13
Manipulating Grouping Dynamics of Nanoscale Boron Particles as Basis for Environmentally Friendlier Combustion and Efficient Filtration
David Katoshevski1 and Levan Chkhartishvili2,3
1Ben-Gurion University of the Negev, Environmental Engineering Unit, P.O.B. 653, Beer-Sheva 8410501, Israel
2Georgian Technical University, Department of Engineering Physics, 77 Merab Kostava Avenue, Tbilisi 0175, Georgia
3Ferdinand Tavadze Institute of Metallurgy and Materials Science, Laboratory for Boron-Containing & Composite Materials, 10 Elizbar Mindeli Street, Tbilisi 0186, Georgia
13.1 Boron Particles and Powders: A Review
13.1.1 Introduction
The synthetic hydrocarbons are known to have the highest energy density among commonly available fuels, about 40 MJ l−1. It can be increased by loading a liquid hydrocarbon fuel with particles of a high energy density solid material. As for solid propellants, they are typically composed of a dense solid fuel and its oxidizer, held together with an organic binder. Boron is interesting as only practical solid material with both volumetric and gravimetric energy densities, 135.8 MJ l−1 and 58.5 MJ kg−1, respectively, which are substantially greater than those of hydrocarbons. Frequently, the efficient engine performance requires rapid fuel ignition and combustion that can be difficult to achieve for a complex hydrocarbon fuel. One approach to speeding up the kinetics is to add to the fuel a fuel-soluble catalyst as well.
Summarizing the advantages of burning recyclable boron as fuel [1], we can state the following:
- Boron can be a powder or pellet. It burns only in a very high oxygen environment. With oxygen boron is ∼4 times denser by energy than gasoline.
- Without concentrated oxygen, boron is safe and inert to store for years, and it does not corrode the storage tank. Boron will not leak out into the atmosphere or explode.
- Boron can be recycled by melting it down and stripping the rust off. Nuclear power could provide all the energy to recycle boron so it can be burned again.
- In the future, the customers will be able to transfer used boron for recycling. It is so safe and cheap to transfer that even with the cost of mail and recycling, it would still be cheaper than oil.
Thus, boron fuel together with nuclear power would greatly assist in reducing energy security, climate change, air pollution and associated health costs, and deliver clean driving with a safer fuel.
Potential of boron as a fuel or fuel additive has not been realized due to the difficulty inigniting and burning it efficiently. On the one hand, boron is a refractory material (with melting temperature around 2800 K); thus, its combustion is inherently a heterogeneous process, which tends to be slow and diffusion limited. In principle, the heterogeneous chemistry can be accelerated by using nanoparticulate boron leading to large surface-area-to-volume ratios. But, on the other hand, the native oxide layer of ∼0.5 nm in thick that forms on air-exposed boron surfaces limits this approach, both by inhibiting combustion and by reducing the energy density for small particles.
Using boron nanoparticles as a carrier for a combustion catalyst is one possible way for simultaneously enhancing ignition of the hydrocarbon carrier fuel (or the binder in a solid propellant), and the total energy density. It is also possible that particles be coated with a material that directly enhances boron ignition. In the combustion, it is desirable to favor B2O3 rather than HOBO products.
Amorphous boron powder has a comparative edge over the conventional solid fuels/solid additives to liquid fuels such as aluminum, magnesium, and so on owing to its high heat of combustion for use in fuel-rich propellants for integrated rocket ramjets. The low atomic weight, high heat output, ready ignitability (e.g., with KNO3), releasing large amount of heat, and persistent burning even at low pressure make boron-based pyrotechnic composition a very attractive igniter composition.
Below, briefly describing the issue of utilization of boron powders for fuel additives, we mainly follow the H.C. Starck GmbH's view [2].
Elementary boron can be obtained either as a black product – amorphous boron (a-B) or in two crystalline forms – red α-rhombohedral boron (α-B) and more stable, dark, shiny β-rhombohedral boron (β-B). Fine boron powder ignites when heated to about 700 °C in air. It burns with a red flame to borontrioxide. At high temperatures, boron also bonds with nitrogen, chlorine, bromine, and sulfur. This element reacts relatively easily with nitric acid, which oxidizes it to boric acid.
The pyrotechnical industry used elementary boron in powder form as an additive in solid rocket propellants to achieve a higher thrust force. The central feature of the solid-propellant ducted rocket is a propellant system, which is referred to as the ramjet engine. In this case, a regulating valve is placed between the gas generator and the combustion chamber to regulate propulsion. Since the gas generator of the system is under high pressure, the opening of the regulating valve has to be appropriately narrow. The reliable grain-size distribution of the solid boron additive has to be calculated, because it is feared that coarse boron grains would interfere with the ability to regulate the engine. Usually, customers demand amorphous boron with a 2% maximum of grains larger than 2 µm and no grains larger than 5 µm.
Ramjet engines have significant advantages compared to conventional rocket motors concerning specific impulse, maneuverability, and range. Boron particle addition to the propellant of ducted rockets further increases this potential due to a very high heating value. However, the combustion of boron particlesis a very complex process because of mentioned inhibiting oxide layer covering the particles. This layer has to be removed before vigorous combustion can start. Consequently, the boron particle combustion process runs in two distinct stages.
A neighboring area of application for a-B is production of the airbag igniters to equip the gas generators of these lifesaving automobile safety systems. Corresponding ignition mixtures are both reliable and nontoxic. For example, a densified powder mixture of sodium nitrate and a-B is triggered by an electric impulse in such airbag igniters. With the energy created by the primary ignition, sodium azide in a fraction of a second decomposes to nitrogen and sodium. While the sodium is removed with the help of silicates, the released nitrogen inflates the airbag. This entire process occurs in just a few microseconds. So, boron has proved to be highly successful as a lifesaving component in this system.
Not only amorphous but also crystalline boron is able to develop into an interesting business. Besides designating the purity, a customer usually prescribes the exact grain-size distribution, which results in the grain sizes of less than 20 µm.
A review of the characteristics of boron particles and powders given in this chapter serves as basis for the focus of the study on the tendency of submicrometer and nanosized boron particles to group/cluster in an oscillating flow. Analysis of the grouping behavior is the first step for describing particle–oscillation interactions' effect on the combustion process, flame characteristics, and pollutants' emission. Then, the combined effect of grouping due to flow oscillation governed by the Brownian motion dynamics of submicrometer particles will be discussed. And finally, some conclusions will made about how controlling flow oscillations can lead to a more environment-friendly combustion process.
13.1.2 Boron Powders
In previous reviews on micro- and nanostructured boron [3,4], we already discussed some aspects of combustion of boron powders. A review of boron particle combustion also was given in the PhD dissertation [5] devoted to the experimental (by single-droplet combustion method) study of ignition and combustion of boron and boron–iron composite nanoparticles in a low energy density biofuel (ethanol) flame. Here, we intend to present more up-to-date data on their production technologies, characterization, and fuel applications.
Let us start with some examples of early patents utilizing amorphous boron powder as fuel. The patent [6] describes a boron-containing fuel and fuel igniter developed for ramjet and rocket. Patent [7] proposed a liquid fuel with a solid additive based on boron and, consequently, with a high calorific power. As for the patent [8], it deals with boron-fuel-rich propellant compositions for use in air-augmented rocket propulsion, containing a high-energy fuel component in the form of finely-divided boron, where in the boron is present in large excess of the amount oxidizable during combustion of the propellant.
The paper [9] describes the studies on optimization of two processes – oxidative roasting of boron in air and roasting boron with zinc in an inert medium – for preparing high-purity amorphous boron: ∼92 and >93%, respectively. Oxidative roasting has a comparative edge over the other processes owing to its ease of scale-up and simplicity. It was concluded that, amorphous boron powder of high purity, 92–94%, with a particle size of l–2 mm would be preferred as a fuel for fuel-rich propellants for integrated rocket ramjets and for igniter formulations.
The high concentration of paramagnetic centers in 95% a-B powders, when the main impurity is carbon, ∼0.9%, highlights the high defectiveness of this material in comparison with crystalline powders [10] and must be related to the broken bonds. The impurity paramagnetic centers associated with unpaired electrons on carbon atoms are also observed. In the interval 720–1270 K, the degree of oxidation of the surface of a-B powder of purity 98% increases from 0.04 to 20.00% [11]. It was noted that the adsorption processes occurring in oxygen mixtures with nitrogen and water vapor on the surface of fine powders of brown and black a-B lead to an increase in the mass of the particles by 10.0 and 5.1%, respectively [12]. Consequently, the powders a-B can contain so many adsorbed gases that they are able to influence the quality of the product obtained.
