25
Nanotechnology: Greener Approach for Sustainable Environment
Ambika1 and Pradeep Pratap Singh2
1University of Delhi, Hansraj College, Department of Chemistry, Delhi 110007
2University of Delhi, Swami Shraddhanand College, Department of Chemistry, Delhi 110036
25.1 Introduction
Nanotechnology is a branch of science that deals with the different types of materials in nanoscale range. It offers opportunities for the development of new technologies to produce new products, to substitute existing production equipment, and to reformulate new materials and chemicals with improved performance resulting in less consumption of energy and materials, reduced harm to the environment, and environmental remediation. Nanotechnology presents an opportunity to develop a new technology and a new industry in a sustainable way from the outset. Nanomaterials (NMs) are employed in diverse fields such as electronics and photonics, catalysis, information storage, chemical sensing and imaging, environmental remediation, drug delivery, and biological labeling [1,2]. NMs have been synthesized using a number of methods such as wet method, UV irradiation, aerosol, and lithography that involves hazardous and toxic substances. Thus, there is an urgent need of methods that are clean, biocompatible, nontoxic, and environment-friendly. Recently, green nanotechnology has drawn the attention of scientists and researchers to design new methods for joint economic, social, and health/environmental benefits [3]. This nanotechnology involves the design of NMs by eliminating or minimizing pollution without using hazardous and toxic chemicals harmful to the environment or human health at low temperatures using less energy and renewable inputs wherever possible. In this way, green nanoscience guides materials development, processing, and application design throughout the life cycle, starting with raw material selection through end of life. In this chapter, we highlight some of the most promising and important nanotechnology applications for a clean and sustainable environment (Figure 25.1).

Figure 25.1 Basic principles and applications of green nanotechnology.
25.2 Classification of Nanomaterials
Nanomaterials can be classified into several different categories that are discussed as follows:
- Nanoclusters are semicrystalline nanostructures with dimensions within 1–10 nm with a narrow size distribution.
- Nanopowders result from the aggregation of noncrystalline NMs with dimensions between 10 and 100 nm.
- Nanocrystals are single crystalline NMs with dimensions between 100 and 1000 nm.
Few more examples of NMs include nanorods, nanocups, nanospheres, nanodiamonds, nanostars, and the quantum dots [4]. Some of the nanoparticles (NPs) used in various fields of nanoscience are discussed next.
25.2.1 Dendrimers
Dendrimers are the highly branched molecules, symmetric around the core, and often adopt a spherical three-dimensional morphology. The surface groups of dendrimers are amenable to modification and can be tailored for specific applications. Polymer growth starts from a central core molecule and occurs in an outward direction by a series of polymerization reactions. Hence, precise control over size can be achieved by the extent of polymerization, starting from a few nanometers. Cavities in the core structure and folding of the branches create cages and channels. Therapeutic and diagnostic agents are usually attached to surface groups on dendrimers by chemical modification. High-density surface groups of dendrimers make this system ideally suited for multifunctional use of dendrimers. Dendritic encapsulation of functional molecules allows for the isolation of the active site, a structure that mimics that of active sites in biomaterials [5]. Also, it is possible to make dendrimers water soluble, unlike most polymers, by functionalizing their outer shell with charged species or other hydrophilic groups.
25.2.2 Liposomes
A liposome is a spherical vesicle having at least one lipid bilayer that form on hydration of dry phospholipids above their transition temperature. Based on their size and number of bilayers, liposomes are classified into three types. Multilamellar vesicles consist of several lipid bilayers separated from one another by aqueous spaces. These entities are heterogeneous in size, often ranging from a few hundreds to thousands of nanometers in diameter. On the other hand, both small unilamellar vesicles (SUVs) and large unilamellar vesicles (LUVs) consist of a single bilayer surrounding the entrapped aqueous space. SUVs are less than 100 nm in size whereas LUVs have diameters larger than 100 nm. They have also been utilized in various selective bioseparations and contrast enhancing agents for MRI in drug delivery systems, magnetic hyperthermia, and magnetically assisted transfection of cells [6].
25.2.3 Carbon Nanotubes and Fullerenes
Carbon nanotubes belong to the family of fullerenes and consist of graphite sheets rolled up into a tubular form. These structures can be obtained either as single or multiwalled nanotubes. The diameter and the length of single-walled nanotubes (SWNTs) may vary between 0.5–3.0 nm and 20–1000 nm, respectively, whereas multiwalled nanotubes (MWNTs) are 1.5–100 nm and 1–50 µm, respectively. CNTs possess unique mechanical and electrical properties due to which they have been utilized in the field of biomedical applications, environmental remediation, and so on.
25.2.4 Quantum Dots
Quantum dots (QDs) are NPs having particle size in the range of 2–10 nm in diameter and are made of semiconductors, such as cadmium selenide, which can transform the color of light [7]. The optical and electronic properties of QDs differ from those of larger particles. Their optoelectronic properties changes as a function of both size and shape. When electricity or light is applied to QDs, many types of them emit light of specific frequencies that can be precisely tuned by changing the dots' size, shape, and material, giving rise to many applications. Larger QDs (radius of 5–6 nm) emit longer wavelengths resulting in emission colors such as orange or red, whereas smaller QDs (radius of 2–3 nm) emit shorter wavelengths resulting in colors like blue and green. QDs have been widely used in transistors, solar cells, light-emitting diodes (LEDs), diode lasers, inkjet printing, spin coating, and medical imaging [8,9]. These processing techniques result in less expensive and less time-consuming methods of semiconductor fabrication.
25.3 Synthesis of Nanoparticles
Nanoparticles can be synthesized by using different methods (Scheme 25.1).

Scheme 25.1 Different approaches for the synthesis of nanoparticles.
25.3.1 Conventional Approach for the Production of Nanoparticles
This approach generally involves two types of methods that are discussed next.
25.3.1.1 Top-Down (Physical Method)
In this process, bulk materials are used as the starting material and are broken down into smaller pieces either by using chemicals and/or by using physical means such as mechanical grinding, mechanical alloying, and sputtering techniques [10–14], followed by subsequent stabilization of the resulting nanosized metal particles by the addition of colloidal protecting agents to synthesize NMs.
25.3.1.2 Bottom-Up (Chemical Method)
In wet chemical synthesis, the metal ions are allowed to grow into clusters or NPs by using different organic and inorganic reducing agents, such as sodium borohydride, sodium citrate, ascorbate, elemental hydrogen, Tollen's reagent, N,N-dimethyl formamide, and so on in aqueous or nonaqueous solutions and capping agents are also used for size stabilization of the NPs [15,16]. Most of the chemicals used for preparing NPs are toxic and hazardous leading to noneco-friendly products.
25.3.2 Green Approach for the Synthesis of Nanoparticles
Nowadays, biosynthesis of NPs has attracted the attention of researchers and scientists for the development of environment-friendly processes. Green synthesis of NPs involves the use of biological entities such as microorganisms, plant extracts, or plant biomass for the production of NPs. Thus, they provide an alternative to chemical and physical methods that involve the use of hazardous chemicals and solvents [17]. Some of the green methods for the synthesis of NPs have been discussed in Scheme 25.2.
