14
Nanosensor in Gas Monitoring: A Review
Nurhidayatullaili Muhd Julkapli and Samira Bagheri
University Malaya, Nanotechnology and Catalysis Research Centre (NANOCAT), IPS Building, 50603 Kuala Lumpur, Malaysia
14.1 Introduction
It has been demonstrated that exposure to toxic gases at high concentrations has profound health effects on the human respiratory system and could induce unconsciousness with attendant neurological sequelae and even death [1–3]. Even at relatively low concentrations, it can cause a malodor nuisance problem [2]. Although the chromatography-based systems have been currently and most frequently employed in petroleum industries due to their reliability of high detectability and precision, they are not convenient enough to track down short-term variations in behavior since their application involves a multistage protocol from the initial sampling to final determination [1–4]. In addition, offline analysis of toxic gases can be time-consuming and making accurate measurements is a big challenge.
The chemical sensitivity of nanosensors based on conductive nanomaterials and composites has been currently used for toxic gas detection due to their many benefits such as low cost, fast response, miniaturization, simple operation, and easy networking [3,5]. However, there are major obstacles to take this technology to an industrial scale including lack of high-quality, reproducible nanomaterials, nonuniform physical and chemical properties, relative inertness to the atmosphere due to lack of active groups, and sensitivity to humidity [6]. Depending on the level of humidity that can be obtained up to thousands of parts per million in the real-world environment in which the sensors are to be operated, their ability to detect gases can be completely screened. Hence, the success of a sensor largely depends on its sensitivity to humidity, ability to sense nonpolar and large molecules, and accessibility to be used under dry and vacuum conditions.
14.2 Sensing Technologies in Petroleum Industries
The development of gas sensors to monitor combustible gases is imperative due to concerns for safety, especially for liquefied petroleum gas [7,8]. Due to its high potential to produce hazardous gases, the detection is particularly important, because explosions may be caused when they leak out accidently or by mistake. Thus, demand for developing simple yet reliable gas sensors is huge for petroleum industries. The detection of gas molecules, including mononitrogen oxides (nitric oxide, NO), nitrogen dioxide (NO2), ammonia (NH3), carbon monoxide (CO), and others is important due to their toxicity and associated risks to ecosystem (Figure 14.1) (Table 14.1).

Figure 14.1 Optimized structures of the interaction of small gases (including highly toxic ones) with the open metal sites of [Cu3(btc)2]: (a) CO, (b) CO2, (c) O2, (d) OCS(O), (e) OCS(S), (f) PH3, (g) NH3, (h) N2, (i) NO, (j) N2O(O), (k) N2O(N), and (l) NO2 [1].
Table 14.1 Gaseous pollutants, sources, and effect on environment.
| Gases | Sources | Effect on environment | References |
| NO2 | Combustion sources and automobile | Harmful to plant and respiratory system of beings and animals | [9] |
| CO2 | Colorless, tasteless, toxic gas generated from the burning of fossil fuels and malfunctioning equipment Used as fault characteristic gas in evaluating the insulation performance of power transformers |
Induces greenhouse gases and global warming | [10] |
| NH3 | Irritating and corrosive gases from industries | Inhalation of even low concentrations causes coughing as well as nose and throat irritation Contact with concentrated NH3 solutions may cause corrosive injury, including skin burns, permanent eye damage, or blindness |
[11] |
| H2S | Produced in large quantity from industrial activities of petroleum/gas drilling and refining, wastewater treatment, coke ovens, tanneries, and landfills | Involved in a wide range of physiological functions, and its abnormal level are associated with a series of disease, including diabetes, hypertension, stroke, and Alzheimer's disease | [12] |
| CO | Colorless, tasteless, toxic gas mainly generated from the burning of fossil fuels and malfunctioning equipment | Effect on respiratory system | [13] |
Therefore, the detection of gases in domestic appliances must be no false alarm, which requires to be done before reaching the explosive limits. A lower explosive limit is the lowest concentration (percentage) of a gas or a vapor in the air capable of producing flash of fire in the presence of ignition sources including arc, flame, and heat [10–12].
14.3 Nanosensor Technology
Nanostructured materials can improve the performance of gas sensors because of their much greater surface to volume ratio compared to micrograined materials. The large surface to volume ratio of nanomaterials can be used as an advantage as the number of defect sites for the reaction is increased [14–16]. The large surface area to volume ratio of the nanocrystalline structures increases the opportunity for this surface reaction to occur. This in turn will increase the sensitivity and response rate of the gas sensor [15]. There are several points that need to be considered to improve the nanosensor technology, including easy and low-cost nanomaterial synthesis, minimizing substrate effect in order to avoid undesired surface contamination produced during microfabrication processes, incorporating new functional molecules with specific interaction designed for improving the sensitivity and selectivity of the adsorbates, and the impact of humidity on the performance of sensors should be quantified and controlled [9,17].
14.3.1 Nanomaterials
Highly effective, stable, and sensitive gas sensors can be achieved by modifying materials at the nanoscale (Figure 14.2). First approach is by achieving a high nonequilibrium amount of O2 vacancies in a nanometal oxide sensor [18]. A high nanoequilibrium amount of O2 vacancies serves to contribute in more effective movement of charge across the sensor material. The nonequilibrium state is used because thermodynamics at room temperature does not predict the amount of vacancies required for a low operating temperature [19]. Second, by well-dispersed ion conductivity in polymeric composites that could increase the number of surface sites for gas interaction, even a low concentration of gas can be detected at room temperature. Both approaches are applied to changing the defect chemistry within the space charge layer and surface of materials making it possible to get maximum signal change in the presence of targeted gases (Table 14.2).

Figure 14.2 Structure and surface modification of nanomaterial for sensor properties improvement (reproduced with permission from Ref. [20]. Copyright 2014, Elsevier).
Table 14.2 Positive and negative aspects of material selection for sensor applications.
| Nanomaterials | Positive view | Negative view | References |
| Nanometal oxide | Low response times and high sensitivity Act as catalyst to improve speed of the detection process |
Require post-treatment such as annealing High operation temperature High power consumption Limited for explosive gas detection |
[20,21] |
| Ion conductive polymeric composites | Lower working temperature Conductive nanoparticle consumption is less |
Advanced technique for synthesis to control the homogeneity and dispersion | [22,23] |
| Nanocarbon | High sensitivity with shorter response, recovery, and sensitivity Mechanically flexible Its two-dimensional structure is compatible with traditional planer process technology Large surface area Ballistic conductivity and high electron mobility at room temperature |
Required aging and thermal process to increase the sensitivity Additional purification process required Lack of selectivity and relatively high cost |
[24,25] |
14.3.1.1 Metal Oxide
The synthesis of nanometal oxides has become a critical point of research activities due to their highly conductive properties. The charge carrier on the surface of metal oxides is sensitive to the composition of surrounding atmospheres and, therefore, considerable research has been carried out on novel solid-state gas sensors based on semiconductor metal oxides [20,26]. The selection of metal oxides is based on their excellent characteristics of higher mobility, high conductivity, and transparent and low free carrier absorbance. Furthermore, in the case of metal oxide gas sensing materials, it is generally a surface controlled process that is responsible for sensitivity [21,27]. The limitations of these gas sensors include high cost, sensitivity in parts per billion level is rare, selectivity is poor, lifetime is limited, repeatability is poor, miniaturization is difficult, and power consumption is high.
14.3.1.1.1 ln2O3
Lanthanum oxide (ln2O3) has gained potential application in nanosensors due to its high sensing ability for different chemical compounds including H2S, NH3, CO, O3, and Cl2; formaldehyde, acetone, and toluene; and H2, CO, and NO2 [27–29]. On account of different surface areas and potential properties, the nanostructure morphology of ln2O3 plays a significant role in determining their electrochemical and optical properties. ln2O3-based sensors' performance toward toxic gases produced from petroleum industries has been much studied and received much attention due to its accurate and selective sensing at high temperature (>268 °C) among the normal gas molecules [28,30].
Different fabrication techniques will produce different morphologies and nanophysical structures, which consequently affect the final sensor prophesies of ln2O3 (Table 14.3). These synthesis techniques produced several morphologies of ln2O3, converted from indium hydroxide, including nanodots, nano- and microspheres, nanocubes, multipods, lotus roots-like nanocrystals, and one-dimensional nanocrystals [27,31]. However, in these studies on the sensing performance at room temperature, the responses of the ln2O3-based sensors to H2S could not reach a stable and maxima stage that is not restored to the baseline unless heated subsequently. Thus, the ultrahigh sensing of ln2O3 fabricated mainly originated from the ambient humidity induced by hydrolyzation of toxic gases [32]. Such hydroxylation will lead to desorption of the chemisorbed O2 and formation of H2O thin film on the surface of ln2O3 significantly increasing the conductance of the sensor and hence the sensitivity.
