20
Biopolymers: A Natural Support for Photocatalysts Applied to Pollution Remediation
Diseko Boikanyo, Ajay Kumar Mishra, Shivani B. Mishra and Sabelo D. Mhlanga
University of South Africa, College of Engineering, Science and Technology, Nanotechnology and Water Sustainability Research Unit (NanoWS), Florida, Johannesburg 1709, South Africa
20.1 Introduction
20.1.1 Wastewater Treatment Methods
Industrialization is inextricably linked to the generation and disposal of innumerable toxic pollutants in quantities difficult to measure. The solid-, liquid-, and gas-phase effluents discharged into water bodies are hazardous to humans, wildlife, and the environment. These effluents contain refractory substances that, by virtue of being difficult to degrade by natural means, would persist in the environment and continue to cause harm long after they have been discharged.
The fact that water covers nearly 70% of the Earth's surface makes it easy to think that it is an infinite resource. However, nearly 97% of this is salt water in the oceans and the remaining 3% is freshwater [1]. About 68% of the freshwater on earth is frozen in ice caps, glaciers, and permafrost, 30% is groundwater and the remaining 2% is surface water that is available to us, distributed in lakes, rivers, and the atmosphere [2].
Exponential population growth and consequent industrialization has put tremendous pressure on this finite and already burdened resource. Industries require large volumes of water for production processes, and as such postproduction wastewater discharged from industries left unregulated poses an imminent threat to an already scarce resource. The concept of sustainable development was found to be the solution to environmental degradation problems as discussed during the Brundtland Commission as far back as 1987. A widely accepted definition of sustainable development is “development that meets the needs of the present without compromising the ability of future generations to meet their own needs” [3]. The key to sustainable development is the generation of new “green” technologies that lessen harmful waste and the conscientious exploitation of alternative sources of renewable raw materials. One such avenue is the use of products made not only from natural products but also from products that degrade into environmentally benign constituents.
The nature of wastewater releasing activities dictates the type of pollutants present in the wastewater. Heavy metals, nitrates, sulfates, phosphates, fluorides, chlorides, oxalates, pesticides, polyaromatic hydrocarbons, dyes, phenols, polychlorinated biphenyls, halogenated aromatic hydrocarbons, formaldehyde, polybrominated biphenyls, biphenyls, detergents, oils, greases, and so on are some of the inorganic and organic pollutants commonly found in wastewater [4]. Furthermore, the effluent characteristics may vary daily and seasonally and this increases the difficulty of treatment [5].
Industrial wastewater treatment systems currently in use (Figure 20.1 [4]) were built for primary and secondary treatment because most publicly owned municipal treatment works (POTWs) were designed to handle domestic effluent and therefore use concentration-based standards and aggregate quality parameters such as chemical oxygen demand (COD), salinity, and pH, with little consideration for pollutant loading of the industrial effluent [6]. This means that primary and secondary treatment is rather inadequate for treating industrial wastewater with a high organic load, and there is a greater incidence of water pollution by industrial effluent discharged into POTWs and ultimately surface waters. This requires treatment facilities to be fitted with additional systems for the handling of recalcitrant organic pollutants and toxic residuals present in these waters. Following secondary treatment, there are several techniques that can be used for the treatment of refractory organic pollutants present in wastewater. These are classed as tertiary treatment processes and can be broadly divided into biological, chemical, and physical techniques (Figure 20.2 [7]). All these have their advantages and disadvantages and will not be discussed in detail here.

Figure 20.1 Illustration of the classification of chemical treatment and water recycling technologies (reproduced with permission from Ref. [4]. Copyright 2012, the Royal Society of Chemistry).
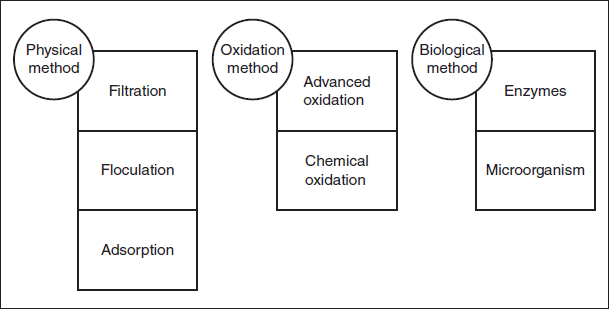
Figure 20.2 Treatment methods for the degradation of organic pollutants in textile wastewater (reproduced with permission from Ref. [7]. Copyright 2016, Elsevier).
Biological treatment is reported to be the most economical and widely applicable method, in comparison to the physical and chemical treatment methods. However, its sensitivity to numerous environmental factors, day-to-day variations in temperature and sunlight, and the necessity for large land area for treatment ponds restricts its use [8]. Biotreatment methods such as fungal and microbial decolorization and adsorption by microbial biomass, for example, are applied to industrial effluents because various microorganisms such as fungi, bacteria, and algae, through bioaccumulation are able to degrade some pollutants. However, bioremediation is often slow, with unpredictable results, but more so, because of the xenobiotic nature of certain persistent organic pollutants, and especially azo dyes, it is inadequate for the removal of COD and total organic carbon (TOC) in industrial effluents characterized by a high fraction of organic pollutant load [9].
Physical treatment techniques include separation and filtration via membranes and adsorption onto different solid substrates such as activated carbon, peat, wood chips, fly ash, silica gel, natural mineral clays, agricultural biomass waste including corn cobs, wheat husks, rice hulls, and so on, ion-exchange resins, irradiation, incineration, and sonolysis [10]. Depollution by adsorption functions based on two mechanisms: adsorption and ion exchange. Therefore it is restricted by its dependence on physicochemical parameters such as the pollutant–sorbent interaction, particle size, temperature, pH, and contact time [10]. Physical treatment techniques are ideal for the removal of the more stable pollutants that prove refractory for conventional methods. However, adsorbent and filtration methods are nondestructive and transfer pollutants to another phase or location and result in secondary pollution with its own disposal requirements [9]. Sonolysis and incineration yield no sludge and minimal secondary pollution; however, these physicochemical techniques have not yet proven to be energy efficient and economically viable beyond the laboratory and reactor development phases [11].
Chemical methods traditionally refer to coagulation, flocculation, and precipitation, electroflotation, electrokinetic coagulation, oxidative processes, irradiation, and electrochemical processes [8]. The massive doses of chemicals required in these methods make them expensive and despite the fact that target pollutants are removed, the accumulation of sludge presents a secondary pollution and disposal problem [12].
Among the chemical methods of wastewater treatment is a class commonly referred to as advanced oxidation processes (AOPs). AOPs typically involve the photochemical activation of already oxidizing substances such as ozone [13], hydrogen peroxide [14], Fenton's reagent [15], and so on to yield highly reactive and oxidizing free radicals. Reports frequently state that the use of photoactivated oxidizing agents for the treatment of effluents with high organic load [16] is largely superior to using only ultraviolet radiation or the oxidants on their own, primarily because photochemical processes completely destroy organic pollutants as opposed to converting them and transferring them to another phase as is the case with physical treatment processes. However, these types of treatments, where UV light is used to photoactivate oxidants, have limitations. For example, by its very nature, textile wastewater is highly UV absorbing and this may inhibit the concentration of radicals required to degrade azo dyes; ozone radicals have very short half-lives and this necessitates rapid reaction times; removal of pollutants depends on pH, target pollutant concentration, and radical concentration; the maintanence of UV reactors and the quartz sleeves may require pretreatment of effluents, the specialized handling and storage of ozone and hydrogen peroxide; and the energy requirements for the generation and sustenance of radicals may prove commercially unsustainable [9,11].
20.1.2 Photocatalysis in Wastewater Treatment
Heterogeneous photocatalysis is listed as one of the most viable advanced oxidation processes for the elimination of organic pollutants in wastewater. Its viability, relative to other wastewater treatment processes, is based on the premise that there is little-to-no mass transfer, it can be carried out under prevailing ambient conditions (extreme pressure and temperature are not required), atmospheric oxygen can be used as the oxidant, and complete mineralization of target pollutants can be achieved [17]. It presents an alternative mechanism to those already listed to generate free radicals. Briefly, in a heterogeneous photocatalytic system, photoinduced molecular transformations and reactions take place at the surface of a catalyst, and depending on where the initial excitation occurs, the process could be a catalyzed photoreaction or a sensitized photoreaction. In the former, the initial photoexcitation occurs in the adsorbed molecule, which subsequently interacts with the catalyst substrate; in the latter, the initial photoexcitation takes place in the catalyst substrate and the electronically excited catalyst then transfers electrons (or energy) to the adsorbed molecule and thereby inducing a reaction [18].
Semiconductor-mediated photocatalytic oxidation mechanisms have been detailed previously in literature [9,18–20]. The process is initiated by the generation of electron–hole pairs in the semiconductor when electrons are excited from the valence band (VB) to the conduction band (CB) by the absorption of photons with energy equal to or greater than the bandgap (Ebg) of the semiconductor (Figure 20.3). Upon excitation, the electron–hole pair has several deexcitation pathways it may follow. The photogenerated electrons (e−cb) could react with electron acceptors such as O2 adsorbed onto the catalyst surface or dissolved in water, reducing them to superoxide radical ions (![]() ). The photogenerated holes (h+vb) can react with surface bound H2O, oxidizing it to •OH− radicals and H+ ions. These in combination with other highly oxidizing species such as peroxide radicals are reported to be responsible for the semiconductor-mediated photocatalytic decomposition of organic contaminants.
). The photogenerated holes (h+vb) can react with surface bound H2O, oxidizing it to •OH− radicals and H+ ions. These in combination with other highly oxidizing species such as peroxide radicals are reported to be responsible for the semiconductor-mediated photocatalytic decomposition of organic contaminants.

Figure 20.3 Schematic diagram illustrating the principle of TiO2 photocatalysis (reproduced with permission from Ref. [9]. Copyright 2015, Elsevier.
The generalized possible reactions occurring at the semiconductor surface are outlined as follows:
Titanium dioxide (TiO2) remains the most studied semiconductor photocatalyst because it demonstrates high activity under UV irradiation, photostability, biological inertness, relative chemical stability, low operational temperature and low energy consumption, water insolubility under typical environmental conditions, a disinclination to photocorrosion, and it is naturally abundant and therefore cheaper than other semiconductor photocatalysts [9]. In an ideal situation, a catalyst to be employed in a photocatalytic system would be photostable, chemically and biologically inert in natural systems, abundant, and cheap. Therefore, efforts are being directed toward the development of TiO2-based photocatalysts that would retain all the favorable properties of TiO2 as a photocatalyst but impart visible-light activity by decreasing the bandgap of parent titania, decrease the electron–hole recombination rate, and enhance interfacial charge transfer efficiency. Titania still remains the most studied metal oxide semiconductor photocatalyst; therefore, this chapter will focus on immobilization supports utilized for this compound.
