Chapter 13
Laser Printing of Chemical and Biological Sensors
Ioanna Zergioti
National Technical University of Athens, Department of Physics, Heroon Polytehneiou 9, 15780 Zografou, Athens, Greece
13.1 Introduction
Today, there is a considerable necessity for depositing high-quality biological, organic, polymer thin films, multilayers, and composite materials. Furthermore, there is a need to find a method that could preserve the structural, morphological, and chemical composition of the film to be transferred. To be more specific, in biological and chemical sensor applications, high-quality thin films of bioselective or chemoselective materials must be processed so that the physiochemical properties are maintained. To take full advantage of the bulk properties of natural and synthetic polymers, the thin film must mimic these features. Various techniques have been established for the fabrication of biological and chemical sensors. For example, matrix-assisted pulsed laser evaporation (MAPLE) [1], dip-pen nanolithography [2], microcontact printing [3], or screen printing [4] and other techniques have been developed; however, some limitations exist in the aforementioned techniques.
Laser direct-write (LDW) techniques based on laser-induced forward transfer (LIFT) of functional materials offer unique advantages and capabilities for the rapid prototyping of electronic, optical, and sensor elements as opposed to other digital printing processes. The development of LIFT has grown steadily over a period of 30 years, and this chapter describes how the technique is used in many applications, especially for the fabrication of biological and chemical sensors. The ability to laser transfer and deposit a variety of materials without limitations at virtually any surface and conditions (ambient or vacuum) makes this technique an appropriate “tool” for the fabrication of sensor devices.
At this point, a brief introduction to the general principle of operation and some examples of chemical and biological sensors will be presented. One commonly cited definition by Higson et al. [5] regarding biosensors is: “a biosensor is a sensing device in which a biologically derived recognition entity is coupled to a transducer, to allow the quantitative development of some complex biochemical parameter.” This sensing device is a combination of a recognition element and a sensor element. A general principle of biosensors operation is that, a recognition element, such as enzymes, nucleic acids, antibodies, whole cells, recognizes a specific analyte and the sensor element transduces the change in the biomolecule into an electrical signal. The recognition element is very specific to the analyte to which it is sensitive, and it does not recognize other elements. Depending on the transducing mechanism used, the biosensors can be of many types such as resonant biosensors, optical detection biosensors, thermal-detection biosensors, ion-sensitive field-effect transistor (ISFET) biosensors, and electrochemical biosensors. The electrochemical biosensors, based on the parameter measured, can be further classified as conductimetric, amperometric, and potentiometric [6].
A chemical sensor can be defined as a device or instrument that detects the presence and quantity, in terms of concentration, of a given analyte, while the complexity of such a device is related to the technical difficulties associated with the specific nature (i.e., elemental or molecular) of the chemical substance to be analyzed. The chemical information may originate from a chemical reaction of a biomaterial, chemical compound, or a combination of both, attached onto the surface of a physical transducer, toward the analyte. According to the working principle, the chemical sensor can be classified into many types such as optical, electrochemical, mass, magnetic, and thermal. The optical chemical sensor is based on the changes in optical phenomena (reflection, deflection, refractive index variation) arising from the interaction between the analyte and the receiver. The electrochemical sensor utilizes the electrochemical effect among the analytes and the featured electrodes. The working principle of the mass sensor depends on the quality change induced by the mass loading, from the adsorption of the analyte on the special modification of the sensor surface. The magnetic device is based on the magnetic properties in analyte adsorption, whereas the thermal sensor utilizes the thermal effect generated by the specific chemical reaction or adsorption process [7, 8].
The following paragraphs briefly present various nonlaser printing techniques that have been investigated for the development of chemical and biological sensors. Furthermore, there is an extended analysis, especially for the LIFT technique as well as other laser-based techniques for the microdeposition of various materials with regard to biological and chemical sensor fabrication.
13.2 Conventional Printing Methods for the Fabrication of Chemical and Biological Sensors
Over the past years, there is an expanding need for the fabrication of simple, rapid, cost-effective, and field-deployable sensors. Sensors play an important role in industrial and safety-based applications. The aim of this chapter is to review and examine various challenges that are mentioned in the literature in the past decade related with the fabrication of chemical and biological sensors.
Various printing techniques have been investigated for the development of chemical and biological sensors, which may be separated into two major categories: contact and noncontact printing techniques, and they could also be classified into serial or parallel deposition. Specifically, in contact printing methods, the printing device requires direct contact with the substrate while depositing materials. On the other hand, noncontact printing methods involve no physical contact between the device and the substrate but involve the transfer of the material to a receiving substrate via an ejection event (e.g., photolithography, inkjet printing, and laser writing). All printing methods have the same object: efficient deposition of uniform, dense arrays of small droplets of probe molecules.
13.2.1 Contact Printing Methods
Contact printing methods are utilized to fabricate microarrays by means of physical contact between the substrates. Contact printing approaches comprise solid pins, split pins, nanotips, and microcontact (or microstamps) technologies.
13.2.1.1 Pin Printing Approach
Over the past years, the pin printing approach has been employed as an appropriate technique for microfabrication and especially for sensor fabrication [9, 10], and the development of pin-printed sensor arrays has attracted great interest especially in the field of biological and chemical sensor applications. Generally, the methodology of pin printing approach relies on the transfer of one or more metallic pins from a microwell plate to a solid substrate, and the simplest approach is the solid pin contact printing, where the microarray spotter is being widely used. Pin printing method is ruled by the surface tension of the solution and the wettability of the solution on the substrate, while typical substrates for microarray printing include microscope slides, plastics, and polymer-coated glass. At this point, it is important to notice that the dispensed volume is also affected by the temperature. Additionally, contaminations and dust must be controlled if high-quality arrays are to be produced while the risk of split pin clogging should be minimized as well [11].
Many studies have been reported regarding the fabrication of sensors. For example, Cho et al. [12] were the first researchers who have fabricated biosensor arrays based on pin printing protein-doped xerogels for the simultaneous detection of glucose and O2. They have observed that the combination of two major processes, namely (i) pin printing and (ii) sol–gel technique, demonstrates a simple approach to fabricate reusable biosensor arrays, which show good sensing properties and pave the way to integrated light source/biosensor arrays. Another work by the same research group presented the development of a reusable multianalyte chemical sensor for the simultaneous detection of O2 and pH variations in aqueous samples using again the pin printing technology and the sol–gel process. They have reported that by utilizing the pin printing method, they could prepare reproducible chemical sensors with the following characteristics: 100 µm diameter, 1–2 µm thickness, at a rate of approximately 1 sensor element per second with a single pin. The aforementioned results have indicated the accuracy of the pin printing method for the fabrication of reusable chemical sensor arrays [13]. As mentioned earlier, except for solid pins, there are other printing techniques that are utilized in pin printing technology for the fabrication of sensors, that is, split pins and silicon pins.
Despite being an accurate printing technique, pin printing also faces various shortcomings. Specifically, pins are difficult to manufacture, also during machining, a pin experiences stresses that leave it vulnerable to corrosion and deformation. Furthermore, the mechanical fabrication procedure is not an appropriate method to form uniform surfaces, which are necessary for drop printing. Moreover, during machining, grind marks and polish lines are being created, affecting the pin surface area and the adhesive properties. In addition, these processes often leave residual surface impurities and contaminants.
13.2.1.2 Microcontact Printing (or Microstamping) Technique
An alternative method of the pin printing technique is the microcontact printing (or microstamps) for the fabrication of sensors [14]. The operating principle of this technique is simple, and the process may be characterized as simple and cost-effective since it can be conducted in a laboratory without the need for special or advanced equipment. To be specific, different types of materials are first adsorbed on the patterned large surface area of a polymer stamp, and then they are deposited on a substrate by physical contact. As a result, a large number of spots are printed in parallel, allowing high-throughput microarray fabrication. The most widely used materials to construct the stamps are the elastomeric ones, since they provide good contact. Polydimethylsiloxane (PDMS) is a commonly used material due to the fact that it is simple to be molded and it becomes a soft polymer. Consequently, this enables a well-defined contact between the stamp and the area to be modified, and the stamp is subsequently soaked in a molecular “ink” that is imprinted on the surface.
Many research groups have been involved with the fabrication of sensors using the microcontact printing. For example, Huang et al. [15] have designed and implemented a fully integrated humidity sensor device by using the microcontact printing for the transfer of sensing elements on top of a CMOS device. Analytically, the Fe–Al–polyaniline polymeric film was formed by the microstamping method so as to be deposited onto the integrated electrodes on top of the fabricated CMOS circuit and was selected due to the fact that this multisolution-based polyaniline humidity sensing material is a good candidate to achieve a stable and adequately selective humidity sensor. They have concluded that the developed sensor has exhibited low power consumption, fast response time of about 5 s, and high sensitivity of about 30 mV/%.
Despite the efficiency of this technique for the fabrication of sensors, it also presents some drawbacks. The main disadvantage of microcontact printing is based on the use of soft polymers. During the “stamping” process, swelling of the stamp is frequently observed and, as a result, an increased pattern size. Another aspect of soft lithography is the contamination of the patterns with unpolymerized low-molecular-weight siloxane from the stamp, while an excess of ink often results in enhanced diffusion of the imprinted molecules on the patterned surface. Finally, a major disadvantage is the reproducibility of the pattern, where an important parameter is the influence from the exerted forces, which derive from the contact of the stamp with the surface. In recent years, efforts were made to shrink the size of the structures to the nanoscale [10].
