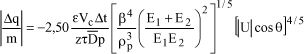Chapter 5
Electrification 1
5.1. Introduction
It is now well known that setting a charged body in motion (in the case of solid particles in a flowing gas) creates currents and electric potentials which can sometimes reach significant values.
All the research already undertaken confirms the important role played by the nature and surface state of the materials involved in the transfer and accumulation of charges. In the next section, we shall attempt to analyze the processes which cause these phenomena.
5.2. Electrification of solid bodies by separation/contact
The generation of charges between two bodies which have been in contact is the fruit of the process known as electrification, i.e. the separation of charges with an opposite sign, combined by displacement or transport. Next, we shall examine the electrification process.
Three types of electrification exist, contact/separation electrification, electrification by influence, electrification by corona effect.
5.2.1. The process
The goal of this section is to recall the process of electrification of two solids during their contact, then their separation and to understand the mechanisms which come into play in order to take all the necessary precautions from a practical point of view to obtain reproducible results.
The contact electrification process always appears at an interface. It is due to the different nature of the two constituents. In all cases, a transfer of ions, due to their physico-chemical difference, or a transfer of peripheral electrons due to the contact potential difference between the surfaces, is produced.
Thus, two large categories can be distinguished according to whether the charge transfer is made by electrons or by ions which flow from one material to the other. The transfer by electrons allows most of the electrification process which appears from contact between solids to be explained. Ionic theory is better suited to contacts between solids and liquids.
Let us take two different materials, and assume they have a perfectly smooth surface (Figure 5.1).
Before being in contact along this surface, the two materials are electrically neutral. When they are in contact, a charge transfer occurs, then again during their separation if the materials are sufficiently insulated (so that the total transferred charge does not relax through the last contact point); each of them conserves the same amount of electricity but with an opposite sign (see Figure 5.1). We shall look more closely at this process.
The outbreak of electrostatic charges on a given solid medium is often imputed to the process of electrification by friction. The first electrostatic experiments confirmed this: if we rub a glass stick with cat’s fur, for example. In reality, even when we simply place two bodies of different materials in contact and then separate them, we observe a separation of electrical charges (electrification) even though there has not been any friction (tangential motion from one contact surface to the other). In this case, the intensity of the process is always much less than that observed for objects rubbed against each other. Friction, however, does not add anything fundamental causing the electrification; it only amplifies the process which is already present in the simple contact between two neutral bodies of different materials.
Electrification by friction, often called triboelectrification, is in fact a derivative of contact electrification. Friction increases the contact surface and temperature. The deformations generated by the friction in a polymer material create vacant energy levels in which electrons from the contact surface can be captured. In general, friction favors the charge transfer process.
Electrification by impact derives from the two previous types. The impact adds additional parameters to the process, such as pressure.
By a misuse of language, all of these charge generation processes are often called triboelectrification.
We shall now develop a theory which will permit the origins of electrification (of electron transfer) resulting from the simple contact (without friction) of two bodies of a different nature to be understood. We have to look for an explanation for the outbreak of electrification in the electronic characteristics of the materials, and it is these that we shall now study, with the aid of theories stemming from quantum mechanics and the composition of atoms. Several cases have to be distinguished.
5.2.2. Charge transfer mechanism by the separation contact of two different conductors
The electrons in conductors (metals) are free; no force acts on them, and they form an “electron gas”. There are, however, at the surface of the metal, forces which prevent them from escaping and send them back inside (see Figure 5.2). The electrons in a metal are in a potential well [COE 93]. The kinetic energy of the electrons is lower than the Fermi energy level of the metal which would allow them to leave. An additional energy is required to get over the metal surface. This additional energy, called work function or extraction energy, is of a few electronvolts.
We can remove the electrons by several processes:
– by photoelectric effect;
– by electron beam (secondary emission);
– by cold emission, creating an electric field.