Table 14.3 Fabrication technique of ln2O3 and its variation on surface and morphological properties.
| Fabrication technique | Structure of ln2O3 | Sensor properties | References |
| Hydrothermal | Nanoparticles | Strong and selective sensing to H2S at 268 °C among the normal gas molecules | [33] |
| Spray dry hydrolysis | Nanostructured thin film | Good sensitivity toward H2S at low temperature of 50 °C | [34] |
| Chemical vapor deposition | Nanowires | High sensitivity to H2S with low detection limit of 1 ppm at room temperature | [35] |
| Electrospinning | Highly porous nanowires | Good response to the dilute H2S at room temperature | [36] |
| Chemical solution method | Self-assembled nanoparticles | High sensitivity to H2S down to 20 ppb in the low detection limit at room temperature | [37,38] |
| Electrospinning and calcining technique | Fabrication of NiO-ln2O3 nanofibers | Excellent sensing properties at room temperature | [39,40] |
| Solution dipping monolayer organic colloids templates | Micro/nanostructured orderly porous ln2O3 films | Possess ultrahigh sensitivity to H2S at room temperature and the ambient humidity | [41] |
| Low-temperature wet chemical method | Cracked cube and maize-corn nanostructured | H2S detection at room temperature | [42] |
Investigations of gas sensor performance with ln2O3 morphologies concluded that pore size and specific surface area of a particular morphology can be correlated with sensing properties to establish basic morphology sensing relation.
14.3.1.1.2 TiO2
Titanium oxide (TiO2) has proven itself as one of the competitive and promising compounds in gas sensor applications due to its optical and electronic properties. The anatase phase of TiO2 is found to exhibit interesting stable and low-temperature sensing properties [43–45]. The relatively high mobility of anatase in comparison to rutile and relatively few trapping centers and compensating levels are considered as reasons for the sensing response [46]. The development of TiO2 thin-film sensors will contribute to the higher sensing properties related to the material surface [44]. Synthesis of TiO2 films has been reported by many researchers using different chemical methods including chemical deposition, electrodeposition, spray pyrolysis deposition, sol–gel, successive ionic layer adsorption, and reaction (Figure 14.3).
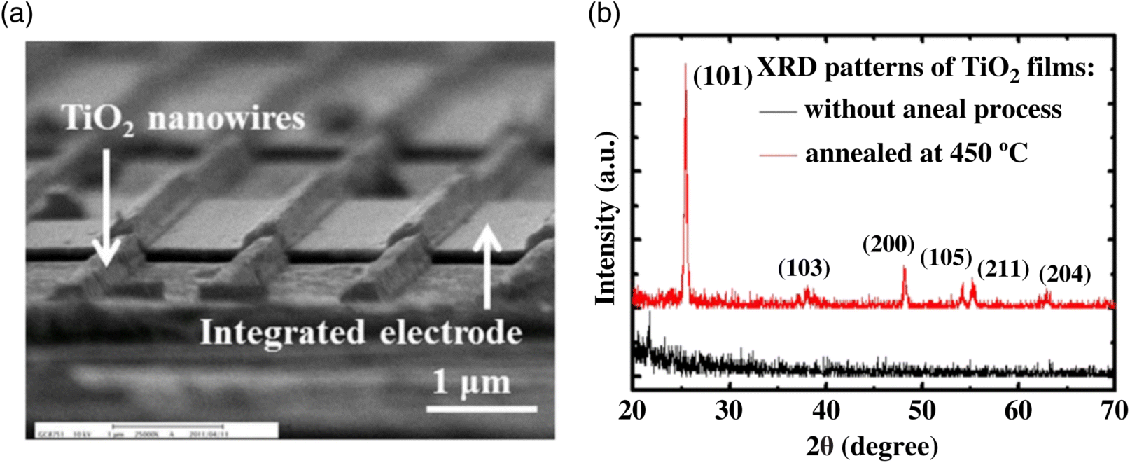
Figure 14.3 (a) SEM image of TiO2 nanowire gas sensor. (b) XRD patterns of TiO2 thin film with 450 °C annealing for 1 h and without the annealing process [44]. Source: http://www.mdpi.com/1424-8220/13/1/865. Licensed under CC BY 3.0.
The selection of synthesis methods is based on the simplicity of the apparatus and good productivity on a large scale (Table 14.4). Surface functionalization of TiO2 with gold nanoparticles has been successfully used as a sensor to identify hydrogen peroxide and glucose [47]. The sensing mechanism is generally based on either an influence of gold nanoparticles on electrical properties of TiO2 or a change in optical features of the localized surface plasmon resonance upon interaction with target analyst. A combination of TiO2 and doped alumina increases the sensing properties up to 400 ppm of liquefied petroleum gas at an operational temperature of 448 K.
Table 14.4 Synthesis methods of TiO2 and its effect on sensor properties.
| Synthesis methods | Features of TiO2 | Sensor properties | References |
| Anodization process | TiO2 nanotubes | H2 sensor at room temperature | [48] |
| Spray pyrolysis | Porous TiO2 | UV sensor | [49] |
| Sputtering and screen printing | TiO2 thin and thick films | NO2 and CO gas sensor | [50] |
| Screen printing process | Nanostructured Nb-doped TiO2 thick film | CO gas sensor | [51] |
14.3.1.1.3 ZnO
Znic oxide (ZnO) is a typical n-type semiconductor with a direct wide bandgap of 3.37 eV and large excitation binding energy of 60 meV [52,53]. The use of ZnO-based gas sensor showed improved gas sensing properties including higher gas response to a variety of reducing or oxidizing gases, low cost, being environment-friendly, and low operating temperature (350 °C). The most attractive features of ZnO is its ability to make the crystal structure O2 deficient, giving it the chemistry possible to change the resistance based on adsorbed surface species (Figure 14.4) [54]. The sensor properties of ZnO can be further improved by addition of small amounts of noble metal catalyst such as palladium or platinum that not only promote gas response but also decrease the operating temperature up to 250 °C. Heterojunction of ZnO with p-type quaternary kesterite makes this sensor applicable in room temperature with 19.3% achievement in gas sensing less than 1200 ppm of liquefied petroleum gas. The compact morphology of p-type quaternary kesterite effectively reduces the surface area that severely hampers the gas response [55–57]. Meanwhile, their nanoheterojunction effectively improves the surface area and results in improved gas response.

Figure 14.4 Sensor properties of ZnO nanowires toward different types of toxic gases (reproduced with permission from Ref. [56]. Copyright 2012, Elsevier).
14.3.1.1.4 SnO2
Tin dioxide (SnO2) is an n-type semiconductor oxide widely used as a gas sensor due to its high level of sensitivity, simple design, low weight, and low cost. With a bandgap of 3.62 eV at 298 K, SnO2 has promising gas sensing properties for various types of toxic gases and organic vapor [58–61]. The sensing mechanism of SnO2 sensor is based on the fact that adsorbed oxygen (O2 or O) on the SnO2 surface interacts with target gas molecules and results in changing SnO2 conductivity [59]. The sensitivity and selectivity have been tuned by using different catalysts and promoters and by varying the operating temperature. The role of different additives including Al, Pt, Pd, Sb, Fe, Cu, Zn, and others on the gas sensing behavior of SnO2 has been studied to develop high-performance sensors [60–62]. Meanwhile, the conductivity and sensor of SnO2 could be improved by doping with other metal elements. Doping creates more of the desired O2 vacancies by substitution of the dopant on Sn lattice site. Doping increases the number of intermediate energy levels created in the forbidden energy region [60,63]. Among the doping elements that have been found to impart sensitivity and selectivity to SnO2 for gas sensing, CuO is found to have an outstanding promoter action. The p–n heterojunction with grain size of 20 nm from this combination has an outstanding sensitivity and selectivity to H2S. Indium and ruthenium dopants in SnO2 not only improve sensing properties toward NO2 but also lower the operating temperature [64]. The high sensitivity is also attributed to the tiny size of SnO2 due to its high surface to volume ratio, which leads to ample adsorption site in sensing process.
CuO has been applied as an outstanding promoter to increase the surface conductance to gas adsorption behavior of SnO2. Subsequently, various materials based on SnO2-CuO have been synthesized and extensively studied for sensing including CuO-SnO2 heterostructures, CuO-doped SnO2 nanorods, and CuO-doped SnO2 nanoribons [65,66]. Their gas sensing mechanism has been attributed to the formation of p–n junctions between n-type SnO2 and p-type CuO, which makes it applicable for high-performance gas sensor [65–68].