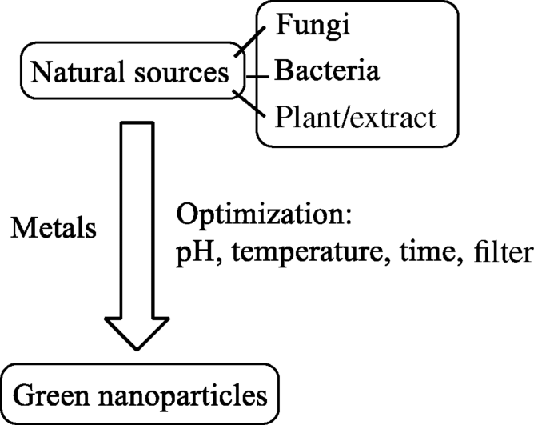
Scheme 25.2 Synthetic methodologies involved in nanoparticle synthesis.
25.3.2.1 Synthesis of Nanoparticles Using Bacteria
Microorganisms such as bacteria and fungi have the potential to synthesize NPs such as Au, Ag, and Cd sulfide. Escherichia coli, Pseudomonas stutzeri, P. aeruginosa, Plectonema boryanum, Salmonella typlus, Staphylococcus currens, and Vibrio cholerae were used for the formation of extracellular and intracellular metal NPs [18–20]. Many microorganisms can produce nanostructured mineral crystals and metallic NPs with properties similar to chemically synthesized materials; few examples include magnetotactic bacteria for the formation of magnetic NPs, P. stutzeri can form AgNPs within the periplasmic space, and sulfate-reducing bacteria in the presence of an exogenous electron donor can form palladium NPs [21]. Some microorganisms can reduce the metal ions via nonenzymatic bioreduction processes, for example, dried cells of Bacillus megaterium D01, Lactobacillus sp. A09 can reduce Ag ions by the interaction of Ag ions with the groups on the microbial cell wall [22]. Treatment of dried cells of Corynebacterium sp. SH 09 with diamine Ag complex produces AgNPs, but the rate of reaction is slow that can be accelerated in the presence of OH− [23]. The ionized carboxyl group of amino acid residues and the amide of peptide chains were the main groups trapping [Ag(NH3)]2+ on to the cell wall and some reducing groups such as aldehyde and ketone were involved in subsequent bioreduction. A thermophilic bacterium Bacillus licheniformis was used for the synthesis of AgNPs [24]. Geobacter sulfurreducens was used for the formation of nanoscale elemental Ag particles through enzymatic reduction [25]. AgNPs were also synthesized using bacteria B. licheniformis, isolated from sewage collected from municipal wastes, and ultrasonically lyzed bacterial cell [26]. AuNPs were synthesized using two P. aeruginosa isolates [27]. The interaction of bacterial cells of Rhodopseudomonas capsulate with HAuCl4 solution results in the generation of AuNPs [28]. The shape of AuNPs depends on the pH of the solution. Also, a novel alkalo-thermophilic actinomycete, Thermomonospora sp., can be utilized to synthesize monodisperse AuNPs both extracellularly and intracellularly under alkaline conditions and slightly elevated temperature [29,30].
25.3.2.2 Synthesis of Nanoparticles Using Fungi
Fungi can produce a large amount of NPs compared to bacteria, which can be attributed to the fact that fungi secrete more amounts of proteins [31]. Baker's yeast (Saccharomyces cerevisiae) was used for the transformation of Sb2O3 NPs with a size range of 2–10 nm. Fungi Verticillium luteoalbum has been employed for the intracellular synthesis of Au NPs. The rate and size of the formed NPs depend on pH, temperature, concentration of metal, and exposure time. The extra- and intracellular biosynthesis of AuNPs by fungus Trichothecium sp. has been reported [32]. Extracellular AuNPs of various morphologies, such as spherical, rod-like, and triangular could be obtained upon reaction of Trichothecium sp. with Au ions under stationary phase, whereas under shaking conditions, it forms intracellular AuNPs. This could be attributed to the fact that under shaking conditions, fungi secretes enzymes and proteins into the medium; however, in stationary phase, no such enzymes and proteins are released. Volvariella volvacea (edible mushroom) can produce Au and AgNPs through metal-reducing compounds. The mushroom biomass also prevents NPs aggregation after their formation [33]. Monodisperse AgNPs were obtained using Aspergillus flavus and a protein from the fungi acted as a capping agent on the NPs [34]. Rhizopus oryzae and Aspergillus fumigatus fungal strains were used for AuNPs [35]. Extracellular AgNPs were synthesized by utilizing mycelia free spent medium of the fungus, Cladosporium cladosporioide. The shape of the crystals depends on proteins, polysaccharides, and organic acids released by the fungus [36]. The extracellular synthesis of AgNPs has been reported using different types of fungus such as marine fungus Penicillium fellutanum, isolated from costal mangrove sediment, Penicillium brevicompactum WA2315, and Fusarium oxysporum [37–39]. However, F. moniliformae was not able to produce NPs either intracellularly or extracellularly even though similar to F. oxysporum; they both had intracellular and extracellular reductases [37]. F. oxysporum can also be used for the synthesis of zirconia NPs [40]. Verticillium sp. on exposure to silver ions resulted in an intracellular growth of AgNPs [41].
25.3.2.3 Synthesis of Nanoparticles Using Plants
Microbiological methods generate NPs at a much slower rate compared to different plant extracts. The plants and their extracts are readily available, safe, environment-friendly, and consist of a wide range of metabolites that may assist in reduction (Scheme 25.3) [42–44].

Scheme 25.3 Synthesis of nanoparticles using plant extract.
Different phytochemicals such as alkaloids, phenolic acids, polyphenols, proteins, sugars, and terpenoids, and so on plays an important role in the metal ions' reduction and their stabilization during NP synthesis. The quality, size, and morphology of the synthesized NPs depend on the nature of metal ions and their concentration, plant extract, reaction time, pH, and temperature [45,46]. AuNPs with a size range of 2–20 nm have been synthesized using the live alfalfa plants [47]. The extracts of Magnolia kobus and Diopyroskaki leaves were employed for the synthesis of AuNPs. The size of the NPs depends on the temperature because at lower temperature AuNPs of 5–300 nm were obtained, while at higher temperature the formation of smaller and spherical particles takes place. AgNPs were synthesized using the leaves of the Parthenium hysterophorus and Mentha piperita leaf extract in short reaction time [48]. The secondary metabolites present in Bryophyllum sp. (xerophytes), Cyprus sp. (mesophytes), and Hydrilla sp. (hydrophytes) were used for the synthesis of AgNPs within a range of 2–5 nm [49]. Azadirachta indica leaf extract was employed for the synthesis of Ag, Au, and bimetallic (Ag and Au) NPs in which the reducing components also served as capping and stabilizing agents [50].
25.4 Applications of Green Nanotechnology
Green nanotechnology has the potential to produce new NMs for the treatment of water, soil, air, and other environmental materials contaminated by toxic metal ions, organic and inorganic solutes, and microorganisms by using nontoxic and eco-friendly methods.