20.2 Biopolymers: Introduction and Definition of Terms
As a whole, polymers are one of the most pervasive materials on the planet, where virtually everything around us is wholly or partially comprised of polymers [21]. Fundamentally, polymers are classified as either synthetic or naturally occurring, and in both instances they are made of repeated monomer units. Traditionally, synthetic polymers have been petroleum based and nonbiodegradable. This practice is rapidly becoming unsustainable as fossil fuels are a finite and nonrenewable resource and this has necessitated the transition to the use of renewable resources such as biomass and naturally occurring polymers for the production of polymers and plastics.
Yarsley and Couzens predicted the benefits of synthetic polymers well over 70 years ago. It was supposed to be a world free of rot and rust and bright with color. They highlighted the durability of plastics and their potential for widespread use as disposable items, where on the first page of their book titled Plastics, it is written “the possible applications [of polymers] are almost inexhaustible” [22]. However, what was not anticipated were the problems associated with these durable materials, namely, accumulation of the plastics in landfills and nature and the difficulty of their disposal, the additives used to improve their performance are potentially toxic (e.g., phthalates and bisphenol A), and these chemical additives may leach from the plastic products and be transferred to humans and wildlife [23]. Nearly 4% of the world's oil production is used as raw material to make plastics and nearly as much is used as energy in the process; combined with declining fossil fuel reserves and the limited capacity of landfills has rendered the use of synthetic polymers unsustainable [24].
The drive toward sustainable living and development has necessitated our “return to nature.” Synthetic polymers have been heavily relied upon to the point of indispensability. However, synthetic polymers originating from petroleum-based products and coal have proven incompatible with the environment and thus fail to meet the ends of sustainable development, because they do not “return to nature.” One solution is material reduction, reuse, and recycling. However, this solution requires the combined efforts and cooperation of the public, governments, industries, and scientists.
Natural polymers and their derivatives have been used for millennia by humans for survival. These include wood, cotton, silk, animal skin, natural rubber, and so on [25]. Unlike modern humans, the ancients did not use them with the acuity of the chemistry and physics of contemporary synthetic polymers but to meet their rudimentary daily material needs. Examples include ink prepared by ancient Egyptians made by mixing carbon black with Arabic gum and native Amazon Indians using vulcanized natural rubber extracted from hevea trees more than 25 centuries ago to make boots [26].
More recently, nature-derived plastics such as Bois durci, an early plastic composite, dating back to the 1850s, give validation to the use and robustness of natural polymers in contemporary societies preoccupied with the longevity and indestructability of materials. Technically, Bois durci is a composite made of sawdust from a hardwood such as ebony or rosewood mixed with carbon black, metal particles, vegetable oils, and blood. Others like casein, a milk protein that was popular in the early 1900s, was made via enzymatic action, where the resultant curds could be molded and treated with a hardening agent [27].
History shows that humans had a long-standing relationship with naturally derived polymers until the advent of synthetic polymers that dominated the twentieth century with the rise of petrochemical industries. The rapid development of synthetic polymers and the migration away from natural polymers has been ascribed to the scarcity of natural polymers during the world war years [28]. In addition to this, the high cost of extracting natural monomers from plants, animals, and microorganisms relative to petroleum-based counterparts has also been cited as motivation for the great development and popularity of synthetic polymers [29].
An imminent problem we are faced with now is the depletion of fossil fuel resources and resilient polymer wastes that persist in the environment. Therefore, it stands to reason that (i) the development and use of polymers that are produced from resources that can be replenished at rates comparable to their consumption and (ii) would biodegrade to harmless and environmentally benign products is inevitable.
“Bio-based” is a term that describes a material that is made of or derived from renewable raw material. These raw materials are considered “renewable” if they are replaced in nature at rates comparable to or faster than the rate at which they are consumed by humankind [30]. The American Society for Testing and Materials (ASTM) in their ASTM D6866 standard testing method for determining the biogenic carbon content of solid, liquid, and gaseous samples using radiocarbon analysis has defined biobased materials as “organic materials in which carbon is derived from a renewable resource via biological processes; where bio-based materials include all plant and animal mass derived from carbon dioxide (CO2) recently fixed via photosynthesis, per definition of a renewable resource” [31].
Literature shows that there are no strict definitions of the terms used to describe biopolymers such as “biobased” and “biodegradable” as well as “biopolymer” and “bioplastic polymer.” Instead, it appears that they have several and intersecting meanings. The synonymous and interchangeable use of the terms “biobased plastic,” “biopolymer,” and “biodegradable plastic” is erroneous. Not all biobased polymers are biodegradable and not all biodegradable materials are biobased. That is, the terms “biobased” and “biodegradable” are related; however, they are not synonymous and should not be used interchangeably. Thus, it is important to define these terms with regards to their physicochemical properties and observed phenomena in order to avoid ambiguity.
The International Union of Pure and Applied Chemistry (IUPAC) has proposed the following terminology that can be used across scientific disciplines [32]:
- Biomass refers to living systems and collection of organic substances produced by living systems that are exploitable as materials, including recent postmortem residues.
- Biobased refers to materials composed or derived in whole or in part from biological products issued from the biomass (including plant, animal, and marine or forestry materials).
- Biodegradable qualifies macromolecules or polymeric substances as being susceptible to degradation by biological activity by lowering of the molar masses of macromolecules that form the substances.
- Biomacromolecule refers to macromolecules (e.g., proteins, nucleic acids, and polysaccharides) formed by living organisms.
- Biopolymer refers to macromolecules (including proteins, nucleic acids, and polysaccharrides) formed by living organisms.
The need for the definition of terms becomes apparent when different scholars have attempted to delineate biopolymers and bioplastics as per the source of the feedstock material and/or biodegradability.
Niaounakis [33] uses two criteria to define biopolymer (or bioplastic): (1) the source of the raw material and (2) the biodegradability of the polymer. The author differentiates between type A: biopolymers made of renewable raw materials (biobased) and being biodegradable; type B: biopolymers made of renewable raw materials (biobased) and not being biodegradable; and type C: biopolymers made of fossil fuels and being biodegradable. Further, type A biopolymers can be produced by biological systems or chemically synthesized from biological starting materials. Type B biopolymers are those that can be produced from biomass or renewable resources and are nonbiodegradable. Those produced from fossil fuels and are biodegradable and compostable fall into the type C category [33].
Reddy et al. classify bioplastics based on their production method (Figure 20.4 below). They are distinguished between (i) renewable resource-based bioplastics, (ii) petroleum-based bioplastics, and (iii) bioplastics from mixed sources. Type (i) includes those bioplastics synthesiszed naturally from plants and animals, or entirely from renewable resources; type (ii) refers to polymers synthesized from petroleum resources and are biodegradable at the end of their functionality; and type (iii) describes those bioplastics made from combinations of biobased and petroleum monomers [34].
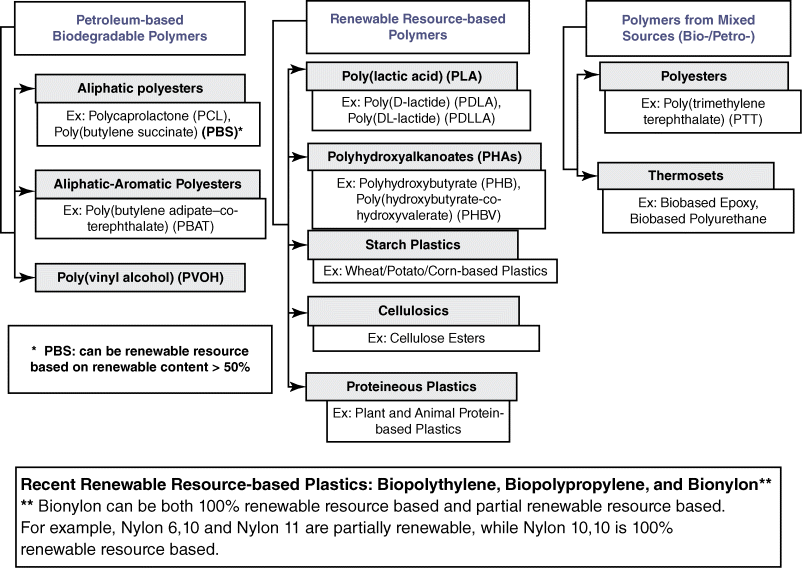
Figure 20.4 Classification of bioplastics based on their production routes. (reproduced with permission from Ref. [34]. Copyright 2013, Elsevier).
Thomas et al. gives a simpler, more direct definition: “biopolymers are macromolecules derived from natural sources and are usually biodegradable” [35]. In a different publication, John and Sabu describe natural polymers as those which are present in, or created by living organisms, including polymers from renewable resources that can be polymerized to create bioplastics. The authors classify natural polymers into two categories: those coming from living organisms and those which must be polymerized, but come from renewable resources. The author makes no mention of “biodegradability” being a requirement [28].
The drive to protect the environment has necessitated the research and development of high-performance polymers that are eco-friendly. These eco-friendly materials have been referred to, using popular buzzwords, as “renewable,” “recyclable,” and “triggered biodegradable” [36]. There are a plethora of such terms used to describe environmentally harmless polymers, especially those synthesized from renewable resources. Teramoto describes the extant conundrum where Web searches on the “environmentally benign” polymers yields a multitude of terms that seem to describe polymers from renewable sources. One term that is rather obscure, but quiet fitting, is “green polymer,” as it includes polymers from renewable biological resources and biodegradable polymers, and is more cognizant of the instinctual relation between the terms [29].
At present, there are no standards on what can be called a biopolymer; however, there are intuitive ways we could qualify the “bio” element of biopolymers: naturally occurring, those derived from biomass, and biodegradability seem useful delimitations under the context of environmental decontamination and sustainability
This chapter will focus on renewable resource-based biopolymers, that is, those polymers produced by plants, animals, and microorganisms or chemically synthesized from biological starting materials and are biodegradable. Biopolymers that are produced from fossil fuels and are biodegradable are included. In general, “green polymers” are described. It is important, however, to note the fact that biobased polymers are neither always and necessarily environment-friendly nor biocompatible or biodegradable, but there are fossil fuel-based polymers that are biodegradable. The objective is to provide recent information about biopolymers that have been used as immobilization supports for photocatalytic systems used for the treatment of wastewater.
20.3 Immobilization of Photocatalysts on Supports
20.3.1 The Necessity for Immobilization
TiO2-mediated heterogeneous photocatalysis demonstrates high potential as an alternative method for the decontamination of wastewaters. However, there are significant challenges in as far as its realization as an industrial technology is concerned: it is fundamentally inadequate for harnessing energy or for the treatment of large volumes of water, because its light energy density is primarily low and TiO2 can utilize only the minute amounts of UV light contained in solar radiation [37]. Another obstacle that has to be overcome is the retention, recycling, and recovery of the photocatalyst powders, because commercial-grade TiO2 has agglomerate particles in the micrometer size (>0.5 µm) range and nanomaterial elementary particles in the nanometer (10–100 nm) range [38].