The neighboring fuel area of use of a-B is the production of environmentally sound signaling missiles with emission in the green spectral region [13].
In addition, a-B powders are widely used in the synthesis of various compounds. For example, boron suboxide B6O can be obtained at room temperature by oxidation of boron together with its own B2O3 oxide or some metal oxides [14]. Amorphous boron in the synthesis of boron nitride nanostructures plays an especially important role as well [15–21]. A different use of a-B is to synthesize ceramic materials. So, for example, Al-based composites containing a-B and ceramics as fillers can be produced [22]. Pyrolysis of precursors that contain a-B provides the technology of obtaining the boron carbide-based metal-ceramic materials [23].
Powders of a-B are often used to form wear-reducing coatings. For example, by the method of high-temperature thermal diffusion saturation, the boron nitride coating was sputtered onto a titanium [24] and iron-boron coating onto a steel [25] surface. The same goal is achieved by thermochemical borating from a powder mixture of a-B and a boron-containing activator of iron-chromium alloys and chromium steels [26].
Powdered a-B can serve as a precursor for various metal borides. For example, a-B with a purity of 99.6% was used in the process of borothermic decomposition of Li2CO3 in argon or vacuum to obtain LiB2 and LiB10 phases [27]. The AlB10 nanoparticles encapsulated in BN layers were grown by arc melting of powder compacts of a-B and Al in a N2/Ar gas mixture [28].
High-strength aluminum materials can be manufactured by mechanical alloying with the participation of a-B [29]. The Ti-Al-B alloys were obtained by mechanochemical synthesis of the initial composite powder containing a-B, followed by compaction [30–33]. The thermochemical treatment of chrome-plated steels in a-B powder gives boride layers at the steel–boron interface [34]. Mechanochemical synthesis with reaction between B2O3 and Mg powders was used to prepare amorphous boron nanoparticles [35]. By this method, boron powders with purity about 91 wt.% can be prepared. The leached boron powders had an amorphous structure with average particle size smaller than 32 nm and the yield of synthesized nanoboron more than 74%.
Thus, amorphous boron powders with small particle size, narrow size distribution, and high purity are very important in the high-tech fields. In particular, such amorphous boron powder has a higher energy density than the fuels such as magnesium, hydrocarbon and carbon.
It was established that the structure of the core of fine particles of a-B is basically α-rhombohedral. There are also reports directly about crystalline α-B powders.
Several types of nanodispersed products are obtained by laser evaporation of boron carbide B4.3C immersed in ethanol [36]. One such product consists of spheres with carbon cores encapsulated in layers of nanocrystalline α-B. It is also possible to isolate the pure α-B-phase powder. Samples of α-B, prepared by the method of crystallization of a-B, are fine crystalline masses, which contain an admixture of boron suboxide B12O2. For this reason, their morphology is similar to an aggregated powder. The paramagnetic centers in this case are inorganic boron radicals and oxygen centers.
In their study, Wang et al. [37] synthesized BN nanotubes in significant amount by high-temperature annealing in the NH3 stream of a precursor containing α-B.
The α-B and β-B crystal structures of pure elemental boron powders have been synthesized via gas phase thermal dissociation of boron trichloride BCl3 by hydrogen on a quartz substrate [38]. Boron powders have been synthesized with a minimum purity of 99.99% after repeated HF leaching. Submicrometer- and micrometer-scale boron particles were obtained in sizes varying between 500 nm and 10 µm.
Powders of the most stable β-rhombohedral modification of boron are usually obtained as a result of mechanical grinding of fused or sintered β-B ingots at high temperatures, ∼2000 °C.
The β-B powders are often used in the study of the phase states of boron-containing systems. For example, in order to construct the phase diagram of elementary boron, a series of experiments were undertaken in which a commercially available high-purity (99.9995%) polycrystalline β-B powder served as a boron source [39]. Chemical interaction and phase transformations in the B-BN system were investigated using β-B powders 99.99% purity of industrial production [40] and with an impurity content of no more than 0.2% [41]. A number of BN structures were synthesized from β-B powders [42–44]. A technology has been developed for the production of galvanic powder metalloceramic coatings, which contain micro- or nanodispersed boron powders as the hardening and consolidating component [45].
For obtaining β-B crystalline powders, we have developed some mechanical [46–48], mechanochemical [49], and arc-discharge [50] production technologies.
Coating of boron powders with titanium was described in Ref. [51].
A simple, scalable, and one-step process for generating air-stable boron nanoparticles that are unoxidized, soluble in hydrocarbons, and coated with a combustion catalyst was also presented [52]. Ball milling was used to produce ∼50 nm particles.
Amorphous boron powder granulated with hydroxyl-terminatedpolybutadiene (HTPB) was prepared by mechanical mill method [53]. Amorphous boron powder could be granulated with HTPB binder to form BHTP B-particles, whose median particle diameter and specific surface area are in the range of 125.0–431.0 µm and 0.02–0.10 m2 g−1, respectively. The content of boron particles in a fuel-rich solid propellant could be increased by addition of BHTPB and its combustion characteristics improved.
At present, boron powders are produced in a variety of structures, dispersity, purity, and so on. For example, Alfa Aesar offers a wide range of boron powders, both amorphous and crystalline and mixed amorphous crystalline [54].
13.1.3 Combustion Mechanisms
The effects of fluorinated graphite CFx with different fluorine contents (x = 0.08, 025, and 1.17) on the combustion of boron powder and boron-based fuel-rich propellants were experimentally investigated in Ref. [55].
The ignition of electrically heated boron filaments in air and argon/oxygen mixtures was studied in Ref. [56]. Boron filament resistance, temperature, and emissions from the BO and BO2 bands were monitored. The experimental data were also obtained to characterize the phases formed inside burning boron particles produced and ignited from the same filament material by feeding a vibrating boron filament into an oxygen–acetylene flame. The liquid boron particles so formed and ignited burned in room air where their combustion was recorded. Samples of both filaments and particles quenched at different times during their combustion were analyzed to characterize their internal structures and compositions. The filaments “burned” in two distinct stages. The onset of the first stage was characterized by a local minimum in the filament resistance, a sharp spike in boron oxide radiation emission, and a rapid rise in temperature. It occurred at a temperature of 1500 ± 70 °C, independent of the preheating history and oxygen content (5–40%) in the gas environment. These data and changes in the filament physical characteristics suggest that a phase transition occurs in the filaments at this temperature and triggers stage-one combustion. A transition from α- to β-rhombohedral boron structures has been reported in this temperature range. The burning boron particles exhibited periodic brightness oscillations that arise from asymmetric particle combustion associated with internal phase changes. Analyses indicated significant amounts of oxygen contained within both quenched filaments and particles. In addition, quenched filament samples collected during the second stage of combustion exhibited large spherical voids. These observations indicated that in-depth heterogeneous processes play important roles in boron ignition and combustion.
The agglomerated boron, which has good roundness and particle size of 0.105–0.190 mm, was obtained [57] through a dry process, and the fuel-rich propellant sample containing 32% of agglomerated boron and with a heat value of 32 MJ kg−1 was successfully prepared. The burning rate and pressure index of propellant samples, with different AP gradation and Mg/Al (particle size) combination and agglomerated boron with different particle size, has been determined by strand burner method. It was found that the processing of fuel-rich solid propellant becomes less difficult when the agglomerated boron is introduced for replacing ordinary boron powder. That would make it realized to increase the content of boron, fine AP, and other solid ingredients in composition so as to improve the heat value and combustion characteristics of the propellant. Especially, increasing the content of fine AP in composition has led to the significant enhancement of burning rate and pressure index of propellant samples, but the effects of particle size of Mg/Al and agglomerated boron were rather minor.
The study by Demirbas [58] aimed to obtain the energy from elemental boron burning as solid fuel, which was synthesized from boron minerals, aluminum, iron, and their substances. In particular, to obtain the elemental boron first from borax decahydrate was obtained boric acid H3BO3 by using HCl or H2SO4. Then the boric acid was converted to boron oxide by dehydration process. For reducing the boric acid into elemental boron, the elemental magnesium, butyl nitrite C5H9NO2, sawdust, charcoal, and cellulose were used. Elemental boron obtained by these methods was tested by burning to see whether C5H9NO2 obtaining was more reasonable than the others. The metallic aluminum powder burns to result in a strong exothermic reaction in the presence of the magnesium stripe. The manufacture of hydrogen on an industrial scale involves the reaction between steam and iron. The alkali metals, lithium, sodium, and potassium react violently with water at the ambient temperature, yielding hydrogen.