An electron could escape from the influence of the atom to which it belongs if it acquires a higher energy than the extraction energy. This extraction energy ![]() is the energy which a peripheral electron must acquire to escape from the influence of the atom (to become free). Two different metals have different Fermi levels (see Figure 5.3).
is the energy which a peripheral electron must acquire to escape from the influence of the atom (to become free). Two different metals have different Fermi levels (see Figure 5.3).
Extraction energy is an important characteristic of metal, and ranges between 4 and 5 eV for many metals. (1 eV is an energy unit equal to 1.6 10−19 joules).
In Figure 5.3 we have represented the work functions ![]() 1 and
1 and ![]() 2 of Metals 1 and 2. When the two metals are separated, no electron transfer can be made from one metal to the other. If, on the other hand, the two metals are in contact, we shall observe on one side of the interface an excess of positive charges and on the other side an excess of the same amount of negative charges (the interface obviously remained neutral as a whole). Indeed, when both metals are in contact, their respective surfaces are separated only by a few Angströms. Their energy levels then become equal through quantum tunneling. Consequently, a potential difference settles; this is the contact potential. In other words, the explanation of this transfer lies in the fact that, in this contact zone, the electrons of Metals 1 and 2 easily acquire a sufficient energy to escape from the influence of the metal to which they belong. The electrons of Metal 1, once freed from the influence of 1, could flow into 2 (there is contact), and vice versa. In this ceaseless motion of electrons between Metals 1 and 2, knowing that it is easier (less energy is required) for an electron 1 to escape 1 than for an electron 2 to get out of 2, the net overall results of the exchanges will show a higher number of electrons in 2 than in 1, hence polarization. Equilibrium will occur when the maximum energetic level occupied by an electron is identical in both materials. For this purpose we need a transfer of electrons from 1 to 2; therefore, there is an outbreak of a negative charge on 2 and a positive charge on 1 (see Figure 5.4).
2 of Metals 1 and 2. When the two metals are separated, no electron transfer can be made from one metal to the other. If, on the other hand, the two metals are in contact, we shall observe on one side of the interface an excess of positive charges and on the other side an excess of the same amount of negative charges (the interface obviously remained neutral as a whole). Indeed, when both metals are in contact, their respective surfaces are separated only by a few Angströms. Their energy levels then become equal through quantum tunneling. Consequently, a potential difference settles; this is the contact potential. In other words, the explanation of this transfer lies in the fact that, in this contact zone, the electrons of Metals 1 and 2 easily acquire a sufficient energy to escape from the influence of the metal to which they belong. The electrons of Metal 1, once freed from the influence of 1, could flow into 2 (there is contact), and vice versa. In this ceaseless motion of electrons between Metals 1 and 2, knowing that it is easier (less energy is required) for an electron 1 to escape 1 than for an electron 2 to get out of 2, the net overall results of the exchanges will show a higher number of electrons in 2 than in 1, hence polarization. Equilibrium will occur when the maximum energetic level occupied by an electron is identical in both materials. For this purpose we need a transfer of electrons from 1 to 2; therefore, there is an outbreak of a negative charge on 2 and a positive charge on 1 (see Figure 5.4).
In equilibrium, there is a potential difference ΔV between the two materials, equal to:
This is the contact potential difference (e is the charge of the electron).
When the contact breakdown is simultaneous at all points on the surface common to both materials, and if we assume that both metals remain insulated after separation from the ground, Metal 2 conserves the excess of electrons gained from Metal 1 and is negatively charged ( q2 <0), while 1 has lost electrons and is positively charged ( q1 <0). Harper [HAR 51] showed that the transferred charge is proportional to this potential difference ΔV:
where K is a factor which depends on the contact surface area and the experimental conditions.
Coste [COS 84] showed that this theory is also applicable to the case of metal/semi-conductor and semi-conductor/semi-conductor contacts.
