23
Engineered Nanoparticles' Toxicity: Environmental Aspects
Neetu Talreja1 and Dinesh Kumar2
1Indian Institute of Technology Kanpur, Centre for Environmental Science and Engineering, Kanpur, Uttar Pradesh 208016, India
2Central University of Gujarat, School of Chemical Sciences, Gandhinagar, Gujarat 382030, India
23.1 Introduction
Nanotechnology and emerging nanomaterials (ENMs) are rapidly developing an intriguing area of research, globally. Nanoparticles or ENMs have a size less than 100 nm and might be zero-dimensional (0D), one- (1D), two- (2D), or three-dimensional (3D) [1–3]. These engineering nanoparticles (ENPs) are being spotlighted as a major concern for rapidly growing industries. ENMs are generated by the fabrication of matter at a nanometric scale. These nanosized range particles have unique physiochemical characteristics such as high surface to volume ratio, small size, and possess more active sites for interaction. Therefore, ENPs are used in various applications such as environmental remediation, energy storage devices, catalyst, sensor, and biological applications including drug delivery system, clinical diagnosis and therapy, food safety, and agricultural applications. The high surface area and more reactive sites of the ENPs make them a suitable candidate for the removal of environmental contaminants such as heavy metal ions, pesticides, pharmaceutical contaminants, radioactive elements, volatile organic compounds, and biological contaminants. These ENPs show anticancerous and antioxidant properties, enhance proliferation of various cell lines, increase immunity, and have antibacterial activity against various multidrug-resistant and extensively drug-resistant bacterial strains; therefore, they can be effectively used in various biomedical applications [1–10]. On the other hand, ENPs also augment seed germination, various photosynthetic pigments, protein content, and plant growth. ENPs also have interesting photocatalytic applications because of their efficient catalytic properties in the degradation of several environmental contaminants, including volatile organic compounds, pesticides, and dyes. ENPs are effectively used in the generation of fuels, including hydrogen production, and generation of electricity [3,11–13].
Despite various positive aspects of ENPs, there are still concerns regarding their adverse effect on environment, agriculture, and human health. ENPs are largely responsible for contamination of the environment at their end applications due to the interaction with dissolved organic matter, multivalent cations, aggregation, transportation ability, and chemical transformation, which leads to heterogeneity by their size, shape, and biological functions. The hysterical release of nanomaterials into the environmental system has raised concerns regarding toxicity of these nanomaterials [2–9]. Various studies suggest that these nanomaterials have an adverse impact on both the environment and the human health. The risk of nanomaterials might be through inhalation and ingestion of water. Therefore, ENPs possess potential risks to biotic and abiotic flora and fauna. The environmental system is active and undergoes various physiochemical changes by using ENPs and consequent reactions in the system [5], which are complicated, and it is difficult to understand the possible risks associated with the release of ENPs. Several organisms in the aquatic system or salt water are important to maintain balance in the environmental system. Interpretation of any adverse effect on microbial species will undoubtedly increase our understanding of ENPs' effects and help develop a useful monitoring tool [14].
In general, environmental applications of ENPs are indispensable to understanding the hazardous effects on human and plants [15]. We lack knowledge of different types of ENPs' transformation and toxicity in environmental and biological systems [16,17]. This chapter emphasizes various synthesis routes of ENPs, their applications, and their adverse environmental and health effects. This chapter gives a fast, forward thinking approach for the synthesis of nanomaterials to strike a balance between the advancement in the nanotechnology with their applications and the safety issues.
23.2 Distribution of Nanoparticles Based on Composition
Nanostructured materials are classified into four types: (1) zero-dimensional such as fullerene and quantum dots; (2) one-dimensional, mainly multilayers; (3) two-dimensional, mainly ultrafine layers; and (4) three-dimensional nanomaterials, mainly crystal structures. Nanomaterials are of different compositions such as purely metal-based, carbon-based, and hybrid nanomaterials.
23.2.1 Metal-Based Nanomaterials
Metal-based nanomaterials include metals, metal sulfide, metal oxides, and quantum dots. Metal-based nanomaterials are widely used in various end applications and there are several consumer products already in use. On the other hand, these metal-based nanomaterials are also synthesized to produce energy and other newer applications.
23.2.2 Carbon-Based Nanomaterials
Carbon-based nanomaterials are an important part of nanomaterials as they provide an intriguing research platform. Several carbon-based nanomaterials have been synthesized such as fullerenes, graphene, carbon nanotubes, carbon nanocones, carbon nanofibers, carbon nanohorn, and nanodiamonds. The unique properties of carbon-based nanomaterials allow interaction with organic molecules through hydrogen bonding, hydrophobic interaction, and electrostatic forces. These nanomaterials are composed mostly of carbon, most commonly taking the form of hollow spheres, ellipsoids, or tubes. Spherical and ellipsoidal carbon nanomaterials are referred to as fullerenes, while cylindrical ones are called nanotubes. These particles have many potential applications, including improved films and coatings, stronger and lighter materials, and applications in electronics [18–21].
23.2.3 Hybrid Nanomaterials
Hybrid nanomaterials composed of both organic and inorganic constituents provide promising platforms for various biological applications including drug delivery system, bioimaging, disease diagnosis, and therapeutic applications. Hybrid nanomaterials are developing into a newer class of nanomaterials that can not only provide beneficial characteristics of both components (organic and inorganic) but also tune up the properties of hybrid materials by adjusting ratio or combination of desired functional groups.
23.3 Common Methods of Engineering of Nanoparticles
23.3.1 Gas Condensation
One of the widely used old methods is gas condensation. The process involves a thermal source (usually high-pressure electron beam and heat refractory) to vaporize metallic or inorganic materials. Gas molecules collide with evaporated atoms due to high pressure (3 MPa) to subsequently form ultrafine particles. However, the gas condensation process for synthesizing nanomaterials is slow and has some limitations of the incompatibility of source precursor, temperature ranges, and different rates of evaporation in an alloy.
23.3.2 Chemical Precipitation
The chemical precipitation is an intriguing route of synthesis of nanomaterials. The method provides a controlled size of nanomaterials due to the arrested precipitation. This method is a simple process to develop and study nanomaterials within the colloidal system, which avoids the aggregation and physical changes in the solution. Sharma and Ghose synthesized mixed metal oxide using the precipitation method [22]. The synthesis process involves a reaction between the solvent and the essential materials. The doping material was also mixed with the parent solution at the incipient time of precipitation reaction, while surfactant was used to form the dispersion of the particles or avoid agglomeration, thereby allowing to easily isolate nanocrystals by using centrifugation, which were subsequently vacuum dried. The vacuum-dried nanomaterial was exposed to UV treatment for the polymerization of surfactant with nanocluster.
23.3.3 Sol–Gel Techniques
The sol–gel process has also been widely used for the formation of silica, glass, and ceramic materials. It involves the formation of the network through the polycondensation process. Network building takes place by agglomeration of colloidal particles, which are much larger in size than normal molecules or nanoparticles. However, upon mixing with liquid colloids appear bulky, whereas the nanosized molecules always look clear.
Silica nanoparticles are formed by the process involving hydrolysis and polycondensation of metal alkoxides (Si(OR)4) such as tetraethylorthosilicate (Si(OC2H5)4) or inorganic salts such as sodium silicate (Na2SiO3) in the presence of a catalyst, mainly a mineral acid (e.g., HCl) or a base (e.g., NH3). Alkoxides are immiscible in water [23]. The other organometallic precursors are aluminum, titanium, zirconium, and many others. As mentioned, the process is initiated with a homogeneous solution of alkoxides. A catalyst is used to start the reaction and control the pH. There are four stages in the sol–gel process:
- Hydrolysis
- Condensation
- Growth of particles
- Agglomeration of particles
23.3.4 Electrodeposition
The electrodeposition method is simple and widely used for the preparation of 1D nanomaterials. The nanomaterials prepared by electrodeposition have high mechanical strength and are uniform and strong film-like materials. Electrodeposition of metals can be achieved by using either two separate electrolytes or much more conveniently from one electrolyte by an appropriate control of agitation and electrical conditions.
