15
Plasmonic Nanomaterials for SERS Detection of Environmental Pollutants
Mengke Su and Honglin Liu
Hefei University of Technology, School of Food Science and Engineering, Hefei 230009, P.R. China
15.1 Introduction
Over the past few decades, the chemo-biosensing in environmental field has been greatly expanded because of the developed preparation and functionalization of nanomaterials, the discovery of new nanostructures, the rapid development of spectral detection means, and the chemical concept of bionic immunization. It has brought a new revolution for the toxicity or risk of trace material detection, environmental monitoring, and clinical diagnosis, which injects new vitality into the development of analytical chemistry. Nowadays, the environmental pollutants, such as pesticide residues, are one of the most serious challenges that the natural environment, food safety, and human health are facing. With the greater requirements of a better life quality, the voice is rising for establishing the food (especially fruit and vegetable) safety tracking and environmental real-time monitoring and management system. Therefore, it has an important academic significance and potential application to develop the advanced sensing materials and detection technology for rapidly detecting the trace environmental pollutants, which also has a great value for the environment protection, clinical symptoms of poisoning diagnosis, and human health care.
Laboratory analysis and detection of environmental pollutants mainly depend on the completion of large-scale instruments such as gas chromatography, liquid chromatography, chromatography-mass spectrometry combined technology, and so on. Although these methods have a high accuracy and sensitivity, they need expensive equipment, professional operators, a long testing time, and cumbersome and time-consuming sample pretreatment, and are easy to create secondary pollution. These limit the application in high throughput, rapid trace pesticide monitoring and field real-time detection. Biological bioassay, enzyme inhibition method, including colorimetric method, quick test card method, and so on, which have been developed to overcome these shortcomings, also cannot be generalized because of low sensitivity and selectivity, no uniform inhibition rate, and other shortages. Enzyme-linked immune sorbent assay (ELISA) has special charm in the rapid detection of pesticide residues, but this method is affected by many factors, such as solvent, pH, molecular structure of pesticides, and the position of carrier protein. Moreover, the animal immune cycle is long, the operation is complex, the cost is high, and the strict process quality control is needed. Today, the development of nanotechnology, especially the emergence of a variety of special properties of nanomaterials and artificial bionic immune design development, put forward new test principles and detection technology to open a new world in this field. Chemical sensing techniques are developing rapidly that can be used for molecular detection of environmental pollutants, such as explosives, pesticides, and drugs.
Raman technique is a promising technique for rapid analysis in the field of environment and food safety. The bottleneck in the practical application of Raman technology is how to identify, separate, and enrich the analytes in complex system, and realize the sensitive, stable, and accurate multichannel detection. The discovery of the plasmonic “hot spot” for Raman enhancement has led to the in-depth development of surface-enhanced Raman scattering (SERS) technique and various plasmonic nanostructures. However, robust SERS identification of chemicals in real environmental samples remains a frightful challenge because of the complex unknown sample, low molecular affinity for metal surface, or inefficient use of hot spots in 1D or 2D geometries. Furthermore, analytes frequently disperse in various phases (e.g., air, organic, and aqueous), thus making identification of chemical species really challenging. This problem is amplified when trace analyte detection is required, as signals from background molecules can swamp the signal of the analyte. Although a practical analyzer, SERS technique still faces three main challenges. The first one is the capability to produce an SERS-active substrate with large number of hot spots that can give rise to high enhancement factors (EF), the second is the efficiency to place the targeted molecules in hot spots, and the third is the reproducibility of the first two issues.
To date, several reviews have comprehensively discussed recent progress in the fabrication of highly ordered SERS substrates [1], rational designs of nanostructures [2], tailoring plasmonic substrates [3], and creating, characterizing, and controlling chemistry with SERS hot spots [4]. This chapter will focus on the development tendency of plasmonic hot spot design and SERS analysis for environmental pollutant detection. We summarize the reported pollutants that have been detected by SERS and mainly discuss recent progress in recent years on the fabrication of three-dimensional (3D) SERS-active hot spots, the various capping agent-directed fabrication of novel 3D plasmonic nanostructures, and the chemical sensing strategies of the “fixed” and “flexible” designs of plasmonic hot spots. This chapter will give a good introduction to understand the strategies on fabricating SERS hot spots with various geometric architectures and the corresponding optical properties, illustrate the benefits of 3D spatial hot spots for SERS detection in comparison with 1D and 2D arrays of hot spots, convey an idea of continually developing new strategy to resolve practical problems of SERS detection, and will discuss the outlook and challenge of this new hot volume for combining portable Raman spectrometer and resolving the problem of SERS practical analysis on various surfaces of targeted objects. We also enumerate the two phase transition-induced dynamic hot spots at liquid–liquid interfaces. Finally, we present a forward-looking overview of SERS “hot volume” for controlling the chemistry of hot spots in 3D space. Although not exhaustive, this chapter presents some of the most interesting methodologies for fabricating and controlling 3D SERS hot volume.
15.2 About SERS
In the early 1970s, efforts were made to study how to observe monolayer molecules on metal surfaces. At that time, it was possible to achieve this goal through some optical techniques (e.g., infrared spectroscopy). However, due to the weak Raman signal and the low sensitivity of Raman system, initially the researchers argued that it was impossible to observe the Raman scattering of monolayer molecules [5]. In 1974, Fleischmann et al. observed a significant enhancement of the Raman signal of pyridine molecules on the roughened silver electrode [6]. They believed that the rough electrode can increase the number of pyridine molecules adsorbed on the effective surface area, which can significantly enhance the Raman signal. Increasing the adsorption of molecules on the surface seems to be the most plausible explanation for signal enhancement. However, there are many unanswered questions that cannot be explained simply by this theory. One notable phenomenon is that in some cases, the signal becomes weaker with the increase in the surface roughness of the electrode, and there is no capacitive effect on the electrochemical electrode. If the signal is attributed to the increase in the number of adsorbed molecules, there is an additional capacitance in the system, which should form an easily detectable layer on the electrode. Creighton [7] and van Duyne [8] almost simultaneously published studies to demonstrate that the observed signal strength cannot be attributed to an increase in surface area. So, they posit that some effect on surface roughness leads to a great enhancement of the Raman signal than that under normal circumstances, and they called this SERS effect.
Over the next 10 years, the discovery of SERS is still a hot research topic. In fact, most of the important theories of SERS originated from these “early works.” In 1985, Moskovits [9] made a landmark review, providing a large number of great progress studies in the field. Subsequently, the enthusiasm for the research in this field seems to have faded, but there is still a lot of potential ongoing research. SERS enhancement is usually divided into two parts: electromagnetic enhancement (EM) and the chemical enhancement (CM) [10,11]. At present, these two mechanisms widely accepted are the long-range EM effect and short-range CM effect; usually, these two effects exist simultaneously. Among them, the EM mechanism is suitable for all analytes and the CM effect is closely related to probe molecules. If the two mechanisms exist at the same time, they will accumulate (i.e., multiplying the enhancement factor). Over the years, the relative contribution of their size has been a controversial topic, which has not yet been fully resolved. It is generally believed that the enhancement of SERS mainly comes from electromagnetic field enhancement. Therefore, it is important to understand the contribution of electromagnetic field enhancement to the SERS effect.
15.2.1 Electromagnetic Enhancement
At present, it is generally accepted that the electromagnetic field enhancement model is a surface plasmon resonance (SPR) model. The electromagnetic field enhancement mechanism contributes the most to the enhancement factor (EF); in the excitation of incident light, the surface of a metal substrate with a certain degree of nanometer roughness produces the SPR, thereby enhancing the local electromagnetic field (Figure 15.1) [12–14]. Plasma resonance is the fluctuation of free electrons on the surface of the metal. As the intensity of the Raman scattering is proportional to the square of the electric field intensity (E2), the increase in the local electric field leads to the enhancement of the Raman scattering intensity. The mechanism of electromagnetic field enhancement is mainly concerned with the contribution of the local electric field enhancement on the Raman scattering of the adsorbed molecules. The electric field intensity produced by SPR decreases exponentially with the increase of the distance between the adsorbed molecules and the metal surface. So, the mechanism belongs to long-range effect. Many theoretical models and experimental results show that the nanotip can not only generate large local field enhancement but also produce high spatial resolution. Most of the SERS signals are generated in the tip region [15–17]. And not all metals can produce SPR; only gold, silver, other precious metals, and alkali metals can produce SPR. Therefore, the shape, size, and composition of SERS substrate have a great influence on the activity of SERS [18].
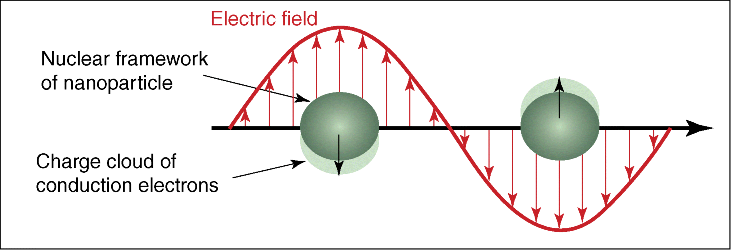
Figure 15.1 Schematic diagram of local SPR excited by spherical particles.
15.2.2 Chemical Enhancement
The chemical enhancement mechanism is related to the electronic coupling of the molecules adsorbed on the atomic rough surface. The charge transfer occurs between the analyte and the metal surface [19,20]. However, the fundamental cause of chemical effects has not been explained clearly. It is an urgent task to reveal the mechanism of chemical effects. At present, it is considered that the chemical enhancement is the chemical interaction between the adsorbed molecules and the metal surface, which is a short-range effect, the molecular polarizability is increased, and the Raman signal is enhanced [21]. Taking the chemical effects into account, it is similar to the heterogeneous catalytic reactions, related to the interaction between adsorbed molecules and solid surfaces (Figure 15.2). It is generally known that the crystal face of the semiconductor oxide catalyst determines its catalytic oxidation performance and plays an important role. Therefore, the catalyst with good activity site can be synthesized by controlling the morphology of the nanomaterials [22].