An experimental investigation of the combustion characteristics of boron nanoparticles in the postflame region of a flat flame burner has been conducted in [59]. Ignition and combustion characteristics were studied in the postflame region of a fuel lean CH4/air/O2 flame, with burner temperatures ranging from 1600 to 1900 K and oxygen mole fractions ranging between 0.1 and 0.3. As in earlier investigations on boron combustion, a two-stage combustion phenomenon was observed. Ensemble averaged burning times of boron nanoparticles were obtained, while the ignition time measurements for boron nanoparticles were extended into a previously unavailable lower temperature range. The measured burning times were between 1.5 and 3.0 ms depending on both the temperature and the oxygen fraction. The ignition times were relatively insensitive to oxygen concentration in the range studied and were affected only by temperature. The measured ignition times were inversely related to the temperature, ranging from 1.5 ms at 1810 K to 6.0 ms at 1580 K.
The combustion characteristics of nanofluid fuels containing additions of boron and iron particles were investigated by Gan et al. [60]. The effects of particle materials, loading rate, and type of base fuel on suspension quality and combustion behavior were determined. The burning behaviors of dilute and dense suspensions were compared, and the results for dense nanosuspensions showed that most particles were burned as the large agglomerate at a later stage when all the liquid fuel had been consumed. Sometimes, this agglomerate may not burn if the energy provided by the droplet flame is insufficient. For dilute suspensions, the burning characteristics were characterized by a simultaneous burning of both the droplet and the particles, which integrated into one stage. The fundamental mechanism responsible for bringing the particles out of the droplet, which is a prerequisite for them to burn, is different for n-decane- and ethanol-based fuels. For the former, the particles are brought out of the droplet by a disruptive behavior of the primary droplet, which was characterized by multiple-time disruptions and with strong intensity. This was caused by the different boiling points between n-decane and the surfactant. For ethanol-based fuels having no added surfactant, the particles are also brought out of the droplet by disruptive behavior, but characterized by continuous disruptions of mild intensity. This was very likely caused by a continuous water absorption by the ethanol droplet during its burning process.
An experimental study was conducted [61] to understand the combustion behavior of polytetrafluoroethylene (PTFE)/boron-based solid fuels for future hybrid rocket motor applications. Fuels were loaded with 10–40% boron powder (w/w). Two different types of PTFE were examined. FTIR experiments show no significant differences in the decomposition mechanisms for PTFE and a candidate solid fuel mixture. Diffusion flame studies between solid fuels and gaseous oxygen were carried out to measure regression rates. The fuels with the lowest boron content readily extinguished upon removal of the supplemental oxygen flow. The fuels with the highest loadings of boron self-propagated after ignition. Graphite and boron carbide revealed as the remaining products, while particles captured leaving the surface of the fuel under normal burning conditions were found to be mostly boric acid. Boron oxidation and magnesium fluorination were observed in the flame zone of the diffusion flame by UV–vis emission spectroscopy (because magnesium is the major impurity in the elemental boron powder used). The results of this study suggest that solid fuels comprising PTFE and boron show promise for improving the energy density of hybrid rockets.
In order to extend the burning rate of boron-based, fuel-rich solid propellants with agglomerated boron powder, the effects of the boron content, the agglomerated particles content, and of the magnesium powder content on the burning rate and pressure exponent were studied systematically [62]. It was shown that when the agglomerated particles content is constant, the burning rate of the propellants increases with an increase in the agglomerated boron content. Furthermore, the burning rate and pressure exponent increase with increasing contents of agglomerated particles and magnesium powder. By means of single-color frame amplification photography and combustion wave tests, the combustion mechanism of these propellants was investigated. It was shown that the agglomerated boron powder is partially oxidized on the combustion surface, and the heat released from it may be beneficial to the combustion of the propellants.
The morphology and particle size of condensed-phase primary combustion products of boron-based fuel-rich propellants under different chamber pressures were analyzed [63]. In addition, organics in condensed-phase products and gaseous products were analyzed. The results show that the condensed-phase products mainly consist of B, C, B4C (or B12C2), BN, Mg, MgO, MgAl2O4, Al, Al2O3, AlCl3, NH4[Mg(H2O)6]Cl3, NH4Cl, and Fe3O4. There are large amounts of boron oxide and boron carbide in the condensed-phase products, which indicates that elemental boron is highly active during the primary combustion process; a higher chamber pressure may be disadvantageous to the secondary combustion efficiency because more inactive boron carbide, graphite, and h-BN are produced.
A study of gas generator fuel-rich propellant for air-breathing propulsion system was performed Won et al. [64]. General solid propellant comprises a mean of 60% or more oxidizing agents. But, to develop the fuel-rich solid propellant, they increased the content of the boron fuel and reduced the content of the oxidizing agents by approximately 30%. Very high amount of heat per volume of fuel into the boron was used. Amorphous boron powder was applied to the propellant as beads type (with diameters up to 40 µm and size distribution maximum near 0.5 µm) and it allowed to design more amount of boron fuel (40–50 wt.%) in the fuel-rich propellant. And the combustion characteristics and properties (viscosity, burning rate, pressure exponent, etc.) of fuel-rich solid propellant according to the boron bead sizes were confirmed.
The paper [65] presented the results of an experimental study of the high-energy materials' combustion at subatmospheric pressures. Systems containing powders of micrometer-sized aluminum, ultrafine aluminum, boron, and their mechanical mixtures were investigated. Effect of the replacement of aluminum by aluminum-boron mixtures in propellant systems on the burning rate was determined.
13.1.4 Combustion Models
According to the literature review presented in the paper [66], two types of combustion models for single boron particles are outstanding. A very detailed model by the Princeton/Aerodyne group features hundreds of elementary reactions and considers all physical processes in the particle environment. It is very elaborate and, thus, not suitable for incorporation into three-dimensional calculations. The second model developed at Penn State University takes a global approach with only a few reactions that makes it promising for computational fluid dynamics applications. However, a careful analysis of this model revealed some inconsistencies, errors, and drawbacks that gave rise to the modified model. This new model comprises a consistent formulation of the heat and mass transfer processes in the particle environment based on a quasi-steady approach, accounts for boron evaporation that is a relevant process despite the high boiling point of boron, and it considers the influence of forced convection on the particle conversion. The chemical reaction rates adopted from the original model were revised and slightly changed, the differential equations to be solved were corrected, and an iterative solution algorithm was introduced. A careful validation of the model was presented in Ref. [67] showing that the new model is suitable for boron particle sizes relevant for ramjet combustion chambers. The results of the new extended model were compared with experimental data from literature and opposed to results of the other two models. They show reasonable agreement with measured data. A more complex transient model is also derived that serves as a means of scrutinizing the principal assumption of the new model that are quasi-steady state changes.
A practical scheme for selecting characterization parameters of boron-based fuel-rich propellant formulation was put forward [68] by establishing and validating a calculation model for primary combustion characteristics of boron-based fuel-rich propellant based on backpropagation (BP) neural network. Then, it was used to predict primary combustion characteristics of such propellant. Calculation error of burning rate is less than 7.3%. The simulation results showed that BP neural network model is superior to multiple linear regression, radial basis network, and generalized regression neural network. The established model was used to predict primary combustion characteristics of boron-based fuel-rich propellant. In the adjustable formulation range, the calculation results of different formulations under different pressures can be directly used to optimize the design of propellant formulations, which can reduce the experimental work load, shorten the research cycle, and improve reproducibility of the research.
13.2 Clustering of Particles in Oscillating Flow: From the Nanometric to the Hundred-micrometer Size Range
The focus of this section of the chapter is on the tendency of submicrometer and nanosized particles, and boron in specific, to group/cluster when in an oscillating flow, although particles up to the order of 200 µm are considered as well. Flow is represented by a wave configuration, representing oscillations within a combustion chamber, and particle motion is considered to be governed by drag and Brownian motion. It is found that in general, as particle size decreases, the tendency for particles to cluster increases, but closer examination reveals that there are two size ranges in which this is more likely. In the first, particles will cluster due to controlled flow oscillations (“oscillatory grouping”) and in the other, due to combined flow oscillations and Brownian motion. At intermediate sizes, the tendency for clustering of particles is weaker. Controlling the grouping of submicrometer boron particles may lead to their coagulation and hence decrease number concentrations and increase average particle size. Such results are desirable, for example, for reducing the health and environmental risks associated with small particles due to engine emissions and the greater ease with which larger clusters may be captured. Also, manipulating the flow for controlling grouping of boron particles in the combustion chamber will lead to a more efficient combustion.