23.3.5 Chemical Vapor Deposition
Chemical vapor deposition (CVD) involves deposition of hydrocarbon on a solid surface in the presence of a catalyst under a high-temperature condition. Gaseous or liquid hydrocarbons such as benzene, acetylene, methane, and so on burn at high temperatures and get deposited over the substrate. Various kinds CVD methods exist such as thermal CVD, low-pressure CVD, laser CVD, photolaser CVD, and plasma-enhanced CVD. In thermal CVD, the reaction is activated by using a high temperature above 600 °C. In plasma CVD, the reaction is a low-temperature process in which nanostructure preparation is activated by plasma at temperature ranging between 300 and 600 °C. In laser CVD, when an absorbing surface is heated by laser, thermal energy causes pyrolysis to occurr. In photolaser CVD, ultraviolet radiation is the source of inducing a chemical reaction, to break the chemical bond in the reactant molecules. In this process, the reaction is photon activated and deposition occurs at room temperature. The process provides a uniform growth of the nanostructure on commercial scale. This method is used for the bulk preparation of nanomaterials having high strength and overall good quality [9–16].
23.4 Toxicity Based on Physicochemical Properties of NPs
23.4.1 Size
The size of nanoparticles is the major factor influencing the toxicity. Depending on particle size and surface structure, nanoparticles penetrate the cell through various pathways, such as phagocytosis and pinocytosis. Nanoscale structure has a very large surface curvature to provide necessary conformational rigidity to allow for multivalent binding with receptors, thereby making it a very active material from toxicity point of view, as very small nanomaterials (diameter ≥6 nm) are difficult to excrete by the kidneys and accumulate in specific organs such as the liver and spleen. These accumulated nanoparticles cause serious side effects. Hence, it can be concluded that size is an important factor for determining the toxicity as shown in Figure 23.1 [24–33].
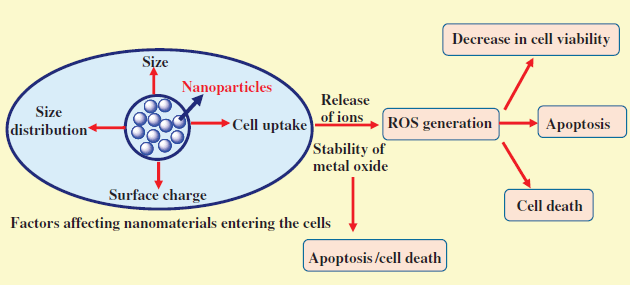
Figure 23.1 Factors influencing the toxicity of nanoparticles.
23.4.2 Surface Area
A common concept is that by decreasing the particle size, surface area increases and hence biological reactivity increases. A high surface area may cause higher reactivity with nearby particles, but it also results in possibly harmful effects when used in fillers, cosmetics, and as drug carriers. It can be said that by decreasing the particle size, its biological activity increases substantially. As the size of particle decreases, the volume occupied by particle also decreases, resulting in increased pathophysiological toxicity mechanisms such as oxidative stress, reactive oxygen species (ROS) generation, mitochondrial perturbation, and so on. It is presumed that the size of the nanoparticle alone may not be responsible for toxicity, but the total number per unit volume may be important. Several human disorders such as pulmonary toxicity may be induced by ultrafine and fine materials having a large surface area as shown in Figure 23.1 [34–39].
23.4.3 Surface Electrostatic Status
Despite the size and surface area, one important factor, that is, electrostatic status, also plays a crucial role in the toxicity of nanoparticles. Due to their small size, nanoparticles are usually used as a drug carrier via either passive or active transport. External properties of surface electronic status are critical to cellular uptake and may also be involved in cytotoxicity. Traditionally, surface charge such as cations and anions play a significant role. Higher uptake efficiency in a cell is achieved by replacing the surface functional moiety, inducing sudden changes in particles' surface charge. Changes in surface charge result in considerable differences in in vivo biodistribution of nanoparticles. Particles show different degrees of toxicity depending on their surface charges. Nanoparticles with a positively charged surface tend to have much higher toxicity than the negatively charged nanoparticles. The higher numeric value is also responsible for significant damage as shown in Figure 23.1 [39–41].
23.4.4 Morphology
Morphology is also a big issue in the nanomaterials that plays an important role in cytotoxicology. Like other well-established inhalable fibers (e.g., asbestos), nanoscale fibers (e.g., carbon nanotubes) are reported to have a serious risk of lung inflammation. Furthermore, prolonged exposure may cause several cancers. It is difficult to determine whether there is a certain toxic effect of single nanotubes or an ensemble of such tubes. Some studies have shown that carbon nanotubes are more toxic than other ultrafine carbon black or silica dust. It is also known that in industry workers directly exposed to single-walled carbon nanotubes beyond the permissible exposure limit lung lesions developed. Interestingly, CNTs have been shown to cause death of targeted kidney cells via inhibiting cell growth induced by decreased cell adhesiveness. Fullerene, a zero-dimensional material, is a well-known agent causing severe lung cancer in human beings, destruction of fish brain, and death of water flea as shown in Figure 23.1.
23.4.5 Agglomeration Status
Besides the other physiochemical properties, agglomeration of nanoparticles inside the human body results in potential toxicity. Severe chronic diseases such as fibrosis and cancer are attributed to higher exposure to nanomaterials [41,42].
23.5 Toxicity of Some Widely Used ENPs to Environmental Organisms
The unintentional exposure of ENPs in the environment occurs via different routes, including solid and liquid waste from domestic sources and industrial activity, accidental spillage, and atmospheric emissions. Other sources of ENPs that need to be assessed are those consequences of the use of consumer products that contain ENPs (e.g., sunscreens and paints). These pathways may allow ENPs to disperse throughout the environment. Some published studies have focused on the fate of ENPs in the microbial community of aquatic and terrestrial habitats [43]. As is known, water is the ultimate source of transport of ENPs into the ecosystem. In natural water ecosystems, water travels over the surface of the land, through ground or underground, passing through different environmental conditions such as increasing salinity, ionic concentration, and differences in pH. During this phenomenon, ENPs are transformed and accumulated in very toxic ways and form colloidal suspensions by association with substances through the process of self-aggregation and appear in a different form that can be harmful to animals or humans. Other factors such as chemical perturbations, for example, ionic strength, temperature, and pH, also affect the behavior of ENPs. ENPs have adsorption properties due to high specific surface area and binding constants, but they tend to separate out from the water column by differences in settling velocity, resulting in transfer of chemicals (e.g., metals and polymers) into the water bodies, where the ENPs can also be ingested by organisms. They are also degraded by the biotic and abiotic factors in soil and transported to water bodies through runoff, leaching, and drain flow, and hence colloidal surface properties are key aspects for tracking the fate and behavior of ENPs in the environment [33–39].
23.5.1 Toxicity of Metal Nanoparticles
Nanotechnology and nanobiotechnology are emerging areas of research, which have potential for wider end applications. Several nanomaterials such as metal nanoparticles, carbon-based hybrid nanomaterials, and polymeric nanoparticles are used in various applications. Among all of them, metal-based nanoparticles have drawn increasing attention toward various applications, mainly environmental remediation, and biological applications including drug targeting, regeneration of tissues, nanomedicine, cells/tissue targeting, and as plant growth stimulants. Despite these useful applications, ENPs also cause toxic effects on both terrestrial and aquatic life [44,45].