5.2.3. Polymer-metal contact
Contact between metals and insulators is still very poorly analyzed, at the present time. Different viewpoints exist on the nature of charge carriers, which is due, on the one hand to the presence of impurities in insulators, and on the other hand, to the difficulty for a direct measurement of the potential difference [COE 93] between a metal and an insulator, when this latter has a resistivity higher than 1012Ω.m.
For these diverse reasons, current studies are restricted to polymer–metal contact and could give an analysis similar to the previous ones in certain atmospheric conditions (in the case of relatively weak humidities) and for certain polymers whose surface state densities are uniformly spread out.
5.2.4. Contact between two polymers
The analysis in this case is not made directly. The technique used consists of first undertaking two studies on the polymer–metal contact for both polymers, and then in deducing the polymer contact between them.
5.2.5. Triboelectric series
During contact between two materials, the charge transfer is of an electronic nature when one of the materials is a metal or a semi-conductor. The transferred charge generally increases as a function of the extraction work difference of the materials in contact; it is linear in certain cases of contact (two metals, semiconductor metal and polymer metal) and seems to have an exponential growth as a function of the apparent extraction works when both materials are polymers. In any case, the transfer is always made from the material with weak extraction energy to the material with strong extraction energy. In most of these cases, the only published results [LÜT 97] consist of triboelectric series (see Figure 5.5), i.e. a listing of the differents bodies with respect to one another concerning the charges they produce in contact. Unfortunately, these series are often subjected to caution and they depend heavily on the experimental conditions in which they have been made.
After separation, the charge present on both constituents depends on the ability of the produced charges to flow on the surface towards the last contact point. Indeed, as the bodies get separated, the mobile charges of an opposite sign present at the surface of both bodies tend to recombine. For two metals, the electrons in excess on a surface will tend to move back to the zone where they are lacking (the other surface). The more the charges are mobile at the surface of the materials, the easier this could happen. This is the case for electrons in metals; it is why the total charge after separation of two metals is always weak. On the other hand, when one of the materials (or both) are insulators (polymers, for example), the charges migrate with great difficultly to the last contact point between the surfaces because they are trapped on (or inside) the insulating material. It is this ability of insulators to trap the charge excess transferred to their surfaces for a long period of time which causes certain electrification issues.
Industrial electrification generally come from phenomena involving friction. The collected charges are then more numerous than those obtained by simple contact. This is explained because surface friction favors contact and improves its quality, thus increasing the transfer of electrons. Most investigations made on this subject show that the transferred charge increases with pressure between both materials in contact and with friction speed. On the other hand, it decreases with the roughness of the surfaces. Nevertheless, the process causing the phenomenon remains the same: electrification by contact separation.
5.3. Electrification of solid particles
Let us assume a solid particle in displacement within a solid pipe. At instant t the particle has never been in contact with the solid wall, and is then electrically neutral, just like the solid wall. After the particle hits the solid wall, there is a charge on the particle and an opposite charge on the solid wall; this is the charge transferred during the impact (see Figure 5.6).
A lot of research has been undertaken on the electrification of particles during their impact with walls. We will now examine some of this.
5.3.1. Theoretical work by Masuda et al.
Masuda et al. [MAS 78] proposed equations to estimate the charge exchanged during an impact. They considered two types of contact, elastic and plastic contact, and proposed an expression for each case.
For an elastic impact:
where ε is the permittivity of the medium in which the contact occurs (air, in most cases), z the thickness of the equivalent capacitor during contact, At the duration of contact, τ the time constant of the equivalent capacitor (much greater than the contact time), Vc the contact potential, ![]() the diameter of the sphere having the same volume as the particle, ρp the volumic mass of the particles,
the diameter of the sphere having the same volume as the particle, ρp the volumic mass of the particles, ![]() (where ro is the radius of curvature of the contact surface of the particle), E1 Young’s modulus of the plate, E2 that of the particle,
(where ro is the radius of curvature of the contact surface of the particle), E1 Young’s modulus of the plate, E2 that of the particle, ![]() the velocity modulus and 6 the impact angle with respect to the normal of the plate.
the velocity modulus and 6 the impact angle with respect to the normal of the plate.