13.2.1.3 Nanotip Printing
The deposited feature spot size is an important parameter, which should be investigated in order to advance to more complex arrays for the fabrication of devices, and nowadays, the trend in the scientific community is heading toward the minimization of the printing resolution. The nanotip printing technique has many similarities with the pin printing technique, but it is based on atomic force microscopy (AFM) nanotips to transfer probes to the array substrate [2, 16]. This technique is also called “dip-pen lithography.” One of the advantages of AFM printing is that the same tip is used to both print and detect so that the molecular and physical properties can be directly measured. When an AFM tip is brought in close proximity to the substrate, the probe solution flows from the coated tip to the substrate. The AFM tip also has the property (also known as AFM grafting) to eliminate parts of self-assembled monolayers (SAM) on a specific region (i.e., molecules resisting biomolecule adsorption). Using the nanotip printing technique, a solution that binds biological materials (e.g., proteins or other biomolecules), which is then transferred from the tip to the substrate, can achieve nanopatterns as well as more complex shapes [17, 18]. The rest of the substrate is blocked with molecules that do not bind the biomolecules under investigation, and the substrate is then exposed to the biomolecule solution. AFM printing technology is a serial printing method and, for that reason, is a slow-moving technique, compared to the microstamping method. Eventually, this technique tends to limit the device functionality because sample volumes are very small and they evaporate quickly.
13.2.2 Noncontact Printing Methods
According to the aforementioned, contact printing methods include a broad range of techniques, but all methods undoubtedly involve contact between the substrate surface and a stamp or pin. On the other hand, noncontact printing techniques vary between photochemistry-based methods to laser writing and fluid droplet dispensing, while the use of noncontact printing techniques presents two major benefits, which are the absence of contaminants and the higher throughput.
13.2.2.1 Photochemistry-Based Printing
This printing method relies on the chemical treatment of the substrate and the exposure with UV light through photomasks. In the photochemistry-based printing technique, the two major categories are photolithography [19–21] and direct photochemical patterning [22, 23]. These fabrication methods are a common practice to the design of protein and DNA arrays, although cell adhesion regions can also be fabricated in this way [24]. In the same way, as all the parallel printing methods, photochemistry features high throughput.
Despite the efficiency of this technique for patterning different samples, it also presents some drawbacks. The disadvantages involve the risk of biomolecule deterioration by photoresist solutions and the difficulty in patterning different samples in a single step [25].
13.2.2.2 Inkjet Printing Technique
This printing technique presents localized deposition and is offered readily to pattern structures without the need of a mask, providing a significant potential for the fabrication of reproducible patterns (especially of sensors). Among the many advantages of the technique, one may find the reduction of waste volumes and cross-contamination, as it is a noncontact deposition method, while it also provides high spatial resolution (<30 µm). Moreover, it is a promising method for depositing features on various substrates and transfer mainly biomaterials (i.e., nucleic acid and protein arrays), while in the last few years, it has been used as a free-form fabrication method for building three-dimensional parts and is currently being explored as a method of printing devices. This printing method is intrinsically compatible with flexible electronics, such as flexible printed circuit boards, and is expected to reduce the deterioration of the sensor characteristics. Its ability to deliver a precise amount of material in a quick, reproducible manner to predetermined locations under computer control is a desirable feature for applications, as in the production of sensors. In addition, it is scalable to large arrays and can coat arbitrary structures in a noncontact way.
In the work by Allain et al. [26], it was highlighted that biological materials (DNA) can be deposited using inkjet printers and remain intact; however, proteins that are more unstable may be more sensitive in the case that extreme conditions of inkjet printing are applied. The technique has also found application in patterning microarrays of in situ synthesized oligonucleotides, which is typically performed by photochemical means. In detail, different piezoelectric heads fabricated in various substrates (i.e., glass, silicon, or ceramics) have been used to synthesize DNA arrays [27, 28], and other solutions that have low volatility, high surface tension, and high viscosity are more desirable since they impede evaporation and contaminations between adjacent sites.
However, there are some significant limitations in this technique, which will not be discussed, thoroughly, in this chapter. Some significant disadvantages are (i) inkjet nozzles have the tendency to develop undesirable droplets (satellites). As a result, these surrounding spots reduce printing resolution [29] and (ii) the droplets experience high shear rates while passing through the nozzle, affecting the substrate surface. Under these shear rates or high temperatures, there is a risk of denaturing the biomolecules in the solution [25].
13.2.2.3 Electrospray Deposition (ESD)
Electrospray deposition (ESD) is, also, an alternative technique that allows for the parallel fabrication of microarrays. According to the vast number of studies that have been already reported, it is often used to transfer biological materials, semiconducting ceramics, and other materials [30–33] as well, as it provides the advantage of producing very small spots. However, there are some drawbacks as with inkjet printing. In more detail, the electrical field that is applied may result in degradation of the transferred material properties during the process, since, for example, the pH levels of the solution can be affected from the electrochemical reactions that occur. Finally, using this method, biological materials could deform under such conditions, because of the charging of the solution.
In summary, there is a variety of new technologies that are promising for faster, more efficient, less expensive microarray fabrication. In order for competing technologies to be viable in the long term, the microarray quality (e.g., spot uniformity and size) must also be evaluated alongside throughput and cost considerations. Although none of these new methods have yet supplanted the conventional method of contact pin printing, the continuing development of new materials and micromachining techniques may soon lead to an alternative technology, making the technique of biomolecular microarray analysis even more accessible to the scientific community. An ideal printing system should be capable of creating uniform, dense arrays of small droplets using minimum volumes of solutions, while preventing solution contamination and biomolecular damage. Furthermore, the system should be inexpensive, reliable, and durable. The aforementioned techniques do not meet all the required criteria, and this is why laser-based techniques are gaining increased interest for sensor applications.
13.3 Laser-Based Printing Techniques: Introduction
Modern laser micromachining offers several advantages in a wide field of applications, such as sensor devices, for chemical analysis as well as in the field of medicine. Owing to their great flexibility, laser-based techniques have been widely applied in many industrial applications, namely microelectronics and integrated devices such as those found in lab-on-a-chip and 3D printing. Nearly all applications require high resolution and high speed, while achieving high flexibility and full control of the quality, that is, morphology and size, of the deposited materials. Among the laser-based processes, laser ablation is widely used for selective material removal, both for laser cutting and drilling applications as well as for surface texturing. while laser printing has gained increased interest during the last decade due to its unique advantages since it provides fast, low-cost, and contact-free deposition in ambient conditions. Moreover, along with the variety of demanding applications that have been mentioned, a wide range of different materials may be transferred using laser printing. So far, several laser-based techniques have been developed to address the challenges of high resolution and printing speed, and given the continuous progress in additive manufacturing technologies, the fabrication of complex three-dimensional structures has also been achieved, as it will be presented in the next paragraphs.
13.3.1 Laser-Induced Forward Transfer
LIFT is a high-resolution 3D direct-write method that was first demonstrated in 1986 [34]. LIFT technique has a high potential for printing of various materials [35–38], mostly metals [39–42]. In this section, the general process of LIFT is discussed, where a more detailed description may be found in Chapters 1 and 2.
The fabrication of device structures requires the formation of complex high-resolution patterns in two-dimensions as well as three-dimensions. The success of any microfabrication technique depends on its ability to direct-write a wide variety of materials over many different types of surfaces. A broad variety of device applications have been described in the literature involving organic and inorganic materials. Since the LIFT technique allows the deposition of complex and multicomponent materials in a direct way from a separately prepared donor film and without further development process of materials, this technique appears suitable for the fabrication of microdevices such as chemical and biological sensors, where functional and sensitive biomaterials such as proteins, enzymes, cells, nucleic acids, and microorganisms have to be accurately deposited in a controlled manner without degrading their desirable properties.
Despite the successful applicability of the LIFT technique, for the direct-laser printing of different types of metals, the actual uses of the LIFT technique are limited due to several shortcomings. In order to overcome several drawbacks of the traditional LIFT technique, novel approaches/modifications based on the original LIFT technique that enable a solvent-free (or “dry”) direct-write deposition of solid and liquid thin films, necessary for the fabrication of devices with sensitive functional materials, have been developed.
One first variation to overcome some of LIFT's drawbacks was to deposit a multilayer film on the donor substrate. This modification has been called “laser ablation transfer” (LAT), a term that was first introduced by Tolbert et al. [43] in 1993 and was originally intended for high-speed color printing applications. In this approach, a thin absorbing layer, usually a metal, is firstly deposited on the transparent support followed by the deposition of a second layer that comprises the material under transfer. The thin absorbing layer was called “dynamic release layer” (or DRL) [43]. During laser printing, the laser pulse interacts with the laser-absorbing layer, causing vaporization of the metallic layer as in the case of traditional LIFT. DRL-LIFT is a highly flexible transfer technique, whose restrictions are mainly determined by the properties of the involved materials. So far, various materials [44, 45] including polymers [46] as well as liquids [47] and living cells [48, 49] have been successfully transferred for sensing applications. A more detailed analysis of LIFT using an intermediate laser-absorbing film has been described in Chapter 3. The following paragraphs present a compilation of “LIFT technique” examples for the microdeposition of various materials regarding the fabrication of biological and chemical sensors.