14.3.1.1.5 Fe3O4
Magnetic particles with smaller size become single domain in contrast with the usual multidomain structures for bulk magnetic material exhibiting superparamagnetization. Iron oxide (Fe3O4) exhibiting superparamagnetic behavior displays greater saturation magnetization and low coercivity having potential application as a sensor [69–71]. The magnetic properties and gas sensing efficiency of the material depends on its microstructural properties related to its method of synthesis including coprecipitation, sol–gel, standard double-sintering method, microwave-induced combustion, microwave sintering method, sol–gel autocombustion method, reverse micelle as reaction process, ceramic method, and oxalate method [70,72]. An advanced sputtering technique has been used to increase the purity and directly assemble onto the substrate with which no further experimental step is required to control the size and yield of nanoferrite.
Ferrite alloys including magnesium ferrite, zinc ferrite, copper ferrite, and nickel ferrite has been used to increase its variations in electrical resistance that in turn improves the selectivity and sensitive toward a variety of toxic gases such as CH4, H2S, and C2H5OH [73,74]. Addition of zinc element to ferrite in nanorod composites resulted in highly selective and sensitive sensors toward liquefied petroleum gas than CO2 gas [75]. This also contributed to the mesoporous structure of the composites in enhancing the sorption capability of the sensor.
14.3.1.1.6 CdO
Cadmium oxide (CdO) has been synthesized by physical and chemical methods including sputter deposition, metal organic chemical vapor deposition, chemical bath deposition, and pulsed laser deposition [76–78]. These techniques apply a small degree of supersaturation of the solution (such as cadmium hydroxide) to cause the heterogeneous nucleation of the metal oxide on the substrate. The solubility of the solutes changes as a result of chemical reaction in the solution. Once the solution reaches supersaturation points, the solid particles are formed through the nucleation and crystal growth process [77,79] (Figure 14.5).

Figure 14.5 (a) Typical N2 adsorption–desorption isotherm and (b) pore-size distribution curve of the 3D hierarchical CdO nanostructures (reproduced with permission from Ref. [77]. Elsevier).
14.3.1.1.7 WO3
Tungsten oxide (WO3) as an important n-type semiconductor has attracted considerable interest due to its promising physical and chemical properties [80]. Taking into account its small bandgap (2.59 eV) and stable physicochemical properties, nanostructured WO3 is generally considered a feasible candidate for chemical and gas sensors [81]. A deeper understanding of the effect of temperature on Raman spectral characteristics of a metal oxide such as WO3 has helped to extend our knowledge regarding the behavior of metal oxide–gas interactions for sensing applications [80,82,83] (Figure 14.6).

Figure 14.6 Determination of the existence of a correlation between the thermal effects and the changes in Raman spectra for multiple WO3 structures. We have obtained results utilizing Raman spectroscopy for three different structures of WO3 (monoclinic WO3 on Si substrate, nano powder, and nanowires) that were subjected to temperatures in the range of 30–160 °C (reproduced with permission from Ref. [80]. Copyright 2013, American Chemical Society).
14.3.1.1.8 Co3O4
Spinel cobalt (II) oxide (Co3O4) is rich in O2 content and thus exhibits p-type semiconducting properties [84,85]. Its gas sensing properties have been explored in which sensing is usually based on catalytic properties of the surface of the oxide and the sensors are usually operated at elevated temperatures above 200 °C [85,86].
14.3.1.2 Glassy Carbon Electrode
Different nanocarbon materials including carbon nanotubes, charcoal, graphene, and graphene oxide have been demonstrated to be useful as gas sensors due to ease in tailoring their sensitivity by simple chemical treatments. In CNTs, the point defect plays a dominant role as a sensing active site. Line defects and edges seem to be the main contribution to the sensing properties of graphene. Adsorption from the gaseous phase resulted in ion exchanges between the adsorbate layer and the materials, meaning a variation of free electron concentration.
14.3.1.2.1 CNT Electrode
Sensing materials based on CNT network have shown high resistance, fast response time, rapid recovery, and good reproducibility [87–89]. CNTs have shown great potential for detection of volatile organic compounds and nonpolar molecules. The sensitivity of CNT sensors is contributed by their Π-Π stacking and can reach 1 ppm in case of dimethyl methylphosphate [88,90]. Besides, it has been demonstrated that cross-reactive arrays of synthetically designed polycyclic aromatic hydrocarbon and single-walled CNT bilayers can discriminate between polar and nonpolar volatile organic compounds as well as between the different volatile organic compounds from each subgroup [91]. The incorporation of gold nanoparticles onto the surface of CNTs has reported high sensitivity and low response/recovery time for detection of methane at room temperature.
14.3.1.2.2 Graphene/graphene Oxide Electrode
In recent years, graphene and its derivatives such as pristine graphene, graphene oxide, and reduced graphene oxide have been reported to show sensing applications due to their exceptional properties including low cost, high surface to volume ratio, ease of processing, high mechanical strength, good thermal stability, ballistic conductivity, high carrier mobility at room temperature, low electrical noise due to unique two-dimensional honeycomb lattice, and large surface area (92 630 m2 g−1) [92–95]. Graphene's two-dimensional structure can screen charge fluctuations better than one-dimensional materials. The planar structure of graphene eases Hall pattern fabrication and four probe measurements, limiting the contact resistance impact and helping to focus only on the active area compared to its one-dimensional counterpart [93]. With that, micrometer-size sensors made of graphene are capable of detecting individual gas molecules that attach to or detach from graphene surfaces. It demonstrated that the adsorbed molecules change with the local carrier concentration in grapheme, one electron by one electron, which leads to step-like changes in resistance [94,96]. Graphene nanoplanar could be synthesized by different methods including micromechanical (Scotch tape), chemical vapor deposition, epitaxial growth on epitaxial matched, chemical exfoliation, and thermal exfoliation unzipping CNTs (Table 14.5).
Table 14.5 Series on graphene synthesis method.
| Synthesis method | Parameters | References |
| Mechanical cleaving of graphite | A repeated stripping of graphite with adhesive tape is involved to obtain single layer These isolated layers can be resolved using optical microscope, atomic force microscopy, or Raman spectroscopy |
[98,99] |
| Chemical exfoliation of graphite | A strong acid solution has been employed to introduce O2 into graphene interlayers and form graphene oxide Graphene oxide can be easily separated into single layers after dispersion into aqueous solution Heating of hexagonal silicon carbide crystal to 1200 °C to produce epitaxial graphene |
[100,101] |
| Chemical vapor deposition | Allow graphene growth on metal substrate like copper and nickel from hydrocarbon gases at 700–1000 °C | [102,103] |
| Combination of chemical treatment and thermal exfoliation | Synthesis process above 800 °C by incorporation of nickel nanoparticles that substantially eliminates the O2-containing impurities | [104,105] |
The gas-induced changes in resistivity had different magnitudes corresponding to different types of gases and the sign of the change indicated whether the gas was an electron acceptor like NO2, H2O, and iodine or an electron donor including NH3, CO, and ethanol (Figure 14.7). The interaction between graphene sheets and adsorbents could vary from weak van de Waals to strong covalent bonding. The above-mentioned interactions tune the electronic structure of graphene, which can be readily monitored by convenient electronic methods [106–108]. The level of interactions between target gas and vapor molecules may reach the lower limit of even single molecules. With that high sensitivity of sensor could be obtained even at low gas concentrations.

Figure 14.7 Transient gas-sensing characteristics of graphene device. (a) Responses to NO2 gas from 1.2 to 5 ppm. (b) Responses to NH3 gas from 5 to 100 ppm. All gas-sensing tests were performed at the operating temperature of 150 °C (reproduced with permission from Ref. [97]. Copyright 2014, Royal Society of Chemistry).
However, there are some limitations regarding the graphene sensor including not producible in large scale, do not have functional groups required for adsorption of gas and vapor and have no bandgap. Therefore, researchers are looking for the surface modification of graphene by doping, hybridization, functionalization, nanomesh formation, FET modulation, and others [109–111]. The reduced graphene oxide is produced by reduction that contains many functional groups and defects (Table 14.6). The decoration of graphene sheets with n-type metal oxides can lead to the formation of n–p junction and the resulting novel nanostructure may exhibit performance far better than that of individual materials. The n–p junction also resulted in a depletion zone by introducing more electrons to be attached from graphene oxide and shifting the Fermi level of graphene oxide toward the valence band. This consequently improves the conductivity of graphene oxide. The metal oxide-loaded graphene hybrid architecture has produced sensors that are highly sensitive, selective, and cost effective applicable to be used at room temperature. In general, graphene sensors combined with SnO2, ZnO2, WO3, Cu2O, Co3O4, ln2O3, and NiO. Nanocrystal decoration on the graphene oxide surface increases the overall active sensing surface area and subsequently increases the gas molecule adsorption during sensing properties. The attachment of nanocrystal onto surface of graphene/graphene oxide leads to more active sites including vacancies, defects, O2 functional groups, and sp2-bonded carbon for adsorption of gas molecules. Meanwhile, there are some report claims that nanocrystal served both as a nanospacer between dried graphene sheets and as a primary sensing transducer for gas sensing applications. This provides a net gas sensing mechanism and not the individual components, and hence the improvement of room temperature response with good selectivity to electron donor gases such as CO and NH3. Indeed, some of the nanocrystals such as Co3O4 behave like the nanopillars resulting in extra macroporous structures between graphene oxide layers that further improve the gas diffusion to the surface of graphene oxide driven by the capillary force leading to the improvement in gas responses. The improvement in O2 reduction ability could be achieved by a strong coupling between O2 ions in the graphene oxide and nanocrystals making them ionic in nature. This consequently serves as extra adsorption sites for NO2 gases and electrons would be extracted indirectly from p-type of graphene oxide through the bridging of O2, leading to an extra decrease in resistance in the presence of NO2.
