plant fibers as reinforcement for green composites. In: Mohanty A.K., Misra M., Drzal L., eds. Natural fibers, biopolymers and biocomposites. Boca Raton: CRC Press, 2005.
Bismarck, A., Springer, J., Mohanty, A.K., Hinrichsen, G., Khan, M.A. Characterization of several modified jute fibers using zeta-potential measurements. Colloid and Polymer Science. 2000; 278:229–235.
Bledzki, A.K., Gassan, J. Composites reinforced with cellulose based fibres. Progress in Polymer Science. 1999; 24(2):221–274.
Collet, F., Bart, M., Serres, L., Miriel, J. Porous structure and water vapour sorption of hemp-based materials. Construction and Building Materials. 2008; 22(6):1271–1280.
Dhakal, H.N., Zhang, Z.Y., Richardson, M.O.W. Effect of water absorption on the mechanical properties of hemp fibre reinforced unsaturated polyester composites. Composites Science and Technology. 2007; 67(7–8):1674–1683.
Eichhorn, S.J., Baillie, C.A., Zafeiropoulos, N., Mwaikambo, L.Y., Ansell, M.P., Dufresne, A., Entwistle, K.M., Herrera-Franco, P.J., Escamilla, G.C., et al. Review: Current international research into cellulosic fibres and composites. Journal of Materials Science. 2001; 36(9):2107–2131.
EU, Directive 1999/31/EC of the European Parliament and of the Council 26 April 1999 on the Landfill of Waste. Official Journal of the European Communities. 1999. [(16/7/1999): L 182/1].
EU, Directive 2000/53/EC of the European Parliament and of the Council of 19 September 2000 on end-of-life vehicles. Official Journal of the European Communities. 2000. [(21/10/2000): L 269/34].
EU, Directive 2002/96/EC of the European Parliament and of the Council of 27 January 2003 on waste electrical and electronic equipment (WEEE). Official Journal of the European Union. 2003. [(13/2/2003): L 37/24].
Frilette, V.J., Hanle, J., Mark, H. Rate of exchange of cellulose with heavy water. Journal of the American Chemical Society. 1948; 70(3):1107–1113.
Geethamma, V.G., Joseph, R., Thomas, S. Short coir fiber-reinforced natural-rubber composites-effects of fiber length, orientation, and alkali treatment. Journal of Applied Polymer Science. 1995; 55(4):583–594.
Gouanve, F., Marais, S., Bessadok, A., Langevin, D., Monvor, C., Métayer, M. Study of water sorption in modified flax fibers. Journal of Applied Polymer Science. 2006; 101(6):4281–4289.
Hansen, N.M.L., Plackett, D. Sustainable films and coatings from hemicelluloses: a review. Biomacromolecules. 2008; 9(6):1493–1505.
Hernandez, R.E., Pontin, M. Shrinkage of three tropical hardwoods below and above the fiber saturation point. Wood and Fiber Science. 2006; 38(3):474–483.
Hill, C.A.S., Norton, A.J., Newman, G., Natural fibre insulation materials–the importance of hygroscopicity in providing indoor climate control. Proceedings of the 11th international conference on non-conventional materials and technologies, 6–9 September 2009 Bath, UK. 2009.
Hill, C.A.S., Norton, A.J., Newman, G. The water vapor sorption behavior of natural fibers. Journal of Applied Polymer Science. 2009; 112(3):1524–1537.
Horikawa, Y., Sugiyama, J. Accessibility and size of Valonia cellulose microfibril studied by combined deuteration/rehydrogenation and FTIR technique. Cellulose. 2008; 15(3):419–424.
Huda, M.S., Drzal, L.T., Mohanty, A.K., Misra, M. Effect of fiber surface treatments on the properties of laminated biocomposites from poly(lactic acid) (PLA) and kenaf fibers. Composites Science and Technology. 2008; 68(2):424–432.
Huda, S., Reddy, N., Karst, D., Xu, W., Yang, W., Yang, Y. Nontraditional biofibers for a new textile industry. Journal of Biobased Materials and Bioenergy. 2007; 1(2):177–190.
Juntaro, J., Pommet, M., Kalinka, G., Mantalaris, A., Shaffer, M.S.P., Bismarck, A. Creating hierarchical structures in renewable composites by attaching bacterial cellulose onto sisal fibers. Advanced Materials. 2008; 20(16):3122–3126.
Juntaro, J., Pommet, M., Mantalaris, A., Shaffer, M., Bismarck, A. Nanocellulose enhanced interfaces in truly green unidirectional fibre reinforced composites. Composite Interfaces. 2007; 14(7–9):753–762.