There are several examples in the literature on how the LIFT technique (with the intermediate-absorbing laser film DRL) is adapted for printing materials associated with sensor applications. For example, intermediate-absorbing titanium layers of about 50–60 nm thickness have been used in a LIFT setup to successfully deposit droplet of viscous DNA dispersions with the 355 nm emission of a frequency-tripled Nd:YAG laser [50]. For biosensor fabrication, where small amounts of delicate materials have to be manipulated, this modified LIFT approach opens up a promising orifice-free and noncontact deposition technique for the gentle and accurate direct-write of “bioinks” [51–53]. A number of bio-LIFT experiments were also reported with approximately 50–100 nm thick silver layers as absorbing layers in conjunction with 248 nm pulses (30 ns duration) using a KrF excimer laser and known as absorbing film-assisted laser-induced forward transfer (AFA-LIFT) [54] for biosensor applications. Moreover, the accurate LIFT deposition of thin polymer films has recently been demonstrated for microsensor applications. Homogeneous and hole-free polyethyleneimine (PEI) pixels with a low surface roughness were deposited in the same way with a triazene photopolymer DRL after process optimization [55]. Meanwhile, LIFT of PEI thin films to sensing chip units allowed measurment of chemoresponsive signals [56]. The potential of photopolymers as laser-absorbing layers in LIFT applications was demonstrated with various highly sensitive materials: assisted by a approximately 100 nm thick sacrificial triazene polymer, DRL living mammalian neuroblast cells were transferred and gently deposited on a receiver substrate using a 193 nm ArF nanosecond excimer laser [57]. This opens up new possibilities for the manufacturing of biosensors, in which, living cells should be precisely deposited onto microchips.
13.3.2 LIFT of Liquid Films
The ability of LIFT to deposit materials from liquid films makes this technique especially suitable for the preparation of miniaturized biological and chemical sensors, since in this way material-containing solutions can be spotted with precision onto a solid substrate where the materials are immobilized to enable the subsequent sensing measurements. The potential of the LIFT technique to print a variety of liquid materials, such as inorganic inks, pastes, and biological solutions, has prompted many research groups to investigate the transfer process that takes place during the LIFT of liquids. The dynamics of the laser-induced ejection process have been studied through time-resolved imaging, and the mechanism of liquid-phase LIFT can be summarized as follows: Below a threshold laser fluence (low laser fluence), a bubble of vaporized material displaces the free surface but subsequently collapses back to the donor without releasing any material. Within an intermediate laser fluence range, the ejected material is confined to a narrow jet [58] suggesting an appropriate window of controlled and reproducible printing. As the laser fluence increases (high laser fluences), the deposition could be described as uncontrollable, presenting satellite droplets and material debris. Since the transferred material remains in the liquid state during the whole process, there is no biomolecule decomposition, and therefore, the deposited material preserves its bioactivity. The volume of the ejected material is determined by the dimensions of the laser beam; the high focusing power of lasers allows to obtain micron-sized droplets, and that provides this technique with a high degree of spatial resolution.
Barron et al. [59] have demonstrated time-resolved images of the LIFT technique for protein solution printing. In this experiment, a 355 nm Nd:YAG laser was used for the deposition of a protein solution from a metallic DRL substrate onto a glass-receiving substrate. It was assumed, using modeling calculations, that the ejection of protein solution from the donor occurs as a result of heat transfer through the intermediate laser-absorbing substrate and subsequent vaporization of a portion of the protein solution that is in direct contact with the heated layer. More recently, our group has studied the ejection behavior of complex solutions (silver nanoparticles (NP) inks) [60]. For the low-viscosity silver NP inks, it was found that the use of a metallic DRL (titanium) resulted in smooth and low-velocity (9–77 m/s) jetting behavior, for a wide laser processing window, presenting well-defined transfer of silver droplets without undesirable satellite drops. For higher-viscosity inks, the printing behavior is similar to the behavior of solid-phase LIFT. The irradiated part of the viscous film that was ejected from the donor substrate was propelled slowly on the receiving substrate, which minimizes its deformation and fragmentation upon impact to the receiving substrate. In general, the understanding of the printing behavior of metallic NP inks provides an enhancement of the capabilities of the LIFT technique in industrial applications, especially on sensors fabrication. A further review on the LIFT technique of liquid transfer can be found in Chapters 4 and 5.
13.4 Applications of Direct Laser Printing
The continuing evolution of the laser transfer process, as presented in the previous sections, has been driven by the wide range of applications ready to benefit from the use of direct-write processes. In the following section, some selected examples in application areas ranging from biological arrays to chemical sensors are presented to indicate the versatility of the laser-based microfabrication techniques.
13.4.1 Biosensors
13.4.1.1 Background
In the past, sensors and biosensors were complex systems regarding the different types of parts and techniques that were required to fabricate them and more importantly their reusability. Nowadays, a variety of techniques have been developed for the fabrication of biosensors, which allow the controlled patterning of biological molecules and other related materials. Two procedures should be implemented in order to achieve fully functional sensors: (i) the transfer of the material from a donor substrate to the receiver substrate under specific circumstances and (ii) the precise and defined localization of the material on the receiver substrate. Two kinds of techniques have been developed to fabricate these sensors. Firstly, mass printing technologies: (i) gravure printing [61, 62], (ii) flexography [63], (iii) screen printing [64, 65], and (iv) roll-to-roll process, which are well-known approaches from the graphic techniques and enable roll-to-roll or high-speed sheet-to-sheet processing [66]; and secondly, the noncontact printing methods [67–70]. These methods have the main characteristic that involves an automated production system, based on a flexible, planar manufacturing technology inspired by the traditional print media industry.
The aforementioned (conventional) techniques, for printing sensors, present a number of drawbacks, where some of them have been described in Section 13.2. In the following paragraphs, we will focus on the laser-based printing techniques, which as we have already indicated present a number of advantages for the development of sensors.
13.4.1.2 Printing of Biological Materials for Biosensors
A rapidly growing field is considered for the fabrication of microarrays of biological molecules for the development of biosensors. Pharmaceutical companies, academia, and diagnostic firms employ massive analysis in order to develop products that improve the quality of life since the fabrication of advanced biological sensors will provide better health studies and faster detection of contagious and rare illnesses.
Different types of methods are currently established for depositing biopolymers in biosensor systems. As stated by most of the published works, biological materials are applied onto their binding or adsorbing substrates in solution by simple dispensing or soaking techniques. A number of methods, such as microfluidic, gel micro-casting, and localized activation methods, have been already reported for the successful localized deposition. The majority of these methods are liquid deposition processes. At this point, it is important to mention some critical parameters for the successful transfer of liquid microarrays. Among them, one may find the laser fluence and the beam-focusing parameters, which should be taken into consideration when it comes to laser printing and its suitability for biomaterial depositions [71].
A large number of biological materials have already been transferred by means of nanosecond and femtosecond LIFT, such as proteins [72, 73]. It should be noticed that both laser pulse regimes could be used for the transfer of proteins without considerable deterioration of their functionality.
Among the first works that have been published on the direct laser printing of biomaterials are those by Ringeisen et al. [74] as well as by Pique and Chrisey [75], who employed a direct-write technique for the laser writing of active protein patterns, viable Escherichia coli, and mammalian Chinese hamster ovary cells using nanosecond pulses. The crucial point during biological laser printing is the survival of the transferred cells and the preservation of their structural and physical properties upon irradiation. After the revolution of sensor fabrication through laser-based techniques and especially LIFT, many research groups have been involved with the development of biological sensors.
Serra et al. [76] proposed a protein-based biological device for protein detection and identification. This research group fabricated a single-protein-based microarray through the LIFT method in order to show the effect of the transfer technique on the protein integrity and reactivity. Specifically, an Nd: YAG laser (355 nm wavelength, 10 ns pulse duration) was utilized to deposit droplets of a solution containing the Treponema pallidum 17 kDa protein antigen on a nylon-coated glass slide, and they mentioned that the solution preserved its antigenic reactivity and diagnostic properties. The results obtained from the immunostaining assay when the array was incubated with a human serum positive for syphilis indicated that the antigen conserved its immunological reactivity after having been transferred. This study revealed that the LIFT method is an appropriate “tool” for the deposition of a diagnostically relevant antigen onto solid substrates. This work proved that the LIFT technique is a promising method for manufacturing protein microarrays of diagnostic interest.
Our group first studied the laser microprinting of different biomaterial patterns, such as DNA and proteins (bovine serum albumin), with spatial resolution, which was achieved due to the use of femtosecond laser pulses, down to 50 µm, and we showed that they were also functional after printing and, thus, could be used as biosensors. This work demonstrated that ultrashort laser pulses reduce the thermal effects, allowing the effective deposition of sensitive biomaterials at high spatial resolution for microfabrication patterns [77, 78]. Figure 13.1, depicts a scanning electron microscopy (SEM) picture of the printed features of lambda phage DNA on glass substrate by LIFT using 500 fs pulses.
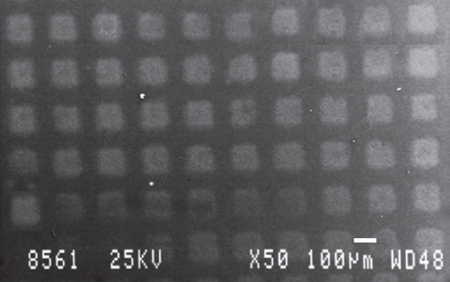
Figure 13.1 Scanning electron microscopy (SEM) picture of the printed features of lambda phage DNA on glass substrate by laser-induced forward transfer (LIFT) using 500 fs.