Research endeavors focus mainly on improving both the photocatalytic activity and the visible-light activation of TiO2 in order to circumvent the expenses incurred from powering UV light sources used for the treatment of massive volumes of wastewater. These strategies involve the use of both physical and chemical methods. Examples of physical techniques include microwave or ultrasound irradiation and chemical methods refer to the use of metal and nonmetal dopants, and coupling TiO2 with different semiconductors [39]. Traditionally, photocatalyst particles had been applied as powder and were found to demonstrate a large surface area and efficiency [40]. However, in order to achieve full-scale industrialization of this technology, certain shortcomings inherent to powdered photocatalysts have to be overcome. First, immobilization of the photocatalyst is critical simply because “decontaminated” water cannot be allowed to contain particles of catalyst. Second, the very essence of photocatalysis is light; therefore, critical to the efficacy of a heterogeneous photosystem is the optimal use of radiation. The pros and cons of immobilized and suspended photocatalyst systems are given in Table 20.1.
Table 20.1 Summarizes the advantages and disadvantages of immobilized and suspended photocatalyst reactor systems [9,41].
| Immobilized photocatalyst | Suspended photocatalyst |
Advantages
|
Advantages
|
From Table 20.1, it can be seen that the most important factors affecting the process performance is the photoactivity of the catalyst. Therefore, from a practical viewpoint, immobilized photoreactor systems are preferred as postdepollution catalyst recovery and retention are circumvented. However, it can be countered that slurry photoreactor systems could be more efficient due to greater reactant accessibility by the suspended catalyst. As such, neither immobilized nor suspended photoreactor systems present themselves as a cure-all for the decontamination of wastewater. Based on the listed advantages and disadvantages of the different photosystems, it seems that a single universally applicable end-of-pipe photocatalytic reactor system is unrealistic. However, toward the ends of attaining sustainable development and advancing photochemical reactor systems beyond laboratory scale to industrialized commercially viable technologies, risk versus potential benefit assessments are critical. Despite the fact that suspended photocatalyst is more efficient at harnessing freely available solar energy, it does not compensate the expenses incurred for the post-treatment filtration of the oxide particles [42]. Overall, immobilization of photocatalysts outweighs the disadvantages.
20.3.2 Features of an Immobilizing Support
The physicochemical principles and the parameters affecting the degradation of pollutants using titania-mediated photocatalysis such as the influence of pH and temperature, generation of free-radicals and oxidizing agents, catalyst loading effects, dopant content, methods of TiO2 preparation that affect reactive surface area, concentration and generation of charge carriers, and incident radiation have been well understood for quite some time [19]. However, a major challenge in the development of highly efficient photocatalytic reactors is the establishment of effective reactor designs that are appropriate for industrial and commercial applications. To realize the industrialization of this technology, photoreactor design parameters such as photoreactor geometry, photocatalyst selection, and efficient utilization of radiation have to be optimized [43].
The design of photoreactors is based on three fundamental features: (i) state of the photocatalyst: suspended or immobile; (ii) type of irradiation: UV polychromatic lamps or solar light; and (iii) positioning of the light source: immersed, external, or distributed light sources [43]. Irrespective of the source and type of illumination, the effective utilization of light is highly sought after because light is rapidly diminished in water; therefore, reactors should be designed with a view to minimize the problem of low light utilization efficiency that is typically associated with conventional slurry photocatalytic reactors [44].
Optimum photocatalyst activity and utilization of radiation are key factors in the design of a photoreactor system. Therefore, it has been oberved that the deposition of the photocatalyst on an inert support presents two obvious problems. First is the reduced ability of photons to reach the catalyst surface and reactants [45]. Thoughtful consideration of reactor design parameters presents a solution, namely, suitable irradiation appropriate for a given geometrical configuration and adequate mixing to allow the water and photocatalyst sufficient interaction time [43]. Second, fixation of the catalyst determines liquid to solid mass transfer limitations of the pollutants to the surface of the catalyst [46] that ultimately affect the photocatalytic rate of reaction.
The choice and development of a catalyst support is generally informed by its role. Since the photocatalyst requires maximum access to light and target contaminants, a good support must demonstrate the following features [40,47]:
- There must be a strong adherence of the photocatalyst to the support in order to provide anchorage.
- The photocatalytic activity must remain unaffected by the method of fixation.
- It must provide a large specific surface area.
- It must have a strong capacity for adsorption of pollutants in order to improve their removal.
- The contact mass must be stable for extended periods of time.
- The immobilizing support must be oxidative degradation resistant when it is subjected to radiation and radicals.
- The photocatalyst must not leach from the support under various reaction conditions.
- Light transparency and low light scattering by the support are a boon, but not always possible.
20.3.3 Approaches Used for Immobilization of the Photocatalyst
Anchorage of photocatalysts onto a support can be achieved via physical surface forces or chemical bonds. Physical forces usually involve at least one thermal step and include all procedures that utilize previously manufactured TiO2, while chemical forces refer to the “in situ” generation of catalyst deposited straight onto a suitable support, often from a precursor [48]. Most important is that regardless of the deposition means used, the photocatalytic activity of the catalyst must remain unhindered or at least retain as much as possible the catalytic activity of the powder form. In addition to this, the support–catalyst junction must be resilient to the strain caused by particle–particle and particle–fluid mechanical interactions inside the reactor environment in order to avoid the shedding of catalyst particles from the support [49].
Compared to the volumes of studies conducted on the optimization of the photocatalytic process, new photocatalytic materials, rate kinetics, and mechanistics of the photocatalysis of various target compounds, immobilization of photocatalysts onto supports is not a flourishing field of research. However, when the target is the realization of photocatalysis as an economically feasible and ultimately industrialized technology, this aspect has garnered the attention it deserves [50].
The wide varieties of substrates that have been tested as supports for the immobilization of TiO2 attest to this. Some of these reported in the literature include, but are not limited to, glass, SiO2 beads, activated carbon, aluminum, Teflon, fiberglass, and polymeric materials, fly ash, vycor glass, hollow glass spheres, polyethylene sheets, reactor walls, silica gel, fabric or wool, microporous cellulose membranes, quartz, optical fibers, alumina clays, ceramic membranes and monoliths, stainless steel, zeolites, and anodized iron [48,51]. A critical aspect in the choice of the method of immobilization used and the support is the photoreactor configuration. The support has a dual function, wherein it (i) provides macroscopic structure to the photocatalytic material and (ii) plays an active role within the reactor where flow distribution is determined by static mixer phenomena, thus requiring high water–photocatalyst interaction [43].
The techniques that have been used for the deposition of TiO2 onto solid supports can be roughly categorized into those using (i) either TiO2 powders or TiO2 precursors and those (ii) employing binders or not. Any of these approaches could be used alone or in combination, but the choice depends on the nature of the support employed. Multiple reviews detail some of the methods typically employed for the immobilization of photocatalyst and will not be detailed here. These methods are predominantly binderless and can be carried out via physical surface forces and include, but are by no means limited to, sol–gel involving spread and dip coating, thermal treatment methods, hydrothermal methods, sol-spray methods, electrophoretic deposition, and various types of chemical vapor deposition (CVD) such as atmospheric pressure CVD, plasma-enhanced CVD, hybrid physical CVD, and metal-organic CVD [40,47,50].
A credible solution to the challenges listed such as catalyst decantability post-depollution and recovery, mass transfer limitations inherent to heterogeneous catalysis systems, the substrates' ability to concentrate intended pollutants onto the contact surface, stable contact mass over extended periods of time, high specific surface area, and strong adhesion between the catalyst and the support may present itself via the use of a suitable stabilizing matrix that could be found in biopolymers. The methods mentioned for anchoring TiO2 are inappropriate for use in coating the biopolymer surface substrate with titania as they require high-temperature calcinations, complex processing, and costly instruments and this presents a limitation in as far as the use of polymeric materials as supports is concerned.
20.4 Survey of Biopolymer-Supported Photocatalysts for Pollution Remediation
This section discusses biopolymer-supported photocatalysts applied to the photocatalytic degradation of organic pollutants such as azo dyes, phenol, and natural organic matter (NOM) under UV irradiation, and where possible, visible and solar irradiation is discussed.
20.4.1 Biopolymers as Immobilization Supports
In 1995, Tennakone et al. conducted one of the first studies using a polymeric matrix for the immobilization of titania applied to the photocatalytic degradation of phenol [52]. The TiO2 was supported on a polythene (PE) film, where commercial anatase-TiO2 was ironed onto the PE support. Albeit rudimentary, the method proved effective and cheap. The photodegradation of phenol under both UV and solar irradiation was monitored by CO2 gas generated using gas chromatography (GC). The results showed that the phenolic solution had more than 50% degradation in ∼2.5 h under nonstirred and nonaerated conditions.
The work by Tennakone et al. proved that the photocatalyst could retain its photoactivity and degrade phenol under solar irradiation while being submerged just below the surface of a pond, pool, or running water, and this opened up avenues for the use of PE as an immobilizing substrate. Along with achieving photomineralization of phenol, what the researchers also illustrated was the current challenge with the use of synthetic polymers: in one study, the CO2 generated was 9 ml more than the calculated values, and this was attributed to either impurities in the PE film or the partial degradation of the polymer.
The problem with the use of synthetic polymers as immobilizing supports is that various compounds such as plasticizers and antioxidants are added to polymers to enhance their durability. Direct exposure to sunlight causes toxic by-products such as phthalates to start leaching out and these have been shown to be endocrine disruptors in mammals [53]. The intention is to use solar radiation for the degradation of organic pollutants; thus, it stands to reason that the use of synthetic polymers is not ideal for purposes of environment-friendly and sustainable processes, because the degradation of the polymer by the oxidizing radical species generated during the photocatalytic process would also cause a potential secondary pollution problem.
Biopolymers present themselves as a candidate solution. They have been applied due to their remarkable features such as an ability to stabilize nanoparticles and prevent particle sintering and agglomeration, low toxicity and cost, and easy availability [54]. Stabilization and the prevention of agglomeration of the photocatalyst nanoparticles is particularly important with regard to photocatalytic activity, because variables such as crystal and particulate sizes have been cited as giving rise to discernible changes in catalyst activity, that is, photocatalysts with a higher surface area are likely to reduce mass transfer limitations and ultimately the rate of degradation of the organic contaminants [20].