Figure 15.2 A typical energy-level diagram of a molecule adsorbed on a metal surface and possible electron transfer excitation. The occupied molecular orbital and unoccupied molecular orbital interact with the metal to expand the resonance phase. Orbital occupation determined by Fermi level.
In recent years, SERS has attracted extensive attention again, which may be attributed to the following reasons: improvements in Raman instrument made this technique to be widely used in physics, chemistry, biology, and engineering; the development of nanoscience and nanotechnology provides more possibility for SERS substrate design and manufacturing. More studies on plasma resonance can indirectly develop the basic research and application of SERS. Both theoretical and experimental results show that SERS can realize single-molecule detection, which further promotes the development of SRES [23–25]. SERS visibility in many disciplines has been significantly improved. Since 2003, the application of SERS has stimulated a new round of research. A lot of work is being done on the fundamental theory of the enhancement effect on SERS. At the same time, there are more and more applied studies and relevant research tools are being developed for its commercial application [26–29].
Interestingly, compared to the total research output, the output is still less than in the early 1980s. Judging from the development of other technologies, the development level of SERS is similar to that of nuclear magnetic resonance (NMR) in the early 1970s. At that stage, NMR had great potential as an analytical tool with many branches (including imaging). However, many of the fundamental aspects of this technology have not changed (especially for imaging capabilities), but some of the basic science and instrumentation are still evolving. SERS has to be developed out of the current state of NMR, so that it does not have to experience the fate of NMR 40 years ago. With the development of analytical studies, SERS will become an analytical tool with a wide range of practical applications for detection at single-molecule levels. As far as NMR is concerned, many results are closely related to the improvement of the instrument, which is difficult to be used for actual detection when it is discovered. As far as SERS is concerned, Raman instruments have made great progress in the past few decades, and there may be more progress in the future, such as in the field of substrate manufacturing (one of the most active areas in recent years). But it should be noted that some of the basic scientific problems of SERS still need to be further studied, and many important problems remain to be solved.
15.3 Environmental Pollution and SERS Detection
The environmental pollution is becoming more and more a serious problem. For example, pesticide residues have made potential threat to food security, to the global ecological system, and to all human beings. Pesticides can be divided into four categories: organochlorine, organophosphorus, carbamate, and pyrethroid. Many of them contain nitro, chlorine, and other groups, about 70% or more contain aromatic or heterocyclic structure. The average pesticide use in China is 931.3 g per mu (equal to about 79 square yards), one time higher than in the developed countries, and 50%–60% of that become the environmental residues. These persistent organic pesticide residues migrate and spread through atmosphere and water to the high level area or other areas, and the contaminated area continues to expand. Organic pesticides (especially aromatic nitro compounds) are characterized by rapid absorption by the skin, and the toxicity will further increase if its molecular structure contains chlorine atom. Moreover, trace toxic pollutants are endangering human health through the food chain, especially the more serious phenomenon of poisoning of fruits and vegetables. The Ministry of Agriculture of China sampled 2110 samples of 14 economically developed provincial cities across the country and found that the pesticide residues in vegetables exceeded 31.3%. Surprisingly, some of the pesticide residues that severely exceeded in fruit and vegetable crops were from the soils where those pesticides had been applied 40 years ago and these residues were almost undetectable in the soil. Up to 2005, the United Nations had set a total of 3574 pesticide limit standards, the United States 8669, and Japan 9052. Nowadays, more and more strict requirements for health indicators such as pesticide residues in foods are put forward across the world. These data and results indicate the need of trace pesticide detection methods that are quick, accurate, sensitive, convenient, and suitable for on-site operation.
The rapid development of nanocomposite material synthesis and theory of biomimetic immune technology has brought revolutionary changes in SERS detection of environmental pollutants (Table 15.1). With the advantages of fingerprint feature, high sensitivity, and short detection time, SERS technology can realize the rapid detection of trace substances. Through the special probe, the active recognition and enrichment of the detected object, the SERS signal of the analyte can be greatly enhanced and even single molecules can be detected. The rapid development of the new nanocomposite materials provides a wide range of probes, which are rich in shape, are controllable, and are easy to use for SERS. SERS probe assembled with functionalized nanomaterials and bionic immune materials make full use of the high sensitive signal output of nanomaterials and molecular recognition ability of biomolecules and can produce sensitive signal output to target molecules. In addition, designing the characteristics of nanoparticles can achieve rapid enrichment in order to achieve high sensitivity, high selectivity, and rapid molecular detection. These not only provide a broad space for the development of SERS technology but also broaden the scope of application of SERS. At present, the combination of nanoparticles and synthetic antibody materials for SERS detection is attracting chemists' strong interest in research. The challenge of chemical and biological detection in the future is to detect in situ and fast and real-time, and the development trends show high selectivity, high sensitivity, and high degree of nanoscale.
Table 15.1 SERS detection of environmental pollutants (adapted from Jing's review [30]).
| The kinds of pollutants | Environmental pollutants detected by the SERS technique |
| Metal ions and inorganic anions | Hg2+ [31–36], Pb2+ [35], Cd2+ [32], Zn2+ [37], Cr6+ [38], Cu2+ [33,39], Co2+ [40], Ag+ [35], uranium [41], |
| Polychlorinated biphenyls (PCB) | PCB-77 [50–53], PCB-29 [54], PCB-101 [54], PCB-1 [55,56], PCB-15 [57], PCB-40 [58], PCB-54 [58], PCB-65 [58], PCB-80 [58], and PCB-47 [59] |
| Polycyclic aromatic hydrocarbon (PAH) | Pyrene [60–70], anthracene [60,64–66,68,70–72], fluoranthene [70,73,74], naphthalene [62,66,72,74,75], benzo[a]pyrene [66], fluorene [66,74], acenaphthene [74], chrysene [64,65,69], 9,10-benzene phenanthrene [64–76], benzo (c) phenanthrene [65,76,77], coronene [65,76], benzene [66], perylene [66,72], and 3,4-benzopyrene [70] |
| Pesticides | Thiram [78–81], methyl parathion [81–84], carbaryl [85–87], imipenem [86], guthion [86], tricyclic azole [88], paraquat [89], atrazine [90,91], prometryn [90], simetryne [90], malathion [92,93], methamidophos [93], ferbam [80], ziram [80], 2,4-dichlorophenoxyacetic acid [83,94], carbofuran [87], isopropyl [87], propoxur [87], paraoxon [93], dipterex [95], glyphosate [95], chlorotoluron [91], diuron [91], terbuthylazine [91], chlorpyrifos [81], dimethoate [96], omethoate [96], aldrin [97], endosulfan [98], and 2-mercaptobenzothiazole [99] |
| Phenols | P-chlorophenol [83,100], bisphenol propane [101], 4-aminothiophenol [43,84,102], p-aminothiophenol [103], 2,6-dichlorophenol [100], 2,4,6-trichlorophenol [100], pentachlorophenol [104], 4-ABT [105], 2-naphthol [72], and catechol [106] |
| Explosives | Trinitrotoluene [107–113], pentaerythritol nitrate [114], thylene glycol dinitrate (EGDNE) [114], hexogen (RDX) [114], and dinitroanisole (DNAN) [115] |
| Antibiotics | Hydrated ampicillin [116], 6-aminopenicillanic acid [116], and benzylpenicillin [116] |
| Toxins | Tetrodotoxin [117] and clam toxins [118] |
| Dyes | Peacock green [119], methylene blue [120], and crystal violet [121] |
| Amine compounds | Thioacetamide [122], benzidine [100,106], aniline [100,106,123], and acrylamide [122] |
| Food additives | Melamine [124], rosin arsenic acid [125], and ammonium phenylarsinic acid [125] |
The combination of nanotechnology and bionic immune technology is an effective way to solve the difficult application of ultrasensitive and rapid SERS technology in pesticide residue detection. The general cross section of a pesticide molecule is small, and the direct adsorption probability with the unmodified nanoparticles is very small, so the application of SERS technology to detect pesticide residues faces many bottlenecks, resulting in the related literature being not so rich, and has always been ultrasensitive. Despite its advantages, SERS technology is facing an application problem. However, the preparation of nanomaterials has made rapid development in the past 10 years, and the concept of artificial bionic immune and molecular imprinting has matured, which has injected new vitality into the development of SERS technology. By introducing the antigen-specific recognition design, the active recognition sites of the pesticide residues were introduced on the surface of the nanoparticles to improve the adsorption capacity, and the magnetic nanoparticles could be easily enriched and concentrated so as to provide a new approach to improve the sensitivity of detection of pesticide residues. At present, SERS based on nanotechnology is widely used in surface adsorption electrochemistry, chemical and biosensor biomedical detection, and trace detection and analysis, among other fields. And the study of magnetic nanoparticle immunochemical detection is also in full swing. Our group has made certain contribution to the SERS detection and probe preparation of trace residual substances such as pesticide residues [126], drugs [113,127–131], and explosives [113,127].
With the development of nanotechnology, magnetic nanomaterials are promising in the detection of trace pesticides. The combination of magnetic nanoparticles and biomimetic immune chemistry has great potential and advantage in the field of pesticide detection. Magnetic nanoparticles can be combined with organic matter, macromolecule polymer, or inorganic material to form core–shell structure magnetic composite microspheres by surface copolymerization and surface modification. It not only has magnetic properties but also has surface active groups, which can be further identified and coupled with biological molecules or some organic and inorganic molecules. Under the action of the external magnetic field, the magnetic particles can be easily separated from the bottom liquid, which has the advantage of simple operation and high separation efficiency. In addition, the magnetic microspheres have a large specific surface area, which provides the basis for modifying many kinds of high-density molecular probes and also shows a wide application prospect in the field of detection. EI-Boubbou et al. reported that the use of magnetic glycerol nanoparticles not only could detect Escherichia coli in 5 min but also could remove the 88% target from the environment under test [132]. Lu et al. used dual-functional magnetic silica nanoparticles as a label for human stem cells [133,207]. Lin et al. used functional magnetic nanoparticles as a solid-phase extraction probe to enrich and detect small molecules [134]. Lee et al. have compounded magnetic core–shell nanoparticles and applied them in the magnetic separation of labeled proteins [135]. Li et al. have designed functionalized magnetic nanoparticles to enrich histidine-labeled proteins and phosphorylated peptides [136]. Fu et al. reported the use of surface protein-functionalized diamagnetic nanoparticles to detect biomolecules with a sensitivity of 10 pM [137]. Magnetic nanostructured materials with high specific surface area, high activity, strong adsorption, and other characteristics can make it possible to achieve high selective recognition, adsorption, and rapid enrichment of trace pesticide residues in the environment with the help of biomimetic immunochemical techniques.
15.4 Plasmonic Materials for Raman Enhancement
The plasmonic hot spot predominantly determines the capability of SERS technique [138]. Many reports have focused on the fabrication of 3D SERS-active nanostructures; nevertheless, we consider that 3D architectures of nanostructures do not necessarily mean the generation of 3D hot spots; sometimes the SERS-active regions of 3D architectures are still limited to a single Cartesian plane. First, when the interparticle gap (g) is smaller than 1 nm, quantum tunneling arises and dramatically reduces the electromagnetic enhancement ability. Second, as the particles touch each other, charge-exchange phenomena occur, leading to a decrease in the electric field enhancement. Finally, solid contacts among particles cause the entire aggregate to behave as an equipotential body and thus give rise to an electrostatic shielding phenomenon that further decreases the electric field enhancement. From this perspective, most of the reported SERS hot spots have been shown to exist in zero-dimensional (0D) point-like, one-dimensional (1D) linear, or two-dimensional (2D) planar geometries that can potentially achieve high SERS enhancement factors, while the maximum number of SERS hot spots that can be achieved for such substrates are limited to a single Cartesian plane. Since the laser confocal volume in SERS apparatus is a 3D space, indicating that 1D and/or 2D arrays are underutilizing the active confocal volume even though large-area hot spots are precisely fabricated in a planar geometry. Another limitation of 1D and 2D SERS substrates is that the incident laser should be tightly focused on the correct plane to achieve optimal Raman enhancement, which reduces the versatility of such substrates, especially for on-site applications. 3D SERS hot spots should refer to the concept that only the extension of a SERS substrate from 2D to 3D brings about a larger number of hot spots in three dimensions, especially along the z-axis. These kinds of 3D hot spots exist in a 3D space that can be imagined as a “hot-volume.” The 3D SERS hot spots in space overcome the longs-tanding limitations of SERS for the ultrasensitive characterization of various substrates and analytes, and it not only greatly increases the number of hot spots but also increases the service efficiency of hot spots.
To address the issues on 3D geometry of hot spots, various surfactants, DNA, peptide, amphiphilic polymers, or even proteins have been used to construct “fixed” hot spots on solid substrates by preparing oriented assemblies of functional nanoparticles, such as SHINERS, core–shell monomers, dimers, trimers, chains, or arrays. On the other side, “flexible” designs utilizing electronic, ferroelectric, thermal, mechanical strain, or capillary effects have allowed for the tunability of plasmonic hot spots. Three-dimensional SERS-active substrates with considerable extension in the third dimension are actively pursued to increase the versatility of a 3D SERS platform, by increasing the number and utility of SERS hot spots in all three dimensions. In addition, the extension of a SERS substrate from 2D to 3D brings about a larger overall surface area that, in turn, enables more target molecules to be adsorbed and detected in the third dimension, especially along the z-axis. Therefore, 3D architecture can achieve higher tolerance in focus misalignment along the z-direction, helping to transform SERS into a practical analytical technique.
15.4.1 SERS Hot Spot
Noble metal nanoparticles exhibit optical excitation effect, that is, SPR, the local electric field enhancement in external irradiation. Particularly, the dense structure of nanoparticle arrangement will produce a more intense enhancement effect; this effect of the nanogap is called hot spot [139]. The gaps have an important role in the enhancement of the electromagnetic field, so that tightly packed nanoparticles can be applied to the ultrasensitive detection, such as SERS and nonlinear optical applications [19].
It has been proved that hot spots caused by the nano-scale intersections and gaps can enhance the electromagnetic field and produce a large SERS effect. Therefore, it is desirable to create a metal nanostructure with a nanogap to form a large number of SERS hot spots (Figure 15.3) [140]. Nevertheless, how to adjust the distance between particles and how to use the simple method to prepare excellent SERS active nanostructures is still a major challenge. In future, it is a pressing need to prepare nanoparticles with specific size and morphology and to well control the assembly of nanoparticles.
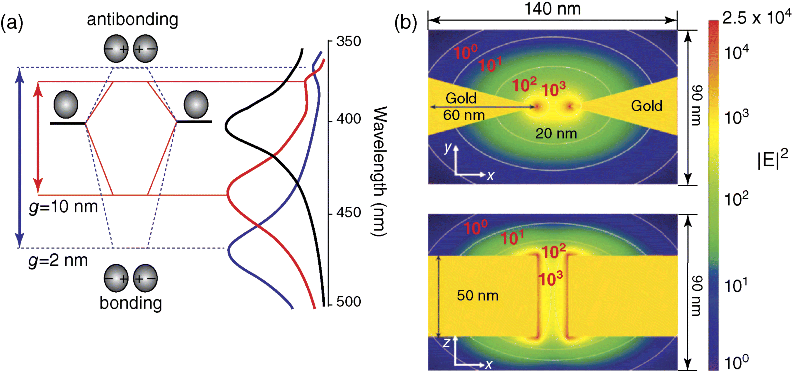
Figure 15.3 Schematic diagram of plasma coupling between adjacent nanoparticles. (a) Both the energy level and the SPR peak change with the nanoparticle gap. (b) The finite difference time domain method is used to simulate the enhancement effect of nanoparticle gap (reproduced with permission from Ref. [140]. Copyright 2011, John Wiley & Sons).
15.4.2 Development Trend of SERS Hot Spot Structures
Noble metal nanoparticles have received great attention from the scientific and industrial fields. They have excellent physical and chemical properties and are widely used in optics, catalysis, SERS, biology, and chemical sensing [19,141–146]. All of these intrinsic properties and applications depend on the size, morphology, composition, crystallinity, and structure of the metal nanostructures. Therefore, these parameters can be adjusted to achieve the desired performance. In the past decades, many researches have focused on the fabrication of monodisperse noble metal nanoparticles with tunable size and variable shape, and their physicochemical properties have been studied systematically. In recent years, scientists have gradually shifted from the synthesis of unique noble metal nanoparticles to 1D, 2D, and 3D assembly systems [147,148]. These systems usually have new optical, electrical, or synergistic photoelectric properties, which are not of independent nanoparticles but generated by the interaction between neighboring nanoparticles.
A variety of techniques have been used to study the self-assembly of nanocrystals into ordered polymers or superlattices. The top-down nanoimitation techniques such as electron beam lithography [149] and focused ion beam [150] can achieve good reproducibility and uniformity. However, due to technical difficulties, production capacity, cost, and other related constraints, their practical application is still difficult to achieve. In addition, it is still difficult to create a few small gaps of several nanometers between the metal nanostructures; a large number of effective hot spots generated by these small gaps are very important for obtaining high signal intensity SERS substrates. The bottom-up self-assembly technique is a good alternative and can be widely used in the production of highly ordered 3D particles [151]. Although the direction of this research has been greatly developed, the progress in the control of the compactness, size, morphology of polymer, and the corresponding SERS activity in the assembly is still slow.
The crystallization of nanocrystals into 2D [152] and 3D [153] lattices has been studied for nearly 20 years. However, the fabrication of arbitrarily shaped nanocrystals (e.g., cubes, prisms made of nanocrystals, etc.) is still a serious scientific challenge. As for metal nanocrystals, one of the purposes of obtaining such an assembly structure is to generate tunable optical resonance materials, which will also promote the development of plasma. SPR can control the light below the diffraction limit and provide potential application platforms for chemo/biosensing [154], material manufacturing [155], nanometer circuit [156], subwavelength waveguide [157], nonlinear optics [158], and so on. When the interval is less than 5 nm, the coupling of surface plasmon model is the strongest. The silver nanoparticle-assembled nanospheres were used as SERS-enhanced substrates, which can realize the highly sensitive detection of hazardous chemicals and organic dye molecules, highlighting the importance of the assembly. In addition, the assembly of noble metal nanoparticles can also significantly enhance the surface reaction activity, such as catalytic performance (Figure 15.4) [159], mainly because they have a larger surface area and specific surface area. Therefore, the efficient assembly of noble metal nanoparticles into good assemblies is essential for their potential applications. However, even with high-resolution electron beam lithography, it is still challenging to manufacture such a structure through a top-down lithography. On the contrary, the particles spontaneously gather in a dense, irregular structure with a very small interval (1–5 nm), which depends on the use of stabilizer properties [160]. Therefore, colloidal assembly is a very important method for manufacturing plasma structures.