(Karaiskou et al. 2003 [78]. Reproduced with permission of Elsevier.)
Figure 13.2, shows the scanning laser confocal fluorescence microscopy of the Alexa 594 labeled lambda phage DNA pattern.

Figure 13.2 Scanning laser confocal fluorescence microscopy of the Alexa 594 labeled lambda phage DNA pattern.
(Karaiskou et al. 2003 [78]. Reproduced with permission of Elsevier.)
In another study we demonstrated [79] the development of a capacitive DNA biosensor, where single-stranded DNA droplets have been transferred using the LIFT technique. Furthermore, in the same work, in order to enhance the immobilization of the thiol-modified oligonucleotides, 3-glycidoxypropyl-tri-methoxy silane (GOPTS) has been used, for the surface functionalization of a low-temperature oxide (LTO)/silicon substrate. Finally, validation tests after laser irradiation showed that the GOPTS-functionalized surfaces were stable when stored at 40 °C over a period of 1 month.
In another work, we have also reported the development of an amperometric biosensor, sensitive toward phenolic compounds, using the enzyme laccase as recognition element [80]. The enzyme was successfully immobilized through the LIFT technique in active form onto nonfunctionalized screen-printed electrodes. The immobilized laccase was characterized toward catechol in solution, which is a typical phenolic compound. It was observed that this biosensor permits the detection of catechol in aqueous solution, without the need of any type of functionalization, at concentrations in the nanomolar range. In Figure 13.3, we can discern the wetting variation in relation to the laser printing energy that leads to the direct immobilization of the biomaterial on the screen-printed electrodes. This type of biosensor exhibited good sensitivity toward the catechol compound and preserved its activity for 35 days.
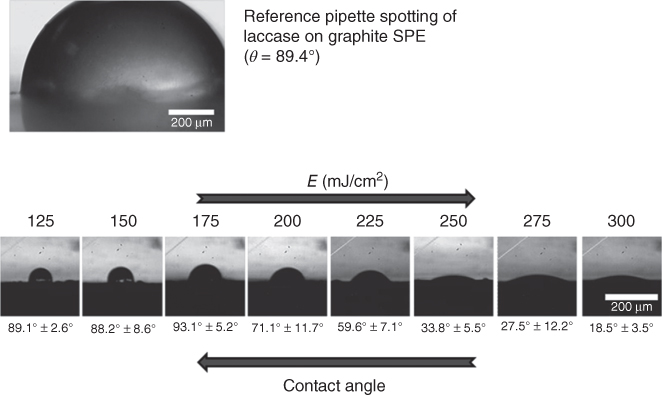
Figure 13.3 Wetting variation in relation to the laser printing energy that leads to the direct immobilization of the biomaterial on the screen-printed electrodes.
(Touloupakis et al. 2014 [80]. Reproduced with permission of Elsevier.)
Palla-Papavlu et al. [81] presented the development of surface acoustic wave (SAW) biosensors for odor detection in the food industry. Specifically, a wild-type bovine odorant-binding protein was laser-transferred on the SAW biosensor, while evaluation of the sensor performance under exposure to different concentrations of octenol presented high sensitivity and a detection limit of 2 ppm. Fernández-Pradas et al. [58] demonstrated, through the LIFT technique and using the third harmonic (λ = 355 nm) of an Nd: YAG laser, regular microarrays of double-stranded DNA of salmon sperm onto poly-l-lysine-coated slides. To achieve this, a laser intermediate-absorbing layer (titanium) was used between the laser-transparent substrate and the solution film. In another work, they compared the fabrication of cDNA microarrays onto poly-l-lysine-treated glass slides by LIFT and pin microspotting [52]. The produced microarrays were hybridized with the complementary strands of the spotted cDNAs, each one tagged with a different fluorochrome. Analysis protocols revealed that, through LIFT, the transferred microarrays are equivalent to those transferred through pin microspotting in terms of signal intensity and gene discrimination capacity.
In another study, Duocastella et al. [82] have demonstrated immunoglobulin (IgG) microarrays prepared through the LIFT technique by varying the laser pulse energy. During their experiment, they have tested the bioactivity of the transferred proteins, showing no loss of their activity along the whole laser pulse energy range. In Figure 13.4, an optical microscopy image of a microarray of the IgG solution at different laser pulse energies is presented.
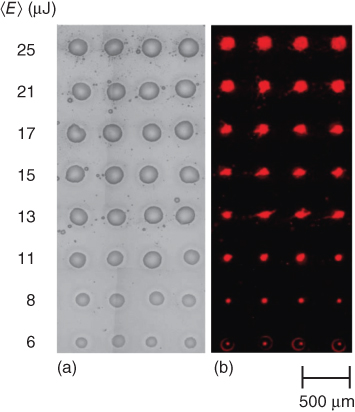
Figure 13.4 Optical microscopy image of microarray (IgG solution) at different laser pulse energies.
(Duocastella et al. 2008 [82]. Reproduced with permission of National Technical University of Athens.)
It is important to mention that the optical absorption characteristics of the material under deposition is a critical step, and in the case where a specific wavelength is absorbed, thermal effects that can cause degradation of the deposited material should be taken into consideration. The plurality of biological moieties are dissolved in aqueous media, which means that the solutions, which are mainly utilized, are transparent to many laser wavelengths that are commonly used, thus laser printing under such terms is considered prohibitive. On this occasion, to bring this restriction under control, especially on biological material printing as well as other materials, a DRL should be used between the donor substrate and the thin material to be transferred during LIFT.
In case of biological material printing, the term DRL LIFT in the literature is also called biological laser printing (BioLP) technique [83] or AFA-LIFT [84]. In the modified LIFT techniques, BioLP and AFA-LIFT, the donor substrate is coated with a (1–100 nm) thick metal or metal oxide layer. In the past, one of LIFT variations, namely BioLP, was employed for the laser printing of human osteosarcoma cells [85].
A recent work for the detection of heavy metal ions using a DNAzyme-functionalized capacitive micromechanical sensor has been presented by our group [86]. The fabricated sensor operation is based on the catalytic activity of the “8–17” DNAzyme, which undergoes self-cleavage in the presence of Pb+2 and is capable of selectively detecting the aforementioned heavy metal. For the immobilization of the catalytic strand with high spatial resolution onto the micromembrane surfaces, the LIFT technique was employed. Following the deposition of the catalytic strand, the substrate strand was then applied with the use of a pipette, thus covering a number of micromembrane sensors as shown in Figure 13.5.
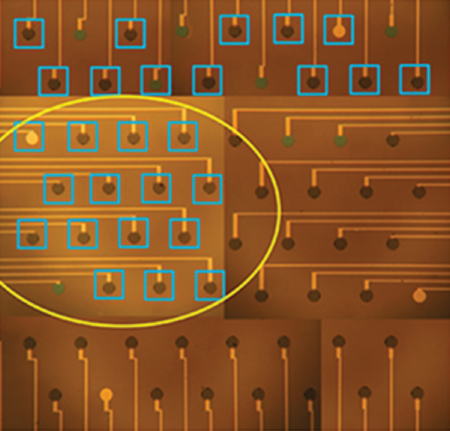
Figure 13.5 Optical microscope image of the micromembrane array following modification with the biological material.
(Tsekenis et al. 2015 [86]. Reproduced with permission of Elsevier.
Furthermore, in Figure 13.6, we can observe the sensor capacitance variation for those sensing elements that were first spotted with the catalytic strand and then allowed to hybridize with their complementary substrate strand.

Figure 13.6 Heavy metal ion detection using the capacitive sensor. (a) Sensors that were first spotted with the catalytic strand and then allowed to hybridize with their complementary substrate strand (red) increase their capacitance upon the insertion of heavy metal, contrary to the sensors with only the catalytic strands immobilized on their surface (blue). (b) After cleavage in the presence of heavy metal, all the sensing elements decrease their capacitance.
(Tsekenis et al. 2015 [86]. Reproduced with permission of Elsevier.)
Recently, Duocastella et al. [87] reported an alternative approach on laser printing for the transfer of DNA microarrays for biosensor fabrication, similarly to LIBT [88]. The idea of this technique is based on the transparency of the receiver substrate to the laser wavelength. In addition, when using this modified technique, some substantial differences appeared (i.e., no donor film is utilized for the coating of the material under transfer, the deposition takes place in the direct opposite to the incident laser beam direction), compared to the original LIFT. A more thorough description of film-free laser printing may be found in Chapter 6.
In other works [78, 89], we have also demonstrated how the deposition of biomaterials, avoiding the use of any matrix material, through laser direct transfer could be performed. This method reaches high spatial resolution given the ultrafast laser pulses that it employs, avoiding melting and vaporization and allowing material transfer with narrow angular divergence. Therefore, sensitive biomaterials are deposited without significant damage due to the use of ultrafast laser pulses, which minimize the adverse thermal effects.
Another interesting study for a low-cost biosensor using a paper platform was initially proposed by Whitesides et al. [90]. After that, Eason et al. [91] motivated by the previous work, investigated the development of such biosensors due to the fact that paper is available in a range of different grades, also it is biocompatible and presents a variety of promising properties such as wettability and porosity to liquid solutions, factors that contribute in making paper an interesting tool for the fabrication of biosensing devices. This research group presented a point-of-care paper-based diagnostic sensor using the LIFT printing technique to transfer a solution that contained either enzyme-tagged or enzyme-untagged antibodies. To test the effectiveness of the functionality and the immunological reactivity of the LIFT-printed structures, an ELISA (Enzyme-Linked Immunosorbent Assay) protocol was developed and confirmed the validation of the LIFT-printed solution. This work highlighted that LIFT is an accurate method to transfer biological solutions onto a paper substrate with minimal loss of their biochemical functionality for the fabrication of point-of-care diagnostic sensors. Finally, it was presented that the immobilized enzymes were maintained during the whole wet-bench process, which is something that confirms the successful deposition of the LIFT-printed antibodies.