25.4.1 Nanomaterials for Water Treatment
25.4.1.1 Nanofibers
Nanofibers have been used in the remediation of pollutants from wastewater. The sorption of the pollutants depends on the functional groups and the polarity of NFs. Nanosilver-based nanofibers and nanocomposites were used to disinfect drinking water [51–54]. AgNPs embedded in cellulose acetate fibers are effective biocides against S. aureus, E. coli, Klebsiella pneumonia, and P. aeruginosa [55]. Cellulose acetate functionalized with poly(methacrylic acid) exhibits enhanced removal of mercury (Hg(II)) from water [56]. Nanofibrous membranes prepared by using blend of wool keratose and silk fibroin (WK/SF) displayed a significant Cu(II) adsorption capacity compared to pure silver wool and filter paper due to their large specific area [57]. Recently, mesoporous ZnO nanofiber mats were utilized in an UV radiation-assisted photodecomposition of polyaromatic hydrocarbons (PAHs) dyes, for example, naphthalene and anthracene [58].
25.4.1.2 Nanomembranes
Nanomembranes have been used for the purification of water (Scheme 25.4). Water filtration membranes consisting of AgNPs possess good antimicrobial activities against E. coli and Pseudomonas [59–61]. Cellulose acetate/silica composite-based membranes were used for Cr(VI) adsorption [62]. Functionalized oxidized cellulose nanofibers embedded in an electrospun polyacrylonitrile nanofibrous membrane with thiol groups was employed for adsorption of heavy metals such as Cr(VI) and Pb(II) [63]. Cellulose acetate membrane incorporated with ZnO NPs exibits enhanced antibacterial efficiency against S. aureus, E. coli, and Citrobacter freundii [64]. Nanomembranes containing AgNPs displayed enhanced antibacterial efficiency [65]. Electrospun chitosan membranes can adsorb heavy metal ions, and the efficiency depends on pH, degree of deacetylation, morphology, and fiber structure [66,67]. A quaternized chitosan membrane displayed inhibition of bacterial growth on the mat. Blending biopolymers with good antibacterial efficiency was also significantly reduced by the biocidal activity of the resulting membrane [68]. The functionalization of the electrospun membrane enhances the biocidal efficiency [69,70]. It has high mineralization and reaction rates and provides more active sites with high specific surface photoactivity.

Scheme 25.4 Purification of water using nanomembranes.
25.4.1.3 Metal Nanoparticles
Zero-valent iron NPs (nZVI) have been used in the reductive disposal of a wide array of environmental contaminants such as organochlorine pesticides and polychlorinated biphenyls in the treatment of contaminated groundwater [71,72]. They have been employed for removing Cr(VI), As(V), and uranium from groundwater effectively [73,74]. nZVI NPs have been used in the remediation. Titanium dioxide NPs (TiO2NPs) have been used in self-cleaning surfaces, water purification, deodorization of environment, and water remediation [75,76]. Recently, TiO2 NPs were employed as a photocatalyst in water treatment. The photocatalytic activity of TiO2 NPs can be enhanced by the modification of its surface with the introduction of polymers or metal ions [77]. TiO2 NPs have been utilized in degradation of organic compounds such as trazines and organochlorine pesticides and adsorption of heavy metal ions such as Pb, Cd, Cu, Zn, and Ni from wastewater [78]. Iron oxide magnetic NMs can effectively adsorb heavy metals such as Pb(II), As(III), and As(V) ions from wastewater [79,80]. Stabilization of the NPs can be achieved by surface modifications, which may enhance the removal of heavy metal ions and organic contaminants from wastewater [81,82]. Magnetic NPs have been used as efficient adsorbents for the removal of Cr(VI), Cu2+, and Cd2+from aqueous solution from aqueous solutions to extract trace PAHs [83,84].
25.4.1.4 Nanoclays
Naturally occurring nanoclays such as montmorillonite, saponite, fluorine hectorite, and fluorine mica and their modified organoclays can be used as adsorbents for the removal of organic pollutants and metal ions from wastewater [85,86]. TiO2 clay nanocomposites were used for water remediation [87]. Allophane nanoclays were used to remove phosphorus (P) from aqueous solutions and meatwork effluent [88].
25.4.2 Nanotechnology for Renewable Energy
Nanomaterials are employed in the development of new technologies that involve the use of eco-friendly methods and sources such as solar cells, batteries, hydrogen as fuel, and so on.
25.4.2.1 Dye-sensitized Solar Cells
The dye-sensitized solar cell (DSSC) uses an insulator (often TiO2) that is “sensitized” by a photosensitive molecular dye. Recently, natural dyes such as carotenoids (e.g., crocetin or bixin) as photosensitizers for DSSCs have been employed due to their low cost, abundant supply, and sustainability (Figure 25.2) [89–91]. The highest ɳ with single carotenoids is 2.6% but it can be further increased by combining it with chlorophyll [92,93]. Sicilian prickly pear extract possess a high ɳ of 2.06% [94]. The optimal length of carotenoids consists of seven conjugated pi bonds [95]. Several extracts have been utilized as efficient photosensitizers such as black rice extract and mangosteen pericarp extract [96,97]. The stability of anthocyanins and betalains when protected from direct sunlight is more than 1 year [94]. An alternative approach to this issue of depleting dyes is mimicking nature where an annual exchange of dyes might be the solution.

Figure 25.2 Schematic representation of a dye-sensitized solar cell.
25.4.2.2 Quantum Dots in Renewable Energy Sources
Quantum dots have been employed in development of resources of nonrenewable energy like in energy-efficient lighting and solar photovoltaic [98,99]. Recently, QD-based LEDs (QDLEDs) have been used in high-power devices. QDLEDs offer several advantages such as color purity, broad wavelength range, stability, low production cost, and similar levels of efficiency with that of conventional LED and organic LED (OLED) technologies. QDLED devices for light harvesting have significant potential in terms of saving energy with a projected reduction of 50% in the electricity consumption occurring due to lighting [100]. Recently, QDs have been employed in solar photovoltaics for the development of QD-based solar cells (QDSCs) [101]. In QDs, the bandgap can be tailored to absorb light in the whole solar spectrum range. Also, the enhanced impact ionization leads to multiple electron generation for each photon absorbed, leading to high and stable photocurrent generation [102]. Greener approaches, such as CuInE2 (E = S, Se) QDs, have been reported; in spite of high absorbing capacity and low toxicity, these solar cells have shown lower efficiencies (<6.79%) [80,103]. Photovoltaic applications of QDs open the possibility of development of light-harvesting structures. QD-sensitized solar cells can have a maximum efficiency of 44% (theoretical), which is higher in comparison to DSSCs using organic sensitizers (33.5%) and for traditional Si solar cells (35%) [104].
25.4.2.3 Fuel Cell
In addition to solar cells, nanotechnology has made big impact on fuel cells, with devices able to convert chemical energy directly into electricity [105]. Nanoporos metals such as Pt-NPs, multi (bi and tri) metallic Pt alloys were utilized in the development of electrode for oxidation/reduction reactions in fuel cells [106–108]. Recently, CNTs and graphene have been used as metal-free catalysts in fuel cells due to their high surface area, mesoporosity, good electrical conductivity, stronger mechanical strength, light weight, and excellent corrosion resistance [109–111].