Several metal nanoparticles and their oxides are used in end applications, but they demonstrate toxic response against human health. For instance, copper (CU) is a well-known antibacterial agent and is also used as an additive in lubricants, metallic coating, and ink. Different physiological properties are associated with this cytotoxicity. Several studies have reported that the Cu is highly toxic against various bacterial strains. Another study showed the toxic effect of CuO on the freshwater insects, which suggests that CuO is toxic at higher concentrations. On the other hand, Cu nanoparticles exhibit a toxic effect on mammalian cells and any aquatic organism [46]. The toxicity of CuO NPs, core–shell CuO NPs, and ionic copper was determined in the aquatic macrophyte Lemna gibba. This study suggested that polymeric coating changes surface characteristics of materials, which affects the mechanism of toxicity. Some studies also reported that the metal nanoparticles also change the enzymatic activity of soil [47].
The zinc NPs also exhibit antibacterial effects and are used in various applications, such as sun creams, and lotions and have medicinal applications such as dental filling materials and wound healing. The numerous applications of Zn are finally reaching the environment system inadvertently. Several studies have been carried out on the toxicity of ZnO nanoparticles [45], suggesting that Zn nanoparticles eventually pose a hazard to fauna, flora, and humans. Therefore, it is necessary to distinguish their outcomes in the ecosystem and their biocompatibility with biological systems. Nations et al. suggested that nano-ZnO suppresses the growth of Xenopus laevis and decreases the food sources of algae [48]. The study also reported that the release of Zn nanoparticles at higher concentrations into aquatic ecosystems causes detrimental effects mainly on the health of amphibians. The exposure significantly increased the mortality in zebrafish. Several studies also suggested that Zn nanoparticles decrease the seed germination rate and development of root. Lin and Xing observed that ZnO nanoparticles might be translocated from apoplast and protoplast via endodermis of root surface [49]. ZnO shows negative impacts on the growth of the plant, bioaccumulation, and antioxidative enzyme activity in Brassica juncea [50]. Yoon et al. also observed similar trends on soybean [51]. It was observed that ZnO nanoparticles unfavorably influenced the various developmental stages of soybean plants. Some study also suggested that ZnO nanoparticles showed to be the most toxic nanoparticles compared to other metal oxides. On the other hand, ZnO was noncytotoxic to human dermal fibroblasts. ZnO nanoparticles cause membrane damage in bacterial strains because of the generation of oxidative stress. Several studies show that it is not necessary that metal nanoparticles enter the cells and damage cellular membrane. Solubilization of metal nanoparticles also causes the toxicity of cell lines. The cytotoxic effects of ZnO nanoparticles on mouse dermal fibroblast cells and human periodontal ligament fibroblast cells were also observed. Bradfield et al. studied the effect of Zn and Cu NPs on sweet potato and observed an adverse impact on the yield at the higher exposure concentrations of these metal ions [52].
Ag nanoparticles are known as antibacterial agent and possess the highest antibacterial activity among all metallic nanoparticles due to their large surface area and strong oxidizing ability; therefore, they are extensively used in various products such as creams, textiles, surgical prosthesis, and cosmetics, and as bactericides in fabrics. Ag nanoparticles also inhibit various enzymatic pathways, block the transcription of DNA, and the production of adenosine triphosphate (ATP) [53]. Ag NPs react with proteins that interact with –SH groups leading to the activation of proteins and interruption of respiration of bacteria. Ag NPs cause damage to membrane integrity, cell cycle arrest, ROS generation, disruption of energy metabolism, and gene transcription [54]. Their wider end applications have led to their by-products to have emerged as a source of contamination of the environment. Ag NPs inhibit the viability of human epidermal keratinocyte. They have also exhibited phytotoxicity against Phaseolus radiates and sorghum bicolor crop plants. The study suggested that Ag inhibited the growth of seedlings. Another study suggested that Ag nanoparticles decrease the growth of seedlings, reduction in root elongation, and total chlorophyll and carotenoid content of Oryza sativa L. Ag nanoparticles stimulate modification in root tip [55].
Another study demonstrates the toxicity of citrate (cit-Ag NPs) or humic acid (HA-Ag NPs) capped silver nanoparticles (Ag NPs) and Ag on exposure of eggs, larvae, juveniles, and adults of Platynereis dumerilii. The data suggested that cit-Ag NPs and HA-Ag NPs were found to be more toxic than Ag. Ag nanoparticles were also found to have cytotoxic effects on fish catla and Labeo rohita. Ag nanoparticles might inhibit the erythrocyte acetylcholinesterase activity and Na+/K+-ATPase in adult zebrafish (Danio rerio).
Besides above-mentioned studies on ENPs, some other studies have also reported toxicity. Fent et al. demonstrated the toxic effects of fluorescent silica nanoparticles (FSNPs) on early life stages of zebrafish [56]. It was observed that the ∼60 and ∼200 nm-sized FSNPs were adsorbed on the chorion of eggs. Zhou et al. reported the toxicity of Ni NPs on aquatic life. Nickel has been found to induce oxidative damage in the benthic copepod Tigriopus japonicas [57].
23.5.2 Toxicity of Polymeric Nanoparticles
The toxicity evaluation of inorganic NPs or biodegradable nanomaterials considered safe is limited. Polymeric nanoparticles such as poly-D,L-lactide-co-glycolide, polylactic acid, poly-caprolactone, gelatin, chitosan, and poly-alkyl-cyano-acrylates (PAC), poly(ethyl)cyanoacrylate are widely used in drug or gene delivery system as a carrier. Several studies suggested that chitosan-based nanoparticles had adverse effect on the nucleus of cells and are responsible for autophagic or necrotic cell death. The cell death might be caused by damaged cellular membrane, thereby causing leakage of the various cellular enzymes [58]. The polymeric nanoparticles also increase the rate of cell death, generation of reactive oxygen species, and overexpression of heat shock protein that causes physiological stress. Research on polymeric nanoparticles suggests that toxicological impacts of biodegradable nanoparticles on specific cell lines must be determined, particularly when they are used in vivo.
In general, metal nanoparticles possibly cause antagonistic effects on human health, mainly organ, tissue, cellular and subcellular, and protein levels because of their unique properties. Metal nanoparticles are drawing increasing interest because of their potential applications in different fields such as biomedical, industrial, military, and consumer products. However, they might interfere with antioxidant mechanism leading to the generation of reactive oxygen species, stimulating an inflammatory response, destroying mitochondria, and subsequently causing cell death. Therefore, overcoming the toxicological issues associated with nanoparticles is a serious concern.