In the case of a plastic impact:
Cole and Baum [COL 69] established a relationship for when a particle undergoes several successive collisions with a wall. We can then establish the following relationship:
where kc is a coefficient, qt is the charge transferred at the moment of the impact nc, qs is the saturation charge of the particle (i.e. after an infinite number of impacts) and qi is the initial charge of the particle (that it had before impact nc).
5.3.2. Experimental work by Touchard et al. [TOU 91]
Touchard et al. analyzed the impact charge generated on copper, black polyethylene and yellow polyethylene according to:
– the speed U of the particles at the moment of the impact;
– their original charges Q0;
– the angle θ under which they hit the surface of a material;
– the nature of the material;
– the diameters Dp of the particles.
The physical quantity measured was the electric current generated by impact of a steady flux of particles on a material; this measurement allowed the impact charge of the particles to be obtained, by unit mass. In the case of copper, the material directly constituted the electrode. In the case of polyethylene, the electrodes clung to one side of the material (that opposed to the impact); thus, in this case, the current measured was capacitive.
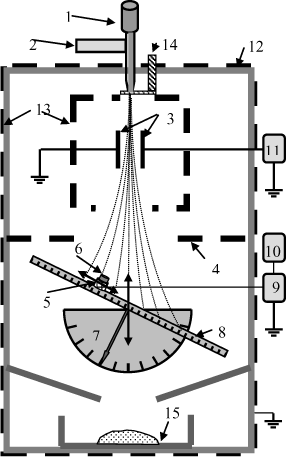
The device used is represented in Figure 5.7. It is inside a cage (12) surrounded by a tank (13). An injection pipette (1) provided with a vibrator (2) and a tap (14) permits particles to be dropped by gravity. By means of friction on the injection pipette, the particles are ejected with an initial charge Q0.
These particles then pass through an electric field perpendicular to their trajectory. This field is supplied by a high tension supply (11) connected to two plane electrodes (3). The whole assembly is inside a tank. The field plays the role of charged particle deflector. Thus, coming out of the field, the particles have several trajectories depending on their initial charge. An electromagnetic screen (4) separates the deflector device from the target. A target electrode (6) is situated on a PTFE support (5) which is mounted on a support permitting adjustment of the angle of the target (7) and, at the same time, its position on a tilted ruler (8) vertically.
The current due to the impact charge is measured with the aid of a Keithley 610C electrometer (9) connected to a data acquisition system (10). The particles are then collected in a receptacle (15).
5.3.2.1. Experimental device
Three target electrodes were used, one in copper, one in black polyethylene (with 2% black carbon) with electrical resistivity of ρ ≈ 0,8 10−15 Ωm and one in yellow polyethylene (without black carbon), with resistivity ρ ≈ 2 10−15 Ωm. The particles were sodocalcic glass microbeads with different diameters (with electrical resistivity ρ ≈ 1010 Ωm and volumic mass ![]() ). Depending on their original charge Q0 the particles were deviated differently by the electric field existing between the electrodes (3). Particles of 4 different sizes were used: 500 µm, 340 µm, 200 µm, 110 µm.
). Depending on their original charge Q0 the particles were deviated differently by the electric field existing between the electrodes (3). Particles of 4 different sizes were used: 500 µm, 340 µm, 200 µm, 110 µm.