Table 14.6 Modification of graphene for improving sensing properties.
| Modification technique | Sensing properties | References |
| Introduction of positive gate potential (n-type conductance) | Instantaneous response and fast recovery for NH3 sensing Far superior to the performance in p-mode at zero or negative gate potential |
[112] |
| Hybrid of graphene oxide, tin oxide, and platinum | Room temperature H2 sensor Fast response and recovery time |
[113,114] |
| Nanomesh patterned from a CVD-grown large area graphene | Exhibit sensitivity at about of about 4.32% per ppm in NO2 and 0.71% per ppm in NH3 with a detection limit of 15 and 160 ppb | [115,116] |
| Lateral confinement of graphene into sub-10 nm wide nanoribbon | Induce a bandgap by the quantum confinement and edge effect | [117,118] |
| Nanocrystal SnO2-graphene-gold interdigital electrodes | Excellent response to target gases at room temperature with detection limit of 1 ppm for NO2 However, weakened the signal for NH3 Sensor sensitivity to NO2 increased from 2.16 to 2.87 Sensor sensitivity to NH3 decreased from 1.46 to 1.1.2 |
[119,120] |
| Alumina substrates supplied with comb-like Pt on surface of graphene oxide | Good sensing characteristics to NO2 at low temperature (50 °C) | [121,122] |
| SnO2-graphene on ceramic plate coated with gold electrodes and ruthenium oxides as heater | The target NO2 gases directly adsorbed on the surface of SnO2 and modify the depth of the first depletion layer, which in turn alters the depletion at SnO2-graphene layers | [123,124] |
| Indium-doped SnO2-graphene nanohydrid | NO2 sensing properties at room temperature with detection limits as low as 0.3 ppm. Excellent selectivity to several gases Recovery process slow and takes overnight to completely recover to the initial state |
[125] |
| SnO2-graphene gold | Good gas-sensing properties toward NH3 of 10–50 ppm at room temperature, high response magnitude, fast response/recovery, and good reversibility and repeatability | [126] |
| ZnO-graphene oxide | Detection of common industrial toxins such as CO, NH3, and NO for concentrations as low as 1 ppm at room temperature Shorter response and recovery time compared to neat graphene oxide for NO2 detection |
[127,128] |
| ZnO-graphene oxide-supported ceramic substrate | Response and recovery of sensing to 5–25 ppm NO2, respectively | [129] |
| WO3-graphene oxide | 2.5 times higher NO2 sensing response than pure WO3 film | [130,131] |
| Pd-doped WO3-graphene oxide | Good sensitivity to NO2 with recovery time less than 1 min | [132] |
| CuO2 nanowire mesocrystal on graphene oxide sheet | Response was 67.8% for 2 ppm NO2, much higher than graphene oxide (22.5%) at room temperature | [133,134] |
| CuO-functionalized graphene oxide | H2S gas detection at room temperature with sensitivity of 11% even at the exposed concentration of 5 ppb | [135] |
| Intercalation of Co3O4-graphene oxide nanocrystal | Co3O4 effectively attaches to the surface of graphene oxide to result in longer response than pure graphene oxide Co3O4 acts as nanopillar to form extra macroporous structures between graphene oxide layers leading to enhanced responses |
[136] |
| Ir functionalized Co3O4 nanofibers/graphene oxide sheet | Highly sensitive and selective acetone sensor | [137] |
14.3.2 Properties of Nanosensor Technology
The sensor performance strongly depends on the microstructural features including crystallite size and size grain boundaries' characteristics. Sensor properties can be controlled by controlling the grain size and surface morphology. The effect of the different surface treatments on active sensor surface causes the change in morphological properties of materials and hence on the sensor performance. Mesoporosity plays a crucial role in improving both the rate of response and sorption capability of nano sensors.
14.4 Conclusions
In recent year, gas detection and monitoring in petroleum industries has created an increasing concern regarding the awareness of environmental protection and human health. Toxic gases including CO2, NO2, and H2S are flammable and hazardous to both humans and environment. During the petroleum synthesis process, combustion accidents might be caused due to leakage or by mistakes. Thus, the requirement for reliable and sensitive gas detecting instruments for safety has increased. Therefore, nanosensor has become the subject of intense research due to its quick response and recovery periods and extremely small detection level capacity and it is reliable, efficient, simple, and cost-effective for good sensitivity. Many effort have being devoted for synthesizing novel sensing nanomaterials of various conducting nanomaterials including metal/metal oxide, ionic liquids, and nanocarbon for improving the gas detection level. Metal oxide nanomaterials have been fabricated because of their abundant nature, low synthesis cost, and availability in various appearances. Ion conductive polymer metal composites have been envisaged in nanosensors for petroleum industries due to their remarkable electronic and optical properties, high electrical conductivity, highly sensitive to external environment, wide bandgap energy, and high free carrier mobility. However, it has been noted here that these achievements are still in the stage of laboratory investigation and much research is needed to take these developments to an industrial scale. For real-life applications, it is noted that the performance of these sensors may strongly depend on the relative humidity of the environment.
Acknowledgment
The authors would like to thank Grand Challenge (GC001B-14SBS) of University Malaya, Malaysia, for their cordial support in completing this work.
References
- 1 Barea, E., Montoro, C., and Navarro, J.A. (2014) Toxic gas removal: metal–organic frameworks for the capture and degradation of toxic gases and vapours. Chem. Soc. Rev., 43 (16), 5419–5430.
- 2 Hu, L., Qiu, A., and Qian, X. (2015, July) Dynamic simulation method of toxic gas diffusion in complex geographic environment. Agro-Geoinformatics (Agro-geoinformatics), 2015 Fourth International Conference on IEEE, pp. 163–167.
- 3 Barton, L.L., Fardeau, M.L., and Fauque, G.D. (2014) Hydrogen sulfide: a toxic gas produced by dissimilatory sulfate and sulfur reduction and consumed by microbial oxidation, in The Metal-Driven Biogeochemistry of Gaseous Compounds in the Environment, Springer, The Netherlands, pp. 237–277.
- 4 Youssef, I.B., Sarry, F., Nysten, B., Alexieva, G., Strashilov, V., Kolev, I., and Alem, H. (2016) Growth and toxic gas sensing properties of poly (urethaneimide) thin films. Talanta, 153, 145–151.
- 5 Jeoung, J.H., Fesseler, J., Goetzl, S., and Dobbek, H. (2014) Carbon monoxide. toxic gas and fuel for anaerobes and aerobes: carbon monoxide dehydrogenases, in The Metal-Driven Biogeochemistry of Gaseous Compounds in the Environment, Springer, The Netherlands, pp. 37–69.
- 6 Qian, Z., Charlton, J., Agnew, B., and Thompson, E. (2015) A11 Towards an integrated evaluation of the effects on health from smoke or toxic gas dispersion in the evacuation of subway tunnels. J. Transp. Health, 2 (2), S10–S11.
- 7 Shyla, M.V., Naidu, K.B., and Kumar, G.V. (2013, December) Optimization of sensor position on different surfaces using CFD analysis for reducing accidents caused by emission of toxic gas in industries. Advanced Computing (ICoAC), 2013 Fifth International Conference on IEEE, pp. 196–204.
- 8 Cai, Y.W., Tang, H.M., Wang, X.G., Wang, L.Y., and Zhu, H.Y. (2015) Simulation assessment of dangerous consequence caused by toxic gas products during KCN decontamination process. Adv. Mater. Res., 1092, 907–911.
- 9 Zhou, R., Hu, G., Yu, R., Pan, C., and Wang, Z.L. (2015) Piezotronic effect enhanced detection of flammable/toxic gases by ZnO micro/nanowire sensors. Nano Energy, 12, 588–596.