23.6 Effect of ENP Toxicity on Plants
Nanotechnology might be finding a newer application in agricultural biotechnology, but plant tissues are under continual threat of toxicity associated with engineered nanoparticles causing various types of abiotic stresses. ENP toxicity is a major environmental concern as it restricts plant growth. Plants absorb nanoparticles as essential elements from the soil. Agrochemicals and pesticides are usually applied to crops by spraying, with a low concentration of chemicals that are below the minimum effective concentration required for the crops. The main drawbacks of such agrochemicals are mainly microbial degradation, photolysis, hydrolysis, and leaching. Frequent uses are required for effective control that causes undesirable effects on the environmental system [59–63]. Therefore, researchers continue to develop such nanomaterials as not only combat these issues but also augment the growth of crops. In this context, several nanomaterials have been synthesized that are effectively used in agricultural applications, to enhance germination of seed, growth of plants, and subsequently crop yields, but unintentionally they also act as a toxic source. Plants possibly offer a path for the transportation of ENPs. Several studies have been done on the effects of ENPs such as Cu, Zn, Au, Mg, Fe, and their oxides and carbon-based materials such as CNTs, CNFs, graphene, and fullerenes, which augment the growth of plants, as well as their toxicological aspects. The cell wall of plants acts as a barrier for any external agent. The pore diameter of cell wall ranges from 5 to 20 nm, and pores of plasmodesmata range 50–60 nm; so, only ENPs with less than 20 nm easily translocate from the cell wall to the plasma membrane through these pores and those with less than 60 nm size easily translocate from plasmodesmata, while less than 500 nm ENPs translocate through plastic and symplastic routes of plants [64–67]. Based on the above discussion, the size of ENPs is an important factor for translocation ability. On the other hand, surface charge of the ENPs is also an important parameter for translocation, as plant surface is negatively charged. The negatively charged surface ENPs easily translocate within the plant, while positively charged surface ENPs accumulate on the root surface and show an adverse effect. The translocation of ENPs in plants occurs by two different ways: root to shoot to leaf/fruit or leaf to shoot to root pathways [68–74]. These translocation pathways show increase or decrease in various physiological processes such as seed germination, root or shoot elongation, and water content. Besides, they can regulate up and down several chemical processes, including antioxidant activities of enzymes, H2O2 level, and lipid peroxidation, agronomic parameters (such as micro/macro element values, food quality, etc.), and photosynthetic activities such as the content of the chlorophyll, the number of chloroplasts, and the rate of photosynthesis. Therefore, toxicological determination of these ENPs is a major concern nowadays [75–79].
The germination of seeds and seedling growth are frequently measured to determine the effect of ENPs. However, researchers do not consider it as an appropriate parameter of phytotoxicity. Several studies suggested conflicting results on phytotoxicity of metal and carbon-based nanomaterials in plants. Inconstancy may arise due to the poor dispersion, agglomeration, and robust release. Functionalized CNTs with the different functional groups and nonfunctionalized CNTs exposed to different crops (cabbage, Brassica oleracea; carrot, Daucus carota; cucumber, Cucumis sativus; lettuce, Lactuca sativa; onion, Allium cepa; and tomato, Solanum lycopersicum) determine their toxicity. CNTs increase root growth in onion and cucumber, while cabbage and carrot did not show any significant effect upon exposure. The growth of roots decreases with exposure to CNTs. However, some studies also suggested that germination of seed and plant growth increase with exposure to CNTs. Similarly, nano-Cu and nano-ZnO have some positive and some negative effects on plant growth.
In general, ENPs are potentially able to translocate from root to shoot to a leaf or vice versa. The translocation ability of these ENPs is due to surface charge and size. The toxicity of ENPs mainly depends on the dispersion in the media, soil, and plant species.
23.7 Effect of ENP Toxicity on Humans
There are two perceptions of the development of engineering nanoparticles: (1) One is based on technological innovations for different applications. (2) The other is potential health hazards related to their wide applicability. Several nanoparticles such as silver (Ag), copper (Cu), zinc (Zn), nickel (Ni), magnesium (Mg), and cobalt (Co) have various applications and health hazards. Their cytotoxicity is based on solubility, size, shape, and surface charge of the prepared nanomaterials. Graff et al. [80] reported the size-dependent toxic effect of Ag nanoparticles that are widely used in various biomedical applications such as wound dressings, catheters, nanoantibiotics, and various household products due to their antimicrobial activity. Toxicity of Ag nanoparticles has been studied in different cell lines suggesting that silver nanoparticles' toxicity is mediated by the stimulation of a signaling cascade that generates ROS and inflammatory markers, cause mitochondrial dysfunction, and subsequently induce apoptosis [80]. Chuang et al. investigated the effect of ZnO nanoparticles on human coronary artery endothelial cells [81]. In the study, the human coronary artery endothelial cells were exposed to different sizes of ZnO nanoparticles to ascertain the viability of cells and regulation of cardiovascular disease-related genes. Exposure of cell lines to zinc oxide NPs resulted in decreased cell viability and increased levels of NO. Armand et al. reported the toxicological effect of TiO2 nanoparticles on epithelial alveolar cells. They suggested that ultrafine particles (less than 2.5 µm in diameter) induce oxidative stress, inflammation, genotoxicity, apoptosis, and cytotoxicity [82]. In short, a long-term exposure to Ti-NPs alters cell cycle progression and genome segregation during mitosis due to interference with mitotic spindle assembly and centrosome maturation. It causes ROS accumulation in exposed cells as well as chromosomal instability and cell transformation. MgO nanoparticles were used as a food additive (E530), ceramic material, fire retardant, and corrosion inhibitor. Ge et al. reported that MgO nanoparticles significantly triggered the NO release of the human umbilical vein endothelial cells [83]. Occupational exposure to cobalt can lead to various diseases, such as interstitial pneumonitis, fibrosis, and asthma. The study reported that cobalt nanoparticles induce genotoxicity in humans. In addition, cobalt/tungsten carbide composite exhibited the synergistic effect of ROS generation and induced DNA fragmentation. ENPs reduce the cell viability, trigger cellular damage, and may lead to mitochondrial membrane potential depletion and ROS generation. These ENPs also cause cell cycle distribution and modulate the expression of antioxidant enzymes and stress response through cellular morphological damage.
In general, ENPs are beneficial to humans and have been developed into different therapeutic applications. However, the risks to human health associated with ENPs might be significantly higher. Therefore, a cytotoxic profiling of ENPs is necessary before they are used in human health application.
23.8 Metal Toxicity Mechanism
23.8.1 Microbial Cells
The mode of action of the metal nanoparticles that inhibit microbial cells is not clearly recognized. However, there are several mechanistic theories on the mode of action that causes antimicrobial effects on microorganisms [84–87]. These toxicity theories of metal may not be excluded; it is assumed that the toxic effect of metal nanoparticles is because of the combination of various mechanisms and the surface chemistry of metals. Figure 23.2 shows the schematic representation of toxicity mechanism. Depending upon the behavior of metal nanoparticles, there are few mechanistic theories described as follows:
- Protein dysfunction:
The amino acid residue in a protein is susceptible to metal-catalyzed oxidation. The oxidation of amino acid might cause the loss of catalytic activity that triggers the degradation of protein, thereby causing metal-catalyzed damage at a specific site to a cellular protein, which is the main cause of metal toxicity. The mononuclear metalloenzymes depend on the metal ions for their structure, function, and binding affinity. Mononuclear enzymes such as peptide deformylase (PDF) (PDF), threonine dehydrogenase (Tdh) (Tdh), and cytosine deaminase are sensitive to Fenton chemistry. The PDF and Tdh use cysteine residue to coordinate metal ions and this residue is oxidized by ROS generation. The cell increases the uptake of metal. The ability to cause displacement of these metalloenzymes [87–90] results in dysfunction of protein, activation of various enzymatic processes, and subsequent cell death.
- Production of ROS and antioxidant depletion:
Several studies reported that toxicity is associated with the production of ROS and antioxidant depletion. The addition of hydrogen peroxide or exogenous agents that initiate the generation of superoxide is able to cause damage to DNA and inhibit the various enzymatic activities resulting in cell death. The metal-catalyzed oxidation increases the reduced form of oxygen, such as hydrogen peroxide and superoxide, within the microorganism; this process is known as Fenton chemistry. The redox cycling of metal possibly consumes antioxidants present in the cells [85,86,91].