13.2.1 Introduction
When particles are carried by an oscillatory flow field, they may, under certain conditions, approach each other and form groups. This grouping phenomenon, illustrated in Figure 13.1, has ramifications for various natural and industrial systems, and our focus is on the case of boron particles that either enter a combustion chamber or exit such a chamber as part of a smoke jet. Grouping-like phenomena have been reported in the literature with respect to several fundamental flow fields such as shear layers [69], jets [70], and vortices [71]. It is important to distinguish between grouping, a process that brings particles into proximity, and coagulation, which deals with the actual attachment of such particles once they are at very close range.

Figure 13.1 Grouping of particles in an oscillating flow. The oscillations are in time and in space. The arrows mark downstream velocity variation. Grouping may lead to coagulation and reduce the number of smaller particles.
A basic mathematical model has been developed and analyzed by Katoshevski and coinvestigators [72–75] where oscillating flows in the form of either a moving or a standing wave were considered (“oscillatory grouping”). These investigations have presented the regime, in terms of operating conditions, where grouping is expected. Three grouping modes were identified and are denoted as “stable grouping,” “weak grouping,” and “nongrouping.” Stable grouping is characterized by the formation of particle groups and subsequent stable conjoint motion. Under weak grouping, particles approach each other, forming temporary groups that later break up, and nongrouping is defined as a regime in which there is little tendency, if any, to aggregate. Also, a nondimensional parameter, denoted as NG (for nongrouping) was introduced, where NG ≫ 1 represents weak or nongrouping [73].
The above-mentioned analysis has set the basis for analyzing particle/droplet grouping in three distinct systems: (1) suspended sediment particles in water [76], (2) fuel spray droplets in an internal combustion engine [77], and (3) smoke particles in diesel exhaust [78,79]. The latter study has led to the design of an exhaust system that enhances grouping and subsequent coagulation of smoke particles, thus reducing health and environmental risks associated with emission of such particles. One such environmental risk is the deposition of diesel exhaust particles in the human respiratory tract [80]. Aerosols may also affect global warming and the greenhouse effect [81].
Note that these studies accounted for particles larger than 1 µm. The dynamics of submicrometer and nanometric particles, which were not considered, are significantly affected by Brownian motion [82,83], as is their grouping behavior. The current study fills this gap in a qualitative manner, and the main focus is on the relative effects of Brownian motion and oscillatory grouping on particle clustering tendency.
Since Brownian motion is a stochastic process, it may be addressed in either of the two ways. The first is a statistical one for the probability to find a particle in a specific location and time. The Fokker–Planck equation describes this probability [84]. The second approach, which we use here, is to obtain the trajectories of specific particles [75], with an additional random force term accounted for in the particle equation of motion.
Previous modeling studies, which have dealt with particle sizes of above 1 µm, have indicated that, at sizes of approximately 50 µm, there is an inverse relation between particle size and grouping tendency. However, this tendency should be examined in more detail, as done here. Another aspect to be explored here is the conditions under which clustering may be manipulated or controlled and in what particle size ranges this is not feasible.
To summarize, in the course of this section of the chapter, we will first present the model for particle motion in oscillating flow, including Brownian effects. This will be followed by analyzing the effect of particle size on clustering tendency, from relatively large particles of about 100 µm down to the nanometric scale.
13.2.2 Mathematical Model
We consider a standing wave flow [74,78]
where U is the carrier fluid velocity, t time, x longitudinal distance, Ua time-averaged velocity, Ub velocity amplitude, ω angular velocity of the wave (ω = 2π/T, where T is the wave period), and κ wave number (κ = 2π/L, where L is the wave length).
- Assuming that particle size is much smaller than the characteristic length scale of the flow and that gas density is much lower than that of the particle (ρg ≪ ρp), the main effects contributing to particle motion are drag and Brownian motion [85], stated as follows:
where Fd is the drag force and Fb is associated with the force induced by Brownian effects on the particle. Thus, as in previous studies, in the frame of this study, we do not account for gravit, or for added mass force, Basset force, and the Faxen force [75].
The drag force is estimated as follows:
where ρg is gas density, Dp particle diameter, V particle velocity, and CD the drag coefficient that depends on particle Reynolds number, Re:
(13.4)
where µ is the fluid dynamic viscosity. For low Reynolds number in the Stokes regime, the drag coefficient may be estimated as
(13.5)
When particle diameter is not much greater than the fluid molecules' mean free path (λ), a correction is required:
(13.6)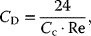
where Cc, the Cunningham Correction Factor, is defined as
(13.7)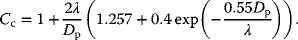
In addition, Brownian force is modeled as a zero-mean Gaussian white noise process with constant power spectral intensity [86,87]:
- Zero-mean force:
(13.8)

- Power spectral intensity:
(13.9)

where again Fb is the force exerted on the particle as a result of Brownian motion, δ the Dirac delta function, and S0 the power spectral density of the noise.
The Brownian force and power spectral density terms are described as follows:
where Gi is a Gaussian random number, Δt the time step size used in the numerical simulation, Kb the Boltzmann constant, and T the absolute temperature [83,88–90].
Substituting the drag force (Equation 13.3) and Brownian force (Equation 13.10) into the particle equation of motion (Equation 13.2) leads to the following:
where τ is Stokes relaxation time, defined as
Substituting the carrier fluid velocity (Equation 13.1) into the equation of motion (Equation 13.12) and normalizing leads to
where
and
Equation 13.14 was solved numerically via a finite difference scheme, with time steps significantly smaller than drag relaxation time, assuring that time resolution is fine enough to account for oscillatory response, yet significantly larger than the time between molecular collisions. For the following calculation, the time step was taken as Δt* = 1 · 10−7, a time step which would fit the smallest particle size in our calculations. Initial conditions for the calculations were location x0 and particle velocity V0, which was taken as V0 = Ua, the time-averaged velocity.
As previously shown [73,74], two parameters, α and β, govern the conditions for the onset of oscillatory grouping. Significant grouping is expected for β < 1 and α ≫ β, where
and
To analyze the grouping tendency as a function of particle size, we keep the value of β constant in the β < 1 range, while the value of α, which is a function of particle diameter, is varied. α is inversely proportional to particle diameter; hence, as particle size is reduced, α is made larger.
13.2.3 Results
Results present trajectories and grouping tendencies of particles of various size ranges. Results are based on a numerical solution of the nondimensional particle momentum equation (Equation 13.14). We are aware that one-dimensional analysis has its drawbacks in such a configuration, but on the other hand it shows the trends and captures the essence of the particle grouping dynamics even in realistic and practical cases [76,78] and can certainly serve for validation of comprehensive numerical simulations. Simulations were performed for air under standard conditions such that
These values are identical to those of the diesel exhaust system used in previous studies [78], which corresponds to Ua = 10 m s−1, Ub = 3 m s−1, ω = 471 1 s−1, and κ = 50 1 m−1.
We begin with a study of particles of diameters ranging from 1 to 50 nm. This section is followed by the results that concern cases that relate to particle sizes of up to 200 µm. We also deal with the time it takes for two particles to possibly achieve contact for different particle sizes. Finally, we will show the optimal size for particle grouping for the entire size range.
13.2.3.1 Grouping of Nanoparticles
Trajectories of 10 particles in close proximity were calculated for several particle sizes. Diameters ranged from 1 to 50 nm (St = 6 × 10−7 − 8 × 10−6). This is the range where Brownian motion is expected to be significant. Particle trajectories were studied with and without flow oscillations. In all cases, flow conditions were the same, such that β remained constant.
Particle trajectories resulting from Brownian motion only (when no flow is present) are described in Figure 13.2 in terms of nondimensional location and time, x* and t*, respectively.

Figure 13.2 Trajectories of 10 particles without flow oscillations. Four sizes are considered in the nanometric region of 1–50 nm. The 1 nm particles (a) move in random motion, while the 50 nm (d) show only a minor effect of Brownian motion.
