
chapter 1
Historical Review
In this chapter we very briefly sketch out four of the main ideas that led to the development of quantum theory. These are Planck’s solution to the blackbody radiation problem, Einstein’s explanation of the photoelectric effect, the Bohr model of the atom, and the de Broglie wavelength of material particles.
CHAPTER OBJECTIVES
In this chapter, you will
• Learn about the origins of quantum theory
• Learn about blackbody radiation and how the ultraviolet catastrophe was solved with Planck’s formula
• Learn about the photoelectric effect and how it led Einstein to postulate that light could be described in terms of a stream of particles
• The Bohr theory of the atom
• Wave-particle duality and de Broglie’s hypothesis
Blackbody Radiation and Planck’s Formula
A blackbody is an object that is a perfect absorber of radiation. In the ideal case, it absorbs all the light that falls on it, no light is reflected by it, and no light passes through it. While such an object doesn’t reflect any light, if we heat up a blackbody, it can radiate light. The study of this radiated light generated a bit of controversy in the late 19th century. Specifically, there was a problem explaining the spectrum of the thermal radiation emitted from a blackbody.
Simply put, a spectrum is a plot, at a fixed temperature, of the amount of light emitted at each wavelength (or, if we choose, at each frequency). A plot of the amount of light (specifically, the energy density) emitted versus wavelength looks something like the curve in Fig. 1-1.

FIGURE 1-1 • Spectral irradiance profile for a blackbody at temperature T = 5400 K. The shaded area corresponds to the spectrum of visible light.
As the temperature is increased, more light is emitted at higher frequencies. This means that the peak in this plot would shift more to the right. Classical theory was not able to explain the high-frequency behavior of blackbody emission. Spectra like the one shown here were found experimentally.
An attempt to explain these results using classical theory was codified in the Rayleigh-Jeans formula, which is an expression that attempts to give us the energy density u(n, T) of radiation in the cavity, where v is frequency and T is the temperature. Qualitatively, it is formed as a product of two quantities:
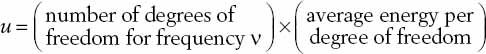
Using classical physics, the average energy per degree of freedom can be calculated in the following way. Let’s call the energy E, Boltzmann’s constant k, and the temperature T. The average energy E is given by

Both of these integrals are easy to do. The integral in the denominator can be done immediately by using the substitution y = −E/(kT):
In the numerator, we use integration by parts. The integration by parts formula is
We let u = E, then du = dE. Using the previous result, dv = e−E/kT and so v = −kTe−E/kT. We then have
Now
And so the evaluation at the upper limit of kTe−E/kT E vanishes. Also, as E → 0, this term clearly vanishes, and so
And so we find that
The other term in the Rayleigh-Jeans formula is the number of degrees of freedom per frequency. Using classical theory, the number of degrees of freedom was found to be
All together the Rayleigh-Jeans formula tells us that the energy density is

You can see from this formula that as v gets large, it’s going to blow sky-high. Worse—if you integrate over all frequencies to get the total energy per unit volume, you will get infinity. The formula only works at low frequencies. Obviously this is not what is observed experimentally, and the prediction that the energy density at high frequencies would go to infinity became known as the “ultraviolet catastrophe” (since ultraviolet is light of a high frequency).
Planck fixed the problem by examining the calculation of E, a calculation that gave us the simple result of kT and seems so reasonable if you’ve studied thermodynamics. Consider the implicit assumption that is expressed by the way the formula is calculated. The formula is computed using integration, which means that it has been assumed that energy exchange is continuous. What if, instead, only certain fixed values of energy exchange were allowed?
Planck’s Radical Assumption
A practical blackbody is made of a metallic cavity with a small hole through which radiation can escape. Planck made the assumption that an exchange of energy between the electrons in the wall of the cavity and electromagnetic radiation can only occur in discrete amounts. This assumption has an immediate mathematical consequence. The first consequence of this assumption is that the integrals above turn into discrete sums. So when we calculate the average energy per degree of freedom, we must change all integrals to sums:
The second important piece of data that Planck told us was that energy comes in little bundles, which we will call the basic “quantum of energy.” According to Planck, the basic quantum of energy ε is given by
ε = hv
where v is the frequency of the radiation. Furthermore, energy can only come in amounts that are integer multiples of the basic quantum:
E = nε = nhv, n = 0, 1, 2, …
The constant h = 6.62×10−34 (jouleseconds) is called Planck’s constant. It is frequently convenient to use the symbol  = h/2π.
= h/2π.
Incorporating this assumption with the change from integrals to discrete sums, we now have
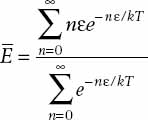
To evaluate this formula, we recall that a geometric series sums to