25.4.2.4 Hydrogen Gas
Hydrogen gas is an endless source of clean fuel for many applications [112]. Semiconductor NMs, for example, TiO2 and cadmium sulfide nanostructures, are utilized as catalysts for water conversion into oxygen and hydrogen [113,114]. For the storage of hydrogen and transportation for high hydrogen capacity and minimal deterioration during hydrogenation, various NMs such as metal-organic frameworks and polymers as well as metal hydrides and related complex hydrides have been developed [115–117].
25.4.3 Nanomaterials for Construction Industry
Manufactured NMs and nanocomposites offer great opportunities in the construction and related infrastructure industries [118]. Strength, durability, and lightness of various materials as well as heat-insulating, self-cleaning, fire-retardant, anti-fogging and sensing structural health properties may be improved by NMs [119–121]. Thus, CNTs, SiO2, TiO2, Fe2O3, and magnetic nickel NPs can remarkably improve mechanical durability and compressive and flexural strength of cement products [122–125]. Highly water-repellent coatings incorporating silica, alumina NPs, and hydrophobic polymers are used for wood [126]. TiO2 NPs were used in glasses with self-cleaning technology due to their photocatalytic and antifouling properties [127]. Fire protective glass is obtained using silica (nano)layers, which may also function as antireflection coatings for exterior light for contributing in energy and air conditioning conservation [128]. AgNPs can be embedded in paint to inactivate pathogenic microbes and provide antimicrobial properties to surfaces [129].
25.4.4 Other Nanoenhanced Green Applications
Several other sustainable nanotechnology applications have been investigated [130]. Nanoporous zeolites may be used as a slowly releasing carrier of fertilizers or as a permanent water reservoir due to their property to hold water molecules that may help plants to withstand dry spells [131]. Different varieties of NMs have been developed for new packaging to obtain films with good exfoliation, barrier, fireproofing, and mechanical properties [132]. This application may increase the shelf life of the food and its safety for consumers. Nanosensors can improve the quality and reduce the cost of continuous environmental monitoring [133].
25.5 Prospects
Green nanonotechnology can play an important role in areas such as generation, conversion, transmission, harvesting, storage, recovery, and transportation of energy. The risks associated with the development of green nanotechnologies should be compared against current technologies already in use. NMs may have significant, still unknown, hazardous properties related to their unique physicochemical properties, which can pose risks to a wide range of population exposed through the overall life cycle of NMs. Thus, there is a huge scope in improving the understanding of toxicity associated with metrics, such as size, surface area, functionalization, or particle number concentration utilized in preparing NMs. With the aim to reach an adequate risk assessment, research should be focused to thoroughly define the hazardous impact of NMs that can provide important information to adopt appropriate safety measures in a widespread risk management program. Overall, green nanotechnology should not only provide green solutions but also “become green” in terms of the attention paid to occupational safety and health. The research methodologies should be designed to overcome practical barriers related to the exposure of green NMs and their problems related to health and practical instrumentation, which will maximize their acceptance in terms of environmental and societal benefits, health gains, and cost savings.
25.6 Conclusions
Green nanotechnology exploits the physical and chemical properties of NMs to develop new methods and products in an environment-friendly and cost-effective manner, which can be easily scaled up for large-scale synthesis of NPs at low temperature and pressure. This technique either eliminates or minimizes the use of hazardous and toxic chemicals. It involves the use of natural resources such as plants and microorganisms for the synthesis of NMs. However, plant extracts for the synthesis of NMs are advantageous compared to microorganisms due to the ease of availability, less biohazard, and providing natural capping agents for the stabilization of metal NPs. These NMs have the potential to clean hazardous waste sites, treat pollutants, or sense and monitor environmental pollutants. This technology also offers the development of alternative energy systems such as light-emitting diodes (LEDs) that could reduce pollution from energy generation and help fossil fuels' conservation. The self-cleaning surface coatings could reduce or eliminate many cleaning chemicals used in regular maintenance. The use of light-weight nanocomposites for transportation could save fuel and reduce materials used for production.
References
- 1 Guo, J.Z., Cui, H., Zhou, W., and Wang, W. (2008) Ag nanoparticle-catalyzed chemiluminescent reaction between luminal and hydrogen peroxide. J. Photochem. Photobiol. A, 193, 89–96.
- 2 Daniel, M.C. and Astruc, D. (2004) Gold nanoparticles: assembly, supramolecular chemistry, quantum-size-related properties, and applications towards biology, catalysis, and nanotechnology. Chem. Rev., 104, 293–346.
- 3 Hutchison, J.E. (2008) Greener nanoscience: a proactive approach to advancing applications and reducing implications of nanotechnology. ACS Nano, 2, 395–402.
- 4 Chakrabarti, S., Fathpour, S., Moazzami, K., Phillips, J., Lei, Y., Browning, N., and Bhattacharya, P. (2004) Pulsed laser annealing of self-organized InAs/GaAs quantum dots. J. Electron. Mater., 33, L5.
- 5 Hecht, S. and Frechet, J.M. (2001) Dendritic encapsulation of function: Applying nature's site isolation principle from biomimetics to materials science. Angew. Chem. Int. Ed., 40, 74–91.
- 6 Aptekar, J.W., Cassidy, M.C., Johnson, A.C., Barton, R.A., Lee, M.Y., Ogier, A.C., Vo, C., Anahtar, M.N., Ren, Y., Bhatia, S.N., Ramanathan, C., Cory, D.G., Hill, A.L., Mair, R.W., Rosen, M.S., Walsworth, R.L., and Marcus, C.M. (2009) Silicon nanoparticles as hyperpolarized magnetic resonance imaging agents. ACS Nano, 3, 4003–4008.
- 7 Chong, E.J., Phan, T.T., Lim, I.J., Zhang, Y.Z., Bay, B.H., Ramakrishna, S., and Lim, C.T. (2007) Evaluation of electrospun PCL/gelatin nanofibrous scaffold for wound healing and layered dermal reconstitution. Ada Biomaterialia, 3, 321–330.
- 8 Victor, R. and Irina, K. (2000) Electron and photon effects in imaging devices utilizing quantum dot infrared photodetectors and light emitting diodes. Proc. SPIE, 3948, 206–219.
- 9 Anikeeva, P., Halpert, J., Bawendi, M., and Bulovic, V. (2009) Quantum dot light-emitting deices with electroluminescence tunable over the entire visible spectrum. Nano Lett., 9, 2532–2536.
- 10 Gubin, S.P., Koksharov, Y.A., Khomutov, G.B., and Yurkov, G.Y. (2005) Magnetic nanoparticles: preparation, structure and properties. Russ. Chem. Rev., 74, 489–520.
- 11 Gaffet, E., Tachikart, M., Kedim, O.E., and Rahouadj, R. (1996) Nanostructural materials formation by mechanical alloying-morphologic analysis basis on transmission and scanning electron microscopic observations. Mater. Charact., 36, 185–190.
- 12 Chung, B.X. and Liu, C.P. (2004) Synthesis of cobalt nanoparticles by DC magnetron sputtering and the effects of electron bombardment. Mater. Lett., 58, 1437–1440.
- 13 Suzuki, S., Suzuki, T., Tomita, Y., Hirano, M., Okazaki, K., Kuwabata, S., and Torimoto, T. (2012) Compositional control of Au-Pt nanoparticles synthesized in ionic liquids by the sputter deposition technique. Cryst. Eng. Comm., 14, 4922–4926.