The use of biologically derived polymers as supports to immobilize the photocatalyst provides an obvious advantage for environmental reasons. In addition, by their very nature, polysaccharides such as chitin and its derivative chitosan (CS), starch, and cyclodextrin, for example, have physicochemical characteristics, chemical stability, high reactivity, and outstanding selectivity toward aromatic compounds [55]. It has been observed that preadsorption of reactants onto the surface of TiO2 during the photocatalytic reaction can expedite the electron transfer process; therefore, it stands to reason that these features make biopolymers inherently suitable adsorbents that have potential to be used in conjunction with TiO2 to facilitate reactant adsorption at the catalyst surface. An added benefit is that natural polymers are abundant, renewable, and biodegradable.
20.4.2 Removal of Azo Dyes
The textile industry is potentially one of the biggest threats to the environment, where water is used for the dissolution and application of chemicals onto the textiles and rinsing. The fabric dyeing process involves steps such as scouring, bleaching, dyeing, dye-fixing, and fabric softening, which are all very water-intensive processes and require large volumes of water [56]. It has been estimated that between 80 and 150 m3 of water are used during the wet process to manufacture 1 kg of fabric and this subsequently translates to 1000–3000 m3 of wastewater generated after processing 12–20 tons of textiles daily [16]. Like many industrial processes, textile dyeing has inherent inefficiencies. The water that remains after the dyeing process is heavily laden with unfixed dye, and depending on the dye type and the fabric, it is estimated that 1–50% of the dye remains unfixed in fabrics, which amounts to nearly 200 000 tons of dyestuff lost in residual liquor annually [16,57].
Removal of organic dyes from wastewater is often considered more urgent than other organic contaminants because colourants present in water, even at minute concentrations, are clearly visible and affect water quality. This wastewater has highly variable content and characteristics depending on the textiles and dyes applied thereto. This variation stems from the fact that numerous other substances are used before and after dyeing: lubricants, stabilizers, caustic soda, peroxide, acetic acid, and detergents are just a few [5]. The multitude of substances used in textiles' manufacture create a situation where, in combination with the dyestuff already present in the water, the wastewater shows notable fluctuation in physicochemical parameters such as color, pH, temperature, salinity, COD, and biochemical oxygen demand (BOD) [57]. Discharging this water into the environment poses major risks to aquatic and terrestrial wildlife because of the resultant eutrophication of water bodies, the limitation of light penetrating contaminated waters, and the harmful byproducts formed when the wastewater is subjected to biotic and abiotic processes that occur in the environment [19].
Azo dyes are strictly of anthropogenic origin; however, some substances containing N-oxide azo (azoxy) are known to occur in nature [58]. They are the outcome of the work of Peter Griess who discovered diazo compounds and went on to prepare the dyes from them [59]. Azo dyes contain at least a single azo group (-N = N); however, they may contain two, three, and in rare cases four azo groups. Figure 20.5–20.7 show typical azo dye structures. Commercially, azo dyes constitute the most important class of dyestuffs as they account for over 60% of the total number of dye manufactured [60,61]. After Griess' discovery in 1858, the first commercially viable azo dye called Bismarck Brown (Figure 20.5) was synthesized by Martius in 1863 [60]. The structure was unknown until Griess established the azo coupling reaction between diazonium ions and amines shortly thereafter [62]. Other structures are shown in Figures 20.6 and 20.7.

Figure 20.5 Structure of Bismarck Brown.

Figure 20.6 Structure of C.I. Acid Red 73.

Figure 20.7 Structure of C.I. Direct Blue 71.
In addition to depreciating the esthetic quality of water bodies, azo dyes and their intermediates have long been established as carcinogens. In 1895, German clinician Ludwig Rehn found that several individuals working in factories producing N-substituted aryl compounds used as intermediates in the dye manufacturing industry had bladder cancer likely caused by exposure to polycyclic aromatic amines such as 2-naphtol thylamine [63]. Other risks of chronic interaction with azo colorants and their intermediates are acute toxicity, causing methemeglobinemia, acute hemolysis, dermatitis, and allergies [64].
The biodegradation of azo dyes occurs in both aerobic and anaerobic bacteria; however, synthetic dyes are generally xenobiotic and the microorganisms occurring in humans, terrestrial and aquatic life, and in rivers and lakes seldom possess enzymes designed by nature to fully mineralize the synthetic dyes [65]. Dyes are manufactured to be thermally, chemically, and photolytically stable; therefore, their photodecomposition in nature is generally slow and depends on factors such as oxygen levels, pH, light intensity, and the chemical structure of the azo dye [66]. The inability of biotic and abiotic processes to fully degrade azo dyes discharged into the environment creates a situation where the compounds will be transformed into aromatic amines that are generally recalcitrant and ultimately, through processes such as sorption into sediment, will bioaccumulate in nature and have a lasting effect on the environment, humans, and aquatic and terrestrial life.
The reasons stated vide supra inform the use of dyes as primary pollutants to study, but another factor informs this decision: in addition to being the target compound for degradation, organic dyes are able to act as photosensitizers, such that when coupled to the semiconductor photocatalyst, they are able to extend the response of the photocatalyst into the visible and near-infrared regions [67]. Azo dyes demonstrate relatively large photoabsorption coefficients and thus measurements in solution are easy even at very low concentrations; as a consequence, they are ideal as model pollutants [68]. Furthermore, the fact that dyes absorb light indicates that the photoreaction could be induced both by visible-light photoabsorption (dye sensitization) and by photoabsorption of a photocatalyst [69].
The urgent need for the sustainable industrialization of heterogeneous photocatalysis has necessitated the development of cheaper and more effective supports. Among them, polysaccharide derivatives such as chitosan have received attention in multiple applications [70]. Polysaccharide biopolymers are an attractive alternative to traditional and synthetic supports, because they are abundant, renewable, and biodegradable [33]. More importantly, their physical and chemical characteristics stemming from the presence of reactive chemical moieties (hydroxyl and acetamido functions) on the polymer chains, endow them chemical stability, high reactivity toward aromatic hydrocarbons and metals, and a capacity to associate by physical and chemical interactions with a wide variety of molecules [55]. Their capacity for physical and chemical association serves as an added advantage in photocatalytic decontamination systems because a good support must have a strong affinity for the adsorption of pollutants in order to concentrate them onto the contact surface [40].
Chitosan is one such polysaccharide derivative that has been used by a number of researchers as an immobilizing support for semiconductors in water purification applications. It was in 2009 that Zainal et al. first reported the combined use of TiO2-chitosan/glass in the photodegradation of methyl orange (MO) under the illumination of visible light [71]. These authors took advantage of the adsorption capacity of chitosan and combined it with the photocatalytic activity of TiO2 to form thin films of TiO2 and chitosan supported on glass. The chitosan–TiO2 composite was prepared by solution blending, and the thin films were made by repeated dip coating and drying in a low-temperature oven (100 °C). They achieved MO removal from 47.9 to 87.0% by increasing the TiO2:chitosan ratios. Their results indicated that catalyst loading plays an important role in the removal efficiency of MO. Furthermore, the removal efficiency was attributed to the reactive NH2, -OH, and metal oxide contents of the composite. It demonstrated a combined adsorption–photodegradation effect stemming from both the chitosan and the increased TiO2 loading; since TiO2 particle surfaces are mainly oxygen atoms with a high electron density, the negative charge readily adsorbs the cationic methyl orange molecules. Their study, however, was yet to evaluate the effects of various important parameters such as initial pollutant concentration, the heat treatment applied to the photocatalyst, light intensity, and pH dependence of the photodegradation–adsorption process. Data on the end products and intermediates was lacking, and thus there was no way of (i) quantifying whether total mineralization of the MO was achieved or if it was merely bleached and (ii) determining if there were any harmful or toxic byproducts formed. The study conducted by Zainal et al. was simple. Development and design of supports has since come a long way since then and more groups have engineered more elegant and sophisticated supports, where pertinent issues such as the polysaccharides' susceptibility to photooxidation by UV light have been interrogated [72].
Nawi et al. made simple yet effective improvements to a similar TiO2-chitosan photosystem [73]. These authors recognized chitosan's vulnerability to photooxidative degradation, where powerful oxidizing radicals generated during photocatalytic processes attack the β-D-(1 → 4) glycosidic linkages (C-O-C) and lead to the formation of carbonyl groups in the chitosan oligomers. The chitosan oligomers obtained from chitosan photooxidative chain scission retained the backbone of the chitosan macromolecular structure and demonstrated improved chemical and physical properties relative to chitosan [72]. Nawi et al. exploited this behavior exhibited by chitosan: exposure to UV light was found to alter not only the chemical structure of chitosan but also its optical properties, such that the spectral response of chitosan was extended to higher wavelengths [74].
By taking advantage of chitosan's susceptibility to photodegradation in combination with its adsorptive capacity, they were able to create a bilayer system by incorporating CS with TiO2. The two-layer system was formed by immobilizing CS on glass followed by TiO2 mixed with epoxidized natural rubber (ENR). They used the bilayer system for the removal of reactive red 4 (RR4) by exploiting the photocatalysis–adsorption synergy extant in the bilayer system. It was found that chitosan demonstrated strong adhesion to the glass substrate as is required of an immobilizing support, and this was attributed to the electrostatic attraction between the positive chitosan biopolymer and the negative silica functional moieties present on the glass plate. In this extensive study, the kinetic, isothermic, and thermodynamic adsorption characteristics of the CS-glass system were evaluated. After optimizing the CS/glass adsorptive system, it was combined with the TiO2 in ENR for the degradation of RR4. The TiO2/glass system performed poorly, where only 11.05% of the dye was removed during 2 h of photocatalytic treatment. This study proved the importance of a support beyond just the immobilization aspect. They showed how the presence of an adsorbent creates a cooperative effect with the photocatalytic system to significantly improve the percentage removal of the RR4 dye by photocatalytic process using the TiO2/CS/glass to have only 1.31% of the dye remaining after 2 h of treatment. The authors attribute this considerable increase in the removal of the dye to the adsorption and photocatalytic processes happening simultaneously, where chitosan adsorbs the RR4 dye molecules and increases their diffusion toward the titania's reactive sites. The kinetics of the system were elucidated using the Langmuir–Hinshelwood model. A rapid decolourization rate by the TiO2/CS/glass was observed and the rate was largely dependent on the TiO2 loading and initial pH of the reaction medium. In subsequent cycles, the TiO2/CS/glass system photocatalytic efficiency decreased due to the saturation of the chitosan adsorption sites. What this study did not address is the issue of the end products of the photocatalytic process. That is: what is the fate of the end products of the RR4 dye. In addition to this is the issue of the robustness and longevity of both the CS substrate and the epoxidized natural rubber used for enhancing the deposition properties of TiO2 powder, that is, what becomes of CS and ENR and if there is leaching of the titania when CS and ENR degrade due to prolonged exposure to UV light.