Figure 15.4 (a) Reaction scheme showing morphological and structural changes involved in the fabrication of Au/Pt/Au core/shell nanoraspberries and (b) SEM image of the product (Au/Pt/Au) (inset: photo of the colloids during different synthesis steps) [159].
The ultrasensitive SERS hot spot that can be used to detect single molecule has stimulated the research on the plasma resonance properties of metal nanostructures [161,162]. It is a frontier research direction in the field of SERS to construct a fixed hot spot on a solid substrate by directional assembly of functional nanoparticles. In addition, the use of electronic, ferroelectric, thermal, mechanical, or capillary forces can also adjust the plasma resonance hot spots [163–167]. However, the influence of gaps on the electric field enhancement is very large. In order to maximize the electric field of the molecule, the key problem is to control the size of the space on the nanometer level [168]. The nanosize gap can be obtained by particle agglomeration, breaking point, or plate printing but there are problems of poor reproducibility, low yield, and high cost. In addition, even if the gap is precisely controlled, so that nanoparticles on solid surface modified by surfactant self-assembly can produce nanogaps of less than 10 nm, but the steric effect may also prevent the molecules into the gap, especially when the metal surface is modified using a different stabilizer [169]. It is still a challenging research to control the orientation and position of nanoparticles in 3D space. The research on the hot spot phenomenon is a systematic study, which not only focuses on proving the existence of hot spots but also explains their dependence on the number of nanoparticles theoretically.
15.4.2.1 Zero-dimensional Structure
The optical properties of noble metal nanoparticles depend on the size, morphology, composition, and structure of metal nanostructures. A large number of monodisperse noble metal nanoparticles can form hot spots, resulting in an enhancement of the electric field. Nanoparticles with sharp tips are often able to form hot spots at the tip, producing up to 108 SERS enhancement; the characteristics of the nanoparticle tip also promote the development of the tip-enhanced Raman spectroscopy (Figure 15.5) [170,171]. Research shows that the angle and edge of the nanoparticles can form hot spots, so that electromagnetic field is greatly enhanced, such as the vertex of nanocube [172], nanostar (Figure 15.6) [173,174], nanotriangle [22], and nanowires and nanorods [175]; a lot of single particles can also be formed at the edges of hot spots [176,177].

Figure 15.5 Schematic diagram of tip-enhanced Raman [170,171].
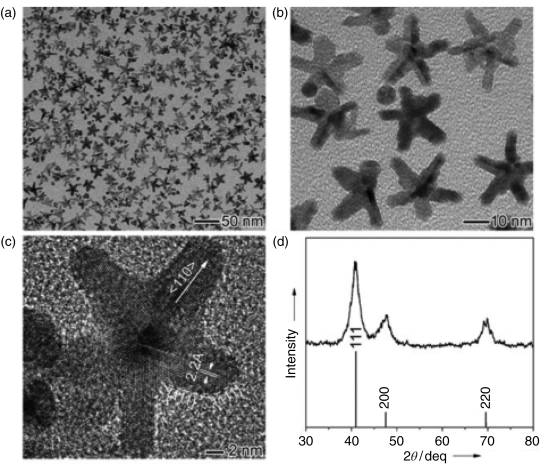
Figure 15.6 (a and b) TEM image, (c) high-resolution TEM image, and (d) XRD of gold nanostars [173,174].
In addition to the single-component particles, the single layer and multilayer core–shell nanoparticles can also form a hot spot (Figure 15.7) [178–180]. This kind of structure has the properties of both the core and the shell at the same time, among which the metal core–shell structure is easily synthesized, such as Au@Ag synthesized by Yanyun Ma et al. [181]. The thickness of the shell can be controlled by adjusting the ratio of AgNO3 to Au. At the same time, the material has the optical properties of gold and silver surface affinity and has a strong SERS effect. In addition to gold and silver complex, the noble metal core–shell structure is common such as SiO2@Au [182], Au@Pt [183], Au@Pd@Pt [184], and so on (Figure 15.8). Lu et al. [185] synthesized Au@Pd core–shell structures with different shapes, such as triangle, octahedron, and icosahedron.
Figure 15.7 Raman detection of surface adsorbed on gold core–shell nanoparticles coated with Pt and Si [178–180].

Figure 15.8 (a–h) TEM-EDS analysis and schematic diagram of Au@Pd core–shell with different shapes.
15.4.2.2 One-dimensional Structure
Adjacent nanoparticles usually form hot spots, resulting in SERS effects. As a result, the preparation of 1D structural substrates has attracted wide attention. Here, we illustrate some 1D structures. Nanodumbbell is a typical 1D structure; in the nanodumbbell structure, the gap between the particles will be formed when the size of the gap in the appropriate range produces a strong Raman enhancement [186]. Lee et al. [187] used DNA to connect Au-Ag core–shell nanoparticles to form nanodumbbells with different gap sizes, and the effect of gap size on the enhancement of SERS signal was studied. The results show that the signal can be enhanced when the gap is smaller than 1 nm (Figure 15.9).

Figure 15.9 (a) Ultraviolet spectra of nanodumbbell structure formed by different silver shell thickness. (b) AFM map for Raman studies of nanodumbbell (scale = 50 nm). (c) Raman spectroscopy corresponding to the nanodumbbell structure (the excitation wavelength = 514. 5 nm; laser energy = 100 W; acquisition time = 1 s).
The gap of the fracture of the mechanical breakage of the nanowire [188] and on-wire lithography (OWL) produced gold nanostructure material [189,190], which can form the SERS hot spot; this is also the hot spot of 1D structure. The gold nanostructures are obtained by the OWL method (Figure 15.10), where the gap in the structure is 10 nm between two 3 µm gold nanorods. In addition, there is a 30 nm gap between three pairs of gold nanoparticle plates. SERS studies show that the gap of structure will produce a strong SERS signal.

Figure 15.10 (a) A schematic of OWL method for the fabrication of nanostructured Ni before and after etching. (b) The Raman scattering study of the produced nanostructures. (c) Optical micrographs of nanostructures in Figure 15.10b.
Nanospheres, nanorods, nanocubes, and other particles can form aggregates, and the structure of the dimer and trimer tend to form hot spots. The nanocube was assembled into a dimer that can produce SERS hot spots, and the existence of hot spots was proved by plasma etching by Camargo's team (Figure 15.11) [191].

Figure 15.11 (a) Schematic diagram of silver nanoparticles dimers' hot spot. The nanocubes are modified by 4-MBT (4-methylbenzenethiol), followed by the method of plasma etching to remove the 4-MBT molecules in non hot spot region. Then, the nanocube aggregates into 1,4-BDT (1,4-benzenedithiol) solution, the 4-MBT molecules are completely replaced by 1,4-BDT. (b–d) SERS spectra of nanocubic dimers, the top is modified by 4-MBT, in the middle of the plasma etching for 2 min, the lower is immersed in 1,4-BDT solution.
The assembly chain of nanoparticles is also a 2D structure and the length of the chain will affect the SERS effect. Barrow et al. [192] assembled nanolinear chains of different lengths, up to six nanoparticles, with a gap of nanoparticles about 1 nm (Figure 15.12). Research on the nanochain light scattering properties showed that the SPR occurred redshifted with increasing chain length.

Figure 15.12 SEM image of self-assembled nanoparticle chains (a) and their corresponding normalized spectrum (b). The average diameter of the nanoparticles is 64 nm and the gap between the particles is about 1 nm. Spectra were collected in a glass substrate covered with ITO; the spectrum sequence number according to the number of particles in the chain.
In addition, the nanoparticles were placed on the gold and silver metal substrates and the contact point between the nanoparticles and the substrate will form a hot spot, resulting in electromagnetic field enhancement, such as the contact point of the cube and the gold substrate will become hot spots [193]; however, the contact between the nanosphere and the gold substrate will also form a hot spot (Figure 15.13). When the nanoparticles are in contact with the nanowires, the plasmon resonance of the nanoparticles is coupled with the nanowires, which leads to a strong electric field enhancement [194].

Figure 15.13 Electric field-enhanced simulation of silver nanocubes or silver nanospheres. (a and b) Field-enhanced distribution system of cubic and spherical nanoparticles, respectively, gap between nanoparticles and metal substrate is 2 nm. Gray area represents the simulation plane, 1 nm apart with the bottom surface. For the nanocube, polarization along the green line. Enhanced distribution calculated by DDA method: (c) on gold substrate; (e) on a silicon wafer; (g) in the air, the same for nanosphere; (d) on gold substrate; (f) on a silicon wafer; and (h) in the air.
15.4.2.3 Two-dimensional Structure
Due to the long range-effect and the increase in hot spots, the overall SERS effect is much stronger than the one-dimensional structure. Common 2D structures are planar monolayer ordered array structures or membrane structures of the substrate, nanoparticle assembly chains, tetramer [195], and so on. Nanoparticle-ordered array structure can be formed by self-assembly method, as shown in Figure 15.14, while gold octahedron nanoparticles and gold cube nanoparticles can be assembled into ordered array structure by self-assembly method [196]. In addition, a variety of morphologies of nanoparticle planar monolayer-ordered array can be formed by self-assembly method, such as nanotriangle [197], nanosphere [152], and so on [148,198].