- 14 Bouchat, V., Feron, O., Gallez, B., Masereel, B., Michiels, C., Borght, T.V., and Lucas, S. (2011) Carbon nanoparticles synthesized by sputtering and gas condensation inside a nanocluster source of fixed dimension. Surf. Coat. Technol., 205, S577–S581.
- 15 Guzman, M.G., Dille, J., and Godet, S. (2009) Synthesis of silver nanoparticles by chemical reduction method and their antibacterial activity. Int. J. Chem. Bio. Engineer., 2, 104–111.
- 16 Khanna, P.K. (2007) Reduction of transition metal salts by SFS: synthesis of copper and silver sulphides. J. Synth. React. Inorg. Metal-Organ. Nano- Met. Chem., 37, 805–808.
- 17 Srikar, S.K., Giri, D.D., Pal, D.B., Mishra, P.K., and Upadhyay, S.N. (2016) Green synthesis of silver nanoparticles: a review. Green Sustain. Chem., 6, 34–56.
- 18 Klaus, T., Joerger, R., Olsson, E., and Granqvist, C.G. (1999) Silver-based crystalline nanoparticles, microbially fabricated. Proc. Natl. Acad. Sci. USA, 96, 13611–13614.
- 19 Beveridge, T.J. and Murray, R.G.J. (1976) Uptake and retention of metals by cell walls of Bacillus subtilis. Bacteriol. Sep., 127, 1502–1518.
- 20 Southam, G. and Beveridge, T.J. (1994) The in vitro formation of placer gold by bacteria. Geochim. Cosmochim. Acta, 58, 4527–4530.
- 21 Gericke, M. and Pinches, A. (2006) Biological synthesis of metal nanoparticles. Hydrometallurgy, 83, 132–140.
- 22 Fu, J.K., Liu, Y., Gu, P., Tang, D.L., Lin, Z.Y., Yao, B.X., and Weng, S.Z. (2000) Spectroscopic characterization on the biosorption and bio reduction of Ag(I) by Lactobacillus sp. A09. Acta Physico-Chim. Sin., 16, 770–782.
- 23 Fu, M., Li, Q., Sun, D., Lu, Y., He, N., Deng, X., Wang, H., and Huang, J. (2006) Rapid preparation process of silver nanoparticles by bioreduction and their characterizations. Chin. J. Chem. Eng., 14, 114–117.
- 24 Kalishwaralal, K., Deepak, V., Ramkumarpndian, S., Nellaiah, H., and Sangiliyandi, G. (2008) Extracellular biosynthesis of silver nanoparticles by the culture supernatant of Bacillus Licheniformis. Mater. Lett., 62, 4411–4413.
- 25 Law, N., Ansari, S., Livens, F.R., Renshaw, J.C., and Lloyd, J.R. (2008) Formation of nanoscale elemental silver particles via enzymatic reduction by Geobacter sulfurreducens. Appl. Environ. Microbiol., 74, 7090–7093.
- 26 Kalimuthu, K., Babu, S.R., Venkataraman, D., Bilal, M., and Gurunathan, S. (2008) Biosynthesis of silver nanoparticles by Bacillus licheniformis. Colloid Surf. B, 65, 150–153.
- 27 Husseiny, I.M., El-Aziz, A.M., Badr, Y., and Mahmoud, T. (2007) Biosynthesis of gold nanoparticles using Pseudomonas aeruginosa. Spectrochim. Acta A, 67, 1003–1006.
- 28 He, S., Guo, Z., Zhang, Y., Zhang, S., Wang, J., and Gu, N. (2007) Biosynthesis of gold nanoparticles using the bacteria Rhodopseudomonas capsulate. Mater. Lett., 61, 3984–3987.
- 29 Sastry, M., Ahmad, A., Khan, I.M., and Kumar, R. (2003) Biosynthesis of metal nanoparticles using fungi and actinomycete. Curr. Sci., 85, 162–170.
- 30 Ahmad, A., Mukherjee, P., Senapati, S., Mandal, D., Khan, M.I., Kumar, R., and Sastry, M. (2003) Extra cellular biosynthesis of silver nanoparticles using the fungus Fusarium oxysporum. Colloid Surf. B, 28, 313–318.
- 31 Mohanpuria, P., Rana, K.N., and Yadav, S.K. (2008) Biosynthesis of nanoparticles: technological concepts and future applications. J. Nano Res., 10, 507–517.
- 32 Absar, A., Satyajyoti, S., Khan, M.I., Rajiv, K., and Sastry, M. (2005) Extra-/intracellular biosynthesis of gold nanoparticles by an alkalotolerant fungus Trichothecium sp. J. Biomed. Nanotechnnol., 1, 47–53.
- 33 Philip, D. (2009) Biosynthesis of Au, Ag and Au-Ag nanoparticles using edible mushroom extract. Spectrochim. Acta A, 73, 374–381.
- 34 Vigneshwaran, N., Kathe, A.A., Varadarajan, P.V., Nachane, R.P., and Balasubramanya, R.H. (2007) Silver-protein (core–shell) nanoparticle production using spent mushroom substrate. Langmuir, 23, 7113–7117.
- 35 Bhainsa, K.C. and D'Souza, S.F. (2006) Extracellular biosynthesisof silver nanoparticles using the fungus Aspergillus fumigatus. Colloid Surf. B, 47, 160–164.
- 36 Balaji, D.S., Basavaraja, S., Deshpande, R., Mahesh, D.B., Prabhakar, B.K., and Venkataraman, A. (2009) Extracellular biosynthesis of functionalized silver nanoparticles by strains of Cladosporium cladosporioides fungus. Colloid Surf. B, 68, 88–92.
- 37 Kathiresan, K., Manivannan, S., Nabeel, A.M., and Dhivya, B. (2009) Studies on silver nanoparticles synthesized by a marine fungus Penicillum fellutanum isolated from coastal mangrove sediment. Colloid Surf. B, 71, 133–137.
- 38 Shaligram, S.N., Bule, M., Bhambure, R., Singhal, S.R., Singh, K.S., Szakacs, G., and Pandey, A. (2009) Biosynthesis of silver nanoparticles using aqueous extract from the compactin producing fungal. Process Biochem., 44, 939–943.
- 39 Duran, N., Marcato, D.P., Alves, L.O., DeSouza, G., and Esposito, E. (2005) Mechanical aspect of biosynthesis of silver nanoparticles by several Fusarium oxysporum strains. J. Nanobiotechnol. doi. 10.1186/1477-3155-3-8
- 40 Bansal, V., Rautaray, D., Ahmad, A., and Sastry, M. (2004) Biosynthesis of zirconiana noparticles using the fungus Fusarium oxysporum. J. Mater. Chem., 14, 3303–3305.
- 41 Mukherjee, P., Ahmad, A., Mandal, D., Senapati, S., Sainkar, S.R., Khan, M.I., Parischa, R., Ajaykumar, P.V., Alam, M., Kumar, R., and Sastry, M. (2001) Fungus mediated synthesis of silver nanoparticles and their immobilization in the mycelia matrix: a novel biological approach to nanoparticle synthesis. Nano Lett., 1, 515–519.