For the smallest particles (1 nm), Brownian motion will induce random motion, which subsides as we move toward larger particles, due to the reduction in the ratio between the molecular mean free path and particle size. Hence, from O (10 nm) and above (as later demonstrated), flow oscillations are expected to come into play and induce oscillatory particle grouping. We now set the flow oscillations “on” and examine their effect on the same four sizes as in Figure 13.2. Results are presented in Figure 13.3.

Figure 13.3 Trajectories of 10 particles of four sizes as in Figure 13.2, but with addition of flow oscillations. The flow oscillations induce grouping tendency for particles of 5 nm (b) and over. Grouping is weak for 5 nm (b) and is more pronounced for 10 nm (c) and 50 nm (d). Flow conditions:  ,
,  , ω = 471 1 s−1, κ = 50 1 m−1, and β = 0.38.
, ω = 471 1 s−1, κ = 50 1 m−1, and β = 0.38.
The above figures show that at particle size of 1–5 nm, Brownian motion plays a major role in particle dynamics, while the effect of flow oscillations is negligible. For larger particles, paths are smoother, suggesting that Brownian effect is less significant. This suggests that flow oscillations induce grouping to some extent from a small particle size of approximately 10 nm.
13.2.3.2 Brownian and Drag-induced Particle Velocities
The velocities of different sized particles were simulated (Figure 13.4). Particle motion is affected by Brownian force, which gives it a random form, and by the drag force that gives the motion a sinusoidal form. As before, 5 nm and even 10 nm particles are mainly controlled by Brownian force, but at closer look larger particles are also influenced by Brownian force. This leads to conclude that particles larger than 10 nm are also affected by Brownian motion while grouping. The next section will present the affect of Brownian motion on fine and ultrafine particles.
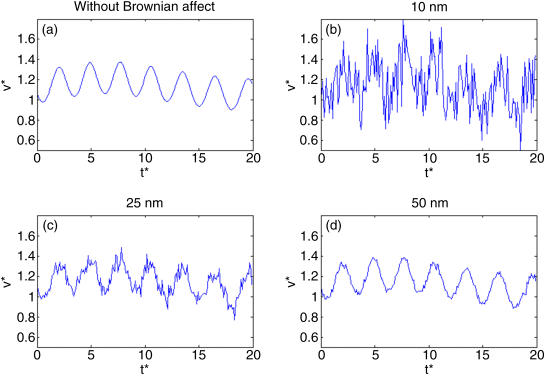
Figure 13.4 Particle velocity for a certain time. The velocity for the nanosize particle is stochastic as a result of Brownian force (b). This effect decreases when the particles are larger (c–d).
13.2.3.3 Time for Attachment
In order to quantify the extent of oscillatory grouping, we introduce here a new parameter, Θ100, defined as “time for attachment.” Time for attachment is the normalized time it takes for the distance between two particles, initially at a distance of 100 Dp, to reduce to 1 Dp, which is actually the distance of contact between particles. Similarly, Θ1000 is the same parameter for an initial distance of 1000 Dp. For example, Θ1000 for two 1 µm-sized particles is the time that takes them to get close to each other up to a 1 µm of separation when the initial distance is 1 mm.
The initial location of particles along the velocity wave of the flow determines the probability of grouping [74]. Particles initially adjacent may, under oscillating flow conditions, form a group or, conversely, separate.
Figure 13.5 shows how several particles at equal initial distances apart will form groups with some particles while moving away from others. Whether particles will form a group depends on the initial location relative to the wave. Particles at the interval [0 2π] will group together while particles at the interval [2π 4π] will move to a different group. In order to keep the particles in the same group, the particles were located with relation to the middle of the group, hence x0* = π ± 50 Dp* for Θ100 and hence x0* = π ± 500 Dp* for Θ1000. Normalized diameter, Dp*, defined as Dp* = Dp·κ.

Figure 13.5 2 µm particles form separate groups, depending on initial location. Flow conditions:  ,
,  , ω = 471 1 s−1, κ = 50 1 m−1, and β = 0.38.
, ω = 471 1 s−1, κ = 50 1 m−1, and β = 0.38.
Θ should be affected by oscillatory grouping and by Brownian motion. This means that grouping will result in a decrease in Θ. In addition, Θ will also be affected by the extent of Brownian motion. Thus, we have dual mechanisms that may facilitate aggregation.
To examine the influence of Brownian motion on oscillatory grouping, the time for attachment was double-checked, once when Brownian force and oscillatory velocity were taken into account and once when only the oscillatory velocity was considered. Figure 13.6 shows different behaviors in three size sections: 0.005–1 µm, 1–30 µm, and above 30 µm. We will now focus on each of these size sections separately.

Figure 13.6 Time for attachment, Θ, for different size particles with and without Brownian motion. (a) The initial distance between particles is 100 Dp. (b) The initial distance between particles is 1000 Dp. Time for attachment for calculations with Brownian motion is the average value based on 10 iterations. Error bars are standard deviation.
13.2.3.3.1 Attachment Tendency of 0.005–1 µm Size-range (St = 6 · 10−7−3 · 10−3)
At this size range, simulations that include Brownian effects diverge from those that do not. Brownian motion has a significant reducing effect on Θ.
To examine attachment tendency at this size range, the distance between two particles was evaluated. We studied the case of 0.1 µm at Θ1000 such that the initial normalized distance was 5 × 10−3 and the simulation calculates the distance between particles at every time step until the distance reaches 5 × 10−6 (Figure 13.7).

Figure 13.7 The distance between two 0.1 µm particles approaches zero.
On average, after a certain amount of time, the particles approach up to the distance required for “time for attachment.” However, when we do not consider Brownian motion, once particles have grouped their oscillatory response is virtually identical, and thus the oscillatory mechanism does not facilitate further clustering (and possible subsequent attachment) of grouped particles. Figure 13.8 displays the velocity difference between two particles as a function of interparticle distance. At large distances, relative velocity fluctuates, resulting in large changes in distance (a). As distance decreases, relative velocity fluctuations diminish, resulting in diminished changes in distance as a result of oscillatory flow, and Brownian motion becomes more consequential (b).

Figure 13.8 The acceleration difference between two particles as a function of distance between those particles. When particles are far enough, acceleration difference is affected by oscillatory grouping. When particles are close enough, the change in the acceleration as a result of oscillatory grouping is weakened and Brownian force takes place.
In addition, when particles are in proximity, drag-based modeling may not accurately describe their motion, as particles may be addressed as a cloud and individual particles are not in effect influenced by the unperturbed flow [91]. Thus, at the highest values of t*, Figure 13.7 may not be accurate and distance between particles, influenced by drag alone, may reach a steady-state value. Here, Brownian motion would expedite actual attachment, allowing for particle motion within the cloud (Figure 13.8).
13.2.3.3.2 Attachment Tendency of 1–30 µm Size-range (St = 3 · 10−3–2.6)
Time for attachment in this particle size range increases when particle size is reduced. We assume that this is due to the fact that large particles deviate from the streamlines, a drift that leads to grouping. Figure 13.9 compares the grouping tendencies for two particle sizes: grouping of two 2 µm particles and of two 20 µm particles. The trajectory of 2 µm particles has a sinusoidal shape, which means that particles are following the streamlines. However, the trajectories of the 20 µm particles are less sinusoidal in shape. They do not closely follow the streamlines and grouping is more rapid for the larger particles.

Figure 13.9 Trajectories of 10 particles while grouping. Flow conditions:  ,
,  , ω = 471 1 s−1, κ = 50 1 m−1, and β = 0.15.
, ω = 471 1 s−1, κ = 50 1 m−1, and β = 0.15.
13.2.3.3.3 Attachment Tendency of Particles of Above 30 µm (St > 2.6)
Particles of this size are almost unaffected by the oscillatory nature of the flow. At this size, α is large, which means that particles are in the nongrouping regime [72]. Therefore, the time for attachment rises sharply with particle size. For proper flow conditions, the largest particles that are still influenced by oscillatory grouping are 200 and 85 µm for Θ100 and Θ1000, respectively.
13.2.3.4 Time for Attachment with Particles of Different Sizes
Based on Smoluchowski's equation [92,93], coagulation is size-dependent. Fuchs [94] showed that the coagulation coefficient, K, is
which means that in comparison with equal-size particles, particles differing in size will have a larger coagulation coefficient and thus the rate of coagulation would be higher. Hence, it is worthwhile to examine the clustering of particles of different sizes.