Kanamaru, K. Wasseraufnahme in ihrer Beziehung zur zeitlichen Erniedrigung des Z-Potentials von Fasern in Wasser. Kolloid-Z. 1960; 168(2):115–121.
Khinnavar, R.S., Aminabhavi, T.M. Diffusion and sorption of organic liquids through polymer membranes. 1. Polyurethane versus normal-alkanes. Journal of Applied Polymer Science. 1991; 42(8):2321–2328.
Lee, K.-Y., Quero, F., et al. Surface modification of bacterial cellulose nanofibrils with organic acids, 2010. [Submitted for publication].
Lu, Y.F., Pignatello, J.J. Demonstration of the "conditioning effect" in soil organic matter in support of a pore deformation mechanism for sorption hysteresis. Environmental Science & Technology. 2002; 36(21):4553–4561.
Lu, Y.F., Pignatello, J.J. History-dependent sorption in humic acids and a lignite in the context of a polymer model for natural organic matter. Environmental Science & Technology. 2004; 38(22):5853–5862.
Marrinan, H.J., Mann, J. A study by infrared spectroscopy of hydrogen bonding in cellulose. Journal of Applied Chemistry. 1954; 4:204–211.
Mukherjee, P.S., Satyanarayana, K.G. Structure and properties of some vegetable fibers. 1. Sisal fiber. Journal of Materials Science. 1984; 19(12):3925–3934.
Perry, R.H., Green, D.W. Perry’s Chemical Engineering Handbook. New York: McGraw-Hill; 1997.
Pommet, M., Juntaro, J., et al. Surface modification of natural fibers using bacteria: depositing bacterial cellulose onto natural fibers to create hierarchical fiber reinforced nanocomposites. Biomacromolecules. 2008; 9(6):1643–1651.
Pott, G.T. Natural fibers with low moisture sensitivity. In: Wallenberger F.T., Weston N., eds. Natural fibers, plastics and composites. Norwell: Kluwer Academic; 2004:105–122.
Rashdi, A.A.A., Sapuan, S.M., et al. Water absorption and tensile properties of soil buried kenaf fibre reinforced unsaturated polyester composites (KFRUPC). Journal of Food Agriculture & Environment. 2009; 7(3–4):908–911.
Sahoo, S., Nakai, A., et al. Mechanical properties and durability of jute reinforced thermosetting composites. Journal of Biobased Materials and Bioenergy. 2007; 1(3):427–436.
Satyanarayana, K.G., Arizaga, G.G.C., et al. Biodegradable composites based on lignocellulosic fibers-an overview. Progress in Polymer Science. 2009; 34(9):982–1021.
Segal, L., Creely, J.J., et al. An empirical method for estimating the degree of crystallinity of native cellulose using the x-ray diffractometer. Textile Research Journal. 1959; 29:786–794.
Sepall, O., Mason, S.G. Hydrogen exchange between cellulose and water. II. Interconversion of accessible and inaccessible regions. Canadian Journal of Chemistry. 1961; 39:1944–1955.
Sreekala, M.S., Thomas, S. Effect of fibre surface modification on watersorption characteristics of oil palm fibres. Composites Science and Technology. 2003; 63(6):861–869.
Stamboulis, A., Baillie, C.A., et al. Environmental durability of flax fibres and their composites based on polypropylene matrix. Applied Composite Materials. 2000; 7(5–6):273–294.
Stamboulis, A., Baillie, C.A., et al. Effects of environmental conditions on mechanical and physical properties of flax fibers. Composites Part A-Applied Science and Manufacturing. 2001; 32(8):1105–1115.
Stana-Kleinschek, K., Ribitsch, V. Electrokinetic properties of processed cellulose fibers. Colloids and Surfaces A-Physicochemical and Engineering Aspects. 1998; 140(1–3):127–138.
Stana-Kleinschek, K., Strnad, S., et al. Surface characterization and adsorption abilities of cellulose fibers. Polymer Engineering and Science. 1999; 39(8):1412–1424.
Tiemann, H.D., Effect of moisture upon the strength and stiffness of wood. 70. US Forest Service Bulletin. 1906.
Tvardovski, A.V., Fomkin, A.A., et al. Hysteresis phenomena in the study of sorptive deformation of sorbents. Journal of Colloid and Interface Science. 1997; 191(1):117–119.
Wada, M., Okano, T., et al. Synchrotron-radiated x-ray and neutron diffraction study of native cellulose. Cellulose. 1997; 4(3):221–232.
Walker, J.C.F. Primary Wood Processing: Principles and Practice. The Netherlands: Springer; 2006.