- 42 Kulkarni, N. and Muddapur, U. (2014) Biosynthesis of metal nanoparticles: a review. J. Nanotechnol. doi. 10.1155/2014/510246
- 43 Khan, A.A., Fox, E.K., Gorzny, M.L., Nikulina, E., Brougham, D.F., Wege, C., and Bittner, A.M. (2013) pH control of the electrostatic binding of gold and iron oxide nanoparticles to tobacco mosaic virus. Langmuir, 29, 2094–2098.
- 44 Kale, A., Bao, Y., Zhou, Z., Prevelige, P.E., and Gupta, A. (2013) Directed self-assembly of CdS quantum dots on bacteriophage P22 coat protein templates. Nanotechnology. doi 10.1088/0957-4484/24/4/045603
- 45 Mittal, A.K., Chisti, Y., and Banerjee, U.C. (2013) Synthesis of metallic nanoparticles using plants. Biotechnol. Adv., 31, 346–356.
- 46 Dwivedi, A.D. and Gopal, K. (2010) Biosynthesis of silver and gold nanoparticles using Chenopodium album leaf extract. Colloids Surf. A, 369, 27–33.
- 47 Torresday, J.L.G., Parsons, J.G., Gomez, E., Peralta-Videa, J., Troian, H.E., Santiago, I.P., and Yacaman, M.J. (2002) Formation and growth of Au nanoparticles inside live alfa alfa plants. Nanoletters, 2, 397–401.
- 48 Parasar, U.K., Saxena, P.S., and Srivastava, A. (2009) Bioinspired synthesis of silver nanoparticles digest. J. Nanomater. Biostruct., 4, 159–166.
- 49 Jha, A.K. and Prasad, K. (2009) A green low-cost biosynthesis of Sb2O3 nanoparticles. J. Biochem. Eng., 43, 303–306.
- 50 Tripathi, A., Chandrasekaran, N., Raichur, A.M., and Mukherjee, A. (2009) Antibacterial applications of silver nanoparticles synthesized by aqueous extract of Azadirachta indica (Neem) leaves. J. Biomed. Nanotechnol., 5, 93–98.
- 51 Balogh, L., Swanson, D.R., Tomalia, D.A., Hagnauer, G.L., and McManus, A.T. (2001) Dendrimer-silver complexes and nanocomposites as antimicrobial agents. Nano Lett., 1, 18–21.
- 52 Botes, M. and Cloete, T.E. (2010) The potential of nanofibers and nanobiocides in water purification. Crit. Rev. Microbiol., 36, 68–81.
- 53 Chen, C.Y. and Chiang, C.L. (2008) Preparation of cotton fibers with antibacterial silver nanoparticles. Mat. Lett., 62, 3607–3609.
- 54 Vimala, K., Sivudu, K.S., Mohan, Y.M., Sreedhar, B., and Mohana Raju, K. (2009) Controlled silver nanoparticles synthesis in semi-hydrogel networks of poly(acrylamide) and carbohydrates: a rational methodology for antibacterial application. Carbohydr. Polym., 75, 463–471.
- 55 Son, W.K., Youk, J.H., Lee, T.S., and Park, W.H. (2004) Preparation of antimicrobial ultrafine cellulose acetate fibers with silver nanoparticles. Macromol. Rapid Commun., 25, 1632–1632.
- 56 Tian, Y., Wu, M., Liu, R., Li, Y., Wang, D., Tan, J., Wu, R., and Huang, Y. (2011) Electrospun membrane of cellulose acetate for heavy metal ion adsorption in water treatment. Carbohydr. Polym., 83, 743–748.
- 57 Ki, C.S., Gang, E.H., Um, I.C., and Park, Y.H. (2007) Nanofibrous membrane of wool keratose/silk fibroin blend for heavy metal ion adsorption. J. Membr. Sci., 302, 20–26.
- 58 Singh, P., Mondal, K., and Sharma, A. (2013) Reusable electrospun mesoporous ZnO nanofiber mats for photocatalytic degradation of polycyclic aromatic hydrocarbon dyes in wastewater. J. Coll. Inter. Sci., 394, 208–215.
- 59 Zodrow, K., Brunet, L., and Mahendra., S. (2009) Polysulfone ultrafiltration membranes impregnated with silver nanoparticles show improved biofouling resistance and virus removal. Water Res., 43, 715–723.
- 60 Chou, W.L., Yu, D.G., and Yang, M.C. (2005) The preparation and characterization of silver-loading cellulose acetate hollow fiber membrane for water treatment. Polym. Adv. Technol., 16, 600–607.
- 61 Lee, S.Y., Kim, H.J., Patel, R., Im, S.J., Kim, J.H., and Min, B.R. (2007) Silver nanoparticles immobilized on thin film composite polyamide membrane: characterization, nanofiltration, antifouling properties. Polym. Adv. Techol., 18, 562–568.
- 62 Taha, A.A., Wu, Y.N., Wang, H., and Li, F. (2012) Preparation and application of functionalized cellulose acetate/silica composite nanofibrous membrane via electrospinning for Cr(VI) ion removal from aqueous solution. J. Environ. Manag., 112, 10–16.
- 63 Yang, R., Aubrecht, K.B., Ma, H., Wang, R., Grubbs, R.B., Hsiao, B.S., and Chu, B. (2014) Thiol-modified cellulose nanofibrous composite membranes for chromium (VI) and lead (II) adsorption. Polymer, 55, 1167–1176.
- 64 Anitha, S., Brabu, B., Thiruvadigal, D.J., Gopalakrishnan, C., and Natarajan, T.S. (2012) Optical, bactericidal and water repellent properties of electrospun nano-composite membranes of cellulose acetate and ZnO. Carbohydr. Polym., 87, 1065–1072.
- 65 Shalumon, K.T., Anulekha, K.H., Nair, S.V., Chennazhi, K.P., and Jayakumar, R. (2011) Sodium alginate/poly(vinyl alcohol)/nano ZnO composite nanofibers for antibacterial wound dressings. Int. J. Biol. Macromol., 49, 247–254.
- 66 Desai, K., Kit, K., Li, J., Davidson, P.M., Zivanovic, S., and Meyer, H. (2009) Nanofibrous chitosan non-wovens for filtration applications. Polymer, 50, 3661–3669.
- 67 Zhao, Z.P., Wang, Z., and Wang, S.C. (2003) Formation, charged characteristic and BSA adsorption behavior of carboxy-methyl chitosan/PES composite MF membrane. J. Membr. Sci., 217, 151–158.
- 68 Ignatova, M., Manolova, N., and Rashkov, I. (2007) Novel antibacterial fibers of quaternized chitosan and poly(vinyl pyrrolidone) prepared by electrospinning. Eur. Polym. J., 43, 1112–1122.
- 69 Kangwansupamonkon, W., Tiewtrakoonwat, W., Supaphol, P., and Kiatkamjornwong, S. (2014) Surface modification of electrospun chitosan nanofibrous mats for antibacterial activity. J. Appl. Poly. Sci., 131, 1–9.
- 70 Ignatova, M., Starbova, K., Markova, N., and Rashkov, I. (2006) Electrospun nano-fibre mats with antibacterial properties from quaternised chitosan and poly (vinyl alcohol). Carbohydr. Res., 341, 2098–2107.