5.3.2.2. Results
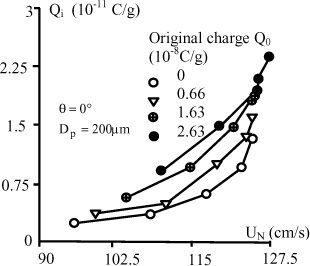
5.3.2.2.1. Influence of the impact angle
For these experiments, we determined the positions of the target in a way that the original charge of the particles which hit it take 4 different values: 0C/g, 0,66 10−8C/ g, 1,6310−8C/g and 2,6310−8C / g (a charge with no origin corresponds to the target positioned vertically below the pipette). We also position the target in a way that the modulus ![]() of the impact speed is constant; it must also be noted that the positions of the three zones traversed by the particles (2 cm before the field, 4 cm in the field and 11 cm after the field) are practically on the same horizontal axis. The modulus of the impact speed obviously corresponds to normal impact components UN varying according to the impact angle.
of the impact speed is constant; it must also be noted that the positions of the three zones traversed by the particles (2 cm before the field, 4 cm in the field and 11 cm after the field) are practically on the same horizontal axis. The modulus of the impact speed obviously corresponds to normal impact components UN varying according to the impact angle.
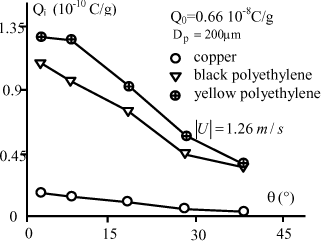
For the three targets, the impact charge strongly decreases as a function of the impact angle. An example is given in Figure 5.8 for the copper target. This decrease is greater than the reduction of the normal component of speed. The reason of this fast decrease is probably due to rebounds more frequent on the target when the impact angle is small.
5.3.2.2.2. Influence of the impact speed on the normal component
The evolution of the charge by unit mass generated as a function of the speed of the particles is similar for the three targets; an example is presented for the black polyethylene target in Figure 5.9. As predicted by Masuda et al., we see that for a given sample and for particles having the same original charge, the charge generated by unit mass increases with the normal component of the speed UN. This increase is, however, more important than that predicted by Masuda et al.
5.3.2.2.3. Influence of the size of the particles
The samples of particles used for this study have respective average diameters of 110, 200, 340, and 500 µm. The results are presented in Figure 5.10. The normal component of the impact speed is obviously different because air friction is dependent on the size of the particles. Although the impact speed is less for smaller particles, we see that the impact charge decreases with the dimensions of the particles.
5.3.2.2.4. Comparison of results obtained on the three targets
We see in Figure 5.11 a comparison for the three targets. It is clear that the impact charge is weakest for copper and highest for yellow polyethylene.
5.3.2.2.5. Evolution of the total charge of a particle according to the number of impacts
It is possible to calculate the different parameters which play a part in the expression proposed by Cole et al. [COL 69] from these experimental results. We can then deduce the evolution of a particle charge according to the number of impacts on a given target. In the case of a particle of diameter 110µm and for a copper target, we see, in Figure 5.12, this evolution for 3 different original charges. In the three cases, the final charge of the particle tends towards the same value. Its total charge decreases when the particle is initially charged to a value greater than the limit value, whereas it increases when it is initially charged to a weaker value.
5.4. Conclusion
The charge generated by separation contact still remains difficult to predict for most materials. Consequently, the charge generated by the impact of particles on a target and, even more certainly, that generated in the flows of dusty gases or pneumatic transports, can only be obtained experimentally in most cases (see bibliography, section 5.5).
5.5. Bibliography
[ART 97] ARTANA G., TOUCHARD G., MORIN M.F., “Contribution to the analysis of the flow electrification process of powders in pneumatic conveyers”, Journal of Electrostatics, vol. 40 & 41, p. 277–282, 1997.
[BAI 01] Bailey A., “The charging of insulator surfaces”, Journal of Electrostatics, vol. 51– 52, p. 82–90, 2001.
[BEN 87] BENMADDA M., TOUCHARD G., BORZEIX J., “Etude de la charge engendrée par le choc d’une particle solide sur une plaque métallique”, Rev. Phys. App., 22, p. 1071–1074, 1987.