- 10 Lara-Gil, J.A., Álvarez, M.M., and Pacheco, A. (2014) Toxicity of flue gas components from cement plants in microalgae CO2 mitigation systems. J. Appl. Phycol., 26 (1), 357–368.
- 11 Dong, K.Y., Choi, J., Lee, Y.D., Kang, B.H., Yu, Y.Y., Choi, H.H., and Ju, B.K. (2013) Detection of a CO and NH3 gas mixture using carboxylic acid-functionalized single-walled carbon nanotubes. Nanoscale Res. Lett., 8 (1), 1–6.
- 12 Chouari, R., Dardouri, W., Sallami, F., Rais, M.B., Le Paslier, D., and Sghir, A. (2015) Microbial analysis and efficiency of biofiltration packing systems for hydrogen sulfide removal from wastewater off gas. Environ. Eng. Sci., 32 (2), 121–128.
- 13 Gauthier, P.T., Norwood, W.P., Prepas, E.E., and Pyle, G.G. (2014) Metal–PAH mixtures in the aquatic environment: a review of co-toxic mechanisms leading to more-than-additive outcomes. Aquat. Toxicol., 154, 253–269.
- 14 Zarepour, E., Adesina, A.A., Hassan, M., and Chou, C.T. (2013) Nano sensor networks for tailored operation of highly efficient gas-to-liquid fuels catalysts. Chemeca 2013: Challenging Tomorrow, p. 91.
- 15 Yousefi, A.T., Ikeda, S., M., RusopMahmood., and Yousefi, H.T. (2014) Simulation of nano sensor based on carbon nanostructures in order to form multifunctional delivery platforms. Adv. Mater. Res., 832, 778–782.
- 16 Jokar, M., Hassan Safaralizadeh, M., Hadizadeh, F., Rahmani, F., and Reza Kalani, M. (2016) Apta-nano-sensor preparation and in vitro assay for rapid diazinon detection using a computational molecular approach. J. Biomol. Struct. Dyn. 35 (2), 1–47.
- 17 Jokar, M., Safaralizadeh, M.H., Hadizadeh, F., Rahmani, F., and Kalani, M.R. (2015) Design and evaluation of an apta-nano-sensor to detect acetamiprid in vitro and in silico. J. Biomol. Struct. Dyn. 34 (11), 1–26.
- 18 Zhang, Y.M., Zhu, L.Q., Luo, F., and He, W. (2014) Study of fiber laser micro-nano sensor based on colloidal crystal. Optoelectron. Lett., 10, 27–29.
- 19 Lin, Z.H., Zhu, G., Zhou, Y.S., Yang, Y., Bai, P., Chen, J., and Wang, Z.L. (2013) A self-powered triboelectric nanosensor for mercury ion detection. Angew. Chem. Int. Ed., 52 (19), 5065–5069.
- 20 Miller, D.R., Akbar, S.A., and Morris, P.A. (2014) Nanoscale metal oxide-based heterojunctions for gas sensing: a review. Sens. Actuators B, 204, 250–272.
- 21 Burgos, S.P., Yokogawa, S., and Atwater, H.A. (2013) Color imaging via nearest neighbor hole coupling in plasmonic color filters integrated onto a complementary metal-oxide semiconductor image sensor. ACS Nano, 7 (11), 10038–10047.
- 22 Wang, X., Ding, B., Sun, G., Wang, M., and Yu, J. (2013) Electro-spinning/netting: a strategy for the fabrication of three-dimensional polymer nano-fiber/nets. Prog. Mater. Sci., 58 (8), 1173–1243.
- 23 Hangarter, C.M., Chartuprayoon, N., Hernández, S.C., Choa, Y., and Myung, N.V. (2013) Hybridized conducting polymer chemiresistive nano-sensors. Nano Today, 8 (1), 39–55.
- 24 Zheng, M., Xie, Z., Qu, D., Li, D., Du, P., Jing, X., and Sun, Z. (2013) On–off–on fluorescent carbon dot nanosensor for recognition of chromium (VI) and ascorbic acid based on the inner filter effect. ACS Appl. Mater. Interfaces, 5 (24), 13242–13247.
- 25 Qu, S., Chen, H., Zheng, X., Cao, J., and Liu, X. (2013) Ratiometric fluorescent nanosensor based on water soluble carbon nanodots with multiple sensing capacities. Nanoscale, 5 (12), 5514–5518.
- 26 Xia, X., Tu, J., Zhang, Y., Wang, X., Gu, C., Zhao, X.B., and Fan, H.J. (2012) High-quality metal oxide core/shell nanowire arrays on conductive substrates for electrochemical energy storage. ACS Nano, 6 (6), 5531–5538.
- 27 Trakhtenberg, L.I., Gerasimov, G.N., Gromov, V.F., Belysheva, T.V., and Ilegbusi, O.J. (2012) Effect of composition on sensing properties of SnO2+In2O3 mixed nanostructured films. Sens. Actuators B, 169, 32–38.
- 28 Ayeshamariam, A., Kashif, M., Raja, S.M., Sivaranjani, S., Sanjeeviraja, C., and Bououdina, M. (2014) Synthesis and characterization of In2O3nanoparticles. J. Korean Phys. Soc., 64 (2), 254–262.
- 29 Zhu, Y., Zhai, X., and Wang, L. (2013) Hydrothermal synthesis of Ln (OH)3 nanorods and the conversion to Ln2O3 (Ln = Eu, Nd, Dy) nanorods via annealing process. J. Nanomaterials, 2013, 146.
- 30 Thangadurai, V., Pinzaru, D., Narayanan, S., and Baral, A.K. (2015) Fast solid-state Li ion conducting garnet-type structure metal oxides for energy storage. J. Phys. Chem. Lett., 6 (2), 292–299.
- 31 Tishchenko, Y.S. (2014) Physicochemical interaction in the Al2O3–HfO2–Ln2O3 systems. Powder Metall. Met. Ceram., 53 (7–8), 469–478.
- 32 Courtin, E., Boy, P., Rouhet, C., Bianchi, L., Bruneton, E., Poirot, N., and Sanchez, C. (2012) Optimized sol–gel routes to synthesize yttria-stabilized zirconia thin films as solid electrolytes for solid oxide fuel cells. Chem. Mater., 24 (23), 4540–4548.
- 33 Zhu, Y., Zhai, X., and Wang, L. (2013) Hydrothermal synthesis of Ln (OH)3 nanorods and the conversion to Ln2O3 (Ln = Eu, Nd, Dy) nanorods via annealing process. J. Nanomaterials, 2013, 146.
- 34 Fernández-Carrión, A.J., Ocaña, M., Florian, P., García-Sevillano, J., Cantelar, E., Fitch, A.N., and Becerro, A.I. (2013) Crystal structure and luminescent properties of Eu3+-doped A-La2Si2O7 tetragonal phase stabilized by spray pyrolysis synthesis. J. Phys. Chem. C, 117 (40), 20876–20886.
- 35 Belaya, S.V., Bakovets, V.V., Boronin, A.I., Koshcheev, S.V., Lobzareva, M.N., Korolkov, I.V., and Stabnikov, P.A. (2014) Terbium oxide films grown by chemical vapor deposition from terbium (III) dipivaloylmethanate. Inorg. Mater., 50 (4), 379–386.
- 36 Xia, X., Hu, W., and Shao, Y. (2015) Density functional theory calculations for the structural, electronic, and magnetic properties of (Gd2O3)n0, ±1 clusters with n = 1–10. J. Phys. Chem. C, 119 (15), 8349–8356.
- 37 Bugrov, A.N., Rodionov, I.A., Zvereva, I.A., Smyslov, R.Y., and Almjasheva, O.V. (2016) Photocatalytic activity and luminescent properties of Y, Eu, Tb, Sm and Er-doped ZrO2 nanoparticles obtained by hydrothermal method. Int. J. Nanotechnol., 13 (1–3), 147–157.
- 38 Han, B., Li, Y., Chen, N., Deng, D., Xing, X., and Wang, Y. (2015) Preparation and photocatalytic properties of LnBaCo2O5+δ (Ln = Eu, Gd, and Sm). J. Mater. Sci. Chem. Eng., 3 (04), 17.
- 39 Reitz, C., Haetge, J., Suchomski, C., and Brezesinski, T. (2013) Facile and general synthesis of thermally stable ordered mesoporous rare-earth oxide ceramic thin films with uniform mid-size to large-size pores and strong crystalline texture. Chem. Mater., 25 (22), 4633–4642.
- 40 Oh, I.K., Kim, K., Lee, Z., Ko, K.Y., Lee, C.W., Lee, S.J., and Kim, H. (2014) Hydrophobicity of rare earth oxides grown by atomic layer deposition. Chem. Mater., 27 (1), 148–156.