- 71 Zhang, W.X. (2003) Nanoscale iron particles for environmental remediation: an overview. J. Nanopart. Res., 5, 323–332.
- 72 Quinn, J., Geiger, C., Clausen, C., Brooks, K., Coon, C., and O'Hara, S. (2005) Field demonstration of DNAPL dehalogenation using emulsified zero-valent iron. Environ. Sci. Technol., 39, 1309–1318.
- 73 Crane, R.A., Dickinson, M., Popescu, I.C., and Scott, T.B. (2011) Magnetite and zero-valent iron nanoparticles for the remediation of uranium contaminated environmental water. Water Res., 45, 2931–2942.
- 74 Kozma, G., Ronavari, A., Konya, Z., and Kukovecz, A. (2016) Environmentally benign synthesis methods of zero-valent iron nanoparticles. Chem. Eng., 4, 291–297.
- 75 Liou, J.W. and Chang, H.H. (2012) Bactericidal effects and mechanisms of visible light-responsive titanium dioxide photocatalysts on pathogenic bacteria. Arch. Immun. Therap. Exp., 60, 267–275.
- 76 Wei, C., Lin, W.Y., Zainal, Z., Williams, N.E., Zhu, K., Kruzic, A.P., Smith, R.L., and Rajeshwar, K. (1994) Bactericidal activity of TiO2 photocatalyst in aqueous media: toward a solar-assisted water disinfection system. Envron. Sci. Technol., 28, 934–938.
- 77 Makarova, O.V., Rajh, T., Thurnauer, M.C., Martin, A., Kemme, P.A., and Cropek, D. (2000) Surface modification of TiO2 nanoparticles for photochemical reduction of nitrobenzene. Environ. Sci. Technol., 34, 4797–4803.
- 78 Nakata, K. and Fujishima, A. (2012) TiO2 photocatalysis: design and applications. J. Photochem. Photobiol. C: Photochem. Rev., 13, 169–189.
- 79 Hu, H.B., Wang, Z.H., and Pan, L. (2010) Synthesis of monodisperse Fe3O4@silica core–shell microspheres and their application for removal of heavy metal ions from water. J. Alloys Comps., 492, 656–661.
- 80 Yang, W., Kan, A.T., Chen, W., and Tomson, M.B. (2010) pH-dependent effect of zinc on arsenic adsorption to magnetite nanoparticles. Water Res., 44, 5693–5701.
- 81 Ambashta, R.D. and Sillanpaa, M. (2010) Water purification using magnetic assistance: a review. J. Hazard. Mater., 180, 38–49.
- 82 Zhao, X.L., Wang, J.M., Wu, F.C., Wang, T., Cai, Y.Q., and Shi, Y.L. (2010) Removal of fluoride from aqueous media by Fe3O4@Al(OH)3 magnetic nanoparticles. J. Hazard. Mat., 173, 102–109.
- 83 Bystrzejewski, M., Pyrzynska, K., Huczko, A., and Lange, H. (2009) Carbon-encapsulated magnetic nanoparticles as separable and mobile sorbents of heavy metal ions from aqueous solutions. Carbon, 47, 1201–1204.
- 84 Hu, J., Lo, I.M.C., and Chen, G. (2007) Comparative study of various magnetic nanoparticles for Cr(VI) removal. Sep. Purif. Technol., 56, 249–256.
- 85 Kowalska, M., Guler, H., and Cocke, D.L. (1994) Interactions of clay minerals with organic pollutants. Sci. Total Environ., 141, 223–240.
- 86 Tuzen, M., Melek, E., and Soylak, M. (2006) Celtek clay as sorbent for separation-preconcentration of metal ions from environmental samples. J. Hazard. Mater., 136, 597–603.
- 87 Ponder, S.M., Darab, J.G., and Mallouk, T.E. (2000) Remediation of Cr(VI) and Pb(II) aqueous solutions using supported, nanoscale zero-valent iron. Env. Sci. Technol., 34, 2564–2569.
- 88 Yuan, G. and Wu, L. (2007) Allophane nanoclay for the removal of phosphorus in water and wastewater. Sci. Technol. Adv. Mater., 8, 60–62.
- 89 Eiji, Y., Masaki, M., Naomi, N., Noritsugu, H., Masashi, S., and Osamu, K. (2007) Utilization of natural carotenoids as photosensitizers for dye-sensitized solar cells. Sol. Energy, 81, 512–516.
- 90 Koyama, Y., Miki, T., Wang, X.F., and Nagae, H. (2009) Dye-sensitized solar cells based on the principles and materials of photosynthesis: mechanisms of suppression and enhancement of photocurrent and conversion efficiency. Int. J. Mol. Sci., 10, 4575–4622.
- 91 Koyama, Y., Kakitani, Y., and Nagae, H. (2012) Mechanisms of suppression and enhancement of photocurrent/conversion efficiency in dye-sensitized solar-cells using carotenoid and chlorophyll derivatives as sensitizers. Molecules, 17, 2188–2218.
- 92 Wang, X., Koyama, Y., Nagae, H., Yamano, Y., Ito, M., and Wada, Y. (2006) Photocurrents of solar cells sensitized by aggregate-forming polyenes: enhancement due to suppression of singlet-triplet annihilation by lowering of dye concentration or light intensity. Chem. Phys. Lett., 420, 309–315.
- 93 Wang, X., Matsuda, A., Koyama, Y., Nagae, H., Sasaki, S., and Tamiaki, H. (2006) Effects of plant carotenoid spacers on the performance of a dye-sensitized solar cell using a chlorophyll derivative: enhancement of photocurrent determined by one electron-oxidation potential of each carotenoid. Chem. Phys. Lett., 423, 470–475.
- 94 Calogero, G., Yum, J., Sinopoli, A., Di Marco, G., Gratzel, M., and Nazeeruddin, M.K. (2012) Anthocyanins and betalains as light-harvesting pigments for dye-sensitized solar cells. Sol. Energy, 86, 1563–1575.
- 95 Wang, X., Fujii, R., Ito, S., Koyama, Y., Yamano, Y., and Ito, M. (2005) Dye-sensitized solar cells using retinoic acid and carotenoic acids: dependence of performance on the conjugation length and the dye concentration. Chem. Phys. Lett., 416, 1–6.
- 96 Hao, S., Wu, J., Huang, Y., and Lin, J. (2006) Natural dyes as photosensitizers for dye-sensitized solar cell. Sol. Energy, 80, 209–214.
- 97 Narayan, M.R. (2012) Review: dye sensitized solar cells based on natural photosensitizers. Renew Sustain. Energy Rev., 16, 208–215.
- 98 Shirasaki, Y., Supran, G.J., Bawendi, M.G., and Bulovic, V. (2012) Emergence of colloidal quantum-dot light-emitting technologies. Nat. Photonics, 7, 13–23.
- 99 Emin, S., Singh, S.P., Han, L., Satoh, N., and Islam, A. (2011) Colloidal quantum dot solar cells. Sol. Energy, 85, 1264–1282.