where | r | < 1. Returning to the formula for average energy, we look at the denominator. We set a = 1 and let r = e−nε/kT. Clearly r is always less than 1, and so

In the exercises, you will show that the other term we have can be written as

These results allow us to rewrite the average energy in the following way:

We can put this in a more familiar form by letting ε = hv and doing some algebraic manipulation:

To get the complete Planck formula for blackbody radiation, we just substitute this term for kT in the Rayleigh-Jeans law. The exponential in the denominator decays much faster than v2. The net result is that the average energy term cuts off any energy density at high frequencies. The complete Planck formula for the energy density of blackbody radiation is

The Photoelectric Effect
In 1905, Einstein made the radical proposal that light consisted of particles called photons. Each photon carries energy
E = hv
and linear momentum
where v and λ are the frequency and wavelength, respectively, of the light wave. Using the relation c = vλ, where c is the speed of light in a vacuum, we can rewrite the momentum of a photon as
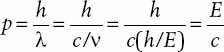
Einstein made this proposal to account for several unexplained features associated with the photoelectric effect. This is a process that involves the emission of electrons from a metal when light strikes the surface. The maximum energy of the emitted electrons is found to be
qV0 = Emax
where q is the charge of the electron and V0 is the stopping potential. Experiment shows that
1. When light strikes a metal surface, a current flows instantaneously, even for very weak light.
2. At a fixed frequency, the strength of the current is directly proportional to the intensity of the light.
3. The stopping potential V0, and therefore the maximum energy of the emitted electrons, depends only on the frequency of the light and the type of metal used.
4. Each metal has a characteristic threshold frequency v0 such that
5. The constant h is found to be the same for all metals, and not surprisingly it turns out to be the same constant used by Planck in his black-body derivation.
Each of these experimental ideas can be explained by accepting that light is made up of particles. For example, consider observation 2, which is easy to explain in the photon picture. If the intensity of the light beam is increased, then the number of photons is increased in turn and there are more photons striking the metal surface. Specifically, suppose we double the intensity of the light. Twice as many photons strike the metal surface and knock out twice as many electrons, making a current that is twice as strong. In the wave picture, however, you would expect that increasing the intensity would increase the energy of the electrons, and not their number. Classical wave theory disagrees with this observation.
The ideas of Planck and Einstein can be summarized by the Planck-Einstein relations.
DEFINITION: The Planck-Einstein Relations
The Planck-Einstein relations connect the particlelike properties of energy and momentum to wavelike properties of frequency and wave vector k. Recalling that frequency v = ω/2π, we have