- Membrane damage:
The cellular membrane of microorganism possesses highly electronegative polymeric chemical groups, which have sorption site of metal cations due to its ability to coordinate metals. The membrane of microorganism might be damaged by exposure to toxic doses. NADH-quinone oxidoreductase (NQR) is part of a respiratory chain of some microorganisms that translocates Na+ and inhibits the NQR activity, thereby altering the membrane potential and causing membrane leakage or damage [84,89,92–94].
- Nutrient uptake interference:
Toxicity of metals is also associated with the growth arrest induced by starvation. Metals inhibit the uptake of sulfate and intracellular sulfur metabolites and induce an enzymatic response [86,89,91–94]. As a result, there is a decrease in the expression of genes and death of cells.
- Genotoxicity:
The lethal DNA damage can be catalyzed by metal-mediated Fenton chemistry. The mutation disrupts homeostasis that increases the amount of metals within cells that increases DNA damage and cause cell death [84,95].
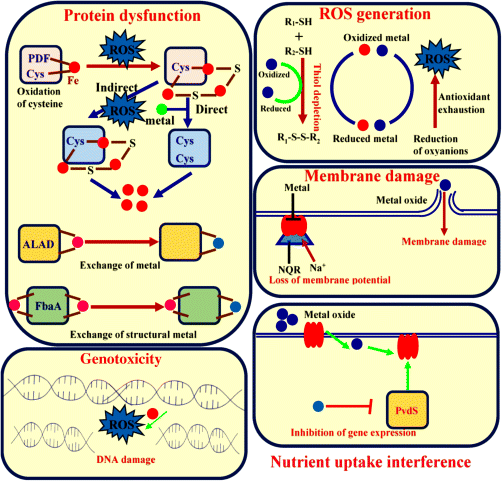
Figure 23.2 Various toxicity mechanisms of metals against microbial cells.
23.8.2 Other Cells
The cytotoxicity of metals in other mammalian cells is also quite like the microbial cells. However, these metals show different cytotoxicity against microbial and mammalian cells because of different metalloproteins and transport system [95–97].
Metals in mammalian cells might interact with membrane proteins that activate the signaling pathways, thereby inhibiting the proliferation of cells. The metal particles might penetrate through diffusion of cells or endocytosis, which leads to dysfunction of mitochondria, production of ROS, protein damage, and subsequent cell death. The generation of reactive oxygen species is the main step of toxicity mechanism of metals for both nano and ionic forms. In this context, we explore the toxicity mechanism that is associated with the generation of ROS [92,94,95,97].
The mitochondria are most sensitive to metals. Mitochondrial cells synthesize ATP by reduction of oxygen to water through electron transfer reaction, thereby generating ROS. ROS play key roles in various processes, mainly cell signaling, and inducing mitogenic responses. However, ROS can also be generated by other biological responses. The introduction of metal particles enhances the ROS formation and leads to overproduction of ROS causing disruption in the cells function, DNA damage, initiation of lipid peroxidation, activation redox transcription factors, and variation in the inflammatory response of signal transduction process, which can lead to cell death and genotoxic effects as shown in Figure 23.3 [89,96,97].
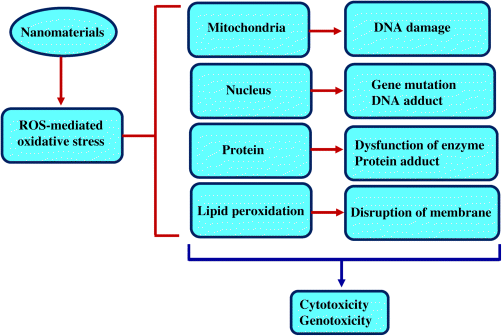
Figure 23.3 Reactive oxygen species-associated toxicity of metals in mammalian cells.
In general, metals are cytotoxic to all types of cells at higher concentrations. However, some metals show antibacterial effects without any adverse effects on the eukaryotic cells because of the presence of different metalloproteins and metal transport systems. Therefore, metal discriminates between microorganism and mammalian targets. Moreover, the cytotoxicity to human beings is prohibited by changes in the administrator routes of nanomaterials.
23.9 Conclusions and Future Perspective
The major reason behind the environmental toxicity is the increasing environmental damage due to increasing population and industrialization. The beginning of environmental remediation, using smarter engineered ENPs, can deliver rapid, cost-effective, and time-saving in situ cleaning procedures for large contaminated sites. Production of a variety of nanoparticles at immensely large scale and their utilization for the benefit of humans will conclusively lead to their entrance into the environment by direct or indirect processes such as disposal, accumulation, and so on. The production, utilization, and disposal of manufactured nanoparticles will inevitably lead to unintentional discharge into air, soil, and aquatic systems, putting the environment at potential risks. With a rapid advancement of this technique, proper evaluation needs to be done to prevent any potential environmental or ecological hazards. This chapter reviewed the complete understanding of ENPs including their synthesis, types, and potential harm associated with nanoparticles, to take the concern a step forward for regulatory models to be established for the safety of engineered nanoparticles.
Acknowledgment
We thank the Ministry of Science and Technology and Department of Science and Technology, Government of India, under the scheme of Establishment of Women Technology Park, for providing the necessary financial support to carry out this study vide letter No. F. No SEED/WTP/063/2014.
References
- 1 Parka, C.M., Chua, K.H., Heo, J., Herb, N., Jang, M., Sond, A., and Yoon, Y. (2016) Environmental behavior of engineered nanomaterials in porous media: a review. J. Hazard. Mater., 309, 133–150.
- 2 Topuz, E. and van Gestel, Cornelis A.M. (2016) An approach for environmental risk assessment of engineered nanomaterials using analytical hierarchy process (AHP) and fuzzy inference rules. Environ. Int., 92–93, 334–347.
- 3 Tiwari, J.N., Tiwari, R.N., and Kim, K.S. (2012) Zero-dimensional, one-dimensional, two-dimensional and three-dimensional nanostructured materials for advanced electrochemical energy devices. Prog. Mater. Sci., 57, 724–803.
- 4 Radad, K., Al-Shraim, M., Moldzio, R., and Rauschc, W.D. (2012) Recent advances in benefits and hazards of engineered nanoparticles. Environ. Toxicol. Pharmacol., 34, 661–672.
- 5 Fernandez, L., Fernandez, A., and Blasco, J. (2012) Nanoecotoxicity effects of engineered silver and gold nanoparticles in aquatic organisms. Trends Anal. Chem., 32, 40–59.
- 6 Ashfaq, M., Verma, N., and Khan, S. (2016) Copper/zinc bimetal nanoparticles-dispersed carbon nanofibers: a novel potential antibiotic material. Mater. Sci. Eng. C, 59, 938–947.
- 7 Ashfaq, M., Singh, S., Sharma, A., and Verma, N. (2013) Cytotoxic evaluation of the hierarchical web of carbon micronanofibers. Ind. Eng. Chem. Res., 52, 4672–4682.
- 8 Ashfaq, M., Khan, S., and Verma, N. (2014) Synthesis of PVA-CAP-based biomaterial in situ dispersed with Cu nanoparticles and carbon micro-nanofibers for antibiotic drug delivery applications. Biochem. Eng. J., 90, 79–89.
- 9 Talreja, N., Verma, N., and Kumar, D. (2016) Carbon bead-supported ethylene diamine-functionalized carbon nanofibers: an excellent adsorbent for salicyclic acid. Clean Soil Air Water, 44 (9999), 1–10.
- 10 Talreja, N., Verma, N., and Kumar, D. (2014) Removal of hexavalent chromium from water using Fe-grown carbon nanofibers containing porous carbon microbeads. J. Water Process Eng., 3, 34–45.