[BOU 91] BOUDALAA M., TOUCHARD G., “Mesure du débit massique de particles dans un transporteur pneumatique travaillant en écoulement en phase dense”, R.G.E., vol. 8, p. 10–14, 1991.
[CAS 95] CASTLE G.S.P., SCHEIN L.B., “General model of sphere-sphere insulator contact electrification”, Journal of Electrostatics, vol. 36, no. 2, p 165–174, 1995.
[COE 93] COELHO R., ALADENIZE B., Les diélectriques – Propriétés électriques des matériaux isolants, Hermès, Paris, 1993.
[COL 69] COLE B.N., BAUM M.R., MOBBS F.R. “An investigation of electrostatic charging effect in high-speed gas–solid pipe flows”, Proc. Instn. Mech. Engrs. 184 (3c), p. 77–83, 1969–1970.
[COS 84] COSTE J., “Idées actuelles sur l’origine des charges électrostatiques – Phénomènes de contact et de frottement”, J.E. SEE Electrostatique, 1984.
[CRO 87] CROSS J.A., Electrostatics, Principles, Problems and Applications, Adam Hilger IOP Publishing, 1987.
[GLO 88] GLOR M., Electrostatic Hazards in Powder handling, Research Studies Press, 1988.
[GRO 01] GROSS F.B., GREK S.B., CALLE C.I., LEE R.U., “JSC Mars-1 Martian Regolith stimulant particle charging experiments in a low pressure environment”, Journal of Electrostatics vol. 53, p. 257–266, 2001.
[HAR 51] HARPER W.R., “The volta effect as a cause of static electrification”, Proc. Roy. Soc. A., 205, p. 83–103, 1951.
[JON 91] JONES T..B., KING J.L., Powder Handling and Electrostatics, Lewis Publishers, 1991.
[KWE 94] KWETKUS B.A., “Contact electrification of coal and minerals”, Journal of Electrostatics, vol. 32, no. 3, p. 271–276, 1994.
[LEO 02] LEON-ESCALANTE S.G., TOUCHARD G., DOMINGUEZ G., “Electrification study in dielectric material fluidized beds for different fluidization regimes”, Proc. IEEE-CEIDP, p. 694–697, 2002.
[LÜT 97] LÜTTGEN G., WILSON N., Electrostatic Hazards, Butterworth-Heinemann, 1997.
[MAS 78] MASUDA H., KOICHI I., IINOYA Y., “Electrification of particles by impact on inclined metal plates”, AIChe Journal, vol. 24, no. 6, p. 950–956, 1978.
[MEH 05] MEHRANI P., BI H.T., GRACE J.R., “Electrostatic charge generation in gas–solid fluidized beds”, Journal of electrostatics, vol. 63, p. 165–173, 2005.
[SCH 99] SCHEIN L.B., “Recent advances in our understanding of toner charging”, Journal of Electrostatics, vol. 46, no. 1, p. 29–36, 1999.
[SUG 95] SUGIHARA M., DASCALESCU L., TOUCHARD G., ROMAT H., GRIMAUD P.O., WATANABE S., “Charge generated by impact of balls on a metallic wall”, Journal of Electrostatics, vol. 35, p. 125–132, 1995.
[TOU 91] TOUCHARD G., ZERGHOUNI A., WATANABE S., “Evolution de la charge électrique d’une particle heurtant une paroi solide”, J. Phys. III France 1, p. 1233–1241, 1991.
[TOU 90] TOUCHARD G., WU Z.H., WANG W.Q., NASANI, A., WATANABE S., “Electrostatic charge generation in pneumatic conveyers”, Materials Science, vol. 16, no. 4, p. 5–10, 1990.
[TOU 99] TOUCHARD G., ARTANA G., PUTIER F., “Electrical characteristics and mechanical behaviour of powders of the animal feeding industry”, Journal of Electrostatics 47 (1–2), p. 3–12, 1999.
1 Chapter written by Gérard TOUCHARD.