Cellulose and its derivatives have also received attention in a wide variety of applications outside the traditional paper and textiles industry such as water filtration membranes, biomedical applications, and in later years as magneto-responsive composites, bioimaging materials, and supports for catalysts [75]. Several groups have tested the ability of cellulosic polymers to act as immobilization supports and coadsorbents for various semiconductor-mediated heterogeneous photocatalysts [76–78].
During the design of immobilizing supports, attention must be given to attaching the photocatalyst particles on a support without compromising its reactivity. However, immobilizing powdered photocatalyst reduces the photocatalyst activity due to the decrease in reactive sites and traditional supports are vulnerable to photooxidative degradation, and thus the current challenge: a convenient synthesis of a photocatalyst that retains the same level of activity as its powder counterpart on a stable support.
Jin et al. fabricated a flexible hybrid film using cellulose acetate (CA) and TiO2 [79]. The composite film was designed for use first as a recyclable photocatalyst and ultimately to be optimized for use as a self-cleaning material or in other applications. Mesoporous anatase TiO2 microspheres were synthesized using a facile solvothermal technique. The as-prepared TiO2 powder was subsequently coated onto the CA gelatum to form the hybrid TiO2-cellulose acetate (TCA) film. The photocatalytic activity was evaluated using methylene blue (MB) as a model dye. It is reported that the TCA film with a 0.01 g TiO2 catalyst load degraded almost all the MB after 3 h, which demonstrated excellent performance when compared with a similar study where 0.1 g of ZnO was used while keeping all the experimental conditions the same [80].
This study also demonstrates the importance of an immobilizing support in concentrating the pollutants onto the substrate for greater accessibility to the photocatalyst, that is, the photocatalysis proccess consists of two parts: adsorption and degradation, where the dye is initially adsorbed onto the surface of the photocatalyst and the photogenerated oxidizing radicals react with the organic compound to mineralize it to carbon dioxide and water. In addition to this, the TCA film exhibits good recyclability, where the cellulose acetate film was stable in water and more so, after being transferred to a new dye system for three cycles, the degradation rates of the MB barely showed a decrease and thus the TCA film remains photocatalytically active and the photocatalyst is retained on the cellulose acetate support. The study further reports that the cellulose acetate film shows some photodegradation effect on the model dye and long-term stability in water during exposure to UV irradiation. The work carried out by Jin et al. was thorough and yielded a proof of concept in our endeavours for sustainable and economically viable green techniques for the fabrication of an efficient photocatalytic system, where the obstacle of photocatalyst retention, recycling, and recovery has been circumvented.
In another study by Sökmen et al., TiO2 was directly immobilized onto and into a biodegradable polymer [81]. This is attractive from a materials' processing point of view in that the method is simple and easily scalable and because biodegradable polymers present a good alternative to synthetic nonbiodegradable polymeric matrices for environmental reasons previously stated at length. Polycaprolactone (PCL) is a readily available, reasonably cheap aliphatic polyester and despite the fact that it is a petroleum-based polymer, it is biodegradable and biocompatible, which has made it suitable for biomedical applications such as drug delivery and tissue engineering [82].
The authors recognized the characteristics of PCL such as its water, oil, solvent, and chlorine resistance, relatively easy processability, good mechanical properties, and the fact that it takes up to 2 years to completely break down making it suitable for longer term applications. However, shortcomings such as low melting temperature present a problem as far as the fabrication of photocatalytically active films is concerned. Physical and chemical methods used to fix photoactive titania powders such as sputtering, electrophoretic deposition, spray pyrolysis, CVD, and thermal oxidation are inappropriate for use on polymeric substrates, because these methods require high-temperature treatments to obtain the desired photoactive titania layer with strong adhesion to the substrate [83].
Sökmen et al. found that wet-coating fixation methods such as spray or dip coating of nanocrystalline sols present a viable avenue for the immobilization of TiO2 onto a polymeric substrate. They were able to immobilize anatase TiO2 nanoparticles into and onto PCL using solvent casting to fabricate a biodegradable and photocatalytically active material that was able to remove a typical model dye, methylene blue, and also possessed antimicrobial properties as demonstrated by its ability to kill Candida albicans, again a model fungus of medical importance. The fabricated materials were successful in the removal of MB, where TiO2 immobilized into PCL (PCL-1) showed an 83.2% MB removal and TiO2 immobilized onto PCL (PCL-2) had a 94.2% MB removal efficiency. This result is credible since it has already been established that direct mixing of the catalyst with the polymer solution significantly reduces the available reactive surface sites, therefore, the photocatalytic activity [75]. PCL-2 also had a killing efficiency of 54% for the C. albicans under near visible-light irradiation after only 60 min of exposure.
The authors took into account the elements important to a good support: capacity of the support to adsorb organic contaminants and the supports' chemical inertness and resistance to photooxidative degradation. PCL exhibited minimal degradative changes after long-term UV and visible-light exposure corresponding to a slight shift in the melting point as revealed by the thermogravimetric analyses. ATR-FTIR spectra of the polymer post-treatment of MB solutions revealed that PCL and TiO2 complement each other in their functions, where MB was adsorbed by PCL and significantly decomposed by TiO2.
A problem with the use of PCL is its low melting point. As seen by morphological changes after UV exposure causing some localized heating that in turn deforms/melts the composite film surface. The SEM analysis of PCL-2 revealed that the addition of TiO2 to PCL creates an uneven surface with TiO2 nanoparticle aggregates, visible cracks, and pinholes on the hybrid film, combined with the low compatibility of TiO2 with PCL. These results highlight the other problem with the use of organo-inorganic metal oxide composite materials is that the degree of homogeneity significantly influences and possibly commands the ultimate properties of the composites. Therefore, in terms of material development and processing, issues such as the scale of mixing and dispersion of the nanoparticles into polymeric matrices could be resolved via the use of alternative techniques such as autoclaving or ultrasonication of the polymer-titania mixture prior to casting.
The TiO2–PCL composite has promise and needs to be explored further because of its demonstrable photocatalytic and antimicrobial efficacy as well as the fact that it requires no additional pH adjustments of the reaction solution but more so because it is simple to manufacture and addresses the cost reduction factor that remains an obstacle to the industrialization of photocatalysis technology.
20.4.3 Removal of Phenolic Compounds
Phenol and its derivatives are a group of substances used as starting materials in many chemical industries, where typical examples include additives for rubber precursors, emulsifiers, dyes, detergents, adhesives, flavors, and impregnating resins [84]. Other phenol derivatives such as chlorophenols form some major sources of environmental pollution and are used in the chlorination of municipal and industrial wastewater, as well as in the chlorination of lignin used in pulp and paper mill plants and some are formed during the degradation of complex chlorinated hydrocarbons and herbicides [85].
Phenolic compounds (Figure 20.8) represent a class of recalcitrant and highly toxic pollutants that are present in industrial effluents discharged from a variety of industries and are known to be carcinogenic, teratogenic, mutagenic, endocrine disrupting, and toxic [86]. Therefore, wastewater containing phenolics must be treated properly in order to prevent the release of these xenobiotic substances into the environment.
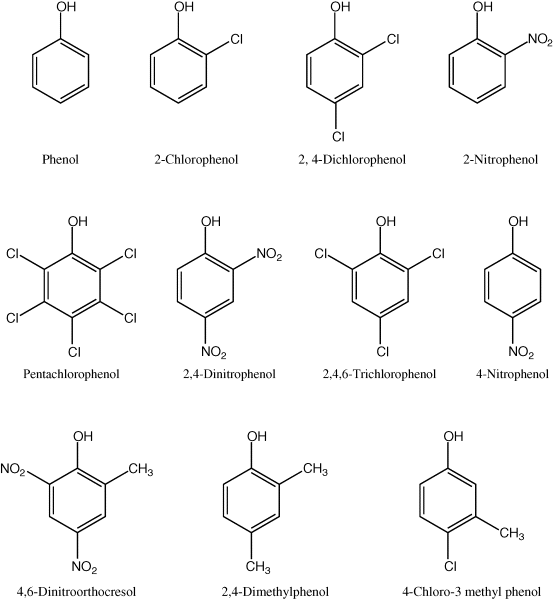
Figure 20.8 Chemical structures of some phenolic compounds classified as priority pollutants.
Several methods including membrane extraction, distillation, adsorption, ozonation, electrochemical methods, pervaporation, liquid–liquid extraction, and biological-based processes have been applied for the removal of phenolics [84]. The problem with these methods, although generally effective, is the operational costs involved on account of high energy inputs, chemicals usage, long-term maintenance, and mechanical complexity [86]. The refractory nature of these contaminants and their inclination to transform into other toxic intermediates and metabolites that accumulate in the environment necessitates their complete and effective removal.
Material scientists and engineers have strived for decades to develop biopolymers that are commercially and technically worthwhile compared to their synthetic petroleum-based counterparts. However, their widespread application remains inundated with limitations inherent to biopolymers such as poor thermal and physical properties that lead to their poor processibility, brittleness, hydrophilicity, poor moisture and gas barrier, subpar compatibility, and electrical properties.
Chemical modification is typically employed to overcome these inherent limitations. Plasticizers, for example, are additives used to reduce brittleness, impart flexibility, improve toughness, reduce crystallinity, and thereby lower the glass transition and melting temperatures. In addition, plasticizers reduce rigidity and thus improve processibility, flexibility, durability, and may reduce the cost of polymers [87]. However, the drawback with plasticizers is that they tend to leach out and migrate from the polymer and be released into the environment during common usage, and this constitutes a major safety risk [88].
Another common form of chemical modification of biopolymers is cross-linking that offers an alternative pathway for the production of biopolymers with increased stability. The term cross-linking means chemically or physicochemically making intramolecular or intermolecular linkages in a polymer material to form a network structure, with the cross-linking agent being a substance having a certain reactivity with said polymer material that is added in order to carry out that cross-linking reaction [89]. Cross-linking is an easy approach that can be used to modify the physicochemical characteristics of biopolymers such as the adsorption and hydration properties due to the alteration of the surface area and surface chemical properties [90]. Polysaccharrides, for example, are relatively easily cross-linked by a reaction between the hydroxyl or amino groups of the chains with a coupling agent to form water-insoluble cross-linked networks [55].
Recently, Jawad and Nawi conducted a study in which they fabricated a cross-linked chitosan-epichlorohydrin (CS-ECH) film (Figure 20.9) and immobilized TiO2 onto the CS-ECH film and photooxidized it by irradiating it with UV light. The TiO2/oxidized CS-ECH film was subsequently evaluated for the photocatalytic degradation of phenol [91]. What makes their study interesting is that they combined relatively simple techniques to tune the properties of a common material.
Unmodified chitosan has limitations such as shrinkage, deformation after drying, compressibility at high operating pressures, and solubility in many organic acids, thus cross-linking is one avenue through which the surface chemistry, morphology, and physicochemical properties of polysaccharides can be tuned such that the mechanical resistance is reinforced, the biopolymer is stable in acidic solutions, and the hydrophobicity and swelling capacity are decreased [92].