Due to the random nature of Brownian motion, Θ was taken as the average over 20 simulations. Figure 13.10 shows that compared to equal-sized particles, time required for particles of different sizes to reach touching distance is between that required for two small particles (20 nm) and two large ones (2000 nm).

Figure 13.10 Time for grouping for different size particles. Θ1000 is the average of 20 iterations.
13.2.4 Summary of Results
Based on results presented in Sections 13.2.3.1–13.2.3.3, it is clear that we may divide the overall size range into four sections in terms of clustering tendency (Figure 13.11). The first is the nanorange, where Brownian effects dominate particle dynamics. The second size section is of particles in the size range of 0.005–1 µm. In this range, both Brownian motion and oscillatory effects come into play. The third size section is of particles that exhibit only oscillatory grouping. The fourth size section, of large particles, does not appear to be affected by either Brownian or oscillatory effects.
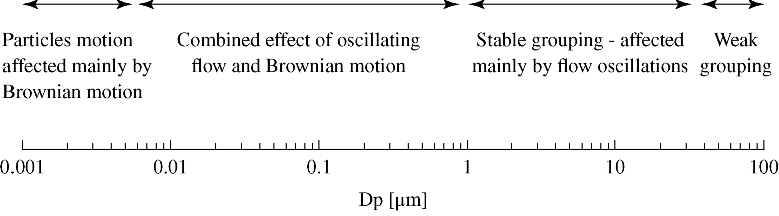
Figure 13.11 Particle behavior at oscillation flow in the whole size range.
If we are interested in an optimal particle size for clustering, the conclusion is that there are two optimal size ranges. The first are nanoparticles, on the order of 5–10 nm, where Brownian effects are appreciable. Second are particles at the size range of 20–30 µm. At this size, as seen in Figures 13.6 and 13.8, the time it takes for particles to cluster is minimal.
13.2.5 Conclusions
A comparative analysis of particle grouping over a wide size range is presented. This has relevance to boron particle dynamics in combustion-related systems and importance with respect to reduction of particle emission. Grouping tendency was estimated while considering the possibility of Brownian effect, and these calculations have pointed out the size range where the Brownian motion dominates grouping. Four size regions were found to reflect the relative importance of the oscillatory and Brownian mechanisms:
- Particles that are less than 10 nm that do not show a tendency to cluster by oscillatory grouping.
- Particles at the size region between 10 nm and 1 µm are influenced by both Brownian motion and oscillatory flow effects, with Brownian motion found to increase the rate of clustering.
- 1 µm–30 µm: Clustering of particles in this size section is governed by oscillatory grouping.
- Particles larger than 30 µm: both Brownian and oscillatory effects appear negligible.
At some point, when distances are sufficiently reduced, particle collisions may occur (see, for example, Figure 13.3). Such collisions may in effect ungroup particles. However, these would occur only once particles are in close proximity and having comparable velocities (both in magnitude and in direction). These would, in essence, present as a diffusional process that may “spread” the group somewhat. However, in comparison with the groups' drag-induced motion, and considering cloud dynamics, we do not expect that these would lead to “degrouping.”
This work allows simulation of grouping at the nanometric size range that was not previously considered. This may be of importance in the study and analysis of particle clustering in such systems as internal combustion engines (ICEs) as well as in their exhaust, complimenting previous studies that have analyzed the oscillatory mechanism only.
Acknowledgments
David Katoshevski gratefully acknowledges Mr. Itamar Hite and Dr. Roee Orland for their assistance. Levan Chkhartishvili gratefully acknowledges the Science and Technology Center in Ukraine for partial support within the Grant STCU #6204.
References
- 1 Blees, T. (2008) Prescription for the Planet. The Painless Remedy for our Energy & Environmental Crises, Ch. 5, pp. 152–166. www.prescriptionfortheplanet.com.
- 2 Starck, H.C. (2001) Boron: From Eye Drops to Rocket Propellants, H.C. Starck GmbH, Laufenburg (Baden, Germany).
- 3 Chkhartishvili, L. (2011) Micro- and nano-structured boron, in Boron. Compounds, Production and Application (ed. G.L. Perkins), Nova Science Publishers, New York, pp. 221–294, Ch. 6.
- 4 Chkhartishvili, L. (2011) Nanoboron (An overview). Nano Studies, 3, 227–314.
- 5 Karmakar, S. (2012) Energetic nanoparticles as fuel additives for enhanced performance in propulsion systems. PhD Dissertation. Louisiana, Louisiana State University, 191 pp.
- 6 Damon, G.H., Herickes, J.A., and Ribovich, J. (18 Dec. 1962) Boron containing fuel and fuel igniter for ram jet and rocket. US Patent 3069300 A.
- 7 Debize, F. (30 Nov. 1965) Fuel with a base of boron and with a high calorific power. US Patent 3220809 A.
- 8 Macri, B.J. (19 Oct. 1976) Boron-fuel-rich propellant compositions. US Patent US 3986909 A.
- 9 Tilekar, K.V., Gajbhiye, V.P., Prasanth, H., and Soman, T. (2005) Preparation of high purity amorphous boron powder. Defense Sci. J., 55 (4), 471–475.
- 10 Kvachadze, N.G., Tomashevskii, E.E., Gurin, V.N., and Nikanorov, S.P. (2010) Paramagnetic centers in boron and aluminum borides. Phys. Solid State, 52 (2), 237–240.
- 11 Kudin, V.G. and Makara, V.A. (2004) Oxidation resistance of B, W2B5 and WC powders. Powd. Metall. Met. Ceram., 43 (1–2), 67–71.
- 12 Zenkov, V.S. (2006) Adsorption-chemical activity of finely-dispersed amorphous powders of brown and black boron used in synthesizing metal borides. Powd. Metall. Met. Ceram., 45 (5–6), 279–282.
- 13 Yayla, S., Özmen, L., Karakulak, F., and Önsipahioğlu, Ö. (2011) Green flare compositions containing elemental amorphous boron and its derivatives. Abs. 17th International Symposium on Boron Borides and Relational Materials. Istanbul, Turkey: Istanbul Technical University, pp. 140–140.
- 14 Herrmann, M., Sigalas, I., Thiele, M., Müller, M.M., Kleebe, H.-J., and Michaelis, A. (2013) Boron suboxide ultrahard materials. Int. J. Ref. Met. Hard Mater., 39, 1, 5–60.
- 15 Zhang, H. and Chen, Y. (2006) Boron nitride nanotubes: synthesis and structure, in Nanomaterials Handbook, Taylor & Francis, pp. 22–22, Ch. 11.
- 16 Tokoro, H., Fujii, Sh., and Oku, T. (2009) Synthesis of boron nitride and carbon nanomaterials through a solid phase reduction process. Mater. Chem. Phys., 114 (1), 204–212.
- 17 Ferreira, T.H., da Silva, P.R.O., dos Santos, R.G., and de Sousa, E.M.B. (2011) A novel synthesis route to produce boron nitride nanotubes for bioapplications. J. Biomat. Nanobiotechnol., 2 (4), 426–434.
- 18 Yu, H., Huang, X., Wen, G., Zhang, T., Zhong, B., and Bai, H. (2011) A facile method for synthesis of novel coral-like boron nitride nanostructures. Mater. Chem. Phys, 129 (1–2), 30–34.
- 19 Yu, Y., Chen, H., Liu, Y., White, T., and Chen, Y. (2012) Preparation and potential application of boron nitride nanocups. Mater. Lett., 80 (1), 148–151.
- 20 Zhang, L., Wang, J., Gu, Y., Zhao, G., Qian, Q., Li, J., Pan, X., and Zhang, Zh. (2012) Catalytic growth of bamboo-like boron nitride nanotubes using self-propagation high temperature synthesized porous precursor. Mater. Lett., 67 (1), 17–20.
- 21 Pakdel, A., Wang, X., Bando, Y., and Golberg, D. (2013) Non-wetting white graphene film. Acta Mater., 61 (4), 1266–1273.
- 22 Gorshenkov, M.V., Kaloshkin, S.D., Tcherdyntsev, V.V., Danilov, V.D., and Gulbin, V.N. (2012) Dry sliding friction of Al-based composites reinforced with various boron-containing particles. J. Alloys Comp., 536, 126–129.
- 23 Antadze, M., Chedia, R., Tsagareishvili, O., Mikeladze, A., Gachechiladze, A., Margiev, B., Gabunia, D., Tsuladze, T., and Khantadze, D. (2012) Metal-ceramics based on nanostructured boron carbide. Solid State Sci., 14 (11–12), 1725–1728.