Figure 15.14 SEM image and schematic diagram of gold octahedron and cube nanoparticles self-assembled structure. (a) Gold octahedron nanoparticles, (b) gold cube nanoparticles, (c) self-assembly diagram of gold octahedron, and (d) self-assembled diagram of gold cube.
The trimer structures formed by the nanoparticles can comprise the symmetrical structure of the different point groups, showing different scattering spectral properties; however, the size of the elementary particles that make up the trimer can affect the formation of hot spots, Therefore, adjusting the size of the elementary particles and controlling the gap of the trimer can provide an excellent hot spot structure for the SERS study (Figure 15.15) [149].

Figure 15.15 Scattering experiment (left), the corresponding TEM image (right: the scale is 50 nm), and simulation of plasma spectral numerical trimer symmetry-damage basic particle composition of different sizes. The green line represents the nonpolarized light excitation, the red line represents the polarized light excitation along the base line of the trimer, the blue line represents the polarized light excitation perpendicular to the basal trimer. For maximum angle, simulation values were 60° (a), 90° (b), 140° (c), and 180° (d).
15.4.2.4 Three-dimensional Structure
One of the most important challenges in current SERS research is to synthesize a three-dimensional structural material with hot spots. Synthesizing simple and regular 3D structure materials has great significance for the development of SERS, which may have unique optical and electrical properties, such as polarization-independent light scattering and strong local field.
15.4.2.4.1 Array Structure
Nanoarray can produce typical 3D structures. For example, Chuanmin Ruan et al. in 2007 used AAO as template and deposited a layer of gold colloid film on the AAO surface by vacuum electron beam evaporation. The growth of the gold acicular structure extended to the AAO gaps. After removing the AAO, the gold nanorod array structure was obtained (Figure 15.16) [199]. Using the SERS substrate for the detection of thionine can obtain a high detection sensitivity and good repeatability. In 2012, the Shanghai Institute of Ceramics used argon ion magnetron sputtering method to synthesize silver nanometer needle arrays and used them for the detection of trace toxic substances. Compared to nanospheres and nanorods, the former has stronger SERS effect (Figure 15.17) [200–205].

Figure 15.16 Comparison of alumina template and gold nanocolumnar array at low resolution (a and c) and high resolution (b and d).

Figure 15.17 SEM images (a) Ag film with a thickness of 500 nm. (b) Nanoneedle array obtained by irradiating a 500 nm Ag film from Ar+ etching at an angle of 45°. (c) Viewed from the top and (d) observed at an angle of 45°.
Nanoparticles aggregation assembled array structure is also a kind of 3D structure (Figure 15.18). Yanying Rao from the Southeast University has synthesized the gold shell array by self-assembly method [182]. First, the gold nanoparticles were prepared and then mixed with the SiO2 nanospheres, after which they were self-assembled into the gold shell arrays under constant stirring. The concentration of H2O2 is positively correlated with the SERS intensity of the array, so it can be used for the detection of H2O2 with a good sensitivity.

Figure 15.18 The SEM of SiO2/gold nanoparticles arrays prepared by 200 µM H2O2 and different concentrations of tannic acid (a) 0 µM, (b) 10 µM, (c) 40 µM, and (d) 100 µM. (e) SERS spectra corresponding to Nile blue A (a) 0 M, (b) 10 M, (c) 40 M, and (d) 100 µM.
Nanowires can produce hot spots with great Raman enhancement. The adjacent silver nanowire tips would be able to gain a strong electromagnetic field enhancement [206]. In the work reported by Lee et al. silver nanowire arrays were fabricated on a highly ordered AAO template by electrochemical deposition (Figure 15.19). SERS study showed that the sensitivity and repeatability of the silver nanowire were very well. In addition, nanoparticle-decorated nanowires assembled into an array structure that is also a 3D structure (Figure 15.20) [133], for example, in situ growth of silver nanoparticles on the synthesized Ag2MoO4 nanowires. A large number of silver nanoparticles are very close to each other, many composite structures are stacked together, and the plasma coupling can be generated between the particles, thus greatly enhancing the SERS signal.

Figure 15.19 SEM image of thiophenol-modified Ag-Al2O3 template. Ag-Al2O3 template was obtained by partial dissolution of Al2O3 in 0.1 M NaOH. The dissolution times were (a) 0 s, (b) 210 s, (c) 270 s, and (d) 450 s.

Figure 15.20 Characterization of Ag2MoO4 and Ag-Ag2MoO4. (a) TEM picture of Ag2MoO4. Inset: electron diffraction and magnification of single Ag2MoO4. (b) TEM picture of Ag2MoO4. (c) TEM picture of single Ag-Ag2MoO4. Inset: the lattice diffraction pattern of Ag and Ag2MoO4. (d) TEM picture of single Ag-Ag2MoO4 after laser irradiation.
15.4.2.4.2 Aggregate Structure
Aggregation of nanoparticles can often form a large number of hot spots. Both theoretical and experimental studies show that multiple aggregate structures are superior SERS substrate materials. DNA-induced self-assembly can produce 3D structure of strongly coupled plasma (Figure 15.21). Three-dimensional mesoscale tetrahedral and octahedral systems and 2D and 3D lattice have been prepared by DNA assembly [150,208]. Although the 3D particle structure of DNA assembly has been obtained, they always collapsed on the sediment of the substrate, and direct images of these structures were not obtained; even using optical study is also impossible [209]. Therefore, only the 3D superlattice structure with electrostatic assembly can be completely represented on the substrate. Barrow et al. [210] reported the synthesis and optical studies of the plasma model of highly ordered 3D gold tetrahedron, pentamer, and hexamer for the first time (Figure 15.22). These assembled structures are stable on the substrate compared to the previous aggregates and can also be observed using scanning electron microscopy (SEM) and atomic force microscopy (AFM). Individual structures of the polarized and unpolarized spectrum are used to illustrate each of the structure of the plasma model and then draw comparisons with calculation model. They used a single-crystal assembly of polarized light scattering spectroscopy as a tool to monitor and analyze the self-assembly process. It is proved that self-assembly can produce highly symmetric and polarization-independent superlattice structures.
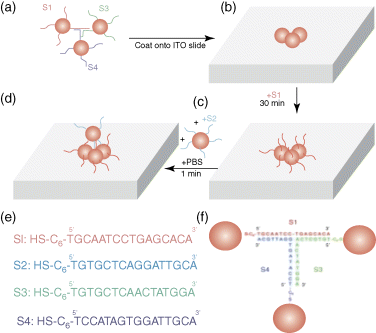
Figure 15.21 DNA guide assembly scheme. (a) Hybrid mechanism for generating 3D nanostructures. (b) The 3D paired DNA is assembled onto the ITO glass coverslip in the aqueous solution. (c) The structure covered on the coverslip is coated with a layer of thiolated DNA. (d) Second-step assembly is covered with a layer of nanoparticles in the precoating structure. (e) Sequence of DNA chains. (f) Complementary schematic diagram of S1, S3, and S4 when making the basic structure.

Figure 15.22 SEM images of assembled nanostructures. Tetramer (top), pentamer (middle), and hexamer (bottom). Each structure can be viewed from the top (left) from the 52° angle (right). Scale bar: 250 nm.
The emulsion template method can also be used to assemble nanoparticles of precious metal to form spherical aggregates with nanoscale particle gaps [130]. In this method, the degree of aggregation of nanoparticles can be controlled by changing the evaporation temperature of the organic solvent in the oil-in-water micelle system (Figure 15.23). The size of nanoparticles has a significant effect on the final form of the aggregation. This is a simple but effective bottom-up assembly route where nanoparticles accumulate into large aggregates in the oil droplets of the emulsion in low boiling solvents. Compared to the nanoparticle sol, 3D spherical aggregates can further improve the properties of nanoparticles, and a series of experiments have demonstrated the versatile properties of the aggregates formed by this method, including the activity of SERS and catalytic properties. The assembly can be used as a highly sensitive SERS substrate for the detection of chemical or biological molecules and exhibits a significantly enhanced SERS activity for drug molecules with high repeatability.
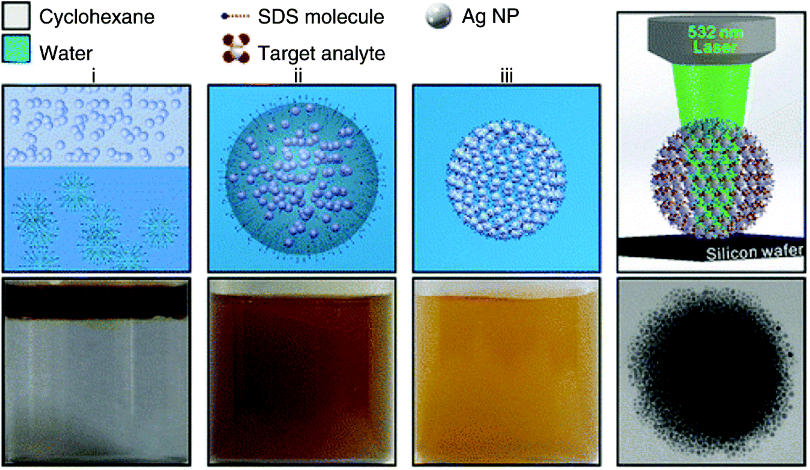
Figure 15.23 Schematic (top) and the corresponding optical images (bottom) of the self-assembly of Ag NPs into spherical Ag colloidal superstructures.
The polymer ligands use electrostatic interactions between the electrolyte and the particles to decorate nanoparticles. The use of some temperature-sensitive molecules, through the control of temperature, can achieve the dynamic control of particle spacing. Polymeric nanoparticles can be used to polymerize highly symmetric nanoparticles up to seven nanoclusters (Figure 15.24) [211]. These nanoclusters have ultrasensitive SERS sensing effects and exhibit three higher order enhancement factors than single particles, thus exhibiting superior optical properties. The final product is a mixture of clusters with different numbers of particles, which generally require the use of a purification process, including sedimentation [212], electrophoresis [213], and density gradient centrifugation [214].

Figure 15.24 Preparation of high coordination number of gold nanoclusters. (a) Density gradient separation method from the initial mixture to get a specific group. (b) the SEM images of the different coordination numbers obtained after separation. (c) HR-TEM images of the dimers and trimers obtained by this method and the HR-TEM images of corresponding particle gap.
Due to difficulty in adjusting the random polymerization and structural balance of the final polymer, it is relatively challenging to obtain small clusters of closely packed nanoparticles. The nanoparticles that make up the clusters need to reach the lowest energy state and are sufficiently stable to prevent them from being decomposed or polymerized in subsequent studies. Urban et al. [215] used an amphiphilic block copolymer, polystyrene block of polyacrylic acid, to solve this problem by preparing a spherical cluster with a clear internal structure (icosahedral structure), including large clusters from dimers to polyhedrahedral (Figure 15.25). Combining nanoparticles into clear boundaries is an important way to create and study the optical properties of materials. So far, most of the research on metal materials is based on the structure of making 2D planar geometry. Using this method, polyvinylpyrrolidone, polyvinyl alcohol, and polymethyl methacrylate can be effectively assembled in a 3D plasma structure. The combination of polymer nanoparticles not only can effectively connect colloidal particles to control the spacing of nanoparticles in a very small range [148,216,217] but also can allow the analytes to accumulate effectively [218]. Since the polymer has a very low Raman scattering cross section, interference with the vibration signal can be avoided near the plasma surface [219].

Figure 15.25 Fabrication and characterization of 3D plasma nanoclusters. (a) Schematic diagram of self-assembly of polystyrene-stabilized metal nanoparticles. (b) TEM image of 3D nanoclusters composed of small nanoparticles. The color box marks the clusters consisting of 5–10 nanoparticles, icosahedron (13 nanoparticles), and double icosahedron (19 nanoparticles). The scale is 50 nm. The amplificative characterization of these clusters is shown in (c) and (d) and are compared with the 3D model. The scale corresponds to 20 nm. (e) SEM images of nanoclusters composed of large silver nanospheres. The scale is 100 nm.
Nanoparticles are often assembled on the substrate by capillary action. By dropping the nanoparticle solution onto the substrate, a large amount of aggregate structure is produced by capillary action during its simple drying process [220,221]. This technique has been widely used in SERS research and it also provides some help for LSPR-based studies of dimers, trimers, and other aggregates [222–225]. Recently, our research group has found a new 3D hot spot matrix structure, each of the two adjacent particles can produce a hot spot in the 3D pace during the evaporation of silver sol on the silicon wafer (Figure 15.26) [226]. In a drop of silver sol, the particle size distribution and the variation of the particle spacing are significantly different from those in the dry state. According to the theoretical simulation, the cohension of the liquid promotes the close accumulation of the nanoparticles; the distance between the particles is not fixed but can be balanced by van der Waals gravitational force and electrostatic repulsion in a small range. The “trapped” and fixed-in-the-3D-space particles can form a large number of 3D hot spots. Both theoretical and experimental studies show that the 3D hot spots are predictable and there is a time variation in evaporation process. The 3D hot spot matrix not only produces giant Raman enhancement at least two orders of magnitude larger than that of the dried substrates but also provides the structural basis for trapping molecules. With a portable Raman spectrometer, the detection capability is also strikingly improved for various analytes with different nature. This 3D hot spot matrix overcomes the long-standing limitations of SERS for the ultrasensitive characterization of various substrates and analytes and promises to transform SERS into a practical analytical technique.