Despite it being considered a hazardous environmental pollutant, epichlorohydrin is one of the popular cross-linking agents finding use in industry as a chemical intermediate in the synthesis of various products ranging from epoxy-resins to drugs [55]. Epichlorohydrin is a bifunctional molecule and is highly reactive with hydroxyl groups. Its popularity as a cross-linking agent in polysaccharides such as chitosan stems from the fact that it does not react with the free amine groups in chitosan that are responsible for its cationic function and thus its major pollutant adsorption sites would remain available [93]. After forming the homogeneously cross-linked chitosan-ECH film, the authors oxidized the said film through photomodification by using the immobilized TiO2 and irradiating the film with UV light in water and air under ambient conditions at solution pH 6.6 and 25 °C (Figure 20.9). This study is interesting in the context of green chemistry and from the materials processing perspective in that it is facile and yields a material that has enhanced optical properties, ionic conductivity, hydrophobicity, and chemical stability.

Figure 20.9 Schematic representation for the homogeneous cross-linking reaction of CS with ECH and the most likely structures of the oxidized CS-ECH (reproduced with permission from Ref. [91]. Copyright 2012, Elsevier).
After irradiation, the CS-ECH film showed signs of oxidation such as a change in color from a clear transparent film, to a dark brown color that was attributed to the formation of carbonyl chromophore in the polymer structure of the oxidized CS-ECH film, which is typical of photooxidized polysaccharides such as chitosan. The qualitative visual findings were confirmed by diffuse reflectance UV–visible spectroscopy and photoluminescence (PL) analysis, where postirradiation, absorption bands assigned to the amine groups for the fresh CS-ECH films had decreased as a result of the elimination of the amine groups. A new absorption band appeared that was attributed to a newly formed carbonyl chromophore in the oxidized CS-ECH film. It is the formation of this carbonyl chromophore in the oxidized CS-ECH film that was responsible for the remarkable reduction in emission intensity and the observed low photoluminescence yield.
For the purposes of using biopolymers in water treatment applications, an important characteristic is swelling. The swelling of cross-linked polymeric substrates is a phenomenon in which a polymer is able to absorb water as a result of its hydrophilic functional groups attached to its backbone, but it remains resistant to dissolution as a result of the cross-links between the network chains [94]. Biopolymers such as chitosan contain basic amine groups that form part of the basis of acid-induced swelling or rather the protonation of the amino group. The study found that the oxidized CS-ECH film had a lower swelling index (SI) than the fresh CS-ECH film and it had become more hydrophobic. This decreased swelling index and related increase in hydrophobicity can be attributed to the decrease (32%) in the basic amino groups. Furthermore, the oxidized CS-ECH film showed lower bulk impedance and higher ionic conductivity than its unirradiated counterpart. The authors attribute this phenomenon to the new carbonyl group in the polymeric structure of the irradiated CS-ECH film. Improved ionic conductivity is a boon in a photocatalytic system where rapid charge carrier mobility is critical for sustained effective photocatalysis.
The TiO2/oxidized CS-ECH system was evaluated for the photocatalytic removal of 10 mg·l−1 phenol. Adsorption studies undertaken to investigate the adsorption–photocatalysis synergistic effects indicated no adsorption of phenol to be taking place on the photocatalyst system. Instead, with exposure to light, the phenol degradation increased with time, and thus the removal of phenol was mainly due to photocatalysis. The TiO2/oxidized CS-ECH film was able to completely mineralize the phenol within 240 min.
The TiO2/oxidized CS-ECH system was found to be superior to its unoxidized TiO2/CS-ECH counterpart and the systems from a previous study by the same group where chitosan was not cross-linked with epichlorohydrin (TiO2/CS), which is more evidence that cross-linking does reinforce the chemical stability of chitosan. An important outcome of this study is the higher photocatalytic activity observed for the TiO2/oxidized CS-ECH system relative to the TiO2/oxidized-CS system. This marked difference in performance is due to a decrease in the photoinduced electron–hole pair recombination rate on the TiO2 surface. The authors qualify this result based on the PL analysis, where the photoluminescence intensity of the TiO2/oxidized CS-ECH was lower than that of the TiO2/oxidized-CS. This is a demonstration of the usefulness of cross-linking in biopolymers, not only to improve physical characteristics but also to improve the charge carrier separation, overcome swelling inherent to natural chitosan, and thereby prevent the leaching of the photocatalyst from the polymeric matrix. This study is one example of a highly viable immobilized photocatalytic system, where in addition to the characteristics previously listed, the support is physically strong as it does not suffer from photooxidative degradation during the depollution process.
20.4.4 Removal of Natural Organic Matter
The inclusion of NOM, or rather attempts at its photocatalytic degradation, deserves a mention because of the complex and dynamic nature of NOM. That is (i) unlike other synthetic pollutants, NOM characteristics and properties differ according to the origin of water sources, climate, hydrological regime, and other environmental factors; (ii) NOM and its analogs have remained difficult to define because of their complex composition and structure and thus equally difficult to quantify; and (iii) the effective and complete photocatalytic degradation of target pollutants is adversely affected by the presence of NOM and its analogs (e.g., humic and fulvic acids) because they are known scavengers of the oxidizing radicals such as -OH• responsible for the degradation of the target pollutants [95]. In addition to this, NOM itself is harmless; however, its conversion, especially humic acid, into potentially carcinogenic disinfection by-products (DBPs) in the form of trihalomethanes, haloacetic acids, and other halogenated compounds when chlorine is used is a major concern regarding NOM in water sources [96].
NOM is present in all surface, ground-, and soil waters and is formed from the decomposition of plant and animal residues and from microbial activity [96]. It is a complex mixture of organic compounds with highly variable chemical composition and molecular sizes [97]. Natural organic matter found in natural waters can be divided into fractions of TOC, which in turn consists of dissolved organic carbon (DOC) and particulate organic carbon (POC). The DOC fraction present in natural water is mainly comprised of hydrophobic and hydrophilic components – the hydrophobic component represents about 50% of DOC, while the hydrophilic component forms between 25 and 40% – and the remaining fraction is transphilic organic matter. The hydrophobic and hydrophilic components can be further split into three different classes, namely, acid, base, and neutral that have different chemical groups where the hydrophobic class is rich with aromatic carbon, phenolic structures, and conjugated double bonds, while the hydrophilic class contains more aliphatic carbon and nitrogenous compounds. Examples of hydrophobic acids are humic and fulvic acids, while carboxylic and polyuronic acids fall within the hydrophilic acids' category [97,98].
Over the years, several researchers have made attempts at the delineation of NOM and its analogs. A literature survey shows that NOM categories are generally operationally defined, and this is based on fractionation procedures and application or research reviews specificity [99,100]. Figure 20.10 presents a simplistic relationship between NOM fractions and the chemical analogs.
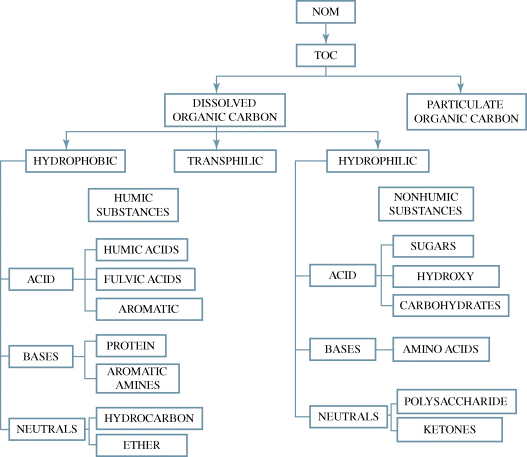
Figure 20.10 Relationship between NOM fractions and its chemical groups. Source: http://dx.doi.org/10.12983/ijsres-2014-p0094-0106. Licensed under CC BY 3.0.
Methods that have been used to remove NOM from water include ion exchange, sorption, coagulation, flotation, bioflocculation, and advanced oxidation processes such as O3/H2O2, O3/UV, UV/H2O2, H2O2/catalyst, Fenton, and photo-Fenton processes. However, these methods suffer from certain drawbacks such as relatively high operational costs, instability, and safety as a result of the large volumes of chemicals required [101]. In light of this, TiO2-mediated heterogeneous photocatalysis technology has been explored as a potential solution in the effective destruction of natural organic matter [95].
Recently, two reviews were published in which advanced oxidation processes, in particular, titania-mediated heterogeneous photocatalysis technology, were applied for the removal and decomposition of natural organic matter and its chemical analogs from water [95,97]. Considering the exhaustive nature of these reviews, what stood out was that of the immobilized TiO2 photocatalyst systems evaluated, there was none that used biopolymers as supports. Further literature searches did not yield results. However, in as far as endeavours for facile, green methods of commercializing photocatalysis technology in the application of pollutants decontamination are concerned, work that deserves mention is the one conducted by Remoundaki et al. [102]. They developed solar concentric parabolic collectors (CPC) and a UV 254 nm reactor in which they evaluated the photolytic and photocatalytic alterations of humic substances by TiO2 in both suspended and immobilized forms. TiO2 was immobilized using a commercial 1049 Ahlstrohm paper loaded with 20 g·m−2 of TiO2 Degussa P25. The adsorption results conducted in the dark showed that 60% of the humic acid (HAA) was adsorbed onto the catalyst surface, which in turn assists the photocatalysis process by concentrating contaminants onto the surface. Furthermore, the immobilized TiO2 in the CPC reactor exposed to solar light had a 90% phototransformation of commercially purchased humic acid at accumulated energy of 2 MJ·m−2 l−1. The photocatalysis experiments carried out in the CPC reactor, however, had a significant problem of residual TOC the authors were yet to investigate further.
The study itself was rather rudimentary; however, it is an illustration of how simple materials can be applied to solving potentially complex problems, with the case in point being the photodegradation of NOM. The commercial nonwoven paper, referred to herein as the “media 1049” produced by AHLSTROM is composed of varying percentages of cellulose and synthetic fibers coated with silica as an inorganic binder and titanium dioxide [103]. The efficacy of cellulose-based and paper pulp photoactive paper has been illustrated by its use in various other pollutant decontamination studies [104–106].
20.5 Conclusions
Nanotechnology has afforded scientists and technologists vast scope and opportunity for the design and development of materials and technologies that would remediate existent wastewater and polluted water sources. The engineering of materials at the atomic level has opened up avenues for scientists to not only mitigate pollution but also find preventative solutions, where production of undesirable, harmful by-products in manufacturing processes can be reduced.