- 11 Khare, P., Talreja, N., Deva, D., Sharma, A., and Verma, N. (2013) Carbon nanofibers containing metal-doped porous carbon beads for environmental remediation applications. Chem. Eng. J., 229, 72–81.
- 12 Saraswat, R., Talreja, N., Deva, D., Sankararamakrisnan, N., Sharma, A., and Verma, N. (2012) Development of novel in-situ nickel-doped, phenolic resin-based micro-nanoactivated carbon adsorbents for the removal of vitamin B-12. Chem. Eng. J., 197, 250–260.
- 13 Kumar, V., Talreja, N., Deva, D., Sankararamakrishnan, N., Sharma, A., and Verma, N. (2011) Development of bi-metal doped micro and nano multi-functional polymeric adsorbent for the removal of fluoride and arsenic in waste-water. Desalination., 282, 27–38.
- 14 Patil, S.S., Shedbalkar, U.U., Truskewycz, A., Chopaded, B.A., and Ball, A.S. (2016) Nanoparticles for environmental clean-up: a review of potential risks and emerging solutions. Environ. Technol. Innovation, 5, 10–21.
- 15 Srivastava, V., Gusain, D., and Sharma, Y.C. (2105) Critical review on the toxicity of some widely used engineered nanoparticles. Ind. Eng. Chem. Res., 54, 6209–6233.
- 16 Zaytseva, O. and Neumann, G. (2016) Carbon nanomaterials: production, impact on plant development, agricultural and environmental applications. Chem. Biol. Technol. Agr., 3, 17.
- 17 Du, W., Tan, W., Videa, J.R.P., Torresdey, J.L.G., Ji, R., Yin, Y., and Guo, H. (2016) Interaction of metal oxide nanoparticles with higher terrestrial plants: physiological and biochemical aspects. Plant Physiol. Biochem., 110, 1–16.
- 18 Canas, J.E., Long, M., Nations, S., Vadan, R., Dai, L., Luo, M., Ambikapathi, R., Lee, E.H., and Olszyk, O. (2008) Effects of functionalized and non- functionalized single-walled carbon nanotubes on root elongation of select crop species. Environ. Toxicol. Chem., 27, 1922–1931.
- 19 Khodakovskaya, M. (2009) Carbon nanotubes are able to penetrate plant seed coat and dramatically affect seed germination and plant growth. ACS Nano, 3, 3221–3227.
- 20 Lin, D. and Xing, B. (2007) Phytotoxicity of nanoparticles: inhibition of seed germination and root growth. Environ. Pollut., 150, 243–250.
- 21 Georgakilas, V., Perman, J.A., Tucek, J., and Zboril., R. (2015) Broad family of carbon nanoallotropes: classification, chemistry, and applications of fullerenes, carbon dots, nanotubes, graphene, nanodiamonds, and combined superstructures. Chem. Rev., 115, 4744–4822.
- 22 Sharma, R.K. and Ghose, R. (2016) Synthesis of Co3O4eZnO mixed metal oxide nanoparticles by homogeneous precipitation method. J. Alloys Compd., 686, 64–73.
- 23 Danks, A.E., Hall, S.R., and Schnepp, Z. (2016) The evolution of ‘sol–gel’ chemistry as a technique for materials synthesis. Mater. Horizon, 3, 91.
- 24 Shin, S.W., Song, I.H., and Um, S.H. (2015) Role of physicochemical properties in nanoparticle toxicity. Nanomaterials, 5, 1351–1365.
- 25 Parka, M.V.D.Z., Neigh, A.M., Vermeulen, J.P., Fonteyne, L.J.J., Verharenb, H.W., Briedéa, J.J., Loverena, H.V., and Jong, W.H. (2011) The effect of particle size on the cytotoxicity, inflammation, developmental toxicity and genotoxicity of silver nanoparticles. Biomaterials, 32, 9810–9817.
- 26 Park, Y.H., Bae, H.C., Jang, Y., Jeong, S.H., Lee, H.N., Ryu, W.I., Yoo, M.G., Kim, Y.R., Kim, M.K., Lee, J.K., Jeong, J., and Son, S.W. (2013) Effect of the size and surface charge of silica nanoparticles on cutaneous toxicity. Mol. Cell. Toxicol., 9, 67–74.
- 27 Shang, L., Nienhaus, K., and Nienhaus, G.U. (2014) Engineered nanoparticles interacting with cells: size matters. J. Nanobiotechnol., 12, 5.
- 28 Chithrani, B.D., Ghazani, A.A., and Chan, W.C.W. (2006) Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano Lett., 6, 662–668.
- 29 Wang, S.H., Lee, C.W., Chiou, A., and Wei, P.K. (2010) Size-dependent endocytosis of gold nanoparticles studied by three-dimensional mapping of plasmonic scattering images. J. Nanobiotechnol., 8, 33.
- 30 Lu, F., Wu, S.H., Hung, Y., and Mou, C.Y. (2009) Size effect on cell uptake in well suspended, uniform mesoporous silica nanoparticles. Small, 5, 1408–1413.
- 31 Varela, J.A., Bexiga, M.G., Aberg, C., Simpson, J.C., and Dawson, K.A. (2012) Quantifying size dependent interactions between fluorescently labeled polystyrene nanoparticles and mammalian cells. J. Nanobiotechnol., 10, 39.
- 32 Huang, J., Bu, L., Xie, J., Chen, K., Cheng, Z., Li, X., and Chen, X. (2010) Effects of nanoparticle size on cellular uptake and liver MRI with polyvinylpyrrolidone-coated iron oxide nanoparticles. ACS Nano, 4, 7151–7160.
- 33 Huang, K., Ma, H., Liu, J., Huo, S., Kumar, A., Wei, T., Zhang, X., Jin, S., Gan, Y., and Wang, P.C. (2012) Size-dependent localization and penetration of ultra-small gold nanoparticles in cancer cells, multicellular spheroids, and tumors in vivo. ACS Nano, 6, 4483–4493.
- 34 Schmid, O. and Stoeger, T. (2016) Surface area is the biologically most effective dose metric for acute nanoparticle toxicity in the lung. J. Aerosol Sci., 99, 133–143.
- 35 Suttiponparnit, K., Jiang, J., Sahu, M., Suvachittanont, S., Charinpanitku, T., and Biswas, P. (2011) Role of surface area, primary particle size, and crystal phase on titanium dioxide nanoparticle dispersion properties. Nanoscale Res. Lett., 6, 27.
- 36 Sager, T.M. and Castranova, V. (2009) Surface area of particle administered versus mass in determining the pulmonary toxicity of ultrafine and fine carbon black: comparison to ultrafine titanium dioxide. Part. Fibre Toxicol., 6, 15.
- 37 Wittmaack, K. (2007) In search of the most relevant parameter for quantifying lung inflammatory response to nanoparticle exposure: particle number, surface area, or what? Environ. Health Perspect., 115 (2), 187–194.
- 38 Sager, T., Kommineni, C., and Castranova, C. (2008) Pulmonary response to intratracheal instillation of ultrafine versus fine titaniumdioxide: role of surface area. Part. Fibre Toxicol., 5, 17.
- 39 Pujalté, I., Passagne, I., Brouillaud, B., Tréguer, M., Durand, E., Ohayon-Courtès, C., and L'Azou, B. (2011) Cytotoxicity and oxidative stress induced by different metallic nanoparticles on human kidney cells. Part. Fibre Toxicol., 8, 10.