Figure 15.26 3D hot spot matrix of noble-metal sols generated in the water evaporation process. (a) Sketches representing (I) a droplet of Ag sols on a hydrophobic surface, (II) the adhesive-force-constructed closely packed particles in 3D space formed in the water-evaporation process, and (III) the aggregation and deposition of particles on the dried substrate, which quenches the 3D hot spot matrix. (b) (I) Interaction energy between citrate-Ag particles as a function of g at I = 0.6 mM, (II) total interaction energy as a function of g at different I, and (III) schematic of SERS measurements on a droplet. (c) Theoretical simulations. (I) The calculated number of hot spots as a function of the number of particles (left) and the average g (right) within an ideal spherical droplet of 1 µm in diameter, where a hot spot is defined by g ≤ 3 nm or g ≤ 2 nm, respectively, (II) distribution of electric-field intensity |E(r)|2 for 35 Ag particles of 50 nm in diameter dispersed randomly in a water drop of 1 µm in diameter, and (III) that for the randomly placed Ag aggregates. The color bar indicates normalized values for convenience of comparison.
15.5 Future Perspective
The hot spots have important influences on the sensitivity and repeatability of SERS detection. Most of the enhancement effects of SERS come from the contribution of hot spots. Ideal SERS substrates should satisfy three important requirements including sufficiently high enhancement factors, uniformity and reproducibility, and facile synthesis of the substrate [227,228]. Since it is now believed that the enhancement factors above 107 [227,228] or even above 106 [229] would be sufficient for single-molecule detection, the issues of uniformity, reproducibility, and practical synthesis become more important. The concept of 3D hot spots can further develop the potential of Raman technology's practical detection, to achieve the effective detection of major issues such as food safety and environmental safety; therefore, the preparation of 3D hot spot plasma structure as the base of Raman will become the development trend of Raman technology. Therefore, it is very important to prepare close and efficient 3D hot spot plasma structures for SERS detection.
However, bottom-up approaches face significant challenge of producing reproducible and uniform Raman response due to the difficulty in making uniform SERS active sites. On the other hand, top-down approaches could produce more uniform and reproducible nanostructures. But these techniques not only require time-consuming serial processing but also have limitation in fabricating versatile 3D nanostructures with interparticle gaps of less than 10 nm, which prevents to exploit the full advantages of surface plasmons. At present, the integration of top-down fabricated microstructures with self-assembly of NPs is an alternative approach to reliably engineer 3D SERS substrates with nanoscale interparticle gaps, although the most efficient 3D architecture as well as the optimal fabrication route remains to be uncovered. And an impartial comparison of structural variations in three dimentions on the SERS performance of a 3D SERS substrate has yet to be fully investigated. Nevertheless, the universality of these platforms in this chapter can extend the use of 3D SERS-active substrates to other fields by permitting the incorporation of either different materials, such as core–shell NPs and nanoshells, or different spectroscopic methods, such as surface-enhanced fluorescence, UV, or IR. By virtue of superhydrophobic surface, the concept of 3D architectures can allow easy access to manipulate the targeted molecules in 3D space. These 3D SERS-active substrates can enable electromagnetic characteristics that are not yet achievable in currently extant nanostructures as well as novel approaches to current technological challenges, such as high-throughput chemical and biological sensing.
References
- 1 Ko, H., Singamaneni, S., and Tsukruk, V.V. (2008) Nanostructured surfaces and assemblies as SERS media. Small, 4 (10), 1576–1599.
- 2 Banholzer, M.J. et al. (2008) Rationally designed nanostructures for surface-enhanced Raman spectroscopy. Chem. Soc. Rev., 37 (5), 885–897.
- 3 Lal, S. et al. (2008) Tailoring plasmonic substrates for surface enhanced spectroscopies. Chem. Soc. Rev., 37 (5), 898–911.
- 4 Kleinman, S.L. et al. (2013) Creating, characterizing, and controlling chemistry with SERS hot spots. Phys. Chem. Chem. Phys., 15 (1), 21–36.
- 5 Haynes, C.L. et al. (2005) Surface-enhanced Raman sensors: early history and the development of sensors for quantitative biowarfare agent and glucose detection. J. Raman Spectrosc., 36 (6–7), 471–484.
- 6 Fleischmann, M., Hendra, P., and McQuillan, A. (1974) Raman spectra of pyridine adsorbed at a silver electrode. Chem. Phys. Lett., 26 (2), 163–166.
- 7 Albrecht, M.G. and Creighton, J.A. (1977) Anomalously intense Raman spectra of pyridine at a silver electrode. J. Am. Chem. Soc., 99 (15), 5215–5217.
- 8 Cotton, T.M., Schultz, S.G., and van Duyne, R.P. (1980) Surface-enhanced resonance Raman scattering from cytochrome c and myoglobin adsorbed on a silver electrode. J. Am. Chem. Soc., 102 (27), 7960–7962.
- 9 Moskovits, M. (1985) Surface-enhanced spectroscopy. Rev. Mod. Phys., 57 (3), 783.
- 10 Sajanlal, P. et al. (2010) Electric field enhancement and concomitant Raman spectral effects at the edges of a nanometre-thin gold mesotriangle. J. Mater. Chem., 20 (11), 2108–2113.
- 11 Lee, Y.R., Kim, M.S., and Kwon, C.H. (2013) Surface-enhanced Raman scattering and DFT study of 4,4′-biphenyldithiol on silver surface. Bull. Korean Chem. Soc., 34 (2), 471.
- 12 Barnes, W.L., Dereux, A., and Ebbesen, T.W. (2003) Surface plasmon subwavelength optics. Nature, 424 (6950), 824–830.
- 13 Samanta, A. et al. (2014) Wavelength and shape dependent SERS study to develop ultrasensitive nanotags for imaging of cancer cells. RSC Adv., 4 (4), 12415–12421.
- 14 Haynes, C.L., McFarland, A.D., and Duyne, R.P.V. (2005) Surface-enhanced Raman spectroscopy. Anal. Chem., 77 (17), 338A–346A.
- 15 Yano, T.a. and Kawata, S. (2014) Tip-enhanced Raman spectroscopy (TERS) for nanoscale imaging and analysis, in Frontiers of Surface-Enhanced Raman Scattering: Single Nanoparticles and Single Cells, (eds Y. Ozaki, K. Kneipp, and R. Aroca), John Wiley & Sons, Ltd, Chichester, UK pp. 139–161.
- 16 Kelly, K.L. et al. (2003) The optical properties of metal nanoparticles: the influence of size, shape, and dielectric environment. J. Phys. Chem. B, 107 (3), 668–677.
- 17 Sun, M. et al. (2014) Plasmonic gradient effects on high vacuum tip-enhanced Raman spectroscopy. Adv. Opt. Mater., 2 (1), 74–80.
- 18 Dintinger, J. et al. (2005) Strong coupling between surface plasmon-polaritons and organic molecules in subwavelength hole arrays. Phys. Rev. B, 71 (3), 035424.
- 19 Willets, K.A. (2014) Super-resolution imaging of SERS hot spots. Chem. Soc. Rev., 43, 3854.
- 20 Lai, Y.H. et al. (2014) Mesostructured arrays of nanometer-spaced gold nanoparticles for ultrahigh number density of SERS hot spots. Adv. Func. Mater., 24, 2544–2552.
- 21 Campion, A. and Kambhampati, P. (1998) Surface-enhanced Raman scattering. Chem. Soc. Rev., 27 (4), 241–250.
- 22 Zhang, J. et al. (2005) Surface enhanced Raman scattering effects of silver colloids with different shapes. J. Phys. Chem. B, 109 (25), 12544–12548.
- 23 Le Ru, E., Meyer, M., and Etchegoin, P. (2006) Proof of single-molecule sensitivity in surface enhanced Raman scattering (SERS) by means of a two-analyte technique. J. Phys. Chem. B, 110 (4), 1944–1948.
- 24 Zhang, R. et al. (2013) Chemical mapping of a single molecule by plasmon-enhanced Raman scattering. Nature, 498 (7452), 82–86.
- 25 Kneipp, K. et al. (1997) Single molecule detection using surface-enhanced Raman scattering (SERS). Phys. Rev. Lett., 78 (9), 1667–1670.
- 26 Lorena, S.E. et al. (2014) Current attitudes regarding the use of perioperative analgesics in dogs and cats by Brazilian veterinarians. Vet. Anaesth. Analg., 41 (1), 82–89.
- 27 De Bleye, C. et al. (2014) Quantitative approaches based on surface-enhanced Raman scattering (SERS) and surface-enhanced Raman chemical imaging (SER-CI).
- 28 Kadam, U.S. and Schulz, B. (2014) Detection and quantification of alternative splice sites in Arabidopsis genes AtDCL2 and AtPTB2 with highly sensitive surface enhanced Raman spectroscopy (SERS) and gold nanoprobes. FEBS Lett.
- 29 Ma, P. et al. (2014) Highly sensitive SERS probe for mercury (II) using cyclodextrin-protected silver nanoparticles functionalized with methimazole. Microchim. Acta, 181 (9–10), 1–7.
- 30 Liu, W.J., Du, J.J., and Jing, C.Y. (2014) Surfce-enhanced Raman spectroscopy (SERS) for environmental pollutants detection: a review. Environ. Chem. (Chinese), 33 (2), 217–228.
- 31 Grasseschi, D. et al. (2010) Surface enhanced Raman scattering spot tests: a new insight on Feigl's analysis using gold nanoparticles. Anal. Chem., 82 (22), 9146–9149.
- 32 Zamarion, V.M. et al. (2008) Ultrasensitive SERS nanoprobes for hazardous metal ions based on trimercaptotriazine-modified gold nanoparticles. Inorg. Chem., 47 (8), 2934–2936.
- 33 Li, F. et al. (2013) Ultrasensitive and selective detection of copper (II) and mercury (II) ions by dye-coded silver nanoparticle-based SERS probes. Biosens. Bioelectron., 39 (1), 82–87.
- 34 Ren, W., Zhu, C.Z., and Wang, E.K. (2012) Enhanced sensitivity of a direct SERS technique for Hg2+ detection based on the investigation of the interaction between silver nanoparticles and mercury ions. Nanoscale, 4 (19), 5902–5909.
- 35 Kang, T. et al. (2012) Single-step multiplex detection of toxic metal ions by Au nanowires-on-chip sensor using reporter elimination. Lab Chip, 12 (17), 3077–3081.
- 36 Senapati, T. et al. (2011) Highly selective SERS probe for Hg(II) detection using tryptophan-protected popcorn shaped gold nanoparticles. Chem. Commun., 47 (37), 10326–10328.
- 37 Szabo, L. et al. (2011) SERS approach for Zn(II) detection in contaminated soil. Cent. Eur. J. Chem., 9 (3), 410–414.
- 38 Du, J.J. and Jing, C.Y. (2011) Preparation of Fe3O4@Ag SERS substrate and its application in environmental Cr(VI) analysis. J. Colloid Interface Sci., 358 (1), 54–61.
- 39 Yuan, Y.X. et al. (2007) Surface enhanced Raman spectroscopic readout on heavy metal ions based on surface self assembly. J. Raman Spectrosc., 38 (10), 1280–1287.
- 40 Tsoutsi, D. et al. (2013) Simultaneous SERS detection of copper and cobalt at ultratrace levels. Nanoscale, 5 (13), 5841–5846.
- 41 Ruan, C.M. et al. (2007) Surface-enhanced Raman spectroscopy for uranium detection and analysis in environmental samples. Anal. Chim. Acta, 605 (1), 80–86.
- 42 Gajaraj, S. et al. (2013) Quantitative detection of nitrate in water and wastewater by surface-enhanced Raman spectroscopy. Environ. Monit. Assess., 185 (7), 5673–5681.