The Bohr Theory of the Atom
Light again took center stage in 1913 when Bohr worked out the basic structure of the hydrogen atom. He did this by considering the light that atoms emit.
The light emitted by isolated atoms takes the form of a discrete series of lines called spectral lines. It is found that these lines occur at specific frequencies for each type of atom. So a sodium atom has a different line spectrum than a hydrogen atom, and a helium atom has yet another spectrum. Think of a spectrum as the fingerprint of each element. It is found that atoms absorb light at specific, well-defined frequencies as well.
This tells us that like Planck’s blackbody oscillators, atoms can exchange energy only in fixed discrete amounts. Neils Bohr noticed this and proposed two radical ideas about the behavior of electrons in atoms.
Bohr Makes Two Key Assumptions about the Atom
1. An electron can only orbit the nucleus in such a way that the orbit is defined by the relationship
mvr = n n = 1, 2, …
n = 1, 2, …
where v is the velocity of the electron, r is the radius of the orbit, and m is the mass of the electron. The presence of n in the formula restricts the angular momentum of the electron to integer multiples of  , where the angular momentum is given by
, where the angular momentum is given by
L = n
2. Electrons only radiate during transitions between states. A transition from energy state Ei to energy state Ef is accompanied by the emission of a photon of energy:
hv = Ei − Ef
The Coulomb force between the positively charged nucleus and the negatively charged electron is what keeps the electrons in orbit. Setting this equal to the centrifugal force results in the following expressions for the velocity of the electron and the radius of the orbit. We label each quantity with subscript n to conform with assumption 1 above:
 (radius of orbit n)
(radius of orbit n)Derive the energy of an electron in the hydrogen atom, using Bohr’s formulas.
We start by recalling that
Total energy = kinetic energy + potential energy = T + V
For an electron moving in the Coulomb potential of a proton, the potential is just
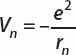
Using the formula for the radius of orbit n, this becomes

For the kinetic energy, we obtain
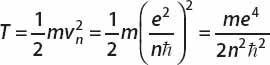
The total energy of an electron in orbit n is therefore
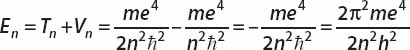
Derive a relation that predicts the frequencies of the line spectra of hydrogen.
Bohr proposed that the frequency of a photon emitted by an electron in the hydrogen atom was related to transitions of energy states as
hv = Ei −Ef
The energy of state n is

Therefore,

Putting this together with Bohr’s proposal, we find the frequency is

This formula can be used to predict the line spectra of hydrogen.
de Broglie’s Hypothesis
In 1923 Louis de Broglie proposed that the Planck-Einstein relations be extended to material particles. A particle with energy E is associated with a wave of frequency ω = E/ . In addition, momentum is related to the wave vector p =
. In addition, momentum is related to the wave vector p =  k. Applying these simple relations to material particles like electrons, de Broglie proposed that a material particle moving with momentum p has a wavelength
k. Applying these simple relations to material particles like electrons, de Broglie proposed that a material particle moving with momentum p has a wavelength

If a particle of mass m is moving with a nonrelativistic energy E, we can write

A thermal neutron has a speed v that corresponds to room temperature T = 300 K. What is the wavelength of a thermal neutron?
At temperature T the average energy is
where k is Boltzmann’s constant. By equating the kinetic energy to this quantity with T = 300 K, we can find the momentum of the neutron:

Using de Broglie’s relation, we obtain the wavelength of the thermal neutron:

Summary
In this chapter we learned how the resolution of several paradoxes in physics around the year 1900 led to the development of quantum theory. The first of these was the ultraviolet catastrophe for blackbodies which was resolved by Planck’s postulate that radiation was emitted and absorbed in individual packets he called “quanta.” This was followed by Einstein’s explanation of the photoelectric effect where he proposed that light could be viewed as a stream of particles called “photons.” Later, in order to explain the structure and behavior of atoms, Niels Bohr proposed that electrons could only occupy certain orbits around the nucleus with specific energy levels. The final piece of the puzzle in the early quantum theory was de Broglie’s hypothesis, which generalized the wave-particle duality of light to matter.
QUIZ
1. For the following definition

write the following series in terms of f′(ε):
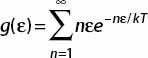
Then use the geometric series result to show that g can be written in the form
2. The lowest energy of an electron in the hydrogen atom occurs for n = 1 and is called the ground state. Show that the ground state energy is −13.6 eV.
3. Using the formula for quantized orbits, show that the ground state radius is 0.529 × 10−8 cm. This is known as the Bohr radius.
..................Content has been hidden....................
You can't read the all page of ebook, please click here login for view all page.