- 41 Mahlambi, M.M., Ngila, C.J., and Mamba, B.B. (2015) Recent developments in environmental photocatalytic degradation of organic pollutants: the case of titanium dioxide nanoparticles – a review. J. Nanomaterials, 2015, 5.
- 42 Li, L., Wang, X., Lan, Y., Gu, W., and Zhang, S. (2013) Synthesis, photocatalytic and electrocatalytic activities of wormlike GdFeO3 nanoparticles by a glycol-assisted sol–gel process. Ind. Eng. Chem. Res., 52 (26), 9130–9136.
- 43 Lou, Z., Li, F., Deng, J., Wang, L., and Zhang, T. (2013) Branch-like hierarchical heterostructure (α-Fe2O3/TiO2): a novel sensing material for trimethylamine gas sensor. ACS Appl. Mater. Interfaces, 5 (23), 12310–12316.
- 44 Tian, W.C., Ho, Y.H., Chen, C.H., and Kuo, C.Y. (2013) Sensing performance of precisely ordered TiO2 nanowire gas sensors fabricated by electron-beam lithography. Biosensors, 13 (1), 865–874.
- 45 Nisar, J., Topalian, Z., De Sarkar, A., Österlund, L., and Ahuja, R. (2013) TiO2-based gas sensor: a possible application to SO2. ACS Appl. Mater. Interfaces, 5 (17), 8516–8522.
- 46 Hazra, A., Das, S., Kanungo, J., Sarkar, C.K., and Basu, S. (2013) Studies on a resistive gas sensor based on sol–gel grown nanocrystalline p-TiO2 thin film for fast hydrogen detection. Sens. Actuators B, 183, 87–95.
- 47 Korotcenkov, G., Brinzari, V., and Cho, B.K. (2016) Conductometric gas sensors based on metal oxides modified with gold nanoparticles: a review. Microchim. Acta, 183 (3), 1033–1054.
- 48 Galstyan, V., Comini, E., Faglia, G., and Sberveglieri, G. (2013) TiO2 nanotubes: recent advances in synthesis and gas sensing properties. Biosensors, 13 (11), 14813–14838.
- 49 Patil, L.A., Suryawanshi, D.N., Pathan, I.G., and Patil, D.G. (2014) Nanocrystalline Pt-doped TiO2 thin films prepared by spray pyrolysis for hydrogen gas detection. Bull. Mater. Sci., 37 (3), 425–432.
- 50 Hussain, T., Salam, A., Malek, F., Jawad, M.S., Ahmed, S.F., Abdullah, A.Z., and Taha, T.A. (2015) Study of TiO2 energy gap based temperature annealing for high performance solar cells. Appl. Mech. Mater., 793, 425–429.
- 51 Chen, H., Liu, Y., Xie, C., Wu, J., Zeng, D., and Liao, Y. (2012) A comparative study on UV light activated porous TiO2 and ZnO film sensors for gas sensing at room temperature. Ceram. Int., 38 (1), 503–509.
- 52 Guo, J., Zhang, J., Zhu, M., Ju, D., Xu, H., and Cao, B. (2014) High-performance gas sensor based on ZnO nanowires functionalized by Au nanoparticles. Sens. Actuators B, 199, 339–345.
- 53 Bagheri, M., Hamedani, N.F., Mahjoub, A.R., Khodadadi, A.A., and Mortazavi, Y. (2014) Highly sensitive and selective ethanol sensor based on Sm2O3-loaded flower-like ZnO nanostructure. Sens. Actuators B, 191, 283–290.
- 54 Wang, L., Wang, S., Xu, M., Hu, X., Zhang, H., Wang, Y., and Huang, W. (2013) A Au-functionalized ZnO nanowire gas sensor for detection of benzene and toluene. Phys. Chem. Chem. Phys., 15 (40), 17179–17186.
- 55 Jia, Q., Ji, H., Zhang, Y., Chen, Y., Sun, X., and Jin, Z. (2014) Rapid and selective detection of acetone using hierarchical ZnO gas sensor for hazardous odor markers application. J. Hazard. Mater., 276, 262–270.
- 56 Chougule, M.A., Sen, S., and Patil, V.B. (2012) Fabrication of nanostructured ZnO thin film sensor for NO2 monitoring. Ceram. Int., 38 (4), 2685–2692.
- 57 Zhang, J., Liu, X., Wu, S., Cao, B., and Zheng, S. (2012) One-pot synthesis of Au-supported ZnO nanoplates with enhanced gas sensor performance. Sens. Actuators B, 169, 61–66.
- 58 Hien, V.X., Lee, J.H., Kim, J.J., and Heo, Y.W. (2014) Structure and NH3 sensing properties of SnO thin film deposited by RF magnetron sputtering. Sens. Actuators B, 194, 134–141.
- 59 Misra, V., Lee, B., Manickam, P., Lim, M., Pasha, S.K., Mills, S., and Bhansali, S. (2015, December) Ultra-low power sensing platform for personal health and personal environmental monitoring. 2015 IEEE International Electron Devices Meeting (IEDM) IEEE, pp. 13–21.
- 60 Miao, Y.E., He, S., Zhong, Y., Yang, Z., Tjiu, W.W., and Liu, T. (2013) A novel hydrogen peroxide sensor based on Ag/SnO2 composite nanotubes by electrospinning. Electrochim. Acta, 99, 117–123.
- 61 Suman, P.H., Felix, A.A., Tuller, H.L., Varela, J.A., and Orlandi, M.O. (2015) Comparative gas sensor response of SnO2, SnO and Sn3O4 nanobelts to NO2 and potential interferents. Sens. Actuators B, 208, 122–127.
- 62 Yamaguchi, M., Anggraini, S.A., Fujio, Y., Sato, T., Breedon, M., and Miura, N. (2013) Stabilized zirconia-based sensor utilizing SnO2-based sensing electrode with an integrated Cr2O3 catalyst layer for sensitive and selective detection of hydrogen. Int. J. Hydrogen Energy, 38 (1), 305–312.
- 63 Lu, H., Yu, S., Fan, Y., Yang, C., and Xu, D. (2013) Nonenzymatic hydrogen peroxide electrochemical sensor based on carbon-coated SnO2 supported Pt nanoparticles. Colloid. Surface B, 101, 106–110.
- 64 Wu, R.J., Lin, D.J., Yu, M.R., Chen, M.H., and Lai, H.F. (2013) Ag@ SnO2 core–shell material for use in fast-response ethanol sensor at room operating temperature. Sens. Actuators B, 178, 185–191.
- 65 Choi, S.W., Katoch, A., Zhang, J., and Kim, S.S. (2013) Electrospun nanofibers of CuOSnO2 nanocomposite as semiconductor gas sensors for H2S detection. Sens. Actuators B, 176, 585–591.
- 66 Joshi, S., Satyanarayana, L., Manjula, P., Sunkara, M.V., and Ippolito, S.J. (2015, March) Chemo-resistive CO2 gas sensor based on CuO-SnO2 heterojunction nanocomposite material. Physics and Technology of Sensors (ISPTS), 2015 2nd International Symposium on IEEE, pp. 43–48.
- 67 Zhao, Y., He, X., Li, J., Gao, X., and Jia, J. (2012) Porous CuO/SnO2 composite nanofibers fabricated by electrospinning and their H2S sensing properties. Sens. Actuators B, 165 (1), 82–87.
- 68 Shen, C.H. and Ke, S.C. (2014) Research on a highly sensitive magnetic-catalytic CMOS-MEMS compatible gas sensor. IEEE Electr. Device Lett., 35 (1), 120–122.
- 69 Capone, S., Manera, M.G., Taurino, A., Siciliano, P., Rella, R., Luby, S., and Majkova, E. (2014) Fe3O4/γ-Fe2O3 nanoparticle multilayers deposited by the Langmuir–Blodgett technique for gas sensors application. Langmuir, 30 (4), 1190–1197.
- 70 Shi, G., Sun, B., Jin, Z., Liu, J., and Li, M. (2012) Synthesis of SiO2/Fe3O4 nanomaterial and its application as cataluminescence gas sensor material for ether. Sens. Actuators B, 171, 699–704.
- 71 Ke, S.C. and Shen, C.H. (2013, October) Fe3O4 magnetic enhanced CMOS MEMS compatible gas sensor. TENCON 2013-2013 IEEE Region 10 Conference (31194) IEEE, pp. 1–4.
- 72 Wang, C., Cheng, X., Zhou, X., Sun, P., Hu, X., Shimanoe, K., and Yamazoe, N. (2014) Hierarchical α-Fe2O3/NiO composites with a hollow structure for a gas sensor. ACS Appl. Mater. Interfaces, 6 (15), 12031–12037.
- 73 Zhai, Q., Du, B., Feng, R., Xu, W., and Wei, Q. (2014) A highly sensitive gas sensor based on Pd-doped Fe3O4 nanoparticles for volatile organic compounds detection. Anal. Methods, 6 (3), 886–892.