- 43 Freye, C.E. et al. (2013) Surface enhanced Raman scattering imaging of developed thin-layer chromatography plates. Anal. Chem., 85 (8), 3991–3998.
- 44 Ye, Y.J. et al. (2012) Sensitive and selective SERS probe for trivalent chromium detection using citrate attached gold nanoparticles. Nanoscale, 4 (20), 6442–6448.
- 45 Xiao, J. et al. (2012) Surface-enhanced Raman scattering for quantitative analysis of perchlorate using poly(diallyldimethylammonium chloride) capped gold nanoparticles. Appl. Spectrosc., 66 (9), 1027–1033.
- 46 Gu, B.H., Ruan, C.M., and Wang, W. (2009) Perchlorate detection at nanomolar concentrations by surface-enhanced Raman scattering. Appl. Spectrosc., 63 (1), 98–102.
- 47 Senapati, D. et al. (2011) A label-free gold-nanoparticle-based SERS assay for direct cyanide detection at the parts-per-trillion level. Chem. Eur. J., 17 (30), 8445–8451.
- 48 Li, J.L. et al. (2011) Highly sensitive SERS detection of As3+ ions in aqueous media using glutathione functionalized silver nanoparticles. ACS Appl. Mater. Interfaces, 3 (10), 3936–3941.
- 49 Xu, Z.H. et al. (2012) Effect of bonding interactions between arsenate and silver nanofilm on surface-enhanced Raman scattering sensitivity. J. Phys. Chem. C, 116 (1), 325–329.
- 50 Xu, W. et al. (2013) Large-scale uniform Ag-NW tip array with enriched sub-10-nm gaps as SERS substrate for rapid determination of trace PCB77. Appl. Surf. Sci., 271, 125–130.
- 51 Tang, H.B. et al. (2012) Arrays of cone-shaped ZnO nanorods decorated with Ag nanoparticles as 3D surface-enhanced Raman scattering substrates for rapid detection of trace polychlorinated biphenyls. Adv. Funct. Mater., 22 (1), 218–224.
- 52 Hu, X.Y. et al. (2012) Large-scale homogeneously distributed Ag-NPs with sub-10 nm gaps assembled on a two-layered honeycomb-like TiO2 film as sensitive and reproducible SERS substrates. Nanotechnology, 23 (38), 385705.
- 53 Zhu, C.H. et al. (2011) Au hierarchical micro/nanotower arrays and their improved SERS effect by Ag nanoparticle decoration. Cryst. Growth Des., 11 (3), 748–752.
- 54 Huang, Z.L. et al. (2013) Large-area Ag nanorod array substrates for SERS: AAO template-assisted fabrication, functionalization, and application in detection PCBs. J. Raman Spectrosc., 44 (2), 240–246.
- 55 Zhu, C.H. et al. (2012) Large-scale well-separated Ag nanosheet-assembled micro-hemispheres modified with HS-beta-CD as effective SERS substrates for trace detection of PCBs. J. Mater. Chem., 22 (5), 2271–2278.
- 56 Zhou, Q. et al. (2010) Rapid recognition of isomers of monochlorobiphenyls at trace levels by surface-enhanced Raman scattering using Ag nanorods as a substrate. Nano Res., 3 (6), 423–428.
- 57 Yuan, J.P. et al. (2012) Synthesis of a beta-cyclodextrin-modified Ag film by the galvanic displacement on copper foil for SERS detection of PCBs. J. Colloid Interface Sci., 365 (1), 122–126.
- 58 Zhou, Q. et al. (2011) Rapid detection of polychlorinated biphenyls at trace levels in real environmental samples by surface-enhanced Raman scattering. Biosensors, 11 (11), 10851–10858.
- 59 Bantz, K.C. and Haynes, C.L. (2009) Surface-enhanced Raman scattering detection and discrimination of polychlorinated biphenyls. Vib. Spectrosc., 50 (1), 29–35.
- 60 Shi, X.F. et al. (2012) An improved self-assembly gold colloid film as surface-enhanced Raman substrate for detection of trace-level polycyclic aromatic hydrocarbons in aqueous solution. J. Raman Spectrosc., 43 (10), 1354–1359.
- 61 Mueller, M. et al. (2012) Large-area organization of pNIPAM-coated nanostars as SERS platforms for polycyclic aromatic hydrocarbons sensing in gas phase. Langmuir, 28 (24), 9168–9173.
- 62 Kwon, Y.H. et al. (2012) Application of calixarene to high active surface-enhanced Raman scattering (SERS) substrates suitable for in situ detection of polycyclic aromatic hydrocarbons (PAHs) in seawater. J. Raman Spectrosc., 43 (8), 1003–1009.
- 63 Kwon, Y.H. et al. (2012) Influence of surface plasmon resonance wavelength on SERS activity of naturally grown silver nanoparticle ensemble. J. Raman Spectrosc., 43 (10), 1385–1391.
- 64 Xie, Y.F. et al. (2011) Selective SERS detection of each polycyclic aromatic hydrocarbon (PAH) in a mixture of five kinds of PAHs. J. Raman Spectrosc., 42 (5), 945–950.
- 65 Lopez-Tocon, I. et al. (2011) Multicomponent direct detection of polycyclic aromatic hydrocarbons by surface-enhanced Raman spectroscopy using silver nanoparticles functionalized with the viologen host lucigenin. Anal. Chem., 83 (7), 2518–2525.
- 66 Du, J.J. and Jing, C.Y. (2011) Preparation of thiol modified Fe3O4@Ag magnetic SERS probe for PAHs detection and identification. J. Phys. Chem. C, 115 (36), 17829–17835.
- 67 Yang, M. et al. (2010) SERS-active gold lace nanoshells with built-in hotspots. Nano Lett., 10 (10), 4013–4019.
- 68 Xie, Y.F. et al. (2010) Sensing of polycyclic aromatic hydrocarbons with cyclodextrin inclusion complexes on silver nanoparticles by surface-enhanced Raman scattering. Analyst, 135 (6), 1389–1394.
- 69 Leyton, P. et al. (2008) Humic acids as molecular assemblers in the surface-enhanced Raman scattering detection of polycyclic aromatic hydrocarbons. Vib. Spectrosc., 46 (2), 77–81.
- 70 Qu, L.L. et al. (2013) Humic acids-based one-step fabrication of SERS substrates for detection of polycyclic aromatic hydrocarbons. Analyst, 138 (5), 1523–1528.
- 71 Shi, X.F. et al. (2013) Trace analysis of polycyclic aromatic hydrocarbons using calixarene layered gold colloid film as substrates for surface-enhanced Raman scattering. J. Raman Spectrosc., 44 (1), 41–46.
- 72 Kasera, S. et al. (2012) Quantitative SERS using the sequestration of small molecules inside precise plasmonic nanoconstructs. Nano Lett., 12 (11), 5924–5928.
- 73 Ossig, R. et al. (2013) SERS signal response and SERS/SERDS spectra of fluoranthene in water on naturally grown Ag nanoparticle ensembles. J. Raman Spectrosc., 44 (5), 717–722.
- 74 Jiang, X.H. et al. (2012) Silver nanoparticle aggregates on copper foil for reliable quantitative SERS analysis of polycyclic aromatic hydrocarbons with a portable Raman spectrometer. Analyst, 137 (17), 3995–4000.
- 75 Peron, O. et al. (2011) Quantitative SERS sensors for environmental analysis of naphthalene. Analyst, 136 (5), 1018–1022.
- 76 Guerrini, L. et al. (2009) Sensing polycyclic aromatic hydrocarbons with dithiocarbamate-functionalized Ag nanoparticles by surface-enhanced Raman scattering. Anal. Chem., 81 (3), 953–960.
- 77 Guerrini, L. et al. (2009) Nanosensors based on viologen functionalized silver nanoparticles: few molecules surface. Enhanced Raman spectroscopy detection of polycyclic aromatic hydrocarbons in interparticle hot spots. Anal. Chem., 81 (4), 1418–1425.
- 78 Zhang, L.L., Jiang, C.L., and Zhang, Z.P. (2013) Graphene oxide embedded sandwich nanostructures for enhanced Raman readout and their applications in pesticide monitoring. Nanoscale, 5 (9), 3773–3779.
- 79 Zheng, X. et al. (2012) High performance Au/Ag core/shell bipyramids for determination of thiram based on surface-enhanced Raman scattering. J. Raman Spectrosc., 43 (10), 1374–1380.
- 80 Saute, B. et al. (2012) Gold nanorods as surface enhanced Raman spectroscopy substrates for sensitive and selective detection of ultra-low levels of dithiocarbamate pesticides. Analyst, 137 (21), 5082–5087.
- 81 Liu, B.H. et al. (2012) Shell thickness-dependent Raman enhancement for rapid identification and detection of pesticide residues at fruit peels. Anal. Chem., 84 (1), 255–261.
- 82 Yazdi, S.H. and White, I.M. (2013) Multiplexed detection of aquaculture fungicides using a pump-free optofluidic SERS microsystem. Anlysist., 138 (1), 100–103.
- 83 Li, X.H. et al. (2010) Multifunctional Au-coated TiO2 nanotube arrays as recyclable SERS substrates for multifold organic pollutants detection. Adv. Funct. Mater., 20 (17), 2815–2824.
- 84 Yang, L.B. et al. (2012) Clean and reproducible SERS substrates for high sensitive detection by solid phase synthesis and fabrication of Ag-coated Fe3O4 microspheres. J. Raman Spectrosc., 43 (7), 848–856.
- 85 Wu, L.N., Wang, Z.J., and Shen, B.Z. (2013) Large-scale gold nanoparticle superlattice and its SERS properties for the quantitative detection of toxic carbaryl. Nanoscale, 5 (12), 5274–5278.
- 86 Liu, B. et al. (2013) Detection of pesticides in fruits by surface-enhanced Raman spectroscopy coupled with gold nanostructures. Food Bioprocess Technol., 6 (3), 710–718.
- 87 Fodjo, E.K. et al. (2012) Cu@Ag/beta-AgVO3 as a SERS substrate for the trace level detection of carbamate pesticides. Anal. Met., 4 (11), 3785–3791.
- 88 Tang, H.R. et al. (2012) Determination of tricyclazole content in paddy rice by surface enhanced Raman spectroscopy. J. Food Sci., 77 (5), T105–T109.
- 89 Gao, R. et al. (2010) Highly sensitive trace analysis of paraquat using a surface-enhanced Raman scattering microdroplet sensor. Anal. Chim. Acta, 681 (1–2), 87–91.
- 90 Bonora, S. et al. (1040) Raman and SERS study on atrazine, prometryn and simetryn triazine herbicides. J. Mol. Struct., 2013, 139–148.
- 91 Carrillo-Carrion, C. et al. (2012) Determination of pesticides by capillary chromatography and SERS detection using a novel silver-quantum dots “sponge” nanocomposite. J. Chromatogr. A, 1225, 55–61.
- 92 Yu, W.W. and White, I.M. (2012) A simple filter-based approach to surface enhanced Raman spectroscopy for trace chemical detection. Analyst, 137 (5), 1168–1173.
- 93 Fathi, F. et al. (2012) Studies of the interaction of two organophosphonates with nanostructured silver surfaces. Analyst, 137 (19), 4448–4453.
- 94 Jia, J.L. et al. (2012) High quality gold nanorods and nanospheres for surface-enhanced Raman scattering detection of 2,4-dichlorophenoxyacetic acid. Nanotechnology, 23 (49), 495710.
- 95 Costa, J.C.S. et al. (2012) Surface-enhanced Raman spectroscopy studies of organophosphorous model molecules and pesticides. Phys. Chem. Chem. Phys., 14 (45), 15645–15651.
- 96 Guerrini, L. et al. (2011) Surface-enhanced Raman spectra of dimethoate and omethoate. J. Raman Spectrosc., 42 (5), 980–985.
- 97 Guerrini, L. et al. (2009) Self-assembly of alpha, omega-aliphatic diamines on Ag nanoparticles as an effective localized surface plasmon nanosensor based in interparticle hot spots. Phys. Chem. Chem. Phys., 11 (34), 7363–7371.
- 98 Guerrini, L. et al. (2008) Functionalization of Ag nanoparticles with the bis-acridinium lucigenin as a chemical assembler in the detection of persistent organic pollutants by surface-enhanced Raman scattering. Anal. Chim. Acta, 624 (2), 286–293.
- 99 Baigorri, R. et al. (2008) Optical enhancing properties of anisotropic gold nanoplates prepared with different fractions of a natural humic substance. Chem. Mater., 20 (4), 1516–1521.
- 100 Song, W. et al. (2011) Raman and surface-enhanced Raman scattering of chlorophenols. Chem. Res. Chinese Univ., 27 (5), 854–856.