In an effort to “return to nature,” scientists and technologists are increasingly focusing their research endeavours on developing bio-based feedstocks and harnessing abundant, renewable naturally occurring polymers in order to substitute and/or complement synthetic polymers made exclusively from petroleum feedstock in a multitude of applications. Water depollution and environmental remediation are applications in which the use of environmentally benign materials is essential. Research efforts have afforded biopolymers technical and commercial success; however, their extensive use remains challenged by their inherent limitations. The challenges that hinder the commercialization of biopolymers as photocatalyst supports must be overcome. One avenue of overcoming these drawbacks is the creation of organic–inorganic hybrid materials and harnessing their combined superior physicochemical properties.
This chapter has reflected on the viability and robustness of biopolymers as an alternative to their traditionally employed synthetic polymer counterparts. However, in all instances, the common thread is the fabrication of robust biopolymer supports via easily scalable routes.
Ackowledgments
The authors are grateful to the National Research Foundation (NRF) of South Africa, the Water Research Commission (WRC), and the University of South Africa for providing financial support.
References
- 1 WWF (Undated) Threats: Water Scarcity. http://www.worldwildlife.org/threats/water-scarcity (accessed 30 October 2016).
- 2 USGS (2016) How Much Water is There On, In, and Above the Earth? http://water.usgs.gov/edu/earthhowmuch.html (accessed on 27 October 2016).
- 3 United Nations (1987) Report of the World Commission on Environment and Development: Our Common Future.
- 4 Gupta, V.K. et al. (2012) Chemical treatment technologies for waste-water recycling-an overview. RSC Adv., 2 (16), 6380–6388.
- 5 Ntuli, F., Ikhu-Omoregbe, D., Kuipa, P.K., Muznda, E., and Belaid, M. (2009) Characterization of effluent from textile wet finishing operations, in World Congress on Engineering and Computer Science, Newswood Limited, San Francisco, USA.
- 6 Eckenfelder, W.W. (2000) Industrial Water Pollution Control, 3rd edn, McGraw-Hill.
- 7 Holkar, C.R. et al. (2016) A critical review on textile wastewater treatments: possible approaches. J. Environ. Manag., 182, 351–366.
- 8 George, Z., Kyzas, M.K., Nikolaos, K. L., and Dimitrios, N. B. (2013) Decolorization of dyeing wastewater using polymeric absorbents: an overview, in Eco-Friendly Textile Dyeing and Finishing (ed. M. Gunay), InTech Open.
- 9 Zangeneh, H. et al. (2015) Photocatalytic oxidation of organic dyes and pollutants in wastewater using different modified titanium dioxides: a comparative review. J. Ind. Eng. Chem., 26, 1–36.
- 10 Robinson, T. et al. (2001) Remediation of dyes in textile effluent: a critical review on current treatment technologies with a proposed alternative. Bioresour. Technol., 77 (3), 247–255.
- 11 Hai, F.I., Yamamoto, K., and Fukushi, K. (2007) Hybrid treatment systems for dye wastewater. Crit. Rev. Environ. Sci. Technol., 37 (4), 315–377.
- 12 Nawaz, M.S. and Ahsan, M. (2014) Comparison of physico-chemical, advanced oxidation and biological techniques for the textile wastewater treatment. Alexandria Eng. J., 53 (3), 717–722.
- 13 Shu, H.-Y. and Chang, M.-C. (2005) Decolorization effects of six azo dyes by O3, UV/O3 and UV/H2O2 processes. Dyes Pigm., 65 (1), 25–31.
- 14 Aleboyeh, A., Aleboyeh, H., and Moussa, Y. (2003) “Critical” effect of hydrogen peroxide in photochemical oxidative decolorization of dyes: acid orange 8, acid blue 74 and methyl orange. Dyes Pigm., 57 (1), 67–75.
- 15 Lucas, M.S. and Peres, J.A. (2006) Decolorization of the azo dye Reactive Black 5 by Fenton and photo-Fenton oxidation. Dyes Pigm., 71 (3), 236–244.
- 16 Ghaly, A.E., Ananthashankar, R., Alhattab, M., and Ramakrishan, V.V. (2014) Production, characterization and treatment of textile effluents: a critical review. J. Chem. Eng. Process Technol., 5 (1), 1–19.
- 17 Konstantinou, I.K. and Albanis, T.A. (2004) TiO2-assisted photocatalytic degradation of azo dyes in aqueous solution: kinetic and mechanistic investigations: a review. Appl. Catal. B, 49 (1), 1–14.
- 18 Linsebigler, A.L., Lu, G., and Yates, J.T. (1995) Photocatalysis on TiO2 surfaces: principles, mechanisms, and selected results. Chem. Rev., 95 (3), 735–758.
- 19 Akpan, U.G. and Hameed, B.H. (2009) Parameters affecting the photocatalytic degradation of dyes using TiO2-based photocatalysts: a review. J. Hazard. Mater., 170 (2–3), 520–529.
- 20 Rauf, M.A. and Ashraf, S.S. (2009) Fundamental principles and application of heterogeneous photocatalytic degradation of dyes in solution. Chem. Eng. J., 151 (1–3), 10–18.
- 21 Ivanova, E.P., Bazaka, K., and Crawford, R.J. (2014) 2-Natural polymer biomaterials: advanced applications, in New Functional Biomaterials for Medicine and Healthcare, Woodhead Publishing, p. 32–70.
- 22 Yarsley, V.E. and Couzens, E.G. (1945) Plastics, Penguin Books Limited, Middlesex.
- 23 Oehlmann, J. et al. (2009) A critical analysis of the biological impacts of plasticizers on wildlife. Philos. Trans. Roy. Soc. B, 364 (1526), 2047.
- 24 Thompson, R.C. et al. (2009) Our plastic age. Philos. Trans. Roy. Soc. B, 364 (1526), 1973.
- 25 Cheremisinoff, P. (1997) Handbook of Engineering Polymeric Materials, Taylor & Francis.
- 26 Rubinstein, M. (2010) Polymer physics – the ugly duckling story: will polymer physics ever become a part of “proper” physics? J. Polym. Sci. B, 48 (24), 2548–2551.
- 27 Osswald, T.A. and Garcia-Rodriguez, S. (2011) History of sustainable bio-based polymers, in A Handbook of Applied Biopolymer Technology: Synthesis, Degradation and Applications, The Royal Society of Chemistry, Ch. 1, pp. 1–21.
- 28 Jacob John, M. and Thomas, S. (2012) Natural polymers: an overview, in Natural Polymers: Volume 1: Composites, The Royal Society of Chemistry, Ch. 1, pp. 1–7.
- 29 Teramoto, N. (2011) Synthetic green polymers from renewable monomers, in A Handbook of Applied Biopolymer Technology: Synthesis, Degradation and Applications, The Royal Society of Chemistry, Ch. 2, pp. 22–78.
- 30 Ravenstijn, J. (2010) Bioplastics in consumer electronics. Ind. Biotechnol., 6 (5), 252–263.
- 31 ASTM (2011) ASTM D6866-11 Standard Test Methods for Determining the Biobased Content of Solid, Liquid, and Gaseous Samples Using Radiocarbon Analysis. ASTM International: West Conshohocken, Pennsylvania USA.
- 32 Vert, M. et al. (2012) Terminology for biorelated polymers and applications (IUPAC Recommendations 2012). Pure Appl. Chem.., 84 (2), 377–410.
- 33 Niaounakis, M. (2015) Biopolymers: Applications and Trends, Elsevier Science.
- 34 Reddy, M.M. et al. (2013) Biobased plastics and bionanocomposites: current status and future opportunities. Prog. Polym. Sci., 38 (10–11), 1653–1689.
- 35 Thomas, S. et al. (eds) (2013) Handbook of Biopolymer-Based Materials: From Blends and Composites to Gels and Complex Networks, Wiley-VCH Verlag GmbH.
- 36 Satyanarayana, K.G., Arizaga, G.G.C., and Wypych, F. (2009) Biodegradable composites based on lignocellulosic fibers: an overview. Prog. Polym. Sci., 34 (9), 982–1021.
- 37 Kazuhito, H., Hiroshi, I., and Akira, F. (2005) TiO2 photocatalysis: a historical overview and future prospects. Jpn. J. Appl. Phys., 44 (12R), 8269.
- 38 CEFIC (2012) About Titanium Dioxide, Titanium Dioxide Stewardship Council, Washington, D.C., 20037 pp. 8.
- 39 Xu, Y.-J., Zhuang, Y., and Fu, X. (2010) New insight for enhanced photocatalytic activity of TiO2 by doping carbon nanotubes: a case study on degradation of benzene and methyl orange. J. Phys. Chem. C, 114 (6), 2669–2676.
- 40 Singh, S., Mahalingam, H., and Singh, P.K. (2013) Polymer-supported titanium dioxide photocatalysts for environmental remediation: a review. Appl. Catal., A, 462–463, 178–195.
- 41 Dionysiou, D.D. et al. (2016) Photocatalysis: Applications, Royal Society of Chemistry.
- 42 Silva, F.V., Lansarin, M.A., and Moro, C.C. (2012) A comparison of slurry and inmobilized TiO2 in the photocatalytic degradation of phenol. Lat. Am. Appl. Res., 42 (3), 275–280.
- 43 de Lasa, H., Serrano, B., and Salaices, M. (2006) Photocatalytic Reaction Engineering, Springer, US.
- 44 Han, H. and Bai, R. (2010) Highly effective buoyant photocatalyst prepared with a novel layered-TiO2 configuration on polypropylene fabric and the degradation performance for methyl orange dye under UV–vis and vis lights. Sep. Purif. Technol., 73 (2), 142–150.
- 45 Bideau, M. et al. (1995) On the “immobilization” of titanium dioxide in the photocatalytic oxidation of spent waters. J. Photochem. Photobiol. A, 91 (2), 137–144.
- 46 Augugliaro, V. et al. (2006) The combination of heterogeneous photocatalysis with chemical and physical operations: a tool for improving the photoprocess performance. J. Photochem. Photobiol. C, 7 (4), 127–144.
- 47 Shan, A.Y., Ghazi, T.I.M., and Rashid, S.A. (2010) Immobilisation of titanium dioxide onto supporting materials in heterogeneous photocatalysis: a review. Appl. Catal. A, 389 (1–2), 1–8.
- 48 Fabiyi, M.E. and Skelton, R.L. (2000) Photocatalytic mineralisation of methylene blue using buoyant TiO2-coated polystyrene beads. J. Photochem. Photobiol. A, 132 (1–2), 121–128.
- 49 Robert, D., Keller, V., and Keller, N. (2013) Immobilization of a semiconductor photocatalyst on solid supports: methods, materials, and applications, in Photocatalysis and Water Purification, Wiley-VCH Verlag GmbH, pp. 145–178.
- 50 Paz, Y. (2010) Application of TiO2 photocatalysis for air treatment: patents' overview. Appl. Catal. B, 99 (3–4), 448–460.
- 51 Tryba, B. (2008) Immobilization of TiO2 and Fe–C–TiO2 photocatalysts on the cotton material for application in a flow photocatalytic reactor for decomposition of phenol in water. J. Hazard. Mater., 151 (2–3), 623–627.