- 40 Hu, G., Jiao, B., Shi, X., Valle, R.P., Fan, Q., and Zuo, Y.Y. (2013) Physicochemical properties of nanoparticles regulate translocation across pulmonary surfactant monolayer and formation of lipoprotein corona. ACS Nano, 7 (12), 10525–10533.
- 41 Li, X., Liu, W., Sun, L., Aifantis, K.E., Yu, B., Fan, Y., Feng, Q., Cui, F., and Watari., F. (2015) Effects of physicochemical properties of nanomaterials on their toxicity. J. Biomed. Mater. Res. A, 103, 2499–2507.
- 42 Gatoo, M.A., Naseem, S., Arfat, M.Y., Dar, A.M., Qasim, K., and Zubair., S. (2014) Physicochemical properties of nanomaterials: implication in associated toxic manifestations. Biomed. Res. Int. doi: 10.1155/2014/498420
- 43 Wang, S., Gao, M., Li, Z., She, Z., Wu, J., Zheng, D., Guo, L., Zhao, Y., Gao, F., and Wang, X. (2016) Performance evaluation, microbial enzymatic activity and microbial community of a sequencing batch reactor under long-term exposure to cerium dioxide nanoparticles. Bioresour. Technol., 220, 262–270.
- 44 Shaw, B.J. and Handy, R.D. (2011) Physiological effects of nanoparticles on fish: a comparison of nanometals versus metal ions. Environ. Int., 37, 1083–1097.
- 45 Maa, H., Williams, P.L., and Diamond, S.A. (2013) Ecotoxicity of manufactured ZnO nanoparticles: a review. Environ. Pollut., 172, 76–85.
- 46 Pradhan, A., Seena, S., Pascoal, C., and Cassio, F. (2012) Copper oxide nanoparticles can induce toxicity to the freshwater shredder allogamus ligonifer. Chemosphere, 89, 1142.
- 47 Josko, I., Oleszczukb, P., and Futa, B. (2014) The effect of inorganic nanoparticles (ZnO, Cr2O3, CuO and Ni) and their bulk counterparts on enzyme activities in different soils. Geoderma, 232–234, 528–537.
- 48 Nations, S., Wages, M., Cañasa, J.E., Maula, J., Theodorakis, C., and Cobb, G.P. (2011) Acute effects of Fe2O3, TiO2, ZnO and CuO nanomaterials on Xenopus laevis. Chemosphere, 83 (8), 1053–1061.
- 49 Lin, D. and Xing, B. (2008) Root uptake and phytotoxicity of ZnO nanoparticles. Environ. Sci. Technol., 42 (15), 5580–5585.
- 50 Rao, S. and Shekhawat, G.S. (2014) Toxicity of ZnO engineered nanoparticles and evaluation of their effect on growth, metabolism and tissue specific accumulation in Brassica juncea. J. Environ. Chem. Eng., 2, 105–114.
- 51 Yoon, S.J., Kwak, J.I., Lee, W.M., Holden, P.A., and An, Y.J. (2014) Zinc oxide nanoparticles delay soybean development: a standard soil microcosm study. Ecotoxicol. Environ. Saf., 100, 131–137.
- 52 Bradfield, S.J., Kumar, P., White, J.C., and Ebbs, S.D. (2016) Zinc, copper, or cerium accumulation from metal oxide nanoparticles or ions in sweet potato: yield effects and projected dietary intake from consumption. Plant Physiol. Biochem., 110, 1–10.
- 53 Rai, M., Ingle, A.P., Gupta, I., and Brandellic, A. (2015) Bioactivity of noble metal nanoparticles decorated with biopolymers and their application in drug delivery. Int. J. Pharm., 496, 159–172.
- 54 Huang, Z., Chena, G., Zenga, G., Guoa, Z., Hea, K., Hua, L., Wua, J., Zhanga, L., Zhua, Y., and Songc, Z. (2017) Toxicity mechanisms and synergies of silver nanoparticles in 2,4-dichlorophenol degradation by Phanerochaete chrysosporium. J. Hazard. Mater., 321, 37–46.
- 55 Vannini, C., Domingo, G., Onelli, E., De Mattia, F., Bruni, I., Marsoni, M., and Bracale, M. (2014) Phytotoxic and genotoxic effects of silver nanoparticles exposure on germinating wheat seedlings. J. Plant Physiol., 171, 1142–1148.
- 56 Fent, K., Weisbrod, C.J., Wirth-Heller, A., and Pieles, U. (2010) Assessment of uptake and toxicity of fluorescent silica nanoparticles in zebrafish (Danio Rerio) early life stages. Aquat. Toxicol., 100, 218–228.
- 57 Zhou, C., Vitiello, V., Casalsc, E., Puntesc, V.F., Iamunnoe, F., Pellegrini, D., Changwen, W., Benvenutoe, G., and Buttinoa, I. (2016) Toxicity of nickel in the marine calanoid copepod Acartia tonsa: nickel chloride versus nanoparticles. Aquat. Toxicol., 170, 1–12.
- 58 Loh, J.W., Yeoh, G., Saunders, M., and Lim, L.-Y. (2010) Uptake and cytotoxicity of chitosan nanoparticles in human liver cells. Toxicol. Appl. Pharmacol., 249, 148.
- 59 Maa, X., Lee, J.G., Deng, Y., and Kolmakov, A. (2010) Interactions between engineered nanoparticles (ENPs) and plants: phytotoxicity, uptake and accumulation. Sci. Total Environ., 408 (16), 3053–3061.
- 60 Damalas, C.A. and Eleftherohorinos, I.G. (2011) Pesticide exposure, safety issues, and risk assessment indicators. Int. J. Environ. Res. Publ. Health, 8 (5), 1402–1419.
- 61 Khot, L.R., Sankaran, S., Maja, M.J., Ehsani, R., and Schuster, E.W. (2012) Applications of nanomaterials in agricultural production and crop protection: A review. Crop Prot., 35, 64–70.
- 62 Parisi, C., Vigani, M., and Rodríguez-Cerezo, E. (2015) Agricultural nanotechnologies: what are the current possibilities? Nano Today, 10, 124–127.
- 63 Husen, A. and Siddiqi, K.S. (2014) Carbon and fullerene nanomaterials in plant System. J. Nanobiotechnol., 12, 16.
- 64 Mukherjee, A., Majumdar, S., Servin, A.D., Pagano, L., Dhankher, O.P., and White, J.C. (2016) Carbon nanomaterials in agriculture: a critical review. Front. Plant Sci., 7 (172), 1–16.
- 65 Lin, D. and Xing, B. (2007) Phytotoxicity of nanoparticles: inhibition of seed germination and root growth. Environ. Pollut., 150, 243–250.
- 66 Chena, H. and Yada, R. (2011) Nanotechnologies in agriculture: new tools for sustainable development. Trends Food Sci. Tech., 22, 585–594.
- 67 Sarlak, N., Taherifar, A., and Salehi, F. (2014) Synthesis of nanopesticides by encapsulating pesticide nanoparticles using functionalized carbon nanotubes and application of new nanocomposite for plant disease treatment. J. Agric. Food Chem., 62, 4833–4838.
- 68 Mondal, A., Basu, R., Das, S., and Nandy, P. (2011) Beneficial role of carbon nanotubes on mustard plant growth: an agricultural prospect. J. Nanopart. Res., 13, 4519–4528.
- 69 Wang, Y., Yang, X., Zhang, Dong, L., Zhang, J., Wei, Y., Feng, Y., and Lu, L. (2014) Improved plant growth and Zn accumulation in grains of rice (Oryza sativa L.) by inoculation of endophytic microbes isolated from a Zn hyperaccumulator, Sedum alfredii H. J. Agric. Food Chem., 62, 1783–1791.