Moreover, in another study by our group [92], we have reported the fabrication of an efficient and sensitive photosynthetic-based biosensor for detecting herbicides as well as an interesting method about how the LIFT technique can enable efficient electron transfer from photosynthetic biomaterials immobilized on screen-printed electrodes, since current electrochemical biosensors present a limitation in the stability and the junctions efficiency between biomaterial and solid-state devices. It was shown that the efficiency of energy production of a photosynthetic system can be strongly enhanced by the LIFT technique (Figure 13.7: SEM analysis of the Au working electrode printed with thylakoid droplets, using (i) pipette and (ii) LIFT).
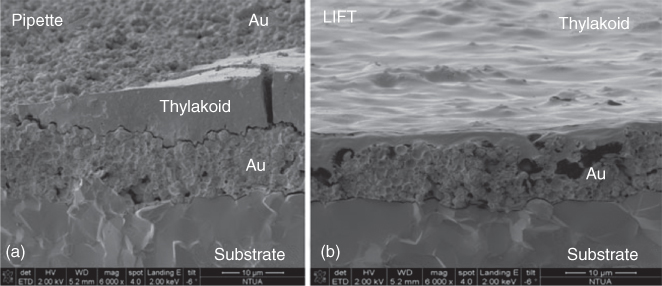
Figure 13.7 SEM analysis of the Au working electrode printed with thylakoid droplets, using (a) pipette and (b) laser-induced forward transfer (LIFT).
(Touloupakis 2012 [92]. Reproduced with permission of Elsevier.)
It is important to notice that the interest for the fabrication of sensors is not limited only in the academia. Companies have also been involved with the fabrication of biological sensors. For instance, a research group from “Fraunhofer Institute for Laser Technology (ILT),” in collaboration with GeSiM GmbH, developed a new system for biological sensing. In more detail, they manufactured a table-top “Protoprinter” system for transferring proteins and DNA on any given surface that can also be used in the manufacturing of microarrays. The utility of the LIFT technique was decisive for this small-scale application. This system is capable of transferring high-viscous and solid materials without damaging them, while achieving a spot size of 10 µm.
13.4.2 Chemical Sensors
The continuing evolution of the laser transfer process and the demand of society for flexible technologies that are useful for manufacturing chemical sensors for the detection of extremely small amounts of chemical vapors have rapidly increased in recent years.
Chemical sensors are available for a broad range of applications including: (i) detection and diagnosis of infections, (ii) identification of spilled chemicals, (iii) quality of foods and drinks, (iv) water and wastewater analysis, and (v) air quality monitoring.
The need for portable, low-cost, and easily deployable chemical sensors has become even more urgent due to the increased number of harmful chemical agents. Recent advances in sensors in conjunction with the use of both organic and inorganic active layers, such as porous metallic oxides, polymers, or metal porphyrins, has led to the development of chemical sensors with improved performances. The recent revolution in laser processing of a wide range of materials such as polymers, carbon nanotubes, and derivatives of graphene proves that laser processing techniques are suitable for the fabrication of accurate chemical sensors.
Recently, a work on the fabrication of chemical sensors using graphene oxide has been presented by our group [93]. This work presented the pulsed laser printing of graphene oxide and a subsequent thermal reduction step, using the LIFT technique. The evaluation of the reduction efficiency was made by Fourier transform reflectance spectroscopy and electrical characterization of the thermally reduced devices. The response of the sensor upon exposure to water vapors was evaluated, and sensitivities down to 0.22%/%RH were recorded.
Apart from the aforementioned biological sensors, LIFT has also been used for the fabrication of chemical sensors using laser-printed oxides (SnO2) [94] for the detection of ethanol, methanol, acetone, and capacitive sensor arrays [95] based on various polymers such as polyacrylic acid (PAA), poly(4-vinylpyridine) (P4VP), poly(hydroxyl styrene) (PHS), poly(methyl methacrylate) (PMMA), and poly(2-hydroxyethyl methacrylate) (PHEMA), as shown in Figure 13.8.

Figure 13.8 Capacitive sensor arrays. (a) Al contacts on the membranes. (b) Sensitive polymers (PVP-, PHEMA-, PAA-, and PVA-) printed in sensor device using LIFT.
(Mattle et al. 2013 [94]. Reproduced with permission of Springer.)
In this study, the sensor device was able to measure 256 sensing sites, with each sensing site comprising a polymer/silicon membrane, passivated with a LTO allowing the use in liquid environments. Assessment of the sensor showed quick response upon exposure to different analytes, emphasizing the potential of the LIFT technique for multisensing purposes.
A recent work on the fabrication of chemical sensors using composite layers has been presented by our group [96]. In this study, the use of LIFT as an alternative and promising technique for the dry printing of polymer/carbon nanotubes (CNTs) composite layers has been presented. These composites have been reported to exhibit significant electrical response toward a wide range of chemical compounds. Furthermore, TEM analysis (Figure 13.9: TEM images of PVP/f-MWCNT and PAA/f-MWCNT composite layers through LIFT) revealed that the LIFT-deposited PVP/f-MWCNT and PAA/f-MWCNT composite layers exhibited a high degree of dispersion of the carbon nanotubes into the polymer matrices.
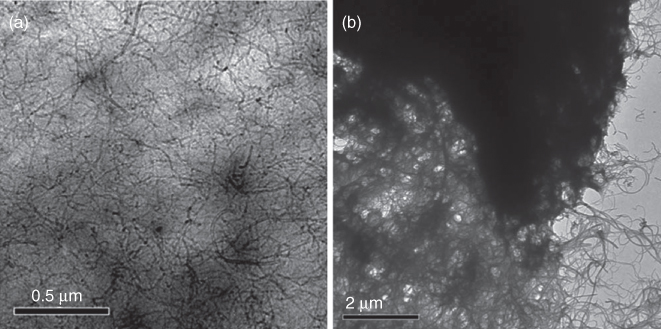
Figure 13.9 TEM images of PVP/f-MWCNT and PAA/f-MWCNT composite layers through LIFT.
(Boutopoulos et al. 2010 [96]. Reproduced with permission of American Institute of Physics.)
Another type of chemical sensors, also using carbon nanotubes, was investigated by Palla-Papavlu et al. [97] The aim of their work was to fabricate a chemoresistor onto different flexible substrates, appropriate for the fabrication of intelligent clothing. Using the LIFT technique, they have transferred single-walled carbon nanotubes (SWCNTs) in a range of different laser fluences, that is, from sufficient conditions to eject the donor films up to high irradiation. Their experiment showed a repeatable response, and they have also made an additional study through Raman spectra where the results were satisfactory. This showed that after the LIFT process, the material (SWCNTs) did not present any decomposition.
Additionally, the work by Dinca et al. [98] revealed how polymers could be used to fabricate chemical sensors. The purpose of their work was to improve the laser printing of active polymer pixels onto surface acoustic waves (SAW) chemical sensors. This kind of devices are able to measure gas, vapor concentration. Specifically, pixels of polyisobutilene (PIB) polymers were transferred through the LIFT technique onto glass at different fluences.
An alternative laser-based direct-write technique, called MAPLE DW, for matrix-assisted pulsed laser evaporation–direct write, which uses all of the benefits associated with LIFT and the primary version of MAPLE-DW, which is known as MAPLE [99–101], has been used for the direct-write of materials for sensor applications, since it operates in air and at low temperatures. The MAPLE-DW technique relies on the transfer of materials in powder form mixed with an organic binder. Such mixtures or matrices are then applied as a uniform coating on the transparent donor substrate. The advantage of this procedure resides in the fact that the transfer of thick films from the donor substrate can be achieved in lower laser fluences than with the LIFT technique. Various types of miniature sensor designs have been fabricated incorporating different materials such as metals, polymers, biomaterials, or composites as multilayers or discrete structures on a single substrate. The MAPLE DW is a computer-controlled process that allows the sensor design to be easily modified and adapted to any specific application.
The first work employing laser printing for sensing applications was mentioned by Pique et al. [102] where chemoresistor gas sensors were fabricated using the MAPLE-DW technique. The operating principle of chemoresistor gas sensors relies on the ability of a chemoselective polymer to reversibly change its volume after exposure to various concentrations of analyte vapors. Then the fluctuation in volume is converted into electrical resistance variations, whose absolute values depend on the analyte concentrations in the sensor chamber. In MAPLE-DW, the laser fluences are below the ablation threshold of the powders under transfer. As a result, no vaporization of the materials takes place, enabling the transfer of complex patterns and avoiding undesirable structural transformations. However, this technique was not developed exactly for biological materials. There has been some concern for the safety of the biological material during the laser transfer and its effects in biological properties after the exposure.