- 52 Tennakone, K., Tilakaratne, C.T.K., and Kottegoda, I.R.M. (1995) Photocatalytic degradation of organic contaminants in water with TiO2 supported on polythene films. J. Photochem. Photobiol. A, 87 (2), 177–179.
- 53 Shahnawaz, M., Sangale, M.K., and Ade, A.B. (2016) Bacteria-based polythene degradation products: GC-MS analysis and toxicity testing. Environ. Sci. Pollut. Res., 23 (11), 10733–10741.
- 54 White, R.J. et al. (2009) Supported metal nanoparticles on porous materials. Methods and applications. Chem. Soc. Rev., 38 (2), 481–494.
- 55 Crini, G. (2005) Recent developments in polysaccharide-based materials used as adsorbents in wastewater treatment. Prog. Polym. Sci., 30 (1), 38–70.
- 56 Şen, S. and Demirer, G.N. (2003) Anaerobic treatment of real textile wastewater with a fluidized bed reactor. Water Res., 37 (8), 1868–1878.
- 57 Chequer, F.M.D. et al. (2013) Textile Dyes: Dyeing Process and Environmental Impact, INTECH Open Access Publisher.
- 58 Hunger, K. (ed.) (2003) Industrial Dyes: Chemistry, Properties, Applications, Wiley-VCH Verlag GmbH, p. 648.
- 59 Griess, P. (1858) Vorläufige notiz über die einwirkung von salpetriger säure auf amidinitro- und aminitrophenylsäure. Ann. Chem. Pharm., 106, 123.
- 60 Gordon, P.F. and Gregory, P. (1983) Organic Chemistry in Colour, Springer-Verlag, Berlin.
- 61 Allen, R.L.M. (1971) Colour Chemistry, Springer, p. 336.
- 62 David, G.H. and Waring, R. (eds) (1990) The chemistry and application of dyes, in Topics in Applied Chemistry, 1st edn, Plenum Press, New York, Springer, US, p. 430.
- 63 Dietrich, H.G. and Golka, K. (2012) Bladder tumors and aromatic amines: historical milestones from Ludwig Rehn to Wilhelm Hueper. Front. Biosci. Elite, 4 (1), 279–288.
- 64 Fishbein, L. (1984) Aromatic Amines, in Anthropogenic Compounds, Springer, Berlin, pp. 1–40.
- 65 Zollinger, H. (1987) Color Chemistry: Syntheses, Properties and Applications of Organic Dyes and Pigments, 1 edn, VCH Publishers, New York, pp. 197.
- 66 Dayan, J. and Trebitz, H. (1995) Health and Environmental Information on Dyes Used in Canada. An Overview to Assist in the Implementation of the New Substances Notification Regulation under the Canadian Environmental Protection Act. Ecological and Toxicological Association of Dyes and Organic Pigments Manufacturers, Canada.
- 67 Cushing, S.K. et al. (2012) Photocatalytic activity enhanced by plasmonic resonant energy transfer from metal to semiconductor. J. Am. Chem. Soc., 134 (36), 15033–15041.
- 68 Otutu, J.O. and Osabohien, E. (2013) Synthesis and absorption spectra of monoazo dyes derived from 2-methoxy-5-nitroaniline. Asian J. Mater. Sci., 5, 1–8.
- 69 Baudys, M. et al. (2012) Notes on heterogeneous photocatalysis with the model azo dye acid orange 7 on TiO2. React. Kinet. Mech. Catal., 106 (2), 297–311.
- 70 Lee, M., Chen, B.-Y., and Den, W. (2015) Chitosan as a natural polymer for heterogeneous catalysts support: a short review on its applications. Appl. Sci., 5 (4), 1272.
- 71 Zainal, Z. et al. (2009) Characterization of TiO2–chitosan/glass photocatalyst for the removal of a monoazo dye via photodegradation–adsorption process. J. Hazard. Mater., 164 (1), 138–145.
- 72 Wang, S.-M., Huang, Q.-Z., and Wang, Q.-S. (2005) Study on the synergetic degradation of chitosan with ultraviolet light and hydrogen peroxide. Carbohydr. Res., 340 (6), 1143–1147.
- 73 Nawi, M.A. et al. (2010) Adsorption of reactive red 4 by immobilized chitosan on glass plates: towards the design of immobilized TiO2–chitosan synergistic photocatalyst-adsorption bilayer system. Biochem. Eng. J., 49 (3), 317–325.
- 74 Sionkowska, A. et al. (2004) The photochemical stability of collagen–chitosan blends. J. Photochem. Photobiol. A, 162 (2–3), 545–554.
- 75 Wittmar, A. et al. (2015) Routes towards catalytically active TiO2 doped porous cellulose. RSC Adv., 5 (45), 35866–35873.
- 76 Zhou, Z. et al. (2016) Electrospun cellulose acetate supported Ag@AgCl composites with facet-dependent photocatalytic properties on degradation of organic dyes under visible-light irradiation. Carbohydr. Polym., 136, 322–328.
- 77 Gadiyar, C., Boruah, B., Mascarenhas, C., and Shetty, V.K. (2013) Immobilized nano TiO2 for photocatalysis of acid yellow-17 dye in fluidized bed reactor. Proceedings of National Conference on ‘Women in Science & Engineering’ (NCWSE 2013), SDMCET Dharwad. Sri Dharmasthala Manjunatheshwara College of Engineering and Technology, Dharwad, India: INPRESSCO.
- 78 Prado, A.G.S. et al. (2005) Ammonium complex of niobium as a precursor for the hydrothermal preparation of cellulose acetate/Nb2O5 photocatalyst. J. Mol. Catal. A, 237 (1–2), 115–119.
- 79 Jin, X. et al. (2014) Flexible TiO2/cellulose acetate hybrid film as a recyclable photocatalyst. RSC Adv., 4 (25), 12640–12648.
- 80 Xu, J. et al. (2011) Zinc-oleate complex as efficient precursor for 1-D ZnO nanostructures: synthesis and properties. CrystEngComm, 13 (7), 2629–2635.
- 81 Sökmen, M. et al. (2011) A new nano-TiO2 immobilized biodegradable polymer with self-cleaning properties. J. Hazard. Mater., 187 (1–3), 199–205.
- 82 Elzubair, A. et al. (2006) The physical characterization of a thermoplastic polymer for endodontic obturation. J. Dent., 34 (10), 784–789.
- 83 Yaghoubi, H., Taghavinia, N., and Alamdari, E.K. (2010) Self cleaning TiO2 coating on polycarbonate: surface treatment, photocatalytic and nanomechanical properties. Surf. Coat. Technol., 204 (9–10), 1562–1568.
- 84 Farhod Chasib, K. (2013) Extraction of phenolic pollutants (phenol and p-chlorophenol) from industrial wastewater. J. Chem. Eng. Data, 58 (6), 1549–1564.
- 85 Igbinosa, E.O. et al. (2013) Toxicological profile of chlorophenols and their derivatives in the environment: the public health perspective. Sci. World J., 2013, 11.
- 86 Ansari, A.A. et al. (2016) Phytoremediation: Management of Environmental Contaminants, Springer International Publishing.
- 87 Mekonnen, T. et al. (2013) Progress in bio-based plastics and plasticizing modifications. J. Mater. Chem. A, 1 (43), 13379–13398.
- 88 Quintana, R. et al. (2012) Recent advances in (reactive) melt processing of cellulose acetate and related biodegradable bio-compositions. Polym. Chem., 3 (3), 591–595.
- 89 Niaounakis, M. (2014) Biopolymers: Processing and Products, Elsevier Science.
- 90 Udoetok, I.A. et al. (2016) Adsorption properties of cross-linked cellulose-epichlorohydrin polymers in aqueous solution. Carbohydr. Polym., 136, 329–340.
- 91 Jawad, A.H. and Nawi, M.A. (2012) Oxidation of crosslinked chitosan-epichlorohydrine film and its application with TiO2 for phenol removal. Carbohydr. Polym., 90 (1), 87–94.
- 92 Jawad, A.H. and Nawi, M.A. (2012) Characterizations of the photocatalytically-oxidized cross-linked chitosan-glutaraldehyde and its application as a sub-layer in the TiO2/CS-GLA bilayer photocatalyst system. J. Polym. Environ., 20 (3), 817–829.
- 93 Robinson, L.N. (2008) Water Resources Research Progress, Nova Science Publishers.
- 94 Ahmed, E.M. (2015) Hydrogel: preparation, characterization, and applications: a review. J. Adv. Res., 6 (2), 105–121.
- 95 Uyguner-Demirel, C.S., Birben, N.C., and Bekbolet, M. Elucidation of background organic matter matrix effect on photocatalytic treatment of contaminants using TiO2: a review. Catal. Today., 284, 202–214.
- 96 Liu, S. et al. (2008) TiO2 photocatalysis of natural organic matter in surface water: impact on trihalomethane and haloacetic acid formation potential. Environ. Sci. Technol., 42 (16), 6218–6223.
- 97 Matilainen, A. and Sillanpää, M. (2010) Removal of natural organic matter from drinking water by advanced oxidation processes. Chemosphere, 80 (4), 351–365.
- 98 Nurazim Ibrahim, H.A.A. (2014) Trends on natural organic matter in drinking water sources and its treatment. Int. J. Sci. Res. Environ. Sci., 2 (3), 94–106.
- 99 Schnitzer, M. and Kahn, S.U. (1972) Humic Substances in the Environment, Marcel Dekker, New York.
- 100 Croue, J.P. et al. (2000) Characterization of Natural Organic Matter in Drinking Water, AWWA Research Foundation and American Water Works, Association.
- 101 Adams, F.V. et al. (2014) Application of polysulfone/cyclodextrin mixed-matrix membranes in the removal of natural organic matter from water. Phys. Chem. Earth. A–C, 67–69, 71–78.
- 102 Remoundaki, E. et al. (2009) Photolytic and photocatalytic alterations of humic substances in UV (254nm) and solar cocentric parabolic concentrator (CPC) reactors. Desalination, 248 (1), 843–851.
- 103 Bouvier, L. et al. (2001) Photocatalytic composition. Google Patents.
- 104 Madani, M.E. et al. (2006) Photocatalytic degradation of diuron in aqueous solution in presence of two industrial titania catalysts, either as suspended powders or deposited on flexible industrial photoresistant papers. Appl. Catal. B 65 (1–2), 70–76.
- 105 Lebullenger, R. et al. (2010) Glass foams for environmental applications. J. Non-Cryst. Solids, 356 (44–49), 2562–2568.
- 106 Hirokazu, M. et al. (1995) Photoactive TiO2 containing paper: preparation and its photocatalytic activity under weak UV light illumination. Chem. Lett., 24 (9), 767–768.