- 101 Xue, J.Q. et al. (2013) Surface-imprinted core–shell Au nanoparticles for selective detection of bisphenol A based on surface-enhanced Raman scattering. Anal. Chim. Acta, 777, 57–62.
- 102 Ding, Q.Q. et al. (2012) Speedy and surfactant-free in situ synthesis of nickel/Ag nanocomposites for reproducible SERS substrates. J. Mater. Chem., 22 (37), 19932–19939.
- 103 Farcau, C. et al. (2013) Reliable plasmonic substrates for bioanalytical SERS applications easily prepared by convective assembly of gold nanocolloids. Analyst, 138 (2), 546–552.
- 104 Contreras-Caceres, R. et al. (2011) Multifunctional microgel magnetic/optical traps for SERS ultradetection. Langmuir, 27 (8), 4520–4525.
- 105 Piorek, B.D. et al. (2007) Free-surface microfluidic control of surface-enhanced Raman spectroscopy for the optimized detection of airborne molecules. Proc. Natl. Acad. Sci. USA, 104 (48), 18898–18901.
- 106 Qu, L.L. et al. (2013) Fabrication of bimetallic microfluidic surface-enhanced Raman scattering sensors on paper by screen printing. Anal. Chim. Acta, 792, 86–92.
- 107 An, Q. et al. (2012) Silver-coated magnetite-carbon core–shell microspheres as substrate-enhanced SERS probes for detection of trace persistent organic pollutants. Nanoscale, 4 (16), 5210–5216.
- 108 Zhou, X. et al. (2013) SERS and OWGS detection of dynamic trapping molecular TNT based on a functional self-assembly Au monolayer film. Analyst, 138 (6), 1858–1864.
- 109 Mahmoud, K.A. and Zourob, M. (2013) Fe3O4/Au nanoparticles/lignin modified microspheres as effectual surface enhanced Raman scattering (SERS) substrates for highly selective and sensitive detection of 2,4,6-trinitrotoluene (TNT). Analyst, 138 (9), 2712–2719.
- 110 Liu, M.M. and Chen, W. (2013) Graphene nanosheets-supported Ag nanoparticles for ultrasensitive detection of TNT by surface-enhanced Raman spectroscopy. Biosens. Bioelectron., 46, 68–73.
- 111 Wen, C.Y. et al. (2013) Bi-functional ZnO-RGO-Au substrate: photocatalysts for degrading pollutants and SERS substrates for real-time monitoring. Chem. Commun., 49 (29), 3049–3051.
- 112 Demeritte, T. et al. (2012) Highly efficient SERS substrate for direct detection of explosive TNT using popcorn-shaped gold nanoparticle-functionalized SWCNT hybrid. Analyst, 137 (21), 5041–5045.
- 113 Liu, H. et al. (2013) Cetylpyridinium chloride activated trinitrotoluene explosive lights up robust and ultrahigh surface-enhanced resonance Raman scattering in a silver sol. Chem. Eur. J., 19 (27), 8789–8796.
- 114 Botti, S. et al. (2013) Trace level detection and identification of nitro-based explosives by surface-enhanced Raman spectroscopy. J. Raman Spectrosc., 44 (3), 463–468.
- 115 Xu, Z.H. et al. (2011) Surface-enhanced Raman scattering spectroscopy of explosive 2,4-dinitroanisole using modified silver nanoparticles. Langmuir, 27 (22), 13773–13779.
- 116 Li, Y.T. et al. (2013) Rapid and sensitive in-situ detection of polar antibiotics in water using a disposable Ag-graphene sensor based on electrophoretic preconcentration and surface-enhanced Raman spectroscopy. Biosens. Bioelectron., 43, 94–100.
- 117 Lin, W.C. et al. (2009) SERS study of tetrodotoxin (TTX) by using silver nanoparticle arrays. Plasmonics, 4 (2), 187–192.
- 118 Pearman, W.F. et al. (2008) Characterization of the Ag mediated surface-enhanced Raman spectroscopy of saxitoxin. Appl. Spectrosc., 62 (7), 727–732.
- 119 Wang, H.H. et al. (2011) Transparent Raman-enhancing substrates for microbiological monitoring and in situ pollutant detection. Nanotechnology, 22 (38), 385702.
- 120 Sinha, G., Depero, L.E., and Alessandri, I. (2011) Recyclable SERS substrates based on Au-coated ZnO nanorods. ACS Appl. Mater. Interfaces, 3 (7), 2557–2563.
- 121 Ye, Y.J. et al. (2013) Sea-urchin-like Fe3O4@C@Ag particles: an efficient SERS substrate for detection of organic pollutants. Nanoscale, 5 (13), 5887–5895.
- 122 Baia, L. et al. (2011) Efficient dual functionality of highly porous nanocomposites based on TiO2 and noble metal particles. J. Alloys Compd., 509 (6), 2672–2678.
- 123 Cecchini, M.P. et al. (2013) Self-assembled nanoparticle arrays for multiphase trace analyte detection. Nat. Mater., 12 (2), 165–171.
- 124 Chen, L.M. and Liu, Y.N. (2011) Surface-enhanced Raman detection of melamine on silver-nanoparticle-decorated silver/carbon nanospheres: effect of metal ions. ACS Appl. Mater. Interfaces, 3 (8), 3091–3096.
- 125 Olavarria-Fullerton, J. et al. (2011) Surface-enhanced Raman scattering (SERS) characterization of trace organoarsenic antimicrobials using silver/polydimethylsiloxane nanocomposites. Appl. Spectrosc., 65 (4), 423–428.
- 126 Li, P. et al. (2014) Polystyrene/Ag nanoparticles as dynamic surface-enhanced Raman spectroscopy substrates for sensitive detection of organophosphorus pesticides. Talanta, 127, 269–275.
- 127 Lin, D. et al. (2012) Ultrasensitive optical detection of trinitrotoluene by ethylenediamine-capped gold nanoparticles. Anal. Chim. Acta, 744 (0), 92–98.
- 128 Dong, R. et al. (2015) Detection and direct readout of drugs in human urine using dynamic surface-enhanced Raman spectroscopy and support vector machines. Anal. Chem., 87 (5), 2937–2944.
- 129 Han, Z. et al. (2015) Portable kit for identification and detection of drugs in human urine using surface-enhanced Raman spectroscopy. Anal. Chem., 87 (18), 9500–9506.
- 130 Han, Z. et al. (2015) Three-dimensional surface-enhanced Raman scattering hotspots in spherical colloidal superstructure for identification and detection of drugs in human urine. Anal. Chem., 87 (9), 4821–4828.
- 131 Ma, Y. et al. (2016) Surface-enhanced Raman spectroscopy on liquid interfacial nanoparticle arrays for multiplex detecting drugs in urine. Anal. Chem., 88 (16), 8145–8151.
- 132 El-Boubbou, K., Gruden, C., and Huang, X. (2007) Magnetic glyco-nanoparticles: a unique tool for rapid pathogen detection, decontamination, and strain differentiation. J. Am. Chem. Soc., 129 (44), 13392.
- 133 Lu, C.W. et al. (2007) Bifunctional magnetic silica nanoparticles for highly efficient human stem cell labeling. Nano Lett., 7 (1), 149–154.
- 134 Lin, P.C. et al. (2007) Functionalized magnetic nanoparticles for small-molecule isolation, identification, and quantification. Anal. Chem., 79 (9), 3401–3408.
- 135 Lee, I.S. et al. (2006) Ni/NiO core/shell nanoparticles for selective binding and magnetic separation of histidine-tagged proteins. J. Am. Chem. Soc., 128 (33), 10658–10659.
- 136 Li, S. et. al. (2007) Rapid, room-temperature synthesis of amorphous selenium/protein composites using Capsicum annuum L extract. Nanotechnology, 18 (40), 405101.
- 137 Fu, A.H. et al. (2009) Protein-functionalized synthetic antiferromagnetic nanoparticles for biomolecule detection and magnetic manipulation. Angew. Chem. Int. Ed., 48 (9), 1620–1624.
- 138 Schlücker, S. (2014) Surface-enhanced Raman spectroscopy: concepts and chemical applications. Angew. Chem. Int. Ed., 53, 4756–4795.
- 139 Tong, L., Xu, H., and Käll, M. (2014) Nanogaps for SERS applications. MRS Bull., 39 (02), 163–168.
- 140 Gellner, M. et al. (2011) 3D self-assembled plasmonic superstructures of gold nanospheres: synthesis and characterization at the single-particle level. Small, 7 (24), 3445–3451.
- 141 Liang, Y.-C., Liao, W.-K., and Deng, X.-S. (2014) Synthesis and substantially enhanced gas sensing sensitivity of homogeneously nanoscale Pd- and Au-particle decorated ZnO nanostructures. J. Alloys Compd., 599, 87–92.
- 142 Li, S. et al. (2014) Preparation and properties of noble metal core/shell nanostructures prepared by excimer laser ablation in liquid solutions. J. Laser Appl., 26 (2), 022001.
- 143 Luo, X. and Yang, S. (2014) Mesoporous nano/micro noble metal particles: synthesis and applications. Nanoscale., 6 (9), 4438–4457.
- 144 Feng, Y., Liu, H., and Yang, J. (2014) Bimetallic nanodendrites via selective overgrowth of noble metals on multiply twinned Au seeds. J. Mater. Chem. A, 2 (17), 6130–6137.
- 145 Hu, Y. et al. (2014) Continuous-flow mass production of silicon nanowires via substrate-enhanced metal-catalyzed electroless etching of silicon with dissolved oxygen as an oxidant. Sci. Rep., 4, 3667.
- 146 Sun, Z. et al. (2014) A versatile designed synthesis of magnetically separable nano-catalysts with well-defined core–shell nanostructures. J. Mater. Chem. A, 2 (17), 6071–6074.
- 147 Gudjonson, H. et al. (2014) Accounting for inhomogeneous broadening in nano-optics by electromagnetic modeling based on Monte Carlo methods. Proc. Natl. Acad. Sci., 111 (6), 201323392.
- 148 Fan, J.A. et al. (2010) Self-assembled plasmonic nanoparticle clusters. Supramol. Sci., 328 (5982), 1135–1138.
- 149 Chuntonov, L. and Haran, G. (2011) Effect of symmetry breaking on the mode structure of trimeric plasmonic molecules. J. Phys. Chem. C, 115 (40), 19488–19495.
- 150 Zhang, Y.X. and Zeng, H.C. (2006) Template-free parallel one-dimensional assembly of gold nanoparticles. J. Phys. Chem. B, 110 (34), 16812–16815.
- 151 Maye, M.M. et al. (2010) Switching binary states of nanoparticle superlattices and dimer clusters by DNA strands. Nat. Nanotechnol., 5 (2), 116–120.
- 152 Giersig, M. and Mulvaney, P. (1993) Preparation of ordered colloid monolayers by electrophoretic deposition. Langmuir, 9 (12), 3408–3413.
- 153 Murray, C., Kagan, C., and Bawendi, M. (1995) Self-organization of CdSe nanocrystallites into three-dimensional quantum dot superlattices. Supramol. Sci., 270 (5240), 1335–1338.
- 154 Lal, S., Link, S., and Halas, N.J. (2007) Nano-optics from sensing to waveguiding. Nat. Photonics, 1 (11), 641–648.
- 155 Smith, D.R. et al. (2000) Composite medium with simultaneously negative permeability and permittivity. Phys. Rev. Lett., 84 (18), 4184.
- 156 Fu, Y. et al. (2012) All-optical logic gates based on nanoscale plasmonic slot waveguides. Nano Lett., 12 (11), 5784–5790.
- 157 Wei, H. et al. (2011) Cascaded logic gates in nanophotonic plasmon networks. Nat. Commun., 2, 387.
- 158 Pacifici, D., Lezec, H.J., and Atwater, H.A. (2007) All-optical modulation by plasmonic excitation of CdSe quantum dots. Nat. Photonics, 1 (7), 402–406.
- 159 Xie, W. et al. (2011) Synthesis of bifunctional Au/Pt/Au core/shell nanoraspberries for in situ SERS monitoring of platinum-catalyzed reactions. J. Am. Chem. Soc., 133 (48), 19302–19305.
- 160 Weitz, D. and Oliveria, M. (1984) Fractal structures formed by kinetic aggregation of aqueous gold colloids. Phys. Rev. Lett., 52 (16), 1433.
- 161 Luk'yanchuk, B. et al. (2010) The Fano resonance in plasmonic nanostructures and metamaterials. Nat. Mater., 9 (9), 707–715.
- 162 Anker, J.N. et al. (2008) Biosensing with plasmonic nanosensors. Nat. Mater., 7 (6), 442–453.
- 163 Alexander, K.D. et al. (2010) Tunable SERS in gold nanorod dimers through strain control on an elastomeric substrate. Nano Lett., 10 (11), 4488–4493.