Moreover, in another work, Piqué et al. [103] have reported the development of a chemical sensor using polyepichlorohydrin (PECH) polymer/carbon. The polymer was successfully deposited through the MAPLE-DW technique onto silver interdigitated electrodes. In order to evaluate the performance of the chemical sensor made by MAPLE DW, similar PECH/carbon pads were fabricated by spray coating onto NaCl substrates and placed side by side and exposed to calibrated concentrations of analyte vapors. The results of the vapors tests showed that the response of the MAPLE DW and that of the spray-coated chemical sensors were similar and showed sensitivities of the order of parts per million (ppm).
13.5 Conclusions
This chapter presented how the LIFT technique has evolved into different LDW techniques and found use in many applications, especially in the sensor fabrication. The ability to laser transfer a variety of materials on virtually any surface without the need of masks or molds, both in ambient and vacuum conditions, shows the potential of the technique in microfabrication processes. LIFT is a noncontact microfabrication technique that offers opportunities for the generation of patterns, structures, and devices, which was not possible with traditional photolithographic tools in the previous years. Due to the fact that LIFT is a nonlithographic microfabrication method, it can be used for many prototyping applications, allowing the design, fabrication, and testing of a given structure to be completed quickly. This chapter demonstrated that LIFT can be utilized for many different types of sensor devices, including various types of materials.
List of Abbreviations
| AFA-LIFT | Absorbing film-assisted laser-induced forward transfer |
| AFM | Atomic force microscopy |
| BioLP | Biological laser printing technique |
| CAD/CAM | Computer-aided design/computer-aided manufacturing |
| CNT | Carbon nanotubes |
| DRL | Dynamic release layer |
| ELISA | Enzyme-linked immunosorbent assay |
| ESD | Electrospray deposition |
| GOPTS | 3-Glycidoxypropyl-tri-methoxy silane |
| IgG | Immunoglobulin |
| LAT | Laser ablation transfer |
| LDW | Laser direct write |
| LIFT | Laser-induced forward transfer |
| LTO | Low-temperature oxide |
| MAPLE | Matrix-assisted pulsed laser evaporation |
| MAPLE-DW | Matrix-assisted pulsed laser evaporation–direct write |
| PDMS | Polydimethylsiloxane |
| SAM | Self-assembled monolayer |
References
- 1 Piqué, A., Wu, P., Ringeisen, B.R., Bubb, D.M., Melinger, J.S., McGill, R.A., and Chrisey, D.B. (2002) Processing of functional polymers and organic thin films by the matrix-assisted pulsed laser evaporation (MAPLE) technique. Appl. Surf. Sci., 186 (1–4), 408–415.
- 2 Lee, K.-B., Park, S.-J., Mirkin, C.A., Smith, J.C., and Mrksich, M. (2002) Protein nanoarrays generated by Dip-Pen nanolithography. Science, 295, 1702–1705.
- 3 Graber, D.J., Zieziulewicz, T.J., Lawrence, D.A., Shain, W., and Turner, J.N. (2003) Antigen binding specificity of antibodies patterned by microcontact printing. Langmuir, 19, 5431.
- 4 Schuler, T., Asmus, T., Fritzsche, W., and Moller, R. (2009) Screen printing as cost-efficient fabrication method for DNA-chips with electrical readout for detection of viral DNA. Biosens. Bioelectron., 24, 2077.
- 5 Higson, S.P.J., Reddy, S.M., and Vadgama, P.M. (1994) Enzyme and other biosensors: evolution of a technology. Eng. Sci. Educ. J., 3 (1), 41–48.
- 6 Swapna, G., Ajaysushanth, K., Erfana Begum, P., Chandrakiran, P., and Bharat, M. (2015) Biosensor general principles and applications a review. Int. J. Curr. Sci. Technol., 3 (10), 90–93.
- 7 Sekhar, P.K., Brosha, E.L., Mukundan, R., and Garzon, F.H. (2010) Chemical sensors for environmental monitoring and homeland security. Electrochem. Soc. Interface, 19 (4), 35–40.
- 8 Hulanicki, A., Glab, S., and Ingman, F. (1991) Chemical sensors definitions and classification. Pure Appl. Chem., 63 (9), 1247–1250.
- 9 Schena, M., Shalon, D., Davis, R.W., and Brown, P.O. (1995) Quantitative monitoring of gene expression patterns with a complementary DNA microarray. Science, 270 (5235), 467–470.
- 10 Sheehan, P.E., Edelstein, R.L., Tamanaha, C.R., and Whitman, L.J. (2003) A simple pen-spotting method for arraying biomolecules on solid substrates. Biosens. Bioelectron., 18, 1455–1459.
- 11 Barbulovic-Nad, I., Lucente, M., Sun, Y., Zhang, M., Wheeler, A.R., and Bussmann, M. (2006) Bio-microarray fabrication techniques—a review. Crit. Rev. Biotechnol., 26, 237–259.
- 12 Cho, E.J., Tao, Z., Tehan, E.C., and Bright, F.V. (2002) Multianalyte pin-printed biosensor arrays based on protein-doped xerogels. Anal. Chem., 74 (24), 6177–6184.
- 13 Cho, E.J. and Bright, F.V. (2002) Pin-printed chemical sensor arrays for simultaneous multianalyte quantification. Anal. Chem., 74 (6), 1462–1466.
- 14 Gopal, A., Hoshino, K., and Zhang, J.X. (2012) Microcontact printing. Encycl. Nanotechnol., 1, 1397–1404.
- 15 Huang, C.-W., Huang, Y.-J., Lu, S.-S., and Lin, C.-T. (2012) A fully integrated humidity sensor system-on-chip fabricated by micro-stamping technology. Sensors, 12, 11592–11600.
- 16 Piner, R.D., Zhu, J., Xu, F., Hong, S.H., and Mirkin, C.A. (1999) “Dip-pen” nanolithography. Science, 283 (5402), 661–663.
- 17 Wadu-Mesthrige, K., Xu, S., Amro, N.A., and Liu, G.Y. (1999) Fabrication and imaging of nanometer-sized protein patterns. Langmuir, 15 (25), 8580–8583.
- 18 Xu, S. and Liu, G.Y. (1997) Nanometer-scale fabrication by simultaneous nanoshaving and molecular self-assembly. Langmuir, 13 (2), 127–129.
- 19 Blawas, A.S. and Reichert, W.M. (1998) Protein patterning. Biomaterials, 19 (7–9), 595–609.
- 20 Lom, B., Healy, K.E., and Hockberger, P.E. (1993) A versatile technique for patterning biomolecules onto glass coverslips. J. Neurosci. Methods, 50 (3), 385–397.
- 21 Mooney, J.F., Hunt, A.J., McIntosh, J.R., Liberko, C.A., Walba, D.M., and Rogers, C.T. (1996) Patterning of functional antibodies and other proteins by photolithography of silane monolayers. Proc. Natl. Acad. Sci. U.S.A., 93, 12287–12291.
- 22 Hengsakul, M. and Cass, A.E.G. (1996) Protein patterning with a photoactivatable derivative of biotin. Bioconjugate Chem., 7 (2), 249–254.
- 23 Pritchard, D.J., Morgan, H., and Cooper, J.M. (1995) Patterning and regeneration of surfaces with antibodies. Anal. Chem., 67 (19), 3605–3607.
- 24 Britland, S., Clark, P., Connolly, P., and Moores, G. (1992) Micropatterned substratum adhesiveness—a model for morphogenetic cues controlling cell behavior. Exp. Cell. Res., 198 (1), 124–129.
- 25 Tseng, F.-G., Lin, S.-C., Yao, D.-J., Huang, H., and Chieng, C.-C. (2005) in Protein Microarrays (ed. M. Schena), Jones and Bartlett Publishers, Inc., Sudbury, MA, pp. 305–338.
- 26 Allain, L.R., Stratis-Cullum, D.N., and Vo-Dinh, T. (2004) Investigation of microfabrication of biological sample arrays using piezoelectric and bubble-jet printing technologies. Anal. Chim. Acta, 518 (1–2), 77–85.
- 27 Butler, J.H., Cronin, M., Anderson, K.M., Biddison, G.M., Chatelain, F., Cummer, M., Davi, D.J., Fisher, L., Frauendorf, A.W., Frueh, F.W., Gjerstad, C., Harper, T.F., Kernahan, S.D., Long, D.Q., Pho, M., Walker, J.A., and Brennan, T.M. (2001) In situ synthesis of oligo nucleotide arrays by using surface tension. J. Am. Chem. Soc., 123 (37), 8887–8894.
- 28 Hughes, T.R., Mao, M., Jones, A.R., Burchard, J., Marton, M.J., Shannon, K.W., Lefkowitz, S.M., Ziman, M., Schelter, J.M., Meyer, M.R., Kobayashi, S., Davis, C., Dai, H.Y., He, Y.D.D., Stephaniants, S.B., Cavet, G., Walker, W.L., West, A., Coffey, E., Shoemaker, D.D., Stoughton, R., Blanchard, A.P., Friend, S.H., and Linsley, P.S. (2001) Expression profiling using microarrays fabricated by an inkjet oligonucleotide synthesizer. Nat. Biotechnol., 19 (4), 342–347.
- 29 Tseng, F.G., Kim, C.J., and Ho, C.M. (2002) A high-resolution high-frequency monolithic top-shooting micro injector free of satellite drops - Part I: concept, design, and model. J. Microelectromech. Syst., 11 (5), 427–436.
- 30 Avseenko, N.V., Morozova, T.Y., Ataullakhanov, F.I., and Morozov, V.N. (2001) Immobilization of proteins in immunochemical microarrays fabricated by electrospray deposition. Anal. Chem., 73 (24), 6047–6052.