- 74 Cao, G.S., Wang, P., Li, X., Wang, Y., Wang, G., and Li, J. (2015) A sensitive nonenzymatic hydrogen peroxide sensor based on Fe3O4–Fe2O3 nanocomposites. Bull. Mater. Sci., 38 (1), 163–167.
- 75 Hoa, N.D., Duy, N.V., El-Safty, S.A., and Hieu, N.V. (2015) Meso-/nanoporous semiconducting metal oxides for gas sensor applications. J. Nanomaterials, 2015, 186.
- 76 Bulakhe, R.N. and Lokhande, C.D. (2014) Chemically deposited cubic structured CdO thin films: use in liquefied petroleum gas sensor. Sens. Actuators B, 200, 245–250.
- 77 Fu, X., Liu, J., Han, T., Zhang, X., Meng, F., and Liu, J. (2013) A three-dimensional hierarchical CdO nanostructure: preparation and its improved gas-diffusing performance in gas sensor. Sens. Actuators B, 184, 260–267.
- 78 Bari, R.H. and Patil, S.B. (2014) Nanostructured CdO thin films for LPG and CO2 gas sensor prepared by spray pyrolisis technique. Int. Lett. Chem. Phys. Astron., 18, 31.
- 79 Nwanya, A.C., Deshmukh, P.R., Osuji, R.U., Maaza, M., Lokhande, C.D., and Ezema, F.I. (2015) Synthesis, characterization and gas-sensing properties of SILAR deposited ZnO-CdO nano-composite thin film. Sens. Actuators B, 206, 671–678.
- 80 Garcia-Sanchez, R.F., Ahmido, T., Casimir, D., Baliga, S., and Misra, P. (2013) Thermal effects associated with the Raman spectroscopy of WO3 gas-sensor materials. J. Phys. Chem. A, 117 (50), 13825–13831.
- 81 Kida, T., Nishiyama, A., Hua, Z., Suematsu, K., Yuasa, M., and Shimanoe, K. (2014) WO3 nanolamella gas sensor: porosity control using SnO2 nanoparticles for enhanced NO2 sensing. Langmuir, 30 (9), 2571–2579.
- 82 Faleh, R., Othman, M., Kachouri, A., and Aguir, K. (2014, March) Recognition of O3 concentration using WO3 gas sensor and principal component analysis. Advanced Technologies for Signal and Image Processing (ATSIP), 2014 1st International Conference on IEEE, pp. 322–327.
- 83 Dey, A., Kantha, B., and Sarkar, S.K. (2016) Sol–gel grown Pd modified WO3 thin film based methanol sensor and the effect of annealing temperatures. Microsyst. Technol., 23 (9), 4195–4201.
- 84 Choi, N.J., Park, H.J., Jung, M.Y., Lee, D.S., Kim, J.Y., Kim, J.M., and Song, H. (2015, November). Ultrasensitive formaldehyde gas sensors based on a hollow assembly and its 3-dimensional network formation of single-crystalline Co3O4 nanoparticles. SENSORS, 2015 IEEE, IEEE, pp. 1–3.
- 85 Lü, Y., Zhan, W., He, Y., Wang, Y., Kong, X., Kuang, Q., and Zheng, L. (2014) MOF-templated synthesis of porous Co3O4 concave nanocubes with high specific surface area and their gas sensing properties. ACS Appl. Mater. Interfaces, 6 (6), 4186–4195.
- 86 Kim, J.Y., Choi, N.J., Park, H.J., Kim, J., Lee, D.S., and Song, H. (2014) A hollow assembly and its three-dimensional network formation of single-crystalline Co3O4 nanoparticles for ultrasensitive formaldehyde gas sensors. J. Phys. Chem. C, 118 (45), 25994–26002.
- 87 Mendoza, F., Hernández, D.M., Makarov, V., Febus, E., Weiner, B.R., and Morell, G. (2014) Room temperature gas sensor based on tin dioxide-carbon nanotubes composite films. Sens. Actuators B, 190, 227–233.
- 88 Mubeen, S., Lai, M., Zhang, T., Lim, J.H., Mulchandani, A., Deshusses, M.A., and Myung, N.V. (2013) Hybrid tin oxide-SWNT nanostructures based gas sensor. Electrochim. Acta, 92, 484–490.
- 89 Dhall, S., Jaggi, N., and Nathawat, R. (2013) Functionalized multiwalled carbon nanotubes based hydrogen gas sensor. Sens. Actuators A-, 201, 321–327.
- 90 Jung, D., Han, M., and Lee, G.S. (2014) Gas sensor using a multi-walled carbon nanotube sheet to detect hydrogen molecules. Sens. Actuators A, 211, 51–54.
- 91 Llobet, E. (2013) Gas sensors using carbon nanomaterials: a review. Sens. Actuators B, 179, 32–45.
- 92 Deng, S., Tjoa, V., Fan, H.M., Tan, H.R., Sayle, D.C., Olivo, M., and Sow, C.H. (2012) Reduced graphene oxide conjugated Cu2O nanowire mesocrystals for high-performance NO2 gas sensor. J. Am. Chem. Soc., 134 (10), 4905–4917.
- 93 Chung, M.G., Kim, D.H., Lee, H.M., Kim, T., Choi, J.H., kyun Seo, D., and Kim, Y.H. (2012) Highly sensitive NO2 gas sensor based on ozone treated graphene. Sens. Actuators B, 166, 172–176.
- 94 Mu, H., Zhang, Z., Zhao, X., Liu, F., Wang, K., and Xie, H. (2014) High sensitive formaldehyde graphene gas sensor modified by atomic layer deposition zinc oxide films. Appl. Phys. Lett., 105 (3), 033107.
- 95 Singh, G., Choudhary, A., Haranath, D., Joshi, A.G., Singh, N., Singh, S., and Pasricha, R. (2012) ZnO decorated luminescent graphene as a potential gas sensor at room temperature. Carbon, 50 (2), 385–394.
- 96 Zou, R., He, G., Xu, K., Liu, Q., Zhang, Z., and Hu, J. (2013) ZnO nanorods on reduced graphene sheets with excellent field emission, gas sensor and photocatalytic properties. J. Mater. Chem. A, 1 (29), 8445–8452.
- 97 Cho, B., Yoon, J., Hahm, M.G., Kim, D.H., Kim, A.R., Kahng, Y.H., and Kim, C.S. (2014) Graphene-based gas sensor: metal decoration effect and application to a flexible device. J. Mater. Chem. C, 2 (27), 5280–5285.
- 98 Liu, Y., Dong, X., and Chen, P. (2012) Biological and chemical sensors based on graphene materials. Chem. Soc. Rev., 41 (6), 2283–2307.
- 99 He, Q., Wu, S., Yin, Z., and Zhang, H. (2012) Graphene-based electronic sensors. Chem. Sci., 3 (6), 1764–1772.
- 100 Basu, S. and Bhattacharyya, P. (2012) Recent developments on graphene and graphene oxide based solid state gas sensors. Sens. Actuators B, 173, 1–21.
- 101 Yang, G., Lee, C., Kim, J., Ren, F., and Pearton, S.J. (2013) Flexible graphene-based chemical sensors on paper substrates. Phys. Chem. Chem. Phys., 15 (6), 1798–1801.
- 102 Suk, J.W., Lee, W.H., Lee, J., Chou, H., Piner, R.D., Hao, Y., and Ruoff, R.S. (2013) Enhancement of the electrical properties of graphene grown by chemical vapor deposition via controlling the effects of polymer residue. Nano Lett., 13 (4), 1462–1467.
- 103 Yu, L., Zhang, L., Song, H., Jiang, X., and Lv, Y. (2014) Hierarchical SnO2 architectures: controllable growth on graphene by atmospheric pressure chemical vapour deposition and application in cataluminescence gas sensor. CrystEngComm, 16 (16), 3331–3340.
- 104 Yuan, W. and Shi, G. (2013) Graphene-based gas sensors. J. Mater. Chem. A, 1 (35), 10078–10091.
- 105 Yuan, W., Liu, A., Huang, L., Li, C., and Shi, G. (2013) High-Performance NO2 sensors based on chemically modified graphene. Adv. Mater., 25 (5), 766–771.
- 106 Zhang, Y., Zhang, L., and Zhou, C. (2013) Review of chemical vapor deposition of graphene and related applications. Acc. Chem. Res., 46 (10), 2329–2339.
- 107 Choi, H., Jeong, H.Y., Lee, D.S., Choi, S.Y., and Choi, C.G. (2013) Flexible NO2 gas sensor using multilayer graphene films by chemical vapor deposition. Carbon Lett., 14 (3), 186–189.