- 100 Irvine-Halliday, D., Peon, R., Doluweera, G., Platonova, I., and Irvine-Halliday, G. (2006) Solid-state lighting: The only solution for the developing. SPIE Int. Soc. Opt. Eng. doi: 10.1117/2.1200601.0056
- 101 Hod, I. and Zaban, A. (2014) Materials and interfaces in quantum dot sensitized solar cells: challenges, advances and prospects. Langmuir, 30, 7264–7273.
- 102 Jun, H.K., Careem, M.A., and Arof, A.K. (2013) Quantum dot-sensitized solar cells-perspective and recent developments: a review of Cd chalcogenide quantum dots as sensitizers. Renew. Sustain. Energy Rev., 22, 148–167.
- 103 Li, W., Pan, Z., and Zhong, X. (2015) CuInSe2 and CuInSe2-ZnS based high efficiency “green” quantum dot sensitized solar cells. J. Mater. Chem. A, 3, 1649–1655.
- 104 Duche, D., Drouard, E., Simon, J., Escoubas, L., Torchio, P., Le Rouzo, J., and Vedraine, S. (2011) Light harvesting in organic solar cells. Sol. Energy Mater. Sol. Cells, 95, S18–S25.
- 105 Chen, X., Li, C., Gratzel, M., Kostecki, R., and Mao, S.S. (2012) Nanomaterials for renewable energy production and storage. Chem. Soc. Rev., 41, 7909–7937.
- 106 Qiao, Y. and Li, C.M. (2011) Nanostructured catalysts in fuel cells. J. Mater. Chem., 21, 4027–4036.
- 107 Zhong, C.J., Luo, J., Fang, B., Wanjala, B.N., Njoki, P.N., Loukrakpam, R., and Yin, J. (2010) Nanostructured catalysts in fuel cells. Nanotechnology, 21. doi: 10.1088/0957-4484/21/6/062001
- 108 Mazumder, V., Chi, M., More, K.L., and Sun, S. (2010) Core/shell Pd/FePt nanoparticles as an active and durable catalyst for the oxygen reduction reaction. J. Am. Chem. Soc., 132, 7848–7849.
- 109 Antolini, E. (2009) Carbon supports for low-temperature fuel cell catalysts. Appl. Catal. B, 88, 1–24.
- 110 Yu, D.S., Nagelli, E., Du, F., and Dai, L.M. (2010) Metal-free carbon nanomaterials become more active than metal catalysts and last longer. J. Phis. Chem. Lett., 1, 2165–2173.
- 111 Qu, X., Alvarez, P.J., and Li, Q. (2013) Applications of nanotechnology in water and wastewater treatment. Water Res., 47, 3931–3946.
- 112 Chen, X., Shen, S., Guo, L., and Mao, S.S. (2010) Semiconductor-based photocatalytic hydrogen generation. Chem. Rev., 110, 6503–6570.
- 113 Chen., X., Liu, L., Yu, P.Y., and Mao, S.S. (2011) Increasing solar absorption for photocatalysis with black hydrogenated titanium dioxide nanocrystals. Science, 331, 746–750.
- 114 Li, Y., Hu, Y., Peng, S., Lu, G., and Li, S. (2009) Synthesis of CdS nanorods by an ethylenediamine assisted hydrothermal method for photocatalytic hydrogen evolution. J. Phys. Chem C., 113, 9352–9358.
- 115 Rosi, N.L., Eckert, J., Eddaoudi, M., Vodak, D.T., Kim, J., O'Keeffe, M., and Yaghi, O.M. (2003) Hydrogen storage in microporous metal-organic frameworks. Science, 300, 1127–1129.
- 116 Germain, J., Frechet, J.M., and Svec, F. (2009) Nanoporous polymers for hydrogen storage. Small, 5, 1098–1111.
- 117 Jeon, K.J., Moon, H.R., Ruminski, A.M., Jiang, B., Kisielowski, C., Bardhan, R., and Urban, J.J. (2011) Air-stable magnesium nanocomposites provide rapid and high-capacity hydrogen storage without using heavy-metal catalysts. Nat. Mater., 10, 286–290.
- 118 Lee, J., Mahendra, S., and Alvarez, P.J. (2010) Nanomaterials in the construction industry: a review of their applications and environmental health and safety considerations. ACS Nano, 4, 3580–3590.
- 119 Sobolev, K. and Gutierrez, M.F. (2005) How nanotechnology can change the concrete world. Am. Ceram. Soc. Bull., 84, 16–20.
- 120 Irie, H., Sunada, K., and Hashimoto, K. (2004) Recent developments in TiO2 photocatalysis: novel applications to interior ecology, materials and energy saving systems. Electrochemistry, 72, 807–812.
- 121 Zhang, W., Suhr, J., and Koratkar, N. (2006) Carbon nanotube/polycarbonate composites as multifunctional strain sensors. J. Nanosci. Nanotechnol., 6, 960–964.
- 122 Li, G.Y. (2004) Properties of high-volume fly ash concrete incorporating nano-SiO2. Cem. Concr. Res., 34, 1043–1049.
- 123 Guskos, N., Zolnierkiewicz, G., Typek, J., Blyszko, J., Kiernozycki, W., and Narkiewicz, U. (2010) Ferromagnetic resonance and compressive strength study of cement mortars containing carbon encapsulated nickel and iron nanoparticles. Rev. Adv. Mater. Sci., 23, 113–117.
- 124 Wang, B., Han, Y., and Zhang, T. (2012) Morphological properties of surface-treated carbon nanotubes in cement-based composites. J. Nanosci. Nanotechnol., 12, 8415–8419.
- 125 Khataee, R., Heydari, V., Moradkhannejhad, L., Safarpour, M., and Joo, S.W. (2013) Self-cleaning and mechanical properties of modified white cement with nanostructured TiO2. J. Nanosci. Nanotechnol., 13, 5109–5114.
- 126 Olar, R. (2011) Nanomaterials and nanotechnologies for civil engineering. Bul. Ist. Polit. Iasit., 4, 109–117.
- 127 Zhu, W., Bartos, P.J.M., and Porro, A. (2004) Application of nanotechnology in construction. Summary of a state of the art report. Mater. Struct., 37, 649–658.
- 128 Rana, AK., Rana, SB., Kumari, A., and Kiran, V. (2009) Significance of nanotechnology in construction engineering. Int. J. Recent Trends Eng., 1, 46–48.
- 129 Kumar, A., Vemula, PK., Ajayan, PM., and John, G. (2008) Silver- nanoparticle-embedded antimicrobial paints based on vegetable oil. Nat. Mater., 7, 236–241.
- 130 Fleischer, T. and Grunwald, A. (2008) Making nanotechnology developments sustainable. A role for technology assessment? J. Clean Prod., 16, 889–898.
- 131 Remesh, K., Biswas, A.S., Somasundaram, J., and Rao, A.S. (2010) Nanoporous zeolites in framing: current status and issues ahead. Curr. Sci., 99, 760–764.
- 132 Pereira de Abreu, D.A., Paseiro Losada, P., Angulo, I., and Cruz, J.M. (2007) Development of new polyolefin films with nanoclays for application in food packaging. Eur. Polymer. J., 43, 2229–2243.
- 133 Andreescu, S., Njagi, J., Ispas, C., and Ravalli, M.T. (2009) JEM spotlight: applications of advanced nanomaterials for environmental monitoring. J. Environ. Monit., 11, 27–40.