- 31 Moerman, R., Frank, J., Marijnissen, J.C.M., Schalkhammer, T.G.M., and van Dedem, G.W.K. (2001) Miniaturized electrospray in gas a technique for the production of microarrays of reproducible micrometer-sizedproteinspots. Anal. Chem., 73 (10), 2183–2189.
- 32 Morozov, V. and Morozova, T.Y. 2002 Electrospray apparatus for mass fabrication of chips and libraries. US Patent 6,350,609.
- 33 Morozov, V.N. and Morozova, T.Y. (1999) Electrospray deposition as a method for mass fabrication of mono-and multicomponent microarrays of biological and biologically active substances. Anal. Chem., 71, 3110–3117.
- 34 Bohandy, J., Kim, B., and Adrian, F.J. (1986) Metal deposition from a supported metal film using an excimer laser. J. Appl. Phys., 60 (4), 1538–1539.
- 35 Fogarassy, E., Fuchs, C., Kerherve, F., Hauchecorne, G., and Perrière, J. (1989) Laser induced forward transfer of high-Tc YBaCuO and BiSrCaCuO superconducting thin films. J. Appl. Phys., 66 (1), 457–459.
- 36 Narazaki, A., Sato, T., Kurosaki, R., Kawaguchi, Y., and Niino, H. (2008) Nano- and microdot array formation of FeSi2 by nanosecond excimer laser-induced forward transfer. Appl. Phys Express, 1, 057001.
- 37 Veiko, V.P., Shakhno, E.A., Smirnov, V.N., Miaskovski, A.M., and Nikishin, G.D. (2006) Laser induced film deposition by lift physical mechanisms and applications. Laser Part. Beams, 24, 203–209.
- 38 Röder, T.C. and Köhler, J.R. (2012) Physical model for the laser induced forward transfer process. Appl. Phys. Lett., 100, 071603.
- 39 Mogyorósi, P., Szörényi, T., Bali, K., Tóth, Z., and Hevesi, I. (1989) Pulsed laser ablative deposition of thin metal films. Appl. Surf. Sci., 36, 157–163.
- 40 Tóth, Z., Szörényi, T., and Tóth, A.L. (1993) Ar+ laser-induced forward transfer (lift): a novel method for micrometer-size surface patterning. Appl. Surf. Sci., 69, 317–320.
- 41 Esrom, H., Zhang, J.-Y., Kogelschatz, U., and Pedraza, A.J. (1995) New approach of a laser-induced forward transfer for deposition of patterned thin metal films. Appl. Surf. Sci., 86, 202–207.
- 42 Yang, L., Wang, C.Y., Ni, X.C., Wang, Z.J., Jia, W., and Chai, L. (2006) Microdroplet deposition of copper film by femtosecond laser-induced forward transfer. Appl. Phys. Lett., 89, 161110.
- 43 Tolbert, W.A., Lee, I.Y.S., Doxtader, M.M., Ellis, E.W., and Dlott, D.D. (1993) High-speed color imaging by laser ablation transfer with a dynamic release layer: fundamental mechanisms. J. Imaging Sci. Technol., 37, 411.
- 44 Fardel, R., Nagel, M., Nuesch, F., Lippert, T., and Wokaun, A. (2007) Laser forward transfer using a sacrificial layer: influence of the material properties. Appl. Surf. Sci., 254, 1322–1326.
- 45 Shaw Stewart, J., Fardel, R., Nagel, M., Delaporte, P., Rapp, L., Cibert, C., Alloncle, A.P., Nuesch, F., Lippert, T., and Wokaun, A. (2010) The effect of laser pulse length upon laser-induced forward transfer using a triazene polymer as a dynamic release layer. Optoelectron. Adv. Mater., 12, 605–609.
- 46 Mito, T., Tsujita, T., Masuhara, H., Hayashi, N., and Suzuki, K. (2001) Hollowing and transfer of polymethyl methacrylate film propelled by laser ablation of triazeno polymer film. Jpn. J. Appl. Phys., 40 (8A), L805.
- 47 Colina, M., Duocastella, M., Fernández-Pradas, J.M., Serra, P., and Morenza, J.L. (2006) Laser-induced forward transfer of liquids study of the droplet ejection process. J. Appl. Phys., 99, 084909.
- 48 Koch, L. (2010) Laser printing of skin cells and human stem cells. Tissue Eng. Part C, 16, 847–854.
- 49 Hopp, B., Smausz, T., Kresz, N., Barna, N., Bor, Z., Kolozsvári, L., Chrisey, D.B., Szabó, A., and Nógrádi, A. (2005) Survival and proliferative ability of various living cell types after laser-induced forward transfer. Tissue Eng., 11 (11–12), 1817–1823.
- 50 Fernandez-Pradas, J.M., Colina, M., Serra, P., Dominguez, J., and Morenza, J.L. (2004) Laser induced forward transfer of biomolecules. Thin Solid Films, 453–454, 27–30.
- 51 Serra, P., Colina, M., Fernandez-Pradas, J.M., Sevilla, L., and Morenza, J.L. (2004) Preparation of functional DNA microarrays through laser-induced forward transfer. Appl. Phys. Lett., 85 (9), 1639–1641.
- 52 Colina, M., Serra, P., Fernandez-Pradas, J.M., Sevilla, L., and Morenza, J.L. (2005) DNA deposition through laser induced forward transfer. Biosens. Bioelectron., 20, 1638–1642.
- 53 Serra, P., Fernandez-Pradas, J.M., Colina, M., Duocastella, M., Dominguez, J., and Morenza, J.L. (2006) Laser-induced forward transfer: a direct- writing technique for biosensors preparation. J. Laser Micro/Nanoeng., 1 (3), 236–242.
- 54 Hopp, B., Smausz, T., Antal, Z., Kresz, N., Bor, Z., and Chrisey, D. (2004) Absorbing film assisted laser induced forward transfer of fungi (Trichoderma conidia). J. Appl. Phys., 96 (6), 3478–3481.
- 55 Dinca, V., Fardel, R., Di Pietrantonia, F., Cannata, D., Benetti, M., Verona, E., Palla-Papavlu, A., Dinescu, M., and Lippert, T. (2010) Laser-induced forward transfer: an approach to single-step polymer microsensor fabrication. Sens. Lett., 8, 1–5.
- 56 Dinca, V., Fardel, R., Shaw-Stewart, J., Di Pietrantonio, F., Cannata, D., Benetti, M., Verona, E., Palla-Papavlu, A., Dinescu, M., and Lippert, T. (2010) Laser-induced forward transfer: an approach to single-step polymer microsensor fabrication. Sens. Lett., 8, 436–440.
- 57 Doraiswamy, A., Narayan, R.J., Lippert, T., Urech, L., Wokaun, A., Nagel, M., Hopp, B., Dinescu, M., Modi, R., Auyeung, R.C.Y., and Chrisey, D.B. (2006) Excimer laser forward transfer of mammalian cells using a novel triazene absorbing layer. Appl. Surf. Sci., 252, 4743–4747.
- 58 Duocastella, M., Fernández-Pradas, J.M., Morenza, J.L., and Serra, P. (2009) Time-resolved imaging of the laser forward transfer of liquids. J. Appl. Phys., 106, 084907.
- 59 Barron, J.A., Young, H.D., Dlott, D.D., Darfler, M.M., Krizman, D.B., and Ringeisen, B.R. (2005) Printing of protein microarrays via a capillary-free fluid jetting mechanism. Proteomics, 5, 4138.
- 60 Boutopoulos, C., Kalpyris, I., Serpetzoglou, E., and Zergioti, I. (2014) Laser-induced forward transfer of silver nanoparticle ink: time-resolved imaging of the jetting dynamics and correlation with the printing quality. Microfluid. Nanofluid., 16, 493–500.
- 61 Li, D.W. and Guo, L.J. (2008) Organic thin film transistors and polymer light-emitting diodes paterned by polymer linking and stamping. J. Phys. D: Appl. Phys., 41, 105115.
- 62 Tuomikoski, M., Suhonen, R., Valimakia, M., Sauer, M., and Rogin, P. (2006) Manufacturing of polymer light-emitting device structures. Org. Optoelectron. Photonics II, 6192, 19204.
- 63 Makela, T., Haatainen, T., Majander, P., and Ahopelto, J. (2007) Continuous roll to roll nanoimprinting of inherently conducting polyaniline. Microelectron. Eng., 84, 877–879.
- 64 Albareda-Sirvent, M., Merkoci, A., and Alegret, S. (2000) Configurations used in the design of screen-printed enzymatic biosensors. A review. Sens. Actuators, B, 69, 153–163.
- 65 Tudorache, M. and Bala, C. (2007) Biosensors based on screen-printing technology, and their applications in environmental and food analysis. Anal. Bioanal. Chem., 388, 565–578.
- 66 Makela, T., Haatainen, T., Majander, P., and Ahopelto, J. (2006) Nickel stamp fabrication using step & stamp imprint lithography. Microelectron. Eng., 83, 948–950.
- 67 Setti, L., Fraleoni-Morgera, A., Ballarin, B., Fillippini, A., and Frascaro, D. (2005) Biosens. Bioelectron., 20, 2019–2026.
- 68 Ngamna, O., Morrin, A., Killard, A.J., Moulton, S.E., Smyth, M.R., and Wallace, G.G. (2007) Langmuir, 23, 8569–8574.
- 69 Fuller, S.B., Wilhelm, E.J., and Jacobson, J.A. (2002) J. Microelectromech. Syst., 11, 54–60.