- 164 Bhattacharjee, S. et al. (2013) Cytotoxicity of surface-functionalized silicon and germanium nanoparticles: the dominant role of surface charges. Nanoscale, 5 (11), 4870–4883.
- 165 Wurtz, G.A. et al. (2007) Molecular plasmonics with tunable exciton-plasmon coupling strength in J-aggregate hybridized Au nanorod assemblies. Nano Lett., 7 (5), 1297–1303.
- 166 Liu, H. et al. (2013) Capillarity-constructed reversible hot spots for molecular trapping inside silver nanorod arrays light up ultrahigh SERS enhancement. Chem. Sci., 4 (9), 3490–3496.
- 167 Chen, H. et al. (2008) Using direct nanoimprinting of ferroelectric films to prepare devices exhibiting bi-directionally tunable surface plasmon resonances. Nanotechnology, 19 (43), 435304.
- 168 Khlebtsov, B.N. et al. (2008) Coupled plasmon resonances in monolayers of metal nanoparticles and nanoshells. Phys. Rev. B, 77 (3), 035440.
- 169 Li, T., Hu, W., and Zhu, D. (2010) Nanogap electrodes. Adv. Mater., 22 (2), 286–300.
- 170 Zhang, Z.-L. et al. (2014) High-vacuum tip enhanced Raman spectroscopy. Front. Phys., 9 (1), 17–24.
- 171 Zhou, J. et al. (2013) Design and theoretical analyses of tip–insulator–metal structure with bottom-up light illumination: formations of elongated symmetrical plasmonic hot spot at sub-10 nm resolution. Plasmonics, 8 (2), 1073–1078.
- 172 Rycenga, M. et al. (2011) Controlling the synthesis and assembly of silver nanostructures for plasmonic applications. Chem. Rev., 111 (6), 3669–3712.
- 173 Zhang, H. et al. (2010) Facile synthesis of five-fold twinned, starfish-like rhodium nanocrystals by eliminating oxidative etching with a chloride-free precursor. Angew. Chem. Int. Ed., 49 (31), 5296–5300.
- 174 Kumar, P.S. et al. (2008) High-yield synthesis and optical response of gold nanostars. Nanotechnology, 19 (1), 015606.
- 175 Wei, W. et al. (2009) Surprisingly long-range surface-enhanced Raman scattering (SERS) on Au-Ni multisegmented nanowires. Angew. Chem. Int. Ed., 48 (23), 4210–4212.
- 176 Xia, Y. et al. (2009) Shape-controlled synthesis of metal nanocrystals: simple chemistry meets complex physics? Angew. Chem. Int. Ed., 48 (1), 60–103.
- 177 Sau, T.K. and Rogach, A.L. (2010) Nonspherical noble metal nanoparticles: colloid-chemical synthesis and morphology control. Adv. Mater., 22 (16), 1781–1804.
- 178 Alvarez-Puebla, R.A. et al. (2009) Au@pNIPAM colloids as molecular traps for surface-enhanced, spectroscopic, ultra-sensitive analysis. Angew. Chem. Int. Ed., 48 (1), 138–143.
- 179 Zhu, J., Li, J.-J., and Zhao, J.-W. (2013) Frequency-dependent polarization properties of local electric field in gold–dielectric multi-nanoshells. Plasmonics, 8 (2), 417–424.
- 180 Wang, H., Kundu, J., and Halas, N.J. (2007) Plasmonic nanoshell arrays combine surface-enhanced vibrational spectroscopies on a single substrate. Angew. Chem. Int. Ed., 46 (47), 9040–9044.
- 181 Ma, Y. et al. (2010) Au@ Ag core– shell nanocubes with finely tuned and well-controlled sizes, shell thicknesses, and optical properties. ACS Nano, 4 (11), 6725–6734.
- 182 Rao, Y. et al. (2011) Growth-sensitive 3D ordered gold nanoshells precursor composite arrays as SERS nanoprobes for assessing hydrogen peroxide scavenging activity. Analyst, 136 (4), 769–774.
- 183 Lu, L. et al. (2004) Fabrication of core–shell Au-Pt nanoparticle film and its potential application as catalysis and SERS substrate. J. Mater. Chem., 14 (6), 1005–1009.
- 184 Wang, L. and Yamauchi, Y. (2010) Autoprogrammed synthesis of triple-layered Au@ Pd@ Pt core– shell nanoparticles consisting of a Au@ Pd bimetallic core and nanoporous Pt shell. J. Am. Chem. Soc., 132 (39), 13636–13638.
- 185 Lu, C.-L. et al. (2010) Au nanocube-directed fabrication of Au– Pd core– shell nanocrystals with tetrahexahedral, concave octahedral, and octahedral structures and their electrocatalytic activity. J. Am. Chem. Soc., 132 (41), 14546–14553.
- 186 Banik, M. et al. (2012) Surface-enhanced Raman trajectories on a nano-dumbbell: transition from field to charge transfer plasmons as the spheres fuse. ACS Nano, 6 (11), 10343–10354.
- 187 Lee, J.-H. et al. (2012) Tuning and maximizing the single-molecule surface-enhanced Raman scattering from DNA-tethered nanodumbbells. ACS Nano, 6 (11), 9574–9584.
- 188 Kleinman, S.L. et al. (2013) Creating, characterizing, and controlling chemistry with SERS hot spots. Phys. Chem. Chem. Phys., 15 (1), 21–36.
- 189 Pedano, M.L. et al. (2010) Periodic electric field enhancement along gold rods with nanogaps. Angew. Chem. Int. Ed., 49 (1), 78–82.
- 190 Zheng, G., Qin, L., and Mirkin, C.A. (2008) Spectroscopically enhancing electrical nanotraps. Angew. Chem. Int. Ed., 47 (10), 1938–1941.
- 191 Camargo, P.H. et al. (2009) Isolating and probing the hot spot formed between two silver nanocubes. Angew. Chem. Int. Ed., 48 (12), 2180–2184.
- 192 Barrow, S.J. et al. (2011) Surface plasmon resonances in strongly coupled gold nanosphere chains from monomer to hexamer. Nano Lett., 11 (10), 4180–4187.
- 193 Rycenga, M. et al. (2011) Generation of hot spots with silver nanocubes for single-molecule detection by surface-enhanced Raman scattering. Angew. Chem. Int. Ed., 50 (24), 5473–5477.
- 194 Wei, H. et al. (2008) Polarization dependence of surface-enhanced Raman scattering in gold nanoparticle– nanowire systems. Nano Lett., 8 (8), 2497–2502.
- 195 Schade, N.B. et al. (2013) Tetrahedral colloidal clusters from random parking of bidisperse spheres. Phys. Rev. Lett., 110 (14), 148303.
- 196 Zhu, Z. et al. (2011) Superstructures and SERS properties of gold nanocrystals with different shapes. Angew. Chem. Int. Ed., 50 (7), 1593–1596.
- 197 Walker, D.A. et al. (2010) Self-assembly of nanotriangle superlattices facilitated by repulsive electrostatic interactions. Angew. Chem. Int. Ed., 49 (38), 6760–6763.
- 198 Hung, A.M. et al. (2010) Large-area spatially ordered arrays of gold nanoparticles directed by lithographically confined DNA origami. Nat. Nanotechnol., 5 (2), 121–126.
- 199 Ruan, C. et al. (2007) Controlled fabrication of nanopillar arrays as active substrates for surface-enhanced Raman spectroscopy. Langmuir, 23 (10), 5757–5760.
- 200 Yang, Y. et al. (2012) Controlled fabrication of silver nanoneedles array for SERS and their application in rapid detection of narcotics. Nanoscale, 4 (8), 2663–2669.
- 201 Kim, K. et al. (2013) Interfacial liquid-state surface-enhanced Raman spectroscopy. Nat. Commun., 4, 2182.
- 202 Edel, J.B., Kornyshev, A.A., and Urbakh, M. (2013) Self-assembly of nanoparticle arrays for use as mirrors, sensors, and antennas. ACS Nano, 7 (11), 9526–9532.
- 203 Cecchini, M.P. et al. (2013) Self-assembled nanoparticle arrays for multiphase trace analyte detection. Nat. Mater., 12 (2), 165–171.
- 204 Petukhova, A. et al. (2012) Standing arrays of gold nanorods end-tethered with polymer ligands. Small, 8 (5), 731–737.
- 205 Alvarez-Puebla, R.A. et al. (2011) Gold nanorods 3D-supercrystals as surface enhanced Raman scattering spectroscopy substrates for the rapid detection of scrambled prions. Proc. Natl. Acad. Sci. USA, 108 (20), 8157–8161.
- 206 Lee, S.J., Morrill, A.R., and Moskovits, M. (2006) Hot spots in silver nanowire bundles for surface-enhanced Raman spectroscopy. J. Am. Chem. Soc., 128 (7), 2200–2201.
- 207 Yang, L. et al. (2010) Ultrasensitive SERS detection of TNT by imprinting molecular recognition using a new type of stable substrate. Chem. Eur. J., 16 (42), 12683–12693.
- 208 Nykypanchuk, D. et al. (2008) DNA-guided crystallization of colloidal nanoparticles. Nature, 451 (7178), 549–552.
- 209 Mastroianni, A.J., Claridge, S.A., and Alivisatos, A.P. (2009) Pyramidal and chiral groupings of gold nanocrystals assembled using DNA scaffolds. J. Am. Chem. Soc., 131 (24), 8455–8459.
- 210 Barrow, S.J. et al. (2012) The surface plasmon modes of self-assembled gold nanocrystals. Nat. Commun., 3, 1275.
- 211 Pazos-Perez, N. et al. (2012) Organized plasmonic clusters with high coordination number and extraordinary enhancement in surface-enhanced raman scattering (SERS). Angew. Chem. Int. Ed., 51 (51), 12688–12693.
- 212 Von White, G., Provost, M.G., and Kitchens, C.L. (2012) Fractionation of surface-modified gold nanorods using gas-expanded liquids. Ind. Eng. Chem. Res., 51 (14), 5181–5189.
- 213 Surugau, N. and Urban, P.L. (2009) Electrophoretic methods for separation of nanoparticles. J. Sep. Sci., 32 (11), 1889–1906.
- 214 Cao, X. et al. (2011) Preparation of novel 3D graphene networks for supercapacitor applications. Small, 7 (22), 3163–3168.
- 215 Urban, A.S. et al. (2013) Three-dimensional plasmonic nanoclusters. Nano Lett., 13 (9), 4399–4403.
- 216 Manoharan, V.N., Elsesser, M.T., and Pine, D.J. (2003) Dense packing and symmetry in small clusters of microspheres. Supramol. Sci., 301 (5632), 483–487.
- 217 Crocker, J.C. (2010) Turning away from high symmetry. Supramol. Sci., 327 (5965), 535–536.
- 218 Abdullin, T.I. et al. (2009) Pluronic block copolymer-mediated interactions of organic compounds with noble metal nanoparticles for SERS analysis. Langmuir, 26 (7), 5153–5159.
- 219 Alvarez-Puebla, R.A. and Liz-Marzán, L.M. (2012) Traps and cages for universal SERS detection. Chem. Soc. Rev., 41 (1), 43–51.
- 220 Lu, L.-Q. et al. (2012) Hydrophobic teflon films as concentrators for single-molecule SERS detection. J. Mater. Chem., 22 (39), 20986–20990.
- 221 Zhou, H. et al. (2011) Trinitrotoluene explosive lights up ultrahigh Raman scattering of nonresonant molecule on a top-closed silver nanotube array. Anal. Chem., 83 (18), 6913–6917.
- 222 Wilson, A.J. and Willets, K.A. (2014) Visualizing site-specific redox potentials on the surface of plasmonic nanoparticle aggregates with super-localization SERS microscopy. Nano Lett., 14 (2), 939–945.
- 223 Cassar, R.N. et al. (2014) Synthesis of size tunable monodispersed silver nanoparticles and the effect of size on SERS enhancement. Vib. Spectrosc., 71 (3), 41–46.
- 224 Pissuwan, D. et al. (2014) Distribution of label free cationic polymer-coated gold nanorods in live macrophage cells reveals formation of groups of intracellular SERS signals of probe nanoparticles. RSC Adv., 4 (11), 5536–5541.
- 225 Lin, X. et al. (2014) Rapid and simple detection of sodium thiocyanate in milk using surface-enhanced Raman spectroscopy based on silver aggregates. J. Raman Spectrosc., 45 (2), 162–176.
- 226 Liu, H. et al. (2014) Three-dimensional and time-ordered SERS hotspot matrix. J. Am. Chem. Soc.
- 227 Jung, K. et al. (2014) Hotspot-engineered 3D multipetal flower assemblies for surface-enhanced Raman spectroscopy. Adv. Mater. doi: 10.1002/adma.201401004
- 228 Nam, J.M. et al. (2011) Highly uniform and reproducible surface-enhanced Raman scattering from DNA-tailorable nanoparticles with 1-nm interior gap. Nat. Nanotechnol., 6 (7), 452–460.
- 229 Park, W.H. and Kim, Z.H. (2010) Charge transfer enhancement in the SERS of a single molecule. Nano Lett., 10 (10), 4040–4048.