- 70 Mahajan, P., Dhoke, S.K., and Khanna, A.S. (2011) Effect of nano-ZnO particle suspension on growth of mung (Vigna radiata) and gram (Cicer arietinum) seedlings using plant agar method. J. Nanotechnology, 2011 (696535), 1–7.
- 71 Tripathi, S., Sonkar, S.K., and Sarkar, S. (2011) Growth stimulation of gram (Cicer arietinum) plant by water soluble carbon nanotubes. Nanoscale, 3, 1176–1181.
- 72 Lee, W., An, Y., Yoon, H., and Kweon, H. (2008) Toxicity and bioavailability of copper nanoparticles to the terrestrial plants mung beans (Phaseolus radiates) and wheat (Triticum aestivum): plant agar test for water insoluble nanoparticles. Environ. Toxicol. Chem., 9 (27), 1915–1921.
- 73 Tiwari, D.K., Schubert, N.D., Cendejas, L.M.V., Villegas, J., Montoya, L.C., and Garcıa, S.E.B. (2014) Interfacing carbon nanotubes (CNT) with plants: enhancement of growth, water and ionic nutrient uptake in maize (Zea mays) and implications for nanoagriculture. Appl, Nanosci., 4, 577–591.
- 74 Tripathi, S. and Sarkar, S. (2015) Influence of water soluble carbon dots on the growth of wheat plant. Appl. Nanosci., 5, 609–616.
- 75 Villagarcia, H., Dervishi, E., Silva, K., Biris, A.S., and Khodakovskaya, M.V. (2012) Surface chemistry of carbon nanotubes impacts the growth and expression of water channel protein in tomato plants. Small, 8, 2328–2334.
- 76 Popescua, B.M., Alib, N., Bastureac, G., Comsaa, G.I., Materond, L.A., and Chipara, M. (2013) One-dimensional nanoparticles: a brief critical review on biological, medical, and toxicological aspects. Appl. Surf. Sci., 275, 2–6.
- 77 Menaa, N.Z., Fernándezd, D.M., Dub, W., Hernandez-Viezcasb, J.A., Bonilla-Bird, N., López-Morenog, M.L., Komárekd, M., Videab, J.R.P., and Gardea-Torresdey, J.L. (2016) Exposure of engineered nanomaterials to plants: insights into the physiological and biochemical responses – a review. Plant Physiol. Biochem., 110, 1–29.
- 78 Mustafa, G. and Komatsu, S. (2016) Toxicity of heavy metals and metal-containing nanoparticles on plants. Biochim. Biophys. Acta Proteins Proteomics, 1864 (8), 932–944.
- 79 Coxa, A., Venkatachalama, P., Sahia, S., and Sharma, N. (2016) Silver and titanium dioxide nanoparticle toxicity in plants: a review of current research. Plant Physiol. Biochem., 107, 147–163.
- 80 Graff, R.M., Rumpkera, R., Richtera, M., Bragab, T.V., Kjeldsenb, F., Brewerc, J., Hoylandd, J., Rubahnd, H.G., and Erdmann, H. (2014) Exposure to silver nanoparticles induces size- and dose-dependent oxidative stress and cytotoxicity in human colon carcinoma cells. Toxicol. In Vitro, 28 (7), 1280–1289.
- 81 Chuang, K.J., Lee, K.Y., Pan, C.H., Lai, C.-H., Lin, L.-Y., Ho, S.C., Ho, K.F., and Chuang, H.C. (2016) Effects of zinc oxide nanoparticles on human coronary artery endothelial cells. Food Chem. Toxicol., 93, 138–144.
- 82 Armand, L., Clier, M.B., Bobyk, L., Faure, V.C., Strub, H.D.J.M., Cianferani, S., Dorsselaer, A.V., Boime, N.H., Rabilloud, T., and Carriere, M. (2016) Molecular responses of alveolar epithelial A549 cells to chronic exposure to titanium dioxide nanoparticles: a proteomic view. J. Proteomics, 134, 163–173.
- 83 Ge, S., Wang, G., Shen, Y., Zhang, Q., Jia, D., Wang, H., Dong, Q., and Yin, T. (2011) Cytotoxic effects of MgO nanoparticles on human umbilical vein endothelial cells in vitro. IET Nanobiotechnol., 5 (2), 36–40.
- 84 Nunoshiba, T., Obata, F., Boss, A.C., Oikawa, S., Mori, T., Kawanishi, S., and Yamamoto, K. (1999) Role of iron and superoxide for generation of hydroxyl radical, oxidative DNA lesions, and mutagenesis in Escherichia coli. J. Biol. Chem., 274, 34832–34837.
- 85 Touati, D., Jacques, M., Tardat, B., Bouchard, L., and Despied, S. (1995) Lethal oxidative damage and mutagenesis are generated by iron in Δfur mutants of Escherichia coli: protective role of superoxide dismutase. J. Bacteriol., 177, 2305–2314.
- 86 Itoh, M., Nakamura, M., Suzuki, T., Kawai, K., Horitsu, H., and Takamizawa, K. (1995) Mechanism of chromium (vi) toxicity in Escherichia coli: is hydrogen peroxide essential in Cr(vi) toxicity? J. Biochem., 117, 780–786.
- 87 Geslin, C., Llanos, J., Prieur, D., and Jeanthon, C. (2001) The manganese and iron superoxide dismutases protect Escherichia coli from heavy metal toxicity. Res. Microbiol., 152, 901–905.
- 88 Stadtman, E.R. and Levine, R.L. (2003) Free radical-mediated oxidation of free amino acids and amino acid residues in proteins. Amino Acids, 25, 207–218.
- 89 Stadtman, E.R. (1993) Oxidation of free amino acids and amino acid residues in proteins by radiolysis and by metal-catalyzed reactions. Annu. Rev. Biochem, 62, 797–821.
- 90 Valko, M., Morris, H., and Cronin, M.T.D. (2005) Metals toxicity and oxidative stress. Curr. Med. Chem., 12, 1161–1208.
- 91 Valko, M., Rhodes, C.J., Moncol, J., Izakovic, M., and Mazur, M. (2006) Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem. Biol. Interact., 160, 1–40.
- 92 Helbig, K., Bleuel, C., Krauss, G.J., and Nies, D.H. (2008) Glutathione and transition-metal homeostasis in Escherichia coli. J. Bacteriol., 190, 5431–5438.
- 93 Hunt, P.R., Marquis, B.J., and Tyner, K.M. (2013) Nanosilver suppresses growth and induces oxidative damage to DNA in Caenorhabditis elegans. J. Appl. Toxicol., 33, 1131–1142.
- 94 Roh, J.-Y., Eom, H.-J., and Choi, J. (2012) Involvement of Caenorhabditis elegans MAPK signaling pathways in oxidative stress response induced by silver nanoparticles exposure. Toxicol. Res., 28, 19–24.
- 95 Ahamed, M., Karns, M., Goodson, M., Rowe, J., Hussain, S.M., Schlager, J.J., and Honga, Y. (2008) DNA damage response to different surface chemistry of silver nanoparticles in mammalian cells. Toxicol. Appl. Pharmacol., 233, 404–410.
- 96 Hou, J.-L., Shuo, G., and Grozova, L. (2013) Reduction of silver nanoparticle toxicity by sulfide. Adv. Mater. Lett., 4, 131–4133.
- 97 Asha Rani, P.V., Mun, G.K., Hande, M.P., and Valiyaveettil, S. (2009) Cytotoxicity and genotoxicity of silver nanoparticles in human cells. ACS Nano, 3, 279–290.