- 24 Pogrelyuk, I.N., Fedirko, V.N., and Samborskii, A.V. (2012) Study of wear resistance of thermal-diffusion boron nitride coatings on titanium. J. Frict. Wear, 33 (5), 388–395.
- 25 Chernega, S.M., Polyakov, I.A., and Medova, I.Y. (2012) Increase of wear resistance of surface layers of steel with coatings based on iron borides with addition of alloying elements. Abs. 3rd International Samsonov Memorial Conference “Materials Science of Refractory Compounds,” 2012, Kyiv, IPMS – KPI, pp. 170–170.
- 26 Dybkov, V.I., Sidorko, V.R., Samelyuk, A.V., Goncharuk, L.V., and Khoruzha, V.G. (2012) Thermochemical boriding of Fe–Cr alloys and chromium steels. Abs. 3rd International Samsonov Memorial Conference “Materials Science of Refractory Compounds,” 2012, Kyiv, IPMS – KPI, pp. 137–137.
- 27 Serebryakova, T.I., Lyashenko, V.I., and Levandovskii, V.D. (1994) Interaction in the system Li–B and some properties of lithium boride phases. Powd. Metall. Met. Ceram., 33 (1–2), 49–53.
- 28 Narita, I. and Oku, T. (2002) Arc-melting synthesis of BN nanocapsules from B/Al, TiB2 and VB2. Diamond Rel. Mater., 11 (3–6), 949–952.
- 29 Lovshenko, G.F. and Lovshenko, F.G. (2007) Phase composition, structure and properties of aluminum materials mechanically alloyed with boron. Powd. Metall. Met. Ceram., 46 (7–8), 338–344.
- 30 Oliker, V.E., Sirovatka, V.L., Gridasova, T.Ya., Timofeeva, I.I., and Bykov, A.I. (2008) Mechanochemical synthesis and structure of Ti–Al–B-based alloys. Powd. Metall. Met. Ceram., 47 (9–10), 546–556.
- 31 Oliker, V.E., Sirovatka, V.L., Gridasova, T.Ya., Grechishkin, E.F., and Kostenk, A.D. (2009) Tribotechnical characteristics of detonation coatings from mechanochemically synthesized Ti–Al–B powders. Powd. Metall. Met. Ceram., 48 (9–10), 522–528.
- 32 Oliker, V.E., Sirovatka, V.L., Gridasova, T.Ya., Timofeeva, I.I., Grechishkin, E.F., Yakovleva, M.S., and Eliseeva, E.N. (2009) Effect of gas media on the structural evolution and phase composition of detonation coatings sprayed from mechanically alloyed Ti–Al–B powders. Powd. Metall. Met. Ceram., 48 (11–12), 620–626.
- 33 Oliker, V.E., Sirovatka, V.L., Grechishkin, E.F., and Gridasova, T.Ya. (2010) Abrasive wear resistance of detonation coatings from mechanochemically alloyed Ti–Al–B powder. Powd. Metall. Met. Ceram., 49 (1–2), 61–65.
- 34 Dybkov, V.I., Sidorko, V.R., Khoruzha, V.G., Samelyuk, A.V., and Goncharuk, L.V. (2011) Interaction of 25% chromium steel with boron powder. Powd. Metall. Met. Ceram., 50 (78), 564–571.
- 35 Seifolazadeh, A. and Mohammadi, S. (2016) Synthesis and characterization of nanoboron powders prepared with mechanochemical reaction between B2O3 and Mg powders. Bull. Mater. Sci., 39 (2), 479–486.
- 36 Liu, C.H., Peng, W., and Sheng, L.M. (2001) Carbon and boron nanoparticles by pulsed-laser vaporization of boron carbide in liquids. Carbon, 39 (1), 144–147.
- 37 Wang, J., Zhang, L., Gu, Y., Pan, X., Zhao, G., and Zhang, Zh. (2013) Self-propagation high-temperature synthesis and annealing route to synthesis of wave-like boron nitride nanotubes. Mater. Res. Bull., 48 (3), 943–947.
- 38 Ağaoğullari, D., Balci, Ö., Duman, İ., and Övçeoğlu, M.L. (2011) Synthesis of α- and β-rhombohedral boron powders via gas phase thermal dissociation of boron trichloride by hydrogen. Metall. Mater. Trans. B, 42 (J6), 568–574.
- 39 Parakhonskiy, G., Dubrovinskaia, N., Bykova, E., Wirth, R., and Dubrovinsky, L. (2011) Experimental pressure-temperature phase diagram of boron: resolving the long-standing enigma. Sci. Rep., 1 (96), 1–7.
- 40 Solozhenko, V.L. and Kurakevych, O.O. (2009) Chemical interaction in the B–BN system at high pressures and temperatures. synthesis of novel boron subnitrides. J. Solid State Chem., 182 (6), 1359–1364.
- 41 Solozhenko, V.L., Kurakevych, O.O., Turkevich, V.Z., and Turkevich, D.V. (2010) Phase diagram of the B–BN system at 5 GPa. J. Phys. Chem. B, 114 (17), 5819–5822.
- 42 Kurdyumov, A.V. (2003) Effect the actual structure of boron and catalytic additions on boron nitride synthesis. Powd. Metall. Met. Ceram., 42 (3–4), 189–194.
- 43 Chen, Y., Gerald, J.F., Williams, J.S., and Willis, P. (1999) Mechanochemical synthesis of boron nitride nanotubes. Mater. Sci. Forum, 312, 173–178.
- 44 Kim, J., Lee, S., Seo, D., and Se, Y.-S. (2012) Synthesis of multiwall boron nitride nanotubes dependent on crystallographic structure of boron. Mater. Chem. Phys., 137 (1), 182–187.
- 45 Luchka, M.V. and Derev'yanko, O.V. (2012) New technology for making of galvanic powder coatings. Proc. 7th Int. Conf. MEE, 2012, Keiv, IPMS. pp. 127–127.
- 46 Gabunia, D.L., Tsagareishvili, O.A., Chkhartishvili, L.S., and Mirijanashvili, Z.M. (2012) Obtaining, structure and properties of β-rhombohedral boron powders. Abs. Conf. “Powder Metallurgy: Its Today and Tomorrow,” 2012, Kyiv, IPMS, pp. 155–155.
- 47 Gabunia, D.L., Tsagareishvili, O.A., and Chkhartishvili, L.S. (2014) Features of grinding of crystalline β-rhombohedral boron, in New Materials and Technologies: Powder Metallurgy, Composite Materials, Protective Coatings, Welding (eds A.F. Ilyushchenko, P.A. Vityaz', M.A. Andreev, V.M. Kantsevich, V.V. Savich, V.G. Smirnov, L.V. Sudnik, and M.L. Kheifets), Belarusian Science, Minsk, pp. 58–61.
- 48 Gabunia, D.L., Tsagareishvili, O.A., Chkhartishvili, L.S., and Mirijanashvili, Z.M. (2014) Structure and properties of β-rhombohedral boron powders produced by mechanical grinding. Powd. Metall. Met. Ceram., 53 (5–6), 251–261.
- 49 Gabunia, D.L., Tsagareishvili, O.A., and Chkhartishvili, L.S. (2013) Mechanochemical aspects of grinding of β-boron, in Proc. 4th Int. Conf. “Fundamental Bases of Mechanochemical Technologies” (eds T.P. Shakhtshneider and I.G. Konstanchuk), Novosibirsk State Univ. Publ. Center, Novosibirsk, pp. 231–231.
- 50 Batsikadze, T.A., Gabunia, D.L., Gabunia, V.M., Gigitashvili, T.G., Tsagareishvili, O.A., and Chkhartishvili, L.S. (2015) Obtaining a mixture of boron and boron carbide in an electric arc. Herald Natl. Acad. Sci. Georgia (Ser. Chem.), 41 (1–2), 160–163.
- 51 Calcote, H.F., Gill, R.J., Berman, Ch.H., and Felder, W. (1990) Production and Coating of Pure Boron Powders. Contract N00014-89-C-0246. AeroChem Res. Lab., Inc., Princeton, pp. 48.
- 52 Devener, B. van, Perez, J.P.L., Jankovich, J., and Anderson, S.L. (2009) Oxide-free, catalyst-coated, fuel-soluble, air-stable boron nanopowder as combined combustion catalyst and high energy density fuel. Energy Fuels, 23 (12), 6111–6120.