- 108 Hu, N., Wang, Y., Chai, J., Gao, R., Yang, Z., Kong, E.S.W., and Zhang, Y. (2012) Gas sensor based on p-phenylenediamine reduced graphene oxide. Sens. Actuators B, 163 (1), 107–114.
- 109 Akbari, E., Ahmadi, M.T., Kiani, M.J., Feizabadi, H.K., Rahmani, M., and Khalid, M. (2013) Monolayer graphene based CO2 gas sensor analytical model. J. Comput. Theor. Nanosci., 10 (6), 1301–1304.
- 110 Hafiz, S.M., Ritikos, R., Whitcher, T.J., Razib, N.M., Bien, D.C.S., Chanlek, N., and Rahman, S.A. (2014) A practical carbon dioxide gas sensor using room-temperature hydrogen plasma reduced graphene oxide. Sens. Actuators B, 193, 692–700.
- 111 Seo, D.H., Rider, A.E., Kumar, S., Randeniya, L.K., and Ostrikov, K. (2013) Vertical graphene gas-and bio-sensors via catalyst-free, reactive plasma reforming of natural honey. Carbon, 60, 221–228.
- 112 Kumar, B., Min, K., Bashirzadeh, M., Farimani, A.B., Bae, M.H., Estrada, D., and Aluru, N.R. (2013) The role of external defects in chemical sensing of graphene field-effect transistors. Nano Lett., 13 (5), 1962–1968.
- 113 Russo, P.A., Donato, N., Leonardi, S.G., Baek, S., Conte, D.E., Neri, G., and Pinna, N. (2012) Room-temperature hydrogen sensing with heteronanostructures based on reduced graphene oxide and tin oxide. Angew. Chem. Int. Ed., 51 (44), 11053–11057.
- 114 Han, J.W., Rim, T., Baek, C.K., and Meyyappan, M. (2015) Chemical Gated field effect transistor by hybrid integration of one-dimensional silicon nanowire and two-dimensional tin oxide thin film for low power gas sensor. ACS Appl. Mater. Interfaces, 7 (38), 21263–21269.
- 115 Paul, R.K., Badhulika, S., Saucedo, N.M., and Mulchandani, A. (2012) Graphene nanomesh as highly sensitive chemiresistor gas sensor. Anal. Chem., 84 (19), 8171–8178.
- 116 Ding, J., Du, K., Wathuthanthri, I., Choi, C.H., Fisher, F.T., and Yang, E.H. (2014) Transfer patterning of large-area graphene nanomesh via holographic lithography and plasma etching. J. Vac. Sci. Technol. B, 32 (6), 06FF01.
- 117 Abbas, A.N., Liu, G., Narita, A., Orosco, M., Feng, X., Müllen, K., and Zhou, C. (2014) Deposition, characterization, and thin-film-based chemical sensing of ultra-long chemically synthesized graphene nanoribbons. J. Am. Chem. Soc., 136 (21), 7555–7558.
- 118 Abbas, A.N., Liu, G., Liu, B., Zhang, L., Liu, H., Ohlberg, D., and Zhou, C. (2014) Patterning, characterization, and chemical sensing applications of graphene nanoribbon arrays down to 5 nm using helium ion beam lithography. ACS Nano, 8 (2), 1538–1546.
- 119 Jayaweera, M.T.V.P., De Silva, R.C.L., Kottegoda, I.R.M., and Rosa, S.R.D. (2015) Synthesis, characterization and ethanol vapor sensing performance of SnO2/graphene composite film. Sri Lankan J. Phys., 15. doi: 10.4038/sljp.v15i0.6345
- 120 Choi, S.J., Jang, B.H., Lee, S.J., Min, B.K., Rothschild, A., and Kim, I.D. (2014) Selective detection of acetone and hydrogen sulfide for the diagnosis of diabetes and halitosis using SnO2 nanofibers functionalized with reduced graphene oxide nanosheets. ACS Appl. Mater. Interfaces, 6 (4), 2588–2597.
- 121 Chatterjee, S.G., Chatterjee, S., Ray, A.K., and Chakraborty, A.K. (2015) Graphene–metal oxide nanohybrids for toxic gas sensor: a review. Sens. Actuators B, 221, 1170–1181.
- 122 Neri, G., Leonardi, S.G., Latino, M., Donato, N., Baek, S., Conte, D.E., and Pinna, N. (2013) Sensing behavior of SnO2/reduced graphene oxide nanocomposites toward NO2. Sens. Actuators B, 179, 61–68.
- 123 Zhang, H., Feng, J., Fei, T., Liu, S., and Zhang, T. (2014) SnO2 nanoparticles-reduced graphene oxide nanocomposites for NO2 sensing at low operating temperature. Sens. Actuators B, 190, 472–478.
- 124 Cui, S., Wen, Z., Mattson, E.C., Mao, S., Chang, J., Weinert, M., and Chen, J. (2013) Indium-doped SnO2 nanoparticle–graphene nanohybrids: simple one-pot synthesis and their selective detection of NO2. J. Mater. Chem. A, 1 (14), 4462–4467.
- 125 Inyawilert, K., Wisitsoraat, A., Sriprachaubwong, C., Tuantranont, A., Phanichphant, S., and Liewhiran, C. (2015) Rapid ethanol sensor based on electrolytically-exfoliated graphene-loaded flame-made In-doped SnO2 composite film. Sens. Actuators B, 209, 40–55.
- 126 Lin, Q., Li, Y., and Yang, M. (2012) Tin oxide/graphene composite fabricated via a hydrothermal method for gas sensors working at room temperature. Sens. Actuators B, 173, 139–147.
- 127 Liu, S., Yu, B., Zhang, H., Fei, T., and Zhang, T. (2014) Enhancing NO2 gas sensing performances at room temperature based on reduced graphene oxide-ZnO nanoparticles hybrids. Sens. Actuators B, 202, 272–278.
- 128 Uddin, A.I., Phan, D.T., and Chung, G.S. (2015) Low temperature acetylene gas sensor based on Ag nanoparticles-loaded ZnO-reduced graphene oxide hybrid. Sens. Actuators B, 207, 362–369.
- 129 Kumar, N., Srivastava, A.K., Patel, H.S., Gupta, B.K., and Varma, G.D. (2015) Facile synthesis of ZnO-reduced graphene oxide nanocomposites for NO2 gas sensing applications. Eur. J. Inorg. Chem., 2015 (11), 1912–1923.
- 130 Esfandiar, A., Irajizad, A., Akhavan, O., Ghasemi, S., and Gholami, M.R. (2014) Pd–WO3/reduced graphene oxide hierarchical nanostructures as efficient hydrogen gas sensors. Int. J. Hydrogen Energy, 39 (15), 8169–8179.
- 131 Srivastava, S., Jain, K., Singh, V.N., Singh, S., Vijayan, N., Dilawar, N., and Senguttuvan, T.D. (2012) Faster response of NO2 sensing in graphene–WO3 nanocomposites. Nanotechnology, 23 (20), 205501.
- 132 Choi, S.J., Fuchs, F., Demadrille, R., Grévin, B., Jang, B.H., Lee, S.J., and Kim, I.D. (2014) Fast responding exhaled-breath sensors using WO3 hemitubes functionalized by graphene-based electronic sensitizers for diagnosis of diseases. ACS Appl. Mater. Interfaces, 6 (12), 9061–9070.
- 133 Wang, C., Zhu, J., Liang, S., Bi, H., Han, Q., Liu, X., and Wang, X. (2014) Reduced graphene oxide decorated with CuO–ZnO hetero-junctions: towards high selective gas-sensing property to acetone. J. Mater. Chem. A, 2 (43), 18635–18643.
- 134 Wang, Z., Xiao, Y., Cui, X., Cheng, P., Wang, B., Gao, Y., and Lu, G. (2014) Humidity-sensing properties of urchinlike CuO nanostructures modified by reduced graphene oxide. ACS Appl. Mater. Interfaces, 6 (6), 3888–3895.
- 135 Uddin, A.I., Phan, D.T., and Chung, G.S. (2015) Low temperature acetylene gas sensor based on Ag nanoparticles-loaded ZnO-reduced graphene oxide hybrid. Sens. Actuators B, 207, 362–369.
- 136 Meng, H., Yang, W., Ding, K., Feng, L., and Guan, Y. (2015) Cu2O nanorods modified by reduced graphene oxide for NH3 sensing at room temperature. J. Mater. Chem. A, 3 (3), 1174–1181.
- 137 Xu, F., Deng, M., Li, G., Chen, S., and Wang, L. (2013) Electrochemical behavior of cuprous oxide-reduced graphene oxide nanocomposites and their application in nonenzymatic hydrogen peroxide sensing. Electrochim. Acta, 88, 59–65.