- 70 Xu, T., Jin, J., Gregory, C., Hickman, J.J., and Boland, T. (2005) Biomaterials, 26, 93–99.
- 71 Dinca, V., Kasotakis, E., Catherine, J., Mourka, A., Mitraki, A., Popescu, A., Dinescu, M., Farsari, M., and Fotakis, C. (2007) Development of peptide-based patterns by laser transfer. Appl. Surf. Sci., 254, 1160–1163.
- 72 Dinca, V., Farsari, M., Kafetzopoulos, D., Popescu, A., Dinescu, M., and Fotakis, C. (2008) Patterning parameters for biomolecules microarrays constructed with nanosecond and femtosecond UV lasers. Thin Solid Films, 516, 6504–6511.
- 73 Dinca, V., Ranella, A., Farsari, M., Kafetzopoulos, D., Dinescu, M., Popescu, A., and Fotakis, C. (2008) Quantification of the activity of biomolecules in microarrays obtained by direct laser transfer. Biomed. Microdevices, 10, 719–725.
- 74 Ringeisen, B.R., Chrisey, D.B., Pique, A., Young, H.D., Modi, R., Bucaro, M., Jones-Meehan, J., and Spargo, B.J. (2002) Generation of mesoscopic patterns of viable Escherichia coli by Ambien tLaser transfer. Biomaterials, 23, 161–166.
- 75 Pique, A. and Chrisey, D.B. (2002) Direct-Write Technologies for Rapid Prototyping Applications, Academic Press, pp. 93–116.
- 76 Serra, P., Fernández-Pradas, J.M., Berthet, F.X., Colina, M., Elvira, J., and Morenza, J.L. (2004) Laser direct writing of biomolecule microarrays. Appl. Phys. A, A79, 949–952.
- 77 Zergioti, I., Karaiskou, A., Papazoglou, D.G., Fotakis, C., Kapsetaki, M., and Kafetzopoulos, D. (2005) Femtosecond laser microprinting of biomaterials. Appl. Phys. Lett., 86, 163902.
- 78 Karaiskou, A., Zergioti, I., Fotakis, C., Kapsetaki, E., and Kafetzopoulos, D. (2003) Microfabrication of biomaterials by the sub-ps laser induced forward transfer process. Appl. Surf. Sci., 208–209, 245–249.
- 79 Tsekenis, G., Chatzipetrou, M., Tanner, J., Chatzandroulis, S., Thanos, D., Tsoukalas, D., and Zergioti, I. (2012) Surface functionalization studies and direct laser printing of oligonucleotides toward the fabrication of a micromembrane DNA capacitive biosensor. Sens. Actuators, B, 175, 123–131.
- 80 Touloupakis, E., Chatzipetrou, M., Boutopoulos, C., Gkouzou, A., and Zergioti, I. (2014) A polyphenol biosensor realized by laser printing technology. Sens. Actuators, B, 193, 301–305.
- 81 Palla-Papavlu, A., Patrascioiu, A., Di Pietrantonio, F., Fernandez-Pradas, J.-M., Cannata, D., Benetti, M., D'Auria, S., Verona, E., and Serra, P. (2014) Preparation of surface acoustic wave odor sensors by laser-induced forward transfer. Sens. Actuators, B, 192, 369–377.
- 82 Duocastella, M., Fernández-Pradas, J.M., Serra, P., and Morenza, J.L. (2008) Laser-induced forward transfer of liquids for miniaturized biosensors preparation. J. Laser Micro/Nanoeng., 3 (1), 1–4.
- 83 Barron, J.A., Wu, P., Ladouceur, H., and Ringeisen, B.R. (2004) Biological laser printing: a novel technique for creating heterogeneous 3-dimensional cell patterns. Biomed. Microdevices, 6, 139–147.
- 84 Hopp, B., Smausz, T., Kresz, N., Barna, N., Bor, Z., Kolozsvari, L., Chrisey, D.B., Szabo, A., and Nogradi, A. (2005) Survival and proliferative ability of various living cell types after laser induced forward transfer. Tissue Eng., 11, 1181.
- 85 Barron, J.A., Krizman, D.B., and Ringeisen, B.R. (2005) Laser printing of single cells: statistical analysis, cell viability, and stress. Ann. Biomed. Eng., 33 (2), 121–130.
- 86 Tsekenis, G., Filippidou, M.K., Chatzipetrou, M., Tsouti, V., Zergioti, I., and Chatzandroulis, S. (2015) Heavy metal ion detection using a capacitive micromechanical biosensor array for environmental monitoring. Sens. Actuators, B, 208 (1), 628–635.
- 87 Duocastella, M., Fernandez-Pradas, J.M., Morenza, J.L., Zafra, D., and Serra, P. (2010) Novel laser printing technique for miniaturized biosensors preparation. Sens. Actuators, B, 145, 596–600.
- 88 Patrascioiu, A., Fernandez-Pradas, J.M., Palla-Papavlu, A., Morenza, J.L., and Serra, P. (2014) Laser-generated liquid microjets: correlation between bubble dynamics and liquid ejection. Microfluid. Nanofluid., 16, 55–63.
- 89 . Fotakis K, Thireos G, Zergioti I, Kafetzopoulos D. (2002) Fabrication of biopolymer patterns by means of laser transfer. Patent: 1004059 (15-NOV-2002). International patent Application PCT/EP 02/14761 (24-DEC-02)
- 90 Martinez, A.W., Phillips, S.T., Butte, M.J., and Whitesides, G.M. (2007) Patterned paper as a platform for inexpensive, low-volume, portable bioassays. Angew. Chem. Int. Ed., 46, 1318–1320.
- 91 Katis, I.N., Holloway, J.A., Madsen, J., Faust, S.N., Garbis, S.D., Smith, P.J.S., Voegeli, D., Bader, D.L., Eason, R.W., and Sones, C.L. (2014) Paper-based colorimetric enzyme linked immunosorbent assay fabricated by laser induced forward transfer. Biomicrofluidics, 8, 036502.
- 92 Touloupakis, E., Boutopoulos, C., Buonasera, K., Zergioti, I., and Giardi, M.T. (2012) A photosynthetic biosensor with enhanced electron transfer generation realized by laser printing technology. Anal. Bioanal. Chem., 402, 3237–3244.
- 93 Papazoglou, S., Tsouti, V., Chatzandroulis, S., and Zergioti, I. (2016) Direct laser printing of graphene oxide for resistive chemosensors. Opt. Laser Technol., 82, 163–169.
- 94 Mattle, T., Hintennach, A., Lippert, T., and Wokaun, A. (2013) Laser induced forward transfer of SnO2 for sensing applications using different precursors systems. Appl. Phys. A, 110, 309–316.
- 95 Tsouti, V., Boutopoulos, C., Goustouridis, D., Zergioti, I., Normand, P., Tsoukalas, D., and Chatzandroulis, S. (2010) A chemical sensor microarray realized by laser printing of polymers. Sens. Actuators, B, 150, 148–153.
- 96 Boutopoulos, C., Pandis, C., Giannakopoulos, K., Pissis, P., and Zergioti, I. (2010) Polymer/carbon nanotube composite patterns via laser induced forward transfer. Appl. Phys. Lett., 96 (4), 041104.
- 97 Palla-Papavlu, A., di Pietrantonio, F., Cannata, D., Benetti, M., Veron, E., Dinca, V., Lippert, T., and Dinescu, M. (2014) Gas sensors fabricated by laser-induced forward transfer. International Conference of Scientific paper AFASES 2014, Brasov, 22–24 May, 2014.
- 98 Dinca, V., Palla-Papavlu, A., Dinescu, M., Shaw Stewart, J., Lippert, T.K., Di Pietrantonio, F., Cannata, D., Benetti, M., and Verona, E. (2010) Polymer pixel enhancement by laser-induced forward transfer for sensor applications. Appl. Phys. A, 101, 559–565.
- 99 McGill, R.A. and Chrisey, D.B. (1997) Method of producing thin film coating by matrix assisted pulsed laser evaporation. Patent W09853767.
- 100 McGill, R.A., Chung, R., Chrisey, D.B., Dorsey, P.C., Matthews, P., Pique, A., Mlsna, T.E., and Stepnowski, J.L. (1998) Performance optimization of surface acoustic wave chemical sensors. IEEE Trans. Ultrason. Ferroelectr. Freq. Control, 45, 1370.
- 101 Pique, A., Auyeung, R.C., McGill, R.A., Chrisey, R.B., Callahan, J.H., and Mlsna, T.E. (1998) Matrix assisted pulsed laser evaporation (maple) of polymeric materials: methodology and mechanistic studies. MRS Proc., 526, 375.
- 102 Pique, A., Auyeung, R.C.Y., Stepnowiski, J.L., Weir, D.W., Arnold, C.B., McGill, R.A., and Chrisey, D.B. (2003) Laser processing of polymer thin films for chemical sensor applications. Surf. Coat. Technol., 163–164, 293–299.
- 103 Piqué, A., Weir, D.W., Wu, P.K., Pratap, B., Arnold, C.B., Ringeisen, B.R., McGill, R.A., Auyeung, R.C.Y., Kant, R.A., and Chrisey, D.B. (2002) Direct-write of sensor devices by a laser forward transfer technique. Proceedings of SPIE LASER 2002, vol. 4637, 19–25 January, 2002, San Jose, CA, pp. 361–368.