- 53 Pang, W., Fan, X., Zhang, W., Xu, H., Li, J., Li, Y., Shi, X., and Li, Y. (2011) Application of amorphous boron granulated with hydroxyl-terminated polybutadiene in fuel-rich solid propellant. Propell. Explos. Pyrot., 36 (4), 360–366.
- 54 Alfa Aesar (2013) High-Purity Metals and Materials, Alfa Aesar – A Johnson Matthey Company, p. 18, www.alfa.com.
- 55 Liu, T.-K., Shyu, I.-M., and Hsia, Y.-Sh. (1996) Effect of fluorinated graphite on combustion of boron and boron-based fuel-rich propellants. J. Propul. Power, 12 (1), 26–33.
- 56 Dreizin, E.L., Keil, D.G., Felder, W., and Vicenzi, E.P. (1999) Phase changes in boron ignition and combustion. Combust. Flame, 119 (3), 272–290.
- 57 Pang, W.-Q., Zhang, J.-Q., Hu, S.-Q., Dang, J.-B., and Guo, J.-Y. (2006) The influence of agglomerated boron on burning rate of fuel-rich solid propellant. Chinese J. Explos. Propel., 3, 20–22.
- 58 Demirbas, A. (2008) Energy from boron and non-nuclear metallic fuels. Energ. Source. A, 30 (12), 1108–1113.
- 59 Young, G., Sullivan, K., Zachariah, M.R., and Yu, K. (2009) Combustion characteristics of boron nanoparticles. Combust. Flame, 156 (2), 322–333.
- 60 Gan, Y., Lim, Y.S., and Qiao, L. (2012) Combustion of nanofluid fuels with the addition of boron and iron particles at dilute and dense concentrations. Combust. Flame, 159 (4), 1732–1740.
- 61 Young, G., Stoltz, Ch.A., Mayo, D.H., Roberts, C.W., and Milby, Ch.L. (2013) Combustion behavior of solid fuels based on PTFE/boron mixtures. Combust. Sci. Technol., 185 (8), 1261–1280.
- 62 Xu, H.-X., Pang, W.-Q., Guo, H.-W., Zhao, F.-Q., Wang, Y., and Sun, Zh.-H. (2014) Combustion characteristics and mechanism of boron-based, fuel-rich propellants with agglomerated boron powder. Central Eur. J. Energ. Mater., 11 (4), 575–587.
- 63 Liu, L.-L., He, G.-Q., Wang, Y.-H., and Hu, S.-Q. (2015) Chemical analysis of primary combustion products of boron-based fuel-rich propellants. RSC Adv., 5 (123), 101416–101426.
- 64 Won, J., Choi, S., Lee, W., Kim, J., Hwang, G., and Park, B. (2014) A study of fuel-rich solid propellant characteristic for boron-bead particle size. J. Korean Soc. Propuls. Eng., 18 (5), 12–18.
- 65 Arkhipov, V., Savel'eva, L., and Zolotorev, N. (2015) Effect of aluminum-boron powders mechanical mixtures on the combustion of high-energy materials at subatmospheric pressures. MATEC Web Conf., 23 (01005), 1–4.
- 66 Hussmann, B. and Pfitzner, M. (2010) Extended combustion model for single boron particles. Part I: theory. Combust. Flame, 157 (4), 803–821.
- 67 Hussmann, B. and Pfitzner, M. (2010) Extended combustion model for single boron particles. Part II: validation. Combust. Flame, 157 (4), 822–833.
- 68 Wan'e, W. and Zh, Zuoming. (2012) Calculation for primary combustion characteristics of boron-based fuel-rich propellant based on BP neural network. J. Combustion, 2012 (635190), 1–6.
- 69 Lazaro, B.J. and Lasheras, J.C. (1989) Particle dispersion in a turbulent, plane, free shear-layer. Phys. Fluids., 1, 1035–1044.
- 70 Longmire, E.K. and Eaton, J.K. (1992) Structure of a particle-laden round jet. J. Fluid Mech., 236, 217–257.
- 71 Yang, X., Thomas, N.H., and Guo, L.J. (2000) Particle dispersion in organized vortex structures within turbulent free shear flows. Chem. Eng.. Sci., 55, 1305–1324.
- 72 Katoshevski, D., Dodin, Z., and Ziskind, G. (2005) Aerosol clustering in oscillating flows: mathematical analysis. Atomization Sprays, 15, 401–412.
- 73 Katoshevski, D. (2006) Characteristics of spray grouping/non-grouping behavior. Aerosol Air Qual. Res., 6, 54–66.
- 74 Katoshevski, D., Shakked, T., and Sazhin, S.S. (2010b) Particle grouping in standing and moving wave velocity fields. Int. J. Eng. Syst. Model. Simul., 2, 177–182.
- 75 Sazhin, S., Shakked, T., Sobolev, V., and Katoshevski, D. (2008) Particle grouping in oscillating flows. Eur. J. Mech. B, 27, 131–149.
- 76 Winter, C., Katoshevski, D., Bartholoma, A., and Flemming, B.W. (2007) Grouping dynamics of suspended matter in tidal channels. J. Geophys. Res. Oceans., 112, C08010 (1–9).
- 77 Katoshevski, D., Shakked, T., Sazhin, S.S., Crua, C., and Heikal, M.R. (2008) Grouping and trapping of evaporating droplets in an oscillating gas flow. Int. J. Heat Fluid Fl., 29, 415–426.
- 78 Katoshevski, D., Ruzal, M., Shakked, T., and Sher, E. (2010a) Particle grouping, a new method for reducing emission of submicron particles from diesel engines. Fuel., 89, 2411–2416.
- 79 Ruzal-Mendelevich, M., Katoshevski, D., and Sher, E. (2016) Controlling nanoparticles emission with particle-grouping exhaust-pipe. Fuel. doi: 10.1016/j.fuel.2016.10.113
- 80 Broday, D.M. and Rosenzweig, R. (2011) Deposition of fractal-like soot aggregates in the human respiratory tract. J. Aerosol Sci., 42 (6), 372–386.
- 81 Pilewskie, P. (2007) Climate change: aerosols heat up. Nature, 448, 541–542.
- 82 Swift, D.L. and Friedlander, S.K. (1964) The coagulation of hydrosols by Brownian motion and laminar shear flow. J. Coll. Sci. Imp. U. Tok., 19, 621–647.
- 83 Abouali, O., Nikbakht, A., Ahmadi, G., and Saadabadi, S. (2009) Three-dimensional simulation of Brownian motion of nano-particles in aerodynamic lenses. Aerosol Sci. Technol., 43, 205–215.
- 84 Gupta, D. and Peters, M.H. (1985) A Brownian dynamics simulation of aerosol deposition onto spherical collectors. J. Colloid Interface Sci., 104, 375–389.
- 85 Crowe, C., Sommerfeld, M., and Tsuji, Y. (1998) Multiphase Flows with Droplets and Particles, CRC Press.
- 86 Chandrasekhar, S. (1943) Stochastic problems in physics and astronomy. Rev. Mod. Phys., 15, 1–85.
- 87 Uhlenbeck, G.E. and Ornstein, L.S. (1930) On the theory of the Brownian motion. Phys. Rev. A., 36, 823–841.
- 88 Li, A. and Ahmadi, G. (1992) Dispersion and deposition of spherical-particles from point sources in a turbulent channel flow. Aerosol Sci. Technol., 16, 209–226.
- 89 Ounis, H. and Ahmadi, G. (1990) A comparison of Brownian and turbulent-diffusion. Aerosol Sci. Technol., 13, 47–53.
- 90 Ounis, H., Ahmadi, G., and Mclaughlin, J.B. (1991) Brownian diffusion of submicrometer particles in the viscous sublayer. J. Colloid Interface Sci., 143, 266–277.
- 91 Broday, D.M. and Robinson, R. (2003) Application of cloud dynamics to dosimetry of cigarette smoke particles in the lungs. Aerosol Sci. Technol., 27, 510–527.
- 92 Norris, J.R. (1999) Smoluchowski's coagulation equation: uniqueness, nonuniqueness and a hydrodynamic limit for the stochastic coalescent. Ann. Appl. Probab., 9, 78–109.
- 93 Cho, Kuk, Chung, Kang-Sup, and Biswas, Pratim (2011) Coagulation coefficient of agglomerates with different fractal dimensions. Aerosol Sci. Tech., 45 (6), 740–743.
- 94 Fuchs, N.A. (1964) The Mechanics of Aerosols, Pergamon, New York.
